Abstract
Circadian clocks have been described in each tissue of the hypothalamo-pituitary-ovarian axis. Although a role for the clock in the timing of ovulation is indicated, the impact of diseases that disrupt fertility on clock function or the clocks' role in the etiology of these pathologies has yet to be fully appreciated. Polycystic ovary syndrome (PCOS) is a particularly devastating endocrinopathy, affecting approximately 10% of women at childbearing age. Common features of PCOS are a polycystic ovary, amenorrhea, and excess serum androgen. Approximately 40% of these women have metabolic syndrome, including hyperinsulinemia, dyslipidemia, and hyperleptinemia. It has been suggested that excess androgen is a critical factor in the etiology of PCOS. We have examined the effects of androgen excess during puberty on the phase of circadian clocks in tissues of the metabolic and hypothalamo-pituitary-ovarian axes. Female period1-luciferase (per1-luc) rats were exposed to androgen (5α-dihydrotestosterone [DHT]) or placebo for 4-6 weeks (short term) or 9-15 weeks (long term). As expected, DHT-treated animals gained more weight than controls and had disrupted estrous cycles. At the end of treatment, tissues, including the liver, lung, kidney, white adipose, cornea, pituitary, oviduct, and ovarian follicles, were cultured, and per1-luc expression in each was recorded. Analysis of per1-luc expression revealed that DHT exposure increased phase distribution of multiple oscillators, including ovarian follicles, liver, and adipose, and altered phase synchrony between animals. These data suggest that excess androgen during puberty, a common feature of PCOS, negatively affects internal circadian organization in both the reproductive and metabolic axes.
Precise timing of physiological events is critical for normal reproductive physiology. In female mammals, the reproductive system exhibits robust circadian rhythmicity, which is normally kept tightly entrained to (synchronized with) the daily 12-hour light, 12-hour dark cycle (L:D cycle) (1, 2). One of the most salient features of the mammalian reproductive cycle is the preovulatory LH surge (3–6). In rats and mice, the LH surge occurs during the late afternoon and early evening of proestrus (3, 7). The timing of ovulation, also occurring in the early evening of proestrus, is thought to be entirely dependent on the timing of the LH surge (6, 8, 9). This rhythm relies on the activity of pacemaker neurons in the central circadian clock or suprachiasmatic nucleus (SCN) (10, 11) and positive feedback from ovarian estradiol (12). Lesions that destroy the SCN or mutations that alter the timing or output of SCN neurons are known to affect the timing and amplitude of the LH surge, block ovulation, and disrupt reproductive cycles (13, 14).
The molecular substrate for circadian rhythms is a feedback loop of interacting transcriptional regulators or clock genes, including the transcriptional enhancer bmal1 and the repressors period (1,2) and cryptochrome (1,2) (15). It is now widely accepted that the mammalian ovary contains cell-autonomous circadian clocks (16). Moreover, circadian clock gene expression has been reported at each level of the hypothalamo-pituitary-ovarian (HPO) axis, including the hypothalamic GnRH neurons and the pituitary gonadotroph (17). However, a functional role for the clock in these tissues, particularly in regard to ovarian physiology and fertility, has yet to be defined (17, 18). We have shown that the clock in the ovary times ovarian sensitivity to endocrine signals, including the rhythmic secretion of gonadotropins (19, 20). Others have linked circadian clock function to reproductive physiology, with particular emphasis on steroid hormone synthesis in rodents (21, 22) and birds (23). Mutations altering or abolishing clock gene expression have dramatic impacts on fertility (1, 2, 24, 25). Clocks have also been described in tissues of both the digestive and cardiovascular systems (26, 27). Disrupting rhythms of clock gene expression in these tissues results in a substantial decline in metabolic function, including altered insulin secretion, glucose homeostasis, and lipid metabolism (28–32).
Although polycystic ovary syndrome (PCOS) is a commonly diagnosed endocrinopathy in women, the etiology of the disease remains largely unknown (33). The primary symptoms of PCOS include abnormal ovarian physiology, specifically anovulation or oligoovulation, and polycystic ovarian morphology (34, 35). PCOS is often comorbid with a metabolic syndrome characterized by hyperinsulinemia, dyslipidemia, decreased insulin sensitivity, obesity, and increased risk of type 2 diabetes and cardiovascular disease (35, 36). Excess testosterone of follicular origin (hyperandrogenemia) is a common feature of PCOS (36–40). Several animal models of androgen-induced PCOS, most often using the nonaromatizable androgen 5α-dihydrotestosterone (DHT), have been developed in rodents. These include the prenatally androgenized (PNA) mouse (41) and, more recently, a pubertal exposure model in rats (42). Each of these models exhibits irregular reproductive cycles, disrupted gonadotropin secretion, abnormal follicular development, and the metabolic features common to clinical PCOS.
Mutations of the core clock genes have been linked to characteristics of the metabolic syndrome commonly associated with PCOS (43). However, the connection between fertility, metabolic homeostasis, and circadian clock function at the cellular level remains poorly understood. Metabolic syndrome and obesity are commonly associated with a decline in reproductive function, disrupted reproductive cycles, and attenuated gonadotropin secretion (44, 45). Circadian clock dysfunction is implicated in obesity and metabolic diseases like type 2 diabetes (46, 47). We hypothesize that disorders that reduce fertility and disturb metabolic function do so in part by altering clock gene expression in target tissues, thus disrupting the organization of the circadian timing system. This internal circadian disruption makes a significant contribution to the progression of the disease. To test this hypothesis, we have exposed period1-luciferase (per1-luc) transgenic rats to excess androgen during development. Our data reveal that excess androgen exposure during puberty significantly disrupts circadian organization. The free-running circadian rhythm of locomotor activity was not affected by excess androgen, indicating that clock function in the SCN, in agreement with the literature, was not affected by DHT exposure. However, the distribution of peak per1-luc expression in peripheral tissues across the 24-hour day, synchronization between peripheral clocks, and the phase relationship between peak per1-luc expression and the L:D cycle were each altered by excess androgen. These data suggest that internal circadian disruption may be a common feature of diseases that affect both reproductive function and metabolism.
Materials and Methods
Animals and pellet implant surgery
Weanling (21 d old) female per1-luc transgenic rats (Japanese Wistar strain) were anesthetized with isoflurane gas anesthesia, and a small incision was made in the intrascapular region. Animals received a sc placebo pellet (vehicle sham) or 90-day continuous release pellet (Innovative Research of America, Sarasota, Florida) containing 7.5 mg of DHT (daily dose, 83 μg) as in Ref. 42. This treatment is intended to produce androgen levels approximately 1.7-fold higher than normal, which is equivocal to the hyperandrogenemia in PCOS patients (42, 48). Animals were placed within our light-tight boxes in a L:D cycle (lights-on, 4 am to 4 pm). In each experiment, temperature and humidity were constant, and food (standard chow) and water were available ad libitum. Body weight was measured weekly, and reproductive cycles were monitored by daily vaginal smear. Only those control animals showing at least 2 consecutive 4-day estrous cycles were included in our experimental analyses. Placebo animals were killed on the afternoon of metestrus to avoid the potential impact of elevated ovarian steroids on internal circadian organization.
