Abstract
Protein concentrates were prepared from underutilized mrigal (Cirrhinus mrigala) roe to produce value added by-products for food applications. Chemical composition and physicochemical properties of protein concentrates prepared from mrigal (Cirrhinus mrigala) roes were determined. The effects of pH and salt concentration on protein solubility were investigated. The protein content of the concentrates was found to be 75, and 91%, respectively for dehydrated and defatted powders. The maximum protein solubility was observed at pH 12, while minimum protein solubility was observed at pH 5, for both dehydrated and defatted protein concentrates. Salt concentration (0.1 to 1 M Nacl) significantly affected the solubility of protein concentrates. The mineral analysis revealed substantial amounts of iron and phosphorus. The emulsifying capacities of dehydrated and defatted protein concentrates were noted as 5.9, and 7.1 ml/g protein, respectively. SDS-PAGE analysis of fresh, dehydrated and defatted roe proteins has revealed the presence of major protein with a molecular weight (MW) of 97 kDa. Addition of defatted fish egg protein concentrate to pasta preparations had improved taste and texture. The results indicated that protein concentrates from underutilized mrigal fish egg may be useful for preparing protein rich food supplements.
Keywords: Mrigal, Cirrhinus mrigala, Fish egg, Protein concentrates, Protein solubility, SDS-PAGE, Sensory analysis
Introduction
India is the second largest producer of fresh water fish next only to Japan. Fresh water fish accounted for 55% of total production of 6.57 MMT of fish and carps contribute more than 87% of the total production in India. Fish eggs (roes) are available in large quantities at low prices during the spawning period, which accounts for about one-third of the weight of total fish. The quality and quantity of roe proteins differ depending on the variety of fish (Mukhopadhyay et al. 1981). Mrigal (Cirrhinus mrigala) commonly known as the Indian major fresh water carp is found to be a good source of protein. Mrigal roe is an abundantly available raw material which goes underutilized source in India and other Asian countries, due to inherent problems related to undesirable odour, texture and highly perishable nature. The roe forms a unique protein source for application in food products to combat protein calorie malnutrition. Fish egg biomass is one of the most valuable food by-products from fishery sources for human consumption (Eun et al. 1994).
Literature on proximate composition and other biochemical characteristics is available on fish roes (Bledsoe et al. 2003, Al-Holy and Rasco 2006). The chemical composition of roes of 18 fish species was reported and the protein content was estimated to be in the range of 11.5–30.2% (Iwasaki and Harada 1985). Protein content of roes from 22 sturgeon species was reported to be in the range of 26.2 to 31.1% (Wirth et al. 2000). Protein content in the range of 39.1–43%, fat content in the range of 14.1–14.8% and elements like Fe, Cu, and Zn were reported in dried and salted roes of hake (Merluccius merluccis) and ling (Molva molva L) (Rodrigo et al. 1998). Fish egg protein concentrate (FEPC) and pickle from rohu (Labeo rohita) roe (Balaswamy et al. 2007, Balaswamy et al. 2010) and protein hydrolysates from mrigal roe (Chalamaiah et al. 2010) were prepared and their proximate composition and functional properties have been studied. Fish protein concentrate from whole fish prepared by isopropyl alcohol extraction has been described, which exhibited a decreased emulsifying capacity and solubility (Sikorski and Naczk 1981).
Solubility is one of the most important characteristic of proteins which have pronounced effects on functional properties such as emulsification and foaming capacities. The solubility of proteins is also influenced by NaCl concentration wherein ion-dipole interactions between water, polar groups on bio-molecules and salt occur. Hydrophobicity plays an important positive role in determining emulsifying properties. Literature is available in abundance on fish proteins as a whole but only meager information is available on the functional properties of fish roes. The objective of the present research work was to prepare protein concentrates from mrigal carp roe, study their chemical composition and functional properties and their application in pasta preparation.
Materials and methods
Materials
Fresh roes from mrigal were collected from a local fish market (Hyderabad, India) immediately after processing and brought to the laboratory and stored at 4 °C for <2 h before carrying out experimental work. The chemicals and solvents used in the present study were of analytical grade and procured from Sd. fine Chemicals, Mumbai, India.
Preparation of protein concentrates from fish roes
Fish roes were separated from blood vessels and skeins and homogenized using a high speed mixer/grinder (Kenstar, Pune, India). The resulting homogenate was dried at 48 ± 2 °C for 8 h in a cabinet tray dryer (Chemida, Mumbai, India) and ground to powder using a high speed mixer-grinder and sieved to pass through a 180 μ mesh to obtain dehydrated fish egg protein concentrate. Defatted fish egg protein concentrate was prepared from fish egg homogenate according to the method of Sikorski and Naczk (1981) with slight modification. The fish egg homogenate was mixed with isopropyl alcohol at a ratio of 1:4 (w/v), and kept at room temperature over night. The supernatant was removed and the residue was further washed thrice with isopropyl alcohol. The defatted meal was dried in a cabinet tray dryer at 45 ± 2 °C for 4 h, ground to powder using a high speed mixer and sieved to pass through a 180 μ mesh to obtain defatted fish roe protein concentrate. Protein concentrates were stored in the refrigerator (5 °C) until used.
