Abstract
Compelling reasons to study the role of sex in the circadian system include the higher rates of sleep disorders in women than in men and evidence that sex steroids modulate circadian control of locomotor activity. To address the issue of sex differences in the circadian system, we examined daily and circadian rhythms in wheel-running activity, electrical activity within the suprachiasmatic nucleus, and PER2::LUC-driven bioluminescence of gonadally-intact adult male and female C57BL/6J mice. We observed greater precision of activity onset in 12-hour light, 12-hour dark cycle for male mice, longer activity duration in 24 hours of constant darkness for female mice, and phase-delayed PER2::LUC bioluminescence rhythm in female pituitary and liver. Next, in order to investigate whether sex differences in behavior are sex chromosome or gonadal sex dependent, we used the 4 core genotypes (FCG) mouse model, in which sex chromosome complement is independent of gonadal phenotype. Gonadal males had more androgen receptor expression in the suprachiasmatic nucleus and behaviorally reduced photic phase shift response compared with gonadal female FCG mice. Removal of circulating gonadal hormones in adults, to test activational vs organizational effects of sex revealed that XX animals have longer activity duration than XY animals regardless of gonadal phenotype. Additionally, we observed that the activational effects of gonadal hormones were more important for regulating activity levels in gonadal male mice than in gonadal female FCG mice. Taken together, sex differences in the circadian rhythms of activity, neuronal physiology, and gene expression were subtle but provide important clues for understanding the pathophysiology of the circadian system.
Daily rhythms in behavior and physiology are adaptive, because they coordinate behavior to environmental resource availability and optimize compatible physiological processes endogenously. In mammals, rhythms in arousal, hormone secretion, and metabolism are coordinated by the hypothalamic pacemaker in the suprachiasmatic nucleus (SCN) (1–3). In the absence of environmental light input or other entraining signals, an endogenous molecular oscillator drives circadian rhythms in SCN neural output with a period of approximately 24 hours. Acting as the body's master pacemaker, the neural output of the SCN temporally controls behavioral rhythms using a combination of paracrine factors and polysynaptic neural connections to regulate arousal and sleep centers in the brainstem (reviewed in Refs. 1, 4, 5). More broadly, with the discovery that many cell populations throughout the body express the molecular clockwork, there is a growing appreciation that the SCN functions to coordinate circadian rhythms throughout the body and that disruption of this timing system produces a wide array of symptoms, including endocrine dysfunction (eg, Refs. 6–9).
Although circadian rhythms are important in health and disease, little research is carried out on female model systems. For example, using a PubMed literature search assessing just the past 5 years of circadian rhythms research, we found that less than 20% of work in this area includes female mouse cohorts in their experimental design. We suspect that this strong bias was spurred by early observations of estrous cycle-dependent ultradian rhythms in the locomotor activity of female hamsters and rats (10–14). However, both male and female circadian rhythms are medically relevant and particularly salient when considered in the context of known sex differences in chronotype and sleep phenotype in humans (15–20). These studies highlight the need to study and understand the neural and molecular underpinnings of sexual dimorphism in circadian rhythms in animal models.
In the first part of this study, we examined possible sex differences in circadian behavior, SCN neural activity, and clock gene expression in a commonly used mouse strain (C57BL/6J). In the second part of this study, we sought to determine the mechanisms underlying sex differences in behavioral patterning. Sexual differentiation of the brain and body occurs at critical points during development and is caused by differences in hormonal environment causing permanent “organizational” changes (21–23). Throughout adulthood, gonadal hormones additionally have nonpermanent “activational” effects that masculinize or feminize (ie, estrogen-dependent ultradian rhythms in rat and hamster) (10–12, 14, 21, 24). The relative contribution of ovarian steroids and androgens to sex differences in sleep and circadian rhythms is an important area of research (reviewed in Ref. 25). Additionally, genetic factors due to differences in sex chromosome complement can cause sex differences in some phenotypes (26). To be able to address the relative contributions of gonadal hormones and sex chromosome complement, we used the 4 core genotypes (FCG) mouse model. The use of gonadally-intact and gonadectomized (GDX) FCG mice allowed us to determine whether sex differences in mouse behavior are caused by the activational effects of gonadal hormones, permanent organizational effects of gonadal hormones, or by sex chromosome complement.
Materials and Methods
Animals and housing
All experimental protocols used in this study were approved by the University of California Los Angeles Animal Research Committee. University of California Los Angeles Division of Laboratory animal recommendations for animal use and welfare, as well as National Institutes of Health guidelines, were followed. Experiments for behavior, physiology, and bioluminescence used 2- to 3-month-old PER2::LUC knock-in mice on a C57BL/6J background, henceforth referred to as Per2Luc male and female mice (minimum 12 generations backcrossed) (27). The period, amplitude, and amount of wheel-running activity of Per2Luc mice have previously been shown to be no different from wild-type (WT) mice (27). Estrous cycle phase was assessed by vaginal smears to confirm that female Per2Luc mice undergo regular estrous cycles. Of an independent cohort of 10 female Per2Luc mice, 8 had regular 4- to 5-day estrous cycles. Vaginal smears were also conducted to determine estrous cycle phase for those female mice whose tissues were harvested for electrophysiology and bioluminescence.
In order to determine whether gonadal hormones or sex chromosomes underlie sex differences in behavior observed in male and female mice, we used the FCG mouse model on a C57BL/6J background for behavioral analysis (28). In FCG mice, the testis-determining Sry gene is deleted from the Y chromosome (Y−), and a Sry transgene is inserted onto an autosome. When the gonadally male XY−Sry animal breeds with a WT XX female, they produce the following FCG offspring: XX, XXSry, XY−, and XY−Sry. Animals carrying the Sry transgene develop testis and are referred to as gonadal males (XXSry and XY−Sry, here referred to as XXM and XYM); whereas animals without the Sry transgene develop ovaries and are referred to as gonadal females (XX and XY−, here referred to as XXF and XYF). At 75 days of age, a cohort of FCG animals were GDX and allowed to recover for 3 weeks before analysis of rhythms in wheel-running activity.
Behavioral analysis
Methods used were similar to those described previously (29, 30). Mice were housed individually from 3 months of age, and their wheel-running activity was recorded as revolutions (rev) per 3-minute intervals. Both the running wheels and data acquisition system were obtained from Mini Mitter Co (Bend, Oregon). Animals were exposed to a 12-hour light, 12-hour dark cycle (LD) (light intensity 300 lux) for 2 weeks and then released in 24 hours of constant darkness (DD) to assess their free-running activity pattern for 2 weeks. Locomotor activity rhythms were analyzed by periodogram analysis combined with the χ2 test with P = .05 significance levels (El Temps, Barcelona, Spain) on the raw data. The periodogram refers to behavioral rhythm amplitude as “power” (%V) or periodicities in the time series. Power values were normalized to the percentage of variance derived from peak significance (P = .05). Fragmentation was calculated using the number of activity bouts (maximum gap, 21 min; threshold, 3 counts/min) per day using ClockLab software (Actimetrics, Wilmette, Illinois). Precision was calculated by determining the difference between the daily onset of activity and lights off under LD conditions from the best-fit line through the daily onsets under DD conditions.