Behavioral analysis
Total cage activity was measured with infrared motion detectors (Quorum, Brookline Station, Missouri) attached to the cage top and recorded using ClockLab (Actimetrics, Evanston, Illinois). To determine the potential effect of androgen excess on the free-running period of activity, a cohort of DHT (n = 3)- and placebo (n = 4)-treated rats was kept under a L:D cycle for at least 3 weeks before being placed in constant darkness. The period (τ) of locomotor activity was determined during the first 2 weeks in constant darkness using a X2 periodogram (ClockLab).
Tissue culture and bioluminescence recording
After short-term (ST) (4–6 wk, ST) or long-term (LT) (9–15 wk, LT) exposure to DHT (ST, n = 7; LT, n = 16) or placebo control (ST, n = 8; LT, n = 15) pellets, rats were euthanized 3 hours before lights-off (Zeitgeber time [ZT], 9–12; lights-off, ZT12) by excess CO2 exposure, and samples of the liver, lung, kidney, perigonadal white adipose tissue (WAT), pituitary, individual ovarian follicles, oviduct, and cornea were collected in sterile Hanks' balanced salt solution. Tissues were placed in 35-mm culture dishes with 1.2 mL of culture medium (DMEM supplemented with B27 [GIBCO, Rockville, Maryland], 10mM HEPES, 352.5-μg/ml NaHCO3, 3.5-mg/ml D-glucose, 25-U/ml penicillin, 25-μg/ml streptomycin, and 0.1mM luciferin (Promega, Madison, Wisconsin)] and sealed with sterile vacuum grease and a glass coverslip. Sealed cultures were maintained at 35°C in a light-tight incubator, and bioluminescence was continuously recorded (counts per second) with an automated luminometer (LumiCycle; Actimetrics).
Data analysis
Raw luminescence data were detrended (24-h moving average) and smoothed (2-h moving average), and the peak of per1-luc expression on the first 24 hours in culture was recorded (Origin Pro 8.5; OriginLabs, Northampton, Massachusetts). The peak phase was recorded as ZT relative to the L:D cycle before killing, where ZT0 is time of lights-on. The peak phases of per1-luc expression for each tissue were converted from relative ZT to angles, plotted on circular Rayleigh plots, and analyzed using circular statistics (Oriana, Kovach Computing Services, Wales, United Kingdom). Phase distribution, commonly referred to as phase clustering or spread, among tissues within a group was analyzed using the Rayleigh test. A distribution of phases was considered “tight” at P < .01. The arithmetic mean phase was calculated for each group and analyzed across groups with the Watson-Williams F test (Oriana, significant at P < .05). Phase synchrony is defined as the spread of tissue phase from any single animal in an experimental group relative to the other animals in a group. It is also defined as the phase of an oscillator or group of tissues relative to the phase of the L:D cycle. Synchrony is heavily influenced by the number of tissues from a given animal peaking at a phase significantly different from the mean, such as a liver clock peaking in the middle of the day when most liver tissues peak during the night. To visualize the impact of androgen on phase synchrony, the phase of per1-luc expression in tissues from the same animal were plotted on horizontal phase “maps” with all other animals in that group (Origin Pro).
Results
Excess androgen during puberty disrupts reproductive cycles and increases body weight but does not affect the free-running period of locomotor activity
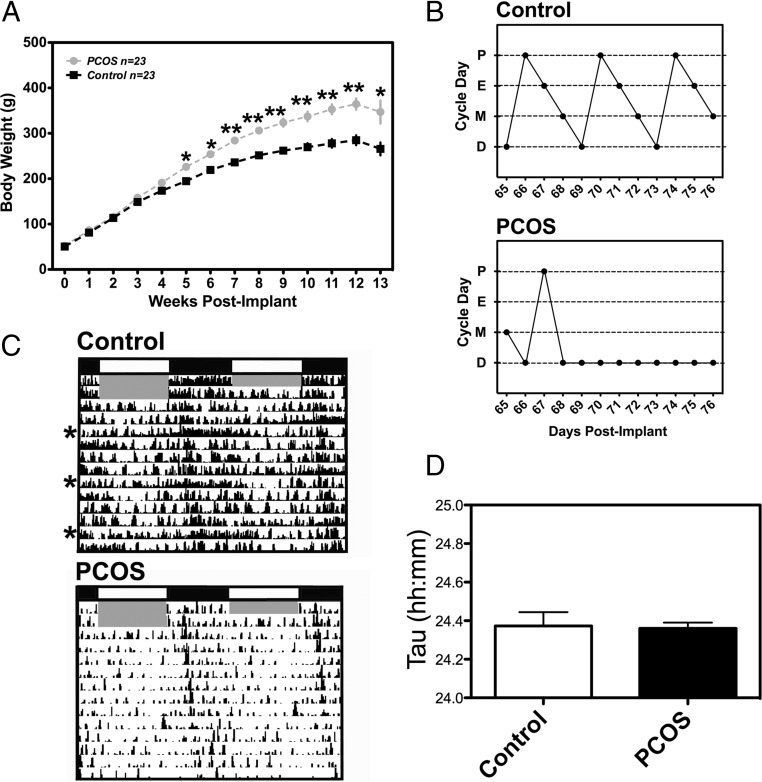
per1-luc rats exposed to both ST and LT DHT gained more weight than controls (n = 23) (Figure 1A). We observed a significant difference between groups as early as 5 weeks after the onset of exposure. On average, animals exposed to DHT for more than 9 weeks were approximately 70–80 g heavier than vehicle controls (∼35% body weight gain). Several animals were collected for tissue culture at 14–15 weeks after initial exposure to DHT or placebo, although weekly body weights were taken only through week 13. Rats exposed to DHT (both ST and LT exposure) failed to show consistent 4-day estrous cycles and remained almost exclusively in diestrus (Figure 1B). Although it appears that peripubertal DHT exposure reduced the overall level of activity (see Figure 1C), this effect was not consistent across all of our DHT-exposed rats. However, it was apparent that DHT exposure eliminated the subtle “scalloping” effect of the reproductive cycle on behavior, an effect most likely due to the abolition of normal reproductive cycles (Figure 1C). We also did not see an effect of DHT on the free-running period of activity (Figure 1D).
Figure 1.
Pubertal DHT exposure produces metabolic and reproductive features of PCOS in per1-luc rats. (A) Weekly body weights from PCOS and healthy control per1-luc rats. Rats with PCOS gained significantly more weight over the course of treatment than their healthy counterparts (all animals fed a standard diet). (B) Estrous cycle patterns from representative per1-luc rats exposed to DHT (PCOS rats) or placebo pellet (9–11 wk after implant). PCOS rats displayed intermittent or irregular cycles marked by persistent diestrus. (C) Representative activity records of per1-luc rats given a placebo pellet (control; n = 3) or a DHT pellet (PCOS; LT exposure, 9-11 wk after implant, n = 4). The gray shaded area indicates the time of lights-on during a L:D cycle. The asterisks to the left of the placebo actogram indicate increased activity on putative “behavioral estrus.” (D) The free-running period of total activity was not affected in animals exposed to DHT (n = 3-4/group). Data in panels A and D are mean ± SEM; *P < .01, **P < .001. In panel B: D, diestrus; P, proestrus; E, estrus; M, metestrus.
