Abstract
Neonatal rats reared on high-carbohydrate (HC) milk formula developed chronic hyperinsulinemia and adult-onset obesity due to programming of islets and the hypothalamic energy circuitry. In this study, calorie restriction by pair-feeding was imposed on HC male rats (HC/PF) to normalize food intake similar to that of mother-fed (MF) rats from weaning until postnatal day 140. A group of HC/PF rats was switched over to ad libitum feeding (HC/PF/AL) from days 90 to 140. Pair-feeding reduced body weight gains and serum insulin and leptin levels in HC/PF rats compared with HC rats, but these parameters were restored to HC levels in the HC/PF/AL rats after ad libitum feeding. Interestingly, the heightened insulin secretory response of isolated islets from adult HC/PF and HC/PF/ AL rats to glucose, acetylcholine, and oxymetazoline were not significantly different from the responses of islets from HC rats. Similarly, the expression of neuropeptide Y and proopiomelanocortin in the hypothalamus was not significantly different among HC, HC/PF, and HC/PF/AL rats. Expression of the leptin receptor in the hypothalami from the HC, HC/PF, and HC/PF/AL rats mirrored that of serum leptin, whereas suppressor of cytokine signaling 3 (Socs3) expression remained high in these three groups. The results indicate that, although calorie restriction resulted in reduction in body weight gain and normalized the serum hormonal pattern, the programed predisposition for the hypersecretory capacity of islets and the hypothalamic hyperphagic response in the HC rats could not be permanently overcome by the pair-feeding imposed on HC rats.
Keywords: obesity, hyperinsulinemia, calorie restriction by pair feeding, metabolic programing, suckling period
in the period 2009–2010, 35.7% of the adult population in the US was classified as obese (BMI >30) (8). The presence of obesity significantly augments the risk for the onset of a number of metabolic disorders such as hypertension, adverse lipid concentrations, and type 2 diabetes (20). Epidemiological data and results from animal models indicate that altered nutritional experiences during early periods in life (fetal and suckling) can predispose the offspring for the development of obesity and metabolic diseases in later life via developmental programming effects (7). These early adaptations enable the organism to endure the nutritional stress but are detrimental in the long run.
Developmental plasticity initiated during fetal development extends into the immediate postnatal period. For example, it has been shown that pancreatic islets and neurons are not fully mature at birth and that their development is completed in the immediate postnatal (suckling) period (10, 12). Hence, an encounter with an altered nutritional experience during this period can independently induce adaptive responses in target organs, resulting in nutritional programming effects. In the context of nutritional programming effects induced in the immediate postnatal period, the long-term consequences of a high-carbohydrate (HC) dietary modification in neonatal rats are being investigated in our laboratory. Artificial rearing of newborn rats on a HC milk formula until the time of weaning resulted in chronic hyperinsulinemia and adult-onset obesity (HC phenotype) in these rats supported by hypersecretory capacity of HC islets and hypothalamic alterations predisposing to hyperphagia (24, 31).
Insulin secretion by pancreatic islets is under the control of peripheral (circulating blood glucose concentrations) and central [autonomic nervous system (ANS)] mechanisms. The parasympathetic nervous system (PNS) and the sympathetic nervous system (SNS) are the two opposing limbs of the ANS that extensively innervate the pancreas and regulate insulin secretion (2, 32). The stimulatory effect of the PNS is exerted via acetylcholine (ACh), whereas the inhibitory effect of the SNS is exerted via norepinephrine (2, 32). In vivo and in vitro studies on the insulin secretory capacity of islets from HC rats indicated an augmented response to cholinergic stimulation and a reduced sensitivity to adrenergic inhibition, suggesting that an altered ANS regulation contributes to the hypersecretory capacity of the HC islet β-cells (19).
The energy circuitry mechanism of the hypothalamus is critical for body weight homeostasis (27). The neurons in the arcuate nucleus produce orexigenic [e.g., neuropeptide Y (NPY)] and anorexigenic [e.g., proopiomelanocortin (POMC)] neuropeptides. They also express receptors for sensing and integration of peripheral signals [such as insulin receptor (Insr) and the long form of the leptin receptor (Lepr)] (4). Our earlier results indicated altered expression of Npy, Pomc, Insr, and Lepr genes in the HC model, suggesting that alterations at the level of the hypothalamic appetite regulatory mechanism supported the development of obesity in HC rats (30).
In the postweaning period, HC rats consumed ∼15% more food compared with age-matched control rats (30). Based on the report that calorie restriction (CR; restriction of energy intake without malnutrition) effectively ameliorated the incidence of obesity and type 2 diabetes (9), two hypotheses were tested in this study: 1) imposition of a pair-feeding regimen on HC rats will normalize the HC phenotype (chronic hyperinsulinemia and adult-onset obesity) to that of control male rats; and 2) moderate CR imposed on HC male rats will permanently reverse the programmed effects in islets and in the hypothalamus. To test these hypotheses, a group of HC male rats were pair-fed (HC/PF) the same amount of rodent chow consumed by control [mother-fed (MF)] rats daily from the time of weaning until postnatal age 140 days. A subgroup of HC/PF rats was given access to rodent chow ad libitum after ∼2 mo of pair-feeding. Our results indicate that the programmed effects on islet function and on the hypothalamic appetite-regulating mechanism were not amenable for permanent reversal by the pair-feeding regimen enforced in this study.
METHODS
Animal protocols.
The University at Buffalo's Animal Care Program and the Laboratory Animal Facilities are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. The Institutional Animal Care and Use Committee at the University at Buffalo approved all animal protocols (protocol no. BCH 06064N). Pregnant Sprague-Dawley rats obtained from Charles River Laboratories (Wilmington, MA) were housed individually with ad libitum access to a standard rodent laboratory chow (Harlan Teklad, Madison, WI) and water and under controlled conditions of temperature, humidity, and a 12:12-h light-dark cycle. One day after the pups were born, the litter size was adjusted to 11 pups/dam. If the litter size was less than 11 pups, additional pups were added to the cage. The bedding material from this cage was spread over the pups so that they acquired the smell of the dam's cage. Our experience over the past several years did not indicate any difference in maternal care of the pups, such as rejection of pups from another dam. The growth rate of the transferred pups was similar within each litter, and the growth rate in the postweaning period did not appear to be influenced by the method that we employed for adjustment of litter size. On postnatal day 4, pups were randomly assigned to the control or experimental group. The control group consisted of pups raised by dams (MF). The calorie composition of rat milk was 8% carbohydrate, 68% fat, and 24% protein. The experimental group consisted of pups artificially reared on the HC milk formula (33). The calorie composition of the HC milk formula was 56% carbohydrate, 20% fat, and 24% protein. Briefly, male pups were mildly anesthetized (isoflurane inhalation), and intragastric cannulas were introduced into them. Buprenorphine (0.02 mg/kg body wt) was administered as an analgesic. Each pup was individually housed in styrofoam cups floating in a 37°C water bath and fed at the rate of 0.45 kcal·g body wt−1·day−1 to match the growth rate of control pups. The pups, cannulas, and the cups were cleaned on a daily basis.
