Abstract
Antioxidant hydrolysates from soybean have the potential as the new antioxidants, but the bitterness limites their application. A study on the debittering of the soybean antioxidant hydrolysates with β-cyclodextrins and the effects of the debittering conditions on the reducing power of the peptides was conducted using response surface methodology (RSM). The coefficient of determination, R2 values for bitterness and reducing power were 0.883 and 0.902 respectively. Reducing power of the soybean hydrolysates varied curvilinearly with increase of temperature, mass fraction of β-cyclodextrin, and incubation time. The optimum conditions to obtain the hydrolysates with the minimum bitterness and the maximum reducing power were: temperature 38.50 °C, the mass fraction of β-cyclodextrin 2.00%, and incubation time 12 min, The resulting response functions under this conditions were the reducing power (OD700 nm) of 0.453 and bitterness of 0.290, which was under the threshold for the detection of bitterness taste.
Keywords: Debittering, Soybean, Antioxidant hydrolysates, β-cyclodextrins, Optimization, Response surface methodology
Introduction
Soybeans protein isolates (SPIs) have high nutritional value, their proteolysis products have been correlated with specific bioactivity and hence the excellent source of bioactive peptides. The researches showed that soybean peptides had angiotensin I-converting enzyme inhibitor activity (Chiang et al. 2006; Wu and Ding 2002), hypocholesterolemic activity (Pak et al. 2005; Zhong et al. 2007), anti-alopecia activity (Tsuruki et al. 2005), immunostimulative activity (Chen et al. 1995), anticancer activity (Mejia 2006) and antioxidant activity (Moure et al. 2006). Antioxidant activity is especially important. Free radicals and other reactive oxygen species are normally produced during the energy production reactions in living organisms, and they may cause destruction to living organisms or the deterioration of food quality when they are in excess. Many artificial antioxidants such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT) and propyl gallate (PG) have potential health hazards and their applications must be under strict regulation (Park et al. 2001). The development of natural antioxidants as alternatives is of great interest for researchers. On the other hand, the hydrolysates produced by proteolytic hydrolysis (Korhonen and Pihlanto 2006; Mao et al. 2007; Rossini et al. 2009; Salampessy et al. 2010; Ma et al. 2010) are frequently accompanied by a bitter taste. The bitter taste limits the utilization of the hydrolysates in the food industry. Methods for debittering of the hyhrolysates include selective separation, masking of bitter taste, and further enzymatic hydrolysis of bitter peptides (Saha and Hayashi 2001; Nishiwaki et al. 2002). However, these methods have some disadvantages such as the loss of essential amino acids, increasing the product viscosity, low industry feasibility and so on. Cyclodextrins (CDs) are cyclic oligosacchrides with a torous-shaped hydrophobic cavity, and can form the inclusion complexes with the hydrophobic animo acids or the functions of the peptides, thus masking the bitter taste of the protein hydrolysates (Linde et al. 2009). Tamura et al. (1990) noticed that cyclodextrins can mask bitterness by wraping the hydrophobic functions of peptides inside their ring structures using model amino acids and peptides. Also, the inclusion complexes with β-cyclodextrin improved the thermal stability of nutraceutical antioxidants (Kalogeropoulos et al. 2010). Thus, treatment with cyclodextrin may have advantages of preserving antioxidant products and modification of disagreeable taste. However, investigation of the debittering of soybean hydrolysates using cyclodextrins and its effect on the antioxidant activity of the hydrolysates has not been previously reported.
For debittering of the hydrolysates with cyclodextrins, several factors, such as mass fraction of cyclodextrins, incubation time and temperature may affect the efficiency of debitterness, and the factors may act independently or interactively. Response surface methodology (RSM) is a desirable tool for optimizing the process when many factors affect the target response. The objectives of the present work were (1) to investigate the effect of mass fraction of cyclodextrins, incubation time, and temperature on the bitterness of the peptides; (2) to study the effect of the debittering conditions on the antioxidant activities of the soybean peptides; and (3) to obtain the optimal conditions to yield the minimum bitterness and the maximum antioxidant activity using RSM;
Materials and methods
Materials
SPIs used in the experiment were supplied by Zhengzhou Yangguang Food Ingredients Co. Ltd. (Zhengzhou, China). The sample contained protein (91.70%), crude fat (1.23%), and water (6.46%).