Mouse circadian time (CT)16 was determined as 4 circadian hours after activity onset in DD (CT12). A light pulse (white light 100 lux, 10 min) was applied at CT16. Phase shifts in activity rhythm were determined by measuring the phase difference between eye-fitted lines connecting the onset of activity for a period 10 days before and 10 days after the CT16 light pulse. Phase shift measurements were made by “blind” investigators, without knowledge of subject genotype. Light intensity (lux) was measured with a light meter (B&K Precision, Yorba Linda, California), and all handling of mice in DD was carried out with the aid of night vision goggles (FJW Industries, Palatine, Illinois).
Whole-cell patch-clamp electrophysiology
Methods used were similar to those described previously (31–33). Per2Luc male and female mice (2–3 mo) entrained on LD or reverse LD cycle were anesthetized in an isoflurane chamber at zeitgeber time (ZT) 2.5 and 12.5 for ZT 4–6 and 14–16 recordings, respectively. Anesthetized mice were decapitated, and brains were extracted and incubated in ice-cold slice solution lution (26mM NaHCO3, 1.25mM NaH2PO4, 10mM glucose, 125mM NaCl, 3mM KCl, 5mM MgCl2, and 1mM CaCl2) for 5 minutes. Coronal slices (300 μm) of mid-SCN were collected in fresh slice solution using a vibratome and then incubated for 30 minutes at 32°C followed by 1 hour at room temperature in artificial cerebrospinal fluid (ACSF) (26mM NaHCO3, 1.25mM NaH2PO4, 10mM glucose, 125mM NaCl, 3mM KCl, 2mM MgCl2, and 2mM CaCl2) while continually being aerated with 95% O2/5% CO2. Slices were placed in a recording chamber (PH-1; Warner Instruments, Hamden, Connecticut) attached to the stage of a fixed stage upright DIC microscope (Olympus, Tokyo, Japan) and superfused continuously (2 mL/min) with room temperature ACSF aerated with 95% O2/5% CO2.
One mid-SCN slice along the rostrocaudal axis was selected based on the third ventricle and optic chiasm shape, as well as further inspection of cell density. At this point in the SCN, retinorecipient ventral region of the SCN (vSCN) and dorsal region of the SCN (dSCN) are relatively discrete neuronal populations (34). Whole-cell patch clamp SCN recordings were made using electrode micropipettes (3–7 MΩ) pulled from glass capillaries, and recording electrodes were filled with standard internal solution: 112.5mM K-gluconate, 1mM EGTA, 10mM HEPES, 5mM MgATP, and 1mM MgCl2. Internal solution pH was adjusted to 7.25-7.3 and osmolarity adjusted to 290-300 mOsm. Recordings were obtained with the AXOPATCH 200B amplifier (Molecular Devices, Sunnyvale, California) and monitored online with pCLAMP (version 10; Molecular Devices). Drug treatments were performed by dissolving either gabazine (GabZ) (10μM) or N-methyl-d-aspartic-acid (NMDA; 25μM; Tocris Bioscience, Minneapolis, Minnesota) in ACSF and delivered via rapid gravity feed system into the slice bath during recording. Spontaneous firing rates (SFRs) were recorded with pCLAMP for 1 minute using current-clamp in the whole-cell patch configuration. Further details of electrophysiology recordings and analysis can be found in the Supplemental Methods, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org.
Bioluminescence
Methods used for real-time monitoring of bioluminescence of PER2::LUC ex vivo tissue explants were similar to those described previously (35). Briefly, 4- to 6-month-old mice were killed between ZT 10 and 11 from mice housed in a LD, and SCN, pituitary, heart, liver, and adrenal explants were collected. Explants were transferred onto Millicell membranes (0.4 μm, PICMORG50; Millipore, Bedford, Massachusetts) resting on 1.2 mL of recording media that contained freshly added 0.1mM luciferin (sodium salt monohydrate; Biosynth, Staad, Switzerland), and the 35-mm dishes were sealed using autoclaved high-vacuum grease (Dow Corning, Midland, Michigan). Tissue explants were immediately inserted in the Lumicycle photometer (Actimetrics), and bioluminescence was monitored at 37°C for 7–10 consecutive uninterrupted days. Raw bioluminescence values were normalized by first subtracting the recorded baseline, then subtracting a running average of 24 hours of this baseline-subtracted bioluminescence, and finally performing a 2-hour smoothing average. Period, amplitude, rate of damping, and first calculated peak for phase relationships were calculated as previously described (35).
Androgen receptor (AR) immunohistochemistry (IHC)
Brains from FCG and GDX FCG mice were collected and processed as described previously (35). Before immunostaining, sections were fixed in 4% paraformaldehyde at room temperature, washed with PBS, and incubated in blocking solution (3% normal goat serum and 0.1% Triton X-100 in PBS). After blocking, sections were incubated with rabbit polyclonal antibody raised against AR (Santa Cruz Biotechnology, Inc, Santa Cruz, California) diluted to 1:150 in blocking solution at 4°C for 6 days. Sections were then washed and incubated with secondary antibody. Sections were again washed with PBS, coverslipped with Vectashield Mounting Media containing 4′6-diamindino-2-phenylindole, dihydrochloride (DAPI) (Vector Laboratories, Burlingame, California), and stored in the dark at 4°C until imaged. In each IHC run, no primary antibody controls were included, along with sections from female Per2Luc brains, which had minimal AR staining, similar to previous reports on female mouse SCN (36). Sections were imaged on an Olympus epifluorescence microscope using Axiovision software (Carl Zeiss Microscopy, Oberkochen, Germany). To determine AR staining, 1 SCN section per animal was selected midway through the rostrocaudal extent of the nucleus based on SCN shape as shown by DAPI staining. AR-positive cells were counted by investigators blind to the genotypes of the sections.
Statistical analysis
For most of the experiments in this study, we applied 2- or 3-way ANOVA followed by the Holm-Sidak post hoc test. Significance of some electrophysiology and bioluminescence experiments was assessed by Student's t tests. All results were reported to be significantly different whether P < .05. All tests were performed using SigmaStat (version 3.5; SYSTAT Software, San Jose, California). Values are shown as mean ± SEM.
