Abstract
The murine neuronal facilitative glucose transporter isoform 3 (Glut3) is developmentally regulated, peaking in expression at postnatal day (PN)14. In the present study, we characterized a canonical CpG island spanning the 5′-flanking region of the glut3 gene. Methylation-specific PCR and bisulfite sequencing identified methylation of this CpG (mCpG) island of the glut3 gene, frequency of methylation increasing 2.5-fold with a 1.6-fold increase in DNA methyl transferase 3a concentrations noted with advancing postnatal age (PN14 vs PN3). 5′-flanking region of glut3-luciferase reporter transient transfection in HT22 hippocampal neurons demonstrated that mCpGs inhibit glut3 transcription. Contrary to this biological function, glut3 expression rises synchronously with mCpGs in PN14 vs PN3 neurons. Chromatin immunoprecipitation (IP) revealed that methyl-CpG binding protein 2 (Mecp2) bound the glut3-mCpGs. Depending on association with specific coregulators, Mecp2, a dual regulator of gene transcription, may repress or activate a downstream gene. Sequential chromatin IP uncovered the glut3-mCpGs to bind Mecp2 exponentially upon recruitment of Creb1 rather than histone deacetylase 1. Co-IP and coimmunolocalization confirmed that Creb1 associated with Mecp2 and cotransfection with glut3-mCpG in HT22 cells enhanced glut3 transcription. Separate 5-aza-2′-deoxycytidine pretreatment or in combination with trichostatin A reduced mCpG and specific small interference RNAs targeting Mecp2 and Creb1 separately or together depleting Mecp2 and/or Creb1 binding of glut3-mCpGs reduced glut3 expression in HT22 cells. We conclude that Glut3 is a methylation-sensitive neuronal gene that recruits Mecp2. Recruitment of Creb1-Mecp2 by glut3-mCpG contributes towards transactivation, formulating an escape from mCpG-induced gene suppression, and thereby promoting developmental neuronal glut3 gene transcription and expression.
Glucose is an essential substrate that fuels brain oxidative metabolism. Circulating glucose crosses the blood brain barrier and enters the different cell types by a family of facilitative glucose transporters (Gluts) (1). Of the various isoforms expressed in brain, Glut3 is the predominant isoform expressed by most neuronal cells (2), notably evident at the synapses, and seen mainly in the human and murine neuropil region (3, 4). During murine development, brain (neuronal) Glut3 mRNA and protein expression are detectable in the late gestation fetus and soon after birth in the immediate postnatal brain, peaking at day 14 when synaptogenesis occurs (5). The mechanism by which Glut3 expression increases postnatally in murine neurons was recently noted to be transcriptional (6). Although our previous studies conducted in N2A neuroblastoma cells demonstrated binding of Sp1/Sp3 and cyclic AMP regulatory element binding protein (Creb) to the glut3 gene rather proximal to the transcription start site (7, 8), their role in postnatal neuronal Glut3 expression remains unknown.
More recently, epigenetic control of gene transcription has surfaced as a mechanism that includes differential DNA methylation fueled by 1 carbon metabolism and histone modifications (9, 10). We hypothesized that glut3 may be a methylation-responsive gene, which recruits various nuclear proteins that may be responsible for developmentally induced postnatal murine neuronal glut3 gene transcription. To test this hypothesis, we examined murine primary neurons isolated at postnatal day (PN)3 (before the peak in Glut3 expression) and PN14 (during peak Glut3 expression) along with HT22 murine hippocampal neurons (11) and compared cis-elements and transactivating factors involved in glut3 transcription during development.
Materials and Methods
Animals
All mouse studies were approved by the Animal Research Committee of the University of California, Los Angeles, in accordance with guidelines set by the National Institutes of Health. C57/BL6 background strain (both male and female) was employed and euthanized by ip phenobarbital (100 mg/kg) injections.
Isolation of primary neurons
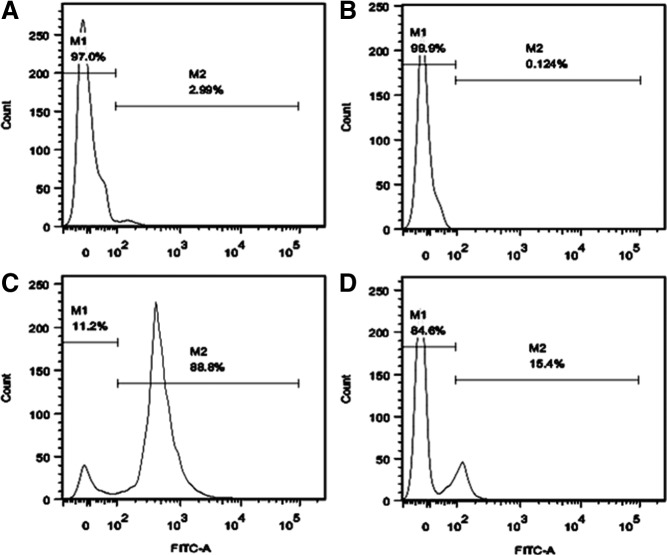
Brains were obtained from postnatal 3- and 14-day-old mice. Single-cell suspensions were prepared using MACS Neural Tissue Dissociation kit (Miltenyi Biotec, Auburn, California) per manufacturer's recommendations with modifications (12). Single cells were incubated with fluorescein isothiocyanate (FITC)-conjugated rat antimouse CD90.2 antibody (eBioscience, Inc, San Diego, California) followed by incubation with magnetically labeled goat antirat microbeads (Miltenyi Biotec), each for 15 minutes at 4°C, and then sorted using MiniMACS separation (Miltenyi Biotec). To determine the purity of magnetically sorted cells, 5 × 106 stained cells were applied to the FACSCalibur flow-cytometer (Becton Dickinson, Franklin Lakes, New Jersey) before and after sorting. Cells incubated with FITC-conjugated goat antirat IgG (heavy and light chains, H+L) (eBioscience, Inc) without the primary antibody set the background fluorescence (Figure 1).
Figure 1.
Percent of CD90+ cells before and after MACS. Single-cell suspension prepared from postnatal mouse brain was magnetically labeled with FITC-conjugated rat antimouse-CD90.2 antibody followed by treatment with goat antirat microbeads and then sorted with the MiniMACS separater. Stained cells were applied to the FACSCalibur flow cytometer with a minimum of 104 events collected per sample. (A) Cells incubated with FITC-conjugated antirat IgG were used to set the isotype negative control (seen in M1). (B) Percent of CD90+ cells before MACS. (C) Percent of CD90+ cells in M2 (positive group) after MACS. (D) Percent of CD90− cells in M1 (negative group) after MACS. M1 and M2 indicate the percent of CD90− vs CD90+ cells, respectively. M, marker for CD90− (M1) and CD90+ (M2); Count, cell count.
Murine hippocampal neuronal cell line
Mouse hippocampal neuronal cell line, HT22 (11), was maintained in DMEM supplemented with 10% fetal bovine serum (Invitrogen, Grand Island, New York) (13). HT22 cells seeded at 1 × 104 cells/mL were treated with varying doses of 5-aza-2′-deoxycytidine (Aza-dC), and 24, 48, 72, 96, and 120 hours after treatment, cell proliferative growth was assessed by the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay using the Cell Growth Determination kit (Sigma-Aldrich Co, St Louis, Missouri).
Epigenetic regulation in HT22 cells
HT22 cells were grown at a density of 1 × 105 cells/mL in 10-cm2 flasks. Twenty-four hours later, cells were treated with Aza-dC at final concentration of 0.5 μM for 5 days preoptimized by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assays, or treated with trichostatin A (TSA) (Sigma-Aldrich Co) at final concentration of 200nM for 24 hours. For treatment with Aza-dC combined with TSA, the latter was added 24 hours before examination of cells on the fifth day after initiation of Aza-dC treatment.
DNA methylation analysis
Genomic DNA (1.5 μg) from neuronal cells was modified with sodium bisulfite using the EpiTect Bisulfite kit (QIAGEN, Valencia, California). CG Genome Universal Methylated and Unmethylated DNA (Millipore, Temecula, California) were also modified to serve as positive and negative controls (100% values). Bisulfite converted DNA (100 ng) was amplified in a methylation-specific PCR (MSP). MethySYBR qMSP primers (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org) specific for fully methylated and unmethylated CpG island of the glut3 gene (mglut3 and uglut3) were designed with the MethPrimer software. Primers for bisulfite converted (ActB) and unconverted (ActG) β-actin DNA sequences containing no CpG sites (14, 15) were used as internal controls necessary for correction of the cycle threshold (CT) values. CT values of mglut3 and uglut3 were normalized using corresponding standard curves and corrected to ActB. Percent of DNA methylation (PMR) and percent of unmethylation ratios of the glut3 gene were calculated assuming mglut3 + uglut3 = 100%.
Bisulfite modification and sequencing
Primers specific for modified DNA that did not contain any CpG sites in their sequence enabled amplification of both methylated and unmethylated DNA by the same primer set. These primer sets were used for bisulfite genomic sequencing PCR. The PCR products were cloned into pCR 2.1 TOPO vector (Invitrogen) following the manufacturer's instructions and the sequence analyzed on an ABI PRISM 310 Genetic Analyzer (Applied Biosystems, Foster City, California).