To rule out any adverse effects of the artificial rearing technique per se on the development of the HC phenotype, in earlier studies neonatal rat pups (referred to as HF pups) were fed a high-fat (HF) milk formula, the macronutrient composition of which was similar to that of rat milk (%calorie composition: 8% carbohydrate, 68% fat, and 24% protein), and were artificially reared away from their nursing dams. The metabolic phenotype (body weight, serum hormone levels, insulin secretory capacity of islets, and hypothalamic characteristics) of the HF rats during both the preweaning and postweaning periods was similar to that of the MF rats, which suggests that the development of the HC phenotype was not influenced by the artificial rearing technique employed in our studies (11, 16, 18, 19, 30, 33, 34). Because of the similarity in the phenotype of MF and HF rats, we used only MF rats as control in the present study.
On postnatal day 24, MF rats were weaned onto a standard rodent laboratory chow (Harlan Teklad) with ad libitum access to food and water. Whereas one group of 24-day-old HC rats was weaned onto ad libitum access to a standard rodent laboratory chow, another group of HC rats was pair-fed (HC/PF) the same quantity of rodent chow consumed by MF rats on a daily basis, and they continued on this dietary regimen until the end of the experiment (day 140). On postnatal day 90, a subgroup of HC/PF rats was given ad libitum access to rodent chow (HC/PF/AL) until the end of the experiment. All rats were euthanized by decapitation on postnatal day 140 after being anesthetized with ketamine-xylazine (75 mg and 10 mg/kg body wt). The brain was dissected out and frozen on dry ice and stored at −80°C. The pancreas was dissected and placed in Hanks' balanced salt solution on ice. Trunk blood was collected, and serum was separated and stored at −80°C for analysis of insulin and leptin. Insulin and leptin concentrations were measured by radioimmunoassay using commercially available kits as per the manufacturer's instructions (Millipore, Billerica, MA). Blood glucose concentrations were determined using a glucometer (Ascencia Elite; Bayer, Mishawaka, IN).
Insulin secretion experiments.
Pancreatic islets were isolated by collagenase (Crescent Chemical, Islandia, NY) digestion at 37°C, as described earlier (38). Islets were handpicked under a dissecting microscope. Insulin secretion was measured by static incubation of islets (38). Briefly, five islets from each pancreatic digestion were incubated with 500 μl of Krebs-Ringer bicarbonate buffer containing 16 mM HEPES, 5.5 mM glucose, and 0.01% bovine serum albumin, pH 7.4, for 30 min at 37°C under an atmosphere of 95% O2 and 5% CO2 in a shaking water bath. At the end of the incubation the buffer was aspirated, and the islets were furthered incubated with 500 μl of fresh Krebs-Ringer bicarbonate buffer containing 1) 5.5 mM glucose, 2) 16.7 mM glucose, 3) stringent calcium deprivation [calcium-free Krebs-Ringer buffer + 5.5 mM glucose + 500 μM EDTA and 1 μM nimodipine (CalBiochem, San Diego, CA)], 4) 5.5 mM glucose + 100 μM ACh (Sigma, St. Louis, MO), 5) 16.7 mM glucose + 100 μM ACh, 6) 5.5 mM glucose + 10 μM oxymetazoline (OM; Sigma), and 7) 16.7 mM + 10 μM OM. Aliquots were withdrawn at 10 and 60 min and frozen at −80°C until they were assayed for insulin.
Determination of mRNA levels.
A hypothalamus-enriched region was dissected from the frozen brains, as described by Reyes et. al. (26). Total RNA was extracted from the hypothalamic blocks with TRIzol reagent (Invitrogen, Carlsbad, CA). Total RNA was quantified, and mRNA samples were reverse transcribed into cDNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) according to the manufacturer's instructions. mRNA levels of Npy, Pomc, Lepr, signal transducer and activator of transcription 3 (Stat3), and suppressor of cytokine signaling 3 (Socs3) were determined via real-time PCR assay using the MyiQ Cycler (Bio-Rad). PCR primers were designed to bridge the exon-intron boundaries within the gene of interest to exclude possible contamination by genomic DNA. Primer specificity was confirmed by visualizing PCR products after electrophoresis in an agarose gel. The SYBR Green primers were designed by the web program Primer 3 input (http://frodo.wi.mit.edu/primer3/) and obtained from Integrated DNA Technologies (Coralville, IA). The sequences of the primers used for assays are shown in Table 1. The mRNA levels detected by SYBR Green (Bio-Rad) were normalized to 18S mRNA levels (QuantumRNA Classic II 18S Internal Standard, 324 bp; Ambion, Austin, TX). PCR efficiency was estimated by serially diluting the template cDNA and analysis of the melting curve data. Each sample was run in triplicate. Relative mRNA levels were determined by using the 2ΔΔCT method (17).
Table 1.
Primer sequences for gene expression
| Genes (bp) | Gene ID | Primer Sequence (5′→3′) | Size of PCR Product |
|---|---|---|---|
| Pomc | NM 139326 | Forward: 5′-TCCATAGACGTGTGGAGCTG-3′ | 174 |
| Reverse: 5′-ACGTACTTCCGGGGATTTTC-3′ | |||
| Npy | NM 012614 | Forward: 5′-AGAGATCCAGCCCTGAGACA-3′ | 236 |
| Reverse: 5′-AACGACAACAAGGGAAATGG-3′ | |||
| Lepr | NM 012596 | Forward: 5′-CCTGAAACATTTGAGCATCTTT-3′ | 370 |
| Reverse: 5′-CGATGCACTGGCTGACAGAA-3′ | |||
| Stat3 | NM 012747 | Forward: 5′-TGATGCGCTCTTATGTGAGG-3′ | 104 |
| Reverse: 5′-GGCGGACAGAACATAGGTGT-3′ | |||
| Socs3 | NM 053565 | Forward: 5′-TTGTCGGAAGACTGTCAACG-3′ | 183 |
| Reverse: 5′-GGCTGGATTTTTGTGCTTGT-3′ |
Pomc, proopiomelanocortin; Npy, neuropeptide Y; Lepr, long form of leptin receptor; Stat3, signal transducer and activator of transcription 3; Socs3, suppressor of cytokine signaling 3.
Statistical analysis.
Results are expressed as means ± SE of six to eight rats per group. One-way analysis of variance (ANOVA) was used for comparisons between the difference of the means of MF, HC, HC/PF, and HC/PF/AL groups of rats, followed by post hoc analysis using the Student-Newman-Keuls method. P < 0.05 was considered significant.
RESULTS
Body weight gains.