Results
Subtle sex difference in diurnal and circadian rhythms of wheel-running behavior of male and female mice
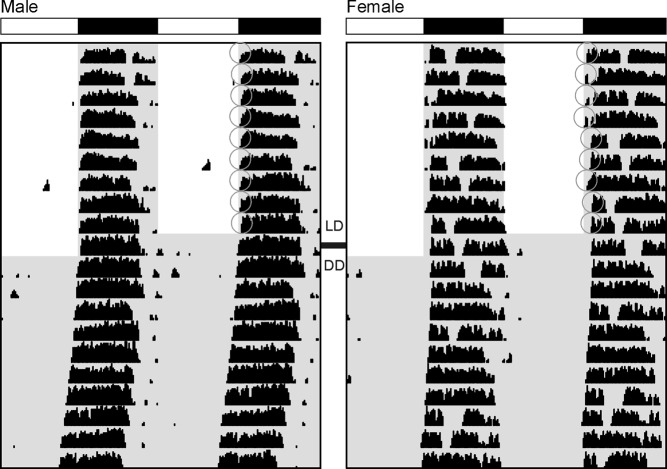
We compared diurnal and circadian rhythms of wheel-running activity in Per2Luc male and female mice (Figure 1). Most daily and circadian parameters of behavioral rhythmicity showed no sex differences (Table 1). Both sexes were behaviorally rhythmic in LD and DD conditions, had similar activity amount and patterning as measured by fragmentation and daytime activity, and had similar free-running period in DD. In LD, activity onset was more precise for males than females (Table 1). In DD, female α (or activity duration) was significantly longer than male (Table 1).
Figure 1.
Sex differences in Per2Luc mouse wheel-running activity rhythms. Representative example of male (left) and female (right) double-plotted actograms in LD (d 1-10) and DD (d 11-20). Male mice have more precise activity onset in LD than females, as indicated by gray circles. Female mice have longer activity duration in DD than males.
Table 1.
Sex Differences in Wheel-Running Activity Rhythms of Per2Luc Mice
| Males n = 10 | Females n = 10 | t | P | |
|---|---|---|---|---|
| LD | ||||
| Power (%V) | 60.2 ± 3.4 | 54.4 ± 4.3 | 0.96 | .35 |
| Activity amount (rev/h) | 383.4 ± 33.3 | 330.9 ± 25.2 | 1.25 | .22 |
| Activity in light (%) | 4.2 ± 0.8 | 4.0 ± 0.8 | 0.50 | .62 |
| α (min) | 560 ± 38 | 648 ± 37 | −1.64 | .12 |
| Precision (min) | −8.3 ± 1.7 | −17.8 ± 3.8a | T = 68 | .006 |
| Fragmentation (bouts/d) | 4.6 ± 0.5 | 5.1 ± 0.3 | T = 101 | .76 |
| Phase angle (min) | −6.5 ± 2.4 | −8.8 ± 3.7 | T = 113 | .56 |
| DD | ||||
| Period (h) | 23.68 ± 0. | 23.68 ± 0.05 | −0.17 | .87 |
| Power (%V) | 49.5 ± 4.9 | 44.93 ± 4.2 | 0.74 | .47 |
| Activity amount (rev/h) | 334.9 ± 37.1 | 321.3 ± 32.1 | 0.49 | .63 |
| α (min) | 483 ± 32 | 590 ± 34a | −2.53 | .02 |
| Precision (min) | −17.3 ± 3.7 | −37.0 ± 11.1 | T = 83 | .10 |
| Fragmentation (bouts/d) | 6.5 ± 0.7 | 6.3 ± 0.5 | 0.33 | .75 |
| LD-DD phase angle (min) | −29.5 ± 5.0 | −21.73 ± 5.8 | T = 103 | .88 |
Sex differences in wheel-running activity rhythms of Per2Luc mice. Most circadian parameters are not sexually dimorphic. Male mice have more precise activity onset in LD (P < .05). Female mice have longer activity duration (α) in DD (P < .05). %V refers to the power of the rhythm as determined by χ2 periodogram analysis. rev/hr refers to the number of wheel revolutions per hour.
P < .05 between males and females.
Sex differences in daytime electrical activity
Neural activity of SCN neurons is a key output, by which the central clock communicates with the rest of the body. Electrophysiological tools were used to measure SFR from SCN neurons in the dorsal region (dSCN) of Per2Luc mice. As expected (Figure 2), daytime (ZT 4–6) firing rates were higher than nighttime (ZT 14–16) in both males and females (Table 2). The males did exhibit higher spontaneous activity levels during the daytime compared with females (Figure 2A). This sex difference in daytime SFR was abolished when γ-aminobutyric acid (GABA)A-mediated synaptic transmission was blocked using the antagonist GabZ (10μM) (Figure 2B). GabZ treatment significantly reduced daytime SFR in dSCN neurons of male but not female mice. SCNs were collected from females in the proestrous and estrous phase, but no difference in SFR was observed (data not shown). Neurons in the vSCN did not exhibit a diurnal rhythm or sex difference in firing rate (Table 2).
Figure 2.
Sex differences in SCN firing rate. Daytime (ZT 4-6) firing rates are higher than nighttime (ZT 14-16) firing rates in male (P < .003) and female (P < .05) dSCN. (A) Daytime firing rates are higher in males than females under baseline conditions (P = .03). (B) GabZ treatment abolishes sex difference in dSCN firing rate (P = .9). (C) No sex difference in early evening vSCN NMDA response. Male and female vSCN neurons firing rate significantly increases after NMDA treatment at ZT 14-16 (male, P = .017; female, P < .00001). The range of firing rates after NMDA treatment is greater in male SCN neurons, but there is no statistically significant interaction between sex and NMDA response (P = .6). Trace examples on the left and corresponding box plots on the right. Bottom and top of box indicate 25th and 75h percentile, respectively, whiskers indicate 10th and 90th percentile, solid line inside box indicates 50th percentile, and dashed lines inside box indicates mean. * indicates day-night difference P < .05, # indicates sex difference P < .05, § indicates effect of NMDA P < .05.
Table 2.
Sex Differences in SFR Rhythms of SCN Neurons
| Males | Females | t | P | ||
|---|---|---|---|---|---|
| dSCN | ZT 4–6 | n = 15 | n = 14 | ||
| Baseline (hz) | 5.7 ± 0.7 | 3.5 ± 0.5 | 2.55 | .02 | |
| GabZ (hz) | 5.0 ± 0.8 | 4.2 ± 0.6 | 0.76 | .45 | |
| ZT 14–16 | n = 10 | n = 13 | |||
| Baseline (hz) | 2.4 ± 0.6a | 1.4 ± 0.4a | 0.99 | .33 | |
| GabZ (hz) | 1.4 ± 0.5ab | 2.0 ± 0.7a | T = 116 | .83 | |
| vSCN | ZT 4–6 | n = 10 | n = 12 | ||
| Baseline (hz) | 3.2 ± 0.7 | 1.9 ± 0.4 | 0.12 | .12 | |
| GabZ (hz) | 2.0 ± 0.5 | 1.7 ± 0.5 | 0.42 | .68 | |
| NMDA (hz) | 3.7 ± 0.7 | 3.1 ± 0.7 | 0.56 | .58 | |
| ZT 14–16 | n = 12 | n = 12 | |||
| Baseline (hz) | 1.5 ± 1.1 | 1.3 ± 0.5 | 0.16 | .88 | |
| GabZ (hz) | 1.8 ± 0.5 | 1.0 ± 0.3 | 1.37 | .18 | |
| NMDA (hz) | 4.1 ± 1.0c | 2.8 ± 0.3c | T = 152 | .93 |
Day-night difference P < .05.