Proximate composition of protein concentrates
Moisture, total ash, crude fat and crude fibre contents were analyzed by standard procedures (AOAC 1965) and nitrogen content was determined using micro Kjeldahl method and protein content was computed as%N × 6.25. Carbohydrates were determined as per the method of Rodrigo et al. (1998). The energy values for protein concentrates were calculated in kilocalories/100 g. Calcium was analysed by the gravimetric method, while iron and phosphorus contents were quantified by spectrophotometric methods (AOAC 1965).
Functional properties
Water holding capacity (WHC) was measured by the method of Johnson (1970) and expressed as grams of water absorbed per 100 g. Oil holding capacity (OHC) was determined according to the method described by Beuchat (1977) and expressed as grams of oil absorbed per 100 g. Foaming capacity (FC) and foam stability (FS) were estimated as per the method of Lawhon et al. (1972). Emulsification capacity (EC) was determined following the method of Gagne and Adambounou (1994) at room temperature.
Protein solubility
The protein solubility was determined by the method of Dev et al. (1986). One gram sample was extracted in water, 0.1, 0.5 and 1.0 M NaCl solutions and pH were adjusted to 2–12 using 0.5 M hydrochloric acid (HCl) or 0.5 M sodium hydroxide (NaOH). After extraction, the suspensions were centrifuged at 4,500 g at 4 °C for 30 min, and the supernatant was estimated for protein content using the Biuret method (Weichselbaum 1946) and percent solubility calculated.
Protein precipitation
Protein precipitation studies were carried out according to Taher et al. (1981). 10 g sample was dispersed in distilled water and the pH was adjusted to 10 using 0.5 M NaOH and stirred using magnetic stirrer for the optimum extraction time (30 min). The suspension was centrifuged at 4,500 g at 4 °C for 30 min to remove undissolved proteins. The clear supernatant (20 ml) was taken in graduated centrifuge tubes and adjusted to the desired pH values of 2.0–6.5 using 0.5 M HCl. The suspensions were centrifuged at 4,500 g at 4 °C for 30 min and the protein content was determined and protein precipitation was calculated using the formula:
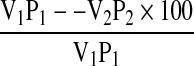 |
where V1 and V2 are the volumes of the supernatant (ml) before and after precipitation, and P1 and P2 are mg protein in 1 ml of V1 and V2, respectively.
Buffer capacity
One gram of sample was dispersed in 40 ml distilled water and known volumes of 0.5 M NaOH or 0.5 M HCl were added and corresponding changes in pH in both alkali and acid ranges were noted. The quantity of alkali and acid added was plotted against pH. Buffer capacity in each range was expressed as mmol of NaOH or HCl per gram of sample required for changing the pH value by one unit.
Bulk density
The bulk density was determined by the method of Wang and Kinsella (1976). Bulk density was expressed as grams per ml.
Moisture sorption isotherms
Protein concentrate powders were exposed to different relative humidity (RH) conditions of 10%, 30%, 50%, 70%, 90% and 100%, and were maintained using sulphuric acid solutions (Landrock and Procter 1951) at room temperature (28 ± 1 °C). Moisture sorption isotherms were drawn by exposing 5 g of samples to the above RH conditions and weights were taken periodically until the samples attained a constant weigh or fungal growth appeared. Samples were observed for fungal appearance, lump formation and decolourisation during exposure.
Sodium dodecyl sulfate –Polyacrylamide gel electrophoresis (SDS-PAGE)
Fresh roe, dehydrated and defatted protein concentrates were examined for molecular weight distribution using SDS-PAGE, following the method of Laemmli (1970). An aliquot (400μL) of the supernatant was taken and mixed with 100μL of 5 X sample loading buffer containing 60 mM Tris–HCl buffer (pH 6.8), 25% glycerol, 2% SDS, 10% β-mercaptoethanol and 0.1% bromophenol blue. The samples were heated in a boiling water bath for 5 min and centrifuged at 4,500 g at 4 °C for 5 min. SDS-PAGE was performed using 4% stacking and 10% separating polyacrylamide gels. Gels were stained in a staining solution containing 0.1% Coomassie brilliant blue R-250, 30% methanol and 20% acetic acid, and de-staining was carried out using a solution containing methanol, glacial acetic acid and water (3:2:5). Molecular masses were estimated using a wide range MW marker (12 to 205 kDa, Sigma Chemicals Co., USA).
Preparation of products and sensory evaluation
The protein concentrate powders from mrigal roes were incorporated in extruded pasta products to evaluate the acceptability of the products. Extruded pasta was prepared using either whole wheat flour or refined wheat flour and enriched with 10 and 20% of protein concentrates. Salt was added at 2% and the dough was exposed to steam in a closed vessel for 10 min for gelatinisation before extrusion. Pasta was prepared using a hand operated home scale extruder (Marcato, Atlas 150 Compack, Campodarsego, Italy) and dried in cabinet tray dryer (Chemida, Mumbai, India) at 50 °C for 4 h. Control samples were prepared without the protein concentrates. Protein content was determined by Kjeldahl method. Pasta were boiled for ten minutes in water, seasoned in oil, and served to the panel. All the products along with control samples were served to a panel of ten judges who were asked to evaluate sensory attributes such as colour, flavour, taste and over all acceptability on a nine point Hedonic scale, wherein 9 stands for like extremely, 5 for neither like nor dislike and 1 for dislike extremely.