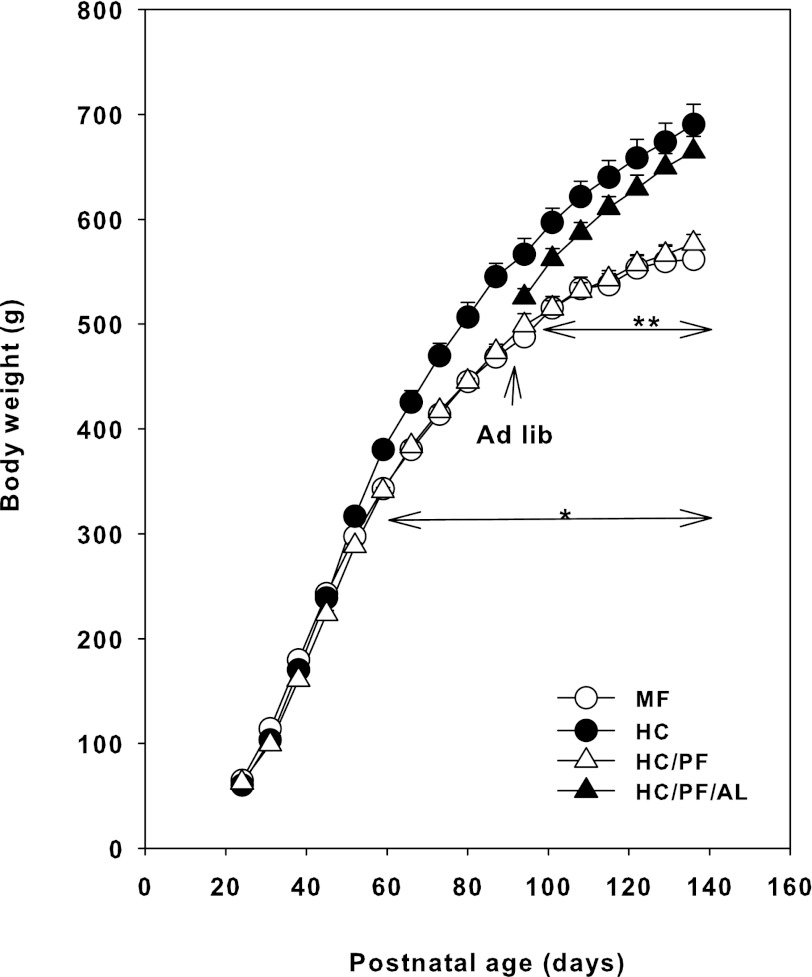
Figure 1 shows the body weights of MF, HC, HC/PF, and HC/PF/AL groups of rats from postnatal days 24 to 140. Up to postnatal day 52 there were no significant differences in the body weights of MF, HC, and HC/PF rats. From postnatal day 52 onward, although the body weight of HC rats was increased significantly (P < 0.05) compared with age-matched MF rats, the body weight of HC/PF rats was similar to that of MF rats. From postnatal day 94 onward (ad libitum feeding was initiated on day 90) the body weight of the HC/PF/AL group of rats demonstrated an increasing trend, and from postnatal day 108 onward there were no significant differences in the body weights of HC and HC/PF/AL groups of rats, indicating catchup growth of these rats.
Fig. 1.
Body weights of mother-fed (MF), high-carbohydrate (HC), high-carbohydrate/pair-fed (HC/PF), and high-carbohydrate/pair-fed/ad libitum (HC/PF/AL) rats from postnatal days 24 to 140. Double arrow indicates initiation of AL feeding of HC/PF rats from postnatal day 90 onward. Results are means ± SE of 8 rats/group. *P < 0.05 for HC compared with MF and HC/PF; **P < 0.05 for HC/PF/AL compared with MF and HC/PF (ANOVA followed by post hoc analysis using the Student-Newman-Keuls method).
Food intake.
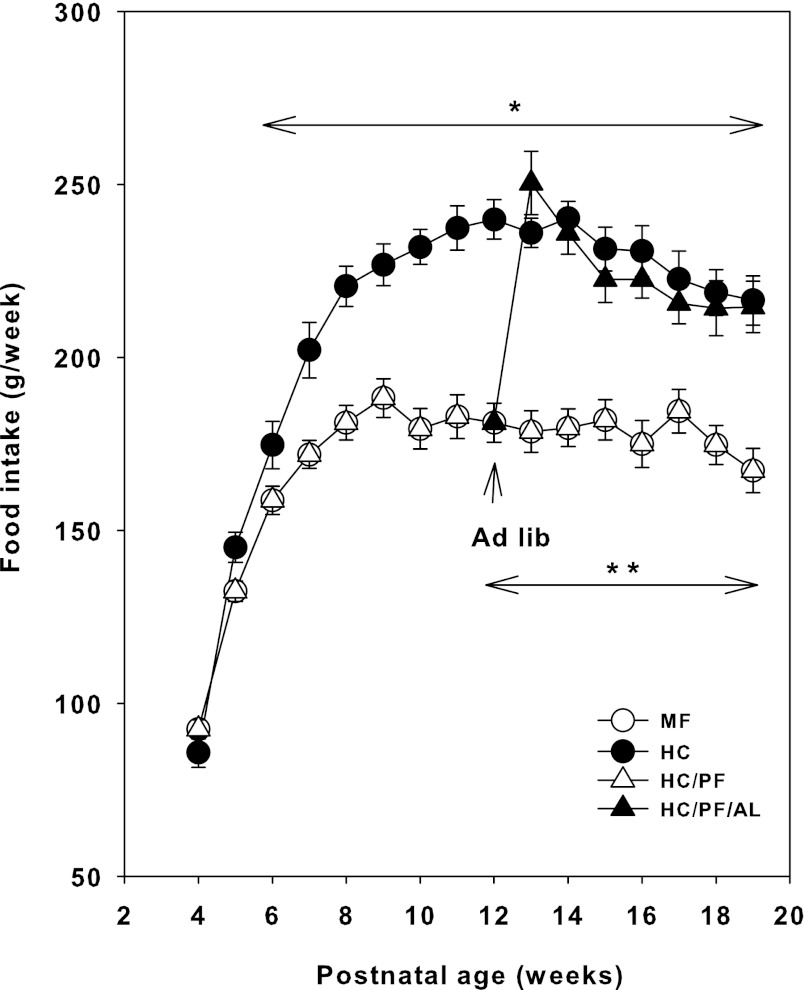
On postnatal day 24, MF and HC rats were weaned onto a standard rodent laboratory chow and water ad libitum, whereas the HC/PF rats were pair-fed the same quantity of chow consumed by age-matched MF rats on a daily basis, with ad libitum access to water. Food intake was measured on a weekly basis. From postnatal week 6 (day 42) onward there was a significant increase (P < 0.05) in the amount of rodent chow consumed by HC rats compared with the amount of rodent chow consumed by age-matched MF rats (Fig. 2). When a group of HC/PF rats were allowed ad libitum feeding on postnatal day 90, within 1 wk the HC/PF/AL rats consumed a significantly increased (P < 0.05) quantity of rodent chow compared with age-matched MF rats and closely approximated the quantity of feed consumed by HC rats during the same period (Fig. 2). From then on, there were no significant differences in the amount of food consumed by the HC and HC/PF/AL groups of rats, whereas there was a marked difference in the amount of rodent chow consumed by HC/PF/AL and HC/PF (and MF) rats (Fig. 2).
Fig. 2.
Weekly intake of rodent chow for MF, HC, HC/PF, and HC/PF/AL in the postweaning period. Double arrow indicates initiation of AL feeding of HC/PF rats from postnatal day 90 onward. The results are means ± SE of 8 rats/group. *P < 0.05 for HC compared with MF and HC/PF; **P < 0.05 for HC/PF/AL compared with MF and HC/PF (ANOVA followed by post hoc analysis using the Student-Newman-Keuls method).
Serum hormone levels.