Excess androgen during puberty differentially affects phase distribution among oscillators of the metabolic axis
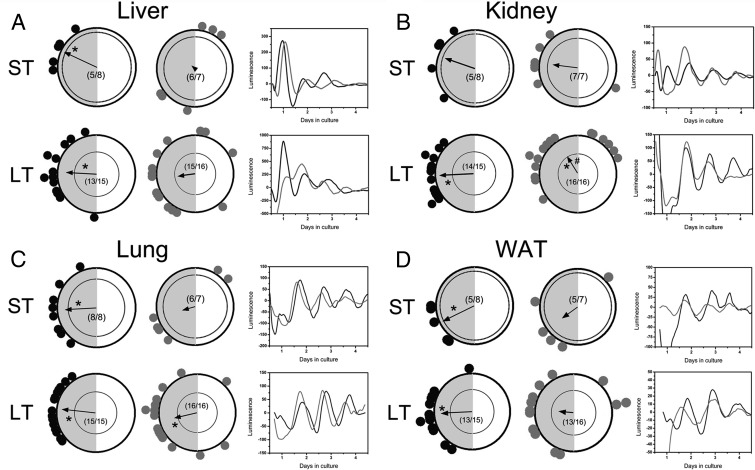
Phase distribution among tissue explants of the metabolic axis, including the liver, lung, kidney, and WAT, was analyzed as a function of treatment (control vs DHT) and exposure duration (ST vs LT). In the liver, there was no effect of exposure time on either the group mean phase or distribution in placebo-treated animals (P < .01) (Figure 2A). However, DHT exposure, both ST and LT, significantly increased the phase distribution of liver tissues without effecting mean phase (Figure 2A). In the kidney, as in the liver, phase distribution was stable over time in kidney cultures from placebo-treated rats (P < .01 in both ST and LT groups) (Figure 2B). We did not detect significant phase clustering in the kidney after ST DHT exposure, most likely due to a single culture peaking near the middle of the light phase (Figure 2B). Although we detected stable phase distribution among kidney cultures from animals exposed to LT DHT (P < .01), there was a significant delay in the mean phase (P < .05) in these animals coincident with a bimodal pattern (Figure 2B). In the lung, we again did not detect an effect of placebo, whereas both LT and ST DHT exposure resulted in a significant increase in phase distribution, although the LT-treated cultures still showed significant clustering of phase (P = .001) (Figure 2C). As in other tissues, placebo treatment did not affect phase distribution in WAT (P < .01 for both). However, both ST and LT DHT exposure substantially reduced clustering among cultures of WAT (Figure 2D).
Figure 2.
ST or LT DHT exposure differentially affects phase distribution of peripheral clocks in the metabolic axis. (A) (left side) Rayleigh plots of peak per1-luc expression in liver explants from animals given (black dots) placebo pellets or (gray dots) DHT pellets. In each plot, the peak phase of per1 expression in a single explant is indicated by a separate dot. The gray shaded area on the left indicates the 12-hour dark phase. The vector in each circle represents the arithmetic mean phase for that group, and the inner circle represents a statistical threshold (P < .01) for phase distribution. Vectors that cross the inner circle indicate a tight distribution (also indicated as asterisks within the circle). The number of rhythmic cultures/total cultures prepared is located in the center of the plot. Boxes on the right show representative luminescence time series of per1-luc expression in tissues from control (black trace) and DHT-treated (gray trace) animals. (B) Rayleigh plots of peak per1-luc expression in kidney explants from animals given (black dots) placebo pellets or (gray dots) DHT pellets. (C) Rayleigh plots of peak per1-luc expression in lung explants from animals given (black dots) placebo pellets or (gray dots) DHT pellets. (D) Rayleigh plots of peak per1-luc expression in WAT explants from animals given (black dots) placebo pellets or (gray dots) DHT pellets. (B–D) Conventions are the same as in A. The # in panel B indicates significant (P < .05) difference in arithmetic mean phase between LT placebo- and LT DHT-treated cultures. ST exposure, 4–6 weeks; LT exposure, 9–15 weeks.
Excess androgen during puberty differentially affects phase distribution among oscillators of the HPO axis
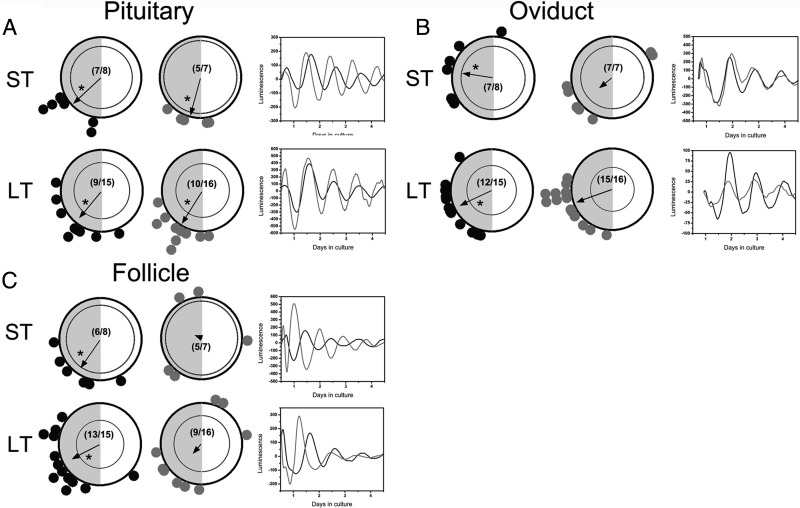
We hypothesized that pubertal DHT exposure, which produces a PCOS phenotype in rats, would disrupt reproductive function in part by altering circadian organization in the HPO axis. We observed a significant decline in phase clustering of follicles after both ST and LT DHT exposure (Figure 3C). In addition to an effect on phase distribution, we also noted a significant decline in the recovery of rhythmic preovulatory follicles (14/23, ∼60%) from animals exposed to DHT, most likely due to the established negative impact of excess androgen on follicular development (Figure 3C) (49). These data suggest that excess androgen may significantly disrupt clock gene expression in follicular cells, although more definitive histological analyses are needed for confirmation. Unlike follicular cultures, peripubertal DHT exposure did not significantly reduce the number of rhythmic cultures from the pituitary gland and oviduct (Figure 3, A and B). Further, DHT exposure did not affect phase clustering among pituitary cultures (Figure 3A). ST DHT exposure did reduce clustering in the oviduct, although this effect did not persist after extended exposure (Figure 3B).
Figure 3.
ST or LT DHT exposure differentially affects phase distribution of peripheral clocks in the reproductive tract. (A) (left side) Rayleigh plots of peak per1-luc expression in pituitary explants from animals given (black dots) placebo pellets or (gray dots) DHT pellets. (Right side) Representative luminescence time series of per1-luc expression in pituitary explants from control (black trace) and DHT-treated (gray trace) animals. (B) (left side) Rayleigh plots of peak per1-luc expression in oviduct explants from animals given (black dots) placebo pellets or (gray dots) DHT pellets. Boxes on the right show representative luminescence time series of per1-luc expression in oviduct explants from control (black trace) and DHT-treated (gray trace) animals. (C) Rayleigh plots of peak per1-luc expression in single ovarian follicle explants from animals given (black dots) placebo pellets or (gray dots) DHT pellets. (Right side) Representative luminescence time series of per1-luc expression in tissues from control (black trace) and DHT-treated (gray trace) animals. (Right side) Representative luminescence time series of per1-luc expression in follicle cultures from control (black trace) and DHT-treated (gray trace) animals. All other details as in Figure 2.