Statistical analysis
Statistical analyses were performed using SPSS 11.0 for Windows (SPSS Inc., Chicago, IL). All experiments were performed in triplicate and the mean values were reported. Comparisons between means were performed with T test. Differences between means were evaluated as significant at P < 0.05.
Results and discussion
Proximate composition
The moisture contents of the protein concentrates were 4.24 and 5.80% for dehydrated and defatted mrigal roe protein concentrates, respectively. The protein content of these roe preparations were 75.4 and 91.1% with a significant difference (P < 0.05) between two protein powders. Protein percent of these fish roe protein concentrates were higher than the protein contents of other roe preparations reported earlier in literature (Eun et al. 1994, Rodrigo et al. 1998, Balaswamy et al. 2007). Iwasaki and Harada (1985) described the proximate composition of raw roes from different species with a wide range in protein content (11.5% in angler fish to 30.2% in crab). Joe et al. (1989) have noticed almost similar values for crude protein in fresh dried mullet (Mugil japonica) roes. Fat content for dehydrated egg protein concentrate was found to be 8.8%, which is comparable with those reported by Iwasaki and Harada (1985). Rodrigo et al. (1998) reported higher values for fat than observed in the present study. Previous studies (Eun et al. 1994) have noticed similar values for fat content in the channel cat fish (Ictalurus punctatus) roe. With reference to the composition of the fat content, it is noteworthy that roe lipids could have preventive effects on heart diseases, improvement of learning ability and plasma lipid content. The total ash content of dehydrated and defatted protein concentrates was found to be 5.95% and 1.95%, respectively. The present findings are in agreement with those of the earlier studies (Rodrigo et al. 1998, Balaswamy et al. 2007). Eun et al. (1994) reported similar values for ash in dried salted roes of hake (Merluccius merluccis) and ling (Molva molva L.). The higher ash content obtained for dehydrated egg protein concentrate as compared to that of defatted fish egg protein concentrate possibly reflecting the presence of phosphorous in the form of phospholipids. The crude fiber was found to be 0.14 and 0.17% in dehydrated and defatted egg protein powders respectively. Energy values were calculated as 402 and 368 kcal/100 g, respectively for dehydrated and defatted fish egg protein concentrates.
Table 1 shows the mineral content of fish egg protein concentrates. Calcium (10.50, 9.49 mg/100 g), iron (2.1, 3.94 mg/100 g) and phosphorus (2228.3, and 1250.7 mg/100 g) were observed in dehydrated and defatted protein concentrates. Calcium values observed in the present study (10.50, 9.49 mg/100 g, respectively) were lower than those reported for roes of Merluccius merluccis and Molva molva (Rodrigo et al. 1998). Phosphorus was found to be higher than other minerals in both protein concentrates. Eun et al. (1994) reported the presence of high quantities of phosphorus (4.7 g/100 g) and calcium (36.0 mg/100 g) in channel cat fish (Ictalurus punctatus) roe as compared to the values reported in the present study. Iron contents obtained in the present study is similar to the iron content of hake and ling roe (2.13 to 4.46 mg/100 g) described by Rodrigo et al. (1998), while Belinsky et al. (1996) reported lower iron content in the range 0.2–0.4 mg/100 g in white fish (Coregonus clupeaformis), cisco (Coregonus artedii) and lake trout (Salvelinus namaycush) raw roe samples. The variation in the mineral composition of marine foods is closely related to seasonal and biological differences (species, size, age, sex and sexual maturity), area of catch, processing methods, food source and environmental conditions (water chemistry, salinity, temperature and contaminant) (Alasalvar et al. 2002).
Table 1.
Mineral content and functional properties of dehydrated and defatted Mrigal egg protein concentrates
| Parameters | Dehydrated egg protein concentrate | Defatted egg protein concentrate |
|---|---|---|
| Calcium, mg/100 g | 10.5 ± 0.50a | 9.4 ± 0.28b |
| Iron, mg/100 g | 2.1 ± 0.10a | 3.9 ± 0.06b |
| Phosphorous, mg/100 g | 2228.3 ± 2.37a | 1250.7 ± 0.69b |
| Foam capacity ml (volume increased) | 33.0 ± 1.10a | 14.0 ± 1.00b |
| Foam stability, after 30 min | 20.0 ± 0.10a | 3.0 ± 0.50b |
| Foam stability, after 60 min | 18.0 ± 0.10a | 2.0 ± 0.50b |
| Emulsification capacity, ml/g protein | 5.9 ± 0.15a | 7.1 ± 0.10b |
| Bulk density, g/ml | 0.77 ± 0.06a | 0.64 ± 0.02b |
(n = 3), a-bDifferent superscript letters in the same row indicate significant difference, P < 0.05
Functional properties
Functional properties of Mrigal egg protein concentrates are shown in Table 1. Water and oil holding capacities were noted as 84.7, 178.5, 85.4 and 88.8 g/100 g for dehydrated and defatted protein concentrates (P < 0.05) respectively. Water holding capacity (WHC) (178.5%) of defatted egg protein concentrate in this study was high compared to those of Labeo rohita fish egg protein concentrate (Balaswamy et al. 2007). The high WHC of mrigal defatted egg protein concentrate may be due to the presence of polar groups such as COOH and NH2. Oil holding capacity (OHC) expresses the quantity of oil directly bound by the protein and is of great interest as it is an important functional characteristic, especially expected by the meat and confectionary industry. The OHC (88.8%) of defatted egg protein concentrate was lower than those reported (98%) for Labeo rohita egg protein concentrate (Balaswamy et al. 2007). The decrease in the OHC might have been due to the hydrophobic interactions of proteins. Decreasing trends were observed for foam capacities and foam stabilities of dehydrated and defatted egg protein concentrates (Table 1). The decrease may be due to denaturation of proteins during processing. Foaming capacity depends on molecular flexibility and physico-chemical properties of proteins. Emulsifying capacity of defatted egg protein concentrate was higher (7.1 ml/g protein) than dehydrated protein concentrate (5.9 ml/g protein). Emulsifying capacity of total protein depends upon the hydrophilic-lipophilic balance. The lower emulsifying capacity of dehydrated egg protein concentrate may be due to its isoelectric point when dispersed in water. Pavlova et al. (1989) reported the preparation of paste like emulsion products by using cod fish protein concentrate as a base material. Bulk density of these protein powders was found to be 0.77 g/ml, and 0.64 g/ml, respectively for dehydrated and defatted egg protein powders.
