in addition to serving as components of nucleic acids and central players in intracellular energy metabolism, adenosine- and guanosine-based metabolites play critical but distinct roles in signal transduction processes. ATP has two major functions in intracellular signaling. It serves as the principal nucleotide phosphate donor for the myriad protein kinases that act as molecular switches for most critical intracellular functions. In addition, ATP is the substrate for adenylyl cyclases and inositol lipid kinases, serving, respectively, as the source of the ubiquitous cyclic AMP second messenger and the various inositol lipid second messengers, e.g., 3,4,5-phosphatidylinositol-tris phosphate or PIP3. Similarly, GTP has two major functions in intracellular signaling. It is the allosteric ligand for the activation of GTP-binding regulatory proteins that comprise the other major group of molecular switches for tuning cell function. Like ATP, GTP also serves as a source of second messengers by providing the substrate for the guanylyl cyclases that catalyze formation of the second messenger cyclic GMP.
Notably, nature has selected adenosine-containing metabolites for a third discrete role in cellular signal transduction: as extracellular agonists for purinergic receptors (8). Purinergic receptors include the P2X-family of ATP-gated ion channel receptors (seven subtypes), the P2Y-family of G protein-coupled nucleotide receptors (eight subtypes), and the P1-family of G protein-coupled adenosine receptors (four subtypes). Some P2Y receptor subtypes additionally (or exclusively) recognize nonpurinergic uridine nucleotides as agonists. In contrast, despite the “purinergic” sobriquet, no members of these three receptor groups recognize the guanosine-containing compounds (the “other” purines) as agonistic or antagonistic ligands. Thus, despite their critical roles in intracellular signal transduction, guanosine-containing compounds have historically been considered as nonentities in terms of extracellular signaling by nucleotides and nucleosides. However, a new study by Jackson et al. (1) in this issue of American Journal of Physiology-Cell Physiology reveals a hitherto unappreciated role for extracellular guanosine (and guanosine nucleotides) in the regulation of extracellular adenosine clearance/metabolism. Thus, these “poor relative” purines may indeed be involved in the complex world of extracellular purinergic signaling.
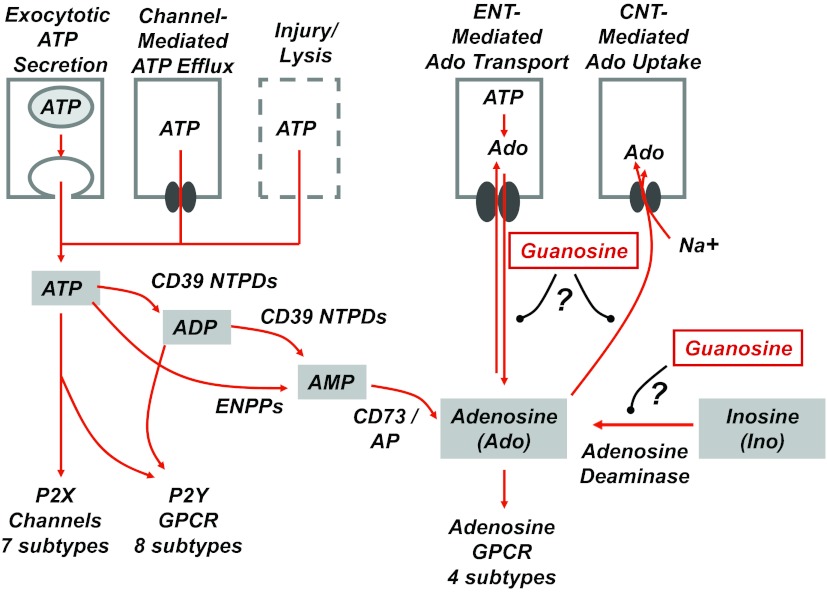
As for any molecules (e.g., neurotransmitters, hormones, cytokines) that function as agonists for cell surface receptors, the extracellular levels of adenosine nucleotides (ATP, ADP, AMP) and adenosine per se are regulated at the levels of production and clearance (2). Cells release intracellular stores of ATP either by traumatic cell lysis or by highly regulated, nonlytic processes that include exocytosis of ATP-containing vesicles and gating of nucleotide-permeable channels (Fig. 1). Released extracellular ATP is efficiently catabolized to ADP and, ultimately, AMP via CD39-family ecto-nucleoside 5' triphosphate diphosphohydrolylases (eNTPDases) or ecto-nucleotide pyrophosphatases/phosphodiesterases (eNPPs) (10). In turn, extracellular AMP is dephosphorylated to adenosine via the actions of the CD73 ecto-5'nucleotidase and/or GPI-anchored ecto-alkaline phosphatases (APs). Extracellular adenosine has several possible fates: 1) metabolism via adenosine deaminase to inosine; 2) active reaccumulation into cells via Na+-coupled concentrative nucleoside transporters (CNTs); or 3) passive reuptake into cells via equilibrative nucleoside transporters (ENTs) (4). Reuptake via CNTs or ENTs links the disposition of extracellular adenosine to the network of intracellular adenosine metabolism that includes (among multiple other reactions) phosphorylation of adenosine to AMP via adenosine kinase or the conjugation of adenosine with homocysteine via S-adenosylhomocysteine hydrolase (SAH).