Excess androgen during puberty disrupts phase distribution of corneal explants
Adverse effects of DHT exposure in tissues of the metabolic and reproductive axes were not entirely unexpected, given the known impacts of pubertal DHT on metabolism and fertility (42, 50). However, in order to determine whether the effects of DHT exposure are specific to the circadian timing system, independent of nonclock-dependent pathophysiology, it was necessary to examine phase distribution in a highly rhythmic peripheral oscillator that is not directly involved in metabolism or reproductive physiology. To that end, we chose to examine the phase distribution of corneal explants after ST or LT DHT exposure. After both ST and LT DHT exposure, we observed a significant reduction in phase clustering among corneal explants (Figure 4). To our surprise, we also observed a slight effect of treatment duration on phase distribution of corneal cultures in the placebo group, such that the overall mean phase was significantly advanced in the cornea after LT treatment (P < .05; ST vs LT control). This advance was also apparent in the LT DHT group, although significance was not attained due to a lack of coherence in this group. Given that there is only a 3-11 weeks of age difference between the groups, it is unlikely that this represents a significant effect of aging on the corneal oscillator.
Figure 4.
Pubertal DHT exposure disrupts phase distribution of corneal explants. Rayleigh plots of peak per1-luc expression in corneal explants from animals given (black dots) placebo pellets or (gray dots) DHT pellets. Boxes on the right show representative luminescence time series of per1-luc expression in corneal cultures from control (black trace) and DHT-treated (gray trace) animals. # indicates a significant difference in arithmetic mean phase between ST and LT placebo pellet-treated groups (P < .05). All other details as in Figure 2.
Phase synchrony among circadian oscillators is disrupted in both the metabolic and reproductive axes after exposure to excess androgen during puberty
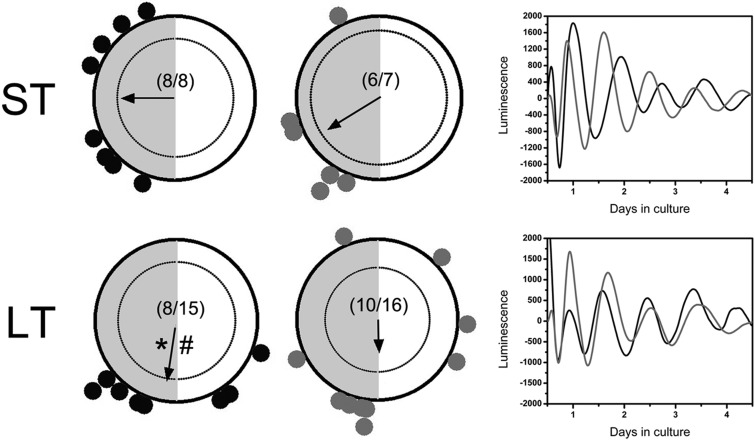
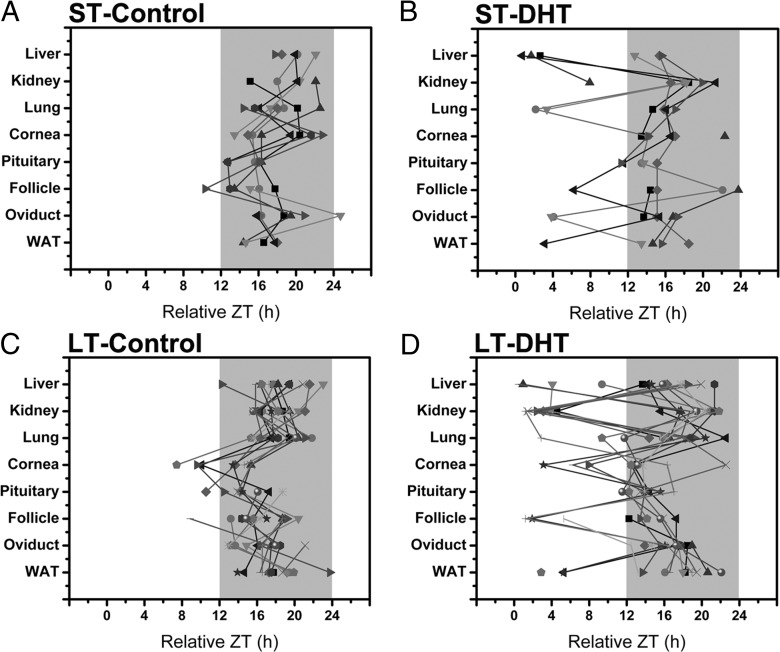
As a consequence of altered or reduced phase distribution in multiple tissues due to DHT exposure, we observed a significant decline in phase synchrony across tissues as a function of treatment. When displayed on horizontal phase maps, with each tissue plotted as a different symbol and tissues from the same animal joined by a solid line, one can clearly see the effects of DHT exposure on the circadian timing system (Figure 5). As shown in Figures 1–4, rats exposed to placebo pellets had fairly consistent and concise phase distribution, with most oscillators peaking in a 6- to 8-hour window between ZT14 and ZT22. This pattern is particularly striking in the lung, liver, kidney, pituitary, WAT, and oviduct (Figure 5, A and C). It is notable that, as shown in Figure 4, the cornea appears to shift its phase distribution to earlier in the dark phase in the LT exposure group. However, with the exception being a small percentage of follicle, oviduct, pituitary, and corneal cultures, most of the tissues display peak per1-luc expression during the dark phase (Figure 5, A and C). This result is in stark contrast with the data form ST and LT DHT-exposed animals. After ST DHT exposure, as indicated on Rayleigh diagrams in Figures 2 and 3, we see a dramatic decline in phase synchrony in the liver, lung, follicles, and WAT (Figure 5B). This apparent decline in phase synchrony is clearly due to reduced phase clustering in several animals. As suggested above, this effect is only enhanced by extended exposure to DHT (Figure 5D).
Figure 5.
LT and ST DHT exposure causes internal circadian disruption. Horizontal phase maps emphasize the impacts of DHT exposure on the organization of the circadian timing system (phase across all tissues and animals as a function of treatment). (A) Phase of per1-luc expression (relative ZT) across all animals given a ST placebo pellet exposure as a function of tissue. Peak phase for each tissue is indicated by a different symbol. All tissues from the same animal are indicated by shading (light gray to black) and are connected by a solid line. Breaks in the line indicate the omission of a tissue. The dark shaded area represents the 12-hour dark phase (as in Figures 2–4). (B) Phase of per1-luc expression (relative ZT) across all animals given a ST DHT pellet exposure as a function of tissue. (C) Phase of per1-luc expression (relative ZT) across all animals given a LT placebo pellet exposure as a function of tissue. (D) Phase of per1-luc expression (relative ZT) across all animals given a LT DHT pellet exposure as a function of tissue.