Protein solubility
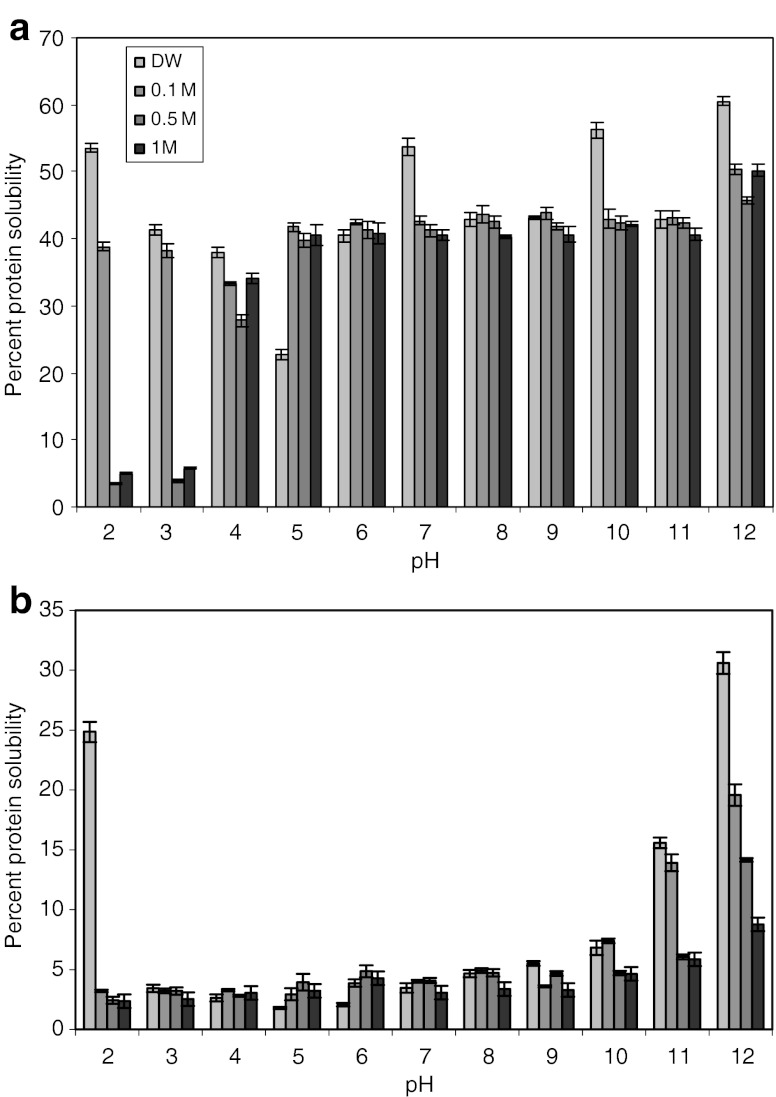
Protein solubility has been the subject of various studies due to its correlation to protein functionality, such as gelation, foaming ability, emulsification, binding ability and whipping properties. Ionic strength and pH of protein environment are the two most important factors affecting protein solubility, conformation and functional properties. The protein solubility of dehydrated and defatted fish egg protein concentrates in distilled water, 0.1, 0.5 and 1 M NaCl concentration with varying pH from 2–12 are shown in Fig. 1. Protein solubilities of dehydrated and defatted egg protein concentrates were found to be higher at pH 2 and 12 in distilled water. The lowest solubility values were obtained from pH 3 to 5 in 0.0 to 1 M Nacl concentration. These results are also in agreement with protein precipitation values (Fig. 2). This is probably due to isoelectric points of dehydrated and defatted egg protein concentrates at pH 5 and 2.5, respectively. The protein solubility of defatted egg protein concentrate at various pH values (2–12) (in 0.0 to 1 M Nacl) was lower (ranging from 2 to 30%) than of dehydrated egg protein concentrate. The lower solubilities observed for defatted protein concentrate could be due to removal of fat using isopropyl alcohol. During isopropyl alcohol extraction, proteins are vulnerable to solvent as well as heat treatment leading to the exposure of the hydrophobic domain and aggregation of proteins and resulting in low protein solubility (Sikorski and Naczk 1981). Venugopal and Shahidi (1995) reported that fish protein concentrate had extremely poor solubility at a wide range of NaCl molarity and pH. Previous studies (Klompong et al. 2007) have also noticed protein solubility of 45% and 19% in mince and defatted mince of yellow stripe trevally (Selaroides leptolepis). The salt concentration (from 0.1 to 1 M Nacl) increased the protein solubility of defatted protein concentrate at pH 5 and 6. While at pH above or below 5 and 6 (0.1 to 1 M Nacl), decreasing trend of protein solubility was observed for defatted protein concentrate. The enhancement of protein solubility by salt has been widely described as the salting-in phenomenon. This contributes to the electrostatic repulsive forces among protein molecules, which results in higher solubility. Thawornchinsombut and Park (2004) reported that protein solubility of Pacific whiting muscle proteins changed significantly over the pH range 2 to 12 and change in ionic strength (10 and 600 mM Nacl).