Fig. 1.
Purinergic signaling pathways: modulation of extracellular adenosine clearance by extracellular guanosine. The pathways for release, extracellular metabolism, and downstream receptor-mediated signaling of ATP and its purine metabolites are indicated. ATP can be serially hydrolyzed to ADP and then AMP via the CD39-family nucleoside triphosphate diphosphohydrolases (NTPDs). Alternatively, ATP can be directly metabolized to AMP via ecto-nucleotide pyrophosphatases/phosphodiesterases (ENPPs). AMP hydrolysis by either the CD73 ecto-5'-nucleotidases or ecto-alkaline phosphatases (APs) generates extracellular adenosine. It is important to note that extracellular GTP, GDP, and GMP are also substrates for these various ecto-nucleotidases. Extracellular adenosine (or guanosine) can be directly released from metabolically stressed cells via efflux through equilibrative nucleoside transporters (ENTs). ENTs can also function as reuptake pathways for adenosine, guanosine, and the pyrimidine nucleosides. Alternatively, these nucleosides can be actively accumulated via the concentrative nucleoside transporters (CNTs) that function as Na+/nucleoside cotransporters. In some tissues, extracellular adenosine is readily converted to inosine via extracellular versions of the adenosine deaminase enzymes. Accumulation of extracellular guanosine acts to sustain higher extracellular levels of adenosine by competition for these or other mechanisms for adenosine clearance. Thus, guanosine may potentiate protective purinergic receptor signaling via adenosine receptors despite its lack of direct agonistic on these receptors. GPCR, G protein-coupled receptor.
Jackson et al. tested whether the levels of extracellular adenosine (added exogenously or generated endogenously) in cultures of rat preglomerular vascular smooth muscle cells (VSMCs) are modulated by the additional presence of either guanosine or guanosine nucleotides. Although high micromolar (10–30 μM) guanosine did not increase accumulation of extracellular adenosine generated from endogenous VSMC sources under basal conditions, the presence of guanosine markedly suppressed the rate at which exogenously added adenosine (in the micromolar range) was cleared. The result was that the extracellular adenosine was sustained at concentrations sufficient to activate adenosine receptors for considerably longer durations in the presence than in the absence of guanosine. When iodoacetate (IAA) treatment was used to induce metabolic stress and injury, the VSMCs generated increased extracellular levels of both adenosine and guanosine from endogenous sources, presumably by ecto-nucleotidases acting on released ATP and GTP. Notably, when the clearance of endogenously generated extracellular guanosine was accelerated by supplementing the culture medium with purine nucleoside phosphorylase (which metabolizes guanosine but not adenosine), the clearance of endogenously generated extracellular adenosine was also accelerated. Exogenous extracellular uridine, thymidine, or cytidine also retarded the rate of adenosine clearance but with reduced efficacies relative to that of guanosine. Moreover, only minor extracellular accumulation of these alternative “competing” nucleosides from endogenous pools was observed in the conditioned media from IAA-treated cells. Thus, based on these observations and data from other experiments, Jackson et al. propose that extracellular guanosine—perhaps uniquely among nonadenosine nucleosides—may indirectly potentiate purinergic signaling via adenosine receptors in metabolically stressed or injured tissues by attenuating the rate and extent of extracellular adenosine clearance (1). Although most experiments utilized the rat preglomerular VSMC model, similar effects of guanosine on extracellular adenosine handling were observed in other rodent and human cell types.