Discussion
We have examined the effects of androgen excess during puberty on the circadian timing system in rats (42, 51). Female per1-luc rats were exposed to DHT at weaning, which produces a phenotype matching several clinical features of PCOS. In agreement with the original report on pubertal DHT exposure (42), our per1-luc rats exposed to DHT gained considerably more weight than our age-matched controls. Further, our animals had severely disrupted reproductive cycles, characterized by persistent diestrus. Moreover, upon recovery, the ovary was clearly hypomorphic and appeared to contain multiple cyst-like structures (data not shown). Tissues were recovered from animals exposed to ST and LT DHT or placebo, and per1-luc expression was monitored in vitro as a marker of in vivo clock function. Although only a single gene reporter, the per1-luc transgenic rat has repeatedly been shown to report the in vivo phase of tissue clocks in explant culture (52). Therefore, we consider the timing of per1-luc expression across multiple peripheral oscillators to be a faithful marker of “internal circadian organization.” As a consequence of DHT exposure, we observed a significant decline in internal circadian organization, characterized by disruption of the phase distribution of per1-luc expression and synchrony among peripheral oscillators. Although most of these tissues showed peak per1-luc expression in a 6- to 8-hour window centered around midnight, DHT-treated animals had more dispersed per1-luc expression rhythms with several tissues peaking during the day. These effects were particularly pronounced among tissues of the metabolic axis, including the liver, lung, and WAT. A decline in phase synchrony and increased phase distribution were observed after both ST and LT exposure to DHT. These effects of excess androgen on the phase of per1-luc expression are even more striking when one considers that these animals were maintained in a L:D cycle throughout the experiment. To our knowledge, this is the first study to examine the impact of developmental androgen excess on circadian clock function and the organization of the circadian timing system.
It is an article of faith that adaptive phase synchrony across the multitude of central and peripheral oscillators is a critical facet of physiological homeostasis (11, 15). Environmental circadian disruption (ECD), in the form of chronic jet-lag or rotating shiftwork, leads to dissociation of oscillators due primarily to differential and tissue-specific rates of resynchronization (53). It has been suggested that a persistent condition of oscillator desynchrony in the presence of a normal L:D cycle underlies the pathophysiology of circadian disruption. Internal circadian disorganization is implicated in the severe negative effects of ECD (54–56). In addition to metabolic, immune, neurophysiological, and behavioral effects, internal disorganization due to circadian disruption is also thought to negatively affect reproductive function in women (57). Recent evidence points to the neuroendocrine and endocrine systems, particularly the timing of glucocorticoid secretion, as critical regulators of internal circadian organization (58–60). Additional evidence suggests that dynamic titers of ovarian steroid hormone secretion across the rodent estrous cycle can also affect the timing of clock gene expression in target tissues (61, 62). Together, these data support the hypothesis that internal circadian organization is, in large part, dependent on normal patterns of both adrenal and gonadal steroid hormone secretion. Thus, any disorder that includes as part of its etiology a significant change or disruption of circulating hormone secretion could be expected to have significant detrimental effects on internal circadian organization. The inverse relationship is already clear, because mutations affecting the circadian clock have been shown to alter steroid hormone synthesis and secretion (22, 24). Further, the circadian clock may directly affect the timing of nuclear hormone receptor expression and activation in target tissues (63). Transgenic reporter models, like our per1-luc rats, have been repeatedly and successfully used to interrogate the effects of in vivo manipulations on internal circadian organization (60, 64). It is possible, although unlikely, that the effects we observed are limited to the phase of period1 gene expression alone. It will be critical to examine the pattern of other clock genes and clock-controlled genes in each of these tissues in order to determine the full impact of excess androgen on the circadian timing system. That said, our results provide further evidence that internal circadian organization (or disorganization) may be a critical factor in the etiology of complex disorders affecting both reproduction and metabolism. Our experiments intended to systematically analyze the effects of excess androgen on the circadian timing system. However, many disorders, including obesity, metabolic syndrome, and diabetes, negatively affect both metabolic function and fertility. It remains to be seen whether altered internal circadian organization is a common feature of each of these conditions.
Several animal models of PCOS, including the PNA mouse, ewe, and rhesus macaque, have been developed and are considered by many to be more representative models of the disease (36, 41, 65). As mentioned above, we did not systematically verify a polycystic morphology in our animals (PCO), although we did observe increased abdominal fat and persistent leukocytic vaginal cytology (“pseudodiestrus”), both features of pubertal androgen excess in female rats (42). Although it is possible that the increase in body weight we observed was due in part to the anabolic effects of steroids on body mass, it appears that this weight gain, as in previous reports, was primarily the result of increased intraabdominal adipose (data not shown and Ref. 42). With that said, it would be an error to assume that the effects we observed on circadian organization after androgen excess exposure is a general feature of PCOS. It may only represent the response of the circadian timing system to excess androgen levels, which by itself does not define the etiology of the disease. It will be necessary to observe reduced internal circadian organization in other animal models of the disease, such as PNA mice, in order to confirm the role of the circadian timing system in PCOS etiology.
Although the impact of excess androgen on tissues of the metabolic axis was somewhat general, we were surprised to observe only limited impacts in the HPG axis, with ovarian follicles being most severely affected. We were only able to recover approximately 60% viable and rhythmic follicles from DHT-treated rats (combined ST and LT exposure). In fact, at dissection, animals exposed to both ST and LT DHT had patently hypotrophic ovaries with pale coloration. Due to the extensive nature of our dissections, we were not able to systematically examine ovarian morphology in our DHT-treated rats. However, our treatment paradigm matches that of previous studies who have confirmed a PCO-like morphology in rats treated with DHT at the same dose and duration (42). Only the largest antral follicles were recovered, which was difficult in DHT-treated animals (42, 50). We cannot rule out the possibility that large ovarian cysts were cultured from our DHT-treated animals. These follicular cysts may or may not have functional circadian clocks. The ovarian cysts common to PCOS patients are fluid filled with an enlarged thecal cell layer and reduced granulosa cell layer (33, 50). Although certainly possible, this alone would not explain the reduced number of rhythmic cultures and increase in phase distribution we observed, because the circadian clock has been shown to be present and rhythmic in both the granulosa and theca cell of the rat ovarian follicle (66, 67). Nevertheless, It will be important to examine the patterns of clock genes and clock-controlled genes in modified theca cells from ovarian cysts to determine the clocks role in the abnormally high androgen secretion common to PCOS (37). Although not quantified in our experiments, previous work using this model indicates that serum testosterone and estradiol levels are not significantly affected by extended exposure to DHT (42). It is unlikely then that the follicles recovered were immature due to suppressed androgen production (68). Our conclusions regarding the nature of our follicular cultures are also supported by evidence that immature ovarian cells do not appear to have functional clocks (69, 70).
Unlike ovarian follicles, we recorded only minor, if any, affects of pubertal DHT on the distribution of circadian clocks in the oviduct and pituitary gland. To our surprise, LT DHT exposure, which is known to significantly affect the rhythm and amplitude of LH secretion, had little effect on the clock in the pituitary (42, 50, 71). Although we cultured only the adenohypophysis, we cannot with any clarity define the myriad pituitary cell types present in our explants. As such, our interpretation of this result must be made with some caution. Circadian clock function has been described in several pituitary cell types, being implicated in the control of cell signaling, gene expression, and hormone secretory activity in both gonadotrophs and lactotrophs (72–74). As with the ovarian follicles, in-depth histological or cell isolation methods must be employed to achieve a more complete understanding of the effects of DHT on circadian clock function in the pituitary.