Effect of GabZ P < .05.
Effect of NMDA P < .05.
As a first step to determining whether intrinsic membrane properties may be sexually dimorphic, we also examined the properties of action potentials (APs) recorded in the SCN. At night in dSCN, females have a higher threshold for evoking an AP than males, and male AP afterhyperpolarization (AHP) area, rhythmic in males only, is larger. At night in vSCN, male APs have a higher threshold, faster rise rate, and greater amplitude than females (Supplemental Figure 1 and Supplemental Table 1).
Finally, NMDA receptor activation in the SCN is a critical part of the signaling cascade by which light-driven release of glutamate onto the SCN generates phase shifts of the circadian system (37, 38). This retinal innervation from the melanopsin-expressing retinal ganglion cells is strongest in the vSCN (39). Therefore, we compared the impact of bath application of NMDA (25μM, 2 min) on firing rate in vSCN from males and females (Figure 2C). During the night (ZT 14–16), both male and female vSCN neurons exhibited significant NMDA-evoked increases in firing rate with no differences between the sexes (Table 2). During the day (ZT 4–6), NMDA treatment did not increase firing rate in vSCN neurons from either male or female mice (Table 2).
Sex differences in PER2 expression of peripheral organs
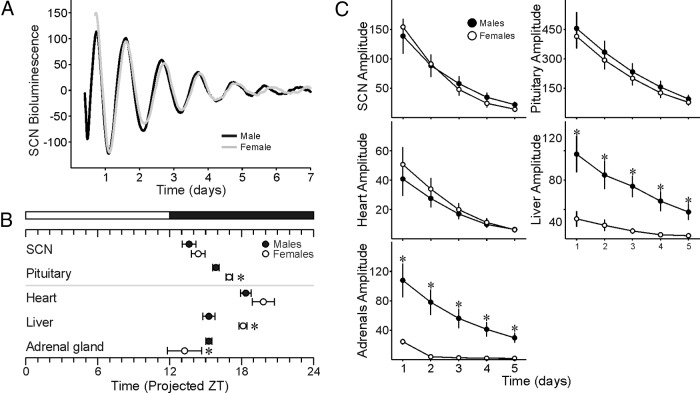
In order to determine whether there are sex differences in the clock-gene transcription/translation feedback loop, we compared male and female PER2::LUC bioluminescence rhythms in explants of SCN as well as peripheral tissues (Figure 3 and Supplemental Table 2). In SCN (Figure 3A), there was no sex difference in bioluminescence phase (Figure 3B) or amplitude (Figure 3C). We also observed sex differences in the peak phase and amplitude of some peripheral tissues. Male pituitary and liver peaks were phase advanced compared with female (Figure 3B). Furthermore, the amplitude of liver and adrenal rhythms was higher in males than females (Figure 3C).
Figure 3.
Sex differences in bioluminescence rhythms of central and peripheral oscillators. (A) No sex difference in bioluminescence phase or amplitude of the SCN. (B) Phase relationship of peripheral oscillators are altered in females relative to male mice. The white/black bar indicates the time of previous lights on and off, respectively. (C) Amplitude of liver and adrenal bioluminescence rhythms are lower in females relative to male mice. *P < .05 between males and females.
Sex differences in diurnal and circadian wheel-running behavioral rhythms of FCG mice
In order to determine whether activational, organizational effects, or sex chromosome complement account for observed sex differences in wheel-running rhythms, we compared the locomotor rhythms of gonadally-intact and GDX FCG mice (XXM, XYM, XXF, and XYF). Gonadally intact FCG animals had similar activity patterning in LD and DD (Supplemental Figure 2, with 2-way ANOVA values reported in Supplemental Table 3). GDX revealed multiple sex differences in diurnal and circadian wheel-running behavior (Supplemental Figure 3 and Table 3, with 2-way ANOVA values reported in Supplemental Table 4). Like female WT mice in DD, FCG XX mice (XXM and XXF) had longer α (activity bout duration) than XY mice in DD (XYM and XYF) (Figure 4A, Table 3, and Supplemental Table 4), suggestive of a sex chromosome effect controlling activity duration. Furthermore, we observed that gonadally male FCG mice (XXM and XYM) had greater deficits in rhythm power (Figure 4B) and activity amount (Figure 4C) than gonadally female FCG after GDX. A 3-way ANOVA, comparing the effects of sex chromosome, gonadal sex, and GDX, revealed an effect of GDX on rhythm power and revealed an effect of both gonadal and chromosomal sex (Supplemental Table 5). A decrease in activity amount was also found by 3-way ANOVA with GDX in FCG mice, and differences due to both gonadal and chromosomal sex were revealed (Figure 4C and Supplemental Table 5).
Table 3.
Sex Chromosome Complement and Gonadal Sex Differences Revealed in FCG Wheel-Running Activity Rhythms After GDX
| GDX |
||||
|---|---|---|---|---|
| XYM n = 8 | XXM n = 8 | XYF n = 8 | XXF n = 8 | |
| LD | ||||
| Power (%V) | 24.8 ± 2.5 | 29.5 ± 3.1 | 36.6 ± 6.3a | 34.5 ± 4.1a |
| Activity amount (rev/h) | 84.3 ± 12.6 | 117.4 ± 24.7 | 166.9 ± 34.5a | 174.3 ± 47.7a |
| Activity in light (%) | 7.7 ± 0.8 | 8.0 ± 2.4 | 6.8 ± 3.8 | 5.6 ± 1.8 |
| α (min) | 712 ± 8 | 638 ± 40 | 640 ± 54 | 727 ± 29 |
| Precision (min) | −25.3 ± 5.8 | −29.4 ± 14.5 | −17.1 ± 5.7 | −86.4 ± 77.7 |
| Fragmentation (bouts/d) | 6.6 ± 0.4 | 15.1 ± 9.6 | 4.8 ± 0.8 | 20.4 ± 1.4 |
| Phase angle (min) | 2.1 ± 5.1 | 1.5 ± 2.0 | 2.6 ± 3.6 | 23.1 ± 21.9 |
| DD | ||||
| Period (h) | 23.8 ± 0.1 | 24.1 ± 0.1 | 23.9 ± 0.1 | 23.9 ± 0.1 |
| Power (%V) | 20.6 ± 2.1 | 18.8 ± 1.9 | 25.7 ± 3.4a | 24.6 ± 2.6a |
| Activity amount (rev/h) | 77.8 ± 16.7 | 79.7 ± 14.0 | 164.0 ± 27.3 | 147.0 ± 41.7 |
| α (min) | 695 ± 47 | 838 ± 36b | 696 ± 39 | 766 ± 14b |
| Precision (min) | −80.5 ± 23.9 | −52.8 ± 13.9 | −27.6 ± 4.2a | −25.3 ± 4.1a |
| Fragmentation (bouts/d) | 6.9 ± 0.8 | 8.2 ± 0.6 | 7.3 ± 0.5 | 7.634 ± 0.3 |
| LD-DD phase angle (min) | 16.2 ± 20.2 | 67.3 ± 61.0 | 73.6 ± 61.9 | 55.3 ± 62.0 |
| Phase shift (min) | −158 ± 17.2 | −117 ± 17.4 | −129 ± 8.9 | −135 ± 17.4 |
Sex chromosome complement and gonadal sex differences revealed in FCG running wheel activity rhythms after GDX. rev/hr refers to the number of wheel revolutions per hour.
