Abstract
While the term “fibrosis” can be misleading in terms of the complex patterns and processes of myocardial extracellular matrix (ECM) remodeling, fibrillar collagen accumulation is a common consequence of relevant pathophysiological stimuli, such as pressure overload (PO) and myocardial infarction (MI). Fibrillar collagen accumulation in both PO and MI is predicated on a number of diverse cellular and extracellular events, which include changes in fibroblast phenotype (transdifferentiation), posttranslational processing and assembly, and finally, degradation. The expansion of a population of transformed fibroblasts/myofibroblasts is a significant cellular event with respect to ECM remodeling in both PO and MI. The concept that this cellular expansion within the myocardial ECM may be due, at least in part, to endothelial-mesenchymal transformation and thereby not dissimilar to events observed in cancer progression holds intriguing future possibilities. Studies regarding determinants of procollagen processing, such as procollagen C-endopeptidase enhancer (PCOLCE), and collagen assembly, such as the secreted protein acidic and rich in cysteine (SPARC), have identified potential new targets for modifying the fibrotic response in both PO and MI. Finally, the transmembrane matrix metalloproteinases, such as MMP-14, underscore the diversity and complexity of this ECM proteolytic family as this protease can degrade the ECM as well as induce a profibrotic response. The growing recognition that the myocardial ECM is a dynamic entity containing a diversity of matricellular and nonstructural proteins as well as proteases and that the fibrillar collagens can change in structure and content in a rapid temporal fashion has opened up new avenues for modulating what was once considered an irreversible event - myocardial fibrosis.
Keywords: fibrosis, collagen, myocardium, extracellular matrix
the clinical presentation of heart failure (HF), which afflicts millions of patients annually, is generally that of a common set of clinical signs and symptoms, whereby fundamental defects in left ventricular (LV) function invariably underlie this clinical syndrome. The structural milestones that contribute to changes in LV function and therefore the development and progression of HF include changes in the structure, composition, and geometry of the LV myocardium, which has been generically termed LV remodeling. There are fairly distinct patterns of LV remodeling that occur and are dependent on the initial pathophysiological stimulus, but once initiated, LV remodeling is an important predictor for the development and progression of HF. LV remodeling entails changes in the structure and function of the cardiocyte, the vascular compartment, and extracellular matrix (ECM), whereby changes within all of these entities occur as a continuum during the initiation and progression to HF. Most certainly, fundamental defects in cardiocyte function and viability as well as vascular structure play critical roles in the development and progression to HF. However, there is growing recognition that the ECM mediates both mechanical and biological signals that contribute to this process (26, 39, 40, 45, 48, 73). For example, the ECM provides the critical interface for force transmission and alignment of myocardial fascicles and also provides the substrate for transmembrane adhesion of cardiocytes (4, 38, 39, 44, 47, 71). Thus, a loss of normal ECM structure and function can directly alter transduction of contractile force and intracellular signaling of cardiocytes, which in turn will change LV systolic function. In contrast, excessive ECM accumulation can directly alter myocardial passive stiffness properties, which will directly affect LV diastolic function (1, 21, 43, 90). In certain instances, significant heterogeneity in ECM remodeling occurs whereby a loss of normal ECM structure and function is accompanied by abnormal ECM accumulation, which can impair both LV systolic and diastolic function (28, 58, 74, 92). The classical approach for evaluating the myocardial ECM has been that of a binary evaluation, whereby histopathological scoring for fibrillar collagen was judged either as apparently “normal” or “increased” in relative content, whereby the latter has been termed myocardial “fibrosis.” These past studies, while providing valuable insight into gross patterns of LV remodeling in terms of fibrillar collagen accumulation, also tended to promulgate the concept that the myocardial ECM is a static structure with a unidirectional response to a pathological stress. However, it is now recognized that the ECM is a dynamic entity containing a diversity of structural and nonstructural proteins and molecules. Through the use of transgenic constructs, imaging modalities, and greater attention to posttranscriptional and posttranslational regulatory pathways, a greater insight into the highly complex and tightly regulated myocardial ECM has become appreciated (1, 4, 21, 25, 28, 38, 39, 43, 44, 47, 58, 71, 74, 90). The overall goal of this themed review is to more closely examine a few of the newer insights regarding specific cellular and posttranslational pathways relevant to the LV myocardial ECM remodeling process, as it relates to the generalized concept of LV myocardial fibrosis. First, generalized patterns of LV remodeling as it relates to ECM accumulation, such as fibrosis, will be briefly described. Second, a reevaluation of the most numerous cell types within the myocardium critical to ECM homeostasis, the fibroblast in relation to LV remodeling, will be discussed. Third, some important posttranslational processing steps in terms of collagen fibril assembly and stability and the relation to LV remodeling will be defined. Fourth, ECM proteolysis will be briefly presented as a dynamic and ongoing process and will also directly determine net ECM accumulation. The ultimate goal of this themed review is to recognize the importance of evaluating a number of posttranslational determinants of collagen biosynthesis/degradation and outline some future directions that would be relevant to a more comprehensive view of the ECM in the context of LV remodeling and HF.
LV Remodeling and the Extracellular Matrix
To provide a foundation for the focus on the ECM and this themed review on myocardial fibrosis, generalities regarding the LV remodeling process that can lead to HF include the following: 1) the pressure overloaded (PO) myocardium, such as that with hypertension or aortic stenosis, which causes LV hypertrophy and can give rise to increased collagen accumulation (i.e., fibrosis); and 2) the injured myocardium, most notably that of myocardial infarction (MI), whereby a heterogeneous remodeling process can occur simultaneously within the LV myocardium, giving rise to myocardial hypertrophy and fibrosis within the remote viable region, as well as mural wall thinning, expansion, and loss of normal collagen matrix structure and function within the MI region. While there are a number of other relevant LV remodeling processes that can lead to HF (i.e., cardiomyopathies, volume overload), PO and MI can serve as prototypical of LV remodeling processes whereby increased ECM accumulation, which has been termed fibrosis, invariably occurs. In PO, the fundamental stimulus for ECM growth and accumulation is the same for cardiocyte growth: increased LV afterload. While initially considered an adaptive process, the LV hypertrophy with PO will cause excessive fibrillar collagen accumulation as well as a number of other ECM proteins and ultimately impair LV filling, resulting in diastolic dysfunction and eventually HF. Following MI, a loss of cardiocyte mass replaced by an unstable ECM, the infarct scar, can eventually lead to LV remodeling, notably characterized by LV dilation and eventually pump dysfunction. The region remote from the MI can undergo remodeling not dissimilar to PO whereby there is growth of both the cellular and extracellular compartments, and the increased ECM can be paralleled by increased regional myocardial stiffness. A brief summary of the LV remodeling phenotype, which occurs in PO or MI, is shown in Table 1. The ECM contains important structural fibrillar collagens, such as collagen I and III, but there is also a large portfolio of other collagen types that can span the interstitial space and comprise the basement membrane (37, 91). While the fibrillar collagens have been a primary focus for structure-function studies in regards to the myocardial ECM, it should be recognized that this is but a small fraction of diverse molecules constituting this interstitial entity. Proteoglycans, such as chondroitin sulfate, dermatan sulfate, and perlecan are only a few examples of a class of complex molecules contained within the ECM. Glycoproteins, such as laminin and fibronectin, are critical constituents of the ECM and basement membrane facilitating cell-ECM adhesion through interaction with the transmembrane ECM receptors, the integrins (71). Many of these ECM constituents are synthesized primarily by the myocardial fibroblast, and yet this cell type is often not rigorously evaluated in the context of LV remodeling and the progression to HF. As detailed in a subsequent section, significant changes in fibroblast growth, proliferation, and functional phenotype occur in the context of both PO and MI, which directly hold relevance to the directionality of the ECM remodeling process. Most intriguing is the concept that there is a population expansion of “transformed” fibroblasts within the myocardial interstitium that occurs following a pathophysiological stimulus. While the measurement of fibrillar collagen mRNA levels can be used as one index of the directionality of ECM remodeling, this measurement alone is not conclusive. Specifically, processing of the newly secreted procollagen molecules is essential for the formation of stable collagen fibrils and ultimately collagen accumulation within the myocardium. As such, some of the newer aspects and determinants of procollagen processing, such as procollagen C-endopeptidase enhancer (PCOLCE) and secreted frizzled-related protein (sFRP), will be discussed in the context of myocardial ECM remodeling. There is a growing list of matricellular proteins secreted into the ECM with a diversity of biological functions, and several of these directly influence collagen stability and assembly. A prototypical example will be briefly presented, which is the secreted protein acidic and rich in cysteine (SPARC). Finally, a highly potent family of ECM degradative enzymes exists within the myocardium, the matrix metalloproteinases (MMPs). These MMPs can proteolytically process newly formed collagen as well as mature collagen, and as such, play a critical role in overall ECM structure, function, and ultimately, the degree of fibrosis that can occur with LV remodeling and HF. Recent insight into how this MMP system is operative in terms of both proteolytic activity as well as the induction of profibrotic pathways in the context of LV remodeling is highlighted.
