Abstract
Starch was phosphorylated through dry-heating in the presence of pyrophosphate at various conditions, and the characteristics of phosphorylated starch (PS) were examined. Starch phosphorylation increases as the pH increases from 3 to 6, but diminishes at pH 7. Increased temperatures enhance phosphorylation. Data from 31P NMR suggests that starch phosphorylation occurs mainly at the C3-OH and C6-OH of the glucose residue. The phosphate linkage is mainly due to monostarch monophosphate. Although starch had almost no calcium phosphate-solubilising capacity, this capacity was markedly enhanced by phosphorylation. X-ray diffraction analysis indicates that the crystal structure of hydroxyapatite was not present in the calcium phosphate-PS complex.
Keywords: Phosphorylation, Starch, Dry-heating, Phosphate linkage, Calcium-phosphate solubilising capacity
Introduction
Phosphate tends to form an insoluble complex with calcium under the alkaline pH conditions that exist in the intestinal environment. The formation of a calcium phosphate precipitate results in reduced calcium absorption at the intestines (Sato et al. 1986, Yamamoto et al. 1992, Nordin and Heaney 1990). Phosphoproteins, such as casein and egg white riboflavin-binding proteins, interact with calcium and phosphate, and solubilize calcium phosphate (Aoki et al. 1986, Aoki 1989, Aoki et al. 1993). Kamasaka et al. (1995) showed that phosphorylated oligosaccharides prepared from potato starch can inhibit the formation of calcium phosphate precipitates by forming soluble complexes with calcium ions. Although starch has no calcium phosphate solubilizing ability, this ability of starch may be given by phosphorylation. In addition, the roles of phosphate groups in physiological (Kitazawa et al. 1996, Kitazawa et al. 1998) and immune (Hata et al. 1998, Otani et al. 2000) functions have also been reported. More recently, Otani et al. (2007) reported that the production of Immunoglobulin A in mice was enhanced by phosphorylated dextrin, which was prepared through dry-heating in the presence of orthophosphate. Thus, the functional properties and physiological functions of starch are expected to be similarly improved by phosphorylation.
Since the beginning of the 20th century, starch phosphorylation has been carried out to improve the functional properties, such as gelling, digestion, and emulsification, of starch. (Sitohy et al. 2000). Sodium trimetaphosphate (STMP) and sodium tripolyphosphate (STPP) have been used to prepare phosphorylated starch (PS) under alkaline conditions. (Kerr and Cleveland 1957, Woo and Seib 1997). Starch can also be phosphorylated through dry-heating in acidic conditions in the presence of orthophosphate (Tarelli and Wheeler 1994a, Tarelli and Wheeler 1994b, Nakano et al. 2003). However, starch phosphorylation through dry-heating in the presence of pyrophosphate has not been studied yet. In addition, little is known about the phosphate groups that are introduced into starch by dry-heating in the presence of pyrophosphate. In previous studies, researchers have shown that the calcium phosphate-solubilizing capacity of certain food proteins is allowed by phosphorylation through dry-heating in the presence of pyrophosphate (Li et al. 2003, 2004, 2005, Enomoto et al. 2007, 2008, 2009, Hayashi et al. 2008). However, there is little information on the calcium phosphate-solubilizing capacity of PS prepared through dry-heating in the presence of pyrophosphate.
The objectives of this study are: (1) to investigate the effects of pH, temperature, and heating time on the phosphorylation of starch through dry-heating in the presence of pyrophosphate; (2) to investigate the type of phosphate linkages formed, as well as the various characteristics of PS; and (3) to examine the calcium phosphate-solubilizing capacity of PS and the calcium phosphate forms in the calcium phosphate-PS complex.
Materials and methods
Materials
Potato starch was purchased from Sigma Chemical Co. (St. Louis, MO, USA). α-Amylase was from Guangdong Huankai Microbial Sci. & Tech. Co. Ltd. (Guangzhou, China). The chemicals were analytical grade.
Preparation of PS
Potato starch was suspended at a 2% concentration in 0.1 M sodium pyrophosphate buffer at various pH values from 3.0 to 7.0. It was gelatinized by heating at 80 °C for 10 min, and then lyophilized. Lyophilized samples were incubated at various temperatures from 70 to130 °C for 1–5 days. Dry-heated samples were dissolved in deionised water and dialysed to remove free pyrophosphate for 2 days, and then lyophilized. For the measurement of browning of PS, the dry-heated samples were not dialysed.
