Abstract
Mast cells regulate intestinal barrier function during disease and homeostasis. Secretion of the mast cell-specific serine protease chymase regulates homeostasis. In the present study, we employ in vitro model systems to delineate the molecular pathways involved in chymase-mediated intestinal epithelial barrier dysfunction. Chymase stimulation of intestinal epithelial (Caco-2 BBe) cell monolayers induced a significant reduction in transepithelial resistance, indicating decreased intestinal epithelial barrier function. The chymase-induced intestinal epithelial barrier dysfunction was characterized by chymase-induced protease-activated receptor (PAR)-2 activation and matrix metalloproteinase (MMP)-2 expression and activation. Consistent with this observation, in vitro analysis revealed chymase-induced PAR-2 activation and increased MAPK activity and MMP-2 expression. Pharmacological and small interfering RNA-mediated antagonism of PAR-2 and MMP-2 significantly attenuated chymase-stimulated barrier dysfunction. Additionally, the chymase/MMP-2-mediated intestinal epithelial dysfunction was associated with a significant reduction in the tight junction protein claudin-5, which was partially restored by MMP-2 inhibition. Finally, incubation of Caco-2 BBe cells with chymase-sufficient, but not chymase-deficient, bone marrow-derived mast cells decreased barrier function, which was attenuated by the chymase inhibitor chymostatin. Collectively, these results suggest that mast cell/chymase-mediated intestinal epithelial barrier function is mediated by PAR-2/MMP-2-dependent pathways.
Keywords: mast cells, epithelial permeability, tight junctions, protease
the intestinal epithelium is a single-cell layer that functions as a selectively permeable barrier, permitting the absorption of nutrients, electrolytes, and water while maintaining an effective defense against intraluminal toxins, antigens, and enteric flora (38). Clinical studies have demonstrated a loss or breakdown of the epithelial barrier during chronic inflammatory diseases, including inflammatory bowel disease (IBD), celiac disease, and food allergies (7, 13, 26, 59, 74, 76, 83, 84). These studies led to the identification of pathways that regulate intestinal epithelial barrier function during chronic disease and revealed a role for exogenous agents, such as bacterial toxins and luminal contents (60), and components of innate and adaptive immunity, including lipid mediators, cytokines (IFN-γ, TNF-α, and IL-13), inflammatory cells (T cells, macrophages, and granulocytes) (1, 78, 81), and leukocytes (46).
Recently, there has been an emergence of clinical data demonstrating increased intestinal epithelial barrier permeability in healthy first-degree relatives of patients with asymptomatic IBD, food allergy, and celiac disease (23, 83). Importantly, these data suggest that altered intestinal permeability may not be a consequence of disease exacerbation but may also predispose to increased susceptibility to diseases including IBD, celiac disease, food allergies, type 1 diabetes, and multiple sclerosis (7, 13, 26, 59, 74, 76, 83, 84). Consistent with this concept, a number of genome-wide association studies have identified susceptibility loci near genes that have been implicated in epidermal and epithelial barrier integrity (FILA, CDSN, PARD-3, MAGI-2, and claudin-1) with atopic dermatitis, atopic sensitization, celiac disease, and IBD. This has led to renewed interest in understanding the relationship between intestinal epithelial barrier integrity and chronic inflammatory disease susceptibility; however, pathways that regulate “steady-state” intestinal epithelial barrier function are not well defined.
Recently, we demonstrated a role for mast cells and the murine mast cell-derived chymase mast cell protease-4 (MCPT-4) in the maintenance of steady-state, or homeostatic, intestinal epithelial barrier function (19). We showed significantly decreased basal small intestinal permeability in mice deficient in mast cells (KitW-sh/W-sh) or mast cell chymase (Mcpt4−/−) compared with wild-type (WT) mice, indicating increased barrier function. Engraftment of KitW-sh/W-sh mice with WT, but not Mcpt4−/−, mast cells restored normal intestinal epithelial barrier function. However, the molecular basis of chymase-mediated regulation of homeostatic intestinal barrier integrity remains undefined. MCPT-4 is a serine protease with chymotrypsin-like specificity, cleaving target peptides and proteins after aromatic amino acids, especially tyrosine and phenylalanine (49). On the basis of tissue distribution, heparin-binding properties, and substrate and cleavage specificity, the β-chymase MCPT-4 has been identified as the murine functional homolog to human chymase (3, 4, 70). MCPT-4 cleaves several physiological substrates important for tissue remodeling and extracellular matrix (ECM) degradation through direct cleavage of ECM proteins and activation of ECM-degrading proteases, including matrix metalloproteinases (MMPs) (31, 69, 75).
