Abstract
Severe injury and infection are often followed by accelerated protein catabolism and acute insulin resistance. This results in several effects that complicate and prolong recovery, including weakness, immobility, impaired wound healing, and organ dysfunction. Recent studies have demonstrated the development of GH resistance during severe inflammation, providing a potential mechanism for the protein loss that follows injury and infection. To understand this GH resistance, we recently developed a murine model of acute injury. Mice were subjected to soft-tissue injury, alone or combined with hemorrhage, and injected iv with GH 30, 60, or 90 minutes later. Hepatic GH signaling was measured via Western analysis. GH-induced signal transducer and activator of transcription 5 phosphorylation was decreased immediately after completion of the trauma procedure, and at 30 and 60 minutes, but further decreased by 90 minutes after trauma. Combined trauma and hemorrhage resulted in severely decreased GH-induced signal transducer and activator of transcription 5 phosphorylation compared with trauma alone, and this was true at all time points studied. Western analysis revealed an apparent decrease in the molecular weight of the hepatic GH receptor (GHR) after trauma and hemorrhage, but not trauma alone. Additional studies determined that the hemorrhage-induced decrease in receptor size was not due to changes in GHR N-linked glycosylation. These results suggest that GH sensitivity is rapidly impaired after acute injury and that trauma combined with hemorrhage results in a more severe form of GH resistance resulting from alteration or inactivation of hepatic GHR.
Recovery after trauma or major surgery is frequently complicated by hyperglycemia, insulin resistance, and metabolic dysfunction. Also often observed is accelerated muscle catabolism, resulting from decreased skeletal muscle protein synthesis and increased protein degradation (1–3). Levels of circulating free amino acids are consequently increased, providing substrate for the acute-phase response as well as gluconeogenesis (4, 5). These metabolic changes may shift substrate away from costly anabolic processes toward those necessary for survival (6). However, prolonged insulin resistance/hyperglycemia and acute-phase responses frequently associated with severe injury adversely impact morbidity and mortality. As urinary nitrogen excretion increases (7, 8), the associated cachexia contributes to weakness, reduced mobility, prolonged ventilator support, and increased risk of thrombus formation (9–11). Other consequences of protein wasting include impaired wound healing and organ dysfunction (12, 13). The catabolic state is not easily reversed, even with aggressive nutritional support, and the causative factors are largely unknown (14). However, GH resistance is often observed after trauma and major surgery, which may play a role in the development of accelerated protein catabolism during intensive care (15–17). Insights into the developmental mechanisms of GH resistance may therefore lead to safer and more effective therapies to aid in recovery from severe injury.
GH is a pituitary hormone with important roles in protein accretion and maintenance of lean body mass (18). GH binding to its receptor (GH receptor [GHR])-(UniProt P16882) results in tyrosine phosphorylation and subsequent activation of receptor-associated Janus kinase 2 (JAK2) (19, 20). Activated JAK2 phosphorylates tyrosine residues within the cytoplasmic domain of GHRs, which serve as docking sites for the src-homology 2 (SH2) domain of signal transducer and activator of transcription 5 (STAT5) (21, 22). Once docked, STAT5 is tyrosine-phosphorylated by JAK2, enabling STAT5 dimerization, nuclear translocation (23–25), and regulation of GH-responsive genes. In particular, GH-activated STAT5 stimulates transcription of IGF-I, which mediates many of the anabolic and anticatabolic effects of GH (26, 27). GH resistance is defined as a reduced response of tissues to GH, including reduced transcription of IGF-I. At the cellular level, this can be due to decreased GH-induced phosphorylation of signaling intermediates (28, 29), impaired STAT5 DNA binding (30), or inhibition of any other GH-dependent signaling pathway.
Clinical GH resistance is characterized by elevated circulating GH and decreased circulating IGF-I and is observed during acute systemic inflammation after major injury or surgery or during infection (15–17). IGF-I is a potent anabolic hormone necessary for maintenance of lean muscle, and reduction of IGF-I likely contributes to protein wasting during critical illness. Several rodent models of sepsis have suggested GH resistance, contributing to decreased hepatic IGF-I gene expression (29–31). However, it is not currently known how quickly GH signaling defects develop in response to stress. In addition, there have been no studies of the effects of surgery or trauma on GH sensitivity. We therefore hypothesized that impaired GH signaling develops within 90 minutes of surgical trauma combined with hemorrhage.
In the current study, we set out to determine whether surgical trauma alone, or combined with hemorrhage, results in GH resistance and to characterize the cellular mechanisms of its development. Here we demonstrate that injury results in impaired GH signaling. In addition, trauma combined with hemorrhage results in a more severe GH resistance, occurring in association with an apparent decrease in size of the GHR.
Materials and Methods
All animal procedures were carried out in accordance with the guidelines set forth in the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health (Bethesda, Maryland). The experimental protocol was approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham.
GH time course study
Mice were anesthetized with isoflurane (Mallinckrodt Veterinary, Mundelein, Illinois) and immediately injected with saline (75 μL) or 1.5 μg/g bovine GH dissolved in 75 μL saline (obtained from Dr. Albert Parlow, National Hormone and Pituitary Program, Torrance, California). Livers were harvested 1, 5, 10, 15, 20, or 25 minutes after GH injection and immediately stored in liquid nitrogen until further processing.
Animal model of trauma and hemorrhage
Mice were treated as described previously (32) with the exception that in the present study mice were not fasted before surgery. Briefly, male C57BL/6 mice (Harlan Laboratories, Inc, Indianapolis, Indiana), approximately 12 weeks old and 23 to 25 g, were allowed food and water ad libitum before experiments. Mice were anesthetized by continuous inhalation of 1.5% isoflurane and 98.5% O2 throughout all procedures. After mice were clipped and shaved, the surgical trauma procedure began with a 2-cm ventral midline laparotomy, representing soft tissue injury. The abdomen was then closed in layers using 6-0 Ethilon sutures (Ethicon, Somerville, New Jersey), and the wounds were bathed with 1% lidocaine (Elkins-Sinn, Cherry Hill, New Jersey) to reduce postoperative pain. Polyethylene-10 catheters (Clay-Adams, Parsippany, New Jersey) were placed in the right and left femoral arteries for monitoring mean arterial pressure (MAP) (Micro-Med, Louisville, Kentucky) and for experimental hemorrhage, respectively. This completed the surgical trauma portion of the procedure. Mice designated for combined trauma and hemorrhage were then immediately hemorrhaged from an MAP of 70 to 75 mm Hg to an MAP of 35 to 40 mm Hg over a 10-minute period by withdrawing approximately 700 μL, equivalent to roughly one-half to two-thirds of the calculated total blood volume in C57BL/6 mice. The hemorrhage period began immediately after MAP dropped below 40 mm Hg. At the conclusion of the study period, the abdominal incision was reopened, and either saline or 1.5 μg/g GH (total volume = 75 μL) were injected directly into the inferior vena cava. Livers were harvested 10 minutes later and stored in liquid nitrogen until further processing (32–35).
Study design
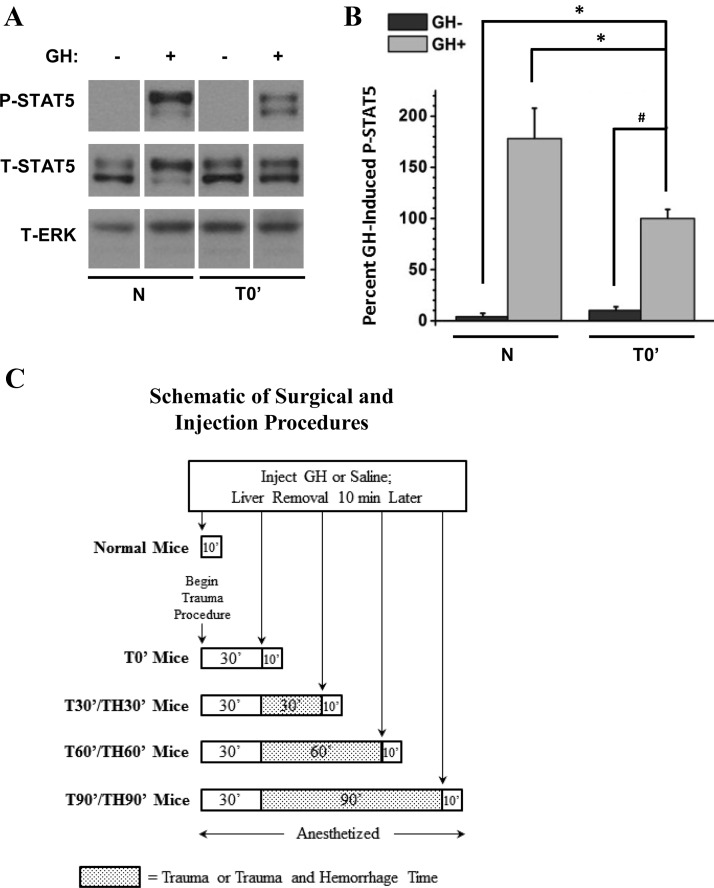
The primary injury control group consisted of mice subjected to the surgical trauma procedure (consisting of anesthesia, laparotomy, wound closure, and arterial catheterization) followed immediately by saline or GH injection (referred to in the text as T0′ mice) (32, 36, 37). To study the effects of trauma on GH signaling, mice were subjected to surgical trauma and injected 30 minutes (T30′), 60 minutes (T60′), or 90 minutes (T90′) later. Trauma and hemorrhage (TH) groups were subjected to surgical trauma, hemorrhaged, and maintained at 35 to 40 mm Hg MAP for 30 minutes (TH30′), 60 minutes (TH60′), or 90 minutes (TH90′) before injection with GH or saline. An additional control group, referred to in the text as normal mice, were anesthetized, subjected to laparotomy, and injected immediately with saline or GH (this procedure was completed in ≤1 minute); livers were removed 10 minutes later. In contrast with T0′ mice, normal mice were not subjected to wound closure or arterial catheterization (see schematic in Figure 2C).