Alkalase was purchased from Novozyme Biotechnology Co., Ltd. (Tianjin, China). Alkalase hydrolyzes peptide bonds in the interior of the protein generating various polypeptides, depending on the extent of hydrolysis (Hamada 2000). The tested enzyme activity of Alkalase was 1286850.00 U/mL. β-cyclodextrin was purchased from Wuhan Yinhe Chemical engineering Co. Ltd. (Wuhan, China). And Kuding Tea was purchase in the Huaruiwanjia Supermarket (Zhengzhou, China). All the other reagents were of analytical grade.
Protein hydrolysis
SPI was added phosphate buffer (0.2 mM, pH 8.0) to reach the final mass fraction of 2.00%, and heated to 70 °C for 15 min. After cooling, the solution was adjusted to pH 8.0 with phosphate buffer (0.2 mM, pH 8.0), and added Alcalase at the mass fraction of 6% (on the basis of weight of SPI) and the final activity of enzyme in reaction system was 1544.20 U/mL. During the hydrolysis performed in a thermostatic water bath shaker (HH-S Model, Jintan Medical Instruments Co., Jintan, China) with constant agitation, the desirable pH value of the solution was kept by addition of 0.2 mol/L NaOH. The reaction was carried out at 55 °C for 120 min, and terminated by putting the reaction vessel into the water bath (100 °C for 10 min) with stirring to inactivate the protease. Degree of hydrolysis (DH) was calculated by measuring the amount of alkali consumed (Adler-Nissen 1986). The degree of hydrolysis, bitterness score and reducing power (OD700 nm) of the soybean hydrolysate were 15.80%, 5.0, and 0.464 respectively, and reducing power of BHT (5 μg/mL) was 0.132.
Treatment of the hydrolysates with β-cyclodextrins
The soybean peptide solutions were treated with the varing mass fractions of β-cyclodextrin (0.50%, 1.00%, 1.50%, 2.00%, 2.50%) at varing temperatures (20 °C, 30 °C, 40 °C, 50 °C, 55 °C,) with constant agitation for either 2 min, 5 min, 10 min, 15 min or 25 min.
Reducing power assay
The determination of the reducing power was performed as described by Athukorala et al. (2006). Soybean peptides samples (1.0 mL) were mixed with 2.5 mL of phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of 1% potassium ferricyanide. The mixture was kept in a 50 °C water bath for 20 min and then cooled quickly in the ice water. Then, samples were kept at room temperature, added with 2.5 mL of 10% trichloroacetic acid (TCA) and after being mixed evenly, centrifuged at 3,000 g for 10 min. Finally, 2.5 mL of the supernatant was mixed with 2.5 mL distilled water and 0.5 mL of 0.1% ferric chloride and incubated for 10 min at room temperature. The absorbance of the samples was read at 700 nm with a Visible spectrophotometer (WFJ7200 model, Shanghai Unico Instruments Co., Shanghai, China). Butylated hydroxytoluene (BHT) (5 μg/mL) was used as the control.
Sensory evaluation
Sensory analysis was performed according to Wang et al. (2007) with little modification. The bitterness of each sample was determined by a panel of ten people. Before the sample was tasted, the mouth was fully rinsed with distilled water. The sample solution was tested in the mouth for 10 s, and the taste of each sample was averaged. Kuding Tea, a traditional Chinese drink, has the thick bitterness and was used as the reference. In the experiment, 1 g of Kuding Tea was added 1 L of the distilled water, boiled for 1 h, and filtered using a Buchner funnel. The volume of the solution was fixed to 1,000 mL, and then diluted with the distilled water to 175 mg/L, 150 mg/L, 125 mg/L, 100 mg/L, 75 mg/L, and 50 mg/L respectively, and the bitterness of the solutions prepared was scored 5.0, 4.0, 3.0, 2.0, 1.0, and 0 accordingly.