Table 1.
Generalized features of LV remodeling with either pressure overload or myocardial infarction in terms of LV structure, function, and extracellular matrix
| Pressure Overload | Myocardial Infarction | |
|---|---|---|
| LV remodeling phenotype | Myocardial hypertrophy | Thinning and expansion of MI region and myocardial hypertrophy in the remote, viable region |
| LV functional phenotype | Abnormal diastolic function primarily characterized by increased myocardial stiffness | LV dilation and systolic dysfunction primarily characterized by infarct expansion process |
| Fibroblast response | Proliferation of fibroblasts and transdifferentiation of fibroblasts primarily characterized by enhanced profibrotic signaling | Fibroblast transdifferentiation within the MI region primarily characterized as an amplification of both matrix degradation and synthesis pathways, and fibroblast proliferation within the remote region primarily characterized by enhanced profibrotic signaling |
| Matrix remodeling response | Enhanced fibrillar collagen synthesis coupled with reduced degradation and turnover results in myocardial collagen accumulation—i.e., fibrosis | Continuous fibrillar collagen turnover within the MI region and amplified degradation pathways results in increased collagen accumulation but an overall abnormal matrix in terms of structure and function. Increased fibrillar collagen synthesis within the remote region (not dissimilar to PO) results in increased collagen accumulation—i.e., fibrosis. |
LV, left ventricular; PO, pressure overload; MI, myocardial infarction.
The Myocardial Fibroblast: A Transformation
Myocardial fibroblasts and fibroblasts in general are poorly defined cells, most simplistically described as connective tissue cells responsible for the production and maintenance of the ECM. Fibroblasts are elongated, spindle-shaped cells, which have a prominent Golgi complex and a rough endoplasmic reticulum necessary for the synthesis and export of collagen and other ECM proteins and lack a basement membrane (73). During development, fibroblasts within the myocardium arise from epicardial cells derived from the proepicardium, which undergo an epithelial to mesenchymal transformation (EMT) (20, 30, 87). In response to injury, fibroblasts in the adult myocardium are thought to arise from a number of sources, including resident fibroblasts, endothelial cell EMT (EndoEMT), pericytes, mesenchymal stem cells, fibrocytes, and myeloid-derived fibroblasts (19, 73, 87). While historically thought of as providing a supporting role within the myocardium, i.e. maintaining ECM homeostasis, communicating with cardiac myocytes, and disseminating mechanical and chemical cues, clearly fibroblasts and the closely related myofibroblast play key roles not only in normal LV function but also in the development of fibrosis in response to pathological stimuli and injury, such as with PO or MI (54, 73).
A recent mouse study examining LV function post-MI revealed that the number of myofibroblasts found within the MI region varies with mouse strain and increased myofibroblast numbers correlated with LV wall thickness (84). Studies in a variety of animal model systems indicate that the temporal appearance and persistence of myofibroblasts within an infarct scar varies widely (15, 26). For example, in Wilm's Tumor 1 (Wt1)-lineage traced mice that were subjected to MI, cells in the newly populated epicardium over the MI region expressed several embryonic epicardial genes and markers, such as Snail-1, associated with EMT (85). These Wt1-positive epicardial cells, via EMT, gave rise to a subepicardial mesenchyme that contributed to fibroblast-myofibroblast populations within the MI. In addition, myofibroblasts also play a prominent role in the fibrosis associated with PO. In vitro studies have demonstrated that cardiac fibroblasts in response to mechanical loading rapidly differentiated into myofibroblasts (89). In vivo, differentiated myofibroblasts can arise from bone marrow-derived cells in addition to differentiation of resident, myocardial fibroblasts (23). As was observed in the MI model, the myofibroblast can also be viewed as a dynamic population within the PO myocardium. In a right ventricular PO model, myofibroblast density increased within the first week after the PO stimulus and had decreased by 2 wk, which paralleled the rate of ECM accumulation (49). Fibroblasts isolated from a similar PO model demonstrated higher collagen deposition in vitro in comparison to non-PO fibroblasts, thereby demonstrating that a phenotype shift had occurred in terms of ECM synthesis (64).
Fibroblasts have long been recognized as the most numerous cell types in the rodent myocardium, and recent work has shown that the number of fibroblasts is dynamic, changing during development and postnatal growth with increased hemodynamic load (6, 59, 96). Following development and under normal physiological conditions, it appears that myocardial fibroblast turnover is limited, and very few myofibroblasts exist within the normal myocardium (7). However, with a pathophysiological stimulus such as PO, fibroblast proliferation occurs (5, 45, 76). For example, PO in rats caused fibroblast proliferation, particularly in the perivascular space within 72 h following PO induction, which was then followed by increased fibroblast proliferation within the entire myocardial ECM (45). In this study, the proliferating fibroblasts costained with α-smooth muscle actin (α-SMA), indicating the presence of myofibroblasts. In a mouse PO model, α-SMA-positive myofibroblasts were observed 3 days post-PO, and the number increased over a 28-day observation period (93). Lineage tracing studies using a Tie1Cre mouse subjected to PO demonstrated that around 25% of fibroblasts and close to 75% of myofibroblasts within the myocardium were derived from a labeled endothelial cell precursor, suggesting that these cells arose from EndoEMT (97). Thus, there is a rapid proliferation of fibroblasts as well as a phenotype shift to myofibroblasts following a PO stimulus. A more robust shift in the number of fibroblasts/myofibroblasts occurs following MI and is likely due to a combination of proliferation/differentiation of existing fibroblasts, infiltration of stem cells, and EndoEMT (19, 87). A summary of potential sources and cell types that may give rise to fibroblast proliferation and transdifferentiation is shown in Table 2. For example, it has been shown in a mouse MI model that fibroblasts within the MI region differentiate from resident CD44+ mesenchymal stem cells within 3 days post-MI; however, CD44+/α-SMA-positive cells were not readily observed until 7 days post-MI (16). Using a bone marrow reporter mouse construct, nearly 25% of fibroblasts and almost 60% of myofibroblasts identified within the MI region were positive for the reporter protein, suggesting these cells originated from the bone marrow cells (57). Thus, there appears to be a significant expansion of nonresident fibroblasts following MI, which may be the result of an EndoEMT type process involving a nondifferentiated population of stem cells. However, whether and to what degree this process contributes to the overall changes in ECM architecture following MI require further study.
Table 2.
Cardiac fibroblast precursors
| Cell Type | Disease Model | Reference |
|---|---|---|
| Bone marrow-derived stem cells | MI, PO | 24, 57 |
| Endogenous mesenchymal stem cells | MI | 16, 19 |
| Endothelium (EndoEMT) | MI, PO | 87, 96 |
| Epicardium | MI | 85, 87 |
| Epithelium (EMT) | MI | 24 |
| Myeloid-derived fibroblasts | MI | 19 |
| Pericytes | Cardiac development | 73 |
| Monocytes | MI | 19 |
| Resident fibroblasts | MI, PO | 24, 94 |
A number of cell types and sources likely give rise to fibroblast transdifferentiation and proliferation, which in turn will contribute to an advanced myocardial matrix structure and function with PO or MI. EndoEMT, endothelial-mesenchymal transformation; EMT, epithelial-mesenchymal transformation.