Figure 2.
Effects of soft-tissue trauma on GH-induced STAT5 tyrosine phosphorylation. Mice were subjected to either laparotomy alone or surgical trauma (laparotomy, wound closure, and catheterization), and GH or saline was immediately injected into the inferior vena cava. Livers were removed 10 minutes later. A, Western analysis of liver lysates was performed, and blots were probed with specific anti-PY694/699-STAT5, anti-STAT5, and anti-ERK antibodies. Representative lanes from the scanned image of a single film were chosen; bands of interest were cropped and rearranged to enhance clarity. ERK was used to control for loading on separate P-STAT5 and T-STAT5 gels. B, Bands of interest were quantified by scanning densitometry, and differences in STAT5 phosphorylation between groups were determined. Data are presented as mean ± SEM percent change in phosphorylation, normalized to total STAT5. T0′ GH+ was arbitrarily set to 100%. *P < .05 vs GH-injected T0′ via 1-way ANOVA; #P < .05 vs GH-injected T0′ via unpaired, two-tailed, Welch-corrected t test; n = 6 mice per group of GH-injected mice; n = 3 mice per group of saline-injected mice. C, Schematic representation of surgical and injection procedures in normal mice and mice subjected to trauma alone or combined trauma and hemorrhage (see Materials and Methods). T, total.
Enzymatic deglycosylation
GHR was deglycosylated as described previously with minor modifications (38). Liver lysates were added to denaturation buffer (New England Biolabs, Ipswich, Massachusetts), and the mixtures were heated to 95°C for 10 minutes. Then, either 1) endoglycosidase H (endoH) (New England Biolabs; 1500 U) or 2) peptide-N-glycosidase (PNGase) F (New England Biolabs; 1000 U) plus neuraminidase (New England Biolabs; 100 U; combination referred to as F/N) were added and the mixtures were incubated at 37°C for 16 hours. Samples were then subjected to SDS-PAGE analysis for qualitative assessment of deglycosylation. Nondeglycosylated controls were treated identically, except that incubation was performed in reaction buffer without enzymes.
Western analysis and immunoprecipitation
Liver tissue from each animal (0.2 g) was homogenized in extraction buffer, as described previously, and stored at −80°C until use (36, 37). Liver lysate protein concentrations were determined by the bicinchoninic acid method (Pierce, Rockford, Illinois). Gel samples containing 22.5 μg protein were heated to 95°C in Laemmli buffer for 5 minutes, which was then resolved via SDS-PAGE (3%–8% gradient gels; Bio-Rad, Hercules, California) and transferred to nitrocellulose paper. Membranes were immunoblotted with the following primary antibodies: anti–phospho (P)-JAK2 (PY1007/1008), anti–total JAK2, anti–P-STAT5 (PY694/699), anti–total STAT5, anti–total ERK, and anti–pan-actin from Cell Signaling (Danvers, Massachusetts), anti-GHR (extracellular domain) from R&D Systems (Minneapolis, Minnesota), and anti-IR-β and anti-epidermal growth factor receptor (EGFR) from Santa Cruz Biotechnology (Santa Cruz, California). An additional anti-GHR antibody (cytoplasmic domain; AL-47) was described previously (39).
Immunoprecipitation was performed using 1 mg of total lysate protein incubated with 2 μg of precipitating antibody, overnight at 4°C with gentle rocking. Sepharose beads conjugated to protein A were added (GE Healthcare, Piscataway, New Jersey), and incubated overnight at 4°C. Beads were precipitated by centrifugation, washed, resuspended in 2× Laemmli buffer, and boiled at 95°C for 5 minutes, and the proteins were resolved via SDS-PAGE as described above.
Densitometric and statistical analysis
Enhanced chemiluminescence images of immunoblots were obtained on CL-X Posure Film (Thermo Scientific, Rockford, Illinois). Films were scanned using a Hewlett-Packard C5280 scanner, and relative band intensities were determined using the Quantity One software package (Bio-Rad). Unless otherwise indicated, analysis of proteins appearing as multiple bands included all bands (for example, densitometric analysis of phosphorylated STAT5 took into account the intensities of both bands). Data are presented as mean ± SEM. Data were analyzed using the InStat statistical program (GraphPad Software, Inc, San Diego, California). Differences between groups were determined using 1-way ANOVA (Tukey posttest) or Student's t test (unpaired, Welch-corrected).
Results
Time course of hepatic GH signaling
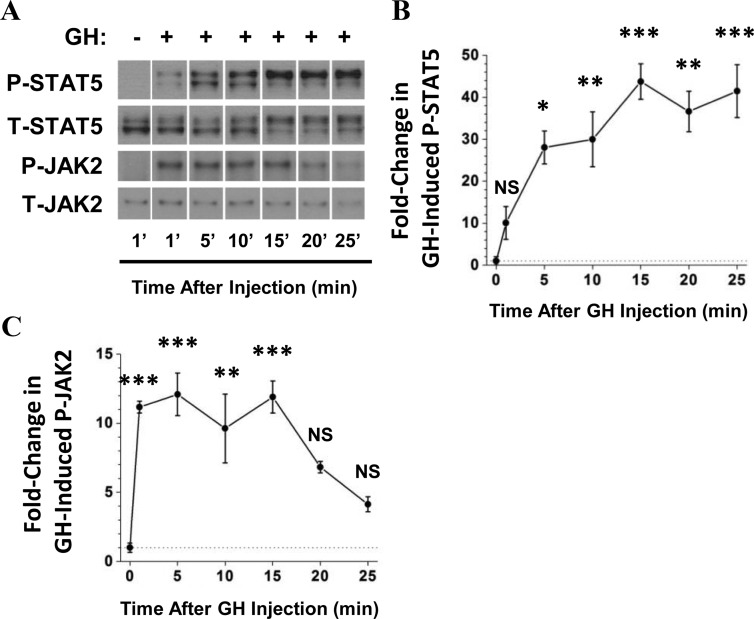
To determine the time course of hepatic GH signaling after injection of GH into the inferior vena cava, livers were harvested from normal mice 1, 5, 10, 15, 20, and 25 minutes after injection with GH or 1 minute after injection with saline. Hepatic STAT5 tyrosine phosphorylation increased 28-fold after GH injection compared with saline-treated mice within 5 minutes of GH injection and remained elevated until at least 25 minutes after injection (Figure 1, A and B).
Figure 1.
Time course of hepatic GH signaling in response to GH injection. Mice were injected with bovine GH or saline, and livers were removed at the indicated times. A, Liver tissue lysates were prepared and subjected to Western blot analysis, with specific anti-PY694/699-STAT5 and anti-STAT5 antibodies. In addition, JAK2 was immunoprecipitated from protein extracts with an anti-JAK2 antibody. JAK2 immunoprecipitates were subjected to Western analysis and probed with specific anti-PY1007/1008-JAK2 and anti-JAK2 antibodies. Representative lanes from the scanned image of a single film were chosen; bands of interest were cropped and rearranged to enhance clarity. B and C, Bands of interest were quantified by scanning densitometry to determine changes in P-STAT5 (B) and P-JAK2 (C) In this figure, the average of 3 measurements of hepatic JAK2 and STAT5 phosphorylation at 1 minute after saline injection was arbitrarily set to a value of 1 and plotted at the t = 0 time point (because GH was not injected in these mice). Data are presented as mean ± SEM fold change in phosphorylation normalized to total protein. *P < .05 vs 0′; **P < .01 vs 0′; ***P < .001 vs 0′, all via 1-way ANOVA; n = 3 mice per time point. NS, no statistically significant difference vs 0 min; T, total.