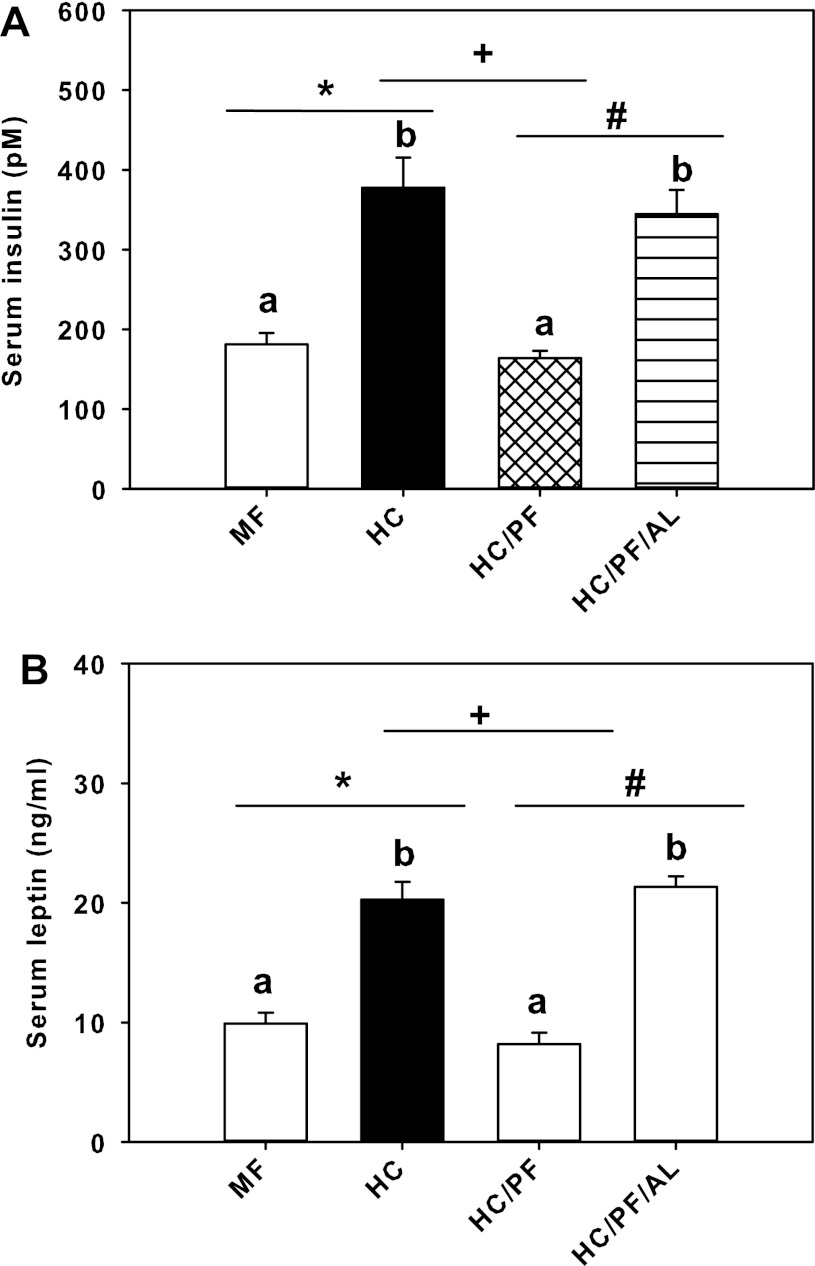
Figure 3 shows the serum levels of insulin and leptin for the four groups of rats. Whereas serum insulin levels were markedly increased (P < 0.05) in HC rats compared with MF rats, pair-feeding resulted in a significant reduction of serum insulin levels in HC/PF rats compared with HC rats and normalization to the levels observed in MF rats (Fig. 3A). The serum insulin levels in HC/PF/AL rats were not significantly different from the levels in HC rats and were increased significantly compared with HC/PF (and MF) rats. A similar trend was observed for serum leptin levels. Pair-feeding resulted in marked reduction of serum leptin levels in HC/PF rats compared with HC rats, and the levels of leptin in HC/PF/AL rats increased to the levels observed in HC rats (Fig. 3B). Additionally, serum glucose levels in randomly fed rats (mg/dl) were not significantly different between the control and experimental groups of rats (MF: 212 ± 9; HC: 220 ± 11; HC/PF: 203 ± 9; HC/PF/AL: 196 ± 4).
Fig. 3.
Serum insulin (A) and leptin levels (B) in 140-day-old MF, HC, HC/PF, and HC/PF/AL rats. The results are means ± SE of 8 rats/group. *P < 0.05 for HC compared with MF; +P < 0.05 for HC/PF compared with HC; #P < 0.05 for HC/PF/AL compared with HC/PF (ANOVA followed by post hoc analysis using the Student-Newman-Keuls method). aNo significant differences between MF and HC/PF groups of rats; bno significant differences between HC ad HC/PF/AL groups of rats.
Insulin secretion from islets.
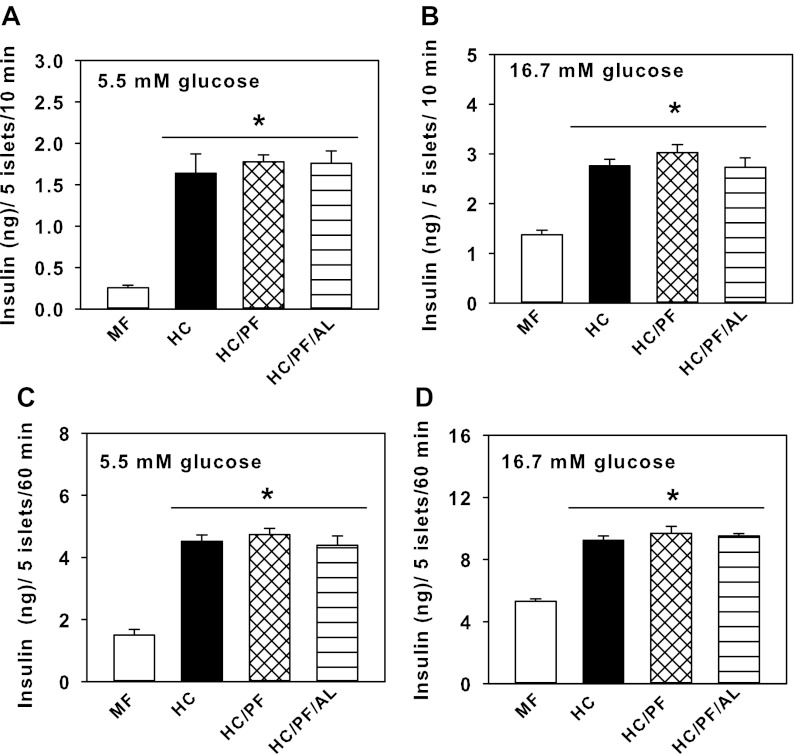
The insulin secretory response to glucose was studied at basal (5.5 mM) and maximally stimulatory (16.7 mM) concentrations at both 10 and 60 min by islets isolated from 140-day-old MF, HC, HC/PF, and HC/PF/AL rats. In the presence of 5.5 mM glucose, HC islets secreted significantly increased amounts of insulin at both 10 and 60 min (Fig. 4, A and B). However, there were no differences in the amount of insulin secreted by HC/PF and HC/PF/AL islets compared with HC islets. Similar observations were found for insulin secreted at 60 min (Fig. 4, C and D).
Fig. 4.
Insulin secretion by islets isolated from 140-day-old MF, HC, HC/PF, and HC/PF/AL rats in the presence of 5.5 (A) and 16.7 mM glucose (B) at 10 min and 5.5 (C) and 16.7 mM glucose (D) at 60 min. Results are means ± SE of 8 rats/group. *P < 0.05 for comparison with MF (ANOVA followed by post hoc analysis using the Student-Newman-Keuls method).