In the present study, we define the mechanistic basis of chymase-mediated regulation of intestinal epithelial permeability. We demonstrate that chymase stimulated an increase in intestinal epithelial permeability, which was associated with chymase-induced protease-activated receptor (PAR)-2 activation and MMP-2 expression and activation. PAR-2 and MMP-2 antagonism significantly attenuated chymase-stimulated barrier dysfunction. The chymase/MMP-2-dependent response was associated with a significant reduction in the tight junction (TJ) protein claudin-5, which was partially restored by MMP-2 inhibition. Finally, we demonstrate that bone marrow-derived mast cells (BMMCs) constitutively secrete chymase, inducing intestinal epithelial barrier dysfunction, which was attenuated by chymase inhibition.
EXPERIMENTAL PROCEDURES
Mice.
C57Bl/6 mice were obtained from Jackson Laboratories; KitW-sh/W-sh and Mcpt4−/− mice on a C57Bl/6 background were kindly provided by Drs. Abrink and Pejler. Mice were bred and maintained on a regular mouse chow diet under specific pathogen-free conditions in our facility in accordance with guidelines of the Cincinnati Children's Hospital Medical Center Institutional Animal Care and Use Committee, which approved the protocol.
Cell culture.
Caco-2 BBe human intestinal adenocarcinoma cells (American Type Culture Collection) were maintained in DMEM supplemented with 10% fetal calf serum, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 10 mM HEPES buffer, and 1× penicillin-streptomycin (Invitrogen, Grand Island, NY) in 75-cm2 culture flasks in a humidified incubator (5% CO2, 37°C). Medium was replaced twice weekly; when the cells reached 80% confluency, they were passaged using 0.05% trypsin-0.02% EDTA.
In vitro epithelial barrier function.
For barrier function experiments, 5 × 105 Caco-2 BBe cells were plated on Snapwell or Transwell filters (12-mm diameter, 0.4-μm pore; Corning, Lowell, MA) and cultured for 10–14 days. Transepithelial resistance (TER) was measured with an EVOM/Endohm or STX2 electrode (Costar), with correction for filter resistance. Only Snapwell filters with TER >250 Ω·cm2 were used. After baseline measurements, vehicle (control-PBS) or 100–1,000 ng/ml human chymase (equivalent to 0.0354–0.354 U/ml; Sigma Aldrich, St. Louis, MO) was added to the basolateral chamber. TER was measured up to 24 h following chymase stimulation. For inhibition studies, cells were stimulated with 0.05 U/ml chymase or 25 ng/ml MMP-2 basolaterally with or without the serine protease inhibitor chymostatin (100 μM; Sigma Aldrich), the MMP-2 inhibitor ARP100 (5 μg/ml; Tocris, Ellisville, MO), or the PAR-2 inhibitor SAM11 (25 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA), and TER was monitored for 24 h. The control group represents the MMP-2 inhibitor ARP100 (5 μg/ml) or the PAR-2 inhibitor SAM11 (25 μg/ml) in medium alone. For permeability studies, Snapwell filters were placed in Ussing chambers following chymase stimulation, and baseline TER measurements were performed. After addition of 2.2 mg/ml FITC-dextran (4.4 kDa; Sigma Aldrich) and 1 mg/ml horseradish peroxidase (40 kDa; Sigma Aldrich) to the apical bath, 0.25-ml aliquots were removed from the basolateral bath and replaced with fresh Krebs solution every 30 min for 3 h. Horseradish peroxidase concentrations were determined by tetramethylbenzidine detection (BD Pharmingen, San Diego, CA) and spectrophotometry at 550–450 nm. Dextran-FITC levels were measured by spectrophotofluorometry (490 nm excitation, 530 nm emission).
Gelatin zymography.
MMP activity in cell lysate and supernatant was assessed by gelatin zymography. Caco-2 BBe cells were stimulated with 0.05 U/ml chymase for up to 24 h, and supernatant and cell lysates were collected. Cell lysate protein concentrations were determined by BCA protein assay (Thermo Scientific, Rockford, IL). Equal amounts of cell protein or equal volumes of supernatant were separated under nonreducing conditions in the presence of SDS on a 10% zymogram gel containing 0.1% gelatin (Invitrogen, Grand Island, NY). Gels were incubated for 90 min in 2.7% Triton X-100 to remove the MMP activity-inhibiting SDS and then overnight in zymogram developing buffer (5 mM Tris, 4 mM HCl, 20 mM NaCl, 0.5 mM CaCl2, and 0.002% Brij 35). Gels were stained with Coomassie blue (Bio-Rad, Hercules, CA) for 3 h and destained with a Coomassie destain mix until clear bands were visible on the dark-blue background.