Experimental design and statistical analysis
In the present work, RSM with three factors and five levels was used in designing this experiment. Design-expert 7.0 software package (Stat-Ease Inc., USA) was employed to generate the experimental design, statistical analysis and regression model. Three main factors namely temperature (χ1), mass fraction of the β-cyclodextrin (χ2), and incubation time (χ3) were chosen as the independent variables. Each independent variables had five levels which were −1.682, −1.000, 0, 1.000 and 1.682. The coded (χ1) and actual (X) levels of variables in the experimental design were shown in Table 1. The responses (y) were bitterness and reducing power. The response functions (y1, y2) were related to the coded variables (χ1, χ2, χ3) by a second order polynomial (Eq. 1) using the method of least squares.
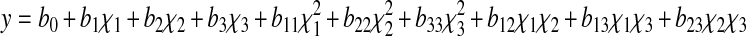 |
1 |
where y is the response variable, χ1, χ2 and χ3 are the coded independent variables, b0, b1, b2, b3, b11, b22, b33, b12, b13, and b23 are the regression coefficients of variables for intercept, linear, quadratic and interaction regression terms, respectively. Analysis of variance (ANOVA) tables were generated, and the effects and regression coefficients of individual linear, quadratic and interaction regression terms were determined. The significances of all terms in the polynomial were tested statistically using Student t-test. The regression coefficients were employed for statistical calculations to generate response surfaces and contour plots.
Table 1.
Independent variables and their levels used in the RSM experimental design
| Levels | Temperature (°C) | Mass fraction of the β-cyclodextrin (%) | Incubation time (min) |
|---|---|---|---|
| X1 (χ1) | X2 (χ2) | X3 (χ3) | |
| −1.682 | 23.182 | 0.659 | 1.591 |
| −1.000 | 30.000 | 1.000 | 5.000 |
| 0.000 | 40.000 | 1.500 | 10.000 |
| 1.000 | 50.000 | 2.000 | 15.000 |
| 1.682 | 56.818 | 2.341 | 18.409 |
Results and discussion
Statistical analysis
RSM was used to develop a prediction model for optimizing the debittering conditions with β-cyclodextrin. The experimental conditions and the corresponding values from the experimental design were presented in Table 2.
Table 2.
Central composite rotatable design (CCRD) and responses
| RUN | Independent variables | Responses (Y) | |||
|---|---|---|---|---|---|
| Temperature (°C) | Mass fraction of the β-cyclodextrin (%) | Incubation time (min) | Y1 (Bitterness) | Y2 (OD700 nm) | |
| 1 | −1 | −1 | −1 | 4.0 | 0.427 |
| 2 | −1 | −1 | 1 | 3.0 | 0.435 |
| 3 | −1 | 1 | −1 | 2.0 | 0.447 |
| 4 | −1 | 1 | 1 | 2.0 | 0.454 |
| 5 | 1 | −1 | −1 | 3.0 | 0.420 |
| 6 | 1 | −1 | 1 | 2.0 | 0.432 |
| 7 | 1 | 1 | −1 | 2.0 | 0.440 |
| 8 | 1 | 1 | 1 | 1.0 | 0.439 |
| 9 | −1.682 | 0 | 0 | 4.0 | 0.447 |
| 10 | 1.682 | 0 | 0 | 2.0 | 0.435 |
| 11 | 0 | −1.682 | 0 | 2.0 | 0.422 |
| 12 | 0 | 1.682 | 0 | 0.0 | 0.459 |
| 13 | 0 | 0 | −1.682 | 2.0 | 0.424 |
| 14 | 0 | 0 | 1.682 | 1.0 | 0.442 |
| 15 | 0 | 0 | 0 | 1.0 | 0.446 |
| 16 | 0 | 0 | 0 | 2.0 | 0.439 |
| 17 | 0 | 0 | 0 | 1.0 | 0.449 |
| 18 | 0 | 0 | 0 | 1.0 | 0.446 |
| 19 | 0 | 0 | 0 | 1.0 | 0.438 |
| 20 | 0 | 0 | 0 | 1.0 | 0.445 |
| 21 | 0 | 0 | 0 | 1.0 | 0.437 |
| 22 | 0 | 0 | 0 | 1.0 | 0.443 |
| 23 | 0 | 0 | 0 | 1.0 | 0.443 |
The regression coefficients of the variables in the models and results of analysis of variance were presented in Table 3.