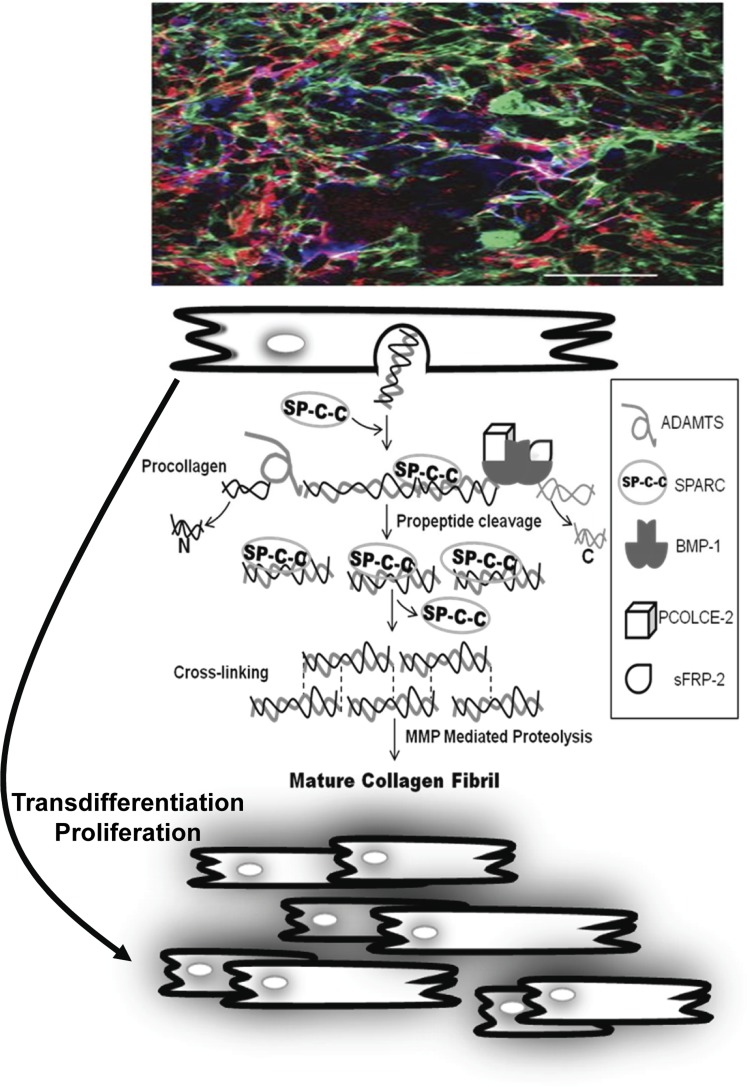
Cardiac myofibroblasts represent an activated phenotype of the fibroblast, associated with a number of functions including increased collagen gel contraction, increased collagen deposition, and ultimately myocardial fibrosis (24, 51, 52). While myocardial fibroblasts can be distinguished by their expression of discoidin domain receptor 2 (DDR2), α8β1 integrin, vimentin, and Thy-1, the most commonly used marker to identify myofibroblasts is the expression of α-SMA, a contractile protein normally associated with smooth muscle cells that imparts an enhanced contractile phenotype to the activated fibroblast (12, 27, 31, 36). In addition to α-SMA, desmin, tropomyosin-1, and non-muscle myosin-heavy chain B are also expressed by myofibroblasts (27, 66). However, previous studies have shown that even after differentiation into myofibroblasts, expression of some fibroblast-associated proteins, such as α8β1 integrin, DDR2, and Thy-1, persists (12, 36, 67). Isolated myocardial fibroblasts plated on rigid culture substrates have been shown to rapidly convert to a myofibroblastic phenotype, evidenced by increased expression of both α-SMA and embryonic smooth muscle myosin (67). Examination of the MI region has revealed positive staining for α-SMA and DDR2, suggesting that myofibroblasts in vitro and in vivo express the same protein markers (67). However, it can be difficult to quantify and localize fibroblasts versus myofibroblasts in terms of these markers. The complexity of expression of these different proteins and markers of myofibroblasts can be appreciated when colocalization of actin, collagen type I, and DDR2 is performed (Fig. 1). As noted above, there are several current markers used to distinguish fibroblasts/myofibroblasts from other cell types within the heart and far fewer that can be used to discern them from one another. This is an important area of further research as the transdifferentiation of fibroblasts, either from a pool of endogenous cells or from a margination of exogenous cells followed by EndoEMT and expansion, could be an important cellular target for the regulation of myocardial fibrosis and adverse ECM remodeling in both PO and MI.
Fig. 1.
Collagen production by cardiac fibroblasts. The ECM is a highly complex and diverse environment, whereby changes in fibroblast phenotype, as defined as changes in cell marker expression as well as function, occur in a dynamic fashion. Top: LV myocardial fibroblasts (rat) were isolated and placed in a three-dimensional fibrin-gel matrix that resulted in significant and detectable changes in both form and function. This confocal photomicrograph of multiple labeled immunohistochemical staining (8, 34), whereby the fibroblasts were stained for DDR2 (blue) and actin (green), exemplifies the complexity in terms of identification of fibroblasts-myofibroblasts in this idealized in vitro environment. These cultured fibroblasts released fibrillar collagen (red) into the culture system, which in turn must undergo a cascade of orchestrated steps to form a mature collagen fibril. Middle: modifying the expression of proteins involved in this highly regulated posttranslational process, such as PCOLCE or SPARC, directly alters the course of collagen assembly and thereby ECM remodeling. One of the more intriguing findings regarding myocardial fibroblast proliferation and adaptation to pathophysiological stimuli, such as PO and MI, is transdifferentiation into a myofibroblast, which may be an EndoEMT-mediated process. Bottom: interruption of this process through altering critical signal cascades, such as TGF or P311 as outlined in this themed review, may be a novel cellular target for interrupting the invariable myocardial fibrosis that occurs with PO or MI.
One fairly recent observation is that there may be an intermediate stage in the differentiation of fibroblasts into myofibroblasts: the proto-myofibroblast (81). Proto-myofibroblasts do not express α-SMA and are characterized by the presence of stress fibers in response to the development of mechanical tension, the formation of focal adhesions, and expression of the ED-A splice variant of fibronectin; however, very little else is known about this particular cell type within the myocardium. It has been reported that P311 [also known as PTZ17, an 8-kDa protein containing several PEST-like domains; structural motifs rich in proline, glutamic acid (E), serine, and threonine that are targets for protein degradation machinery and are typically found in proteins with short half-lives] could induce myofibroblast differentiation in mouse NIH 3T3 fibroblasts (63). Immunohistochemistry demonstrated that P311 was expressed by proto-myofibroblasts and myofibroblasts in vivo but not expressed in fibroblasts (63). More recent studies examining the role of P311 in human fibroblasts derived from hypertrophic scars suggest that this protein stimulates myofibroblast differentiation associated with increased profibrotic signaling pathways, notably transforming growth factor (TGF) (79). While remaining speculative, these observations suggest the intriguing possibility that the proto-myofibroblast may represent the checkpoint in the evolution of the myofibroblast phenotype, and proteins such as P311 could regulate the profibrotic potential of this cellular phenotype.
Critical Steps in Collagen Biosynthesis and Relevance to LV Remodeling
A generalized schematic that highlights just some of the important posttranslational steps in fibrillar collagen processing is shown in Fig. 1, and it serves to underscore not only the complexity of this process but also the potential targets for modulation of collagen accumulation within the myocardium. Fibrillar collagens are secreted as triple helical procollagen molecules with NH2 and COOH-terminal propeptides that must be removed prior to being deposited into stable, mature collagen within the ECM (65). Members of the ADAMTS family, ADAMTS 2 and 3, cleave the NH2-terminal propeptide, whereas BMP-1 (and related family members) clips off the COOH-terminal propeptide (18, 41). Enhancers of BMP-1 activity with regard to fibrillar procollagens expressed in the myocardium include PCOLCE 1 and 2 as well as sFRP 2. In the case of PCOLCE 1 and 2, these proteins bind to the C-propeptide and increase the efficiency of C-propeptide cleavage up to 20-fold (83). Release of the COOH-terminal propeptide is considered a critical event in converting soluble procollagen to insoluble fibrillar collagen. Interestingly, the LV myocardium was found to have the highest levels of expression of PCOLCE 1 and 2 in comparison to other tissues, including lung, liver, and skeletal muscle (75). This observation would imply that regulation of procollagen processing by PCOLCE is particularly relevant within the myocardium and is discussed in a subsequent paragraph.