Determination of phosphorus content of PS
Total phosphorus (Pt) was determined after digestion with perchloric acid by the method of Chen et al. (1956). To determine the inorganic phosphate (Pi) of the PS, 5 mL of 0.1% sample were ultrafiltered using a Centrisart I (Cutoff 10,000 Da, Goettingen, Germany) and the phosphorus in the filtrate was regarded as Pi of PS. Organic phosphate (Po) content was estimated from the difference between Pt and Pi contents. Each value is the mean with its SD (n = 3).
Measurement of browning
PS samples were suspended in deionized water at a concentration of 1% at pH 6.0. To 2 mL of 1% of PS solution, 2 mL of 0.1% a-amylase was added. Absorbance was measured at 470 nm after incubation at 60 °C for 2 h. Data shown are the mean value of the two determinations, with a deviation of <1%.
31P Nuclear magnetic resonance (31P NMR) spectroscopy
31P NMR spectroscopy was carried out by a JNM-ECA 400-NMR spectrometer (JEOL Ltd., Tokyo, Japan) operating at 162 MHz and 20 °C. Phosphoric acid (85%) was used as an external standard. The proton-decoupled 31P NMR spectrum of PS, which was dry-heating at pH 6.0 and 110 °C for 3 days in the presence of pyrophosphate, was performed at pH 8.0 (adjusted with 1 N NaOH/D2O solution). The PS was dissolved in D2O solution at a concentration of 2%. The spectrum of SP solution was obtained with an 80° tipping pulse and a 2-s repetition time.
Measurement of calcium-phosphate solubilizing capacity by PS
The preparation of test solutions was done according to the procedures for artificial casein micelles (Aoki 1989), with the minor modification. Two hundred microliters of 0.2 M potassium citrate, 200 μL of 0.2 M CaCl2 and 240 mL of 0.2 M K2HPO4 were added to 2 mL of 4% starch solution followed by 100 μL of 0.2 M CaCl2 and 50 μL of 0.2 M K2HPO4.
The addition of 100 μL of 0.2 M CaCl2 and 50 μL of 0.2 M K2HPO4 was repeated to give the concentrations of calcium and Pi of 30 and 22 mM, respectively. The interval set for addition was 15 min, and all additions were accompanied by stirring at pH 6.7 with 1 M KOH. The volume was adjusted to 4 mL by measuring the weight of solution. The prepared solutions were centrifuged at 3000 x g for 15 min. The centrifugated solutions were observed by visual inspection before measurements of calcium and phosphate. Calcium was determined with a Shimadzu ICPS-1000 II sequential plasma spectrometer (Kyoto, Japan). Pi was measured by the method of Chen et al. (1956). Each value is the mean with its SD (n = 3).
X-ray diffraction analysis
Calcium phosphate-PS (CaPi-PS) complex was applied to X-ray diffraction analysis. In comparison with the existence form of calcium phosphate in (CaPi-PS) complex, the X-ray diffraction analysis of calcium-phosphate without starch sample and calcium phosphate-starch complexes were also performed. The X-ray analysis was performed with a Rigaku D/Max-3B X-ray Diffractometer (Tokyo, Japan). Monochromatized CuKa radiation generated at 40 kV and 100 mA was used, and the count full scale was 6000 c/s (CPS) for all samples.