Table 3.
Regression coefficients, and the results of analysis of variance (ANOVA) of the regression parametres for the response surface models
| Coefficients of the regression equation | Bitterness | Reducing power (OD700 nm) |
|---|---|---|
| b0 | 0.991 | 0.443 |
| b1 | −0.552b | −0.0053b |
| b2 | −0.727c | 0.009c |
| b3 | −0.347a | 0.004b |
| b11 | 0.762c | −0.003 |
| b22 | 0.055 | −0.001 |
| b33 | 0.356a | −0.003b |
| b12 | 0.113 | −0.002 |
| b13 | 0.213 | −0.001 |
| b23 | 0.088 | −0.002 |
| R2 | 0.883 | 0.902 |
| p | <0.0001c | <0.0001c |
aSignificant at p ≤ 0.05
bSignificant at p ≤ 0.01
cSignificant at p ≤ 0.001
The statistical analysis results showed R2 values for responses were satisfactorily greater than 0.800. Joglekar and May (1987) suggested R2 should be at aleast 0.800 to present a model of a good fit. The R2 values for bitterness and the reducing power were 0.883 and 0.902 respectively. The probability (p) values of all regresssion models were less than 0.001. Therefore, the proposed models were adequate for presenting the real relationship among the parameters chosen.
Effects of independent variables on responses
Bitterness
Table 3 showed that the linear term temperature (p ≤ 0.01), mass fraction of β-cyclodextrin (p ≤ 0.001) and incubation time (p ≤ 0.05) had significantly negative effects on bitterness. The quadratic terms of temperature (p ≤ 0.001) and incubation time (p ≤ 0.05) had significant effects on bitterness, indicating that the two independent variables had non-linear effects on bitterness. All the interaction terms were not significant (p > 0.05).
The response surface plot by the Design-expert software is showen in Fig. 1. It is observed that bitterness decreased at the beginning and then then increased gradually with increase in temperature. These changes in bitterness are probably the results of the following factors: (i) incubation teperature covered the range of 23.18–56.82 °C in the study, some peptides in the hydrolysates might be denatured at their denaturation temperature, start to unfold, expose the hydrophobic residues buried inside the molecules, facilitate the wrapping the hydrophobic groups with β-cyclodextrin; and thus contributing to the lower bitterness; (ii) inclusion of hydrophobic groups with β-cyclodextrin would be a reversible reaction depending on temperature, its kinetic constant might reach the maximum at certain optimum temperature btween the range of 23.18–56.82 °C. Zhong et al. (2009) determined the stability constant of complexation of resveratrol with β-cyclodextrins by phase-solubility measurements.
Fig. 1.
