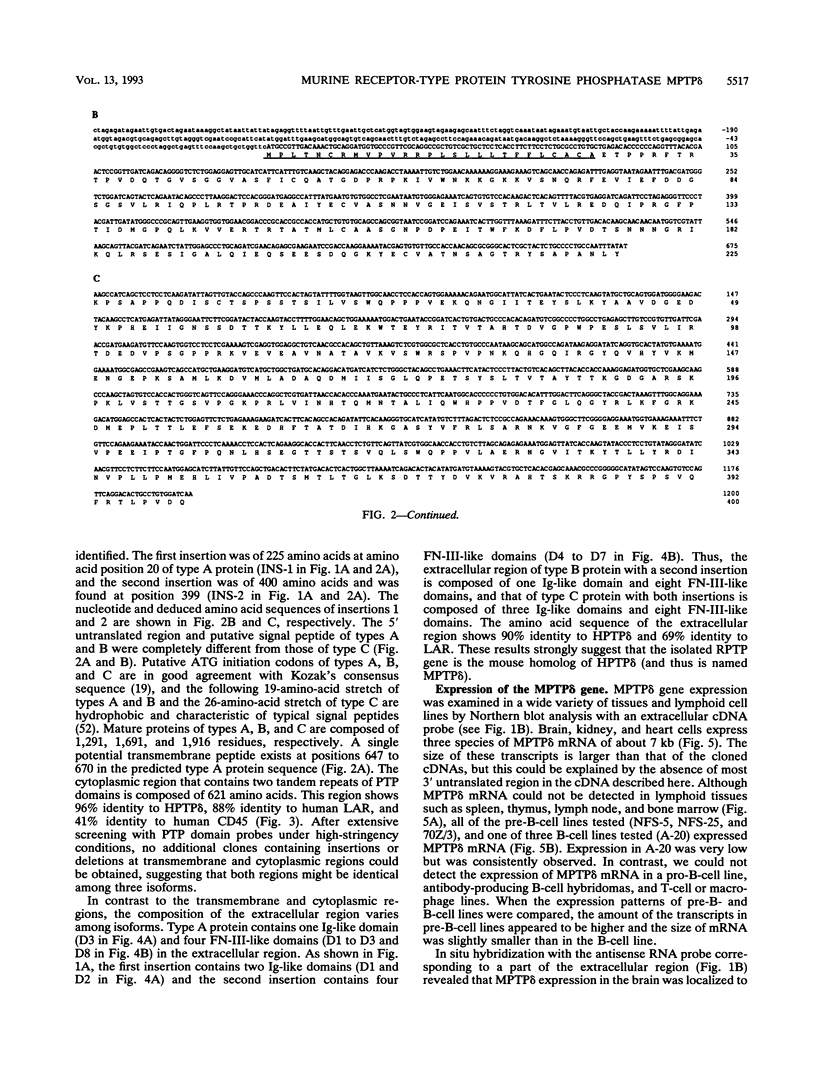
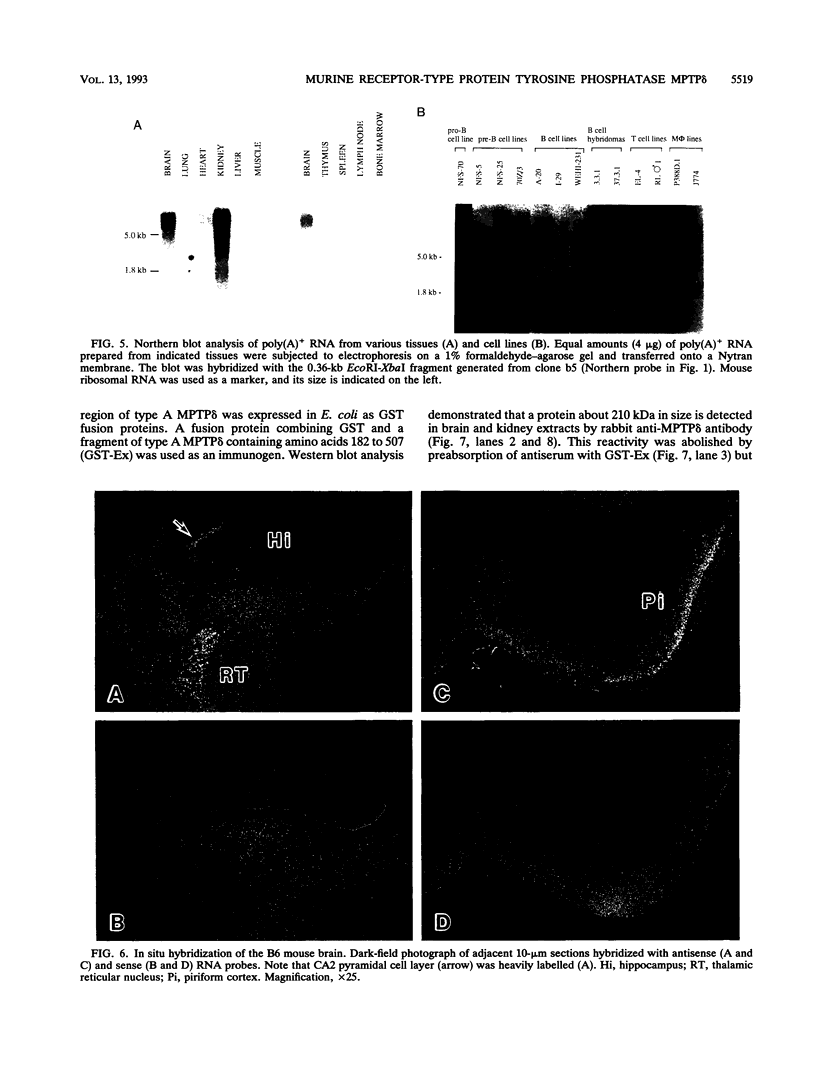
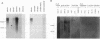Abstract
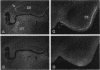
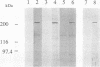
Protein tyrosine phosphatases (PTPs), together with protein tyrosine kinases (PTKs), are involved in the regulation of cell activation, growth, and differentiation. To further elucidate the fine tuning of cell growth and differentiation through tyrosine phosphorylation, we tried to isolate mouse receptor-type PTP (RPTP) cDNA clones by screening mouse brain cDNA libraries with mouse CD45 PTP domain probes under reduced-stringency conditions. Characterization of isolated cDNA clones for RPTP showed that the cytoplasmic region contains two tandem repeats of PTP domain of about 230 amino acids with intrinsic phosphatase activity. The extracellular region was composed of immunoglobulin (Ig)-like domains and fibronectin type III (FN-III)-like domains. The gene was highly homologous to human PTP delta (HPTP delta) and thus was named MPTP delta (murine counterpart of HPTP delta). The MPTP delta gene appeared to generate at least three species of mRNA, which differ in the composition of the extracellular domain: type A, one Ig-like and four FN-III-like domains; type B, one Ig-like and eight FN-III-like domains; and type C, three Ig-like and eight FN-III-like domains. Interestingly, the 5' untranslated region and the leader peptide of types A and B were completely different from those of type C. Northern (RNA) blot analysis demonstrated that brain, kidney, and heart cells express three mRNA species of about 7 kb. Antibody directed against part of the extracellular domain of type A MPTP delta recognized a 210-kDa protein in brain and kidney lysates. In situ hybridization of brain samples revealed that MPTP delta mRNA is present in the hippocampus, thalamic reticular nucleus, and piriform cortex, where some Src family PTKs have been also demonstrated to exist. Although MPTP delta mRNA was not detected in lymphoid tissues, all of the pre-B-cell lines tested and one of three B-cell lines tested expressed MPTP delta mRNA, whereas antibody-producing B-cell hybridomas and T-cell and macrophage lines did not. Finally, the MPTP delta locus was tightly linked to the brown (b) locus on mouse chromosome 4.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander D. R. The role of phosphatases in signal transduction. New Biol. 1990 Dec;2(12):1049–1062. [PubMed] [Google Scholar]
- Barthels D., Santoni M. J., Wille W., Ruppert C., Chaix J. C., Hirsch M. R., Fontecilla-Camps J. C., Goridis C. Isolation and nucleotide sequence of mouse NCAM cDNA that codes for a Mr 79,000 polypeptide without a membrane-spanning region. EMBO J. 1987 Apr;6(4):907–914. doi: 10.1002/j.1460-2075.1987.tb04837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomly K. A functional dichotomy in CD4+ T lymphocytes. Immunol Today. 1988 Sep;9(9):268–274. doi: 10.1016/0167-5699(88)91308-4. [DOI] [PubMed] [Google Scholar]