Results and discussion
Phosphorylation
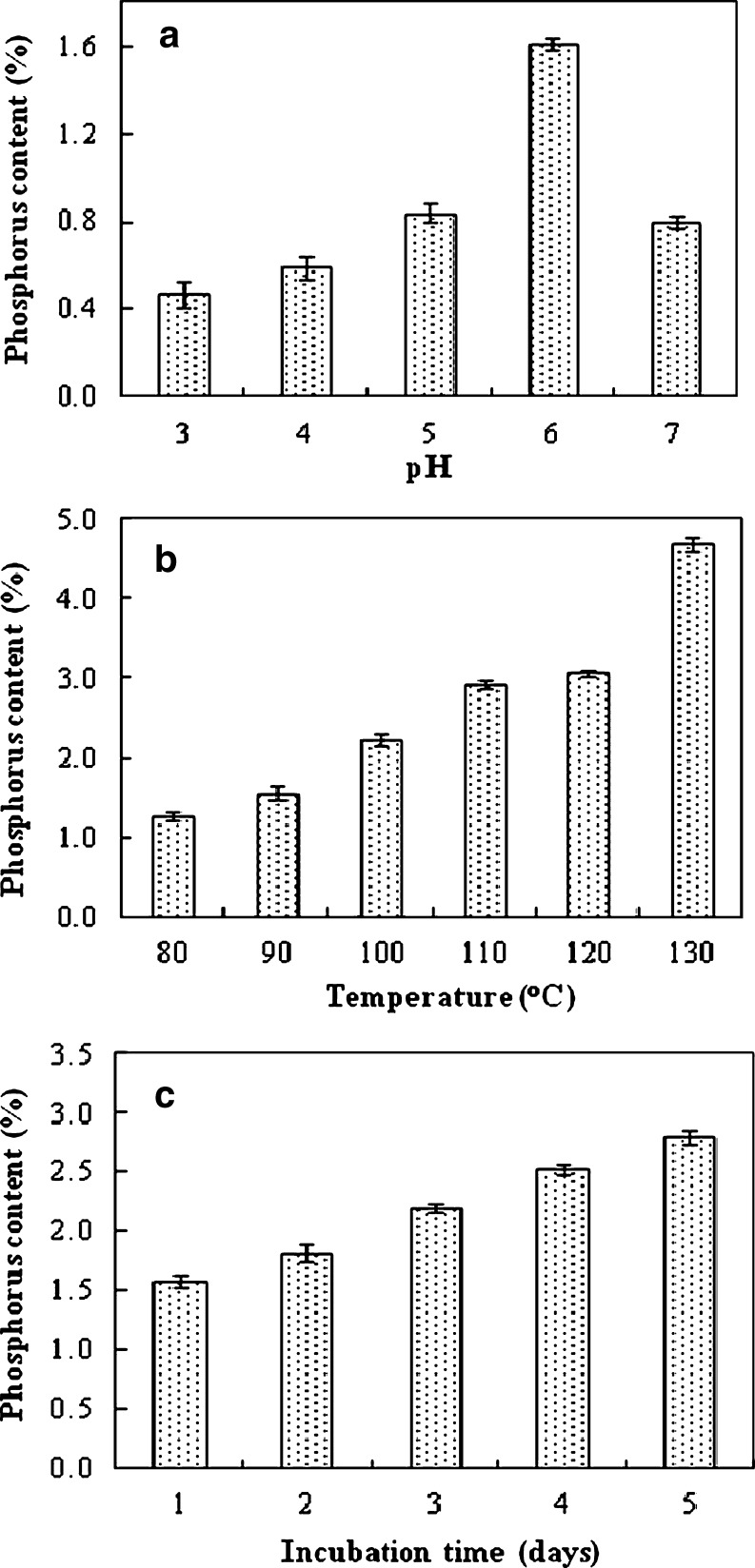
In the previous papers (Li et al. 2003, 2004), we have showed that the phosphorylation of protein by dry-heating in the presence of pyrophosphate was higher than that in the presence of orthophosphate. Based on this result, we speculated that the phosphorylation of starch may be promoted by dry-heating in the presence of PP. Starch was phosphorylated through dry-heating in the presence of pyrophosphate at various pH conditions. Figure 1 shows the introduced Po content of PS at various conditions. The phosphorylation of starch was accelerated as the pH was increased from 3 to 6, but diminished at pH 7 (Fig. 1a). This result is similar to a previous reported by Sitohy et al. (2000). As such, the subsequent phosphorylation of starch was performed at pH 6. Phosphorylation increased as the dry-heating temperature increased from 80 to130 °C (Fig. 1b).
Fig. 1.