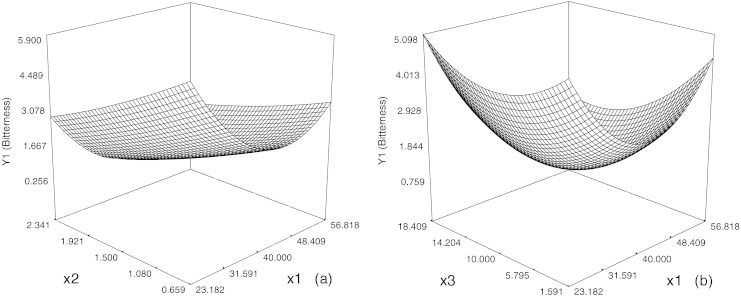
Response surface diagram for the effects of temperature (x1) and mass fraction (x2) (a), and those of temperature (x1) and incubation time (x3) (b) on the bitterness of soybean hydrolysates
At fixed temperature and incubation time, bitterness decreased linearly with the increasing dosage of β-cyclodextrin. Hydrolysate bitterness is closely correlated to the low molecular mass peptides containing hydrophobic amino acid residues (Kim and Li-Chan 2006). β-cyclodextrin are able to form the reversible complexes with the hydrophobic amino acids and small peptide fragments with such amino acids, which may lead to the physicochemical change of peptides, or avoiding the detection of the amino acid taste (Linde et al. 2010). The result could be due to the fact that more hydrophobic functions of the peptides are wrapped in the hydrophobic cavities of β-cyclodextrin with the increases in the mass fraction of β-cyclodextrin, and thus resulting in the lower bitterness.
The variation of the bitterness with temperature and incubation time at the constant dosage of β-cyclodextrin is presented in Fig. 1b. Incubation time showed a quadratic effect on the response, hence bitterness decreased at the start and then increased with incubation time increasing.
Reducing power
The reducing power of a compond is one of the important indicator of its potantial antioxidant capacity (Meir et al. 1995) and indicates the potential of the compound to donoate its electron. The result of Pan et al. (2007) indicated a positive correlation between the resucing power and the antioxidant activity of Polygonum cuspidatum extract.
It is clear from Table 3 that reducing power is negatively affected by the linear term of temperature (p < 0.01) and quadratic term of incubation time (p < 0.01). Table 3 also showed that the linear terms of the mass fraction of β-cyclodextrin (p < 0.001) and incubation time (p < 0.01) had a positive effect on the reducing power.
The variation of the reducing power with temperature and mass fraction of β-cyclodextrin at fixed incubation time is represented in Fig. 2. It may be observed that the reducing power decreased with the increasing temperature and increased linearly when the amount of β-cyclodextrin increased. It is also clear from Fig. 2b that the reducing power decreased slowly at the start and then increased with the incubation time increasing.
Fig. 2.
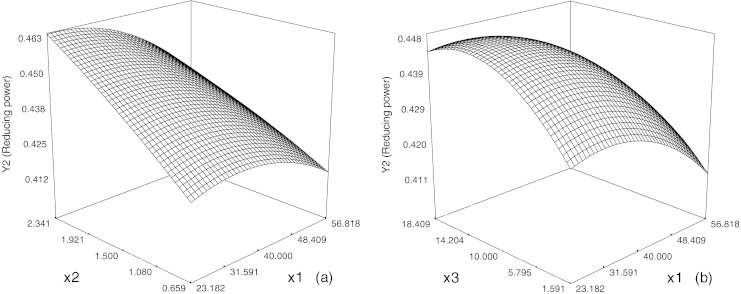
Response surface diagram for the effects of temperature (x1) and mass fraction (x2) (a), and those of temperature (x1) and incubation time (x3) (b) on the reducing power of soybean hydrolysates
Optimum conditions
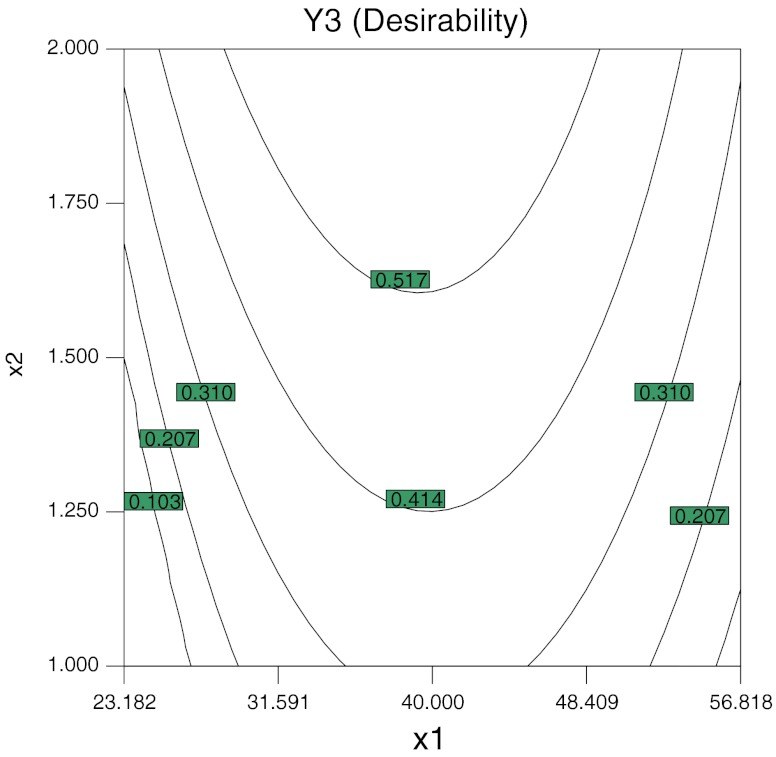