Chromatin immunoprecipitation (ChIP) and sequential ChIP (SeqChIP)
ChIP was performed using the ChIP-IT Express and control kits (Active Motif, Carlsbad, California) according to the manufacturer's instructions. In brief, after cross-linking, cells were homogenized in cell lysis buffer and then sonicated on ice using a Fisher Scientific Sonic Dismembrator 100 (output control 4, duty cycle 40%; Fisher Scientific, Pittsburgh, Pennsylvania) to an average length of approximately 500 bp as determined by 2% agarose gel electrophoresis. A total of 100 μL of sheared cross-linked chromatin for each ChIP reaction was immunoprecipitated overnight at 4°C with 25 μL of protein G magnetic beads, 2 μg of antimethyl-CpG binding protein 2 (Mecp2) antibody (Millipore), and negative IgG and anti-RNA Pol II IgG (positive control) (Active Motif). After IP, chromatin was eluted, and the DNA and histones/other proteins were dissociated by reverse cross-linking. Finally, the dissociated proteins were digested with proteinase K, and the eluted DNA was subjected to PCR.
SeqChIP was performed according to Geisberg and Struhl (16) with modifications of Furlan-Magaril et al (17). After the DNA-chromatin complex was immunoprecipitated by the anti-Mecp2 antibody and dissociated from the secondary antibody linked protein G magnetic beads, re-IP was carried out with the anti-Creb1 antibody (Santa Cruz Biotechnology, Inc, Santa Cruz, California) or anti-HDAC1 antibody (ChIP validated; Millipore) supplemented with magnetic G beads, following the identical procedure described above.
To quantify protein/histone-associated DNA, ChIP-quantitative PCR (qPCR) reactions were performed using SYBR GreenER Assay (Invitrogen) with the eluted DNA serving as the template (18). Protein enrichment was expressed as the ratio of IP-DNA/input (target) to IP-DNA/input (RNA PoI II) followed by standardization to an internal control (glyceraldehyde-3-phosphate dehydrogenase [GAPDH]).
SYBR Green real-time PCR
First-strand cDNA was synthesized from 1 μg of deoxyribonuclease-treated total RNA using Superscript III First-Strand Synthesis System for RT-PCR (Invitrogen Life Technologies, Carlsbad, California) as previously described (19). DNA methyl transferase (Dnmt)1, which is a maintenance methyl transferase, Dnmt3a, and Dnmt3b mRNAs, which catalyze de novo DNA methylation, were quantified using GAPDH as the internal control by RT-PCR. Mecp2 and Glut3 mRNAs were quantified using SYBR GreenER RT-qPCR Assay (Invitrogen Life Technologies) according to the manufacturer's recommendations. PCRs were conducted in independent triplicates for each sample. The gene expression level was standardized using an endogenous control gene GAPDH, and the relative gene expression level was determined using the 2(−ΔΔCT) method (20).
glut3 5′-flanking region luciferase reporter gene assays
Luciferase reporter plasmids of mouse glut3 5′-flanking region harboring (−1553 to +320 bp) or deleting (−573 to +320 bp) the region containing the CpG island were constructed as previously described (7, 8). Cells were cultured in 24-well plates at a density of 1 × 105 per well overnight and were transfected with Lipofectamine LTX (Invitrogen Life Technologies) as per the manufacturer's instructions. Each transfection contained 500 ng/well of glut3-luciferase and 100 ng/well of pRL-TK. Cells were harvested at 48 hours after transfection, and lysates were analyzed for luciferase activity using the Dual Luciferase Reporter Assay (Promega, Madison, Wisconsin) in a Clarity Luminescence Microplate Reader (BioTek, Winooski, Vermont). pGL2-control was used as the negative control. The relative luciferase activity was normalized to pRL-TK activity followed by correction to the negative pGL2-control. Results were expressed as a fold induction over that of pGL2-control activity that did not contain any glut3 sequences.
In vitro DNA methylation and cotransfection of MeCP2 and Creb1 proteins
glut3-luciferase plasmids were methylated in vitro by a CpG-sensitive methylase, M.SssI (2-U/μg DNA) (New England Biolabs, Ipswich, Massachusetts) in the presence of 160μM S-adenosyl methionine for 16 hours at 37°C. The efficacy of M.SssI was pretested by digesting the M.SssI-treated DNAs with restriction enzymes either blocked (SalI) or not blocked (BamHI) by M.SssI treatment. Resistance to SalI and sensitivity to BamHI indicates efficient methylation of DNA by M.SssI (10). Methylated and unmethylated luciferase reporter plasmids of mouse glut3 5′-flanking region harboring −1553- to +320-bp fragment that contains the CpG island along with pRL-Tk-Renilla luciferase (for transfection efficiency) were cotransfected using Lipofectamine LTX (Invitrogen Life Technologies) into HT22 cells (1 × 105 cell density per 24-well plate per grown overnight) with either the empty vector (negative control), Mecp2 with green fluorescent protein (GFP) (GFP-assessed protein expression level) (21), Creb1 (22), or Mecp2 with GFP+Creb1-containing expression vectors (500 ng each). GFP was detected in fixed cells by microscopy, whereas luciferase activity was assessed by the Dual Luciferase Reporter Assay (Promega).
Small interference RNA (siRNA) transfection
Silencer Select predesigned siRNA for mouse Mecp2 gene silencing (target transcript: NM_001081979, 5′-UUACCGUGAAGUCAAAAUCat-3′), mouse Creb1 gene silencing (target transcript: NM_001037726.1, 5′-UUUGGUUUUCAAGCACUGCca-3′), and scrambled control siRNAs were obtained from Ambion (Grand Island, New York). The siRNA transfections were performed using siPORT Amine Transfection Agent (Applied Biosystems) according to the manufacturer's protocol. Cells were harvested 72 hours later for the assessment of RNA interference gene knockdown effect. Experiments were conducted as 6 independent transfections, each one in triplicate.
Coimmunoprecipitation (Co-IP)
Co-IP was performed using Nuclear Complex Co-IP kit (Active Motif). For each Co-IP reaction, approximately 4 mg of total nuclei extracted from neuronal cells were used. Nuclei were immunoprecipitated with 5-μg mouse monoclonal anti-Mecp2 antibody (Millipore), and the immune complexes were bound to a 50% slurry of protein G-Sepharose beads and washed. Samples were separated by SDS-PAGE and immunoblotted as described below with antibodies against Creb1 and HDAC1 (Santa Cruz Biotechnology, Inc). Antirabbit IgG was used as the nonspecific negative control (10).
Coimmunolocalization
Coimmunolocalization was performed as described previously (23). Cells grown on cover slips were initially fixed using acetone and blocked using 0.2% Triton X-100 in 5% normal donkey serum. Double immunofluorescence staining for Mecp2 and Creb1 was carried out by incubation with a mixture of goat antimouse Mecp2 antibody and rabbit antimouse Creb1 antibody at 1:100 or 1:200 dilution (Santa Cruz Biotechnology, Inc) for 1 hour. After washing with PBS, cells were incubated with a mixture of FITC-conjugated rabbit antigoat IgG (Abcam, Inc, Cambridge, Massachusetts), Texas red-conjugated donkey antirabbit IgG (Jackson ImmunoResearch, West Grove, Pennsylvania), and 4′,6-diamino-2-phenylindole dihydrochloride (Sigma-Aldrich Co), a nuclear stain, for 45 minutes. These cells were washed, mounted and visualized by a Nikon E-600 microscope (Nikon, Melville, New York).
Western blot analysis
Nuclear protein extracts (50 μg) were subjected to Western blot analysis (10) using specific antibodies. Polyclonal antimouse Dnmt1 (1:1000; Sigma-Aldrich Co), monoclonal antimouse Dnmt3a and Dnmt3b (each 1:1000; Imgenex, San Diego, California), Mecp2 (1:1000; Millipore), and Creb1 and HDAC1 (1:250 or 1:500; Santa Cruz Biotechnology, Inc) were used. Glut3 antibody was generated and characterized by us (24). Vinculin antibody (1:5000; Sigma-Aldrich Co) was used as an internal loading control.
Statistical analysis
Results are shown as mean ± SEM. All statistical analyses were performed using Sigmastat 3.5 software (Systat, Point Richmond, California). Two-group comparisons were performed by Student's t test in the presence or the nonparametric Mann-Whitney rank sum test in the absence of a normal distribution. When more than 2 groups were compared, ANOVA with Fisher's protected least significant difference (PLSD) test was used. Significance was assigned at P < .05.