LV myocardial fibrosis in response to PO occurs over a time scale distinct from that of myocyte hypertrophy. In other words, past studies have shown that the induction of the PO stimulus first causes an adaptive myocyte growth response, which is subsequently followed by measurable changes in collagen content (4). For example, using a right ventricular PO model, it was demonstrated that robust myocyte hypertrophy occurred within the initial 2 weeks following PO, but observable differences in collagen accumulation were not detected until myocyte hypertrophic growth had plateaued (4). In other studies, a robust increase in mRNA encoding fibrillar collagens occurs early following the induction of PO but actually declines prior to a quantifiable change in myocardial fibrillar collagen (86). The proportion of newly synthesized procollagen that is degraded prior to ECM incorporation is ∼50% in adult myocardium, as detected by measuring incorporation of [3H]proline to hydroxyproline in vivo (55). Along with increases in procollagen synthesis rate after PO, levels of procollagen degradation also decrease significantly (10, 22). These observations clearly suggest that transcriptional control of fibrillar collagens is not the sole regulatory factor influencing collagen deposition and accumulation in response to PO. Thus, when evaluating the initial and subsequent adaptation of a sustained PO or following myocardial injury, such as MI in terms of ECM remodeling and fibrosis, measurement of fibrillar collagen expression levels alone may be insufficient to predict the actual amount and extent of mature collagen within the ECM.
As both the NH2 and COOH-terminal propeptides of fibrillar collagen need to be removed prior to efficient incorporation of soluble monomeric collagen to insoluble fibrillar collagen, propeptide cleavage is one event that controls ECM collagen deposition. Although few studies to date have investigated the functional regulation of N-propeptide cleavage by ADAMTS 2/3 in cardiac interstitium, proteins that influence removal of the C-propeptide have been found to significantly affect collagen deposition within the myocardium. When wild-type and PCOLCE-2-null mice were subjected to PO, a significant reduction in collagen accumulation was observed in the PCOLCE-2-deficient mice (3). This relative reduction in collagen accumulation was associated with decreased myocardial stiffness in comparison to wild-type PO mice. The absence of PCOLCE-2 activity within the myocardium, therefore, would be expected to decrease the removal of the C-propeptide of the procollagen molecule and in turn reduce the formation of the mature collagen fibrils. Similarly, mice deficient in sFRP2 expression, another putative enhancer of BMP-1 activity on procollagen, demonstrated less fibrosis and significantly improved LV pump function following MI (42). LV fibroblasts isolated from both PCOLCE-2-null mice and sFRP2-null mice exhibited inefficient procollagen processing, specifically COOH-terminal propeptide cleavage, in comparison to wild-type fibroblasts (3, 42). These studies suggest that procollagen processing by BMP-1 family members represents a rate-limiting step in collagen deposition in the heart in response to PO or MI. For example, He et al. (35) reported that high concentrations of sFRP2 injected directly into the myocardium following MI reduced the relative degree of collagen accumulation. Kobayashi et al. (42) concluded that the absence of sFRP2, a putative enhancer of BMP-1, resulted in decreased fibrosis. However, the findings regarding sFRP2 on procollagen processing have not been uniform. For example, several studies have reported no observable effect of sFRP2 on BMP-1 activity on procollagen I in the context of a range of concentrations and buffer conditions (9, 88). In contrast, sizzled, a sFRP found in Xenopus and zebrafish, was reported to be an inhibitor of BMP-1 activity in these past reports (9). While the mechanism(s) by which sFRP2 may regulate BMP-1 and procollagen processing remains to be completely understood, it is apparent that sFRP2 can modify myocardial collagen content in a manner relevant to the adverse ECM remodeling, which occurs in the context of both PO and MI.
A growing number of matricellular proteins have been identified within the myocardium and can contribute significantly to critical steps in ECM structural assembly. Some of the matricellular proteins that may play an important role in myocardial collagen biosynthesis are shown in Table 3. For the purposes of illustration, the matricellular protein SPARC, which directly affects procollagen processing and therefore fibrillar collagen assembly and stability, will be briefly presented in terms of relevance to LV remodeling. In SPARC-null myocardial fibroblasts, increased amounts of collagen type I was detected at the cell surface/pericellular locations in comparison to wild-type fibroblasts (34). In this past study, increased amounts of fully processed collagen type I was localized to the cell-ECM interface of SPARC-null fibroblasts, indicative of increased procollagen processing efficiency. These results would suggest that the absence of SPARC would facilitate the fully processed collagen type I to be incorporated into the mature, more insoluble ECM. However, in vivo findings do not support this supposition and surprisingly identified the opposite effect (13). Specifically in a murine model of PO, genetic deletion of SPARC significantly attenuated collagen fibril formation and overall collagen accumulation (13). In addition, impaired collagen maturation and MI scar formation was observed in SPARC-null mice, which was associated with higher post-MI mortality rates compared with wild-type mice (68). These results support the hypothesis that the absence of SPARC greatly reduces the relative rate and efficiency of collagen maturation within the myocardium. These observations hold relevance for future basic and translational studies with respect to regulation of posttranslational ECM processing, but they also underscore the significant difference in terms of ECM accumulation regarding PO and MI. Specifically, inhibition of SPARC in the context of PO may alter the rate and magnitude of myocardial collagen accumulation, which in turn would potentially yield favorable effects on LV diastolic function and delay the progression to HF. In contrast, inhibition of SPARC may cause inadequate fibrillar collagen assembly within the critical wound healing phase post-MI with adverse consequences on LV remodeling and function. Thus, it will be important in future studies to evaluate these critical posttranslational ECM pathways that regulate “fibrosis” in a temporal and regional context. Furthermore, modification of the relative “maturity” and stability of the ECM, the fibrillar collagens in particular in terms of posttranslational processing, will also alter the vulnerability to proteolysis, which is outlined in the next paragraph.
Table 3.
Matricellular proteins expressed within the myocardium and potentially relevant collagen matrix biosynthesis
| Transgenic Null Phenotype | Effects on Collagen Fibril Morphology | Cardiac ECM Homeostasis | ECM Remodeling Post-MI or PO |
|---|---|---|---|
| SPARC | Decreased dermal collagen fibril diameter (14) | Reduced collagen content in SPARC-null hearts (13) | MI: increased incidence of rupture, decreased collagen content and fiber maturity (68) PO: decreased collagen content, decreased diastolic stiffness (13) |
| Periostin | Decreased dermal collagen fibril diameter, reduced collagen cross-links (61) | No differences detected (62) | MI: increased incidence of rupture, reduced collagen fibril diameter, reduced collagen cross-links (62, 72) PO: reduced collagen content (62) |
| Thrombospondin 2 | Increased dermal collagen fibril diameters, aberrant fibrils (46) | Reduced collagen content with aging (78) | MI: increased incidence of rupture (69) PO: increased rupture in response to ANG II infusion, decreased fibrosis (70) |
| Osteopontin | Decreased dermal collagen fibril diameters in wounds (50) | No difference in collagen content, disrupted fibrillar collagen weaves (82) | MI: reduced collagen content, decreased numbers of thin collagen filaments (82) |
| Tenascin C | None reported | No differences detected (60) | MI: decreased collagen content in border zone (60) |
| Tenascin X | Reduced dermal fibril density (53) | Not characterized | Not characterized |
ECM, extracellular matrix; SPARC, secreted protein acidic and rich in cysteine. Reference nos. in parentheses.