Effect of gonadal sex P < .05.
Effect of sex chromosome P < .05, as determined by 2-way ANOVA, with further details reported in Supplemental Table 4.
Figure 4.

Genetic and hormonal influence on wheel-running activity rhythms of FCG mice in DD. (A) XX chromosome complement confers longer activity duration than XY chromosome complement after GDX. (B) After GDX, phenotypic male FCG mice have greater deficits in wheel-running rhythm power than phenotypic female FCG mice. (C) After GDX, phenotypic male mice have greater activity level decrease than phenotypic female mice. (D) Before GDX, phenotypic female mice respond to CT16 light pulse with greater phase shifts than male mice. After GDX, there is no difference in phase shift. * indicates a significant effect of gonadal sex (P < .05), and ^ indicates a significant effect of sex chromosome (P < .05) as determined by 2-way ANOVA. M refers to gonadal and phenotypic males and F refers to gonadal and phenotypic females.
In order to examine the light response of the circadian system, mice were exposed to light in the early subjective night (CT 16, 100 lux, 10 min). Gonadal males displayed smaller magnitude phase shifts than gonadal female FCG mice (Figure 4D and Supplemental Table 3). After GDX, the gonadal sex differences in phase shift magnitude were lost (Figure 4D, Table 3, and Supplemental Table 4), suggesting that they were caused by the activational effects of gonadal secretions in adulthood.
Finally, we used IHC to examine AR within the SCN of FCG mice (Figure 5). We observed strong AR expression within the central core region of the SCN of both XYM and XXM groups. This AR expression in the gonadal males was significantly higher than in females (F = 36.10, P < .001) (Figure 5B). As a control, we confirmed that AR expression in the SCN was absent after GDX (n = 2 per group) (Supplemental Figure 4).
Figure 5.
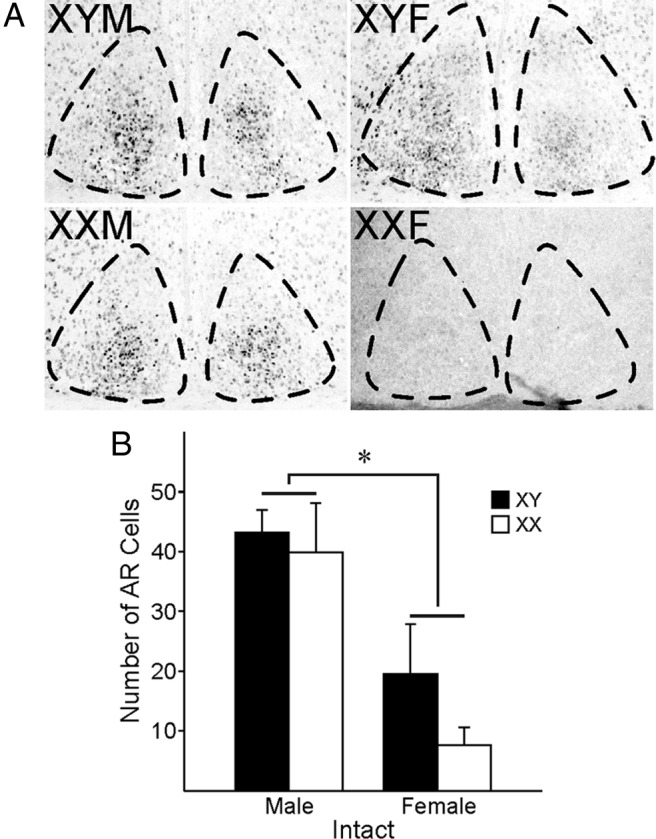
AR expression in FCG mice. Grayscale images from fluorescent anti-AR sections are shown with the area positive for DAPI outlined using dotted lines. (A) (left column) XYM (top) and XXM (bottom); (right column) XYF (top) and XXF (bottom). (B) Gonadal male FCG mouse SCN (left; 40 ± 10 cells) expresses more AR+ cells than gonadal female FCG mouse SCN (right; 12 ± 11 cells, P < .001). *P < .05 between gonadal sexes. Although there appears to be a sex chromosome difference in AR expression within gonadal females, the analysis was underpowered to draw any conclusions (post hoc t = 11.91, P = .08, power = 0.144).
Discussion
There is a strong sex bias in selecting animal models for circadian rhythms research. Perhaps the most common justification for this bias is that key circadian parameters in wheel-running rhythms, such as period and activity amount, are estrogen dependent. Certainly early work in rat and hamster models suggests that the 4-day estrous cycle (10–14) would interfere with the measurement of activity onset, which is so vital for the calculation of many behavioral parameters. Present circadian research makes extensive use the C57BL/6J line of mice expressing the Per2Luc transgene (27). In the present study, we first sought to carefully compare circadian behavior in males and females of this mouse line. Overall, we observed modest sex differences in the circadian rhythms of locomotor activity of male and female Per2Luc mice. There were no sex differences in free-running period (τ) in our cohort of Per2Luc mice as has also been reported in mice on a C57BL/6J congenic background (40). The impact of sex on τ appears to vary with the species, because male hamsters and rats have a longer τ than females, whereas Octagon degus males have shorter τ than females (41–43). We did not find a sex difference in the total amount of activity in mice, similar to Ogawa et al (44), but this contrasts with the finding by Blattner et al (40) that females exhibit greater amounts of activity than male mice. It is likely that these sex differences are subtle and hence could vary in magnitude or detection level under different housing conditions. For example, the study by Blattner et al used a carefully chosen diet low in soy estrogens, which may have revealed differences in activity levels. We also observed a sex difference in the α in the Per2Luc mice, with females displaying longer duration of activity in DD than the males. This suggests that although both sexes have the same amount of activity over the course of a day, the female mice spread their wheel running over a longer duration. One striking sex difference in mouse activity was the greater precision in the daily onset of activity of males in LD, which is consistent with previous reports in hamsters (41). This difference in precision of daily onset of activity was not found in DD. Finally, it is worth stressing that the female C57BL/6J mice do not show evidence of the 4-day estrous-driven variation or “scalloping” of activity onset that would interfere with measurements of circadian behavior.