Effects of pH (a), temperature (b), and heating time (c) on the phosphorylation of starch. a, starch was phosphorylated by dry-heating at pH 3–7 and 85 °C for 1 day in the presence of pyrophosphate; b, starch was phosphorylated by dry-heating at pH 6 and 80–130 °C for 1 day in the presence of pyrophosphate; c starch was phosphorylated by dry-heating at pH 6 and 85 °C for 1–5 days in the presence of pyrophosphate. Each value is the mean with its SD (n = 3)
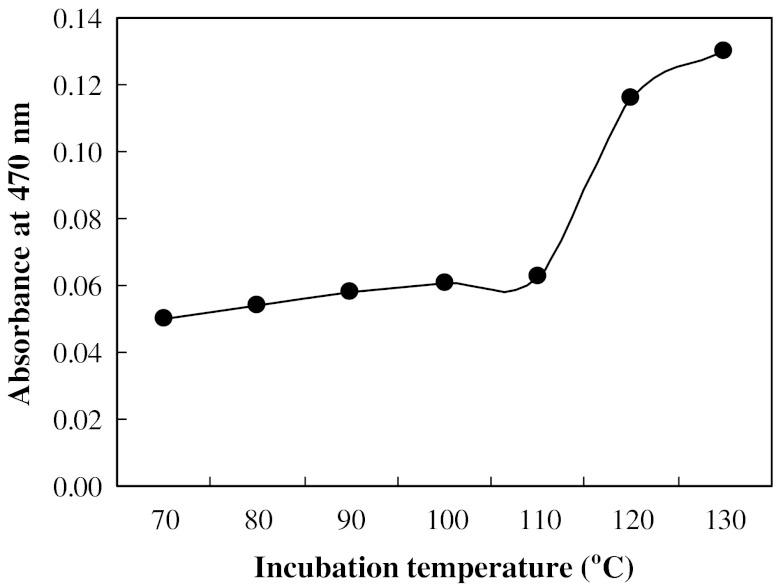
Although the Po content of PS was 4.69% when dry-heated at pH 6 and 130 °C for 1 day, the browning of the PS was also substantial (Fig. 2). Thus, the phosphorylation of PS was performed at 110 °C in the subsequent experiments. The phosphorylation of starch was accelerated as the dry-heating time increased (Fig. 1c). Similarly, the temperature of the phosphorylation of starch was recommended to be carried out at <120 °C to avoid the browning of PS. The phosphorylation level of starch dry-heating in the presence of pyrophosphate is higher than that in the presence of othrophosphate at the same condition.
Fig. 2.
Effect of temperature on the browning of starch. Starch was dry-heated at pH 6 and various temperatures from 70 to 130 °C for 1 day in the presence of pyrophosphate. Data shown are the mean value of the two determinations with a deviation of <1%
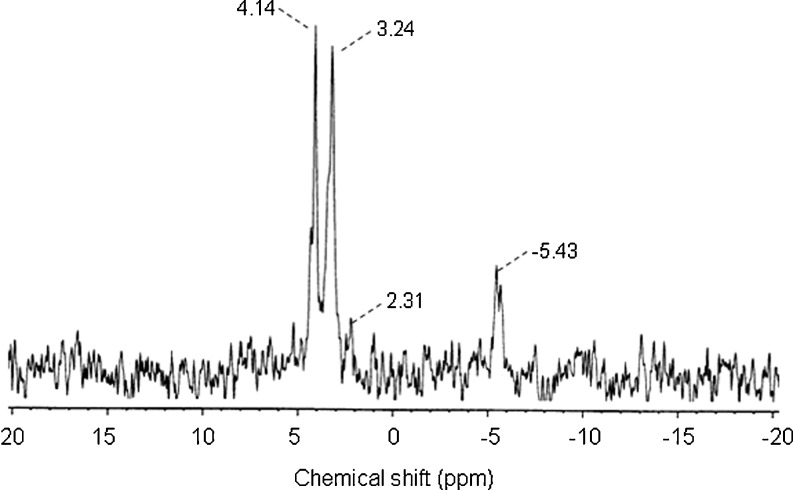
Figure 3 shows the 31P NMR spectrum of PS at pH 8.0. A peak at −5.43 ppm appeared in the PS, and was assigned as pyrophosphate. Peaks at 3.24 and 4.14 ppm were assigned to C6-O-P and C3-O-P (Sang et al. 2007, Sang et al. 2010). These results are similar to those obtained through roasting both α- and β-cyclodextrin with sodium metaphosphate at pH 4.0. (Tarelli et al. 1997). On the other hand, this study shows that distarch monophosphate (DSMP, starch-P-starch) and monostarch diphosphate (MSDP, Starch-P-P) were not observed in the PS preparation. This may be attributed to the fact that the phosphorylation pH is different from that used for starch phosphorylation with STPP (Lim and Seib 1993). A weak peak at 2.31 ppm was assigned to the inorganic orthophosphate (Sang et al. 2010).
Fig. 3.