Matrix Proteolytic Enzymes Facilitate Both ECM Instability and Fibrosis
There are now approximately 23 MMP types expressed within humans, and the distribution, functionality, and substrates are highly diverse as discussed in several broader reviews (11, 17, 33, 40). After approximately two decades of research, there is no question that changes in MMP type and activity directly influence the myocardial ECM and hold particular relevance to LV remodeling processes, such as PO and MI. What is becoming more recently appreciated, however, is that certain MMPs not only facilitate collagen degradation but can also process profibrotic signaling molecules. Thus, MMPs likely contribute to the degradation of relatively normal ECM structure as well as facilitate the deposition of a more “fibrotic” and dysfunctional myocardial ECM. The MMPs can be broadly classified into the “collagenases,” such as MMP-1, MMP-13, and MMP-8; the “gelatinases” would include MMP-2 and MMP-9; the “stromelysins/matrilysins” would include MMP-3 and MMP-7; and the “membrane type MMPs” would include MMP-14. This is hardly the exhaustive list of MMP types that are likely expressed within the mammalian myocardium. For the purposes of highlighting the duality and diversity of the MMP family, MMP-14 will be discussed. The pathways by which MMP-14 contributes to adverse ECM remodeling likely include facilitating proteolysis of interstitial molecules directly (such as integrins), amplification of active MMP-2 causing ECM instability and abnormal architecture, as well as through enhancing profibrotic signaling pathways (2, 32, 48, 74, 80). Of more recent discovery is that an important proteolytic relationship likely exists between MMP-14 and the subsequent activation of the profibrotic signaling molecule TGF, which would hold particular relevance in the context of myocardial fibrosis (48, 80). In animal models of PO, an early and sustained induction of MMP-14 has been identified (95, 98). For example, increased MMP-14 promoter activity and subsequently MMP-14 proteolytic activity has been reported following the induction of PO in mice (98). Moreover, these studies have identified an association between changes in myocardial ECM remodeling, notably increased collagen accumulation to that of MMP-14 induction. In murine models of MI, increased levels of MMP-14 were identified within both the MI and the remote regions but were associated with significantly different effects (74). Precisely, using an MMP-14-specific fluorogenic substrate, it was demonstrated that increased MMP-14 activity occurred within the MI region and was associated with increased MMP-2 activation, which in turn would cause ECM instability. On the other hand, increased MMP-14 activity within the remote region was associated with heightened indices of TGF signaling and was associated with increased collagen accumulation. These past observations underscore that certain MMP types do not only degrade the ECM, but likely play a much larger and diverse role in ECM remodeling. Moreover, assessing MMP expression alone may be insufficient in providing insight into the type of ECM remodeling process that is occurring in either PO or MI.
Summation and Future Directions
The growing recognition that the myocardial ECM is a dynamic entity that contains biological signaling molecules, a diversity of matricellular and nonstructural proteins, and that the fibrillar collagens can change in structure and content in a rapid temporal fashion has opened up new avenues for modulating what was once considered an irreversible event—myocardial fibrosis. Using both metabolic and racemization/isomerization studies, it appears that fibrillar collagen turnover within the LV myocardium is one of the highest of any organ, and thus challenges the historical concept that myocardial fibrosis is a dormant, end-stage process (29, 56). While the term “fibrosis” can be misleading in terms of the complex patterns and processes of ECM remodeling within the myocardium, there is no question that fibrillar collagen accumulation is a common consequence of two relevant pathophysiological stimuli: PO and MI. The fact that transdifferentiation of fibroblasts to a myofibroblast phenotype occurs in both PO and MI, and may be the result of EndoEMT, holds a number of intriguing future directions. First, the novel insights of this transdifferentiation process that have been provided from the large body of cancer research may be applicable to adverse ECM remodeling process with PO and MI. Second, the possibility that the proliferation of a specific stem cell-derived population of myofibroblasts may occur in these cardiac disease states may afford a number of interventional possibilities. Third, since myofibroblasts that populate the MI region can persist for months to years following the initial MI, this certainly affords an important cellular target in terms of therapeutics (77). However, a number of obstacles must be overcome and will need to be addressed in future studies. These would include improved methods for identifying fibroblast-myofibroblast phenotype, understanding the transdifferentiation process to a much greater degree, particularly in terms of a “transitional state” of fibroblast-myofibroblast transdifferentiation, and more specific gene targeting tools (i.e., fibroblast- vs. myofibroblast-specific promoters). While there are a number of profibrotic signaling molecules relevant to myocardial ECM remodeling, most certainly TGF is considered a predominant pathway. More importantly, however, interruption of TGF signaling directly or indirectly, such as through regulation of P311, may afford an opportunity to modify fibroblast transdifferentiation and in turn, the course of myocardial ECM remodeling. It is becoming not only clear that myofibroblasts represent a cellular target for modifying the ECM remodeling process, but also this cell transdifferentiation process can cause changes in posttranslational collagen processing (64). This set of posttranslational events that yield a mature and stable collagen matrix within the ECM is highly orchestrated (Fig. 1). Targeting key steps/proteins in this posttranslational process that are robustly expressed within the myocardium, such as PCOLCE 1 and 2, as well as matricellular proteins, such as SPARC in the context of PO and MI, may be a novel approach in favorably modifying the ECM and as a result slow the progression of adverse LV remodeling (75). Since a number of these ECM posttranslational processing steps are cell surface associated, transmembrane proteases would hold particular relevance in terms of degradation of the immature, poorly processed collagen. The transmembrane protease MMP-14 is a prototypical example of the tight interaction between profibrotic and degradative pathways. For example, MMP-14 degrades fibrillar collagens directly as well as through the activation of other MMPs, which in turn would alter the turnover and stability of the myocardial ECM. On the other hand, MMP-14 facilitates TGF signaling within the ECM and in turn would amplify a profibrotic signaling cascade. Targeting MMP-14, therefore, may provide an avenue to simultaneously prevent the loss of normal ECM structure and abnormal ECM accumulation, both of which occur in the context of PO and MI. These are but a few examples of the highly diverse and dynamic nature of the myocardial ECM and underscore the need for a more thorough understanding of cellular and extracellular pathways that contribute to the changes in myocardial collagen assembly and accumulation, which in turn will likely yield important new therapeutic targets.
GRANTS
This work was supported by National Institutes of Health Grants HL-057952, HL-089944, HL-095608, HL-111090, HL-097214, and HL-094517 and by Merit Awards (5I01BX000168 and 1I01BX001385) from the Veterans' Affairs Health Administration.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
E.C.G. and F.G.S. prepared the figure; E.C.G., A.D.B., and F.G.S. drafted the manuscript; E.C.G., A.D.B., and F.G.S. edited and revised the manuscript; F.G.S. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors express appreciation to Ashley Sapp, University of South Carolina School of Medicine, for editorial assistance.