What are the likely mechanisms that underlie this heretofore unappreciated action of extracellular guanosine? Jackson et al. used pharmacological approaches to test whether guanosine might compete with adenosine for either the metabolic enzymes (adenosine deaminase, adenosine kinase, SAH) or the nucleoside reuptake transporters (CNTs and ENTs) that participate (directly or indirectly) in the regulation of extracellular adenosine homeostasis (1). However, none of these “players” appeared to be the target of guanosine. As noted by the authors, the ability of the pyrimidine nucleosides to mimic this action of guanosine (albeit with reduced efficacy) suggests that a nucleoside transporter rather than an adenosine-metabolizing enzyme is the more likely mediator. The inability of Na+-deficient medium to alter the action of guanosine effectively ruled out participation of the three CNT-family members. Jackson et al. argue against likely roles for one of more of the four ENT-family members based on pharmacology (insensitivity of the guanosine action to drugs that potently block ENT 1 or ENT2 transport function), subcellular localization (ENT3 predominantly traffics to lysosomes rather than the plasma membrane), or specific allosteric requirements (ENT4 function is highly dependent on an acidic extracellular pH) (1, 4). Unequivocal assessment of these ENTs will require genetic approaches and more extensive pharmacological/cell biological characterizations. However, if more stringent experiments also negate roles for the molecularly identified ENTs, this would suggest the existence of a novel nucleoside transporter(s). This is a highly salient issue given the critical roles for such transporters in the uptake of not only physiological nucleosides but also therapeutically relevant nucleoside analogs.
Microdialysis analyses in several types of metabolically stressed tissues have indicated that interstitial concentrations of both guanosine- and adenosine-containing metabolites can rise to micromolar levels (5–7, 9). Localized adenosine receptor stimulation can drive both antiinflammatory signaling and protective ischemic preconditioning responses (3). Thus, this newly identified competitive “duel” between extracellular guanosine and adenosine for nucleoside clearance mechanisms may be highly relevant to understanding and manipulating these protective actions of purinergic signaling in ischemic diseases and tissue trauma.
GRANTS
This work was supported in part by National Institutes of Health Grant R01-GM36387.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
G.R.D. drafted the manuscript; edited and revised the manuscript; approved the final version of the manuscript.
REFERENCES
- 1. Jackson EK, Cheng D, Jackson TC, Verrier JD, Gillespie DG. Extracellular guanosine regulates extracellular adenosine levels. Am J Physiol Cell Physiol (December 12, 2012). doi:10.1152/ajpcell.00212.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol 64: 785–795, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol 41: 775–787, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Loffler M, Morote-Garcia JC, Eltzschig SA, Coe IR, Eltzschig HK. Physiological roles of vascular nucleoside transporters. Arterioscler Thromb Vasc Biol 27: 1004–1013, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Melani A, Turchi D, Vannucchi MG, Cipriani S, Gianfriddo M, Pedata F. ATP extracellular concentrations are increased in the rat striatum during in vivo ischemia. Neurochem Int 47: 442–448, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Ninomiya H, Otani H, Lu K, Uchiyama T, Kido M, Imamura H. Complementary role of extracellular ATP and adenosine in ischemic preconditioning in the rat heart. Am J Physiol Heart Circ Physiol 282: H1810–H1820, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Nishiyama A, Majid DS, Taher KA, Miyatake A, Navar LG. Relation between renal interstitial ATP concentrations and autoregulation-mediated changes in renal vascular resistance. Circ Res 86: 656–662, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492, 1998 [PubMed] [Google Scholar]
- 9. Valen G, Owall A, Takeshima S, Goiny M, Ungerstedt U, Vaage J. Metabolic changes induced by ischemia and cardioplegia: a study employing cardiac microdialysis in pigs. Eur J Cardiothorac Surg 25: 69–75, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol 362: 299–309, 2000 [DOI] [PubMed] [Google Scholar]