Fig. 1.
Percent protein solubility of (a) dehydrated egg protein concentrate and (b) defatted egg protein concentrate at various pH and salt strengths (Distilled water, 0.1, 0.5, and 1 M). *Bars represent standard deviations (n = 3)
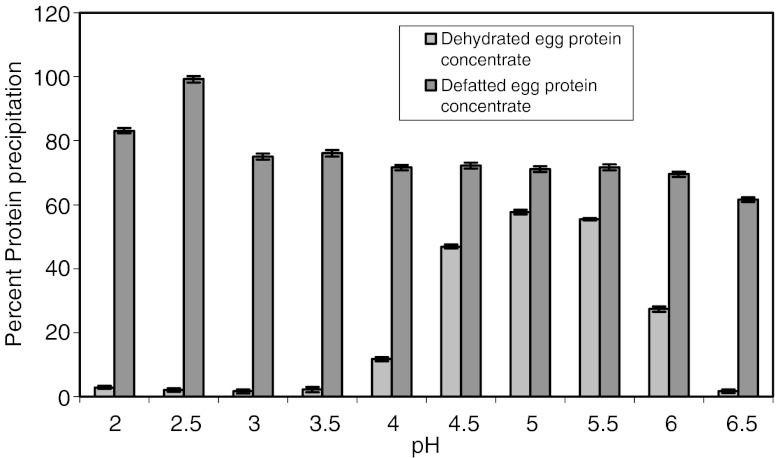
Fig. 2.
Percent protein precipitation of dehydrated and defatted egg protein concentrates at various pH. *Bars represent standard deviations from triplicate determinations
Protein precipitation
Protein precipitation studies at various pH values for dehydrated and defatted egg protein concentrates are presented in Fig. 2. The isoelectric points for dehydrated and defatted egg protein concentrates were found to be at pH 5 and 2.5. These studies are more useful in preparation of protein isolates with 97% proteins by changing pH from alkaline to acidic side.
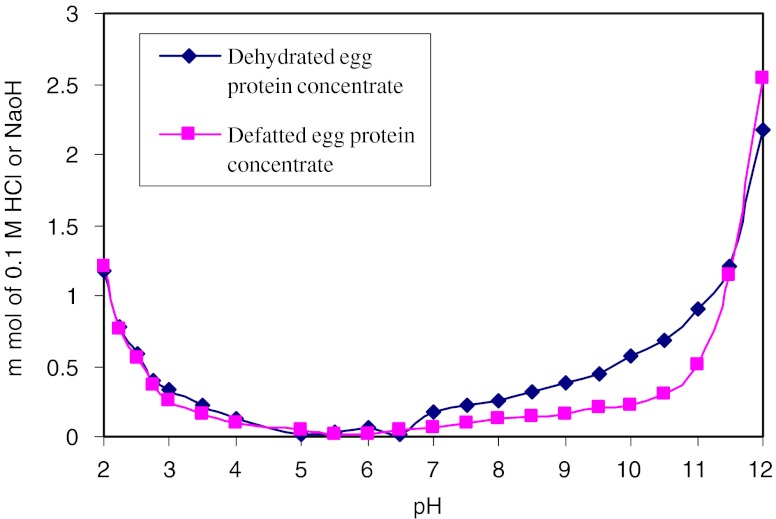
Buffer capacity
Buffer capacity of dehydrated and defatted egg protein concentrates are presented in Fig. 3. Buffer capacity was determined by adding 0.5 N HCl/0.5 N NaOH. Initial pH of dehydrated and defatted egg protein concentrates in distilled water was noted as 5.5 and 5.8, respectively. The buffer capacities of dehydrated egg protein concentrate was higher than that of defatted egg protein concentrate in both acid and alkaline range with an average of 0.65 mmol HCl and 1.22 mmol NaOH/1 g required to change of one pH unit. Higher values for dehydrated egg protein concentrate may be due to presence of fat component which requires more acid or alkali to bring pH change by one unit.
Fig. 3.