A common clinical feature of PCOS is the development of metabolic syndrome or prediabetes (75–77). Our DHT-exposed rats showed a weight gain on average of approximately 30–40%, primarily due to an increase in abdominal and perigonadal fat. It is now appreciated that diet-induced obesity is associated with a significant change in the timing and amplitude of clock gene expression in numerous tissues (47). Likewise, it has been suggested that clock gene expression in the pancreas and liver are critical to normal glucose homeostasis (46, 78). Thus, we were not surprised to see a considerable decline in clock organization among tissues of the metabolic axis. In fact, the most robust response we observed with regard to aberrant internal circadian organization was in the liver, lung, kidney, and adipose. Our data show that a mere 4–6 weeks of DHT exposure during puberty increased adiposity and significantly affected internal circadian organization. Both ST and LT DHT exposure significantly reduced phase clustering in the liver, lung, kidney, and WAT, resulting in a profound drop in phase synchrony. As stated above, circadian disruption is a well-established mediator of metabolic disease in both rodents and humans (56, 79, 80). ECD and abnormal timing of food intake (as in shift work) are both associated with the appearance of obesity and metabolic disease (56, 81). Our results did not define the precise temporal relationship between the appearance of metabolic abnormalities due to DHT exposure and internal circadian disruption. Thus, it will be critical to more closely examine the effects of excess androgen exposure, in both postnatal DHT-treated and PNA animals, because it relates to the severity and nature of internal circadian disruption.
In addition to the HPO and metabolic axes, we also observed a significant effect of pubertal androgen on the phase of per1-luc expression in corneal explants. The cornea is a robust peripheral oscillator that is normally tightly synchronized with the L:D cycle (60). Corneal cells express functional androgen receptors (ARs), and the development of keratoconus in mice is linked to androgen signaling (82, 83). Our data confirm that excess androgen during development in female mice can affect phase distribution among corneal cultures. The role of the circadian clock in the cornea is currently unknown, but these data suggest that the clock in corneal cells may be linked to steroid-dependent visual deficits. These data suggest that the impacts of pubertal androgen on internal circadian organization may not be due to the indirect effects of increased body weight and ovarian physiology on peripheral clock function. Finally, these data indicate that the effects of androgen excess on circadian organization and clock function may be somewhat ubiquitous and represent a general impact of elevated androgen on circadian timing.
Our data reveal a significant decline in internal circadian organization after exposure to excess androgen without a significant effect in free-running circadian rhythms of activity. Several investigators have shown that ARs are expressed in SCN pacemaker neurons and testicular androgens affect circadian rhythms of locomotor activity in males (84–87). Most AR expression in mouse SCN is restricted to a small region in the ventrolateral core of the SCN (88). Androgen depletion after gonadectomy reduces activity levels and produces fragmented activity patterns with highly variable onset in both males and females. Each of these effects of gonadectomy can be reversed by replacement with exogenous androgens (84–86). AR expression in SCN neurons does not appear to be rhythmic but is significantly affected by circulating androgen levels (85). Recently, it was reported that gonadectomy alters functional morphology and the response to a phase-shifting light pulse in the SCN (89). ARs are only sparsely expressed in the SCN of female mice. Moreover, testosterone or DHT treatment after ovariectomy increased activity levels and restored onset precision but had no effect on the period of the free-running activity rhythm (88). These data indicate that the amplitude of clock output and the precision of clock control over behavior may be affected by excess androgen but that circadian rhythms of clock gene expression in the SCN are not. In the present study, we did not detect a significant effect of excess androgen on the period of locomotor activity. Thus, the effects of androgen excess we observed are most likely due to altered clock function in target tissues in the periphery. That said, we cannot at present eliminate the possible impact of elevated androgen levels SCN outputs, such as vasopressin and vasoactive intestinal polypeptide, which if altered would certainly have a dramatic affect on internal timing. Finally, it is possible that AR expression levels are differentially elevated or reduced in the various tissues examined. It will be important to determine the impact of pubertal androgen excess on AR expression and the link between AR signaling and the clock in each tissue.
Our data, for the first time, reveal a significant decline in internal circadian organization associated with the development of a complex condition affecting both reproduction and metabolism. We have shown that excess androgen, a common feature of PCOS, during development disrupts internal circadian organization by significantly altering phase distribution among tissues of the metabolic and reproductive axes. This decline in circadian organization may represent both a novel characteristic of PCOS and an exciting new avenue for translational medicine. To confirm the role of internal circadian organization in this and other diseases, it will be necessary to attenuate the condition by targeted improvement of circadian organization. Eventually, compounds that modify clock gene expression and clock-dependent genes or directly synchronize peripheral clocks may show promise as novel therapies for the improvement of complex endocrinopathies like PCOS.
Acknowledgments
The authors gratefully acknowledge the technical assistance of Denise T. Holmes, Gwendolyn Yao, Pratik Patel and Hing-Kiu Chan (University of Virginia).
This work was supported by National Institutes of Health (NIH) Grant RO1 MH56647 (to M.M.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AR
- androgen receptor
- DHT
- 5α-dihydrotestosterone
- ECD
- environmental circadian disruption
- HPO
- hypothalamo-pituitary-ovarian
- L:D cycle
- 12-hour light, 12-hour dark cycle
- LT
- long term
- PCOS
- polycystic ovary syndrome
- per1-luc
- period1-luciferase
- PNA
- prenatally androgenized
- SCN
- suprachiasmatic nucleus
- ST
- short term
- WAT
- white adipose tissue
- ZT
- Zeitgeber time.
