Abstract
The interface between bone tissue and metal implants undergoes various types of mechanical loading, such as strain, compression, fluid pressure, and shear stress, from daily activities. Such mechanical perturbations create suboptimal environments at the host bone-implant junction, causing an accumulation of wear particles and debilitating osseous integration, potentially leading to implant failure. While many studies have focused on the effect of particles on macrophages or osteoprogenitor cells, differential and combined effects of mechanical perturbations and particles on such cell types have not been extensively studied. In this study, macrophages and osteoprogenitor cells were subjected to physiological and superphysiological mechanical stimuli in the presence and absence of Ti particles with the aim of simulating various microenvironments of the host bone-implant junction. Macrophages and osteoprogenitor cells were capable of engulfing Ti particles through actin remodeling and also exhibited changes in mRNA levels of proinflammatory cytokines under certain conditions. In osteoprogenitor cells, superphysiological strain increased proinflammatory gene expression; in macrophages, such mechanical perturbations did not affect gene expression. We confirmed that this phenomenon in osteoprogenitor cells occurred via activation of the ERK1/2 signaling pathway as a result of damage to the cytoplasmic membrane. Furthermore, AZD6244, a clinically relevant inhibitor of the ERK1/2 pathway, mitigated particle-induced inflammatory gene expression in osteoprogenitor cells and macrophages. This study provides evidence of more inflammatory responses under mechanical strains in osteoprogenitor cells than macrophages. Phagocytosis of particles and mechanical perturbation costimulate the ERK1/2 pathway, leading to expression of proinflammatory genes.
Keywords: ERK, actin, strain, AZD6244, particle, osteolysis
joint arthroplasty is an effective treatment for end-stage osteoarthritis. However, the long-term outcome of joint arthroplasties is limited by implant instability and inflammatory osteolysis (15, 18, 30). As shown in numerous clinical studies, bone implants generate a large amount of wear debris, which alters the mechanical environment of the bone-implant interface and initiates inflammatory osteolysis, potentially through phagocytosis (8, 16, 23, 34).
Wear particle-induced inflammatory osteolysis is heavily influenced by macrophages and osteoprogenitor cells. Macrophages are among the main phagocytic cells that engulf wear debris and secrete inflammatory cytokines such as IL-1β, TNFα, and IL-6 (5, 17, 24, 32). These secreted cytokines significantly affect cell behavior, leading to osteoclastogenesis. Additionally, osteoprogenitor cells/osteoblasts regulate osteoclastogenesis by secreting receptor activator of NF-κB ligand, osteoprotegerin, and macrophage colony-stimulating factor (M-CSF) (20, 38). In our previous study, we noted the innate immune function of osteoprogenitor cells and revealed the potential synergistic effects between these cells and macrophages in inflammatory osteolysis (21).
MAPK pathways are signaling cascades that have pivotal roles in the modulation of cell processes such as cell differentiation and apoptosis. In particular, ERK, a serine/threonine-specific end effector of the MAPK pathway, regulates transcription factors that are involved in cell proliferation and survival (9). In the context of inflammatory osteolysis, previous studies have shown that ERK signaling leads to expression of proinflammatory cytokines, further augmenting the inflammatory response (21, 31).
Bone tissue is exposed to many different types of mechanical loading, such as strain, compression, and shear stress, from daily activities (4, 36). In response to such mechanical perturbations, bone tissue undergoes remodeling through a feedback mechanism (29). Alteration of mechanical stimuli significantly affects osseous integration between host bone and implant, potentially leading to implant failure (6, 14, 22, 35). Although bone cells at the implant junction are exposed to wear particles and mechanical loading at the same time simultaneously (26, 37), the effect of simultaneous exposure to these factors has not been extensively studied. To help fill this gap of knowledge, the inflammatory responses of macrophage- and osteoblast-like cells to concurrent stimuli of wear particles and mechanical perturbation were studied with the long-term goal of enhancing osseous integration.
The objectives of this study were 1) to investigate the effects of mechanical strain, wear particles, and the combination of mechanical strain and wear particles on macrophage- and osteoblast-like cells and 2) to elucidate the intracellular mechanism leading to inflammation. To achieve our objectives, cyclic equibiaxial strain and Ti particles were utilized for this study.
MATERIALS AND METHODS
Study design.
The intracellular mechanism of osteoprogenitor cells and macrophages in the inflammatory cascade was investigated in MC3T3-E1 and RAW 264.7 cells, primary calvarial osteoblasts, and bone marrow-derived macrophages (BMMs). The key inflammatory signaling pathway was investigated using real-time RT-PCR and Western blotting. We used customized immunostaining analysis to examine the effects of equibiaxial strain on phagocytic capacity and cell damage.
Primary cell isolation and cell culture.
Cells from the murine preosteoblastic cell line MC3T3-E1 were purchased from American Type Culture Collection (Manassas, VA) and maintained in MEMα (Invitrogen) supplemented with 10% FBS (Gemini Bio-Products, Woodland, CA) and 1% antibiotic/antimycotic (Gemini BIO-Products) at 37°C and 5% CO2. Murine macrophage-like RAW 264.7 cells were purchased from American Type Culture Collection and maintained in DMEM (American Type Culture Collection) supplemented with 10% FBS (Gemini Bio-Products) and 1% antibiotic/antimycotic (Gemini Bio-Products).
Six-week-old male C57BL/6J mice were purchased from Jackson Laboratory for primary osteoblast and BMM isolation. Primary osteoblasts were isolated from calvaria of 6-wk-old C57BL/6J mice by enzymatic digestion (2). After removal of the suture, calvaria were chopped into small (∼1–2 mm2) fragments. Bone pieces were incubated in collagenase II-trypsin solution for 2 h. The bone pieces were washed with PBS and placed in 10-cm cell culture plates with DMEM supplemented with 10% FBS (GIBCO, Grand Island, NY), antibiotics (1.25 g/ml amphotericin B, 100 U/ml penicillin G, and 100 μg/ml streptomycin), 10 mM β-glycerophosphate (Sigma), and 100 μg/ml l-ascorbic acid (Sigma). The culture plates were kept at 37°C and 5% CO2. Cells are incubated with differentiation medium for 14 days. Differentiated primary calvarial osteoblasts expressed a high level of alkaline phosphatase (2).
BMMs were harvested from long bone of C57BL/6J mice, as described elsewhere (25). Briefly, the nonadherent fraction of mouse bone marrow cells was cultured in a 10-cm culture dish with MEMα (Invitrogen) supplemented with 10% FBS (Gemini Bio-Products), 1% antibiotic/antimycotic (Gemini Bio-Products), and 10 ng/ml recombinant mouse M-CSF (R & D). The resulting adherent purified BMMs were collected and subsequently cultured in serum-free MEMα supplemented with M-CSF.