Total STAT5 levels did not change significantly at any of the time points after GH injection. However, a progressive change in the distribution of STAT5 between the upper and lower bands of the STAT5 doublet was observed for both P-STAT5 and total STAT5, although more pronounced for P-STAT5 (Figure 1A). Maximal phosphorylation of JAK2 was detected even more rapidly, within 1 minute after GH injection (11-fold increase over basal; Figure 1, A and C), and remained elevated until 15 minutes after injection before returning toward control levels.
Acute traumatic injury impairs GH-induced STAT5 phosphorylation
To determine whether GH signaling was affected by the surgical trauma procedure (consisting of laparotomy, wound closure, and bilateral femoral artery catheterization), STAT5 phosphorylation was measured in T0′ mice and normal controls. P-STAT5 was detected only at very low levels in saline-injected normal and T0′ mice, and no significant difference was observed between these groups (Figure 2, A and B). This was true of all subsequent studies of STAT5 phosphorylation after injury; therefore, P-STAT5 graphs in subsequent figures do not include saline-injected groups. However, representative Western blots presented in subsequent figures include lanes from saline-treated mice for clarity. In both normal and T0′ mice, GH injection resulted in a substantial increase in hepatic P-STAT5. However, P-STAT5 in T0′ mice was 45% less compared with normal mice, suggesting a rapid reduction in hepatic GH sensitivity after injury. A total ERK antibody was used as a control to indicate equal protein loading and transfer in each lane. To illustrate the technical differences between normal mice, and mice subjected to trauma alone or combined trauma and hemorrhage, a schematic of the surgical and injection procedures is presented in Figure 2C.
Hemorrhage for 90 minutes results in severe GH resistance
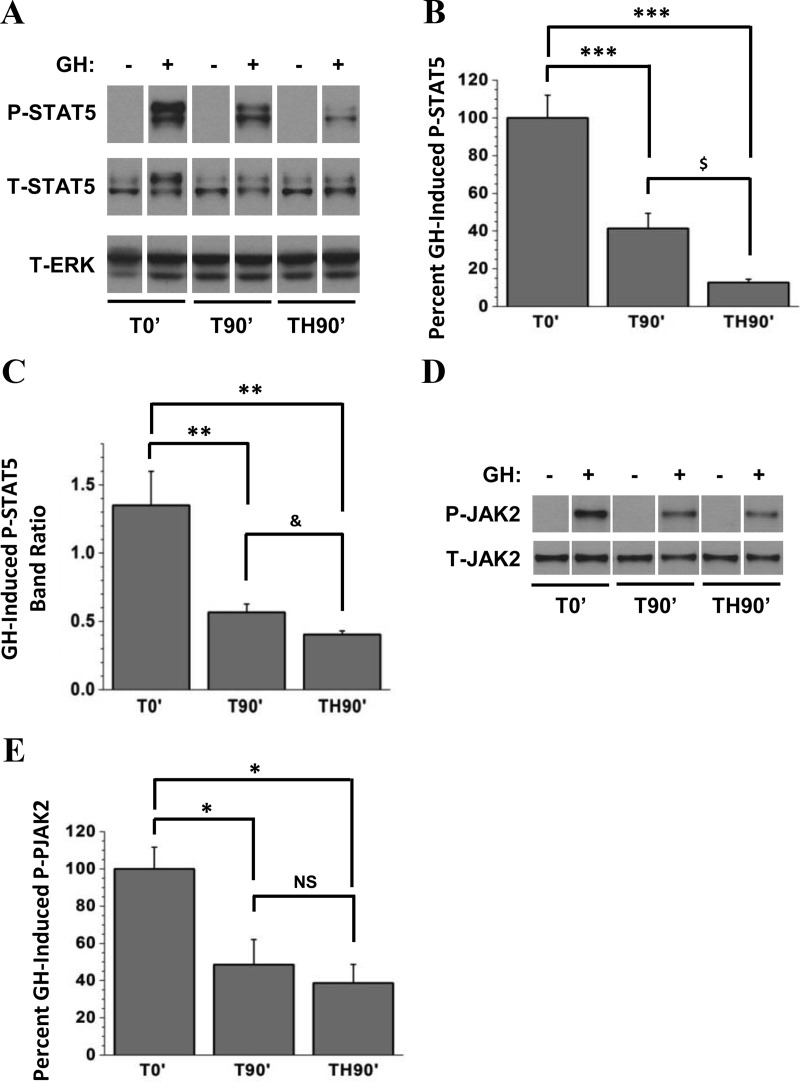
We next asked whether trauma followed by a 90-minute hemorrhage period would result in more severe GH resistance compared with T0′ mice. To determine the effects of trauma and isolate those of hemorrhage, an additional group was generated, consisting of mice subjected to trauma and then maintained on anesthesia without hemorrhage for 90 minutes before injection with GH or saline (T90′ mice). Only low levels of P-STAT5 were detected in saline-injected groups, but a substantial induction of P-STAT5 was observed in all GH-injected groups (Figure 3A; again, use of a total ERK antibody indicated equal protein loading in each lane.). However, GH-injected T90′ mice exhibited a 59% reduction in GH-induced P-STAT5 compared with GH-injected T0′ mice (Figure 3, A and B). Hemorrhage for 90 minutes (TH90′ mice) resulted in even more severe GH resistance evidenced by an 87% reduction in GH-induced P-STAT5 compared with T0′ controls and a significant reduction in GH-induced P-STAT5 compared with T90′ mice (Figure 3, A and B).
Figure 3.
Effect of trauma alone and combined trauma and hemorrhage for 90 minutes on hepatic GH-induced JAK/STAT signaling. Mice were subjected to trauma alone and injected with GH or saline immediately (T0′), 90 minutes later (T90′), or after 90 minutes hemorrhage (TH90′), and livers were harvested 10 minutes later. A, Western analysis of liver lysates was performed, and blots were probed with specific anti-PY694/699-STAT5, anti-STAT5, and anti-ERK antibodies. Representative lanes from the scanned image of a single film were chosen; bands of interest were cropped and rearranged to enhance clarity. ERK was used to demonstrate even loading. B, Bands of interest from Western blot autoradiographs were quantified by scanning densitometry, and differences in STAT5 phosphorylation between GH+ groups were determined. C, Individual P-STAT5 bands were quantified by scanning densitometry, and the ratios of the upper vs lower bands were calculated. D, The same liver lysates as in A were immunoprecipitated with anti-JAK2 antibody. JAK2 immunoprecipitates were subjected to Western analysis and probed with specific anti-PY1007/1008-JAK2 and anti-JAK2 antibodies. E, Bands of interest were quantified by scanning densitometry, and differences in JAK2 phosphorylation between GH+ groups were determined. Except for C, data are presented as mean ± SEM percent change in phosphorylation, normalized to total protein. P-STAT5 after T0′ was arbitrarily set to 100%. *P < .05 vs T0′; **P < .01 vs T0′; ***P < .001 vs T0′; all via one-way ANOVA; $P < .05 vs T90′ via two-tailed, Welch-corrected t test; &P < .05 vs T90′ via one-tailed, Welch-corrected t test; n = 5 mice per group. NS, no statistically significant difference vs T90'; T, total.
Figure 3A also demonstrates a change in the ratio of the upper and lower P-STAT5 bands. Whereas in Figure 1A the ratio increases with increasing time after GH injection, a decrease in the band ratio is observed after injury. In normal mice after GH injection (Figure 2A), the upper-to-lower P-STAT5 band ratio was greater than 1, indicating most P-STAT5 detected was at the higher molecular weight (band ratio = 1.81). Similarly, the P-STAT5 band ratio in T0′ mice (Figure 3A) was also greater than 1 (1.35), indicating that in these mice, most P-STAT5 was also at the higher molecular weight after GH (Figure 3C). However, T90′ mice and TH90′ mice displayed P-STAT5 band ratios that were reduced to 0.57 and 0.40, respectively. Collectively, these results indicate that, in addition to impairing GH-induced STAT5 phosphorylation, increasing trauma time, with or without hemorrhage, impairs GH-induced P-STAT5 band migration.
JAK2 phosphorylation was difficult to detect in saline-injected T0′, T90′, and TH90′ mice (Figure 3D) and was therefore not quantified. However, GH injection resulted in substantial induction of hepatic JAK2 tyrosine phosphorylation in T0′ mice. Compared with T0′ mice, GH-induced JAK2 signaling was impaired in T90′ and TH90′ mice (Figure 3, D and E). GH-induced P-JAK2 was reduced by 49% in T90′ mice and 61% in TH90′ mice compared with T0′ mice. In contrast to GH-induced P-STAT5 (Figure 3B), P-JAK2 levels were not significantly different in T90′ vs TH90′ mice (Figure 3E).