References
- 1. Kennaway DJ. The role of circadian rhythmicity in reproduction. Hum Reprod Update. 2005;11:91. [DOI] [PubMed] [Google Scholar]
- 2. Boden MJ, Kennaway DJ. Circadian rhythms and reproduction. Reproduction. 2006;132:379–392 [DOI] [PubMed] [Google Scholar]
- 3. Bronson FH, Vom Saal FS. Control of the preovulatory release of luteinizing hormone by steroids in the mouse. Endocrinology. 1979;104:1247–1255 [DOI] [PubMed] [Google Scholar]
- 4. Goldman BD. The circadian timing system and reproduction in mammals. Steroids. 1999;64:679. [DOI] [PubMed] [Google Scholar]
- 5. Legan SJ, Karsch FJ. A daily signal for the LH surge in the rat. Endocrinology. 1975;96:57. [DOI] [PubMed] [Google Scholar]
- 6. Moenter SM, DeFazio AR, Pitts GR, Nunemaker CS. Mechanisms underlying episodic gonadotropin-releasing hormone secretion. Front Neuroendocrinol. 2003;24:79–93 [DOI] [PubMed] [Google Scholar]
- 7. Everett JW, Sawyer CH. A 24-hour periodicity in the “LH-release apparatus” of female rats, disclosed by barbiturate sedation. Endocrinology. 1950;47:198. [DOI] [PubMed] [Google Scholar]
- 8. Kriegsfeld LJ, Silver R. The regulation of neuroendocrine function: timing is everything. Horm Behav. 2006;49:557–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karsch FJ, Bowen JM, Caraty A, Evans NP, Moenter SM. Gonadotropin-releasing hormone requirements for ovulation. Biol Reprod. 1997;56:303. [DOI] [PubMed] [Google Scholar]
- 10. de la Iglesia HO, Schwartz WJ. Minireview: timely ovulation: circadian regulation of the female hypothalamo-pituitary-gonadal axis. Endocrinology. 2006;147:1148–1153 [DOI] [PubMed] [Google Scholar]
- 11. Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Legan SJ, Coon GA, Karsch FJ. Role of estrogen as initiator of daily LH surges in the ovariectomized rat. Endocrinology. 1975;96:50. [DOI] [PubMed] [Google Scholar]
- 13. Miller BH, Olson SL, Levine JE, Turek FW, Horton TH, Takahashi JS. Vasopressin regulation of the proestrous luteinizing hormone surge in wildtype and clock mutant mice. Biol Reprod. 2006;75:778–784 [DOI] [PubMed] [Google Scholar]
- 14. Wiegand SJ, Terasawa E, Bridson WE, Goy RW. Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology. 1980;31:147. [DOI] [PubMed] [Google Scholar]
- 15. Albrecht U. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron. 2012;74:246–260 [DOI] [PubMed] [Google Scholar]
- 16. Sellix MT, Menaker M. Circadian clocks in the ovary. Trends Endocrinol Metab. 2010;21:628–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sellix MT, Menaker M. Circadian clocks in mammalian reproductive physiology: effects of the “other” biological clock on fertility. Discov Med. 2011;11:273–281 [PubMed] [Google Scholar]
- 18. Williams WP, III, Kriegsfeld LJ. Circadian control of neuroendocrine circuits regulating female reproductive function. Front Endocrinol (Lausanne). 2012;3:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yoshikawa T, Sellix M, Pezuk P, Menaker M. Timing of the ovarian circadian clock is regulated by gonadotrophins. Endocrinology. 2009;150:4338–4347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sellix MT, Yoshikawa T, Menaker M. A circadian egg timer gates ovulation. Curr Biol. 2010;20:R266–R267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alvarez JD, Sehgal A. The thymus is similar to the testis in its pattern of circadian clock gene expression. J Biol Rhythms. 2005;20:111–121 [DOI] [PubMed] [Google Scholar]
- 22. Alvarez JD, Hansen A, Ord T, et al. The circadian clock protein BMAL1 is necessary for fertility and proper testosterone production in mice. J Biol Rhythms. 2008;23:26–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakao N, Yasuo S, Nishimura A, et al. Circadian clock gene regulation of steroidogenic acute regulatory protein gene expression in preovulatory ovarian follicles. Endocrinology. 2007;148:3031–3038 [DOI] [PubMed] [Google Scholar]
- 24. Ratajczak CK, Boehle KL, Muglia LJ. Impaired steroidogenesis and implantation failure in Bmal1−/− mice. Endocrinology. 2009;150:1879–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ratajczak CK, Asada M, Allen GC, et al. Generation of myometrium-specific Bmal1 knockout mice for parturition analysis. Reprod Fertil Dev. 2012;24:759–767 [DOI] [PubMed] [Google Scholar]
- 26. Yamazaki S, Numano R, Abe M, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682. [DOI] [PubMed] [Google Scholar]
- 27. Yoo SH, Yamazaki S, Lowrey PL, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101:5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kudo T, Horikawa K, Shibata S. Circadian rhythms in the CNS and peripheral clock disorders: the circadian clock and hyperlipidemia. J Pharmacol Sci. 2007;103:139–143 [DOI] [PubMed] [Google Scholar]
- 29. Fontaine C, Staels B. The orphan nuclear receptor Rev-erbα: a transcriptional link between circadian rhythmicity and cardiometabolic disease. Curr Opin Lipidol. 2007;18:141–146 [DOI] [PubMed] [Google Scholar]
- 30. Yin L, Wu N, Curtin JC, et al. Rev-erbα, a heme sensor that coordinates metabolic and circadian pathways. Science. 2007;318:1786–1789 [DOI] [PubMed] [Google Scholar]
- 31. Gomez-Abellan P, Hernandez-Morante JJ, Lujan JA, Madrid JA, Garaulet M. Clock genes are implicated in the human metabolic syndrome. Int J Obes (Lond). 2008;32:121–128 [DOI] [PubMed] [Google Scholar]
- 32. Garaulet M, Madrid JA. Chronobiology, genetics and metabolic syndrome. Curr Opin Lipidol. 2009;20:127–134 [DOI] [PubMed] [Google Scholar]
- 33. Xita N, Tsatsoulis A. Review: fetal programming of polycystic ovary syndrome by androgen excess: evidence from experimental, clinical, and genetic association studies. J Clin Endocrinol Metab. 2006;91:1660–1666 [DOI] [PubMed] [Google Scholar]
- 34. Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800 [DOI] [PubMed] [Google Scholar]
- 35. Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–1236 [DOI] [PubMed] [Google Scholar]
- 36. Dumesic DA, Abbott DH, Padmanabhan V. Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord. 2007;8:127–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gilling-Smith C, Story H, Rogers V, Franks S. Evidence for a primary abnormality of thecal cell steroidogenesis in the polycystic ovary syndrome. Clin Endocrinol. 1997;47:93–99 [DOI] [PubMed] [Google Scholar]
- 38. Homburg R. Androgen circle of polycystic ovary syndrome. Hum Reprod. 2009;24:1548–1555 [DOI] [PubMed] [Google Scholar]
- 39. Balen A, Homburg R, Franks S. Defining polycystic ovary syndrome. BMJ. 2009;338:a2968. [DOI] [PubMed] [Google Scholar]
- 40. Padmanabhan V, Manikkam M, Recabarren S, Foster D. Prenatal testosterone excess programs reproductive and metabolic dysfunction in the female. Mol Cell Endocrinol. 2006;246:165–174 [DOI] [PubMed] [Google Scholar]
- 41. Sullivan SD, Moenter SM. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci USA. 2004;101:7129–7134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Manneras L, Cajander S, Holmang A, et al. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology. 2007;148:3781–3791 [DOI] [PubMed] [Google Scholar]
- 43. Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134:728–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Franks S. Genetic and environmental origins of obesity relevant to reproduction. Reprod Biomed Online. 2006;12:526–531 [DOI] [PubMed] [Google Scholar]
- 45. Balasubramanian P, Jagannathan L, Mahaley RE, et al. High fat diet affects reproductive functions in female diet-induced obese and dietary resistant rats. J Neuroendocrinol. 2012;24:748–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Turek FW, Joshu C, Kohsaka A, et al. Obesity and metabolic syndrome in circadian clock mutant mice. Science. 2005;308:1043–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kohsaka A, Laposky AD, Ramsey KM, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–421 [DOI] [PubMed] [Google Scholar]