AZD6244 (ARRY-142886, AZD), a potent, selective, and ATP-uncompetitive inhibitor of MAPK/ERK kinase-1/2, was purchased from Selleck Chemicals (Houston, TX). AZD was used to treat cells for 1 h prior to the particle treatment, which consisted of 3 h of direct stimulation of cells with Ti particles.
All procedures and animals use were approved by the Institutional Animal Care and Use Committee.
Ti particles.
Commercially available pure Ti particles (0.3–1 μm average diameter) were purchased from Johnson Matthey Chemicals (Ward Hill, MA), as previously described by Ragab et al. (28). To remove endotoxins from particles, 10 cycles of the following two steps were performed: 1) Ti particles were incubated in 25% nitric acid at room temperature for 20 h and then washed three times with PBS, and 2) Ti particles were treated in 0.1 N NaOH-95% ethanol at 30°C for 18–20 h and then washed five times with PBS between cycles. The endotoxin level was determined using a Limulus amebocyte lysate kit (i.e., <0.01 endotoxin units/ml). Prior to use, particles were sonicated for 15 min to avoid particle aggregation. Cells were directly stimulated with 0.05% (vol/vol) Ti particles for 3 h. To flush unattached particles, the plates were rinsed 10 times with PBS and then inverted for 10 min to allow any residue and unattached particles to be removed by gravity.
Mechanical strain.
Cyclical equibiaxial tensile strain was applied to cells, which were cultured on six-well plates with a silicone elastomeric membrane (Flexcell, Hillsborough, NC). Cells were exposed to 5,000 and 30,000 microstrain (με) at 1 Hz for various durations (model FX4000T, Flexcell). Equibiaxial strain of 5,000 and 30,000 με was selected to represent normal physiological [physiological strain (PS)] and superphysiological [superphysiological strain (SPS)] mechanical loading, respectively; these strain levels were previously observed at the periprosthetic interface between host bone and implant (3, 10).
RNA isolation and real-time RT-PCR.
Immediately after the stimulation, total RNA was isolated from cells using the RNeasy Mini Kit (Qiagen). Purity and integrity of RNA were verified by the ratio of absorbance at 260 nm to absorbance at 280 nm and ethidium bromide agarose gel electrophoresis. Single-stranded cDNA was synthesized from the total RNA with the SuperScript III system (Invitrogen). Real-time RT-PCR for each target was performed using LightCycler FastStart DNA MasterPLUS SYBR Green I (Roche) and the MasterCycler realplex system (Eppendorf). Primers sets were as follows: 5′-AGAACATCATCCCTGCATCC-3′ and 5′-AGTTGCTGTTGAAGTCGC-3′ for mouse GAPDH, 5′-AAAGCCAGAGTCCTTCAGAG-3′ and 5′-CTAGGTTTGCCGAGTAGATC-3′ for mouse IL-6, 5′-ACATCGATGTCATGGAACTG-3′ and 5′-GGACACCCCTTCACATTATT-3′ for mouse cyclooxygenase 2 (Cox2), 5′-CCTGTGTAATGAAAGACGGC-3′ and 5′-GGAAGACACAGATTCCATGG-3′ for mouse IL-1β, 5′-GTTCTCTTCAAGGGACAAGG-3′ and 5′-TGACTCCAAAGTAGACCTGC-3′ for mouse TNFα, and 5′-AAGAGAAGTACCAGGGATCG-3′ and 5′-TCCAATGTCTGAGGGTTTCG-3′ for mouse M-CSF. Thermal cycling conditions consisted of preheating (10 min at 95°C), 40 cycles of denaturation (15 s at 95°C), annealing (15 s at 60°C), and elongation (20 s at 72°C). For each sample, mRNA levels of each gene were normalized to GAPDH levels.
Protein isolation and Western blot analysis.
The Nuclear Extract Kit (Active Motif, Carlsbad, CA) was used to isolate nuclear and cytoplasmic extracts. These extracts were further homogenized by sonication with 15 strokes at a 10% duty cycle and 4°C (Sonifier 250, Branson, Danbury, CT). The samples were centrifuged at 10,000 g for 10 min, and the supernatant was collected for analysis. Protein was quantified using the BCA Protein Assay (Pierce, Rockford, IL), and 20 μg of protein extract were used for Western blotting. Samples were run in 4–20% SDS-polyacrylamide gels (Invitrogen) and electrotransferred to polyvinylidene difluoride membranes, which were blocked and probed with primary antibodies overnight at 4°C. Excess antibodies were washed off the blots with Tris-buffered saline containing 0.1% Tween 20 and with 10% FBS. Blots were further probed with a horseradish peroxidase-conjugated secondary antibody (Cell Signaling Technologies, Beverly, MA) and detected using enhanced chemiluminescence reagents (Amersham Pharmacia Biotech, Piscataway, NJ). Membranes were stripped with Restore Western blot stripping buffer (Pierce) and reprobed with GAPDH (Chemicon, Temecula, CA) antibody as loading controls.
Confocal microscopic imaging.