Hemorrhage-induced GH resistance occurs within 30 minutes of hemorrhage onset
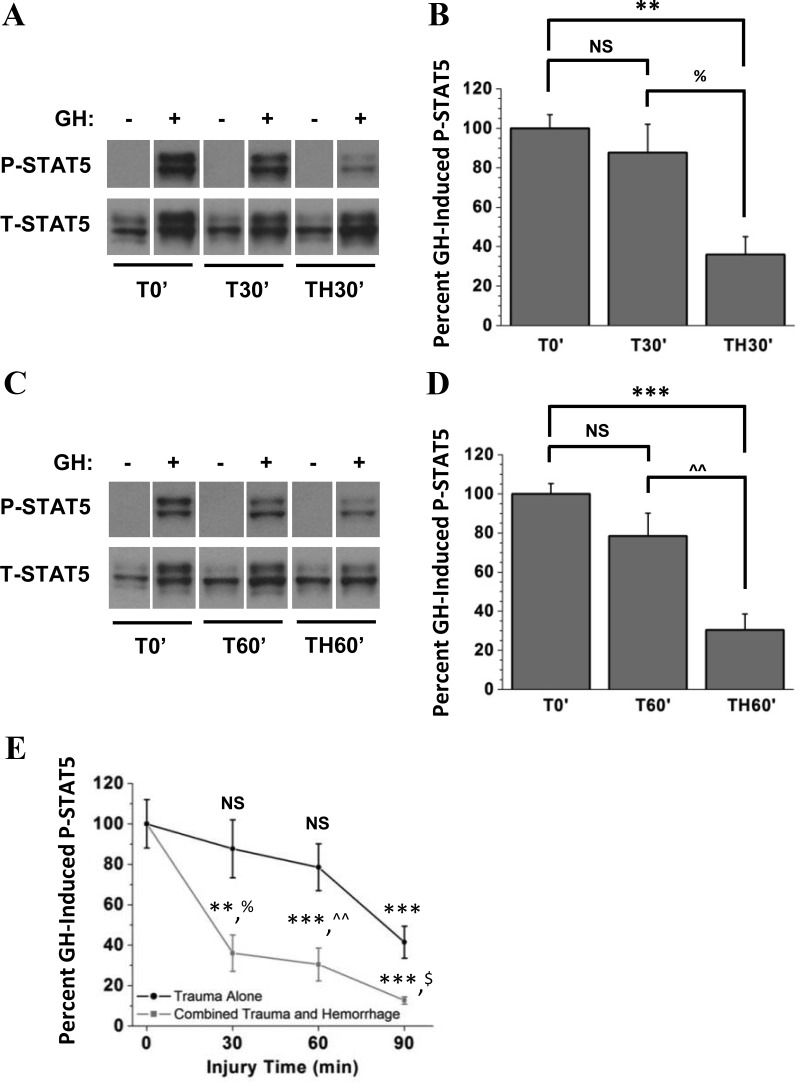
To determine how quickly GH resistance develops after trauma alone or combined trauma and hemorrhage, shorter injury periods were studied. Mice were subjected to trauma alone or trauma combined with 30 minutes hemorrhage followed by injection with GH or saline (T30′ or TH30′ mice, Figure 4A). Additional groups of mice were subjected to trauma alone or combined with 60 minutes hemorrhage followed by injection with GH or saline (T60′ or TH60′ mice, Figure 4C). P-STAT5 levels were negligible in all saline-injected groups (Figure 4, A and C), and were therefore not quantified. Compared with T0′ mice, there was no significant change in GH-induced P-STAT5 in T30′ or T60′ mice (Figure 4, B and D). However, the combination of trauma and either 30 or 60 minutes hemorrhage resulted in significantly impaired GH-induced STAT5 phosphorylation compared with T0′ mice (Figure 4, B and D). Together, these results suggest two distinct mechanisms of GH resistance after injury: a mechanism associated with the soft-tissue injury incurred during the surgical trauma procedure and a second, more rapidly developing and more severe form of GH resistance associated with hemorrhage (see Figure 4E).
Figure 4.
Effect of 30 or 60 minutes of trauma alone or combined trauma and hemorrhage on GH-induced STAT5 phosphorylation. Mice were subjected to trauma alone and injected with GH or saline immediately (T0′), 30 or 60 minutes later (T30′ and T60′), or after hemorrhage for 30 or 60 minutes (TH30′ and TH60′). A and C, Western analysis of liver lysates was performed, and blots were probed with specific anti-PY694/699-STAT5 and anti-STAT5 antibodies. Representative lanes from the scanned image of a single film were chosen; bands of interest were cropped and rearranged to enhance clarity. B and D. Bands of interest were quantified by scanning densitometry, and differences in STAT5 phosphorylation between GH+ groups were determined. Data are presented as mean ± SEM percent change in STAT5 phosphorylation, normalized to total STAT5. E, Comparison of GH-induced STAT5 phosphorylation after acute injury (compiling information from Figures 3 and 4). Data are expressed as mean ± SEM percent change in STAT5 phosphorylation. P-STAT5 after T0′ was arbitrarily set to 100%, and P-STAT5 after trauma alone and combined trauma and hemorrhage for 30, 60, and 90 minutes are expressed relative to T0′. **P < .01 vs T0′; ***P < .001 vs T0′; %P < .05 vs T30′; ^^P < .01 vs T60′, all via 1-way ANOVA; $P < .05 vs T90′ via two-tailed, Welch-corrected t test; NS, no statistically significant difference vs T0′; n = 6 or 7 mice per group. T, total.
Hepatic GHR is altered after hemorrhage
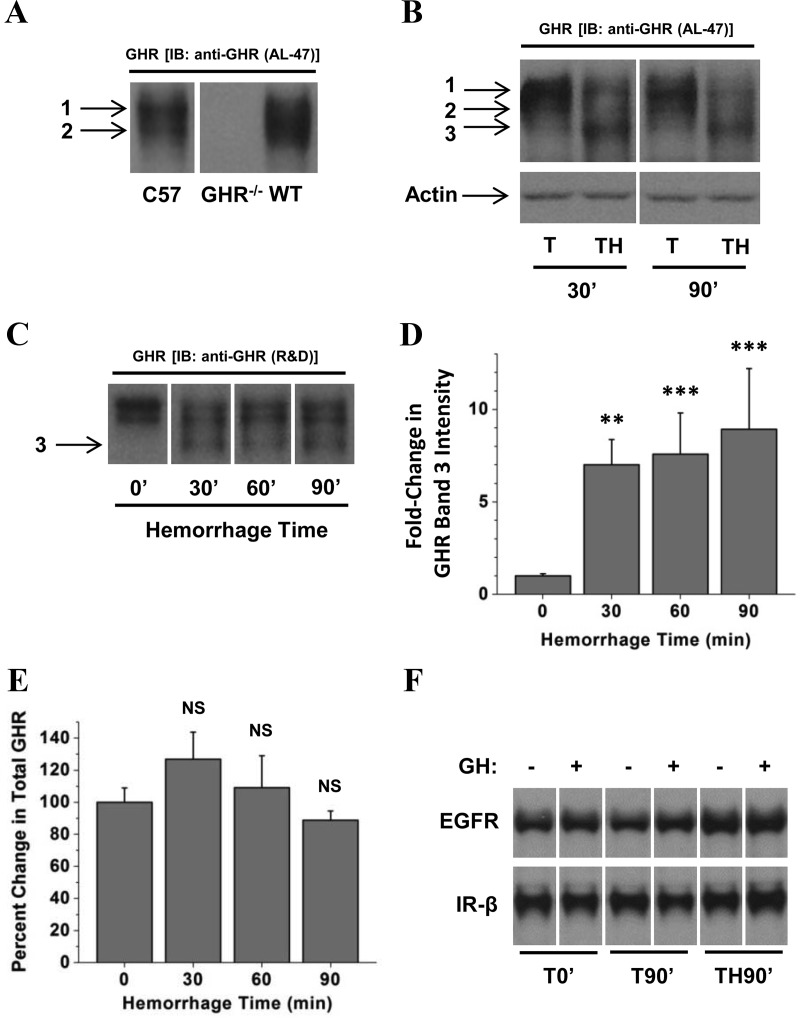
There have been conflicting reports about the role, if any, of the GHR in experimental models of GH resistance (34, 40–42). In addition, these previous reports include models of sepsis and renal failure, but there have been no reported studies of GH resistance after injury. We therefore asked whether changes in total GHR protein might account for the GH resistance observed in our model of injury. Western analysis of liver lysates from normal animals yielded a doublet of approximately 95 and 90 kDa that specifically reacted to the AL-47 antiserum directed to the cytoplasmic domain of GHR (bands 1 and 2, respectively, Figure 5A). The identities of these bands were confirmed by comparison with liver lysates from GHR−/− mice and their wild-type littermates (C57BL/6J GHR−/− mice courtesy of Dr. John Kopchick, Ohio University, Athens, Ohio; Figure 5A). After trauma and hemorrhage for 30 or 90 minutes, a third GHR band appeared at approximately 85 kDa (band 3, Figure 5B). In those same TH30′ or TH90′ mice, the upper band (band 1) was diminished compared with T30′ and T90′ mice, respectively (Figure 5, B and C). The increase in band 3 intensity coupled with the decrease in band 1 intensity in TH30′ and TH90′ mice suggested an apparent reduction of GHR size via Western analysis after hemorrhage.