31P NMR spectrum at pH 8.0 and 20 °C of PS, which was prepared by dry-heating at pH 6 and 110 °C for 3 days. H3PO4 (%) was the external standard. Resonance signals downfield to H3PO4 are positive
From the results of 31P NMR, the phosphate ester in PS was determined to be monostarch monophosphate (C3-O-P and C6-O-P). Thus, the dry-heating phosphorylation process may be explained in simple terms
 |
where S represents starch. The phosphorylation of starch was enhanced with a pH level between 3 and 6, and was inhibited when the pH was increased to between 6 and7. Pyrophosphoric acid has four ionisable hydrogen atoms. Its dissociation constants are as follows: pk1 = 0.91, pk2 = 2.10, pk3 = 6.70, and pk4 = 9.32. At pH 6.7, the two main anionic pyrophosphate species (H2P2O2-7 and HP2O3-7) are equal. If the pH decreases from 6.7 to 6.0, the proportion of H2P2O2-7 increases, while that of HP2O3−7 decreases. Phosphoester formation between hydroxyl groups and H2P2O2−7 may be more favorable than that between hydroxyl groups and HP2O3−7. Therefore, greater phosphorylation may be expected at pH 6.0. On the other hand, H2P2O2−7 may not be the reactive species, as it can be one of the components within an equilibrium mixture (Tarelli and Wheeler 1994a, b). The solution chemistry of phosphates is complex and a variety of phosphorus oxyacid species are known to co-exist in equilibrium. Thus, it is possible that, in all cases, a common component of the equilibrium is the agent responsible for phosphorylation. This may be attributed to the possible interconversion of species.
The calcium phosphate-solubilizing capacity of PS
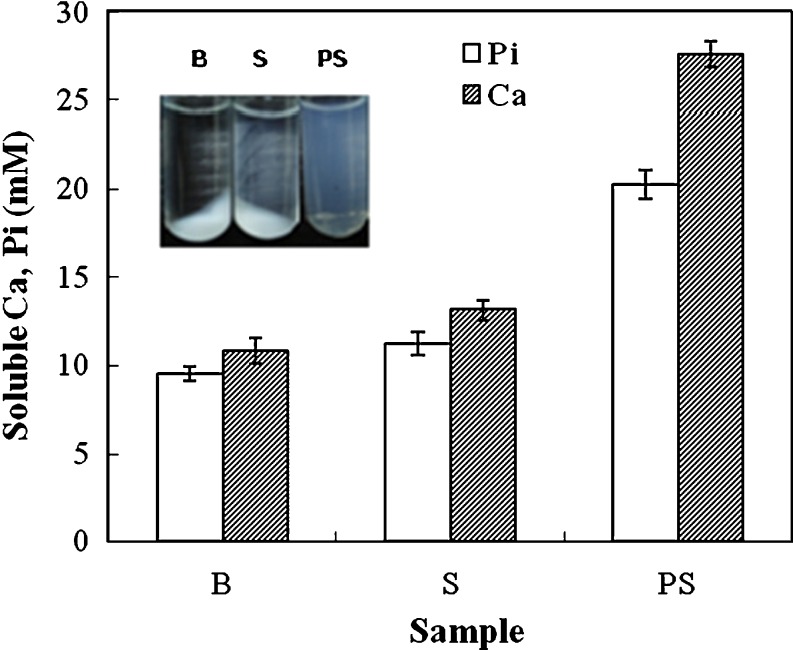
The calcium phosphate-solubilising capacity of PS was examined using the method of artificial casein micelles. As shown in Fig. 4, in the absence of starch, the soluble calcium and Pi were 10.83 and 9.52 mM, respectively. In the presence of 2% starch, the soluble calcium and Pi were 13.12 and 11.23 mM, respectively. However, the soluble calcium and Pi increased to 27.56 and 20.24 mM, respectively, in the presence of 2% PS (Po content, 3.37%). An image of the calcium phosphate-starch solution after centrifugation is shown in Fig. 4. In the absence of PS, a calcium phosphate precipitate forms after centrifugation. However, the CaPi-PS solution was clear. When solutions containing calcium and phosphate are mixed, a precipitate of amorphous calcium phosphate is first formed. The amorphous calcium phosphate is unstable and finally transforms to stable hydroxyapatite (Schmidt 1982). The conversion of amorphous calcium phosphate to hydroxyapatite can be prevented by a number of compounds, including casein, phosvitin, and poly-L-glutamate, among others (Schmidt 1982, Van Kemenade and De Bruyn 1989). Although starch hardly possesses any calcium phosphate-solubilizing capacity, this capacity was markedly enhanced by phosphorylation (Fig. 4). As well-known fact, in milk, calcium phosphate in excess of its solulility forms colloilal calcium phosphate and cross-links the caseins. The caseins are cross-linked through their phosphate linkage by colloidal calcium phosphate (Aoki 1989). In this report, the starch was given to calcium phosphate-solubilising capacity. According to the result reported by Aoki, we believed that the calcium phosphate was solubilised through the introduced phosphate group in PS.