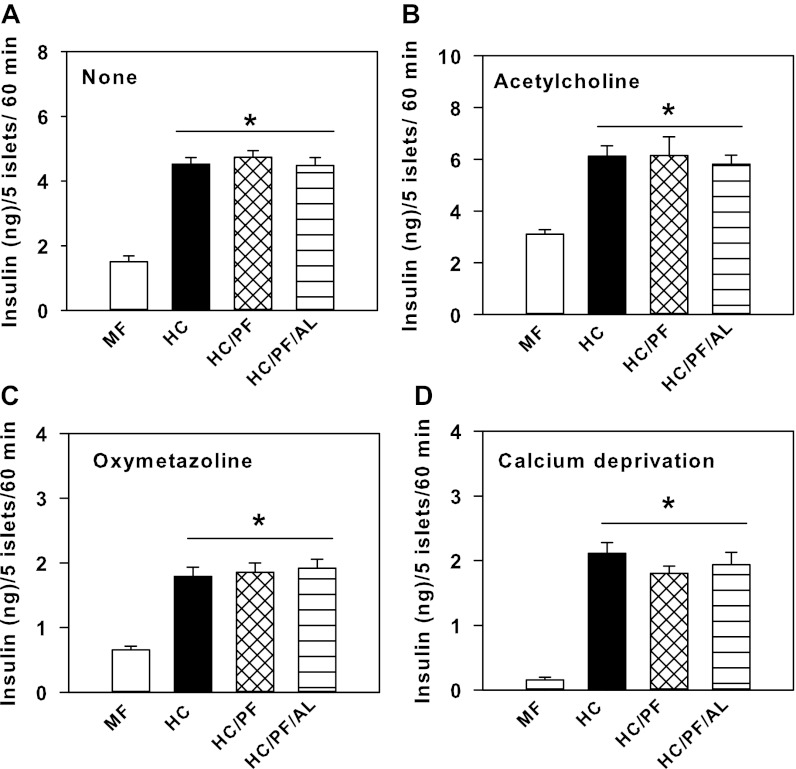
The insulin secretion response of islets from the four groups of rats was further evaluated in the presence of ACh, a muscarinic receptor agonist, and OM, an α1-adrenergic receptor agonist, at both 5.5 and 16.7 mM glucose at 60 min. ACh treatment (100 μM; Fig. 5B) significantly potentiated secretion of insulin by islets from all four groups of rats at 60 min in the presence of 5.5 mM glucose compared with glucose treatment alone (Fig. 5A). However, the stimulation of insulin secretion in the presence of ACh and 5.5 mM glucose was markedly higher in HC islets compared with MF islets (Fig. 5B). The responses of HC/PF and HC/PF/AL islets to ACh in the presence of 5.5 mM glucose were not significantly different from that observed for HC islets (Fig. 5B). OM treatment (10 μM) resulted in significant reductions in the amount of insulin secreted by islets from all four groups of rats (Fig. 5C) compared with their response to 5.5 mM glucose alone (Fig. 5A). However, the inhibitory effect of OM on insulin secretion by HC islets was blunted compared with its effect on MF islets. Furthermore, HC/PF and HC/PF/AL islets responded to OM treatment in a manner similar to that observed for HC islets (Fig. 5C).
Fig. 5.
Insulin secretion by islets isolated from 140-day-old MF, HC, HC/PF, and HC/PF/AL rats in the presence of 5.5 mM glucose plus none (A), 100 μM acetylcholine (B), 10 μM oxymetazoline (C), and stringent calcium-deprived conditions (D) at 60 min. Results are means ± SE of 8 rats/group. *P < 0.05 for comparison with MF (ANOVA followed by post hoc analysis using the Student-Newman-Keuls method).
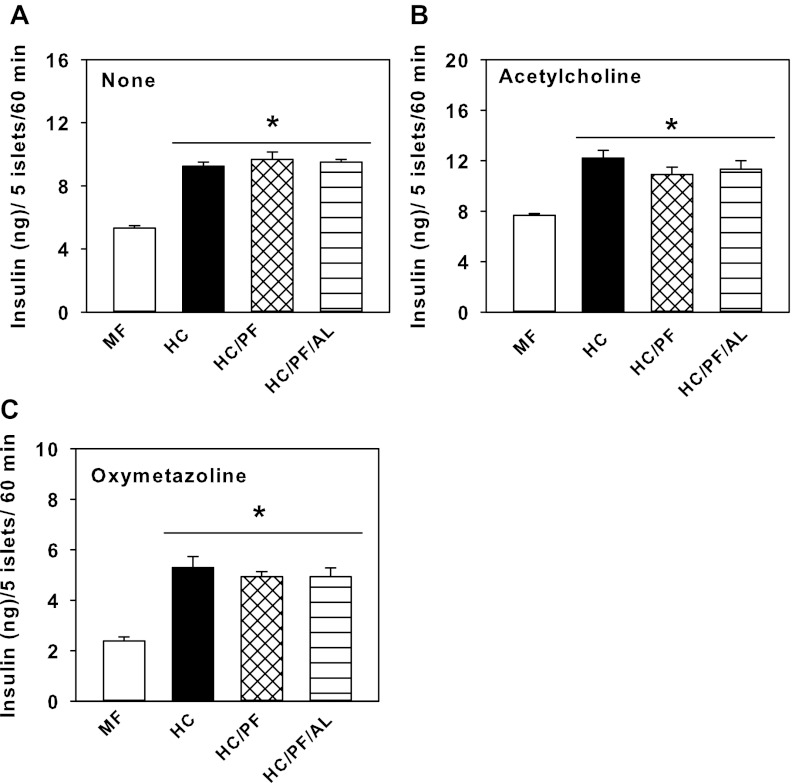
In the presence of 16.7 mM glucose, ACh induced a large potentiation of insulin secretion in HC islets compared with MF islets (Fig. 6, A and B), although there were no differences in its ability to stimulate insulin secretion by HC, HC/PF, and HC/PF/AL islets. OM markedly inhibited insulin secretion in the presence of 16.7 mM glucose by islets from each of the groups of rats, but as observed in the presence of 5.5 mM glucose, its ability to inhibit insulin secretion was blunted in HC islets compared with MF islets (Fig. 6, A and C). OM treatment in the presence of 16.7 mM glucose elicited similar responses in HC, HC/PF, and HC/PF/AL islets (Fig. 6C).
Fig. 6.
Insulin secretion by islets isolated from 140-day-old MF, HC, HC/PF, and HC/PF/AL rats in the presence of 5.5 mM glucose plus none (A), 100 μM acetylcholine (B), and 10 μM oxymetazoline (C) at 60 min. Results are means ± SE of 8 rats/group. *P < 0.05 for comparison with MF (ANOVA followed by post hoc analysis using the Student-Newman-Keuls method).
An earlier study indicated that HC islets secreted significant amounts of insulin under stringent calcium-deprived conditions (29). In this study, a similar investigation was carried out with islets from the four groups of rats. Under stringent calcium-deprived conditions in the presence of 5.5 mM glucose, whereas MF islets secreted minimal amounts of insulin (0.15 ng), HC islets secreted significantly larger amounts of insulin (2.13 ng) at 60 min (Fig. 5D). The ability to secrete insulin under stringent calcium-deprived conditions was maintained in islets from HC/PF and HC/PF/AL rats (Fig. 5D).
mRNA levels in the hypothalamus.