- Charbonneau H., Tonks N. K., Walsh K. A., Fischer E. H. The leukocyte common antigen (CD45): a putative receptor-linked protein tyrosine phosphatase. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7182–7186. doi: 10.1073/pnas.85.19.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Fischer E. H., Charbonneau H., Tonks N. K. Protein tyrosine phosphatases: a diverse family of intracellular and transmembrane enzymes. Science. 1991 Jul 26;253(5018):401–406. doi: 10.1126/science.1650499. [DOI] [PubMed] [Google Scholar]
- Gebbink M. F., van Etten I., Hateboer G., Suijkerbuijk R., Beijersbergen R. L., Geurts van Kessel A., Moolenaar W. H. Cloning, expression and chromosomal localization of a new putative receptor-like protein tyrosine phosphatase. FEBS Lett. 1991 Sep 23;290(1-2):123–130. doi: 10.1016/0014-5793(91)81241-y. [DOI] [PubMed] [Google Scholar]
- Gilbert C. W., Zaroukian M. H., Esselman W. J. Poly-N-acetyllactosamine structures on murine cell surface T200 glycoprotein participate in natural killer cell binding to YAC-1 targets. J Immunol. 1988 Apr 15;140(8):2821–2828. [PubMed] [Google Scholar]
- Gillitzer R., Pilarski L. M. In situ localization of CD45 isoforms in the human thymus indicates a medullary location for the thymic generative lineage. J Immunol. 1990 Jan 1;144(1):66–74. [PubMed] [Google Scholar]
- Hunter T. A tail of two src's: mutatis mutandis. Cell. 1987 Apr 10;49(1):1–4. doi: 10.1016/0092-8674(87)90745-8. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Jove R., Hanafusa H. Cell transformation by the viral src oncogene. Annu Rev Cell Biol. 1987;3:31–56. doi: 10.1146/annurev.cb.03.110187.000335. [DOI] [PubMed] [Google Scholar]
- June C. H., Fletcher M. C., Ledbetter J. A., Schieven G. L., Siegel J. N., Phillips A. F., Samelson L. E. Inhibition of tyrosine phosphorylation prevents T-cell receptor-mediated signal transduction. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7722–7726. doi: 10.1073/pnas.87.19.7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J. D., Ohnishi K., Lebow L. T., Bonavida B. The SJL/J T cell response to both spontaneous and transplantable syngeneic reticulum cell sarcoma is mediated predominantly by the V beta 17a+ T cell clonotype. J Exp Med. 1988 Nov 1;168(5):1553–1562. doi: 10.1084/jem.168.5.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincade P. W. Experimental models for understanding B lymphocyte formation. Adv Immunol. 1987;41:181–267. doi: 10.1016/s0065-2776(08)60032-2. [DOI] [PubMed] [Google Scholar]