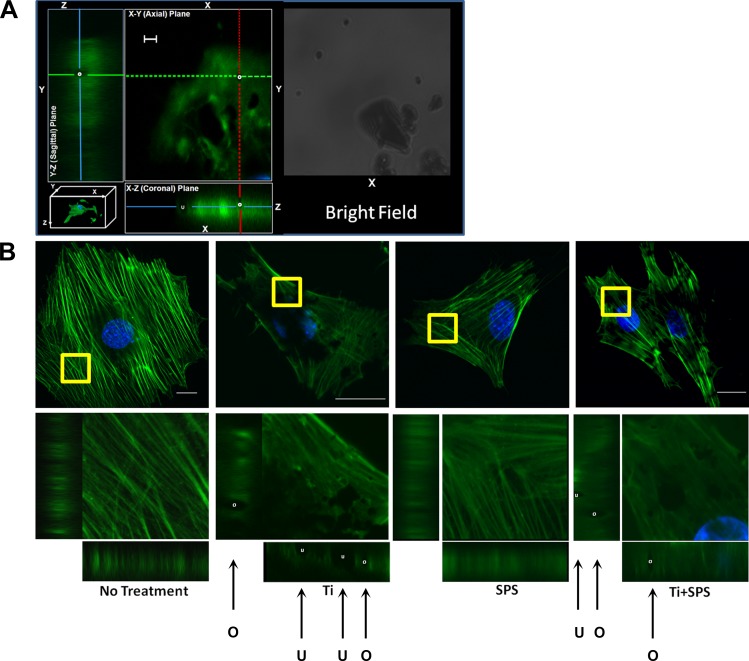
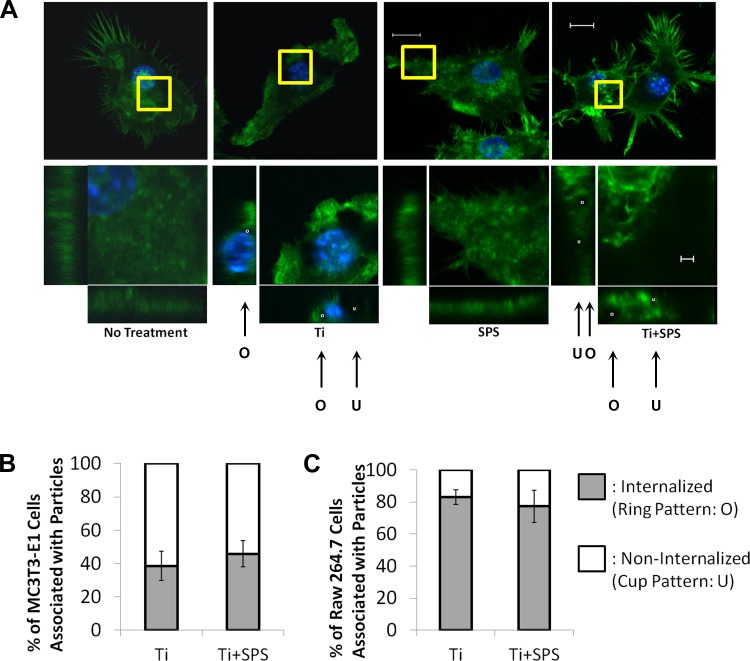
After Ti treatment and strain stimulation, three-dimensional images of green fluorescent protein-positive actin-stained cells were obtained with a confocal microscope (axial slice thickness of 0.22 μm, reformatted in x, y, and z dimensions). To study actin remodeling, actin filaments in a selected area were magnified and reformatted into x-y (axial), x-z (coronal), and y-z (sagittal) planes. Three-dimensional imaging was used to distinguish among completely phagocytosed (internalized), partially phagocytosed, and membrane-bound particles. While the internalized particles are fully covered by actin filaments in all views (ring pattern: O), partially phagocytosed particles are hemispheres of actin filaments in the sagittal and coronal view (see Fig. 4, cup pattern: U). For quantitative analysis of phagocytosis, we selected 100 cells that exhibited Ti attachment and counted the number of cells with internalized particles.
Fig. 4.
Actin remodeling in MC3T3-E1 cells after Ti treatment with and without mechanical strain. A: actin dynamics, including actin ring (O) and cup (U) formations, identified through actin structure in axial, coronal, and sagittal planes and bright field. B: orientation of actin filaments in MC3T3 cells in no-treatment, Ti, SPS, and Ti + SPS groups. Actin filaments of selected area (yellow square box) were magnified and projected into x-y, x-z, and y-z planes to visualize actin remodeling. While the no-treatment group shows actin fibers in alignment, active actin ring (white circle) and cup (white cup) formations were detected in Ti group. In the presence of SPS, MC3T3-E1 cells maintain their actin fiber alignment and active actin ring and cup formations. Scale bars: 10 μm (nonmagnified images) and 1 μm (magnified images).
Detection of cell membrane damage and death.
Membrane damage was evaluated by Sytox green assay. After Ti treatment, 1 μM Sytox green was added to each well of the six-well plate for 1 h with equibiaxial strain. All cells were visualized using the Axiovert imaging system (Zeiss, Thornwood, NY). For quantitative analysis, we randomly selected 100 cells in bright field and counted the number of nucleus-stained cells under a fluorescent microscope. Cell death by wear particles and mechanical perturbation was examined through 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay (Invitrogen). A MTT assay kit was purchased from Invitrogen and utilized according to the manufacturer's instruction. After exposure to Ti and strain, cells were incubated for 4 h at 37°C with 50 μg/ml MTT. SDS-HCl solution was added to the wells. After 4 h of incubation, MTT absorbance was read at 570 nm.
Statistical analysis.
A one-way ANOVA was used to test for the effects of Ti, mechanical strain, AZD, and a combination of these stimuli on phagocytic activity and inflammatory gene expression. The significance for each of the factors was checked by Student-Newman-Keuls multiple (post hoc) comparisons tests. Statistical significance was taken as P < 0.05.
RESULTS
Superphysiological level of cyclic equibiaxial strain exaggerates wear particle-mediated inflammatory gene expression in osteoprogenitor cells.