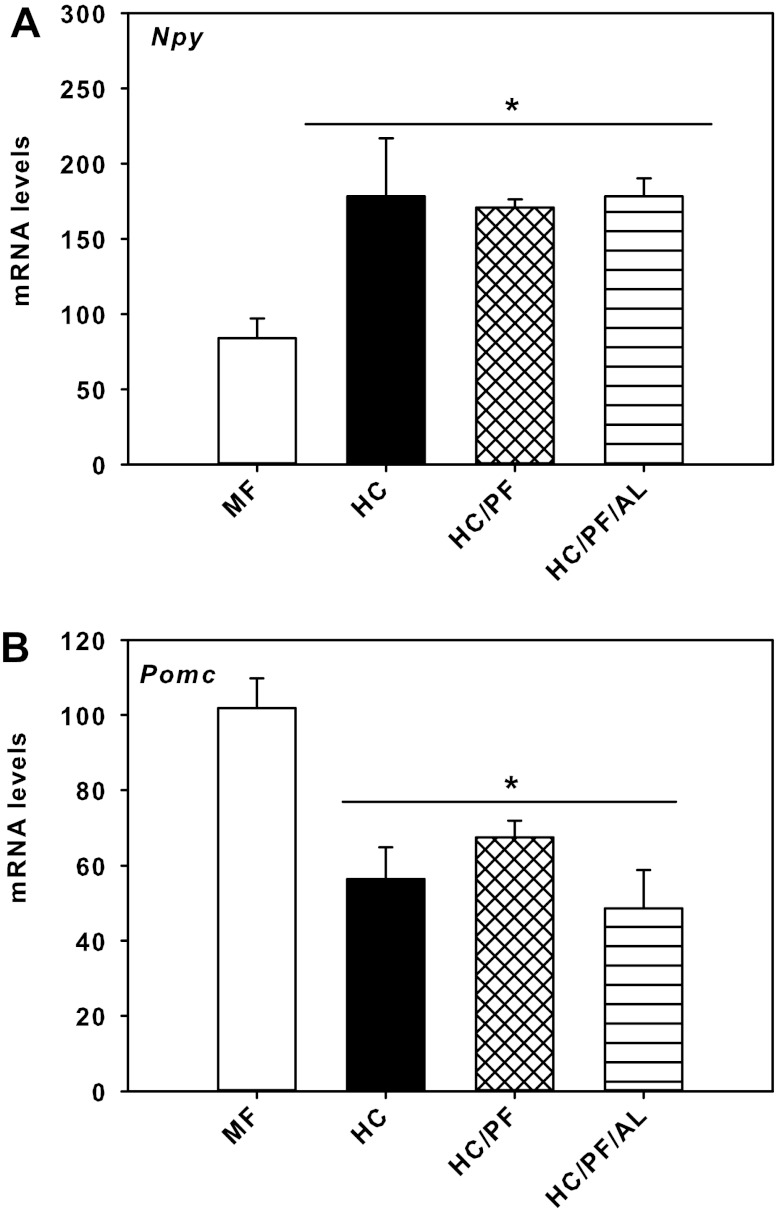
The alterations in the hypothalamic regulation of appetite due to the HC dietary modification, pair-feeding, and switching pair-feeding to ad libitum feeding were determined in the MF, HC, HC/PF, and HC/PF/AL groups of rats. For this purpose, the expression of genes such as neuropeptides Npy and Pomc and those of the Lepr and downstream components of the leptin-signaling pathway were quantified. Figure 7A depicts the mRNA levels of Npy in the hypothalamus of the four groups of rats. mRNA levels of Npy were significantly higher (P < 0.05) in the hypothalamus of HC rats compared with MF rats, but surprisingly, there were no significant differences in the expression of this gene in the hypothalamus of HC, HC/PF, and HC/PF/AL rats, suggesting that pair-feeding did not result in a reduction in the expression of this gene in the HC/PF hypothalamus (Fig. 7A). The mRNA levels of Pomc were reduced significantly (P < 0.05) in the hypothalamus of HC rats (Fig. 7B). As in the case of the expression of Npy, there were no differences in the mRNA levels of Pomc in the hypothalamus of HC, HC/PF, or HC/PF/AL groups of rats.
Fig. 7.
Quantitative real-time PCR analysis of neuropeptide Y (Npy; A) and proopiomelanocortin (Pomc; B) mRNA levels in the hypothalamus-enriched region obtained from 140-day-old MF, HC, HC/PF, and HC/PF/AL rats. The threshold cycle values were normalized using 18s as an internal control. Fold differences were calculated using the ΔΔCT method. Individual samples were run in triplicate, and results are means ± SE of 6 rats/group. *P < 0.05 for comparison with MF (ANOVA followed by post hoc analysis using the Student-Newman-Keuls method).
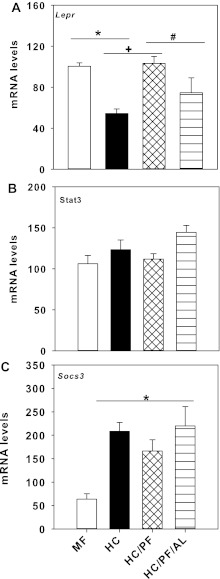
Figure 8A shows the mRNA levels of Lepr in the hypothalamus of the four groups of rats. The mRNA levels of Lepr were significantly reduced (P < 0.05) in the hypothalamus of HC rats compared with MF rats but were normalized to MF levels in the HC/PF rats. In the hypothalamus of HC/PF/AL rats, the mRNA levels of Lepr were significantly reduced (P < 0.05) compared with levels in MF and HC/PF hypothalamus, but levels were not significantly different from levels in HC rats. The expression of Stat3 did not differ significantly in the hypothalamus of the four groups of rats (Fig. 8B). In contrast, the mRNA levels of Socs3 in the hypothalamus of HC, HC/PF, and HC/PF/AL were markedly increased compared with MF rats. Interestingly, there were no differences in Socs3 expression between the three groups of rats (Fig. 8C).
Fig. 8.
Quantitative real-time PCR analysis of the long form of leptin receptor (Lepr; A), signal transducer and activator of transcription 3 (Stat3; B), and suppressor of cytokine signaling 3 (Socs3; C) mRNA levels in the hypothalamus-enriched region obtained from 140-day-old MF, HC, HC/PF, and HC/PF/AL rats. The threshold cycle values were normalized, using 18s as an internal control. Fold differences were calculated using the ΔΔCT method. Individual samples were run in triplicate, and results are means ± SE of 6 rats/group. *P < 0.05 for comparison of HC with MF; +P < 0.05 for comparison of HC/PF with HC; #P < 0.05 for comparison of HC/PF/AL with HC/PF. ANOVA was followed by post hoc analysis using the Student-Newman-Keuls method.
DISCUSSION
Epidemiological findings and results from animal models have demonstrated clearly that altered nutritional experiences during critical periods of development are an important factor in the etiology of obesity and related metabolic diseases (7). Reversal of these early programming effects by application of an appropriate regimen in the postweaning period is a promising avenue for the reduction of the prevalence of overweight/obesity in the population. The results from the present study indicate that pair-feeding transiently improved the gross metabolic phenotype in the HC rats. Importantly, our results indicate that the programmed effects in the islets and the hypothalamus that underlie the development of hyperinsulinemia and hyperphagia in the HC rat could not be permanently erased under our experimental protocol.
With reference to the first hypothesis of this study, pair-feeding of HC rats from the time of weaning resulted in normalization of body weight, food intake, and serum hormone levels in the adult male HC/PF rats, culminating in a MF-like gross metabolic profile in these rats. This observation is in concurrence with the report that CR causes reductions in body weight and normalization of blood glucose and hormone levels in obese animals as well as in nonhuman primates (35). Another study showed that CR reversed the alterations in taste-guided responsiveness that was induced by high-fat diet feeding in rats (28). The pair-feeding regimen imposed on the HC rats involved restriction of rodent chow for the HC/PF rats on a daily basis from the time of weaning, whereas CR in other animal models involved food restriction ranging from 20 to 40% during the period of investigation and in most studies was imposed on older animals. The consensus among these studies is that CR is beneficial for improvement of metabolic parameters in overweight/obese individuals.