Results
CpG island of the glut3 gene is hypermethylated in postnatal mouse neurons
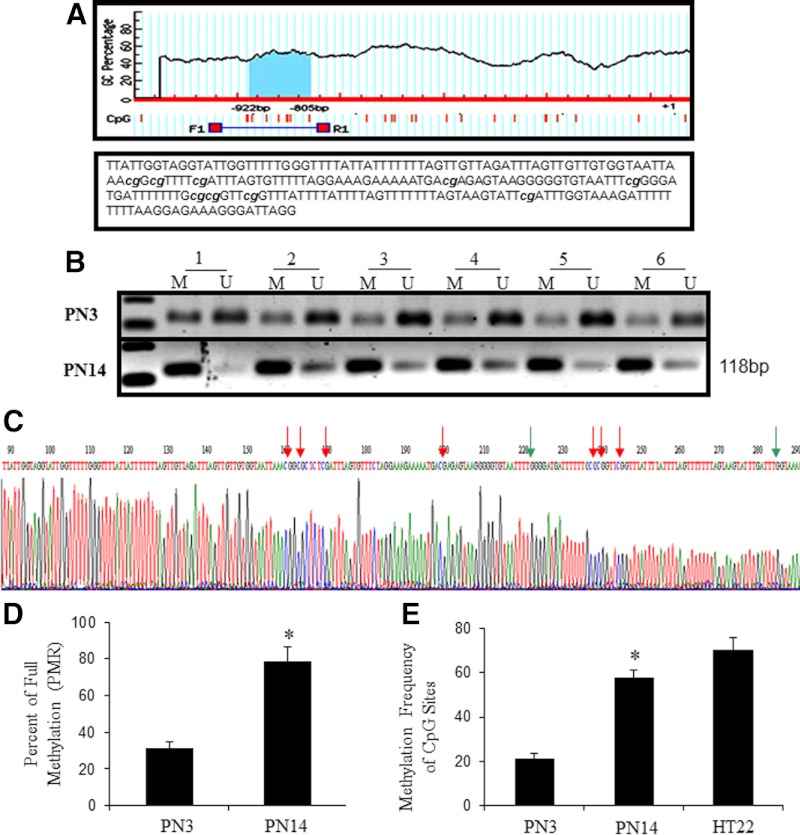
The promoter and 5′-flanking region of the mouse glut3 gene has been previously characterized by our group with a series of deletional analysis of the 5′-flanking region (7, 8). To detect whether glut3 expression is regulated by DNA methylation, we screened for the presence of CpG islands in the 5′-flanking region using MethPrimer (http://www.urogene.org/methprimer/). This search resulted in detection of a canonical CpG island of 118 bp in length containing 9 CpG dinucleotides located in the −922- to −805-bp region (Figure 2A). To precisely determine the methylation status of the glut3 gene, MSP, MethySYBR qMSP, and bisulfite conversion with sequencing were employed in genomic DNA obtained from primary CD90.2+ mouse neuronal cells isolated at PN3 and PN14. In PN3 and PN14 neurons, both methylated and unmethylated DNAs were displayed in MSP with a stronger relative optical density of the methylated form observed in PN14 vs PN3 neurons (Figure 2B). Representative bisulfite conversion and sequencing demonstrates both the methylated and unmethylated CpG dinucleotides (Figure 2C). PMRs calculated from MethySYBR qMSP indicated a value of 31.3% (PN3) and 78.4% (PN14), respectively (Figure 2D). The average methylation frequency of all the 9 CpG dinucleotides of the CpG island in the glut3 gene was 25.6% (PN3) and 59.8% (PN14), respectively. HT22 cells for comparison revealed 67.03% methylation frequency (Figure 2E). These findings suggested that the glut3-CpG island demonstrated increasing frequency of methylation with maturation and development of the postnatal mouse neuron.
Figure 2.
Methylation of the 5′-flanking region-associated CpG island of the glut3 gene in postnatal mouse neurons. (A) (top panel) Schematic representation of the putative CpG island in the 5′-flanking region of the glut3 gene; (bottom panel) bisulfite converted sequence of this particular 5′-flanking region-associated CpG island of the glut3 gene. The 9 CpG dinucleotides capable of being methylated are shown in lower case and italics. (B) DNA methylation detected by MSP. Lanes labeled M and U denote products (118 bp) amplified by primers recognizing methylated and unmethylated glut3 sequences, respectively. (C) Representative bisulfite sequencing of the glut3 5′-flanking region-associated CpG island in postnatal neurons, the CpG dinucleotide indicated by red arrows are methylated and green arrows are unmethylated. (D) PMR values of the glut3 5′-flanking region-associated CpG island as obtained by MethySYBR qMSP is depicted as mean ± SEM of 3 independent experiments, each conducted in triplicate; *P < .05 when compared with PN3. (E) Average methylation frequency of a total of 9 CpG dinucleotides in PN3 and PN14 neurons obtained by bisulfite sequencing were calculated from 6 clones obtained for each sample and shown. *P < .05 vs PN3. The value obtained in mouse hippocampal neuronal cells HT22 is also presented although not statistically compared with the mouse brain neuronal cells.
Functional significance of the hypermethylated glut3-CpG island
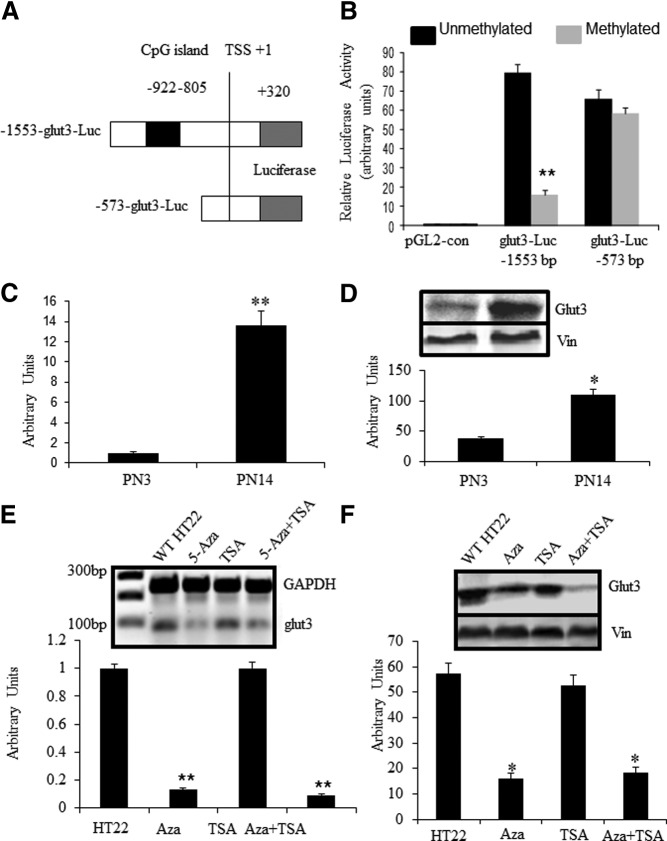
To verify whether the identified CpG island with higher frequency of methylation at PN14 has any regulatory function with respect to glut3 expression, we employed 2 previously constructed and described glut3 5′-flanking recombinants (8) redesigned as pGL2-glut3-luciferase constructs (−1553 to +320 and −573 to +320 bp). Of these DNA constructs, the −1553-bp construct encompassed the CpG island in question, whereas the −573-bp construct served as a deletional construct without the CpG island of the glut3 gene (Figure 3A). The vectors containing these 2 DNA constructs were methylated in vitro before transfection into HT22 cells and assessment of relative luciferase activity. As shown in Figure 3B, the relative luciferase activity was distinctly restrained in methylated pGL2-glut3(−1553)-luciferase (−1553 to +320 bp) compared with the unmethylated intact counterpart (P < .01). In contrast, no difference was observed between the unmethylated and methylated versions of the pGL2-glut3(−573)-luciferase (−573 to +320 bp) constructs (P > .05). This observation assigned a functional significance to the CpG island within the 5′-flanking region of the transfected sequences of the glut3 gene, particularly when methylated. Thus, methylation of this particular exogenously delivered CpG island in of itself reduced glut3 gene transcription.
Figure 3.
5′-flanking region-associated CpG island regulates glut3 expression through methylation. (A) 5′-flanking sequences of glut3-luciferase reporter assay quantifying gene transcription. The diagram demonstrates schematically the −1553-bp-luciferase (Luc) construct containing the CpG island between −922- and −805-bp region (shown as a black box), whereas the −573-bp-luciferase (Luc) construct does not contain this CpG island. TSS, transcriptional start site. (B) Relative luciferase activity in respective unmethylated and methylated glut3-Luc DNA constructs. Glut3 sequences containing plasmids were methylated in vitro by methylase M.SssI (2-U/μg DNA) in the presence of 160μM S-adenosyl methionine for 16 hours at 37°C and transfected into HT22 cells. Relative luciferase activity was normalized to pRL-TK activity (transfection efficiency control) and corrected for nonspecificity using the pGL2-control (negative control). Data are represented as mean ± SEM of 6 independent experiments each performed in triplicate. **P < .01 when compared with the respective unmethylated counterpart. (C and D) Glut3 expression in postnatal mouse neurons was detected by SYBR Green qPCR at the mRNA level (C) and Western blottings at the protein level with vinculin (Vin) serving as the internal control (D). (E and F) Glut3 expression in HT22 cells treated by epigenetic reagents. HT22 cells were treated with Aza-dC (0.5 μM) for 120 hours alone or in combination with TSA (100nM) added during the last 24 hours, or treated with TSA alone for 24 hours. Glut3 expression was determined by qPCR at the mRNA level with GAPDH as the internal control (C, E) and Western blottings at the protein level with Vin as the internal control (D, F). Data are depicted as mean ± SEM of 3 independent experiments; *P < .05, **P < .01 when compared with wild-type HT22.