We used electrophysiological techniques to examine possible sex differences at the level of the central clock in the SCN. Here, we found 2 main differences. First, the frequency of daytime electrical activity was significantly higher in the male dSCN. This difference appears to be driven by GABA, because the sex difference in firing frequency was lost in the presence of the GABAA blocker GabZ. This sex difference in electrical activity has not been previously described and may provide a mechanistic explanation for more precise activity onsets in males. Sex hormones have been shown to alter GABA currents in other brain regions (45–49, reviewed in Refs. 50, 51). The other main sex difference found in dSCN was observed at night when females' AP threshold was higher than males', and rhythmic male AP AHP area was larger than during the daytime. Both a higher AP threshold and larger AHP area would reduce dSCN firing rates, and it appears males and females may use divergent physiological mechanisms to converge on the same firing phenotype. The AHP current in the male SCN has been explored in detail in the rat (52) and is dependent on calcium currents that enter the cell during depolarization and the calcium-sensitive potassium currents. Sex hormones modulate ionic currents genomically and via rapid action on cellular pathways (53–58), although this type of regulation has not yet been described in the SCN, it may influence rhythms in SCN's physiological output. Based on our present study, both GABA currents as well as AHP are strong candidates for regulation by sex hormones in SCN neurons.
At the level of the molecular oscillator, we did not observe sex differences in the phase or amplitude of PER2::LUC-driven rhythms in bioluminescence. The female SCN had faster damping rates in bioluminescence rhythms, which may suggest a lower degree of coupling within the SCN. Interestingly, a variety of evidence suggests that the neuropeptide vasoactive intestinal peptide (VIP) is critical for coupling within the SCN circuit (59, 60). Anatomically, there is evidence that human male SCN expresses a larger VIP cellular population (61), and evidence from rodent models includes sexually dimorphic rhythm of Vip expression in the SCN in the diurnal rodent, Arvicanthus niloticus (62), and the nocturnal rat (63). Differences in VIP-driven coupling may explain the sex differences in damping that we observed in the SCN molecular oscillator. We also found an altered phase angle of entrainment of the oscillators in the pituitary and liver of female mice. We do not know whether these changes in phase alignment are somehow adaptive in the female. Still our bioluminescence data, taken together with the lower baseline SFR, suggest that the female SCN circuit may be less robust than the male circuit. There is some evidence that lower amplitude oscillators are more easily phase shifted (64), and it is that the female is trading a low-amplitude SCN circuit for more sensitivity to environmental signals.
Finally, we made use of the FCG model to help us to determine whether gonadal hormones or sex chromosome complement underlie these circadian sex differences. Gonadally intact FCG mice had few sex differences in circadian behavior under LD and DD conditions. Apart from the phase shift response discussed below, most of the sex differences we observed in the FCG model were revealed upon GDX. The decline in activity amount due to loss of sex steroids after GDX has been previously documented (36), and we additionally observed a dramatic decrease in the power of the rhythm as measured by periodogram analysis. This decline in activity amount and rhythm power may be due to the male-specific dependence on androgens and the expression of AR in the SCN (6, 36, 65). The evidence we have gathered from the FCG mouse model supports this hypothesis of activational effects of sex steroids on activity amount as well as the power of the rhythm. GDX also revealed persistent sex differences in rhythm power and activity amount that depended on previous gonadal sex, suggesting an additional organization effect of sex steroids. The divergent effects of GDX also suggest that sex steroids normally allow male and female circadian rhythms to converge in adulthood. Furthermore, GDX revealed effects of sex chromosome on rhythm power and activity duration, which appear to have been masked in the presence of sex steroids in the intact group. The lengthened activity duration in GDX XX mice could be due to a sex difference in coupling between the oscillating populations within the SCN, of which there is hypothesized to be a morning vs an evening component (66, 67). Further work on sex differences in SCN coupling may elucidate mechanisms by which this occurs and could help explain the sex differences in sleep and circadian rhythms as men and women age and sex steroid levels decline (17, 18).
Light-input pathway
Another critical aspect of the circadian system is to gate and respond to environmental stimuli. This light information is detected by melanopsin-containing retinal ganglion cells, which project to the SCN and release glutamate and pituitary adenylate cyclase activating polypeptide (PACAP) as transmitters (38, 68). The NMDA subtype of glutamate receptor plays a critical role in determining the response of the SCN to this light input (eg, Refs. 69, 70). Behaviorally, the phase angle of entrainment to the LD cycle was not different in male and female Per2Luc mice. Gonadal FCG females had a greater phase delay in response to a discrete photic stimulus than gonadal males, but this sex difference disappeared upon GDX, suggesting that activational effects of sex steroids may modulate circadian entrainment to light. Physiologically, we were unable to detect sex differences in the magnitude of NMDA-evoked responses of SCN neurons, but at baseline, male vSCN neurons had faster AP rise rates and greater amplitude, indicating that male neurons may express more voltage-gated sodium channels than female neurons. Male SCN expresses 4 times the number of AR+ neurons (36), which we also confirmed in FCG gonadal males. It is believed that androgens may be involved in regulating phase shifts in response to light via activation of MAPK/ERK signaling (6, 56), but based on our findings, this may be related to AP properties rather than acute increases in firing rate.
Conclusion
In this study, we found that gonadal hormone activational effects are major factors regulating the response of the circadian system to light as well as rhythm amplitude and power. Sex chromosome complement was relatively minor but did regulate activity bout length. Physiologically, we found sex differences in the levels of daytime neural activity, which is a critical output of the circadian system. Sex differences in both synaptic communication via GABA and intrinsic membrane properties may be responsible. These results argue for increasing study of sex differences in animal models. In addition, the data in the present study demonstrate that female mice of the C57BL/6J line are amenable for detailed circadian analysis and should not be excluded on the basis of subtle complications of estrous cycles. Unlike robust sex differences observed in other rodent models, most daily and circadian parameters are similar between the sexes in mice. Finally, when considered in context of known sex differences in chronotype and sleep disorders (15–20, 25, 71), the impact of circadian dysfunction in mouse models of circadian disease should be examined in both male and female mice.
Acknowledgments
This work was supported in part by an Iris Cantor-University of California Los Angeles Women's Health Center grant awarded to D.H.L. and C.S.C. D.A.K. was supported by the Laboratory of Neuroendocrinology Training Grant T32 HD 07228-26. A.P.A. was supported by the National Institutes of Health Grant NS043196.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACSF
- artificial cerebrospinal fluid
- AHP
- afterhyperpolarization
- AP
- action potential
- AR
- androgen receptor
- CT
- circadian time
- DAPI
- 4′6-diamindino-2-phenylindole, dihydrochloride
- DD
- 24 hours of constant darkness
- dSCN
- dorsal region of the SCN
- FCG
- 4 core genotypes
- GABA
- γ-aminobutyric acid
- GabZ
- gabazine
- GDX
- gonadectomized
- IHC
- immunohistochemistry
- LD
- 12-hour light, 12-hour dark cycle
- NMDA
- N-methyl-d-aspartic acid
- rev
- revolutions
- SCN
- suprachiasmatic nucleus
- SFR
- spontaneous firing rate
- VIP
- vasoactive intestinal peptide
- vSCN
- ventral region of the SCN
- WT
- wild type
- ZT
- zeitgeber time.