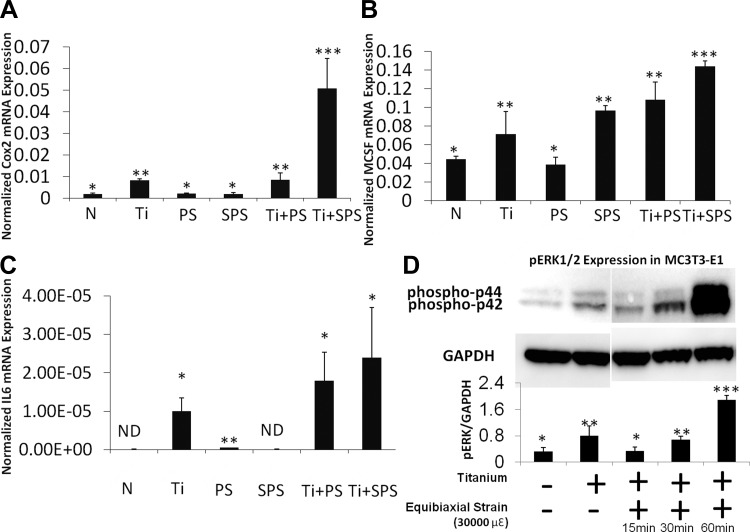
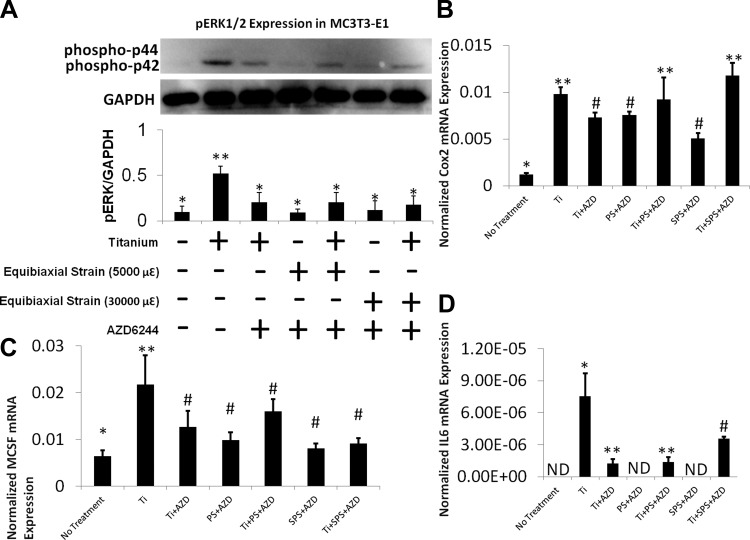
To investigate the effect of equibiaxial strain- on wear particle-induced osteolysis, we used real-time RT-PCR and Western blot analysis to assess the relative mRNA expression of inflammatory and osteoclastogenic genes and phosphorylated ERK (pERK) expression in osteoprogenitor cells. mRNA expression of the inflammatory genes IL-6, Cox2, IL-1β, and TNFα was examined. Ti stimulation of MC3T3-E1 cells significantly increased IL-6 and Cox2 mRNA levels (Fig. 1), while TNFα and IL-1β mRNA levels remained unaffected by Ti (data not shown). Exposure to PS or SPS did not statistically change the level of IL-6 mRNA expression in MC3T3-E1 cells. However, exposure to SPS after Ti treatment resulted in a synergistic increase in IL-6 mRNA expression (Fig. 1C). Similar to IL-6 mRNA expression, Cox2 mRNA expression increased in MC3T3-E1 cells exposed to Ti + PS and Ti + SPS. Furthermore, mRNA expression of M-CSF, an osteoclastogenic gene, was examined. The level of M-CSF mRNA expression significantly increased in the Ti and SPS groups (Fig. 1B). Additional increases were also seen in the Ti + PS and Ti + SPS groups (Fig. 1B). Corresponding to mRNA expression, the level of pERK increased after 1 h of SPS subsequent to Ti exposure (Fig. 1C).
Fig. 1.
Superphysiological strain (SPS) synergistically induces inflammatory gene expression with wear particles in MC3T3-E1 cells. A–C: relative gene expression of cyclooxygenase 2 (Cox2), macrophage colony-stimulating factor (M-CSF), and IL-6 in MC3T3-E1 cells. No treatment (N), Ti particles, physiological strain (PS), superphysiological strain (SPS), Ti + PS, and Ti + SPS groups were compared. Gene expression assay is normalized by GAPDH. Each symbol (*, **, ***) denotes significant difference (P < 0.05); n = 6 per group. ND, not detectable. D: immunoblot of MC3T3-E1 cells showing level of ERK1/2 phosphorylation [phosphorylated p44 and p42 (phospho-p44 and phospho-p42)] after Ti stimulation with and without mechanical strain (30,000 με). Each symbol denotes significant difference (P < 0.05); n = 3 per group.
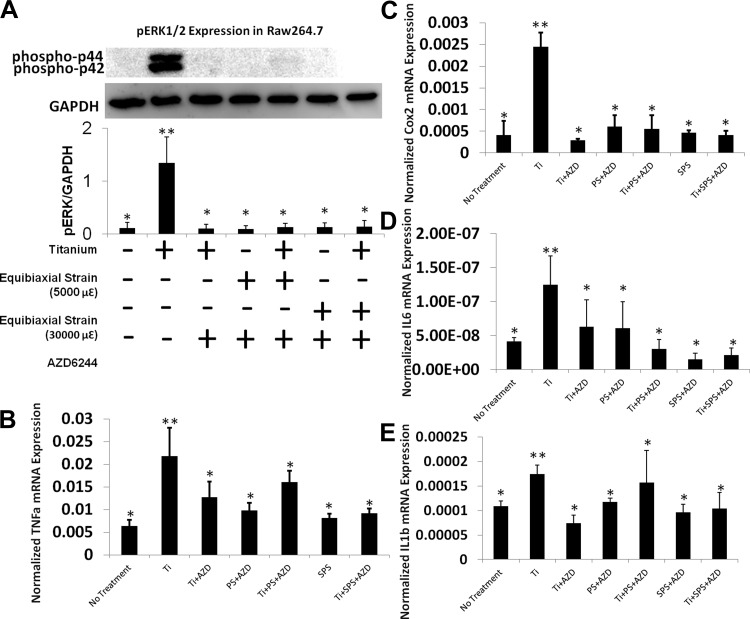
Particle-mediated inflammatory gene expression in macrophages is not affected by mechanical perturbation.
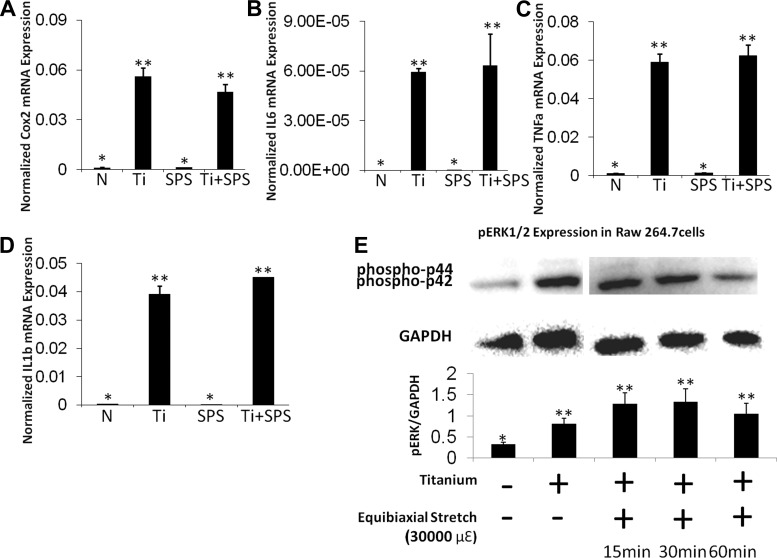
Since macrophages play a crucial role in wear particle-mediated osteolysis, we examined the effects of mechanical perturbation on particle-mediated inflammatory gene expression in macrophages. Expression of all inflammatory genes, including IL-6, Cox2, TNFα, and IL-1β, increased after Ti treatment (Fig. 2, A–D). Unlike the osteoprogenitor cells, expression levels of these inflammatory genes were not amplified by SPS in macrophages. Furthermore, no change was observed in Ti-induced inflammatory gene expression levels between PS- and SPS-treated groups. Similarly, a distinct difference in the level of pERK between the Ti group and the Ti + SPS group was not detected by Western blot analysis (Fig. 2E).