In the context of the second hypothesis of this study, although PF impacted positively in the HC rats, as observed from the metabolic profile of the HC/PF rats, our results on the HC/PF/AL rats indicate clearly that the normalization of the HC phenotype to that of the MF phenotype was a temporary response consequent to reduced food availability. As soon as the HC/PF rats were provided free access to rodent chow, the features of the HC phenotype reappeared within a short span of time. Similar observations have been reported in other animal models for obesity. Subjecting high-fat diet-fed obese rats to CR by 40% of ad libitum consumption for 2 wk resulted in a reduction of ∼12% body weight compared with controls. However, the calorie-restricted rats regained the lost body weight on resumption of ad libitum feeding, indicating defense of their body weight (37). In another study, it was shown that a 40% reduction in daily intake of a high-fat diet in diet-induced obese rats for 4 wk reduced body weight, body fat, and plasma leptin and normalized leptin signaling in the hypothalamus of these rats. When food restriction was lifted for these rats' body weights, body fat and plasma leptin increased rapidly to basal levels (25). The above observations suggest that the effects of CR are transitory in nature.
Although insulin secretion is regulated primarily by circulating glucose concentrations (15), the ANS also controls insulin secretion by islets (2, 32). HC islets demonstrated aberrations at both of these levels of regulation of insulin secretion: 1) a leftward shift in the response to glucose (1, 29) and 2) an increased PNS activity (increased response to ACh stimulation) and a compromised SNS activity (diminished inhibition by OM) (18, 19). Our results indicate that although serum insulin levels were normalized in the adult HC/PF rats, the in vitro insulin secretory capacity of HC/PF islets did not differ from that observed for HC islets. The response of HC/PF islets to glucose, ACh, OM, and stringent calcium deprivation matched the response of HC islets to these agonists/antagonists. These results indicate that the programming effects of the HC dietary modification on the insulin secretory capacity could not be reversed by the pair-feeding regimen imposed on HC rats in the postweaning period. The persistence of the early programmed effects on insulin secretory capacity in the HC/PF rats provides a mechanistic basis for the reappearance of the hyperinsulinemia in the HC/PF/AL rats.
The reduced levels of serum insulin in HC/PF rats are likely due to the reduced amount of food provided to these rats on a daily basis because of the imposition of the pair-feeding regimen. The termination of the pair-feeding regimen resulted in the increase in serum insulin levels in the HC/PF/AL rats because of increased food consumption by these rats. Unlike the results on the gross metabolic features, pair-feeding could not expunge the programmed effects on the insulin secretory ability of HC islets. In the HC/PF rats, there was a suppression of these programmed effects that resurfaced to full capacity in the HC/PF/AL rats. Interestingly, 60% CR imposed on 8-wk-old rodent chow-fed rats for 3 wk resulted in diminished glucose-induced insulin secretion in islets (5). Unlike the HC rats, the rats used in that study were not nutritionally malprogrammed for an altered insulin secretory response.
In the case of hypothalamic programming for development of obesity, our earlier studies demonstrated that the mRNA levels of Npy were increased, whereas the mRNA levels of Pomc were decreased in the hypothalamus of HC rats (30). The results from the present study confirm the earlier observations on the HC rat. The expression of Npy and Pomc was not different among the HC, HC/PF, and HC/PF/AL groups of rats, suggesting that malprogramming of the hypothalamic energy homeostasis mechanism at the level of the orexigenic and anorexigenic neuropeptide expression was not erased by the PF protocol in this study.
Additionally, the expression of Lepr was markedly reduced in the HC hypothalamus compared with control MF rats, which is in agreement with our earlier results (30). Under normal conditions the expression of Lepr in the hypothalamus parallels the circulating leptin levels, but under conditions of leptin resistance there is a reverse correlation between circulating leptin levels and expression of Lepr in the hypothalamus (22). The pair-feeding regimen normalized the mRNA levels of Lepr in the hypothalamus of HC/PF rats, suggesting that the expression of this gene is regulated by circulating levels of leptin. In high-fat diet-fed obese rats, diminished expression of the leptin receptor coupled with attenuated leptin-induced STAT3 phosphorylation was reversed by CR (36). That the pair-feeding approach did not erase the program for hyperphagia is indicated by the decreased expression of Lepr in the hypothalamus of the HC/PF/AL rats. Defects in the expression and protein levels of Stat3 have been observed in the obese state (6, 21). There were no significant differences in the expression of Stat3 in the hypothalami of the four groups of rats in this study. The levels of pSTAT3 (the functional form of STAT3) were not determined in this study. It is possible that the programmed effects are at the level of phosphorylation of STAT3 in this model and not at the level of its expression. SOCS3 is a negative regulator of leptin signaling (14). Increased mRNA levels of Socs3 have been reported in the hypothalamus of obese rodent models (21). In the present study, the mRNA levels of Socs3 were markedly higher in the hypothalami of HC, HC/PF, and HC/PF/AL rats compared with its level in the control hypothalamus, indicating that PF was not efficacious for overcoming the programmed effects in the HC hypothalamus.
Compared with the high-fat diet-induced obese rats wherein the dietary treatment was implemented in the postweaning period, the HC rat model is a typical example of metabolic programming because the HC dietary modification was imposed during the critical period of organ development (pancreatic islets and hypothalamic axonal connections) in the immediate postnatal period. Furthermore, the HC rats were not subjected to any altered dietary treatment in the postweaning period. The results from the present study indicate that although normalization of the food intake of HC rats to that of age-matched control rats from the time of weaning (HC/PF rats) resulted in beneficial effects during the period of its implementation, the programmed effects in islets and the hypothalamus that support chronic hyperinsulinemia and hyperphagia could not be deleted by the pair-feeding approach implemented from postnatal days 24 to 140. (nearly 4 mo). The pair-feeding regimen used in this study, although not as severe as observed in some of the studies on CR of high-fat diet-fed obese rats, was effective in normalizing the gross HC phenotype in the HC/PF rats but was not effective in reversing the mechanisms that support the hypersecretory capacity of islets and hyperphagia in the hypothalamus.
Epidemiological data suggest that breast feeding can protect against later development of obesity and major features of the metabolic syndrome (3, 23), whereas increased body weight gains and adult-onset obesity were observed in infants who were fed only formula in their infancy (13). In the context of the human obesity epidemic, the observations from this study suggest that dietary practices for infants require careful attention. In individuals in whom metabolic programming contributed to the development of obesity, the results from the present study imply that the altered set points in target organs may not be amenable for reversal by measures such as dietary restriction implemented in later life.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-61518 (M. S. Patel).
DISCLOSURES
The authors declare that there are no conflicts of interest, financial or otherwise.
AUTHOR CONTRIBUTIONS
M.S. and M.S.P. contributed to the conception and design of the research; M.S. and S.M. performed the experiments; M.S., S.M., and M.S.P. analyzed the data; M.S. and M.S.P. interpreted the results of the experiments; M.S. and S.M. prepared the figures; M.S. drafted the manuscript; M.S., S.M., and M.S.P. edited and revised the manuscript; M.S., S.M., and M.S.P. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Dr. Suzanne Laychock of the Department of Pharmacology and Toxicology, University at Buffalo, for constructive comments on the manuscript.