References
- 1. Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263 [DOI] [PubMed] [Google Scholar]
- 2. Tonsfeldt KJ, Chappell PE. Clocks on top: the role of the circadian clock in the hypothalamic and pituitary regulation of endocrine physiology. Mol Cell Endocrinol. 2012;349:3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rutter J, Reick M, McKnight SL. Metabolism and the control of circadian rhythms. Annu Rev Biochem. 2002;71:307–331 [DOI] [PubMed] [Google Scholar]
- 4. Morin LP, Shivers K-Y, Blanchard JH, Muscat L. Complex organization of mouse and rat suprachiasmatic nucleus. Neurosci. 2006;137:1285–1297 [DOI] [PubMed] [Google Scholar]
- 5. Mistlberger RE. Circadian regulation of sleep in mammals: role of the suprachiasmatic nucleus. Brain Res Rev. 2005;49:429–454 [DOI] [PubMed] [Google Scholar]
- 6. Karatsoreos IN, Butler MP, Lesauter J, Silver R. Androgens modulate structure and function of the suprachiasmatic nucleus brain clock. Endocrinol. 2011;152:1970–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fonken LK, Workman JL, Walton JC, et al. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci USA. 2010;107:18664–18669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marcheva B, Ramsey KM, Buhr ED, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinemia and diabetes. Nature. 2010;466:627–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morin L, Fitzgerald K, Zucker I. Estradiol shortens the period of hamster circadian rhythms. Science. 1977;96:305–307 [DOI] [PubMed] [Google Scholar]
- 11. Takahashi JS, Menaker M. Interaction of estradiol and progesterone: effects on circadian locomotor rhythm of female golden hamsters. Am J Physiol. 1980;239:R497–R504 [DOI] [PubMed] [Google Scholar]
- 12. Widmaier EP, Campbell CS. Interaction of estradiol and photoperiod on activity patterns in the female hamster. Physiol, Behav. 1980;24:923–930 [DOI] [PubMed] [Google Scholar]
- 13. Albers H, Gerall A, Axelson J. Effect of reproductive state on circadian periodicity in the rat. Physiol Behav. 1981;26:21–25 [DOI] [PubMed] [Google Scholar]
- 14. Wollnik F, Turek FW. Estrous correlated modulations of circadian and ultradian wheel-running activity rhythms in LEW/Ztm rats. Physiol Behav. 1988;43:389–396 [DOI] [PubMed] [Google Scholar]
- 15. Adan A, Natale V. Gender differences in morningness-eveningness preference. Chronobiol Inte. 2002;19:709–720 [DOI] [PubMed] [Google Scholar]
- 16. Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111 [DOI] [PubMed] [Google Scholar]
- 17. Roenneberg T, Kuehnle T, Pramstaller PP, Havel M, Guth A, Merrow M. A marker for the end of adolescence. Curr Biol. 2004;14:R1038–R1039 [DOI] [PubMed] [Google Scholar]
- 18. Roenneberg T, Kuehnle T, Juda M, et al. Epidemiology of the human circadian clock. Sleep Med Rev. 2007;11:429–438 [DOI] [PubMed] [Google Scholar]
- 19. Cain SW, Dennison CF, Zeitzer JM, et al. Sex differences in phase angle of entrainment and melatonin amplitude in humans. J Biol Rhythms. 2010;25:288–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duffy JF, Cain SW, Chang AM, et al. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci USA. 2011;108(suppl 3):15602–15608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee TM, Hummer D, Jechura T, Mahoney MM. Pubertal development of sex differences in circadian function: an animal model. Ann NY Acad. Sci. 2004;262–275 [DOI] [PubMed] [Google Scholar]
- 22. Hagenauer MH, King AF, Possidente B, et al. Changes in circadian rhythms during puberty in Rattus norvegicus: developmental time course and gonadal dependency. Horm Behav. 2011;60:46–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 26:163–174 [DOI] [PubMed] [Google Scholar]
- 24. Bakker J, Honda S, Harada N, Balthazart J. Restoration of male sexual behavior by adult exogenous estrogens in male aromatase knockout mice. Horm Behav. 2004;46:1–10 [DOI] [PubMed] [Google Scholar]
- 25. Mong JA, Baker FC, Mahoney MM, et al. Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones. J Neurosci. 2011;31:16107–16116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gioiosa L, Chen X, Watkins R, et al. Sex chromosome complement affects nociception in tests of acute and chronic exposure to morphine in mice. Horm Behav. 2008;53:124–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yoo SH, Yamazaki S, Lowrey PL, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101:5339–5346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;1:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Colwell CS, Michel S, Itri J, et al. Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R939–R949 [DOI] [PubMed] [Google Scholar]
- 30. Colwell CS, Michel S, Itri J, et al. Selective deficits in the circadian light response in mice lacking PACAP. Am J Physiol Regul Integr Comp Physiol. 2004;1759:1194–1201 [DOI] [PubMed] [Google Scholar]
- 31. Itri JN, Michel S, Vansteensel MJ, Meijer JH, Colwell CS. Fast delayed rectifier potassium current is required for circadian neural activity. Nat Neurosci. 2005;8:650–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Itri JN, Vosko AM, Schroeder A, Dragich HM, Michel S, Colwell CS. Circadian regulation of a-type potassium currents in the suprachiasmatic nucleus. J Neurophysiol. 2010;103:632–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kudo T, Schroeder A, Loh DH, et al. Dysfunctions in circadian behavior and physiology in mouse models of Huntington's disease. Exp Neurol. 2011;228:80–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haam J, Popescu IR, Morton LA, et al. GABA is excitatory in adult vasopressinergic neuroendocrine cells. J Neurosci. 2012;32:572–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Loh DH, Dragich JM, Kudo T, et al. Effects of vasoactive intestinal peptide genotype on circadian gene expression in the suprachiasmatic nucleus and peripheral organs. J Biol Rhythms. 2011;26:200–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iwahana E, Karatsoreos I, Shibata S, Silver R. Gonadectomy reveals sex differences in circadian rhythms and suprachiasmatic nucleus androgen receptors in mice. Horm Behav. 2008;53:422–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morin LP, Allen CN. The circadian visual system, 2005. Brain Res Rev. 2006;51:1–60 [DOI] [PubMed] [Google Scholar]