In order to obtain the desirable product, the minimum bitterness and the maximum reducing power of the hydrolysates were set as the goal (Desirability). And because the unpleasant flavour occurred when mass fraction of β-cyclodextrin was greater than 2.00%, so the range of mass fraction of β-cyclodextrin was set between 1.00% (coded level: −1.0) and 2.00% (coded level: 1.0), the superimposed contour plot was obtained and the optimial conditions were investigated. Figure 3 shows the superimposed contour plot to optimize bitterness and the reducing power keeping the incubation time unvaried at the central point. It is shown in the superimposed contour plot (Fig. 3) that the zone of optimization depicts temperature to be in the range of 35–45 °C and the mass fraction of the β-cyclodextrin between 1.75 and 2.00%.
Fig. 3.
Superimposed contour plots for optimization of bitterness and the reducing power while keeping incubation time constant at central point (10 min)
Similarly, keeping the temperature and the mass fraction of the β-cyclodextrin constant as determined from Fig. 3, the best combination of response function can be determined, and the best combinations of process variables for response functions are found. The process condition for best combination of response functions was found to be 2.0% mass fraction of the β-cyclodextrin at 38.5 °C for 12 min. The resulting response functions under this conditons were reducing power (OD700 nm) of 0.453, and bitterness of 0.290, which was less than 1 and under the threshold for the detection of bitterness taste.
Conclusions
RSM was used to study the debittering condition of the soybean antioxidant hydrolysates with β-cyclodextrins and to sudy the effects of the debittering conditions on the reducing power of the peptides. Reducing power of the soybean hydrolysates varied curvilinearly with increase of temperature, mass fraction of β-cyclodextrin, and incubation time.The optimum conditions to obtain the hydrolysates with the minimum bitterness and the maximum reducing power were: temperature 38.5 °C, the mass fraction of β-cyclodextrin 2.0%, and incubation time 12 min, The resulting response functions under this conditons were the reducing power (OD700 nm) of 0.453 and bitterness of 0.290, which was under the threshold for the detection of bitterness taste.
Acknowledgements
This work was supported by the Program of Imported Brains of Henan Technology University (Grant No. 2007BS034) and the Program for Science and Technology Development of Zhengzhou (Grant No. 083SGYG25121-6).
Contributor Information
Lixia Hou, Email: doctorhou2003@yahoo.com.cn.
Jinshui Wang, Phone: +86-371-67789970, FAX: +86-371-67789817, Email: jinshuiw@163.com.