Buffer capacity of dehydrated and defatted fish egg protein concentrates
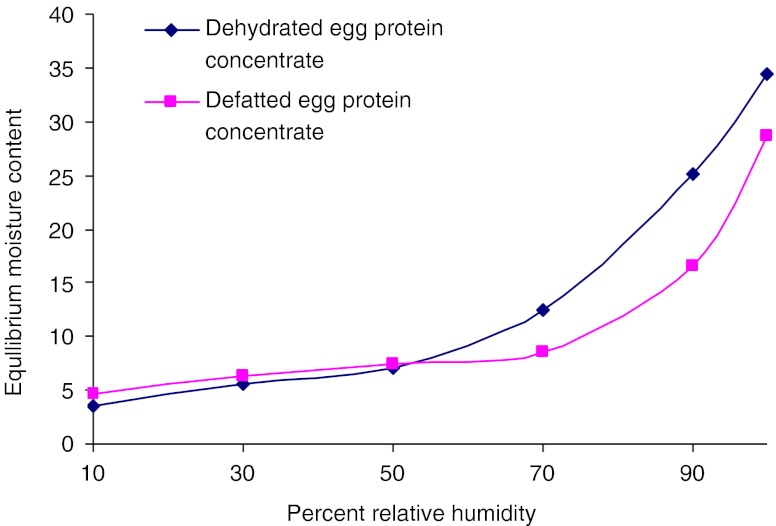
EMC-RH studies
In the present study, moisture sorption isotherms (Fig. 4) indicated that the critical moisture contents were 7.8 and 16.8%, which equilibrated at 65 and 75% RH for dehydrated and defatted egg protein concentrates, respectively. Chau et al. (1982) studied sorption isotherms and drying rates for both fillet and roe of mullet fish and concluded that fillets were dehydrated four times faster than roes and the migration of water was within the roes rather than at the roe surface.
Fig. 4.
Equilibrium moisture isotherm curves for dehydrated and defatted fish egg protein concentrates
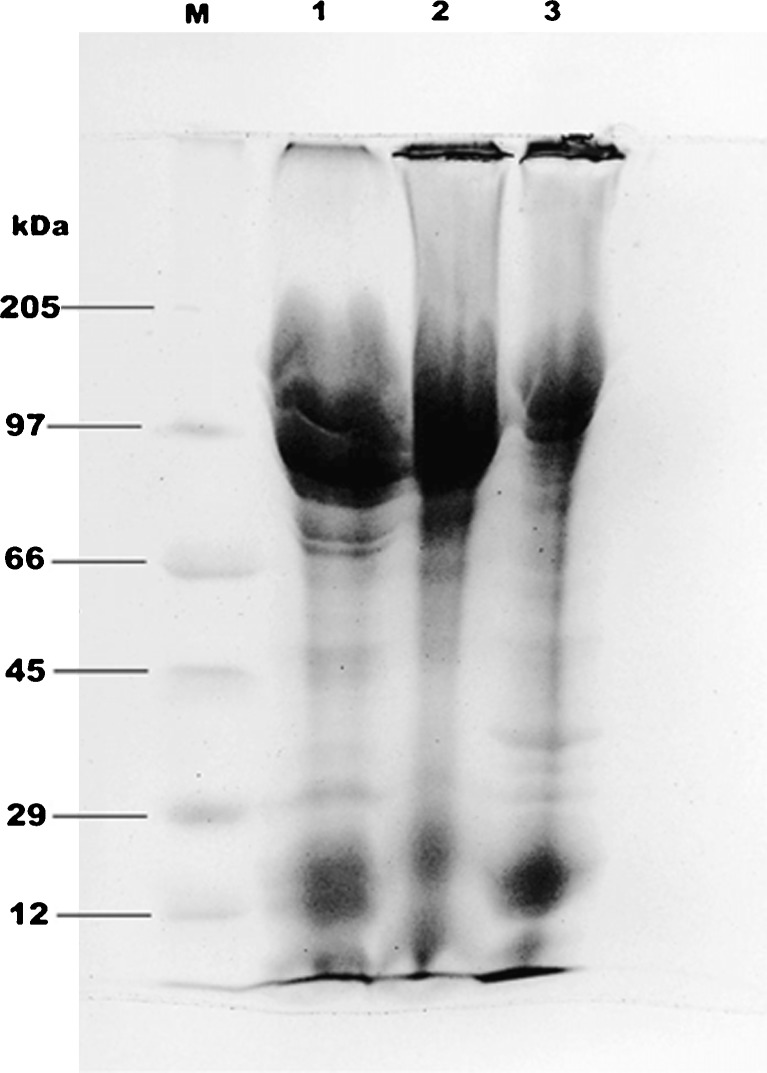
SDS-PAGE
Electrophoretic pattern of extracts of fresh roe, dehydrated and defatted egg protein concentrates of mrigal are shown in Fig. 5. The major bands observed occurred at molecular weight around 97 kDa. Fresh roe exhibited clear protein bands in the range of 66–100 kDa. Protein bands at approximately 97 kDa could be vitellin like protein, which was found in skipjack, tongol, bonita roes (Intarasirisawat et al. 2011) and chicken egg yolk (De Man 1999). Protein bands with MW of ~12-24 might be the phosvette, which was earlier reported in the eggs of Xenopus laevis (Wiley and Wallace 1981) and chicken (Wallace and Morgan 1986). The banding pattern was reduced in case of defatted egg protein concentrate due to the effect of defatting process. The electrophoretic pattern observed for fresh roe, dehydrated and defatted egg protein concentrates were comparable with the results of Al-Holy and Rasco (2006).
Fig. 5.