- 38. Colwell CS. Linking neural activity and molecular oscillations in the SCN. Nat Rev Neurosci. 2011;12:553–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hattar S, Liao H, Takao M, Berson D, Yau K. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blattner MS, Mahoney MM. Circadian parameters are altered in two strains of mice with transgenic modifications of estrogen receptor subtype 1. Genes Brain Behav. 2012;11:828–836 [DOI] [PubMed] [Google Scholar]
- 41. Davis FC, Darrow JM, Menaker M. Sex differences in the circadian control of hamster wheel-running activity. Am J Physiol. 1983;244:R93–R105 [DOI] [PubMed] [Google Scholar]
- 42. Schull J, Walker J, Fitzgerald K, et al. Effects of sex, thyro-parathyroidectomy, and light regime on levels and circadian rhythms of wheel-running in rats. Physiol Behav. 1989;46:341–346 [DOI] [PubMed] [Google Scholar]
- 43. Labyak SE, Lee TM. Estrus- and steroid-induced changes in circadian rhythms in a diurnal rodent, Octodon degus. Physiol Behav. 1995;58:573–585 [DOI] [PubMed] [Google Scholar]
- 44. Ogawa S, Chan J, Gustafsson J, Korach KS, Pfaff W. Estrogen increases locomotor activity in mice through estrogen receptor α: specificity for the type of activity. Endocrinology. 2003;144:230–239 [DOI] [PubMed] [Google Scholar]
- 45. Morrow A, Suzdak P, Paul S. Steroid hormone metabolites potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. Eu J Pharmacol. 1987;142:483–485 [DOI] [PubMed] [Google Scholar]
- 46. McCarthy MM, Perrot-Sinal TS, Auger AP, Sickel MJ. Excitatory GABA as mediator of steroid induced brain sexual differentiation. In: Hand RJ, Hayashi S, Terasawa E, Kawata M, eds. Neuroplasticity, Development, and Steroid Hormone Action. Boca Raton, FL: CRC Press LLC; 2002; 319–337 [Google Scholar]
- 47. Perrot-Sinal TS, Davis AM, Gregerson KA, Kao JPY, McCarthy MM. Estradiol enhances excitatory γ-aminobutyric acid mediated calcium cignaling in neonatal hypothalamic neurons. Endocrinology. 142:2238–2243 [DOI] [PubMed] [Google Scholar]
- 48. Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc Natl Acad Sci USA. 2003;100:14439–14444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Strömberg J, Lundgren P, Taube M, Backstrom T, Wang M, Haage D. The effect of the neuroactive steroid 5β-pregnane-3β, 20(R)-diol on the time course of GABA evoked currents is different to that of pregnenolone sulphate. Eur J Pharmacol. 2009;605:78–86 [DOI] [PubMed] [Google Scholar]
- 50. Woolley CS. Acute effects of estrogen on neuronal physiology. Ann Rev Pharmacol Toxicol. 2007;47:657–680 [DOI] [PubMed] [Google Scholar]
- 51. Maguire J, Mody I. Steroid hormone fluctuations and GABA(A)R plasticity. Psychoneuroendocrinology. 2009;34(suppl 1):S84–S90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cloues RK, Sather WA. Afterhyperpolarization regulates firing rate in neurons of the suprachiasmatic nucleus. J Neurosci. 2003;23:1593–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cato AC, Nestl A, Mink S. 2002 Rapid actions of steroid receptors in cellular signaling pathways. Sci STKE. 2002;re9. [DOI] [PubMed] [Google Scholar]
- 54. Fix C, Jordan C, Cano P, Walker WH. Testosterone activates mitogen-activated protein kinase and the cAMP response element binding protein transcription factor in Sertoli cells. Proc Natl Acad Sci USA. 2004;101:10919–10924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Marino M, Galluzzo P, Ascenzi P. Estrogen signaling multiple pathways to impact gene transcription. Curr Genomics. 2006;7:497–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rahman F, Christian HC. Non-classical actions of testosterone: an update. Trends Endocrinol Metab. 2007;18:371–378 [DOI] [PubMed] [Google Scholar]
- 57. Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol. 2008;70:165–190 [DOI] [PubMed] [Google Scholar]
- 58. Raz L, Khan MM, Mahesh VB, Vadlamudi RK, Brann DW. Rapid estrogen signaling in the brain. Neuro-Signals. 2008;16 140–53 [DOI] [PubMed] [Google Scholar]
- 59. Vosko AM, Schroeder A, Loh DH, Colwell CS. Vasoactive intestinal peptide and the mammalian circadian system. Gen Comp Neuroendocrinol. 2007;152:165–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Maywood ES, Chesham JE, O'Brien JA, Hastings MH. A diversity of paracrine signals sustains molecular circadian cycling in suprachiasmatic nucleus circuits. Proc Natl Acad Sci USA. 2011;108:14306–14311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhou JN, Hofman MA, Swaab DF. VIP neurons in the human SCN in relation to sex, age, and Alzheimer's disease. Science. 1995;16:571–576 [DOI] [PubMed] [Google Scholar]
- 62. Mahoney MM, Romanathan C, Hagenauer MH, Thompson RC, Smale L, Lee T. Daily rhythms and sex differences in vasoactive intestinal polypeptide, VIPR2 receptor, and arginine vasopressin mRNA in the suprachasmatic nucleus of diurnal rodent, Arvicanthis niloticus. Eur J Neurosci. 2009;30:1537–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Krajnak K, Kashon ML, Rosewell KL, Wise PM. Sex differences in the daily rhythm of vasoactive intestinal polypeptide but not arginine vasopressin messenger ribonucleic acid in the suprachiasmatic nuclei. Endocrinology. 1998;139:4189–4196 [DOI] [PubMed] [Google Scholar]
- 64. Abraham U, Granada AE, Westermark PO, Heine M, Kramer A, Herzel H. Coupling governs entrainment range of circadian clocks. Mol Syst Biol. 2010;6:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Karatsoreos IN, Wang A, Sasanian J, Silver R. A role for androgens in regulating circadian behavior and the suprachiasmatic nucleus. Endocrinology. 2007;148:5487–5495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pittendrigh C, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents.V. Pacemaker structure: a clock for all seasons. J Comp Physiol. 1976;106:333–355 [Google Scholar]
- 67. Jagota A, de la Iglesia HO, Schwartz WJ. Morning and evening circadian oscillations in the suprachiasmatic nucleus in vitro. Nat Neurosci. 2000;3:372–376 [DOI] [PubMed] [Google Scholar]
- 68. Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev. 2010;90:1063–1102 [DOI] [PubMed] [Google Scholar]
- 69. Mintz EM, Marvel CL, Gillespie CF, Price KM, Albers HE. Activation of NMDA receptors in the suprachiasmatic nucleus produces light-like phase shifts of the circadian clock in vivo. J Neurosci. 1999;19:5124–5130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang LM, Schroeder A, Loh DH, et al. Role for the NR2B subunit of the N-methyl-D-aspartate receptor in mediating light input to the circadian system. Eur J Neurosci. 2008;27:1771–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Duffy JF, Wright KP. Entrainment of the human circadian system by light. J Biol Rhythm. 2005;20:326–338 [DOI] [PubMed] [Google Scholar]