References
- Adler-Nissen J. Enzymic hydrolysis of food proteins. New York: Elsevier Applied Science Publisher; 1986. [Google Scholar]
- Athukorala Y, Kim KN, Jeon YJ. Antiproliferative and antioxidant properties of an enzymatic hydrolysate from brown alga, Ecklonia cava. Food Chem Toxicol. 2006;44:1065–1074. doi: 10.1016/j.fct.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Chen J, Suetsuna K, Yamauchi F. Isolation and characterization of immunostimulative peptides from soybean. Nutr Biochem. 1995;6:310–313. doi: 10.1016/0955-2863(95)00022-R. [DOI] [Google Scholar]
- Chiang WD, Tsou MJ, Tsai ZY, Tsai TC. Angiotensin I-converting enzyme inhibitor derived from soy protein hydrolysate and produced by using membrane reactor. Food Chem. 2006;98:725–732. doi: 10.1016/j.foodchem.2005.06.038. [DOI] [Google Scholar]
- Hamada JS. Characterization and functional properties of rice bran proteins modified by commercial exoproteases and endoproteases. Food Chem Toxicol. 2000;65:305–310. [Google Scholar]
- Joglekar AM, May AT. Product excellence through design of experiments. Cereal Foods World. 1987;32:857–868. [Google Scholar]
- Kalogeropoulos N, Yannakopoulou K, Gioxari A, Chiou A, Makris DP. Polyphenol characterization and encapsulation in β-cyclodextrin of a flavonoid-rich Hypericum perforatum (St John’s wort) extract. LWT Food Sci Technol. 2010;43:882–889. doi: 10.1016/j.lwt.2010.01.016. [DOI] [Google Scholar]
- Kim HO, Li-Chan ECY. Quantitative structure-activity relationship study of bitter peptides. J Agric Food Chem. 2006;54:10102–10111. doi: 10.1021/jf062422j. [DOI] [PubMed] [Google Scholar]
- Korhonen H, Pihlanto A. Bioactive peptides: production and functionality. Int Dairy J. 2006;9:945–960. doi: 10.1016/j.idairyj.2005.10.012. [DOI] [Google Scholar]
- Linde GA, Junior AL, Faria EV, Colauto NB, Moraes FF, Zanin GM. Taste modification of amino acids and protein hydrolysate by α-cyclodextrin. Food Res Int. 2009;42:814–818. doi: 10.1016/j.foodres.2009.03.016. [DOI] [Google Scholar]
- Linde GA, Junior AL, Faria EV, Colauto NB, Moraes FF, Zanin GM. The use of 2D NMR to study β-cyclodextrin complexation and debittering of amino acids and peptides. Food Res Int. 2010;43:187–192. doi: 10.1016/j.foodres.2009.09.025. [DOI] [Google Scholar]
- Ma Y, Xiong Y, Zhai J, Zhu H, Thomas D. Fractionation and evaluation of radical scavenging peptides from in vitro digests of buckwheat protein. Food Chem. 2010;118:582–588. doi: 10.1016/j.foodchem.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao XY, Ni JR, Sun WL, Hao PP, Li F. Value-added utilization of yak milk casein for the production of angiotensin-I-converting enzyme inhibitory peptides. Food Chem. 2007;103:1282–1287. doi: 10.1016/j.foodchem.2006.10.041. [DOI] [Google Scholar]
- Meir S, Kanner J, Akiri B, Philosoph-Hadas S. Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves. J Agric Food Chem. 1995;43:1813–1817. doi: 10.1021/jf00055a012. [DOI] [Google Scholar]
- Mejia E. Soybean bioactive peptides: a new horizon in preventing chronic diseases. Sex Reprod Meno. 2006;4(2):91–95. doi: 10.1016/j.sram.2006.08.012. [DOI] [Google Scholar]