- Klarlund J. K. Transformation of cells by an inhibitor of phosphatases acting on phosphotyrosine in proteins. Cell. 1985 Jul;41(3):707–717. doi: 10.1016/s0092-8674(85)80051-9. [DOI] [PubMed] [Google Scholar]
- Koretzky G. A., Picus J., Thomas M. L., Weiss A. Tyrosine phosphatase CD45 is essential for coupling T-cell antigen receptor to the phosphatidyl inositol pathway. Nature. 1990 Jul 5;346(6279):66–68. doi: 10.1038/346066a0. [DOI] [PubMed] [Google Scholar]
- Kornblihtt A. R., Umezawa K., Vibe-Pedersen K., Baralle F. E. Primary structure of human fibronectin: differential splicing may generate at least 10 polypeptides from a single gene. EMBO J. 1985 Jul;4(7):1755–1759. doi: 10.1002/j.1460-2075.1985.tb03847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger N. X., Streuli M., Saito H. Structural diversity and evolution of human receptor-like protein tyrosine phosphatases. EMBO J. 1990 Oct;9(10):3241–3252. doi: 10.1002/j.1460-2075.1990.tb07523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaForgia S., Morse B., Levy J., Barnea G., Cannizzaro L. A., Li F., Nowell P. C., Boghosian-Sell L., Glick J., Weston A. Receptor protein-tyrosine phosphatase gamma is a candidate tumor suppressor gene at human chromosome region 3p21. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):5036–5040. doi: 10.1073/pnas.88.11.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Tonks N. K., Fischer E. H., Clark E. A. CD45 regulates signal transduction and lymphocyte activation by specific association with receptor molecules on T or B cells. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8628–8632. doi: 10.1073/pnas.85.22.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustelin T., Altman A. Dephosphorylation and activation of the T cell tyrosine kinase pp56lck by the leukocyte common antigen (CD45). Oncogene. 1990 Jun;5(6):809–813. [PubMed] [Google Scholar]
- Nadeau J. H., Berger F. G., Kelley K. A., Pitha P. M., Sidman C. L., Worrall N. Rearrangement of genes located on homologous chromosomal segments in mouse and man: the location of genes for alpha- and beta-interferon, alpha-1 acid glycoprotein-1 and -2, and aminolevulinate dehydratase on mouse chromosome 4. Genetics. 1986 Dec;114(4):1239–1255. doi: 10.1093/genetics/114.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A., Kauer J. A., Malenka R. C. The current excitement in long-term potentiation. Neuron. 1988 Apr;1(2):97–103. doi: 10.1016/0896-6273(88)90193-6. [DOI] [PubMed] [Google Scholar]
- O'Dell T. J., Kandel E. R., Grant S. G. Long-term potentiation in the hippocampus is blocked by tyrosine kinase inhibitors. Nature. 1991 Oct 10;353(6344):558–560. doi: 10.1038/353558a0. [DOI] [PubMed] [Google Scholar]
- Ogimoto M., Mizuno K., Tate G., Takahashi H., Katagiri M., Hasegawa K., Yakura H. Regulation of lipopolysaccharide- and IL-4-induced immunoglobulin heavy chain gene activation: differential roles for CD45 and Lyb-2. Int Immunol. 1992 Jun;4(6):651–659. doi: 10.1093/intimm/4.6.651. [DOI] [PubMed] [Google Scholar]