Glossary
- ADAMTS
A disintegrin and metalloproteinase with thrombospondin motifs
- ANG II
angiotensin II
- BMP
bone morphogenetic protein
- DDR2
discoidin domain receptor 2
- ECM
extracellular matrix
- EGFP
enhanced green fluorescent protein
- EMT
epithelial-mesenchymal transformation
- EndoEMT
endothelial cell EMT
- FSP1
fibroblast-specific protein 1
- HF
heart failure
- LV
left ventricular
- MI
myocardial infarction
- MMP
matrix metalloproteinase
- mRNA
messenger RNA
- PAB
pulmonary artery banding
- PCOLCE
procollagen C-endopeptidase enhancer
- PO
pressure overload
- sFRP
secreted frizzled-related protein
- shRNA
small hairpin RNA
- SMA
smooth muscle actin
- SPARC
secreted protein acidic and rich in cysteine
- TAC
transverse aortic constriction
- TGF
transforming growth factor
- TSP2
thrombospondin 2
- WT
wild type
- Wt1
Wilm's Tumor 1
REFERENCES
- 1. Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, McClure CD, Spinale FG, Zile MR. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation 113: 2089–2096, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Baciu PC, Suleiman EA, Deryugina EI, Strongin AY. Membrane type-1 matrix metalloproteinase (MT1-MMP) processing of pro-alphav integrin regulates cross-talk between alphavbeta3 and alpha2beta1 integrins in breast carcinoma cells. Exp Cell Res 291: 167–175, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Baicu CF, Zhang Y, Van Laer AO, Renaud L, Zile MR, Bradshaw AD. Effects of the absence of procollagen C-endopeptidase enhancer-2 on myocardial collagen accumulation in chronic pressure overload. Am J Physiol Heart Circ Physiol 303: H234–H240, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baicu CF, Li J, Zhang Y, Kasiganesan H, Cooper G, 4th, Zile MR, Bradshaw AD. Time course of right ventricular pressure-overload- induced myocardial fibrosis: relationship to changes in fibroblast postsynthetic procollagen processing. Am J Physiol Heart Circ Physiol 303: H1128–H1134, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balasubramanian S, Quinones L, Kasiganesan H, Zhang Y, Pleasant DL, Sundararai KP, Zile MR, Bradshaw AD, Kuppuswamy D. Beta-3 integrin in cardiac fibroblast is critical for extracellular matrix accumulation during pressure overload hypertrophy in mouse. PLos One 7: e45076, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol 293: H1883–H1891, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Baum J, Duffy HS. Fibroblast and myofibroblasts: what are we talking about? J Cardiovasc Pharmacol 57: 376–379, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baxter SC, Morales MO, Goldsmith EC. Adaptive changes in cardiac fibroblast morphology and collagen organization as a result of mechanical environment. Cell Biochem Biophys 51: 33–44, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bijakowski C, Vadon-Le Goff S, Delolme F, Bourhis JM, , Lecorche P, Ruggiero F, Becker-Pauly C, Yiallouros I, Stocker W, Dive V, Hulmes DJ, Moali C. Sizzled is unique among secreted frizzled-related proteins for its ability to specifically inhibit bone morphogenetic protein-1 (BMP-1)/tolloid-like proteinases. J Biol Chem 287: 33581–33593, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bishop JE, Rhodes S, Laurent GJ, Low RB, Stirewalt WS. Increased collagen synthesis and decreased collagen degradation in right ventricular hypertrophy induced by pressure overload. Cardiovasc Res 28: 1581–1585, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): positive and negative regulators in tumor cell adhesion. Semin Cancer Biol 20: 161–168, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bouzeghrane F, Mercure C, Reudelhuber TL, Thibault G. Alpha8beta1 integrin is up-regulated in myofibroblasts of fibrotic and scarring myocardium. J Mol Cell Cardiol 36: 343–353. 2004 [DOI] [PubMed] [Google Scholar]
- 13. Bradshaw AD, Baicu CF, Rentz TJ, Van Laer AO, Boggs J, Lacy JM, Zile MR. Pressure overload-induced alterations in fibrillar collagen content and myocardial diastolic function: role of secreted protein acidic and rich in cysteine (SPARC) in post-synthetic procollagen processing. Circulation 119: 269–280, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bradshaw AD, Puolakkainen P, Dasgupta J, Davidson JM, Wight TN, Helene Sage E. SPARC-null mice display abnormalities in the dermis characterized by decreased collagen fibril diameter and reduced tensile strength. J Invest Dermatol 120: 949–955, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Bryant JE, Shamhart PE, Luther DJ, Olson ER, Koshy JC, Costic DJ, Mohile MV, Dockry M, Doane KJ, Meszaros JG. Cardiac myofibroblast differentiation is attenuated by α3 integrin blockade: potential role in post-MI remodeling. J Mol Cell Cardiol 46: 186–192, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Carlson S, Trial J, Soeller C, Entman ML. Cardiac mesenchymal stem cells contribute to scar formation after myocardial infarction. Cardiovasc Res 91: 99–107, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clark IM, Swingler TE, Sampieri CL, Edwards DR. The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol 40: 1362–1378, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Colige A, Colige A, Sieron AL, Li SW, Schwarze U, Petty E, Wertelecki W, Wilcox W, Krakow D, Cohn DH, Reardon W, Byers PH, Lapière CM, Prockop DJ, Nusgens BV. Human Ehlers-Danlos syndrome type VII C and bovine dermatosparaxis are caused by mutations in the procollagen I N-proteinase gene. Am J Hum Genet 65: 308–317, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crawford JR, Haudek SB, Cieslik KA, Trial J, Entman ML. Origin of developmental precursors dictates the pathophysiologic role of cardiac fibroblasts. J Cardiovasc Transl Res 5: 749–759, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dettman RW, Denetclaw W, Ordahl CP, Bristow J. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Dev Biol 193: 169–181, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Díez J, Laviades C, Mayor G, Gil MJ, Monreal I. Increased serum concentrations of procollagen peptides in essential hypertension. Relation to cardiac alterations. Circulation 91: 1450–1456, 1995 [DOI] [PubMed] [Google Scholar]
- 22. Eleftheriades EG, Durand JB, Ferguson AG, Engelmann GL, Jones SB, Samarel AM. Regulation of procollagen metabolism in the pressure-overloaded rat heart. J Clin Invest 91: 1113–1122, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Endo J, Sano M, Fujita J, Hayashida K, Yuasa S, Aoyama N, Takehara Y, Kato O, Makino S, Ogawa S, Fukuda K. Bone marrow-derived cells are involved in the pathogenesis of cardiac hypertrophy in response to pressure overload. Circulation 116: 1176–1184, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Fan D, Takawale A, Lee J, Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair 5: 15–28, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fomovsky GM, Thomopoulos S, Holmes JW. Contribution of extracellular matrix to the mechanical properties of the heart. J Mol Cell Cardiol 48: 490–496, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Frangogiannis NG, Michael LH, Entman ML. Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb). Cardiovasc Res 48: 89–100, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol 200: 500–503, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Gajarsa JJ, Kloner RA. Left ventricular remodeling in the post-infarction heart: a review of cellular, molecular mechanisms, and therapeutic modalities. Heart Fail Rev 16: 13–21, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Gineyts E, Cloos PA, Borel O, Grimaud L, Delmas PD, Garnero P. Racemization and isomerization of type I collagen C-telopeptides in human bone and soft tissues: assessment of tissue turnover. Biochem J 345: 481–485, 2000 [PMC free article] [PubMed] [Google Scholar]