Figure 5.
Effect of combined trauma and hemorrhage on hepatic GHR. Liver lysates were subjected to Western analysis and probed with GHR antiserum raised against the cytoplasmic domain (AL-47; A and B) or a rabbit polyclonal antibody raised against the extracellular domain of GHR (C). Representative lanes from the scanned image of a single film were chosen; bands of interest were cropped and rearranged to enhance clarity. In A–C, the numbers 1, 2, and 3 correspond to GHR bands 1, 2, and 3, respectively, as described in the text. Bands of interest were quantified by scanning densitometry. Data are presented as mean ± SEM fold change in GHR. D, Band 3 densitometric values were normalized to ERK, and this value was arbitrarily set to 1 for the 0′ hemorrhage group. E, Total GHR was calculated by adding the densitometric values for GHR bands 1, 2, and 3 in each lane and normalizing those to the total ERK signal for the same lane. F, Comparison of EGFR and IR-β levels in livers from mice subjected to trauma alone for 0 or 90 minutes or combined trauma and hemorrhage for 90 minutes and injection with GH or saline. The image in A was stretched vertically to improve clarity and to enhance band identification; this change was applied consistently to the entire image. The 0′ groups were arbitrarily set to 1 and 100% in D and E, respectively, and all other groups are expressed relative to 0′ groups in these panels. **P < .01 vs T0′; ***P < .001 vs T0′, both via 1-way ANOVA; NS, no statistically significant difference vs T0′; n = 4 or 7 mice per group.
An additional gel was run to compare the level of band 3 GHR at each TH time point. Whereas band 3 GHR was significantly increased in hemorrhaged mice relative to T0′ controls, there were no significant differences in band 3 intensity between TH30′, TH60′, and TH90′ mice (Figure 5, C and D). In addition, total GHR abundance (all 3 bands) was unchanged in TH30′, TH60′, or TH90′ mice compared with T0′ controls (Figure 5E). These results suggest that after hemorrhage, cellular GHR was rapidly altered in a manner that changed the electrophoretic mobility of a significant fraction of immunoreactive receptors without changing the total number of receptors. Coupled with changes in GH-induced STAT5 phosphorylation, this suggests that hemorrhage-induced changes in GHR diminish GH-induced signal transduction.
To determine whether the hemorrhage-induced change in GHR was specific to GHR or whether other similar growth factor receptors were also altered by hemorrhage, Western blots were probed with antibodies raised against the EGFR, and the β-subunit of the insulin receptor (IR-β). In contrast to GHR, neither EGFR nor IR-β were decreased in size after trauma and 90 minutes hemorrhage, and no new bands were generated that interacted with EGFR or IR-β antisera (Figure 5F).
Increased GHR electrophoretic mobility is not due to changes in N-linked glycosylation
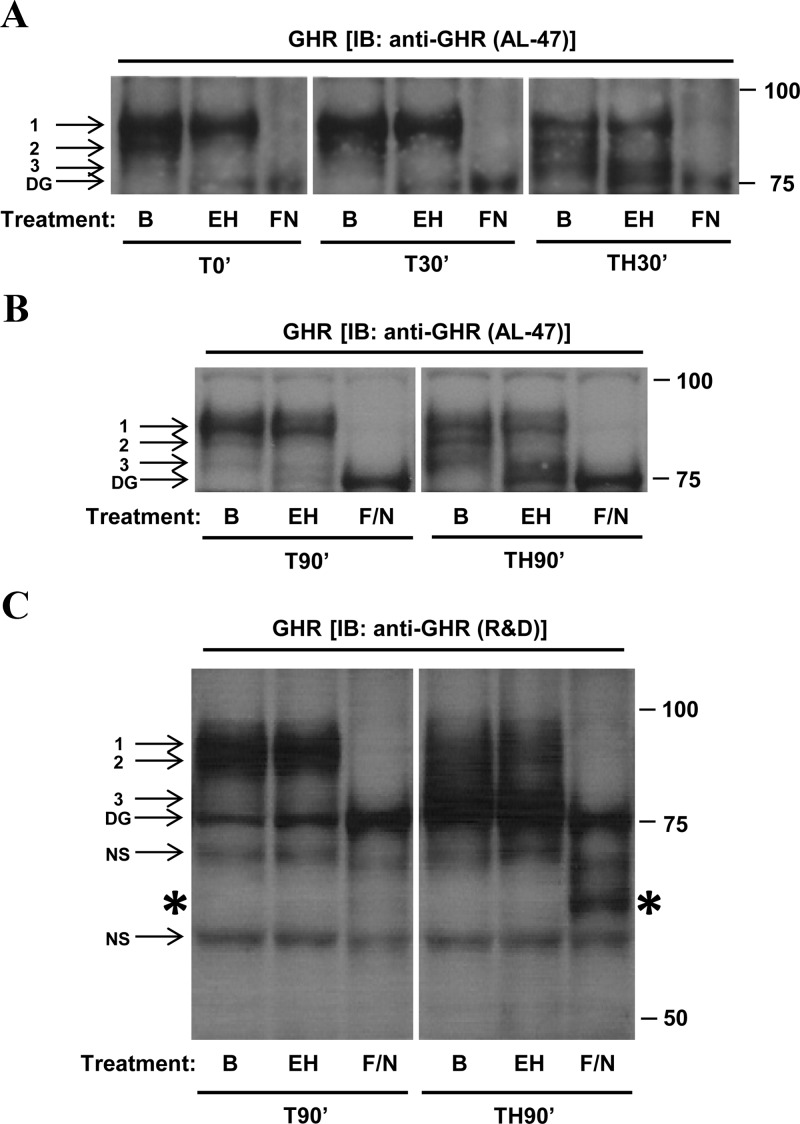
GHR protein is extensively glycosylated during transit to the plasma membrane (43) and has a fairly short half-life in the liver (30–40 minutes) (44). We therefore asked whether band 3 GHR, which was observed only after hemorrhage for 30 minutes or more, might represent newly synthesized GHR protein with an immature pattern of N-glycosylation. To determine whether the appearance of band 3 indicates that hemorrhage interferes with cellular processes, such as receptor maturation, liver lysates were treated with endoH, a combination of PNGase F and neuraminidase (F/N), or a buffer control. EndoH is an endoglycosidase that removes only immature (high-mannose) N-linked oligosaccharides. F/N is a combination of an exoglycosidase (neuraminidase) and an endoglycosidase (PNGase F); this combination removes all N-linked oligosaccharides from glycoproteins, regardless of maturity (mannose content).
After hemorrhage (TH30′ or TH90′), band 3 was not affected by treatment with endoH but was eliminated by treatment with F/N, suggesting that the reduction in GHR size after hemorrhage is not due to an increased proportion of immature receptors (Figure 6, A and B). However, band 2 in TH30′ and TH90′ mice was almost completely absent after treatment with either endoH or F/N, suggesting that band 2 GHR is an immature (high-mannose) glycoprotein. Band 2 was also eliminated in lysates from trauma-alone mice that were treated with endoH (T0′, T30′, and T90′ mice; Figure 6, A and B). Because Band 2 GHR is a prominent feature in livers from normal mice (Figure 5A) as well as injured mice (Figure 6, A and B), these data suggest that band 2 represents a precursor form of hepatic GHR as it traverses the secretory pathway and is not significantly affected by either trauma or hemorrhage. Because Band 3 is not reduced by endoH treatment, the appearance of band 3 GHR in hemorrhaged livers likely results from some other posttranslational alteration, possibly GHR cleavage. After long film exposures of GHR immunoblots, we have frequently observed a low level of band 3 GHR in livers from normal and trauma-alone mice (for example, Figures 5B and 6B). This may suggest that the band 3 observed in livers from hemorrhaged mice actually represents a form of GHR that is naturally present at low levels but is rapidly generated in response to hemorrhage. Finally, band 1 GHR was also unaffected by treatment with endoH, suggesting that band 1 is the mature form of hepatic GHR detected in all experimental groups.
Figure 6.