Fig. 2.
Mechanical strain does not induce significant changes in inflammatory responses in RAW 264.7 cells. A–D: relative gene expression of Cox2, IL-6, TNFα, and IL-1β in RAW 264.7 cells. Gene expression assay is normalized by GAPDH. Each symbol denotes significant difference (P < 0.05); n = 6 per group. E: immunoblot of RAW 264.7 cells demonstrating level of ERK1/2 phosphorylation (phospho-p44 and phospho-p42) after Ti stimulation with and without mechanical strain (30,000 με). Each symbol denotes significant difference (P < 0.05); n = 3 per group.
Inflammatory responses of primary osteoblasts and BMMs to wear particle and mechanical perturbation.
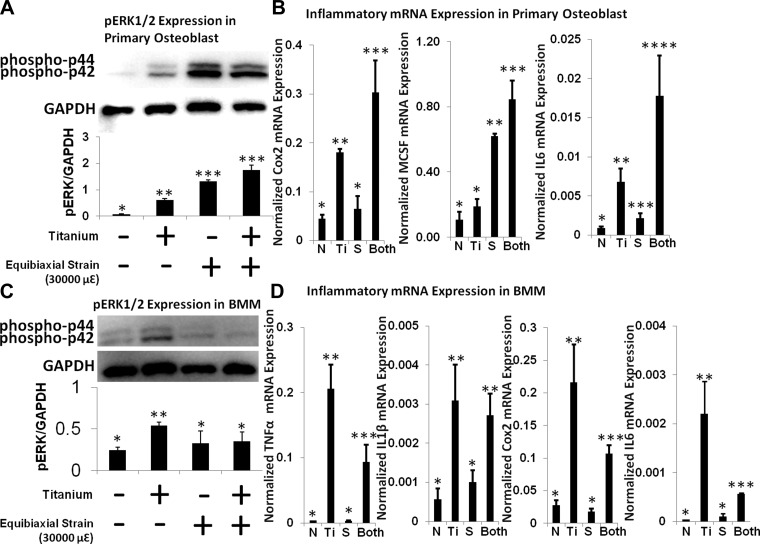
Inflammatory responses of primary calvarial osteoblasts and BMMs to Ti particles and SPS were investigated and compared with responses of MC3T3-E1 and RAW 264.7 cells (Fig. 3). Similar to progenitor cells, inflammatory gene (IL-6, Cox2, M-CSF, IL-1β, and TNFα), as well as pERK, expression was increased in Ti-treated primary calvarial osteoblasts and BMMs. SPS increased pERK protein expression, as well as IL-6, Cox2, and M-CSF gene expression, in primary calvarial osteoblasts, and Ti + SPS further increased gene expression levels. BMMs were less sensitive to SPS and exhibited no additional inflammatory responses under combined stimulation.
Fig. 3.
Inflammatory responses of primary calvarial osteoblasts and bone marrow-derived macrophages (BMMs) to Ti, SPS, and Ti + SPS. A and C: immunoblots of primary calvarial osteoblasts and BMMs showing level of ERK1/2 phosphorylation (phospho-p44 and phospho-p42) after Ti stimulation with and without mechanical strain (30,000 με). Each symbol denotes significant difference (P < 0.05); n = 3 per group. B and D: relative gene expression of Cox2, IL-6, M-CSF, TNFα, and IL-1β in primary calvarial osteoblasts and BMMs. Gene expression assay is normalized by GAPDH. Each symbol denotes significant difference (P < 0.05); n = 6 per group.
Effects of mechanical perturbation on wear particle-induced actin remodeling.
The effect of equibiaxial strain on Ti particle-induced actin remodeling was investigated using confocal microscopy. In our previous study, we used a novel method to quantify actin-mediated phagocytosis (21). Completely internalized particles were identified by their actin ring appearance (O in Fig. 4A), and membrane-bound particles were identified by actin cup appearance (U in Fig. 4A). Using this method, we detected actin cup and ring formation in Ti-treated MC3T3-E1 and RAW 264.7 cells (Figs. 4 and 5). In the no-treatment group, actin fibers in MC3T3-E1 cells were highly organized in a uniform alignment, while alignment of actin fibers was less uniform in RAW 264.7 cells (Figs. 4 and 5). There were no visible changes in the actin structure of cells treated with SPS. Cells exposed to SPS exhibited no clear changes in their actin structure. However, MC3T3-E1 cells exposed to strain and particles exhibited disorganized actin filaments with actin cup and ring formation. Ti particles were internalized in ∼40% of the cells that exhibited Ti attachment in the Ti and Ti + SPS groups (Fig. 5B). Although they showed less organized baseline actin fiber alignment, RAW 264.7 cells exposed to Ti and Ti + SPS showed similar trends of actin stress. These particles were internalized in ∼80% of the cells that showed Ti attachment in the Ti and Ti + SPS groups (Fig. 5, B and C).
Fig. 5.
Actin remodeling in RAW 264.7 cells after Ti treatment with and without mechanical strain. A: actin stress fibers are less organized in RAW 264.7 than MC3T3-E1 cells. Aggressive actin remodeling, such as active actin ring (white circle) and cup (white cup) formations, are detected in the Ti group. SPS does not affect actin structure and remodeling for phagocytosis of wear particles. B and C: quantification of cells associated with Ti particles. Quantification of internalized Ti particles (actin ring counts) shows no significant difference between Ti and Ti + SPS groups in MC3T3-E1 and RAW 264.7 cells. In each group, n = 100.