- 30. Gittenberger-de Groot AC, Vrancken Peeters MP, Mentink MM, Gourdie RG, Poelmann RE. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ Res 82: 1043–1052, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Goldsmith EC, Hoffman A, Morales MO, Potts JD, Price RL, McFadden A, Borg TK. Organization of fibroblasts in the heart. Dev Dyn 230: 787–794, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Guo C, Piacentini L. Type I collagen-induced MMP-2 activation coincides with up-regulation of membrane type 1-matrix metalloproteinase and TIMP-2 in cardiac fibroblasts. J Biol Chem 278: 46699–46708, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Hadler-Olsen E, Fadnes B, Sylte I, Uhlin-Hansen L, Winberg JO. Regulation of matrix metalloproteinase activity in health and disease. FEBS J 278: 28–45, 2011 [DOI] [PubMed] [Google Scholar]
- 34. Harris BS, Zhang Y, Card L, Rivera LB, Brekken RA, Bradshaw AD. SPARC regulates collagen interaction with cardiac fibroblast cell surfaces. Am J Physiol Heart Circ Physiol 301: H841–H847, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. He W, Zhang L, Ni A, Zhang Z, Mirotsou M, Mao L, Pratt RE, Dzau VJ. Exogenously administered secreted frizzled related protein 2 (Sfrp2) reduces fibrosis and improves cardiac function in a rat model of myocardial infarction. Proc Natl Acad Sci USA 107: 21110–21115, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hudon-David F, Bouzeghrane F, Couture P, Thibault G. Thy-1 expression by cardiac fibroblasts: lack of association with myofibroblast contractile markers. J Mol Cell Cardiol 42: 991–1000, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Jenniskens GJ, Veerkamp JH, van Kuppevelt TH. Heparan sulfates in skeletal muscle development and physiology. J Cell Physiol 206: 283–294, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Kassiri Z, Defamie V, Hariri M, Oudit GY, Anthwal S, Dawood F, Liu P, Khokha R. Simultaneous transforming growth factor beta-tumor necrosis factor activation and cross-talk cause aberrant remodeling response and myocardial fibrosis in Timp3-deficient heart. J Biol Chem 284: 29893–29904, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kato S, Spinale FG, Tanaka R, Johnson W, Cooper G, 4th, Zile MR. Inhibition of collagen cross-linking: effects on fibrillar collagen and ventricular diastolic function. Am J Physiol Heart Circ Physiol 269: H863–H868, 1995 [DOI] [PubMed] [Google Scholar]
- 40. Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141: 52–67, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kessler E, Takahara K, Biniaminov L, Brusel M, Greenspan DS. Bone morphogenetic protein-1: the type I procollagen C-proteinase. Science 271: 360–362, 1996 [DOI] [PubMed] [Google Scholar]
- 42. Kobayashi K, Luo M, Zhang Y, Wilkes DC, Ge G, Grieskamp T, Yamada C, Liu TC, Huang G, Basson CT, Kispert A, Greenspan DS, Sato TN. Secreted Frizzled-related protein 2 is a procollagen C proteinase enhancer with a role in fibrosis associated with myocardial infarction. Nat Cell Biol 11: 46–55, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Krayenbuehl HP, Hess OM, Monrad ES, Schneider J, Mall G, Turina M. Left ventricular myocardial structure in aortic valve disease before, intermediate, and late after aortic valve replacement. Circulation 79: 744–755, 1989 [DOI] [PubMed] [Google Scholar]
- 44. Kresh JY, Chopra A. Intercellular and extracellular mechanotransduction in cardiac myocytes. Pflügers Arch 462: 75–87, 2011 [DOI] [PubMed] [Google Scholar]
- 45. Kuwahara F, Kai H, Tokuda K, Kai M, Takeshita A, Egashira K, Imaizumi T. Transforming growth factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overload rats. Circulation 106: 130–135, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Kyriakides TR, Zhu YH, Smith LT, Bain SD, Yang Z, Lin MT, Danielson KG, Iozzo RV, LaMarca M, McKinney CE, Ginns EI, Bornstein P. Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J Cell Biol 140: 419–430, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Leask A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res 106: 1675–1680, 2010 [DOI] [PubMed] [Google Scholar]
- 48. Lehti K, Rose NF, Valavaara S, Weiss SJ, Keski-Oja J. MT1-MMP promotes vascular smooth muscle dedifferentiation through LRP1 processing. J Cell Sci 122: 126–135, 2009 [DOI] [PubMed] [Google Scholar]
- 49. Leslie KO, Taatjes DJ, Schwarz J, von Turkovich M, Low RB. Cardiac myofibroblasts express alpha smooth muscle actin during right ventricular pressure overload in the rabbit. Am J Pathol 139: 207–216, 1991 [PMC free article] [PubMed] [Google Scholar]
- 50. Liaw L, Birk DE, Ballas CB, Whitsitt JS, Davidson JM, Hogan BL. Altered wound healing in mice lacking a functional osteopontin gene (spp1). J Clin Invest 101: 1468–1478, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lijnen P, Petrov V, Rumilla K, Fagard R. Transforming growth factor beta 1 promotes contraction of collagen gel by cardiac fibroblasts through their differentiation into myofibroblasts. Methods Find Exp Clin Pharmacol 25: 79–86, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Lijnen P, Petrov V. Transforming growth factor beta 1-induced collagen production in cultures of cardiac fibroblasts is the result of the appearance of myofibroblasts. Methods Find Exp Clin Pharmacol 24: 333–344, 2002 [DOI] [PubMed] [Google Scholar]
- 53. Mao JR, Taylor G, Dean WB, Wagner DR, Afzal V, Lotz JC, Rubin EM, Bristow J. Tenascin-X deficiency mimics Ehlers-Danlos syndrome in mice through alteration of collagen deposition. Nat Genet 30: 421–425, 2002 [DOI] [PubMed] [Google Scholar]
- 54. Martin ML, Blaxall BC. Cardiac intercellular communication: are myocytes and fibroblasts fair-weather friends? J Cardiovasc Transl Res 5: 768–782, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mays PK, McAnulty RJ, Campa JS, Laurent GJ. Age-related changes in collagen synthesis and degradation in rat tissues. Importance of degradation of newly synthesized collagen in regulating collagen production. Biochem J 276: 307–313, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McAnulty RJ, Laurent GJ. Collagen synthesis and degradation in vivo. Evidence for rapid rates of collagen turnover with extensive degradation of newly synthesized collagen in tissues of the adult rat. Coll Relat Res 7: 93–104, 1987 [DOI] [PubMed] [Google Scholar]
- 57. Mollmann H, Nef HM, Kostin S, von Kalle C, Pilz I, Weber M, Schaper J, Hamm CW, Elsasser A. Bone marrow-derived cells contribute to infarct remodeling. Cardiovasc Res 71: 661–671, 2006 [DOI] [PubMed] [Google Scholar]
- 58. Mukherjee R, Brinsa TA, Dowdy KB, Scott AA, Baskin JM, Deschamps AM, Lowry AS, Escobar GP, Lucas DG, Yarbrough WM, Zile MR, Spinale FG. Myocardial infarct expansion and matrix metalloproteinase inhibition. Circulation 107: 618–625, 2003 [DOI] [PubMed] [Google Scholar]
- 59. Nag A. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios 28: 41–61. 1980 [PubMed] [Google Scholar]
- 60. Nishioka T, Onishi K, Shimojo N, Nagano Y, Matsusaka H, Ikeuchi M, Ide T, Tsutsui H, Hiroe M, Yoshida T, Imanaka-Yoshida K. Tenascin-C may aggravate left ventricular remodeling and function after myocardial infarction in mice. Am J Physiol Heart Circ Physiol 298: H1072–H1078, 2010 [DOI] [PubMed] [Google Scholar]
- 61. Norris RA, Damon B, Mironov V, Kasyanov V, Ramamurthi A, Moreno-Rodriguez R, Trusk T, Potts JD, Goodwin RL, Davis J, Hoffman S, Wen X, Sugi Y, Kern CB, Mjaatvedt CH, Turner DK, Oka T, Conway SJ, Molkentin JD, Forgacs G, Markwald RR. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem 101: 695–711, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, Lorts A, Brunskill EW, Dorn GW, 2nd, Conway SJ, Aronow BJ, Robbins J, Molkentin JD. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res 101: 313–321, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pan D, Zhe X, Jakkaraju S, Taylor GA, Schuger L. P311 induces a TGFbeta1-independent, nonfibrogenic myofibroblast phenotype. J Clin Invest 110: 1349–1358, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Poobalarahi F, Baicu CF, Bradshaw AD. Cardiac myofibroblasts differentiated in 3D culture exhibit distinct changes in collagen I production, processing, and matrix deposition. Am J Physiol Heart Circ Physiol 291: H2924–H2932, 2006 [DOI] [PubMed] [Google Scholar]
- 65. Prockop DJ, Kivirikko KI. Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem 64: 403–434, 1995 [DOI] [PubMed] [Google Scholar]
- 66. Ruhs S, Nass N, Somoza V, Friess U, Schinzel R, Silber RE, Simm A. Maillard reaction products enriched food extract reduced the expression of myofibroblast phenotype markers. Mol Nutr Food Res 51: 488–495, 2007 [DOI] [PubMed] [Google Scholar]