Decreased apparent molecular weight of GHR is not due to changes in N-linked glycosylation. Liver lysates were prepared from livers of mice subjected to trauma alone or combined trauma and hemorrhage. Lysates were incubated for 16 hours at 37°C in reaction buffer alone (B), reaction buffer plus Endo H (EH), or reaction buffer plus the F/N combination (F/N). Representative lanes from the scanned image of a single film were chosen; bands of interest were cropped and rearranged to enhance clarity. The numbers 1, 2, and 3 in this figure indicate GHR bands 1, 2, and 3. DG represents fully deglycosylated GHR; NS represents nonspecific bands. Panel A, Comparison of the effects of enzymatic treatment on liver lysates from T0′, T30′, and TH30′ mice probed with AL-47 antiserum. Panel B, Comparison of the effects of enzymatic treatment on livers from T90′ and TH90′ mice probed with AL-47. Panel C, Comparison of the effects of enzymatic treatment on livers from T90′ and TH90′ mice probed with anti-GHR antibody raised against the extracellular domain of GHR. The large asterisks along the sides of the immunoblot in panel C are intended to draw attention to the band at 65 kDa that is present in the hemorrhaged mouse but absent in the mouse subjected to trauma alone. The image in this panel was stretched vertically to improve clarity and to enhance band identification; this change was applied consistently to the entire image.
Deglycosylated liver lysates were further subjected to Western analysis, with care taken to resolve smaller GHR isoforms (in the 50- to 75-kDa range) that may have been generated by enzymatic treatment. To prevent the high background signal usually observed in this region when probing with AL-47, an anti-GHR antibody raised against the extracellular domain of GHR was substituted for AL-47 (Figure 6C). In lysates from hemorrhaged mice treated with F/N, a dark band was observed at approximately 65 kDa (asterisk in Figure 6C, F/N treated TH90′ lane). This band was absent in lysates from nonhemorrhaged (T90′) mice, indicating that the 65-kDa band in Figure 6C was likely derived from fully deglycosylated band 3 GHR.
Discussion
The clinical consequences of GH resistance after injury have profound implications in critical care. GH resistance after injury contributes to protein/muscle wasting and organ dysfunction. We therefore set out to determine whether after trauma alone or combined trauma and hemorrhage, there was an acute impairment of GH signaling. In the present study, we demonstrated that GH resistance develops immediately in response to surgical trauma and that surgical trauma combined with hemorrhage results in a more severe form of GH resistance compared with trauma alone. It is important to realize that these changes in GH signaling due to trauma and hemorrhage are very rapid, before any measurable pathophysiological changes that occur after injury, such as decreased IGF-I levels and loss of muscle mass. Thus, these changes in GH signaling are the earliest signs yet found of the development of GH resistance after injury or critical illness.
Injection of GH in normal mice resulted in rapid induction of hepatic JAK2 and STAT5, consistent with previous reports (24, 25, 33). In saline-injected mice, STAT5 was detected as a doublet on Western analysis, with the majority localized to the lower band. After injection with GH, most STAT5 was detected in the upper band. We and others have previously described a similar shift in STAT5 banding pattern after GH stimulation in cultured cells as well as rodents (25, 45, 46). The upper band was identified as STAT5 phosphorylated on both serine and tyrosine, and this serine phosphorylation of PY-STAT5 may influence DNA binding and therefore GH-induced transcriptional regulation (47, 48). In the present studies, Western analysis of total STAT5 and P-STAT5 banding patterns indicated that trauma alone or combined trauma and hemorrhage reduced GH-induced STAT5 band migration, regardless of the duration of trauma or hemorrhage. This may suggest a further STAT5 signaling impairment in addition to impaired tyrosine phosphorylation, both likely affecting GH-induced gene expression.
Previous reports are conflicting concerning the role of GHR in experimental models of GH resistance (28, 40–42, 49). In several reports, GH resistance was associated with decreased GHR mRNA, but these measurements were obtained several hours (28, 42) after the initial insult and cannot be used to explain the signaling defects in our model. The GH resistance observed after surgical trauma, alone or combined with hemorrhage, in the present work developed too rapidly to be explained by altered gene expression. However, a previous study reported a reduction of hepatic GHR protein after lipopolysaccharide injection, occurring in as little as 60 minutes (40). Thus, to determine the mechanism of impaired GH signaling after injury, GHR protein was studied via Western analysis.
Rodent GHR is often detected at molecular masses between 100 and 120 kDa on SDS-PAGE (28, 29, 42, 50), and our previous work yielded similar results for rat GHR in cultured hepatoma cells (45, 51). Western analysis of GHR content in the current study, however, yielded bands at 95 and 90 kDa (what we refer to as bands 1 and 2). To confirm the identity of these bands, we obtained livers from GHR-knockout mice (GHR−/− on C57BL/6J background) and wild-type littermates (both kindly provided by Dr. John Kopchick of Ohio University). The 95- and 90-kDa doublet bands were absent in liver lysates from GHR−/− mice but present in livers from their wild-type littermates, confirming the identities of bands 1 and 2 as GHR. We are unsure of the reasons for the discrepancy in GHR molecular weight, but it is possible that GHR in cultured cells is more extensively glycosylated than GHR in vivo, contributing to the increased size of GHR in cultured cell lines.
We next asked whether changes in total GHR protein might account for the observed decreases in signaling. There was no change in the total amount of GHR protein after trauma alone at any of the time points studied. However, the reduction of band 1 GHR and the unexpected and simultaneous appearance of an additional GHR band (band 3) in hemorrhaged mice suggested that the more severe STAT5 signaling impairment after hemorrhage was related to decreased receptor size, although a direct causality needs to be confirmed. GHR is an extensively glycosylated protein (43). Thus, to determine whether band 3 GHR represented precursor GHR lacking a mature glycosylation pattern, N-linked oligosaccharides were enzymatically removed. Enzymatic removal of N-linked oligosaccharides from band 1 GHR resulted in 75-kDa GHR, demonstrating that N-glycosylation accounted for ∼20 kDa of band 1 GHR. Because hepatic GHR turnover occurs rapidly, with a half-life of 30 to 40 minutes (44), we hypothesized that band 3 (at approximately 85 kDa) might represent newly synthesized GHR with incomplete N-glycosylation, possibly due to hemorrhage-induced impairment of receptor maturation. To test this hypothesis, we adapted a technique for use in tissue to distinguish mature from immature GHR N-glycosylation (differential enzymatic deglycosylation). In livers from hemorrhaged mice, neither band 1 nor band 3 GHR was affected by treatment with endoH, suggesting that both forms of GHR have a mature pattern of N-linked glycosylation. The only GHR band affected by treatment with endoH was band 2, indicating that this 90-kDa band represents the precursor (high-mannose) form of GHR. Complete deglycosylation resulted in the absence of all previously observed bands (1, 2, and 3) and the appearance of a fourth band at 75 kDa, representing fully deglycosylated GHR (bands 1 and 2). This indicated that band 1 represented full-length, fully glycosylated GHR and that band 2 represented full-length, incompletely glycosylated (likely Golgi-bound) GHR.
Based on amino acid sequence, the predicted molecular mass of murine GHR is approximately 73 kDa, and deglycosylated GHR is very close in size to that predicted by amino acid sequence alone (75 vs 73 kDa, respectively). This suggests that N-linked glycosylation is the only major postsecondary addition to GHR and that the deglycosylated protein consists primarily of the GHR peptide sequence. Detection of fully deglycosylated band 3 GHR with an R&D Systems anti-GHR antibody revealed that band 3 GHR is approximately 65 kDa upon removal of all N-linked oligosaccharides (vs band 1, which was 75 kDa after deglycosylation). The difference in apparent molecular mass between nondeglycosylated band 1 and band 3 GHR is approximately 10 kDa (95 vs 85 kDa, respectively). The difference in size between band 1 and band 3 GHR was therefore the same (10 kDa) in both the glycosylated and deglycosylated states. Taken together, this suggests that hemorrhage results in a loss of part of the amino acid sequence of GHR, independent of glycosylation. As controls, EGFR and IR-β were studied. Similar to the GHR, these are extensively glycosylated integral membrane growth factor receptors, and no demonstrable hemorrhage-dependent change in size was observed, suggesting that this was specific, and possibly unique, to GHR.
The membrane-proximal Box 1 sequence of GHR is necessary for JAK2 association with GHR (20, 52). JAK2 kinase activity is retained even when large portions of the GHR cytoplasmic domain are removed, as long as Box 1 remains intact (21, 23, 53). However, STAT5 must associate with the C-terminal end of GHR to be tyrosine phosphorylated by JAK2. This is accomplished by interaction of the STAT5 SH2 domain with phosphorylated tyrosines at the cytoplasmic C-terminal end of GHR (21–23). Therefore, it is possible that C-terminal cleavage of GHR might impair GH-induced STAT5 tyrosine phosphorylation without affecting JAK2 phosphorylation. This would explain our finding of reduced STAT5 tyrosine phosphorylation but not JAK2 tyrosine phosphorylation after hemorrhage (TH90′ vs T90′) and may suggest that the ∼10-kDa fragment missing from band 3 actually derives from the C-terminal end of GHR.