Immunofluorescence.
Caco-2 BBe cells grown to confluency on Transwell filters were rinsed with Tris-buffered saline (TBS) and fixed in 4% paraformaldehyde for 45 min on ice. Cells were permeabilized in 0.5% Triton X-100 for 10 min and blocked with 5% goat serum-1% bovine serum albumin-0.05% Triton X-100 for 1 h. Monolayers were incubated overnight at 4°C in a humidified chamber with primary antibodies: rat anti-human E-cadherin (1:500 dilution; Zymed, San Francisco, CA), mouse anti-human MMP-2 (1:400 dilution; Millipore, Billerica, MA), and rabbit anti-human claudin-5 (1:150 dilution; Zymed). The signal was detected by Alexa Fluor 488- and 594-conjugated goat anti-human, anti-mouse, and anti-rabbit antibodies (1:250 dilution; Molecular Probes, Grand Island, NY). Filters were placed on slides, and coverslips were applied using 4′,6-diamidino-2-phenylindole-Supermount G solution (Fluoromount-G). Images were captured using a Zeiss microscope and AxioCam MRc camera.
Western blot analysis.
After stimulation, cell monolayers were rinsed with ice-cold TBS, and protein was extracted with extraction buffer (50 mM Tris·HCl, 150 mM NaCl, 10 mM CaCl2, 0.2 mM NaN3, and 0.01% Triton X-100) containing EDTA-free protease and phosphatase inhibitors. Protein concentrations were determined by BCA assay. Protein (20 μg) was prepared under reducing and denaturing conditions, separated on 4–12% Bis-Tris gels, and transferred to nitrocellulose membranes (Invitrogen). Membranes were blocked in 5% nonfat milk-0.5% Tween-20-TBS overnight at 4°C under gentle agitation. Primary antibodies for actin (1:2,000 dilution; Sigma Aldrich), claudin-1, -2, -3, and -5 (1:250 dilution; Zymed), occludin (1:500 dilution; Zymed), p38, phosphorylated p38, and phosphorylated p44/42 (Cell Signaling, Danvers, MA) were incubated with the membrane for 3 h. Membranes were incubated for 1 h with a goat anti-rabbit IgG peroxidase-conjugated secondary antibody (1:10,000 dilution). Enhanced chemiluminescence (ECL Plus) was used for detection, and densitometric analysis was performed using National Institutes of Health ImageJ.
Lentiviral transduction.
For lentiviral short-hairpin RNA (shRNA) transduction, Caco-2 BBe cells at 70–90% confluence were transduced with lentiviral particles containing shRNAs targeting PAR-2 (PAR-2 shRNA lentiviral particles; catalog no. sc-36188-v, Santa Cruz Biotechnology) or a nontarget control shRNA (82). Lentiviral particles were incubated with Caco-2 BBe cells (multiplicity of infection ∼10) in the presence of Polybrene (4 μg/ml; Sigma) for 24 h and then selected in puromycin at a concentration that killed uninfected cells within 3 days (2 μg/ml). Puromycin-resistant cells were passaged for 14 days and demonstrated stable green fluorescent protein gene expression (results not shown). The lentiviral-transduced Caco-2 BBe cells (passages 1–5) were grown to confluency under puromycin selection pressure until they reached >250 Ω·cm2; electrophysiological and biochemical analyses were performed as described elsewhere (82). PAR-2 knockdown was confirmed by Western blot analysis using the anti-PAR-2 MAb (Santa Cruz Biotechnology).
BMMC generation.
BMMCs isolated from 6- to 8-wk-old mice were maintained in complete medium consisting of RPMI 1640 supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA), glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 mg/ml), pyruvate (1 mM), nonessential amino acids, HEPES (10 mM), and 2-methyl-2-butanol (50 μM). BMMCs were cultured in the presence of IL-3 (20 ng/ml; Peprotech, Rocky Hill, NJ) during weeks 1–3 and with IL-3 (20 ng/ml) and stem cell factor (10 ng/ml; Peprotech) during week 4. After 4 wk of culture, BMMCs were examined for the high-affinity receptor for the Fc region of IgE (FcεRI) and c-Kit expression by flow cytometry analyses, as we previously demonstrated (19), and were found to be double-positive (95–97%) for FcεRI and c-Kit. Four- to 8-wk-old BMMCs were used for all experiments. In some experiments, BMMCs (2 × 106 cells/ml) were stimulated with LPS (10 ng/ml; Sigma Aldrich) for 6 h and subsequently cocultured with Caco-2 BBe cells plated on Transwell filters (TER >500 Ω·cm2) for 24 h, and TER was measured using EVOM/Endohm (see above).