SDS-PAGE of extracts of fresh roe, dehydrated, and defatted protein concentrates from C. mrigala. Lane M: molecular weights of standard protein markers (myosine 205 KDa, phosphorylase B 97 KDa, bovine serum albumin 66 KDa, ova albumin 45 KDa, carbonic anhydrase 29 KDa, cytochrome C 12 KDa); lane 1: fresh egg proteins; lane 2: dehydrated protein concentrate and lane 3: defatted protein concentrate
Product sensory evaluation
Table 2 shows the sensory scores of cooked pasta containing defatted fish egg protein concentrate. The pasta samples tasted good at both 10 and 20% level of addition of mrigal fish egg protein concentrate powder. Difference in taste or flavour was not significant between the control and fish egg fortified products. In the cooked pasta, the fishy odour was not detectable at 10% level, however at 20% level it was only perceptible slightly. The protein analysis indicated an increase in content of the dry pasta by 8 and 15% respectively at 10 and 20% levels of incorporation from the control value of 8.5%.
Table 2.
Sensory scores for cooked pasta containing defatted fish egg protein concentrate
| Parameter | Percentage of defatted fish egg protein concentrate | ||
|---|---|---|---|
| 0 | 10 | 20 | |
| Appearance | 8.2 ± 0.18 | 8.1 ± 0.15 | 7.8 ± 0.19 |
| Colour | 8.0 ± 0.21 | 7.8 ± 0.16 | 7.6 ± 0.20 |
| Flavour | 8.0 ± 0.25 | 7.5 ± 0.11 | 7.2 ± 0.18 |
| Taste | 8.0 ± 0.33 | 7.8 ± 0.22 | 7.5 ± 0.15 |
| Overall quality | 8.0 ± 0.10 | 7.8 ± 0.24 | 7.5 ± 0.25 |
(n = 3)
Conclusion
This study has presented the data on crude protein, crude fat, total ash, moisture, crude fiber, carbohydrates and some functional properties of Cirrhinus mrigala fish roe protein concentrates. The present study indicated protein concentrates from Cirrhinus mrigala roe have significant amount of protein as well as iron and phosphorous contents. Dehydrated and defatted fish roe protein concentrates may potentially serve as a good source of protein with desirable functional properties. Therefore, these protein concentrates could be used as protein supplements and functional ingredients in human diets. This fish roe could be an economic and alternative protein source to alleviate protein malnutrition in developing countries.
Acknowledgement
The authors thank V. Prakash, Director, Central Food Technological Research Institute, Mysore, for his keen interest in the work. The authors would also like to thank Department of Biotechnology (DBT), Government of India for their financial assistance.
References
- Alasalvar C, Taylor KDA, Zubcov E, Shahidi F, Alexis M. Differentiation of cultured and wild sea bass (Dicentrarchus labrax): total lipid content, fatty acid and trace mineral composition. Food Chem. 2002;79:145–150. doi: 10.1016/S0308-8146(02)00122-X. [DOI] [Google Scholar]
- Al-Holy MA, Rasco BA. Characterization of salmon (Oncorhynchus keta) and sturgeon (Acipenser transmontanus) caviar proteins. J Food Biochem. 2006;30:422–428. doi: 10.1111/j.1745-4514.2006.00069.x. [DOI] [Google Scholar]
- AOAC (1965) Official Methods of Analysis of Association of Official Analytical Chemists 10th edn. Washington, DC
- Balaswamy K, Jyothirmayi T, Rao DG. Chemical composition and some functional properties of fish egg (roes) protein concentrate of rohu (Labeo rohita) J Food Sci Technol. 2007;44:293–296. [Google Scholar]
- Balaswamy K, Prabhakara Rao PG, Rao DG, Jyothirmayi T. Effects of pretreatments and salt concentration on rohu (Labeo rohita) roes for preparation of roe pickle. J Food Sci Technol. 2010;47:219–223. doi: 10.1007/s13197-010-0035-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belinsky DL, Kuhnlein HV, Yeboah F, Penn AF, Chean HM. Composition of fish consumed by the james Bay Cree. J Food Comp. Anal. 1996;9:148–162. doi: 10.1006/jfca.1996.0022. [DOI] [Google Scholar]
- Beuchat LR. Functional and electrophoretic characteristics of succinylated peanut flour protein. J Agric Food Chem. 1977;25:258–261. doi: 10.1021/jf60210a044. [DOI] [Google Scholar]
- Bledsoe GE, Bledsoe CD, Rasco BA. Caviars and fish roe products. Crit Rev Food Sci Nutr. 2003;43:317–356. doi: 10.1080/10408690390826545. [DOI] [PubMed] [Google Scholar]
- Chalamaiah M, Narsing Rao G, Rao DG, Jyothirmayi T. Protein hydrolysates from mrigal (Cirrhinus mrigala) egg and evaluation of their functional properties. Food Chem. 2010;120:652–657. doi: 10.1016/j.foodchem.2009.10.057. [DOI] [Google Scholar]
- Chau KV, Heinis JJ, Perez H. Sorption isotherms and drying rates for mullet fillet and roe. J Food Sci. 1982;47:1318–1322. doi: 10.1111/j.1365-2621.1982.tb07677.x. [DOI] [Google Scholar]