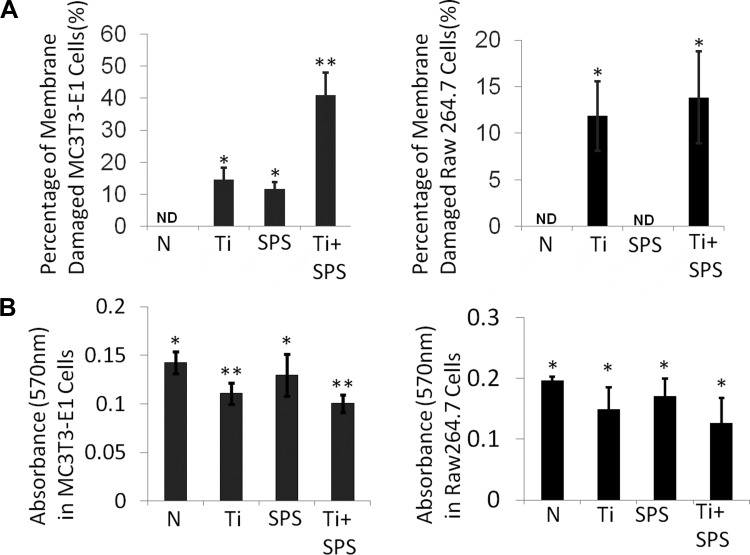
Membrane damage by wear particles and cyclic equibiaxial strain.
Membrane damage in MC3T3-E1 and RAW 264.7 cells was assessed by Sytox green assay. About 15% of MC3T3-E1 and RAW 264.7 cells had membrane damage after Ti treatment. Unlike RAW 264.7 cells, which were not affected by SPS, ∼10% of MC3T3-E1 cells were damaged by SPS. In MC3T3-E1 cells, Ti + SPS increased the percentage of membrane-damaged cells to 40%. However, no significant changes were detected in RAW 264.7 cells treated with Ti + SPS (Fig. 6A). MTT assay of cell viability showed a decreased absorbance level in the Ti group compared with the no-treatment group in MC3T3-E1 and RAW 264.7 cells. Unlike the Sytox green assay, MTT assay of the Ti + SPS group revealed an absorbance level similar to that of the Ti group.
Fig. 6.
Wear particles and strain cause different levels of cell membrane damage in MC3T3-E1 and RAW 264.7 cells. A: Sytox green assay of membrane damage in no-treatment (N), Ti, SPS, and Ti + SPS groups. In MC3T3-E1 cells, membrane damage in the Ti + SPS group was increased compared with control and Ti groups. In RAW 264.7 cells, number of membrane-damaged cells was similar in Ti + SPS and Ti groups. B: in MC3T3-E1 and RAW 264.7 cells, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay shows similar level of metabolic activities in Ti and Ti + SPS groups. Each symbol denotes significant difference (P < 0.05); n = 5.
Wear particle and mechanical perturbation synergistically increase inflammation through the ERK pathway in osteoprogenitor cells.
To further elucidate the role of ERK in inflammatory osteolysis, the effect of wear particle treatment and mechanical perturbation on MC3T3-E1 and RAW 264.7 cells was examined in the presence of AZD. Cells treated with AZD exhibited a selective and drastic inhibition of ERK phosphorylation, which was induced by Ti particles in MC3T3-E1 and RAW 264.7 cells (Western blot analysis). The level of pERK expression correlated well with Cox2, M-CSF, and IL-6 expression in MC3T3-E1 cells. Real-time RT-PCR data verified that Ti particles elevated IL-6, M-CSF, Cox2, IL-1β, and TNFα mRNA expression, while AZD pretreatment dramatically decreased the levels of expression of these inflammatory genes in MC3T3-E1 and RAW 264.7 cells (Figs. 7 and 8). In MC3T3-E1 and RAW 264.7 cells, there was no synergistic increase in these inflammatory markers after AZD treatment in both treated groups.
Fig. 7.
ERK pathway regulates inflammatory responses of MC3T3-E1 cells to wear particles and mechanical strain. A: immunoblot showing selective inhibition of phosphorylated ERK 1/2 (pERK1/2) by AZD6244 (AZD) in MC3T3-E1 cells. AZD blocks phosphorylation of ERK, which is induced by Ti and mechanical strain. Each symbol denotes significant difference (P < 0.05); n = 3 per group. B–D: relative gene expressions of Cox2, M-CSF, and IL-6 in MC3T3-E1 cells after AZD pretreatment. Gene expression assay is normalized by GAPDH. Each symbol (*, **, #, ##) denotes significant difference (P < 0.05); n = 6 per group.
Fig. 8.
Role of the ERK pathway as an inflammatory cascade in RAW 264.7 cells. A: immunoblot showing selective inhibition of pERK1/2 by AZD in RAW 274.7 cells. Each symbol denotes significant difference (P < 0.05); n = 3. B–E: relative gene expression of Cox2, IL-6, TNFα, and IL-1β after AZD pretreatment in RAW 264.7 cells with no treatment, Ti, Ti + AZD, PS + AZD, PS + Ti + AZD, SPS + AZD, or SPS + Ti + AZD. Gene expression assays were normalized by GAPDH. Each symbol denotes significant difference (P < 0.05); n = 6 per group.
DISCUSSION
Osteolysis occurs in clinical scenarios where nonphysiological conditions, such as foreign material perturbations, loading, and atypical hormone releases, interfere with the balance between bone formation and resorption (18). Patients with long-term prognoses of joint arthroplasties suffer from osteolysis due to the joint's exposure to routine mechanical loading and wear particle accumulation (4, 15, 30, 36). While several studies have investigated the individual effect of wear particle or mechanical perturbation on cell behavior, the combined effects of these stimuli were not well studied. In this study, we examined the effects of mechanical strain, Ti particles, and strain + Ti on inflammatory osteolysis. We explored the intracellular inflammatory mechanisms in MC3T3-E1 cells, primary calvarial osteoblasts, BMMs, and RAW 264.7 cells. These cells were selected for this study, since osteoprogenitor cells, osteoblasts, and macrophages are largely responsible for bone remodeling.
Ti-induced inflammatory gene expression was synergistically exaggerated by SPS in MC3T3-E1 cells. However, expression of these genes was not elevated in the Ti + SPS group of RAW 264.7 cells. To investigate inflammatory responses, cells were exposed to Ti particles and equibiaxial strain. As described in other studies, <5,000-με equibiaxial strain was considered PS, and 5,000- to 30,000-με strain was considered SPS (3, 10). Because <5,000-με strain causes instability in loading levels, we defined 5,000-με strain as the minimum PS level. Several studies have indicated that Ti and mechanical loading induce inflammatory gene expression (1, 7, 12, 13, 22). Similar to these findings, we observed increased levels of Cox2, M-CSF, and IL-6 gene expression in MC3T3-E1 cells after Ti treatment. In RAW 264.7 cells, the gene expression of IL-1β and TNFα, as well as Cox2 and IL-6, increased. Cells were exposed to 0.05% (vol/vol) Ti for 3 h, because our dose and time-course experiments revealed that proinflammatory gene expression was maximal under this condition. Our previous studies showed that the ERK pathway is a key inflammatory cascade in the osteoinflammatory response (21, 31). The pERK protein expression analysis conducted in this study reflected a trend similar to that of the inflammatory gene expression data.