REFERENCES
- 1. Aalinkeel R, Srinivasan M, Kalhan SC, Laychock SG, Patel MS. A dietary intervention (high carbohydrate) during the neonatal period causes islet dysfunction in rats. Am J Physiol Endocrinol Metab 277: E1061–E1069, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Ahren B. Autonomic regulation of islet hormone secretion—implications for health and disease. Diabetologia 43: 393–410, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Arenz S, Ruckerl R, Koletzko B, von Kries R. Breast-feeding and childhood obesity—a systematic review. Int J Obes Relat Metab Disord 28: 1247–1256, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci 8: 571–578, 2005 [DOI] [PubMed] [Google Scholar]
- 5. do Amaral ME, Ueno M, Oliveira CA, Borsonello NC, Vanzela EC, Ribeiro RA, Alves PL, Barbosa HC, Carneiro EM, Boschero AC. Reduced expression of SIRT1 is associated with diminished glucose-induced insulin secretion in islets from calorie-restricted rats. J Nutr Biochem 22: 554–559, 2011 [DOI] [PubMed] [Google Scholar]
- 6. El-Haschimi K, Pierroz DD, Hileman SM, Bjørbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest 105: 1827–1832, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fernandez-Twinn DS, Ozanne SE. Early life nutrition and metabolic programming. Ann NY Acad Sci 1212: 78–96, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303: 235–241, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Fontana L, Klein S. Aging, adiposity, and calorie restriction. JAMA 297: 986–994, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Grove KL, Allen S, Grayson BE, Smith MS. Postnatal development of the hypothalamic neuropeptide Y system. Neuroscience 116: 393–406, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Hiremagalur BK, Vadlamudi S, Johanning GL, Patel MS. Long-term effects of feeding high carbohydrate diet in pre-weaning period by gastrostomy: a new rat model for obesity. Int J Obes Relat Metab Disord 17: 495–502, 1993 [PubMed] [Google Scholar]
- 12. Huang HP, Tsai MJ. Transcription factors involved in pancreatic islet development. J Biomed Sci 7: 27–34, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Ip S, Chung M, Raman G, Chew P, Magula N, DeVine D, Trikalinos T, Lau J. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep) 1–186, 2007 [PMC free article] [PubMed] [Google Scholar]
- 14. Isobe A, Takeda T, Sakata M, Yamamoto T, Minekawa R, Hayashi M, Auernhammer CJ, Tasaka K, Murata Y. STAT3-mediated constitutive expression of SOCS3 in an undifferentiated rat trophoblast-like cell line. Placenta 27: 912–918, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Komatsu M, Schermerhorn T, Noda M, Straub SG, Aizawa T, Sharp GW. Augmentation of insulin release by glucose in the absence of extracellular Ca2+: new insights into stimulus-secretion coupling. Diabetes 46: 1928–1938, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Laychock SG, Vadlamudi S, Patel MS. Neonatal rat dietary carbohydrate affects pancreatic islet insulin secretion in adults and progeny. Am J Physiol Endocrinol Metab 269: E739–E744, 1995 [DOI] [PubMed] [Google Scholar]
- 17. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Mitrani P, Srinivasan M, Dodds C, Patel MS. Autonomic involvement in the permanent metabolic programming of hyperinsulinemia in the high-carbohydrate rat model. Am J Physiol Endocrinol Metab 292: E1364–E1377, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Mitrani P, Srinivasan M, Dodds C, Patel MS. Role of the autonomic nervous system in the development of hyperinsulinemia by high-carbohydrate formula feeding to neonatal rats. Am J Physiol Endocrinol Metab 292: E1069–E1078, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Morrison JL, Duffield JA, Muhlhausler BS, Gentili S, McMillen IC. Fetal growth restriction, catch-up growth and the early origins of insulin resistance and visceral obesity. Pediatr Nephrol 25: 669–677, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Munzberg H, Flier JS, Bjørbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145: 4880–4889, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Myers MG, Jr, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab 21: 643–651, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics 115: 1367–1377, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Patel MS, Srinivasan M. Metabolic programming in the immediate postnatal life. Ann Nutr Metab 58, Suppl 2: 18–28, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reidelberger R, Haver A, Chelikani PK, Apenteng B, Perriotte-Olson C, Anders K, Steenson S, Blevins JE. Effects of leptin replacement alone and with exendin-4 on food intake and weight regain in weight-reduced diet-induced obese rats. Am J Physiol Endocrinol Metab 302: E1576–E1585, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reyes TM, Walker JR, DeCino C, Hogenesch JB, Sawchenko PE. Categorically distinct acute stressors elicit dissimilar transcriptional profiles in the paraventricular nucleus of the hypothalamus. J Neurosci 23: 5607–5616, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 404: 661–671, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Shin AC, Townsend RL, Patterson LM, Berthoud HR. “Liking” and “wanting” of sweet and oily food stimuli as affected by high-fat diet-induced obesity, weight loss, leptin, and genetic predisposition. Am J Physiol Regul Integr Comp Physiol 301: R1267–R1280, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Srinivasan M, Aalinkeel R, Song F, Lee B, Laychock SG, Patel MS. Adaptive changes in insulin secretion by islets from neonatal rats raised on a high-carbohydrate formula. Am J Physiol Endocrinol Metab 279: E1347–E1357, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Srinivasan M, Mitrani P, Sadhanandan G, Dodds C, Shbeir-ElDika S, Thamotharan S, Ghanim H, Dandona P, Devaskar SU, Patel MS. A high-carbohydrate diet in the immediate postnatal life of rats induces adaptations predisposing to adult-onset obesity. J Endocrinol 197: 565–574, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Srinivasan M, Patel MS. Metabolic programming in the immediate postnatal period. Trends Endocrinol Metab 19: 146–152, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Thorens B. Brain glucose sensing and neural regulation of insulin and glucagon secretion. Diabetes Obes Metab 13, Suppl 1: 82–88, 2011 [DOI] [PubMed] [Google Scholar]
- 33. Vadlamudi S, Hiremagalur BK, Tao L, Kalhan SC, Kalaria RN, Kaung HL, Patel MS. Long-term effects on pancreatic function of feeding a HC formula to rats during the preweaning period. Am J Physiol Endocrinol Metab 265: E565–E571, 1993 [DOI] [PubMed] [Google Scholar]
- 34. Vadlamudi S, Kalhan SC, Patel MS. Persistence of metabolic consequences in the progeny of rats fed a HC formula in their early postnatal life. Am J Physiol Endocrinol Metab 269: E731–E738, 1995 [DOI] [PubMed] [Google Scholar]
- 35. Wanagat J, Allison DB, Weindruch R. Caloric intake and aging: mechanisms in rodents and a study in nonhuman primates. Toxicol Sci 52: 35–40, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Wilsey J, Scarpace PJ. Caloric restriction reverses the deficits in leptin receptor protein and leptin signaling capacity associated with diet-induced obesity: role of leptin in the regulation of hypothalamic long-form leptin receptor expression. J Endocrinol 181: 297–306, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Woods SC, Seeley RJ, Rushing PA, D'Alessio D, Tso P. A controlled high-fat diet induces an obese syndrome in rats. J Nutr 133: 1081–1087, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Xia M, Laychock SG. Insulin secretion, myo-inositol transport, and Na(+)-K(+)-ATPase in glucose-desensitized rat islets. Diabetes 42: 1392–1400, 1993 [DOI] [PubMed] [Google Scholar]