Increasing expression of glut3 gene is associated with hypermethylation of the 5′-flanking region-associated CpG island in postnatal mouse neurons
Our previous studies have established that glut3 expression in postnatal mouse brains increases with advancing age reaching a peak at PN14 and remaining constant thereafter (4, 5). To determine whether a similar pattern was evident in isolated neurons, glut3 expression profiled in isolated primary neurons by RT-qPCR and Western blot analysis revealed a 13.6-fold increase in Glut3 mRNA (P < .01) (Figure 3C) and an approximately 4-fold increase in Glut3 protein (Figure 3D) at PN14 vs PN3 (P < .05).
To determine the functional link between CpG island methylation and endogenous glut3 gene expression, we next treated HT22 cells with a DNA methylation inhibitor (Aza-dC at 0.5 μM) either alone or in combination with a histone deacetylase (class I and II) inhibitor (TSA at 200nM). After such treatment, HT22 cells at 5 days revealed a 7.69- and 11.11-fold decrease in endogenous Glut3 mRNA concentrations when exposed to Aza-dC alone or in combination with TSA, respectively. Concurrently, Glut3 protein concentrations were also drastically down-regulated in both treatment groups. In contrast, when these cells were exposed to TSA alone in the absence of Aza-dC, no change in Glut3 mRNA or protein was displayed (Figure 3, E and F). These results support a role for diminishing methylation of CpGs in the endogenous glut3 gene to reduce Glut3 expression. In contrast, diminishing histone deacetylation failed to increase or change Glut3 expression significantly.
Dnmts associated with methylation of the CpG island in the glut3 gene in mouse neurons
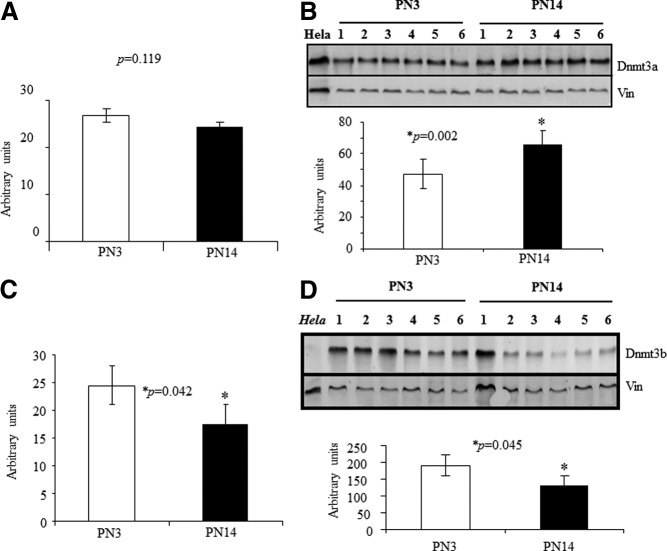
We next explored the Dnmt isoforms responsible for postnatal neuronal glut3 gene methylation. To this end, we assessed Dnmt1, Dnmt3a, and Dnmt3b by RT-PCR and Western blot analysis in nuclear extracts obtained from PN3 and PN14 isolated neurons. No change in Dnmt1 mRNA or protein was noted (negative data not shown). We observed an increase in Dnmt3a protein despite no change in Dnmt3a mRNA at PN14 vs PN3 (Figure 4, A and B). In contrast, a reduction in Dnmt3b mRNA was reflected in the protein at PN14 vs PN3 (Figure 4, C and D). These results suggest that increased nuclear expression of Dnmt3a is associated with increased glut3-mCpG (methylation of CpG island) at PN14 and therefore may play a role in methylating the glut3 gene during postnatal development.
Figure 4.
Dnmt expression in the neuronal cells of postnatal mouse brain. (A–D) Dnmt3a (A) and Dnmt3b (C) mRNAs with GAPDH as the internal control and Dnmt3a (B) and Dnmt3b (D) proteins with vinculin (Vin) as the internal control are shown in representative Western blotting on nuclear extracts of isolated neurons obtained at PN3 and PN14. Hela cell nuclear extracts served as a positive control for Dnmt3a and a negative control for Dnmt3b. Quantification of the respective protein concentrations is shown below as a ratio to Vin (B and D) in bar graphs (n = 6, each).
Mecp2 was up-regulated and enriched at the CpG island of the glut3 gene in mouse neurons
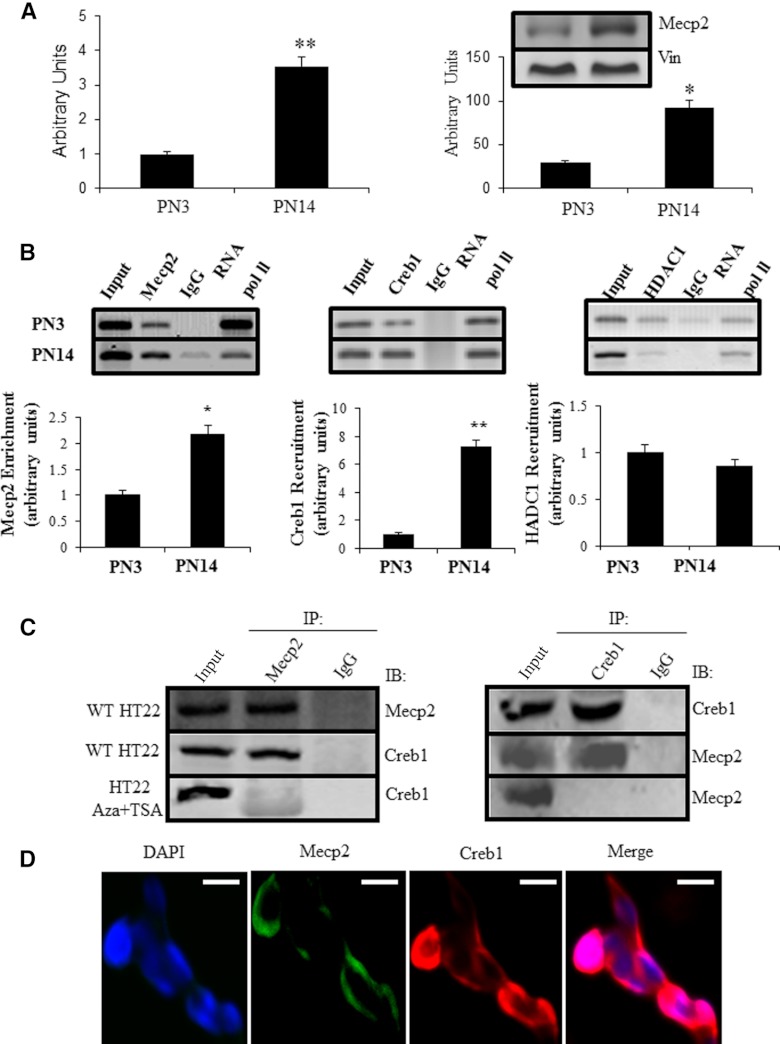
Expression of neuronal Mecp2 mRNA and protein were explored using SYBR Green qPCR and Western blot analysis, respectively. Compared with PN3 neurons, both the Mecp2 mRNA and protein concentrations were significantly higher (3-fold) in PN14 neurons (Figure 5A).
Figure 5.
Glut3 expression was up-regulated upon enrichment of Mecp2 and Creb1 at the glut3 DNA fragment containing the CpG island in mouse postnatal neurons. (A and B) Mecp2 expression in mouse neurons at the mRNA level (A) and protein level with vinculin (Vin) serving as the internal control (B). (C) Co-IP experiments. ChIP assay targeting Mecp2 enrichment at the CpG island of the glut3 gene (left); and SeqChIP assay targeting Creb1 (middle) or HDAC1 (right) recruitment by Mecp2. IgG served as the negative control and RNA Pol II as the positive control. Representative blots are shown above. Protein enrichment/recruitment was expressed as a ratio of IP-DNA/input (target) to IP-DNA/input (RNA PoI II) followed by normalization to the PN3 value (depicted as 1). Data are shown as the mean ± SEM of 3 independent experiments shown below; *P < .05 and **P < .01 when compared with the PN3 value. Nuclei extracted from HT22 cells were IP with antibodies against Mecp2 and subsequently Western blotted with antibodies targeting Creb1 (left). Conversely, they were IP by antibodies against Creb1 and blotted by antibodies targeting Mecp2 (right), IPs performed with rabbit IgG were incorporated into each experiment as nonspecific negative control. Bottom panels show the Co-IP of nuclei obtained from HT22 cells treated with Aza-dC in combination with TSA. (D) Immunocolocalization of Mecp2 with Creb1 in HT22 cells. Cells were prepared, fixed, immunostained, and visualized by microscopy. To detect Mecp2, cells were incubated with goat antimouse Mecp2 antibody and stained with the FITC-conjugated antigoat IgG (green fluorescence), whereas Creb1 was detected by incubating with rabbit antimouse Creb1 antibody and immunostained using Texas red-conjugated antirabbit IgG (red fluorescence), nuclei were stained with 4′,6-diamino-2-phenylindole dihydrochloride (DAPI) (blue). Merged images showed superimposition of Mecp2 and Creb1 in right panel. Images are representative of at least 3 separate experiments yielding similar results. IB, immunoblotted. Scale bars, 10 μm.