- Moure A, Domínguez H, Parajó JC. Antioxidant properties of ultrafiltration-recovered soy protein fractions from industrial effluents and their hydrolysates. Process Biochem. 2006;41:447–456. doi: 10.1016/j.procbio.2005.07.014. [DOI] [Google Scholar]
- Nishiwaki T, Yoshimizu S, Furuta M, Hayashi K. Debittering of enzymatic hydrolysates using an aminopeptidase from the edible Basidiomycete Grifola frondosa. J Biosci Bioeng. 2002;93:60–63. [PubMed] [Google Scholar]
- Pak VV, Koo MS, Kasymova TD, Kwon DY. Isolation and identification of peptides from soy 11S-globulin with hypocholesterolemic activity. Chem Nat Compd. 2005;41(6):710–714. doi: 10.1007/s10600-006-0017-6. [DOI] [Google Scholar]
- Pan Y, Zhang X, Wang H, Liang Y, Zhu J, Li H, Zhang Z, Wu Q. Antioxidant potential of ethanolic extract of Polygonum cuspidatum and application in peanut oil. Food Chem. 2007;105:1518–1524. doi: 10.1016/j.foodchem.2007.05.039. [DOI] [Google Scholar]
- Park PJ, Jung WK, Nam KD, Shahidi F, Kim SK. Purification and characterization of antioxidative peptides from protein hydrolysate of lecithin-free egg yolk. J Am Oil Chem. 2001;78:651–656. doi: 10.1007/s11746-001-0321-0. [DOI] [Google Scholar]
- Rossini K, Noreña CPZ, Cladera-Olivera F, Brandelli A. Casein peptides with inhibitory activity on lipid oxidation in beef homogenates and mechanically deboned poultry meat. LWT Food Sci Technol. 2009;42:862–867. doi: 10.1016/j.lwt.2008.11.002. [DOI] [Google Scholar]
- Saha BC, Hayashi K. Debittering of protein hydrolyzates. Biotechnol Adv. 2001;19:355–370. doi: 10.1016/S0734-9750(01)00070-2. [DOI] [PubMed] [Google Scholar]
- Salampessy J, Phillips M, Seneweera S, Kailasapathy K. Release of antimicrobial peptides through bromelain hydrolysis of leatherjacket (Meuchenia sp.) insoluble proteins. Food Chem. 2010;120:556–560. doi: 10.1016/j.foodchem.2009.10.054. [DOI] [Google Scholar]
- Tamura M, Mori N, Miyoshi T, Koyama S, Kohri H, Okai H. Practical debittering using model peptides and related compounds. Agric Biol Chem. 1990;54:41–51. doi: 10.1271/bbb1961.54.41. [DOI] [PubMed] [Google Scholar]
- Tsuruki T, Takahata K, Yoshikawa M. Anti-alopecia mechanisms of soymetide-4, an immunostimulating peptide derived from soy β-conglycinin. Peptides. 2005;26:707–711. doi: 10.1016/j.peptides.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Wang S, Liu F, Shu K. Enzymatic hydrolysis of soybean protein isolate and Debittering of soybean peptides (in Chinese) Mod Food Tech. 2007;23:54–57. [Google Scholar]
- Wu J, Ding X. Characterization of inhibition and stability of soy-protein-derived angiotensin I-converting enzyme inhibitory peptides. Food Res Int. 2002;35:367–375. doi: 10.1016/S0963-9969(01)00131-4. [DOI] [Google Scholar]
- Zhong F, Zhang X, Ma J, Shoemaker CF. Fractionation and identification of a novel hypocholesterolemic peptide derived from soy protein Alcalase hydrolysates. Food Res Int. 2007;40:756–762. doi: 10.1016/j.foodres.2007.01.005. [DOI] [Google Scholar]
- Zhong L, Cheng B, Hu Y, Zhang Y, Zou G. Complexation of resveratrol with cyclodextrins: solubility and antioxidant activity. Food Chem. 2009;113:17–20. doi: 10.1016/j.foodchem.2008.04.042. [DOI] [Google Scholar]