- Dev DK, Quansel E, Hansen P. Nitrogen extractability and buffer capacity of defatted linseed flour. J Sci Food Agric. 1986;37:199–205. doi: 10.1002/jsfa.2740370215. [DOI] [Google Scholar]
- De Man JM. Proteins. In: Deman JM, editor. Principles in Food Chemistry. Gaithersburg: Aspen Publishers Inc; 1999. pp. 111–162. [Google Scholar]
- Eun JB, Hee JC, Hearnsberger JO. Chemical composition and micro flora of channel catfish (Ictalurus punctatus) roe and swim bladder. J Agric Food Chem. 1994;42:714–717. doi: 10.1021/jf00039a022. [DOI] [Google Scholar]
- Gagne N, Adambounou LT. Physico-chemical and functional properties of roes from autumn spawning herring (Clupea herengus herengus L.) Food Res Int. 1994;27:405–408. doi: 10.1016/0963-9969(94)90197-X. [DOI] [Google Scholar]
- Intarasirisawat R, Benjakul S, Visessanguan W. Chemical compositions of the roes from skipjack, tongol and bonita. Food Chem. 2011;124:1328–1334. doi: 10.1016/j.foodchem.2010.07.076. [DOI] [Google Scholar]
- Iwasaki M, Harada R. Proximate and amino acid composition of the roe and muscle of selected marine species. J Food Sci. 1985;50:1585–1587. doi: 10.1111/j.1365-2621.1985.tb10539.x. [DOI] [Google Scholar]
- Joe SI, Rhee CO, Kim DY. Study of processing and composition of salted and dehydrated mullet roe. Korean J Food Sci Technol. 1989;21:242–251. [Google Scholar]
- Johnson DW. Functional properties of oil seed proteins. J Americ Oil Chem Soc. 1970;47:402–407. doi: 10.1007/BF02632475. [DOI] [Google Scholar]
- Klompong V, Benjal S, Kantachota D, Shahidi F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007;102:1317–1327. doi: 10.1016/j.foodchem.2006.07.016. [DOI] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landrock AA, Procter BE. A new graphical interpolation method for obtaining humidity equlibria data with special reference to its role in food packing studies. Food Technol. 1951;5:332–337. [Google Scholar]
- Lawhon JF, Carter CM, Matil KF. A comparative study of the whipping potential of an extract from several oil seed flours. Cereal Sci Today. 1972;17:240–244. [Google Scholar]
- Mukhopadhyay SK, Sahoo CR, Bose AK. Analysis of egg protein in five carps. Hydrobiologia. 1981;77:97–102. doi: 10.1007/BF00008867. [DOI] [Google Scholar]
- Pavlova ZhP, Boyarkina LG, Mikhaleva VF, Kastornykh MS. Fish protein concentrate. Pishchevaya- Promyshlennost. 1989;12:43–44. [Google Scholar]
- Rodrigo J, Ros G, Periago MJ, Lopez C, Ortuno J. Proximate and mineral composition of dried salted roes of hake (Merluccius merluccis) and ling (Molva molva L.) Food Chem. 1998;63:221–225. doi: 10.1016/S0308-8146(98)00002-8. [DOI] [Google Scholar]
- Sikorski ZE, Naczk M. Modification of technological properties of fish protein concentrates. Crit Rev Food Sci Nutr. 1981;14:201–230. doi: 10.1080/10408398109527305. [DOI] [PubMed] [Google Scholar]
- Taher FS, Abbassy M, El-Nockroshy AS, Shoeb ZE. Counter current extraction – isoelectric precipitation of sunflower seed protein isolates. J Sci Food Agric. 1981;32:166–174. doi: 10.1002/jsfa.2740320212. [DOI] [Google Scholar]
- Thawornchinsombut S, Park JW. Role of pH in solubility and conformational changes of pacific whiting muscle proteins. J Food Biochem. 2004;28:135–154. doi: 10.1111/j.1745-4514.2004.tb00061.x. [DOI] [Google Scholar]
- Venugopal V, Shahidi F. Value-added products from underutilized fish species. Crit Rev Food Sci Nutr. 1995;35:431–453. doi: 10.1080/10408399509527708. [DOI] [PubMed] [Google Scholar]
- Wallace RA, Morgan JP. Chromatographic resolution of chicken phosvitin: multiple macromolecular species in a classic vitellogenin-derived phosphoprotein. Biochem J. 1986;240:871–878. doi: 10.1042/bj2400871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JC, Kinsella JE. Functional properties of novel proteins: alfalfa leaf protein. J Food Sci. 1976;41:286–289. doi: 10.1111/j.1365-2621.1976.tb00602.x. [DOI] [Google Scholar]
- Weichselbaum TE (1946) An accurate and rapid method for the determination of proteins in small amounts of blood serum and plasma. Americ J Clin Pathol 16 (Tech. Suppl.10):40 [PubMed]
- Wiley HS, Wallace RA. The structure of vitellogenin. Multiple vitellogenins in Xenopus laevis give rise to multiple forms of the yolk proteins. J Biol Chem. 1981;256:8626–8634. [PubMed] [Google Scholar]
- Wirth M, Kirschbaum F, Gessner J, Kruger A, Patriche N, Billard R. Chemical and biochemical composition of caviar from different sturgeon species and origins. Nahrung. 2000;44:233–237. doi: 10.1002/1521-3803(20000701)44:4<233::AID-FOOD233>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]