To enhance the relevance of this study, we also tested primary calvarial osteoblasts and BMMs. Interestingly, inflammatory responses from primary cell lines were similar to those from MC3T3-E1 and RAW 264.7 cells. Ti increased inflammatory gene, as well as pERK, expression, in primary calvarial osteoblasts and BMMs. An increase in pERK and inflammatory gene expression was observed in SPS-treated primary calvarial osteoblasts. However, BMMs, which were costimulated with SPS and Ti, responded differently, with no additional inflammatory response compared with the aforementioned cells. Our results imply that osteoprogenitor cells/osteoblasts may play more significant roles than macrophage-like cells/macrophages in responding to mechanical strain and may induce synergistic increases in inflammatory responses through the ERK pathway. We previously reported that wear particles accelerate actin remodeling, which leads to activation of the ERK pathway (21). Thus we further investigated whether wear particle-induced actin remodeling is affected by equibiaxial strain. Contrary to our hypothesis, quantitative analysis of particle internalization showed that actin cup formation was not affected by PS or SPS.
Since wear particle internalization by actin remodeling was not affected by SPS, we further suspected that membrane damage caused by mechanical strain could be another source of increase in inflammatory gene and pERK expression. Membrane damage is known to elevate inflammatory gene expression through the ERK pathway (11, 33). Interestingly, SPS induced cellular membrane damage in MC3T3-E1 cells, while no membrane damage was observed in RAW 264.7 cells. For membrane damage analysis, we utilized the Sytox green assay. Since Sytox green stains the nucleus by entering the perforated membrane, membrane-damaged and dead cells could be detected using this system. To differentiate the true membrane-damaged cells from the dead cells, we performed the MTT assay, which measures the metabolic activity of cells. In MC3T3-E1 and RAW 264.7 cells, metabolic activities were decreased in the Ti and Ti + SPS groups compared with the no-treatment group. However, the Sytox green assay revealed increased cell membrane damage in MC3T3-E1 cells, which were treated with both stimuli. On the other hand, it only took Ti exposure to cause membrane damage similar to that in RAW 264.7 cells. This difference may be due to differences in actin alignment between MC3T3-E1 and RAW 264.7 cells. While the actin fibers in MC3T3-E1 cells were highly organized and aligned and appeared connected to both ends of the cell, the actin fibers in RAW 264.7 cells were less organized. Basal actin stress fibers are known to affect cell morphology by generating tensional forces. Cellular mechanical properties, such as stiffness and viscoelasticity, are regulated by membrane morphology established by actin stress fibers (19, 27, 39). Since actin fibers may need higher tension to maintain cell morphology in MC3T3-E1 than RAW 264.7 cells, they may be more susceptible to mechanical perturbation. We need to further investigate the various roles of actin in membrane damage caused by wear particles and mechanical perturbation.
Furthermore, we examined the role of the ERK pathway in the inflammatory response triggered by the combination of wear particle exposure and strain. AZD significantly decreased pERK expression in the Ti, SPS, and Ti + SPS groups. These low pERK expressions resulted in a significant decrease in inflammatory gene expression in MC3T3-E1 and RAW 264.7 cells. Consistent with previous studies, our data support the idea that the ERK pathway could be an important therapeutic target for preventing inflammatory osteolysis (21).
In summary, this study supports our hypothesis that 1) SPS synergistically increases inflammatory gene expression, which is induced by wear particles in osteoprogenitor cells/osteoblasts and 2) the ERK pathway is a key inflammatory cascade in MC3T3-E1 and RAW 264.7 cells. Our study is limited by the absence of other mechanical stimuli, such as compressive, bending, and torsional forces. Since cells are exposed to various mechanical perturbations in clinical scenarios, in-depth investigations of cellular responses to those mechanical stimuli are needed. However, we observed that osteoprogenitor cells are more susceptible to strain than are macrophages. Specifically, we highlighted the synergistic increase in pERK expression by actin remodeling by wear particles and membrane damage by mechanical strain, which consequently elevates inflammatory gene expression.
GRANTS
This work was supported by National Institutes of Health Research Grants R01 AR-056246 (F. Y. Lee) and R01 EB-006834 (F. Y. Lee).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.G.L., S.H.K., and F.Y.L. are responsible for conception and design of the research; H.G.L., A.H., H.G., and S.N. performed the experiments; H.G.L. and S.H.K. analyzed the data; H.G.L., J.H.L., E.R.C., P.T., C.C., S.H.K., D.S.O., H.A.T., and F.Y.L. interpreted the results of the experiments; H.G.L. prepared the figures; H.G.L. drafted the manuscript; H.G.L., A.H., H.G., S.N., R.S., and F.Y.L. edited and revised the manuscript; H.G.L., D.S.O., H.A.T., and F.Y.L. approved the final version of the manuscript.