The cytoplasmic domain of GHR contains 3 tyrosines (548, 577, and 609) that are especially important for GHR/STAT5 association and appear to be necessary for maximal GH-induced STAT5 phosphorylation (21, 23). The region of mouse GHR containing all 3 tyrosines is a C-terminal domain with a calculated molecular mass of ∼12 kDa; the region containing only tyrosines 577 and 609 is ∼8 kDa. It is therefore possible that cleavage of an approximately 10-kDa C-terminal GHR fragment could disrupt 2 or more tyrosines that are necessary for maximal GH-induced STAT5 phosphorylation, which would explain the severe decrease in STAT5 signaling observed after hemorrhage. Alternatively, some other posttranslational modification, such as a hemorrhage-induced change in mRNA splicing, could result in production of a shortened form of GHR with limited signal transduction capabilities. The appearance of band 3 occurred with timing similar to the development of the more extensive STAT5 signaling impairment observed after the combination of trauma and hemorrhage, suggesting that band 3 is not capable of or interferes with GH signaling, resulting in the more severe form of GH resistance after hemorrhage.
We are not aware of any previous reports describing similar alterations to GHR. Proteases known to cleave GHR, such as TNF-α converting enzyme or γ-secretase, result in fragments that are significantly larger than the ∼10 kDa described in the present report, generally resulting in reduced GHR abundance rather than a simple decrease in GHR size. It is interesting to note that on darker exposures, GHR immunoblots revealed a very low level of band 3 GHR in livers from normal and trauma-only mice. The presence of band 3 GHR in these mice suggests that band 3 represents a physiological form of GHR, normally at low levels but rapidly accumulating in response to hemorrhage. Future studies will attempt to determine whether GHR is cleaved after hemorrhage or whether there is a change in GHR mRNA splicing. Unfortunately, use of techniques such as mass spectrometry sequence analysis may be difficult because there is relatively little GHR in the liver of C57BL/6 mice, and there is no previous evidence of a similar GHR alteration in cultured cell lines that express higher levels of GHR.
In conclusion, the present study demonstrated severe impairments in hepatic GH signaling after either trauma alone or combined trauma and hemorrhage. Trauma alone resulted in biphasic impairment of GH-induced STAT5 phosphorylation. The initial impairment occurred immediately after completion of the trauma procedure and was sustained for at least 60 minutes, with more severe resistance 90 minutes after trauma. Thus, the present studies explore the initial mechanisms that could lead to the development of GH resistance after injury. The mechanisms of GH resistance after trauma alone are still unclear, but the absence of a lower molecular weight GHR (band 3) suggests a separate mechanism from the more severe GH resistance occurring rapidly after the combination of trauma and hemorrhage, which correlated with an apparent structural change to GHR.
Acknowledgments
We thank Dr. John Kopchick and Dr. Darlene Berryman of Ohio University for providing livers from GHR−/− mice and wild-type littermates. We also thank John L. Franklin and Ling Zhou for their technical assistance.
This work was supported by grants from the National Institutes of Health (DK 62071 to J.L.M. and DK 58259 to S.J.F.) and Veterans Administration Merit Review Awards to J.L.M. and S.J.F.
Current address for L.L.: Department of Psychiatry and Behavioral Neurobiology, University of Alabama Birmingham, Birmingham, Alabama 35294.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- EGFR
- epidermal growth factor receptor
- endoH
- endoglycosidase H
- F/N
- PNGase F and neuraminidase combination
- GHR
- GH receptor
- IR-β
- insulin receptor-β
- JAK2
- Janus kinase 2
- MAP
- mean arterial pressure
- P
- phospho
- PNGase
- peptide-N-glycosidase
- SH2
- src-homology 2
- STAT5
- signal transducer and activator of transcription 5.
References
- 1. Askanazi J, Carpentier YA, Michelsen CB, et al. Muscle and plasma amino acids following injury. Influence of intercurrent infection. Ann Surg. 1980;192:78–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gamrin L, Essen P, Forsberg AM, Hultman E, Wernerman J. A descriptive study of skeletal muscle metabolism in critically ill patients: free amino acids, energy-rich phosphates, protein, nucleic acids, fat, water, and electrolytes. Crit Care Med. 1996;24:575–583 [DOI] [PubMed] [Google Scholar]
- 3. Debaveye Y, Van den Berghe G. Risks and benefits of nutritional support during critical illness. Annu Rev Nutr. 2006;26:513–538 [DOI] [PubMed] [Google Scholar]
- 4. Jeschke MG, Herndon DN. Effect of growth factors as therapeutic drugs on hepatic metabolism during the systemic inflammatory response syndrome. Curr Drug Metab. 2004;5:399–413 [DOI] [PubMed] [Google Scholar]
- 5. Pereira C, Murphy K, Jeschke M, Herndon DN. Post burn muscle wasting and the effects of treatments. Int J Biochem Cell Biol. 2005;37:1948–1961 [DOI] [PubMed] [Google Scholar]
- 6. Jenkins RC, Ross RJ. Growth hormone therapy for protein catabolism. QJM. 1996;89:813–819 [DOI] [PubMed] [Google Scholar]
- 7. Frankenfield DC, Smith JS, Cooney RN. Accelerated nitrogen loss after traumatic injury is not attenuated by achievement of energy balance. JPEN J Parenter Enteral Nutr. 1997;21:324–329 [DOI] [PubMed] [Google Scholar]
- 8. Jeevanandam M, Hsu YC, Ramias L, Schiller WR. Mild orotic aciduria and uricosuria in severe trauma victims. Am J Clin Nutr. 1991;53:1242–1248 [DOI] [PubMed] [Google Scholar]
- 9. Delano MJ, Moldawer LL. The origins of cachexia in acute and chronic inflammatory diseases. Nutr Clin Pract. 2006;21:68–81 [DOI] [PubMed] [Google Scholar]
- 10. Taylor BE, Buchman TG. Is there a role for growth hormone therapy in refractory critical illness? Curr Opin Crit Care. 2008;14:438–444 [DOI] [PubMed] [Google Scholar]
- 11. Friedrich O. Critical illness myopathy: sepsis-mediated failure of the peripheral nervous system. Eur J Anaesthesiol Suppl. 2008;42:73–82 [DOI] [PubMed] [Google Scholar]
- 12. Elijah IE, Branski LK, Finnerty CC, Herndon DN. The GH/IGF-1 system in critical illness. Best Pract Res Clin Endocrinol Metab. 2011;25:759–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ruokonen E, Takala J. Dangers of growth hormone therapy in critically ill patients. Curr Opin Clin Nutr Metab Care. 2002;5:199–209 [DOI] [PubMed] [Google Scholar]
- 14. Streat SJ, Beddoe AH, Hill GL. Aggressive nutritional support does not prevent protein loss despite fat gain in septic intensive care patients. J Trauma. 1987;27:262–266 [DOI] [PubMed] [Google Scholar]
- 15. Ross R, Miell J, Freeman E, et al. Critically ill patients have high basal growth hormone levels with attenuated oscillatory activity associated with low levels of insulin-like growth factor-I. Clin Endocrinol (Oxf). 1991;35:47–54 [DOI] [PubMed] [Google Scholar]
- 16. Van den Berghe G, de Zegher F, Veldhuis JD, et al. The somatotropic axis in critical illness: effect of continuous growth hormone (GH)-releasing hormone and GH-releasing peptide-2 infusion. J Clin Endocrinol Metab. 1997;82:590–599 [DOI] [PubMed] [Google Scholar]
- 17. Timmins AC, Cotterill AM, Hughes SC, et al. Critical illness is associated with low circulating concentrations of insulin-like growth factors-I and -II, alterations in insulin-like growth factor binding proteins, and induction of an insulin-like growth factor binding protein 3 protease. Crit Care Med. 1996;24:1460–1466 [DOI] [PubMed] [Google Scholar]
- 18. Isaksson OG, Eden S, Jansson JO. Mode of action of pituitary growth hormone on target cells. Annu Rev Physiol. 1985;47:483–499 [DOI] [PubMed] [Google Scholar]
- 19. Argetsinger LS, Campbell GS, Yang X, et al. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell. 1993;74:237–244 [DOI] [PubMed] [Google Scholar]