The interaction between Mecp2 and the CpG island of the glut3 gene was assessed by ChIP assay. In PN14 neurons, the Mecp2 association with the glut3 CpG island was greater than at PN3. ChIP-qPCR analysis revealed a 2.2-fold enrichment of Mecp2-glut3 DNA (P < .05, Figure 5B, left) at PN14 vs PN3. These findings support enhanced recruitment of Mecp2 to the CpG island of the glut3 gene at PN14 vs PN3.
Mecp2-dependent recruitment of Creb1 to the CpG island of the glut3 gene in mouse neurons
Contradictory observations where hypermethylation of the glut3 CpG island paralleled increasing neuronal glut3 gene expression at PN14 vs PN3 prompted us to examine the potential recruitment of 2 cofactors, namely Creb1 (transactivator) and HDAC1 (repressor). In SeqChIP assays followed by qPCR, a 7.26-fold increased Creb1 recruitment to the glut3 CpG island was evident in PN14 vs PN3 neurons (P < .01) (Figure 5B, middle). In contrast, HDAC1 revealed minimal recruitment by Mecp2 with an opposite pattern, being higher in PN3 vs PN14, although not statistically significant (P > .05) (Figure 5B, right). This observation supports increased Creb1 but not HDAC1 recruitment by Mecp2-glut3-CpG island in PN14 vs PN3 neurons.
Therefore, we investigated whether Mecp2 and Creb1 proteins associate (directly or indirectly) with each other using Co-IP in postnatal mouse neurons. As seen in Figure 5C, Creb1 was immunoprecipitated by the anti-Mecp2 antibody and failed to Co-IP by a nonspecific antibody (negative control) (Figure 5C, left). Accordingly, when antibodies recognizing Creb1 were used for the IP, Mecp2 was also Co-IP (Figure 5C, right). Loss of Mecp2 polymerization with Creb1 is evident upon pretreatment with Aza-dC+TSA (Figure 5C, left and right). Furthermore, the colocalization of Mecp2 with Creb1 was examined by immunocytochemistry. Mecp2 was visualized using FITC-labeled antigoat antibody, and Creb1 was visualized using Texas red-labeled antirabbit antibody. Mecp2 was densely distributed in HT22 cells, immunoreactive Creb1 was almost entirely colocalized with Mecp2 (Figure 5D). These results suggested that Mecp2 and Creb1 colocalize to the same discrete subcellular compartments. This colocalization was lost upon pretreatment with Aza-dC (data not shown). Taken together, these observations indicated that when Mecp2 bound methylated CpGs of the glut3 gene, it selectively recruited Creb1 over HDAC1.
Functional significance of Mecp2 and Creb1
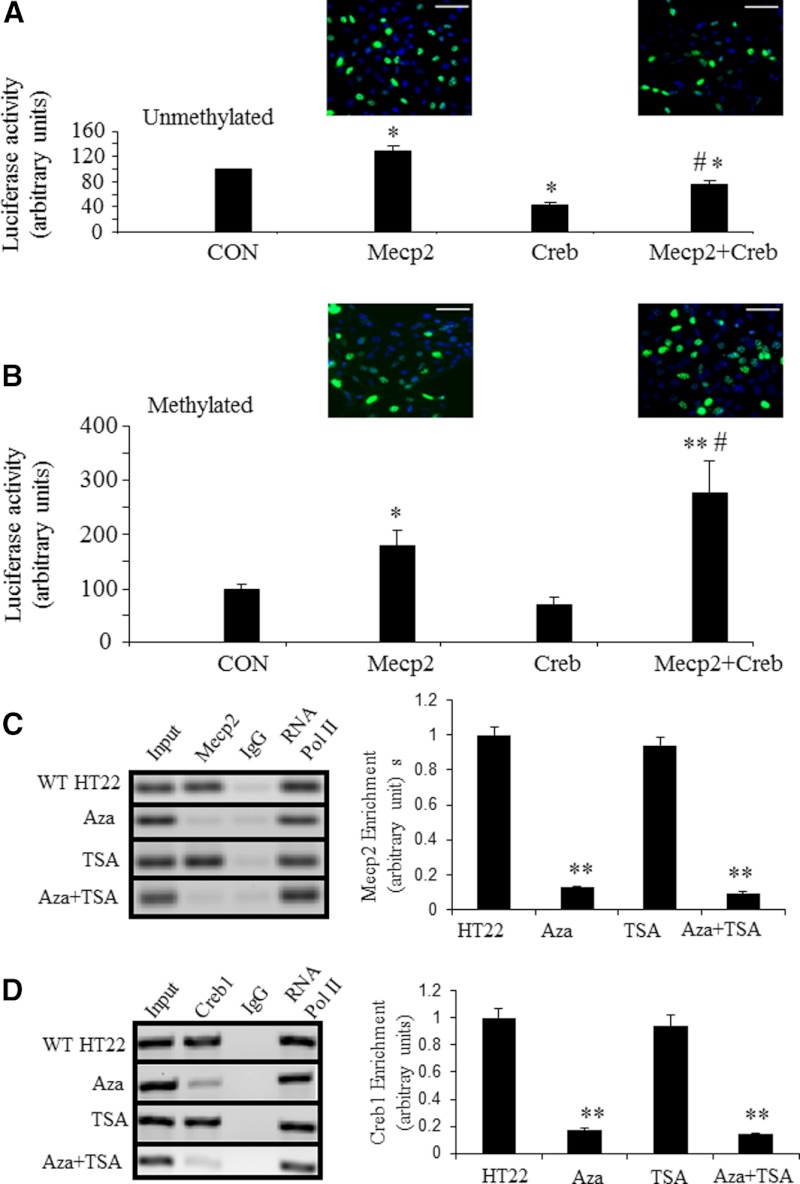
To test the functionality of Mecp2 and Creb1, we undertook cotransfection experiments in HT22 cells employing unmethylated and methylated pGL2-glut3(−1553)-luciferase DNA constructs along with expression vectors containing Mecp2 alone, Creb1 alone, or Mecp2 and Creb1 combined, with the empty vector serving as the baseline control. When unmethylated pGL2-glut3(−1553)-luciferase construct was cotransfected with Mecp2 alone, an approximately 27% increase in glut3-luciferase activity was observed (P < .01), whereas Creb1 alone led to a approximately 60% reduction in glut3-luciferase activity (P < .01). However, a combination of Mecp2+Creb1 cotransfection led to a reduction in glut3-luciferase activity by only approximately 25% (P < .01) (Figure 6A). In contrast, the methylated pGL2-glut3(−1553)-luciferase construct when cotransfected with Mecp2 alone revealed a doubling of glut3-luciferase activity (P < .02, Student's t test), whereas Creb1 alone did not alter glut3-luciferase activity (P = .09, Student's t test; P = .55, Fisher's PLSD). In contrast, Mecp2+Creb1 revealed a 3-fold increase in glut3-luciferase activity (P < .002, Fisher's PLSD) (Figure 6B). These observations attest to Mecp2 along with Creb1 activating glut3 transcription when interacting with methylated glut3-CpG vs a reduction with unmethylated glut3-CpG.
Figure 6.
Interaction of Mecp2 and Creb1 in HT22 cells was enhanced by methylation of the glut3-mCpG and dissociated by epigenetic reagents. (A and B) Cotransfection experiments. Luciferase activity expressed by transiently transfected unmethylated (A) and methylated (B) glut3(−1553)-luciferase (n = 6 each) in HT22 cells along with expression vectors that contain no insert (CON, standardized to 100%), Mecp2 alone, Creb1 alone, and Mecp2+Creb1 combination (500 ng each; n = 6 each), depicted as a percent of control. In the presence of unmethylated glut3(−1553)-luciferase, Mecp2 increased, whereas Creb1 alone and Mecp2+Creb1 combination reduced luciferase activity (*P < .01 vs CON, #P < .001 vs Mecp2) (A). When glut3-(1553)-luciferase was methylated, Mecp2 (*P < .02 vs CON) and Mecp2+Creb1 combination substantially increased (**P < .002 vs CON (B), #P < .05 vs Mecp2) luciferase activity. Creb1 alone demonstrated no change when compared with CON (B). Insets demonstrate GFP in HT22 cells as a measure of Mecp2 expression vector transfection efficiency. A and B, Scale bars, 50 μm. (C) Enrichment of Mecp2 at the glut3 CpG island in HT22 cells treated with Aza-dC and TSA detected by ChIP. IgG served as the negative control and RNA Pol II as the positive control. Representative blots are shown in the left panel and quantification in the right panel. *P < .05 and **P < .01 when compared with wild-type HT22 cells. (D) Recruitment of Creb1 by Mecp2 in HT22 cells treated with Aza-dC in combination with TSA detected by SeqChIP. Representative blots are shown in left panels and quantification in right panels. Data are depicted as the mean ± SEM of 3 independent experiments. *P < .05 and **P < .01 when compared with wild-type HT22 cells. CON, control; WT, wild type.