- 48. Silfen ME, Denburg MR, Manibo AM, et al. Early endocrine, metabolic, and sonographic characteristics of polycystic ovary syndrome (PCOS): comparison between nonobese and obese adolescents. J Clin Endocrinol Metab. 2003;88:4682–4688 [DOI] [PubMed] [Google Scholar]
- 49. Franks S, Hardy K. Aberrant follicle development and anovulation in polycystic ovary syndrome. Ann Endocrinol (Paris). 2010;71:228–230 [DOI] [PubMed] [Google Scholar]
- 50. van Houten EL, Kramer P, McLuskey A, Karels B, Themmen AP, Visser JA. Reproductive and metabolic phenotype of a mouse model of PCOS. Endocrinology. 2012;153:2861–2869 [DOI] [PubMed] [Google Scholar]
- 51. Manneras L, Jonsdottir IH, Holmang A, Lonn M, Stener-Victorin E. Low-frequency electro-acupuncture and physical exercise improve metabolic disturbances and modulate gene expression in adipose tissue in rats with dihydrotestosterone-induced polycystic ovary syndrome. Endocrinology. 2008;149:3559–3568 [DOI] [PubMed] [Google Scholar]
- 52. Yamazaki S, Takahashi JS. Real-time luminescence reporting of circadian gene expression in mammals. Methods Enzymol. 2005;393:288–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Davidson AJ, Castanon-Cervantes O, Leise TL, Molyneux PC, Harrington ME. Visualizing jet lag in the mouse suprachiasmatic nucleus and peripheral circadian timing system. Eur J Neurosci. 2009;29:171–180 [DOI] [PubMed] [Google Scholar]
- 54. Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M, Block GD. Chronic jet-lag increases mortality in aged mice. Curr Biol. 2006;16:R914–R916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Castanon-Cervantes O, Wu M, Ehlen JC, et al. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol. 2010;185:5796–5805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci USA. 2011;108:1657–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mahoney MM. Shift work, jet lag, and female reproduction. Int J Endocrinol. 2010;2010:8137–8164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sujino M, Furukawa K, Koinuma S, et al. Differential entrainment of peripheral clocks in the rat by glucocorticoid and feeding. Endocrinology. 2012;153:2277–2286 [DOI] [PubMed] [Google Scholar]
- 59. Kiessling S, Eichele G, Oster H. Adrenal glucocorticoids have a key role in circadian resynchronization in a mouse model of jet lag. J Clin Invest. 2010;120:2600–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pezuk P, Mohawk JA, Wang LA, Menaker M. Glucocorticoids as entraining signals for peripheral circadian oscillators. Endocrinology. 2012;153:4775–4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nakamura TJ, Sellix MT, Menaker M, Block GD. Estrogen directly modulates circadian rhythms of PER2 expression in the uterus. Am J Physiol Endocrinol Metab. 2008;295:E1025–E1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nakamura TJ, Sellix MT, Kudo T, et al. Influence of the estrous cycle on clock gene expression in reproductive tissues: effects of fluctuating ovarian steroid hormone levels. Steroids. 2010;75:203–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Oiwa A, Kakizawa T, Miyamoto T, et al. Synergistic regulation of the mouse orphan nuclear receptor SHP gene promoter by CLOCK-BMAL1 and LRH-1. Biochem Biophys Res Commun. 2007;353:895–901 [DOI] [PubMed] [Google Scholar]
- 64. Pezuk P, Mohawk JA, Yoshikawa T, Sellix MT, Menaker M. Circadian organization is governed by extra-SCN pacemakers. J Biol Rhythms. 2010;25:432–441 [DOI] [PubMed] [Google Scholar]
- 65. Roland AV, Nunemaker CS, Keller SR, Moenter SM. Prenatal androgen exposure programs metabolic dysfunction in female mice. J Endocrinol. 2010;207:213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Karman BN, Tischkau SA. Circadian clock gene expression in the ovary: effects of luteinizing hormone. Biol Reprod. 2006;75:624–632 [DOI] [PubMed] [Google Scholar]
- 67. Fahrenkrug J, Georg B, Hannibal J, Hindersson P, Gras S. Diurnal rhythmicity of the clock genes Per1 and Per2 in the rat ovary. Endocrinology. 2006;147:3769–3776 [DOI] [PubMed] [Google Scholar]
- 68. Sen A, Hammes SR. Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. Mol Endocrinol. 2010;24:1393–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. He PJ, Hirata M, Yamauchi N, Hashimoto S, Hattori MA. The disruption of circadian clockwork in differentiating cells from rat reproductive tissues as identified by in vitro real-time monitoring system. J Endocrinol. 2007;193:413–420 [DOI] [PubMed] [Google Scholar]
- 70. He PJ, Hirata M, Yamauchi N, Hashimoto S, Hattori MA. Gonadotropic regulation of circadian clockwork in rat granulosa cells. Mol Cell Biochem. 2007;302:111–118 [DOI] [PubMed] [Google Scholar]
- 71. Walters KA, Allan CM, Handelsman DJ. Rodent models for human polycystic ovary syndrome. Biol Reprod. 2012;86:149:1–12 [DOI] [PubMed] [Google Scholar]
- 72. Olcese J, Sikes HE, Resuehr D. Induction of PER1 mRNA expression in immortalized gonadotropes by gonadotropin-releasing hormone (GnRH): involvement of protein kinase C and MAP kinase signaling. Chronobiol Int. 2006;23:143–150 [DOI] [PubMed] [Google Scholar]
- 73. Resuehr HE, Resuehr D, Olcese J. Induction of mPer1 expression by GnRH in pituitary gonadotrope cells involves EGR-1. Mol Cell Endocrinol. 2009;311:120–125 [DOI] [PubMed] [Google Scholar]
- 74. Bose S, Boockfor FR. Episodes of prolactin gene expression in GH3 cells are dependent on selective promoter binding of multiple circadian elements. Endocrinology. 2010;151(5):2287–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Martinez-Bermejo E, Luque-Ramirez M, Escobar-Morreale HF. Obesity and the polycystic ovary syndrome. Minerva Endocrinol. 2007;32:129–140 [PubMed] [Google Scholar]
- 76. Escobar-Morreale HF, San Millan JL. Abdominal adiposity and the polycystic ovary syndrome. Trends Endocrinol Metab. 2007;18:266–272 [DOI] [PubMed] [Google Scholar]
- 77. Hoffman LK, Ehrmann DA. Cardiometabolic features of polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab. 2008;4:215–222 [DOI] [PubMed] [Google Scholar]
- 78. Marcheva B, Ramsey KM, Buhr ED, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Buxton OM, Cain SW, O'Connor SP, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4:129ra143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity. 2009;17:2100–2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Suzuki T, Kinoshita Y, Tachibana M, et al. Expression of sex steroid hormone receptors in human cornea. Curr Eye Res. 2001;22:28–33 [DOI] [PubMed] [Google Scholar]
- 83. Tachibana M, Adachi W, Kinoshita S, et al. Androgen-dependent hereditary mouse keratoconus: linkage to an MHC region. Invest Ophthalmol Vis Sci. 2002;43:51–57 [PubMed] [Google Scholar]
- 84. Daan S, Damassa D, Pittendrigh CS, Smith ER. An effect of castration and testosterone replacement on a circadian pacemaker in mice (Mus musculus). Proc Natl Acad Sci USA. 1975;72:3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Karatsoreos IN, Wang A, Sasanian J, Silver R. A role for androgens in regulating circadian behavior and the suprachiasmatic nucleus. Endocrinology. 2007;148:5487–5495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Morin LP, Cummings LA. Effect of surgical or photoperiodic castration, testosterone replacement or pinealectomy on male hamster running rhythmicity. Physiol Behav. 1981;26:825–838 [DOI] [PubMed] [Google Scholar]
- 87. Kruijver FP, Swaab DF. Sex hormone receptors are present in the human suprachiasmatic nucleus. Neuroendocrinology. 2002;75:296. [DOI] [PubMed] [Google Scholar]
- 88. Iwahana E, Karatsoreos I, Shibata S, Silver R. Gonadectomy reveals sex differences in circadian rhythms and suprachiasmatic nucleus androgen receptors in mice. Horm Behav. 2008;53:422–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Karatsoreos IN, Butler MP, Lesauter J, Silver R. Androgens modulate structure and function of the suprachiasmatic nucleus brain clock. Endocrinology. 2011;152:1970–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]