Fig. 4.
Calcium phosphate-solubilizing capacity of PS. PS was prepared by dry-heating at pH 6 and 110 °C for 3 days. B, blank (in the absence of starch and PS); S, starch. The centrifugated solutions were observed by visual inspection before measurements of calcium and phosphate. Each value is the mean with its SD (n = 3). Pi, inorganic phosphate; Ca, calcium
X-ray diffraction of calcium phosphate-PS complex
X-ray diffraction analysis was performed to examine which type of calcium phosphate forms in the calcium phosphate-PS complex. In the absence of PS or starch, a number of peaks, which appeared to be those of a certain type of crystals, were observed. According to the X-ray diffraction results, peaks 1–6 were identified as having arisen from potassium chloride (KCl), while the other peaks were derived from a hydroxyapatite crystal [Ca10(PO4)6(OH)2]. This reveals that calcium phosphate formed as hydroxyapatite. However, hydroxyapatite peaks were not observed in the calcium phosphate-PS complex, indicating that the hydroxyapatite was not formed in the presence of PS (Fig. 5b). As shown in Fig. 5b, KCl crystal peaks were observed. The formation of the KCl crystal in the CaPi-PS complex is attributable to CaCl2 and KOH used during the preparation of the CaPi-PS complex. Subsequently, to remove the KCl, the CaPi-PS complex solution was concentrated to approximately 1/10 volume through ultrafiltration using a Centrisart I (Cutoff 10,000 Da), and then lyophilised. As shown in Fig. 5c, the KCl-related peaks in Fig. 5b disappeared and steep peaks were no longer observed in the X-ray diffraction patterns of the concentrated CaPi-PS complex.
Fig. 5.
X-ray diffraction patern of calcium phosphate (a), calcium phosphate-PS complex (b), and calcium phosphate-PS complex concentrated by ultrafiltration (c). Count full scale was 6,000 CPS for all samples
Toba et al. (1999) reported that the bioavailability of calcium phosphate-casein phosphopeptide (amorphous form) is better than that of rennet whey (hydroxyapatite form). The bioavailability of calcium from a CaPi-PS complex can be beneficial because the calcium phosphate in the CaPi-PS complex was an amorphous form. Casein phosphopeptide is sufficient for rendering the calcium ion soluble in several kinds of food, but its use may be somewhat restricted because of its bitterness (Kamasaka et al. 1995). PS possesses no bitter taste in sensory tests (data not shown). This may enable PS to be used in many kinds of food without any problems. Furthermore, the physiological and immune functions of PS may be anticipated.
Conclusion
Starch was successfully phosphorylated through dry-heating in the presence of pyrophosphate with little browning. The phosphorylation of starch mainly occurred at the C3-OH and C6-OH of the glucose residue. The phosphate linkages are attributable to monostarch monophosphate. Starch acquired calcium phosphate-solubilizing capacity through phosphorylation. The calcium phosphate-PS complex may be used a new, cost-efficient, and bitterness-free calcium resource.
Acknowledgment
This work was supported by Natural Science Foundation of Yunnan Province (2007C155M), PR China and Major Program of Science Foundation of Educational Commission of Yunnan Province (ZD2009002), PR China.
Contributor Information
Can-Peng Li, Phone: +86-871-5031391, FAX: +86-871-5031391, Email: lcppp1974@sina.com.
Hui Zhao, Phone: +86-871-5031391, FAX: +86-871-5031391, Email: zhaohyau@yahoo.com.cn.