DNA methylation inhibition in HT22 neuronal cells depolymerizes Creb1 and Mecp2 from the 5′-flanking CpG island of the glut3 gene
The enrichment of Mecp2 and recruitment of Creb1 were depleted from the CpG island of the glut3 gene upon exposure of HT22 cells to Aza-dC alone or with TSA (Figure 6, C and D). Interestingly, these observed changes occurred to the same extent in cells treated either with Aza-dC separately or in combination with TSA. In contrast, TSA alone in HT22 cells revealed no effect on depolymerization of Mecp2 and Creb1 recruitment. These findings support a need for glut3 CpG methylation to bring about increased DNA-binding of Mecp2 and the subsequent recruitment of Creb1. No role for histone deacetylases was verified.
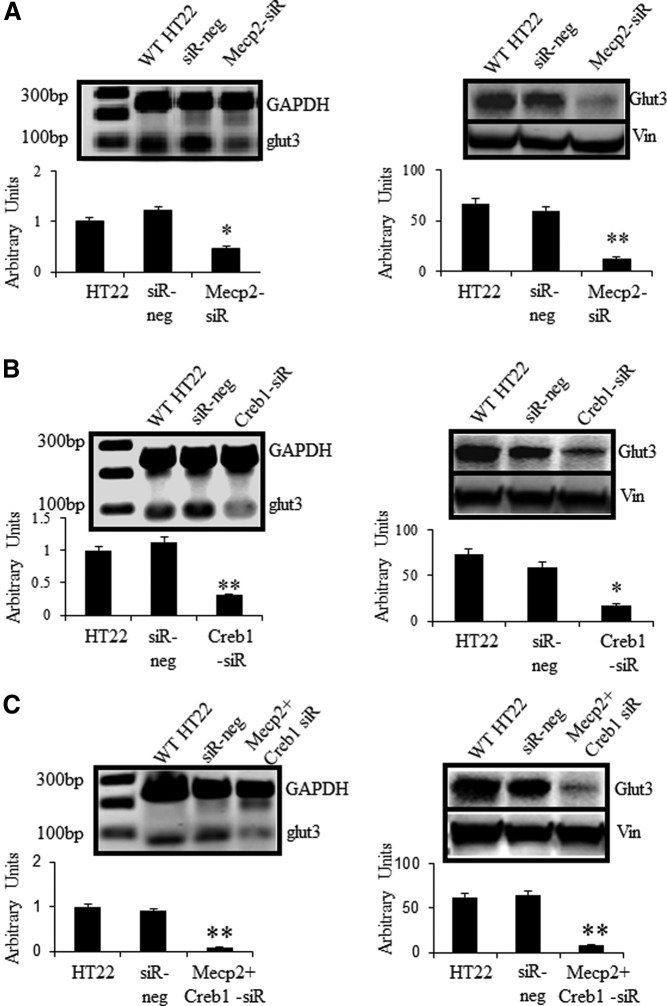
Knockdown of Mecp2 and/or Creb1 significantly suppresses glut3 gene expression in HT22 neuronal cells
To determine whether the Mecp2-dependent recruitment of Creb1 is critical for expression of Glut3, the effect of Mecp2 or Creb1 depletion, either alone or in combination, on Glut3 expression was investigated. HT22 cells were transfected with scrambled siRNA (siR-neg) that served as the negative control or siRNA directed against Mecp2 and/or Creb1, and endogenous Glut3 expression was assessed by RT-qPCR and Western blot analysis. siRNA-induced depletion of Mecp2 and Creb1 was initially confirmed by measuring mRNA concentrations of Mecp2/Creb1 relative to GAPDH by RT with qPCR. Mecp2/Creb1 mRNA were reduced by approximately 60% as compared with the scrambled negative control (data not shown) on day 3 after transfection. Individual depletion of Mecp2 (Figure 7A) or Creb1 (Figure 7B) decreased Glut3 expression (for mRNAs, see Figure 7, A and B, left; for protein, see Figure 7, A and B, right) when compared with wild-type HT22 cells or those containing scrambled siR-neg. Moreover, concurrent depletion of Mecp2 and Creb1 reduced the expression of Glut3 to an extent significantly lower than that obtained for cells depleted of either Mecp2 or Creb1 alone (Figure 7C). This result indicated that Mecp2 and Creb1 cooperatively activated glut3 gene expression.
Figure 7.
siRNAs specifically targeting Mecp2 or Creb1 inhibited Glut3 expression in HT22 cells. 1 × 105 cells were reseeded in 6-well plates, and 10nM siRNA was transfected 24 hours later. Cells were incubated at 37°C for 72 hours and harvested for assessment of the RNA interference knockdown effect. Data are depicted as the mean ± SEM of 6 independent experiments performed in triplicate; *P < .05 and **P < .01 when compared with wild-type HT22 cells. (A) HT22 cells were transfected with scrambled (siR-neg) or Mecp2 siRNA. (B) HT22 were transfected with scrambled or Creb1 siRNA. (C) HT22 cells were transfected with scrambled or Mecp2 siRNA in combination with Creb1 siRNA. Glut3 expression was detected by SYBR Green qPCR and Western blot analysis. Representative blots are depicted above, and quantifications are shown as fold over basal with GAPDH as the internal control (below in left panels), protein concentrations are presented as relative optical density (below in right panels). WT, wild type.
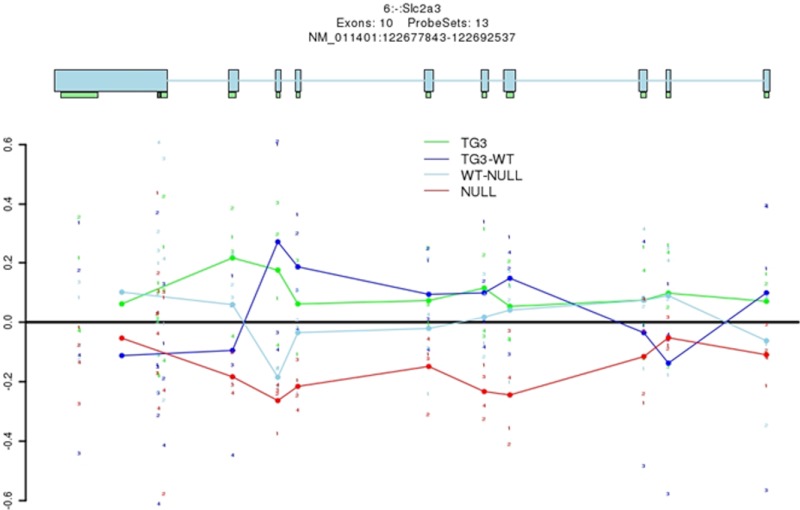
In vivo analysis of brain expression arrays in MeCP2 knockout and transgenic mice
To determine whether there was any validity to our in vitro findings either in isolated primary neurons or HT22 hippocampal neurons within the in vivo context of the whole brain, we computer analyzed the results of previously reported expression arrays conducted in brains obtained from Mecp2 null and transgenic mice vs their corresponding wild-type littermates (25). We noted that in Mecp2 null mice at an exon array level, there was a decrease in glut3 gene expression when compared with wild-type mice. This supports an activating role for Mecp2 in glut3 gene transcription. In contrast, there is less evidence that glut3 gene expression was higher than its wild-type counterpart in Mecp2 transgenic mice. This may relate to the need for cooperative interplay between Creb1 and Mecp2 and not merely an increase in Mecp2 alone as encountered in these transgenic mice (Figure 8). This bioinformatics analysis further supports our experiments demonstrating that Mecp2 plays a vital role in regulating glut3 gene transcription and expression. Reliance on recruitment of Creb1 is necessary to further enhance the transactivation by Mecp2 during the process of postnatal neuronal development.
Figure 8.
Computer analysis of expression arrays revealed glut3 expression in MeCP2 null and transgenic mouse brains and their respective wild-type counterparts. Brain glut3 expression was reduced in Mecp2 null brain (NULL, red) vs wild-type (WT-NULL, light blue) brains (exons 2 to 8 and 10); however, no reciprocal increase in glut3 expression to the same extent was deciphered in Mecp2 transgenic mice (TG3, green) vs the wild-type (TG3, dark blue) counterpart, except for exon-specific increase noted in the case of exon 1, 2, and 9.
Discussion
Transcription of neuronal glut3 gene
We have demonstrated that in isolated CD90.2+ fluorescence-activated cell-sorted murine neurons, glut3 gene expression increases with advancing postnatal age paralleling that seen in ex vivo intact murine brain (5). This postnatal age-related increase in Glut3 is associated with increasing frequency of methylation of the identified CpG island in the 5′-flanking region of the glut3 gene. This increased DNA methylation, which associates with increased nuclear Dnmt3a, attracts increased Mecp2 binding, which in turn recruits Creb1, a coregulator. Complementary in vitro investigations demonstrate that together, these 2 nuclear factors with the methylated CpG island transactivate glut3 gene transcription.