- 67. Santiago JJ, Dangerfield AL, Rattan SG, Bathe KL, Cunnington RH, Raizman JE, Bedosky KM, Freed DH, Kardami E, Dixon IM. Cardiac fibroblast to myofibroblast differentiation in vivo and in vitro: expression of focal adhesion components in neonatal and adult rat ventricular myofibroblasts. Dev Dyn 239: 1573–1584, 2010 [DOI] [PubMed] [Google Scholar]
- 68. Schellings MW, Vanhoutte D, Swinnen M, Cleutjens JP, Debets J, van Leeuwen RE, d'Hooge J, Van de Werf F, Carmeliet P, Pinto YM, Sage EH, Heymans S. Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. J Exp Med 206: 113–123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schellings MW, Pinto YM, Heymans S. Matricellular proteins in the heart: possible role during stress and remodeling. Cardiovasc Res 64: 24–31, 2004 [DOI] [PubMed] [Google Scholar]
- 70. Schroen B, Heymans S, Sharma U, Blankesteijn WM, Pokharel S, Cleutjens JP, Porter JG, Evelo CT, Duisters R, van Leeuwen RE, Janssen BJ, Debets JJ, Smits JF, Daemen MJ, Crijns HJ, Bornstein P, Pinto YM. Thrombospondin-2 is essential for myocardial matrix integrity: increased expression identifies failure-prone cardiac hypertrophy. Circ Res 95: 515–522, 2004 [DOI] [PubMed] [Google Scholar]
- 71. Shai SY, Harpf AE, Babbitt CJ, Jordan MC, Fishbein MC, Chen J, Omura M, Leil TA, Becker KD, Jiang M, Smith DJ, Cherry SR, Loftus JC, Ross RS. Cardiac myocyte-specific excision of the beta1 integrin gene results in myocardial fibrosis and cardiac failure. Circ Res 90: 458–464, 2002 [DOI] [PubMed] [Google Scholar]
- 72. Shimazaki M, Nakamura K, Kii I, Kashima T, Amizuka N, Li M, Saito M, Fukuda K, Nishiyama T, Kitajima S, Saga Y, Fukayama M, Sata M, Kudo A. Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med 205: 295–303, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Souders CA, Bowers SLK, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res 105: 1164–1176, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Spinale FG, Mukherjee R, Zavadzkas JA, Koval CN, Bouges S, Stroud RE, Dobrucki LW, Sinusas AJ. Cardiac restricted overexpression of membrane type-1 matrix metalloproteinase causes adverse myocardial remodeling following myocardial infarction. J Biol Chem 285: 30316–30327, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Steiglitz BM, Keene DR, Greenspan DS. PCOLCE2 encodes a functional procollagen C-proteinase enhancer (PCPE2) that is a collagen-binding protein differing in distribution of expression and post-translational modification from the previously described PCPE1. J Biol Chem 277: 49820–49830, 2002 [DOI] [PubMed] [Google Scholar]
- 76. Stewart JA, Massey EP, Fix C, Zhu J, Goldsmith EC, Carver W. Temporal alterations in cardiac fibroblast function following induction of pressure overload. Cell Tissue Res 340: 117–126, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sun Y, Weber KT. Infarct scar: a dynamic tissue. Cardiovasc Res 46: 250–256, 2000 [DOI] [PubMed] [Google Scholar]
- 78. Swinnen M, Vanhoutte D, Van Almen GC, Hamdani N, Schellings MW, D'hooge J, Van der Velden J, Weaver MS, Sage EH, Bornstein P, Verheyen FK, VandenDriessche T, Chuah MK, Westermann D, Paulus WJ, Van de Werf F, Schroen B, Carmeliet P, Pinto YM, Heymans S. Absence of thrombospondin-2 causes age-related dilated cardiomyopathy. Circulation 120: 1585–1597, 2009 [DOI] [PubMed] [Google Scholar]
- 79. Tan J, Peng X, Luo G, Ma B, Cao C, He W, Yuan S, Li S, Wilkins JA, Wu J. Investigating the role of P311 in the hypertrophic scar. PLos One 5: e9995, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tatti O, Vehviläinen P, Lehti K, Keski-Oja J. MT1-MMP releases latent TGF-beta1 from endothelial cell extracellular matrix via proteolytic processing of LTBP-1. Exp Cell Res 314: 2501–2514, 2008 [DOI] [PubMed] [Google Scholar]
- 81. Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechanoregulation of connective tissue remodeling. Nat Rev Mol Cell Biol 3: 349–363, 2002 [DOI] [PubMed] [Google Scholar]
- 82. Trueblood NA, Xie Z, Communal C, Sam F, Ngoy S, Liaw L, Jenkins AW, Wang J, Sawyer DB, Bing OH, Apstein CS, Colucci WS, Singh K. Exaggerated left ventricular dilation and reduced collagen deposition after myocardial infarction in mice lacking osteopontin. Circ Res 88: 1080–1087, 2001 [DOI] [PubMed] [Google Scholar]
- 83. Vadon-Le Goff S, Kronenberg D, Bourhis JM, Bijakowski C, Raynal N, Ruggiero F, Farndale RW, Stöcker W, Hulmes DJ, Moali C. Procollagen C-proteinase enhancer stimulates procollagen processing by binding to the C-propeptide region only. J Biol Chem 286: 38932–38938, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Van en Borne SW, van de Schans VA, Strzelecka AE, Vervoort-Peters HT, Lijnen PM, Cleutjens JP, Smits JF, Daemen MJ, Jassen BJ, Blankestein WM. Mouse strain determined the outcome of wound healing after myocardial infarction. Cardiovasc Res 84: 273–282, 2009 [DOI] [PubMed] [Google Scholar]
- 85. van Wijk B, Gunst QD, Moorman AF, van den Hoff MJ. Cardiac regeneration from activated epicardium. PLos One 7: e44692, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Villarreal FJ, Dillmann WH. Cardiac hypertrophy-induced changes in mRNA levels for TGF-β1, fibronectin, and collagen. Am J Physiol Heart Circ Physiol 262: H1861–H1866, 1992 [DOI] [PubMed] [Google Scholar]
- 87. Von Gise A, Pu WT. Endocardial and epicardial epithelial to mesenchymal transitions in heart development and disease. Circ Res 110: 1628–1645, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. von Marschall Z, Fisher LW. Dentin sialophosphoprotein (DSPP) is cleaved into its two natural dentin matrix products by three isoforms of bone morphogenetic protein-1 (BMP1). Matrix Biol 29: 295–303, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wang J, Chen H, Seth A, McCulloch CA. Mechanical force regulation of myofibroblast differentiation in cardiac fibroblasts. Am J Physiol Heart Circ Physiol 285: H1871–H1881, 2003 [DOI] [PubMed] [Google Scholar]
- 90. Weidemann F, Herrmann S, Störk S, Niemann M, Frantz S, Lange V, Beer M, Gattenlöhner S, Voelker W, Ertl G, Strotmann JM. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation 120: 577–584, 2009 [DOI] [PubMed] [Google Scholar]
- 91. Widgerow AD. Cellular/extracellular matrix cross-talk in scar evolution and control. Wound Repair Regen 19: 117–133, 2011 [DOI] [PubMed] [Google Scholar]
- 92. Wilson EM, Moainie SL, Baskin JM, Lowry AS, Deschamps AM, Mukherjee R, Guy TS, St, John-Sutton MG, Gorman JH, 3rd, Edmunds LH, Jr, Gorman RC, Spinale FG. Region- and type-specific induction of matrix metalloproteinases in post-myocardial infarction remodeling. Circulation 107: 2857–2863, 2003 [DOI] [PubMed] [Google Scholar]
- 93. Xia Y, Dobaczewski M, Gonzalez-Quesada C, Chen W, Biernacka A, Li N, Lee DW, Frangogiannis NG. Endogenous thrombospondin 1 protects the pressure-overloaded myocardium by modulating fibroblast phenotypes and matrix metabolism. Hypertension 58: 902–911, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yano T, Miura T, Ikeda Y, Matsuda E, Saito K, Miki T, Kobayashi H, Nishino Y, Ohtani S, Shimamoto K. Intracardiac fibroblasts, but not bone marrow derived cells, are the origin of myofibroblasts in myocardial infarct repair. Cardiovasc Pathol 14: 241–246, 2005 [DOI] [PubMed] [Google Scholar]
- 95. Yarbrough WM, Mukherjee R, Stroud RE, Rivers WT, Oelsen JM, Dixon JA, Eckhouse SR, Ikonomidis JS, Zile MR, Spinale FG. Progressive induction of left ventricular pressure overload in a large animal model elicits myocardial remodeling and a unique matrix signature. J Thorac Cardiovasc Surg 143: 215–223, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zak R. Development and proliferative capacity of cardiac muscle cells. Circ Res 32: 17–26, 1974 [PubMed] [Google Scholar]
- 97. Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13: 952–961, 2007 [DOI] [PubMed] [Google Scholar]
- 98. Zile MR, Baicu CF, Stroud RE, Van Laer A, Arroyo J, Mukherjee R, Jones JA, Spinale FG. Pressure overload-dependent membrane type 1-matrix metalloproteinase induction: relationship to LV remodeling and fibrosis. Am J Physiol Heart Circ Physiol 302: H1429–H1437, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]