- Okumoto M., Nishikawa R., Imai S., Hilgers J. Genetic analysis of resistance to radiation lymphomagenesis with recombinant inbred strains of mice. Cancer Res. 1990 Jul 1;50(13):3848–3850. [PubMed] [Google Scholar]
- Olumi A. F., Tsai Y. C., Nichols P. W., Skinner D. G., Cain D. R., Bender L. I., Jones P. A. Allelic loss of chromosome 17p distinguishes high grade from low grade transitional cell carcinomas of the bladder. Cancer Res. 1990 Nov 1;50(21):7081–7083. [PubMed] [Google Scholar]
- Ostergaard H. L., Shackelford D. A., Hurley T. R., Johnson P., Hyman R., Sefton B. M., Trowbridge I. S. Expression of CD45 alters phosphorylation of the lck-encoded tyrosine protein kinase in murine lymphoma T-cell lines. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8959–8963. doi: 10.1073/pnas.86.22.8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingel J. T., Thomas M. L. Evidence that the leukocyte-common antigen is required for antigen-induced T lymphocyte proliferation. Cell. 1989 Sep 22;58(6):1055–1065. doi: 10.1016/0092-8674(89)90504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F., Mason D. Phenotypic and functional heterogeneity of CD4+ T cells. Immunol Today. 1988 Sep;9(9):274–277. doi: 10.1016/0167-5699(88)91309-6. [DOI] [PubMed] [Google Scholar]
- Ross C. A., Wright G. E., Resh M. D., Pearson R. C., Snyder S. H. Brain-specific src oncogene mRNA mapped in rat brain by in situ hybridization. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9831–9835. doi: 10.1073/pnas.85.24.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saga Y., Tung J. S., Shen F. W., Boyse E. A. Alternative use of 5' exons in the specification of Ly-5 isoforms distinguishing hematopoietic cell lineages. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5364–5368. doi: 10.1073/pnas.84.15.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saga Y., Tung J. S., Shen F. W., Boyse E. A. Sequences of Ly-5 cDNA: isoform-related diversity of Ly-5 mRNA. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6940–6944. doi: 10.1073/pnas.83.18.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler U., Sierra F. Alternative promoters in developmental gene expression. Annu Rev Genet. 1987;21:237–257. doi: 10.1146/annurev.ge.21.120187.001321. [DOI] [PubMed] [Google Scholar]