Previous investigations in murine neuroblastic N2A cells demonstrated pCreb1 to transactivate glut3 gene transcription when unmethylated glut3(−203)-luciferase DNA construct was used (8). However, in our present study, Creb1 repressed glut3 gene transcription when unmethylated glut3 (−1553)-luciferase DNA was employed with a Creb1-containing expression vector in HT22 neurons. These findings collectively support the direct or indirect interaction of Creb1 at different cis-elements of the glut3 gene, activating when binding the −203- to −177-bp region (8) and repressing when binding elsewhere between −1553 and +320 bp. However, upon methylation of the glut3-CpG island and enhanced recruitment of the transactivating Mecp2, Creb1 coactivates glut3 gene transcription further.
Such novel molecular mechanisms that sustain postnatal neurodevelopment have the potential of shedding light on abnormal neurodevelopment. Although the timing of murine neurodevelopment is distinct when compared with that of humans (PN10 in mice equates to that of a term human infant at birth) (26), unraveled molecular mechanisms in mouse translate to human neurons and provide the molecular basis of disease processes. Thus, any disruption of the normal molecular process uncovered in mice as in the present study may underlie neurodevelopmental disorders akin to autism or Rett syndrome (RTT) seen in humans (25, 27) and mimicked with mouse models (25, 28–30).
Translation of observations in mice to human neurodevelopmental disorders
Autism
Autism is a neurodevelopmental disorder, the clinical features present during infancy and can be detected as early as 18 months of age in the human (27). Brain imaging has revealed that autism is characterized by neuronal disconnectivity (27, 31), which affects various functions, such as social behavior, language, intellectual development with repetitive behaviors (27). In addition, positron emission tomography has revealed reduced brain glucose uptake (32–34).
Glut3 and autism
In a mouse model that harbors monoallelic mutation of the neuronal Glut3, clinical features of autism spectrum disorder emerged, which consisted of a lack of cognitive flexibility, abnormal spatial learning and working memory, impaired social interaction and social novelty, reduced vocalizations, low frequency stereotypies and electroencephalographic seizures (28). Thus, a direct etiological role for neuronal Glut3 (with associated glucose deficiency) in producing features of autism spectrum disorder was established in this mutant mouse (28). Glut3 is concentrated in synapses (35) and responsible for fueling energy for maintaining connectivity.
Mecp2 and RTT
Mutations of Mecp2 protein are responsible for 95% of the RTT, which demonstrates neuronal disconnectivity (25). RTT is a human progressive neurodevelopmental disorder involving a maturational arrest of brain development and regression in neurobehavior starting at 6 months of age (25). Positron emission tomography in RTT revealed age-dependent and anatomic region-specific changes in brain glucose uptake (36). Diffusion tensor imaging studies revealed loss of gray and white matter volumes with perturbed neuronal connectivity (37). Mutations of Mecp2 result in neuronal disconnectivity and perturbed brain glucose uptake.
Mecp2 knockout mice exhibit severe neurological symptoms that resemble RTT (29, 30). These features include social and behavioral regression at a developmental period that coincides with that of axonal networking in most murine brain regions (38, 39). There is a postnatal reduction of dendritic spines, a hallmark of neuronal disconnectivity (25). On the other hand, overexpression of Mecp2 in transgenic mice also resulted in a severe postnatal neural phenotype (25). Thus, neuronal Mecp2 must be tightly regulated to yield normal neurodevelopment.
Glut3 and Mecp2 in neurodevelopmental disorders
Thus, both Glut3 and Mecp2 are responsible for maintaining normal neuronal energy and connectivity, respectively, mutations of both mediating neuron structural and functional disconnectivity (30, 34, 38), and autism-like (or RTT) clinical features (28–30). These observations prompted our present investigations to detect whether there is any molecular connection between Mecp2 and Glut3, particularly whether Mecp2 orchestrates the epigenetic regulation of neuronal glut3 gene expression.
Coactivators that drive glut3 gene transcription
Methyl-CpG binding protein 2
Mecp2 binds mCpGs in gene promoters and transcriptionally represses downstream gene expression (40–42), such as in the case of brain-derived growth factor III that promotes neuronal connectivity (42). Mecp2 also binds histone deacetylase enzymes and certain key transcription factors towards stabilizing the heterochromatin state (42). Contrary to this silencing action, Mecp2 facilitates binding of key transactivators, such as Sin3A, NCorr, or Creb1, and enhances gene expression (40–42). The exact role of Mecp2 in regulating glut3 gene transcription was unknown until uncovered by our present investigation. Mecp2 binding of the unmethylated (increased by ∼27%) vs methylated (increased by ∼80%) glut3-CpGs was quite different, almost 4-fold higher in the latter.
Creb and Creb-binding protein (CBP)
Mecp2 attracts Creb towards binding and activating gene promoters. Creb mediates synaptogenesis and formation of neuronal networks postnatally after sensory stimulation in mice (43). We observed that Creb1 association with unmethylated glut3 gene repressed transcription. Mutations of Creb1 are found in human chromosome 2 deletion causing RTT-like features despite the absence of Mecp2 mutations (44). Creb1 in turn is regulated by the CBP, a histone acetyl transferase that promotes decondensation of chromatin, recruits other proteins, and activates gene transcription (45). Monoallelic mutations of CBP activate histone methyltransferase that hypermethylates histone 3 lysine 9 residue, causing condensation of pericentromeric heterochromatin provoking gene silencing (40). Human CBP mutations result in intellectual disability as encountered in Rubinstein-Taybi syndrome patients (40). Thus, Mecp2, Creb1, and CBP mutations interfere with murine and human neurodevelopment, pertaining to synaptogenesis. In our present investigation, Creb1 enhanced transactivation of the glut3 gene via the Mecp2-mCpG association.
Conclusion
In the present study, we have demonstrated the novel molecular interaction between Mecp2 and the glut3 gene in murine neurons, an interaction that increases with advancing postnatal age. Further, Mecp2 associated with the glut3-mCpG and recruited Creb1 to form a complex that transactivates the glut3 gene during postnatal synaptogenesis (46, 47). Thus, disruption of any of these players in the Creb1-Mecp2-mCpG-glut3 gene complex can disrupt neurodevelopment. In fact, in expression array analysis of brains from Mecp2 null or transgenic mice, we observed an impact on glut3 gene expression, validating our novel in vitro mechanistic observations. Although previous in vivo studies demonstrated associations between mutations of Mecp2 and brain glucose uptake, a cause-and-effect paradigm was established by our present in vitro investigations that established a molecular link between neuronal Mecp2 and Glut3. Unraveling this novel molecular mechanism paves the way for future investigations targeting the role of neuronal Glut3 in mediating cerebral connectivity induced by Mecp2, Creb1, and CBP either separately or collectively.
Acknowledgments
We thank David Schubert at the Salk Institute (La Jolla, California) for the HT22 murine hippocampal neuronal cell line, Guoping Fan at University of California Los Angeles for the Mecp2-GFP expression vector, and Marc Montiminy at La Jolla (University of California San Diego, California), for the Creb1 expression vector. We also thank Chad Shaw and Huda Zhogbi (Baylor College of Medicine, Houston, Texas) for the help in analyzing brain expression arrays performed in their Mecp2 knockout and transgenic mice.
This work was supported by National Institutes of Health Grants HD33997, HD46979, and HD25024 (to S.U.D.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Aza-dC
- 5-aza-2′-deoxycytidine
- CBP
- Creb-binding protein
- ChIP
- chromatin immunoprecipitation
- CT
- cycle threshold
- Creb
- cyclic AMP regulatory element binding protein
- Dnmt
- DNA methyl transferase
- FITC
- fluorescein isothiocyanate
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GFP
- green fluorescent protein
- Glut
- glucose transporter
- IP
- immunoprecipitation
- mCpG
- methylation of CpG island
- Mecp2
- methyl-CpG binding protein 2
- MSP
- methylation-specific PCR
- PLSD
- protected least significant difference
- PMR
- percent of DNA methylation
- PN
- postnatal day
- qPCR
- quantitative PCR
- RTT
- Rett syndrome
- SeqChIP
- sequential ChIP
- siRNA
- small interference RNA
- siR-neg
- scrambled siRNA
- TSA
- trichostatin A.