REFERENCES
- 1. Akisue T, Bauer TW, Farver CF, Mochida Y. The effect of particle wear debris on NFκB activation and pro-inflammatory cytokine release in differentiated THP-1 cells. J Biomed Mater Res 59: 507–515, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Bakker AD, Klein-Nulend J. Osteoblast isolation from murine calvaria and long bones. Methods Mol Biol 816: 19–29, 2012 [DOI] [PubMed] [Google Scholar]
- 3. Basso N, Heersche JN. Characteristics of in vitro osteoblastic cell loading models. Bone 30: 347–351, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Bergmann G, Graichen F, Rohlmann A. Hip joint loading during walking and running, measured in two patients. J Biomech 26: 969–990, 1993 [DOI] [PubMed] [Google Scholar]
- 5. Bianco C, Griffin FM, Jr, Silverstein SC. Studies of the macrophage complement receptor. Alteration of receptor function upon macrophage activation. J Exp Med 141: 1278–1290, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bikle DD, Halloran BP, Morey-Holton E. Spaceflight and the skeleton: lessons for the earthbound. Endocrinologist 7: 10–22, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Bukata SV, Gelinas J, Wei X, Rosier RN, Puzas JE, Zhang X, Schwarz EM, Song XY, Griswold DE, O'Keefe RJ. PGE2 and IL-6 production by fibroblasts in response to titanium wear debris particles is mediated through a Cox-2 dependent pathway. J Orthop Res 22: 6–12, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Carlsson AS, Gentz CF, Lindberg HO. Thirty-two noninfected total hip arthroplasties revised due to stem loosening. Clin Orthop Relat Res 196–203, 1983 [PubMed] [Google Scholar]
- 9. Cobb MH. MAP kinase pathways. Prog Biophys Mol Biol 71: 479–500, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Decking R, Puhl W, Simon U, Claes LE. Changes in strain distribution of loaded proximal femora caused by different types of cementless femoral stems. Clin Biomech (Bristol, Avon) 21: 495–501, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Decraene D, Smaers K, Gan D, Mammone T, Matsui M, Maes D, Declercq L, Garmyn M. A synthetic superoxide dismutase/catalase mimetic (EUK-134) inhibits membrane-damage-induced activation of mitogen-activated protein kinase pathways and reduces p53 accumulation in ultraviolet B-exposed primary human keratinocytes. J Invest Dermatol 122: 484–491, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Duncan RL, Turner CH. Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int 57: 344–358, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Grottkau BE, Noordin S, Shortkroff S, Schaffer JL, Thornhill TS, Spector M. Effect of mechanical perturbation on the release of PGE2 by macrophages in vitro. J Biomed Mater Res 59: 288–293, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Haapasalo H, Kannus P, Sievanen H, Pasanen M, Uusi-Rasi K, Heinonen A, Oja P, Vuori I. Effect of long-term unilateral activity on bone mineral density of female junior tennis players. J Bone Miner Res 13: 310–319, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Harris WH. Wear and periprosthetic osteolysis: the problem. Clin Orthop Relat Res 66–70, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Harris WH, Schiller AL, Scholler JM, Freiberg RA, Scott R. Extensive localized bone resorption in the femur following total hip replacement. J Bone Joint Surg Am 58: 612–618, 1976 [PubMed] [Google Scholar]
- 17. Hukkanen M, Corbett SA, Batten J, Konttinen YT, McCarthy ID, Maclouf J, Santavirta S, Hughes SP, Polak JM. Aseptic loosening of total hip replacement. Macrophage expression of inducible nitric oxide synthase and cyclo-oxygenase-2, together with peroxynitrite formation, as a possible mechanism for early prosthesis failure. J Bone Joint Surg Br 79: 467–474, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Jacobs JJ, Roebuck KA, Archibeck M, Hallab NJ, Glant TT. Osteolysis: basic science. Clin Orthop Relat Res 71–77, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Kumar S, Maxwell IZ, Heisterkamp A, Polte TR, Lele TP, Salanga M, Mazur E, Ingber DE. Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys J 90: 3762–3773, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93: 165–176, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Lee HG, Minematsu H, Kim KO, Celil Aydemir AB, Shin MJ, Nizami SA, Chung KJ, Hsu AC, Jacobs CR, Lee FY. Actin and ERK1/2-CEBPβ signaling mediates phagocytosis-induced innate immune response of osteoprogenitor cells. Biomaterials 32: 9197–9206, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. MacQuarrie RA, Fang Chen Y, Coles C, Anderson GI. Wear-particle-induced osteoclast osteolysis: the role of particulates and mechanical strain. J Biomed Mater Res B Appl Biomater 69: 104–112, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Maloney WJ, Jasty M, Harris WH, Galante JO, Callaghan JJ. Endosteal erosion in association with stable uncemented femoral components. J Bone Joint Surg Am 72: 1025–1034, 1990 [PubMed] [Google Scholar]
- 24. Merkel KD, Erdmann JM, McHugh KP, Abu-Amer Y, Ross FP, Teitelbaum SL. Tumor necrosis factor-α mediates orthopedic implant osteolysis. Am J Pathol 154: 203–210, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Minematsu H, Shin MJ, Celil Aydemir AB, Seo SW, Kim DW, Blaine TA, Macian F, Yang J, Lee FY. Orthopedic implant particle-induced tumor necrosis factor-α production in macrophage-monocyte lineage cells is mediated by nuclear factor of activated T cells. Ann NY Acad Sci 1117: 143–150, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Okafor CC, Haleem-Smith H, Laqueriere P, Manner PA, Tuan RS. Particulate endocytosis mediates biological responses of human mesenchymal stem cells to titanium wear debris. J Orthop Res 24: 461–473, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Prasain N, Stevens T. The actin cytoskeleton in endothelial cell phenotypes. Microvasc Res 77: 53–63, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ragab AA, Van De Motter R, Lavish SA, Goldberg VM, Ninomiya JT, Carlin CR, Greenfield EM. Measurement and removal of adherent endotoxin from titanium particles and implant surfaces. J Orthop Res 17: 803–809, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Rubin CT, Lanyon LE. Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int 37: 411–417, 1985 [DOI] [PubMed] [Google Scholar]
- 30. Schmalzried TP, Callaghan JJ. Wear in total hip and knee replacements. J Bone Joint Surg Am 81: 115–136, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Seo SW, Lee D, Minematsu H, Kim AD, Shin M, Cho SK, Kim DW, Yang J, Lee FY. Targeting extracellular signal-regulated kinase (ERK) signaling has therapeutic implications for inflammatory osteolysis. Bone 46: 695–702, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shanbhag AS, Jacobs JJ, Black J, Galante JO, Glant TT. Human monocyte response to particulate biomaterials generated in vivo and in vitro. J Orthop Res 13: 792–801, 1995 [DOI] [PubMed] [Google Scholar]
- 33. Subramaniam S, Zirrgiebel U, von Bohlen und Halbach O, Strelau J, Laliberte C, Kaplan DR, Unsicker K. ERK activation promotes neuronal degeneration predominantly through plasma membrane damage and independently of caspase-3. J Cell Biol 165: 357–369, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tanzer M, Maloney WJ, Jasty M, Harris WH. The progression of femoral cortical osteolysis in association with total hip arthroplasty without cement. J Bone Joint Surg Am 74: 404–410, 1992 [PubMed] [Google Scholar]
- 35. Turner CH, Pavalko FM. Mechanotransduction and functional response of the skeleton to physical stress: the mechanisms and mechanics of bone adaptation. J Orthop Sci 3: 346–355, 1998 [DOI] [PubMed] [Google Scholar]
- 36. van den Bogert AJ, Read L, Nigg BM. An analysis of hip joint loading during walking, running, and skiing. Med Sci Sports Exerc 31: 131–142, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Wang ML, Tuli R, Manner PA, Sharkey PF, Hall DJ, Tuan RS. Direct and indirect induction of apoptosis in human mesenchymal stem cells in response to titanium particles. J Orthop Res 21: 697–707, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 95: 3597–3602, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton 60: 24–34, 2005 [DOI] [PubMed] [Google Scholar]