References
- Aoki T. Incorporation of individual casein constituents into casein aggregates cross-linked by colloidal calcium phosphate in artificial casein micelles. J Dairy Res. 1989;56:613–618. doi: 10.1017/S0022029900029137. [DOI] [Google Scholar]
- Aoki T, Kako Y, Imamura T. Separation of casein aggregates cross-linked by colloidal calcium phosphate from bovine casein micelles by high performance gel chromatography in the presence of urea. J Dairy Res. 1986;53:53–59. doi: 10.1017/S0022029900024651. [DOI] [Google Scholar]
- Aoki T, Yamao Y, Yonemasu E, Kumasaki Y, Kako Y. Cross-linking of egg white riboflavin-binding protein by calcium phosphate. Arch Biochem Biophys. 1993;305:242–246. doi: 10.1006/abbi.1993.1417. [DOI] [PubMed] [Google Scholar]
- Chen PS, Toribara TY, Warner H. Microdetermination of phosphorus. Anal Chem. 1956;28:1756–1758. doi: 10.1021/ac60119a033. [DOI] [Google Scholar]
- Enomoto H, Li CP, Morizane K, Ibrahim HR, Sugimoto Y, Ohki S, Ohtomo H, Aoki T. Glycation and phosphorylation of β-lactoglobumin by dry-heating: effect on protein structure and some properties. J Agric Food Chem. 2007;55:2392–2398. doi: 10.1021/jf062830n. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Li CP, Morizane K, Ibrahim HR, Sugimoto Y, Ohki S, Ohtomo H, Aoki T. Improvement of functional properties of bovine serum albumin through phosphorylation by dry-heating in the presence of pyrophosphate. J Food Sci. 2008;73:C84–C91. doi: 10.1111/j.1750-3841.2007.00634.x. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Hayashi Y, Li CP, Ohki S, Ohtomo H, Shiokawa M, Aoki T. Glycation and phosphorylation of alpha-lactalbumin by dry heating: effect on protein structure and physiological functions. J Dairy Sci. 2009;92:3057–3068. doi: 10.3168/jds.2009-2014. [DOI] [PubMed] [Google Scholar]
- Hata I, Higashiyama S, Otani H. Identification of a phosphopeptide in bovine αs1-casein digest as a factor influencing proliferation and immunoglobulin production in lymphocyte cultures. J Dairy Res. 1998;65:569–578. doi: 10.1017/S0022029998003136. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Li CP, Enomoto H, Ibrahim HR, Sugimoto Y, Aoki T. Improvement of functional properties of ovotransferrin by phosphorylation through dry-heating in the presence of pyrophosphate. Asian-Australas J Anim Sci. 2008;21:596–602. [Google Scholar]
- Kamasaka H, Uchida M, Kusaka K, Yoshikawa K, Yamamoto K, Okada S, Ichikawa T. Inhibitory effect of phosphorylatedoligosaccharides prepared from potato starch on the formation of calcium phosphate. Biosci Biotechnol Biochem. 1995;59:1412–1416. doi: 10.1271/bbb.59.1412. [DOI] [PubMed] [Google Scholar]
- Kerr RW, Cleveland FC (1957) Process for the preparation of distarch phosphate and the resulting product. US Patent 2,801,242
- Kitazawa H, Itoh T, Tomioka Y, Mizugaki M, Yamaguchi T. Induction of IFN-γ and IL-1α production in macrophages stimulated with phosphopolysaccharide produced by Lactococcus lactis ssp. cremoris. Int J Food Microbiol. 1996;31:99–106. doi: 10.1016/0168-1605(96)00968-3. [DOI] [PubMed] [Google Scholar]
- Kitazawa H, Harata T, Uemura J, Saito T, Kaneko T, Itoh T. Phosphate group requirement for mitogenic activation of lymphocytes by an extracellular phosphopolysaccharide from Lactobacillus delbrueckii ssp. bulgaricus. Int J Food Microbiol. 1998;40:169–175. doi: 10.1016/S0168-1605(98)00030-0. [DOI] [PubMed] [Google Scholar]
- Li CP, Salvador AS, Ibrahim HR, Sugimoto Y, Aoki T. Phosphorylation of egg white proteins by dry-heating in the presence of phosphate. J Agric Food Chem. 2003;51:6808–6815. doi: 10.1021/jf030043+. [DOI] [PubMed] [Google Scholar]
- Li CP, Ibrahim HR, Sugimoto Y, Hatta H, Aoki T. Improvement of functional properties of egg white protein through phosphorylation by dry-heating in the presence of pyrophosphate. J Agric Food Chem. 2004;52:5752–5758. doi: 10.1021/jf0498259. [DOI] [PubMed] [Google Scholar]