References
- 1. Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27:1766–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Simpson IA, Dwyer D, Malide D, Moley KH, Travis A, Vannucci SJ. The facilitative glucose transporter GLUT3: 20 years of distinction. Am J Physiol Endocrinol Metab. 2008;295:E242–E253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mantych GJ, James DE, Chung HD, Devaskar SU. Cellular localization and characterization of Glut 3 glucose transporter isoform in human brain. Endocrinology. 1992;131:1270–1278 [DOI] [PubMed] [Google Scholar]
- 4. Fields HM, Rinaman L, Devaskar SU. Distribution of glucose transporter isoform-3 and hexokinase I in the postnatal murine brain. Brain Res. 1999;846:260–264 [DOI] [PubMed] [Google Scholar]
- 5. Khan JY, Rajakumar RA, McKnight RA, Devaskar UP, Devaskar SU. Developmental regulation of genes mediating murine brain glucose uptake. Am J Physiol. 1999;276:R892–900 [DOI] [PubMed] [Google Scholar]
- 6. Thamotharan S, Stout D, Shin BC, Devaskar SU. Temporal and spatial distribution of murine placental and brain Glut3-luciferase transgene as a read out of in-vivo transcription. Am J Physiol Endocrinol Metab. 2013;304:E254–E266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rajakumar RA, Thamotharan S, Menon RK, Devaskar SU. Sp1 and Sp3 regulate transcriptional activity of the facilitative glucose transporter isoform-3 gene in mammalian neuroblasts and trophoblasts. J Biol Chem. 1998;273:27474–27483 [DOI] [PubMed] [Google Scholar]
- 8. Rajakumar A, Thamotharan S, Raychaudhuri N, Menon RK, Devaskar SU. Trans-activators regulating neuronal glucose transporter isoform-3 gene expression in mammalian neurons. J Biol Chem. 2004;279:26768–26779 [DOI] [PubMed] [Google Scholar]
- 9. Devaskar SU, Raychaudhuri S. Epigenetics–a science of heritable biological adaptation. Pediatr Res. 2007;61:1R–4R [DOI] [PubMed] [Google Scholar]
- 10. Raychaudhuri N, Raychaudhuri S, Thamotharan M, Devaskar SU. Histone code modifications repress glucose transporter 4 expression in the intrauterine growth-restricted offspring. J Biol Chem. 2008;283:13611–13626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li Y, Maher P, Schubert D. A role for 12-lipoxygenase in nerve cell death caused by glutathione depletion. Neuron. 1997;19:453–463 [DOI] [PubMed] [Google Scholar]
- 12. Yu S, Zhang JZ, Zhao CL, Zhang HY, Xu Q. Isolation and characterization of the CD133+ precursors from the ventricular zone of human fetal brain by magnetic affinity cell sorting. Biotechnol Lett. 2004;26:1131–1136 [DOI] [PubMed] [Google Scholar]
- 13. Suo Z, Wu M, Citron BA, Palazzo RE, Festoff BW. Rapid tau aggregation and delayed hippocampal neuronal death induced by persistent thrombin signaling. J Biol Chem. 2003;278:37681–37689 [DOI] [PubMed] [Google Scholar]
- 14. Lo PK, Watanabe H, Cheng PC, et al. MethySYBR, a novel quantitative PCR assay for the dual analysis of DNA methylation and CpG methylation density. J Mol Diagn. 2009;11:400–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hattermann K, Mehdorn HM, Mentlein R, Schultka S, Held-Feindt J. A methylation-specific and SYBR-green-based quantitative polymerase chain reaction technique for O6-methylguanine DNA methyltransferase promoter methylation analysis. Anal Biochem. 2008;377:62–71 [DOI] [PubMed] [Google Scholar]
- 16. Geisberg JV, Struhl K. Quantitative sequential chromatin immunoprecipitation, a method for analyzing co-occupancy of proteins at genomic regions in vivo. Nucleic Acids Res. 2004;32:e151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Furlan-Magaril M, Rincon-Arano H, Recillas-Targa F. Sequential chromatin immunoprecipitation protocol: ChIP-reChIP. Methods Mol Biol. 2009;543:253–266 [DOI] [PubMed] [Google Scholar]
- 18. Irvine RA, Hsieh CL. Q-PCR in combination with ChIP assays to detect changes in chromatin acetylation. Methods Mol Biol. 2004;287:45–52 [DOI] [PubMed] [Google Scholar]
- 19. Shin BC, Dai Y, Thamotharan M, Gibson LC, Devaskar SU. Pre- and postnatal calorie restriction perturbs early hypothalamic neuropeptide and energy balance. J Neurosci Res. 2012;90:1169–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods. 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 21. Kudo S, Nomura Y, Segawa M, et al. Functional analyses of MeCP2 mutations associated with Rett syndrome using transient expression systems. Brain Dev. 2001;23(suppl 1):S165–S173 [DOI] [PubMed] [Google Scholar]
- 22. Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680 [DOI] [PubMed] [Google Scholar]
- 23. Shin BC, Fujikura K, Suzuki T, Tanaka S, Takata K. Glucose transporter GLUT3 in the rat placental barrier: a possible machinery for the transplacental transfer of glucose. Endocrinology. 1997;138:3997–4004 [DOI] [PubMed] [Google Scholar]
- 24. Ganguly A, McKnight RA, Raychaudhuri S, Shin BC, Ma Z, Moley K, Devaskar SU. Glucose transporter isoform-3 mutations cause early pregnancy loss and fetal growth restriction. Am J Physiol Endocrinol Metab. 2007;292:E1241–E1255 [DOI] [PubMed] [Google Scholar]
- 25. Chahrour M, Jung SY, Shaw C, et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Innocenti GM. Development and evolution: two determinants of cortical connectivity. Prog Brain Res. 2011;189:65–75 [DOI] [PubMed] [Google Scholar]
- 27. Geschwind DH. Advances in autism. Annu Rev Med. 2009;60:367–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao Y, Fung C, Shin D, et al. Neuronal glucose transporter isoform 3 deficient mice demonstrate features of autism spectrum disorders. Mol Psychiatry. 2010;15:286–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet. 2001;27:327–331 [DOI] [PubMed] [Google Scholar]
- 30. Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27:322–326 [DOI] [PubMed] [Google Scholar]
- 31. Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111 [DOI] [PubMed] [Google Scholar]
- 32. Toal F, Murphy DG, Murphy KC. Autistic-spectrum disorders: lessons from neuroimaging. Br J Psychiatry. 2005;187:395–397 [DOI] [PubMed] [Google Scholar]
- 33. Haznedar MM, Buchsbaum MS, Wei TC, et al. Limbic circuitry in patients with autism spectrum disorders studied with positron emission tomography and magnetic resonance imaging. Am J Psychiatry. 2000;157:1994–2001 [DOI] [PubMed] [Google Scholar]
- 34. Haznedar MM, Buchsbaum MS, Hazlett EA, LiCalzi EM, Cartwright C, Hollander E. Volumetric analysis and three-dimensional glucose metabolic mapping of the striatum and thalamus in patients with autism spectrum disorders. Am J Psychiatry. 2006;163:1252–1263 [DOI] [PubMed] [Google Scholar]
- 35. Thoidis G, Kupriyanova T, Cunningham JM, et al. Glucose transporter Glut3 is targeted to secretory vesicles in neurons and PC12 cells. J Biol Chem. 1999;274:14062–14066 [DOI] [PubMed] [Google Scholar]
- 36. Villemagne PM, Naidu S, Villemagne VL, et al. Brain glucose metabolism in Rett syndrome. Pediatr Neurol. 2002;27:117–122 [DOI] [PubMed] [Google Scholar]
- 37. Naidu S, Kaufmann WE, Abrams MT, et al. Neuroimaging studies in Rett syndrome. Brain Dev. 2001;23(suppl 1):S62–S71 [DOI] [PubMed] [Google Scholar]
- 38. Kishi N, Macklis JD. MECP2 is progressively expressed in post-migratory neurons and is involved in neuronal maturation rather than cell fate decisions. Mol Cell Neurosci. 2004;27:306–321 [DOI] [PubMed] [Google Scholar]
- 39. Belichenko PV, Wright EE, Belichenko NP, et al. Widespread changes in dendritic and axonal morphology in Mecp2-mutant mouse models of Rett syndrome: evidence for disruption of neuronal networks. J Comp Neurol. 2009;514:240–258 [DOI] [PubMed] [Google Scholar]
- 40. Urdinguio RG, Sanchez-Mut JV, Esteller M. Epigenetic mechanisms in neurological diseases: genes, syndromes, and therapies. Lancet Neurol. 2009;8:1056–1072 [DOI] [PubMed] [Google Scholar]
- 41. Hite KC, Adams VH, Hansen JC. Recent advances in MeCP2 structure and function. Biochem Cell Biol. 2009;87:219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bogdanovic O, Veenstra GJ. DNA methylation and methyl-CpG binding proteins: developmental requirements and function. Chromosoma. 2009;118:549–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nonaka M. A Janus-like role of CREB protein: enhancement of synaptic property in mature neurons and suppression of synaptogenesis and reduced network synchrony in early development. J Neurosci. 2009;29:6389–6391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pescucci C, Meloni I, Bruttini M, et al. Chromosome 2 deletion encompassing the MAP2 gene in a patient with autism and Rett-like features. Clin Genet. 2003;64:497–501 [DOI] [PubMed] [Google Scholar]
- 45. Dekker FJ, Haisma HJ. Histone acetyl transferases as emerging drug targets. Drug Discov Today. 2009;14:942–948 [DOI] [PubMed] [Google Scholar]
- 46. Wirtz S, Schuelke M. Region-specific expression of mitochondrial complex I genes during murine brain development. PLoS One. 2011;6:e18897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Finlay BL, Darlington RB. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268:1578–1584 [DOI] [PubMed] [Google Scholar]