- 20. Frank SJ, Gilliland G, Kraft AS, Arnold CS. Interaction of the growth hormone receptor cytoplasmic domain with the JAK2 tyrosine kinase. Endocrinology. 1994;135:2228–2239 [DOI] [PubMed] [Google Scholar]
- 21. Smit LS, Meyer DJ, Billestrup N, Norstedt G, Schwartz J, Carter-Su C. The role of the growth hormone (GH) receptor and JAK1 and JAK2 kinases in theactivation of Stats 1, 3, and 5 by GH. Mol Endocrinol. 1996;10:519–533 [DOI] [PubMed] [Google Scholar]
- 22. Sotiropoulos A, Moutoussamy S, Renaudie F, et al. Differential activation of Stat3 and Stat5 by distinct regions of the growth hormone receptor. Mol Endocrinol. 1996;10:998–1009 [DOI] [PubMed] [Google Scholar]
- 23. Yi W, Kim SO, Jiang J, et al. Growth hormone receptor cytoplasmic domain differentially promotes tyrosine phosphorylation of signal transducers and activators of transcription 5b and 3 by activated JAK2 kinase. Mol Endocrinol. 1996;10:1425–1443 [DOI] [PubMed] [Google Scholar]
- 24. Waxman DJ, Ram PA, Park SH, Choi HK. Intermittent plasma growth hormone triggers tyrosine phosphorylation and nuclear translocation of a liver-expressed, Stat 5-related DNA binding protein. Proposed role as an intracellular regulator of male-specific liver gene transcription. J Biol Chem. 1995;270:13262–13270 [DOI] [PubMed] [Google Scholar]
- 25. Ram PA, Park SH, Choi HK, Waxman DJ. Growth hormone activation of Stat 1, Stat 3, and Stat 5 in rat liver: differential kinetics of hormone desensitization and growth hormone stimulation of both tyrosine phosphorylation and serine/threonine phosphorylation. J Biol Chem. 1996;271:5929–5940 [DOI] [PubMed] [Google Scholar]
- 26. Davey HW, Xie T, McLachlan MJ, Wilkins RJ, Waxman DJ, Grattan DR. STAT5b is required for GH-induced liver IGF-I gene expression. Endocrinology. 2001;142:3836–3841 [DOI] [PubMed] [Google Scholar]
- 27. Ohlsson C, Mohan S, Sjogren K, et al. The role of liver-derived insulin-like growth factor-I. Endocr Rev. 2009;30:494–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yumet G, Shumate ML, Bryant P, Lin CM, Lang CH, Cooney RN. Tumor necrosis factor mediates hepatic growth hormone resistance during sepsis. Am J Physiol Endocrinol Metab. 2002;283:E472–E481 [DOI] [PubMed] [Google Scholar]
- 29. Yumet G, Shumate ML, Bryant DP, Lang CH, Cooney RN. Hepatic growth hormone resistance during sepsis is associated with increased suppressors of cytokine signaling expression and impaired growth hormone signaling. Crit Care Med. 2006;34:1420–1427 [DOI] [PubMed] [Google Scholar]
- 30. Chen Y, Sun D, Krishnamurthy VM, Rabkin R. Endotoxin attenuates growth hormone-induced hepatic insulin-like growth factor I expression by inhibiting JAK2/STAT5 signal transduction and STAT5b DNA binding. Am J Physiol Endocrinol Metab. 2007;292:E1856–E1862 [DOI] [PubMed] [Google Scholar]
- 31. Rodriguez-Arnao J, Yarwood G, Ferguson C, Miell J, Hinds CJ, Ross RJ. Reduction in circulating IGF-I and hepatic IGF-I mRNA levels after caecal ligation and puncture are associated with differential regulation of hepatic IGF-binding protein-1, -2 and -3 mRNA levels. J Endocrinol. 1996;151:287–292 [DOI] [PubMed] [Google Scholar]
- 32. Zhai L, Ballinger SW, Messina JL. Role of of reactive oxygen species in injury-induced insulin resistance. Mol Endocrinol. 2011;25:492–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chow JC, Ling PR, Qu ZS, et al. Growth hormone stimulates tyrosine phosphorylation of JAK2 and STAT5, but not insulin receptor substrate-1 or SHC proteins in liver and skeletal muscle of normal rats in vivo. Endocrinology. 1996;137:2880–2886 [DOI] [PubMed] [Google Scholar]
- 34. Beauloye V, Willems B, de Coninck V, Frank SJ, Edery M, Thissen JP. Impairment of liver GH receptor signaling by fasting. Endocrinology. 2002;143:792–800 [DOI] [PubMed] [Google Scholar]
- 35. Hong-Brown LQ, Brown CR, Cooney RN, Frost RA, Lang CH. Sepsis-induced muscle growth hormone resistance occurs independently of STAT5 phosphorylation. Am J Physiol Endocrinol Metab. 2003;285:E63–E72 [DOI] [PubMed] [Google Scholar]
- 36. Ma Y, Wang P, Kuebler JF, Chaudry IH, Messina JL. Hemorrhage induces the rapid development of hepatic insulin resistance. Am J Physiol Gastrointest Liver Physiol. 2003;284:G107–G115 [DOI] [PubMed] [Google Scholar]
- 37. Ma Y, Toth B, Keeton AB, Holland LT, Chaudry IH, Messina JL. Mechanisms of hemorrhage-induced hepatic insulin resistance: role of tumor necrosis factor-α. Endocrinology. 2004;145:5168–5176 [DOI] [PubMed] [Google Scholar]
- 38. Kim SO, Jiang J, Yi W, Feng GS, Frank SJ. Involvement of the Src homology 2-containing tyrosine phosphatase SHP-2 in growth hormone signaling. J Biol Chem. 1998;273:2344–2354 [DOI] [PubMed] [Google Scholar]
- 39. Zhang Y, Guan R, Jiang J, et al. Growth hormone (GH)-induced dimerization inhibits phorbol ester-stimulated GH receptor proteolysis. J Biol Chem. 2001;276:24565–24573 [DOI] [PubMed] [Google Scholar]
- 40. Wang X, Jiang J, Warram J, et al. Endotoxin-induced proteolytic reduction in hepatic growth hormone (GH) receptor: a novel mechanism for GH insensitivity. Mol Endocrinol. 2008;22:1427–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Denson LA, Held MA, Menon RK, Frank SJ, Parlow AF, Arnold DL. Interleukin 6 inhibits hepatic growth hormone signaling via up regulation of Cis and Socs-3. Am J Physiol Gastrointest Liver Physiol. 2003;284:G646–G654 [DOI] [PubMed] [Google Scholar]
- 42. Schaefer F, Chen Y, Tsao T, Nouri P, Rabkin R. Impaired JAK-STAT signal transduction contributes to growth hormone resistance in chronic uremia. J Clin Invest. 2001;108:467–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Harding PA, Wang XZ, Kelder B, Souza S, Okada S, Kopchick JJ. In vitro mutagenesis of growth hormone receptor Asn-linked glycosylation sites. Mol Cell Endocrinol. 1994;106:171–180 [DOI] [PubMed] [Google Scholar]
- 44. Baxter RC. Measurement of growth hormone and prolactin receptor turnover in rat liver. Endocrinology. 1985;117:650–655 [DOI] [PubMed] [Google Scholar]
- 45. Ji S, Guan R, Frank SJ, Messina JL. Insulin inhibits growth hormone signaling via the growth hormone receptor/JAK2/STAT5B pathway. J Biol Chem. 1999;274:13434–13442 [DOI] [PubMed] [Google Scholar]
- 46. Ram PA, Waxman DJ. Interaction of growth hormone-activated STATs with SH2-containing phosphotyrosine phosphatase SHP-1 and nuclear JAK2 tyrosine kinase. J Biol Chem. 1997;272:17694–17702 [DOI] [PubMed] [Google Scholar]
- 47. Weaver AM, Silva CM. S731 in the transactivation domain modulates STAT5b activity. Biochem Biophys Res Comm. 2007;362:1026–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Park SH, Yamashita H, Rui H, Waxman DJ. Serine phosphorylation of GH-activated signal transducer and activator of transcription 5a (STAT5a) and STAT5b: impact on STAT5 transcriptional activity. Mol Endocrinol. 2001;15:2157–2171 [DOI] [PubMed] [Google Scholar]
- 49. Defalque D, Brandt N, Ketelslegers JM, Thissen JP. GH insensitivity induced by endotoxin injection is associated with decreased liver GH receptors. Am J Physiol. 1999;276:E565–E572 [DOI] [PubMed] [Google Scholar]
- 50. Zhou Y, Xu BC, Maheshwari HG, et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc Natl Acad Sci U S A. 1997;94:13215–13220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bennett WL, Keeton AB, Ji S, Xu J, Messina JL. Insulin regulation of growth hormone receptor gene expression: involvement of both the PI-3 kinase and MEK/ERK signaling pathways. Endocrine. 2007;32:219–226 [DOI] [PubMed] [Google Scholar]
- 52. VanderKuur JA, Wang X, Zhang L, et al. Domains of the growth hormone receptor required for association and activation of JAK2 tyrosine kinase. J Biol Chem. 1994;269:21709–21717 [PubMed] [Google Scholar]
- 53. Deng L, Jiang J, Frank SJ. Growth hormone-induced JAK2 signaling and GH receptor down-regulation: role of GH receptor intracellular domain tyrosine residues. Endocrinology. 2012;153:2311–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]