- Li CP, Enomoto H, Ohki S, Ohtomo H, Aoki T. Improvement of functional properties of whey protein isolate through glycation and phosphorylation by dry-heating. J Dairy Sci. 2005;88:4137–4145. doi: 10.3168/jds.S0022-0302(05)73099-X. [DOI] [PubMed] [Google Scholar]
- Lim S, Seib PA. Preparation and pasting properties of wheat and corn starch phosphates. Cereal Chem. 1993;70:137–144. [Google Scholar]
- Nakano T, Salvador AS, Tamochi J, Sugimoto Y, Ibrahim HR, Toba Y. Phosphorylation of starch and dextrin by dry-heating in the presence of phosphate, and their calcium phosphate-solubilizing ability. Nahrung/Food. 2003;47:274–278. doi: 10.1002/food.200390064. [DOI] [PubMed] [Google Scholar]
- Nordin BEC, Heaney RP. Calcium supplementation of the diet: Justified by present evidence. Brit Med J. 1990;300:1056–1060. doi: 10.1136/bmj.300.6731.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani H, Kitamura H, Park M, Kihara Y, Oshida T, Kusuhara S, Sawada K. Enhancement of intestinal IgA levels in piglets by oral administration of a commercially available casein phosphopeptide preparation. Milchwissenschaft. 2000;55:429–432. [Google Scholar]
- Otani H, Morimoto T, Kawahara T. Oral ingestion of phosphorylated dextrin stimulates antibody responses in mice. J Nutr Sci Vitaminol. 2007;53:354–357. doi: 10.3177/jnsv.53.354. [DOI] [PubMed] [Google Scholar]
- Sang Y, Prakash O, Seib PA. Characterization of phosphorylated cross-linked resistant starch by 31P nuclear magnetic resonance (31P-NMR) spectroscopy. Carbohydr Polym. 2007;67:201–212. doi: 10.1016/j.carbpol.2006.05.009. [DOI] [Google Scholar]
- Sang Y, Seib PA, Herrera AI, Prakash O, Shi YC. Effects of alkaline treatment on the structure of phosphorylated wheat starch and its digestibility. Food Chem. 2010;118:323–327. doi: 10.1016/j.foodchem.2009.04.121. [DOI] [Google Scholar]
- Sato R, Noguchi T, Naito H. Casein phosphopeptide (CPP) enhances calcium absorption from the ligated segment of rat small intestine. J Nutr Sci Vitaminol. 1986;32:67–76. doi: 10.3177/jnsv.32.67. [DOI] [PubMed] [Google Scholar]
- Schmidt DG. In: Milk proteins, vol. 1. Fox PF, editor. London: Applied Science Publishers; 1982. pp. 61–68. [Google Scholar]
- Sitohy MZ, Labib SM, EI-Saadany SS, Ramadan MF. Optimizing the conditions for starch dry phosphorylation with sodium mono- and dihydrogen orthophosphate under heat and vacuum. Starch. 2000;52:95–100. doi: 10.1002/1521-379X(200006)52:4<095::AID-STAR95>3.0.CO;2-X. [DOI] [Google Scholar]
- Tarelli E, Wheeler SF. Drying from phosphate-buffered solutions can result in the phosphorylation of primary and secondary alcohol groups of saccharides, hydroxlyated amino acids, proteins, and glycoproteins. Anal Biochem. 1994;222:196–201. doi: 10.1006/abio.1994.1473. [DOI] [PubMed] [Google Scholar]
- Tarelli E, Wheeler SF. The preparation of the four monophosphates of α, α’-trehalose from trehalose and sodium phosphate. Carbohydr Res. 1994;261:25–36. doi: 10.1016/0008-6215(94)80003-0. [DOI] [Google Scholar]
- Tarelli E, Lemercinierm X, Wheeler S. Direct preparation of cyclodextrin monophosphates. Carbohydr Res. 1997;302:27–34. doi: 10.1016/S0008-6215(97)00108-0. [DOI] [PubMed] [Google Scholar]
- Toba Y, Kato K, Takada Y, Tanaka M, Nakano T, Aoki T, Aoe S. Bioavailability of milk micellar calcium phospate-phosphopeptide complex in rats. J Nutr Sci Vitaminol. 1999;45:311–323. doi: 10.3177/jnsv.45.311. [DOI] [PubMed] [Google Scholar]
- Van Kemenade MJJM, De Bruyn PL. The influence of casein on the kinetics of hydroxyapatite precipitation. J Colloid Interface Sci. 1989;129:1–14. doi: 10.1016/0021-9797(89)90411-6. [DOI] [Google Scholar]
- Woo K, Seib PA. Cross-linking of wheat starch and hydroxypropylated wheat starch in alkaline slurry with sodium trimetaphosphate. Carbohydr Polym. 1997;33:263–271. doi: 10.1016/S0144-8617(97)00037-4. [DOI] [Google Scholar]
- Yamamoto K, Kumagai H, Sakiyama T, Song CM, Yano T. Inhibitory activity of alginates against the formation of calciumphosphate. Biosci Biotechnol Biochem. 1992;56:90–93. doi: 10.1271/bbb.56.90. [DOI] [Google Scholar]