- Streuli M., Hall L. R., Saga Y., Schlossman S. F., Saito H. Differential usage of three exons generates at least five different mRNAs encoding human leukocyte common antigens. J Exp Med. 1987 Nov 1;166(5):1548–1566. doi: 10.1084/jem.166.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Ariniello P. D., Tang M., Munro J. M., Blattler W. A., Adler D. A., Disteche C. M., Saito H. Expression of the receptor-linked protein tyrosine phosphatase LAR: proteolytic cleavage and shedding of the CAM-like extracellular region. EMBO J. 1992 Mar;11(3):897–907. doi: 10.1002/j.1460-2075.1992.tb05128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Tsai A. Y., Saito H. A family of receptor-linked protein tyrosine phosphatases in humans and Drosophila. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8698–8702. doi: 10.1073/pnas.86.22.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugrue M. M., Brugge J. S., Marshak D. R., Greengard P., Gustafson E. L. Immunocytochemical localization of the neuron-specific form of the c-src gene product, pp60c-src(+), in rat brain. J Neurosci. 1990 Aug;10(8):2513–2527. doi: 10.1523/JNEUROSCI.10-08-02513.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. L., Reynolds P. J., Chain A., Ben-Neriah Y., Trowbridge I. S. B-cell variant of mouse T200 (Ly-5): evidence for alternative mRNA splicing. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5360–5363. doi: 10.1073/pnas.84.15.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. L. The leukocyte common antigen family. Annu Rev Immunol. 1989;7:339–369. doi: 10.1146/annurev.iy.07.040189.002011. [DOI] [PubMed] [Google Scholar]
- Tonks N. K., Charbonneau H., Diltz C. D., Fischer E. H., Walsh K. A. Demonstration that the leukocyte common antigen CD45 is a protein tyrosine phosphatase. Biochemistry. 1988 Nov 29;27(24):8695–8701. doi: 10.1021/bi00424a001. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Ostergaard H. L., Johnson P. CD45: a leukocyte-specific member of the protein tyrosine phosphatase family. Biochim Biophys Acta. 1991 Oct 16;1095(1):46–56. doi: 10.1016/0167-4889(91)90043-w. [DOI] [PubMed] [Google Scholar]
- Tsai Y. C., Nichols P. W., Hiti A. L., Williams Z., Skinner D. G., Jones P. A. Allelic losses of chromosomes 9, 11, and 17 in human bladder cancer. Cancer Res. 1990 Jan 1;50(1):44–47. [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Yakura H., Kawabata I., Shen F. W., Katagiri M. Selective inhibition of lipopolysaccharide-induced polyclonal IgG response by monoclonal Ly-5 antibody. J Immunol. 1986 Apr 15;136(8):2729–2733. [PubMed] [Google Scholar]
- Yakura H., Shen F. W., Bourcet E., Boyse E. A. On the function of Ly-5 in the regulation of antigen-driven B cell differentiation. Comparison and contrast with Lyb-2. J Exp Med. 1983 Apr 1;157(4):1077–1088. doi: 10.1084/jem.157.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Wake N., Fujimoto S., Barrett J. C., Oshimura M. Multiple chromosomes carrying tumor suppressor activity for a uterine endometrial carcinoma cell line identified by microcell-mediated chromosome transfer. Oncogene. 1990 Aug;5(8):1141–1147. [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]