Statistical analysis.
Values are means ± SE. Data were analyzed using unpaired Student's t-test or one- or two-way ANOVA followed by Bonferroni's post hoc test as appropriate (GraphPad Prism 5). Statistical significance was considered when P < 0.05.
RESULTS
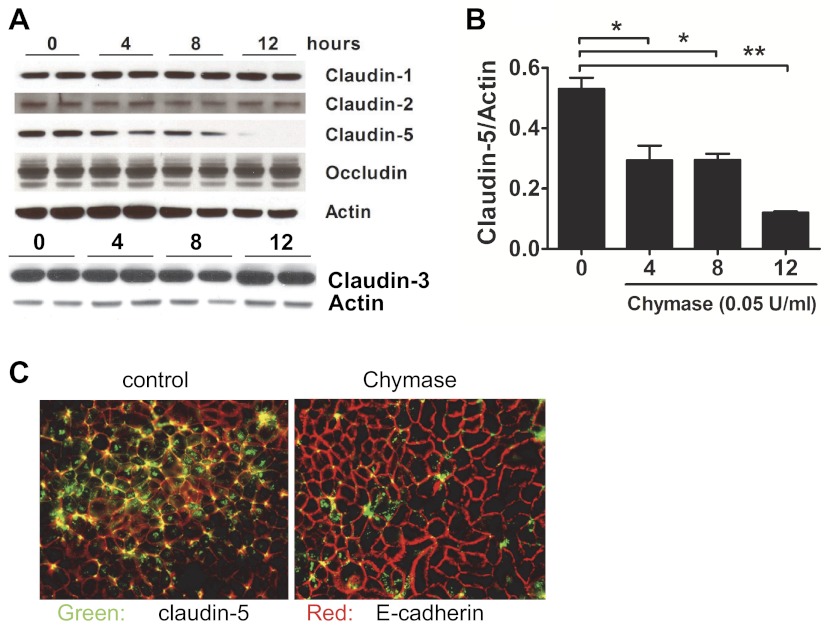
Chymase-induced intestinal epithelial barrier function and dysregulation of the TJ protein claudin-5 in Caco-2 BBe cells.
We previously demonstrated that chymase stimulation of Caco-2 BBe cell monolayers induced a dose-dependent decrease in TER (19). Notably, the decreased TER correlated with increased paracellular macromolecular permeability and was dependent on the proteolytic activity of chymase. To begin to define the molecular pathways involved in chymase-mediated intestinal epithelial barrier dysfunction, we examined Caco-2 BBe epithelial TJs and adherence junction proteins, as these proteins primarily regulate paracellular macromolecular permeability. Western blot analysis of TJ protein expression in Caco-2 BBe cells revealed the presence of claudin-1, -2, -3, and -5 and occludin (Fig. 1A). Chymase stimulation induced a significant decrease in detectable levels of claudin-5 protein (Fig. 1, A and B): claudin-5 levels began to decrease by 4 h and were nearly completely absent 12 h following chymase stimulation (Fig. 1, A and B). Importantly, the effect of chymase was specific to claudin-5, as we observed no changes in the level of claudin-1, -2, and -3, occludin, and E-cadherin protein following 12 h of chymase stimulation (Fig. 1A and results not shown). To assess whether the chymase-induced decrease in claudin-5 protein levels was associated with loss of claudin-5 at epithelial TJs, we used immunofluorescence analysis to examine claudin-5 and E-cadherin expression in Caco-2 BBe cells. Assessment of control-treated Caco-2 BBe cells revealed claudin-5 and E-cadherin colocalization at the cellular junctions (Fig. 1C; yellow signal). We also observed positive claudin-5 staining within the intestinal epithelial cells (green signal), suggesting intracellular pooling of claudin-5 (Fig. 1C). In contrast, chymase-stimulated Caco-2 BBe cells demonstrated a significant decrease in colocalization of claudin-5 and E-cadherin at the cellular junctions (Fig. 1C). Importantly, we observed no change in expression of the adherence junction protein E-cadherin (red signal), indicating TJ specificity (Fig. 1C). Collectively, these data reveal that chymase-induced reduction in intestinal barrier function in Caco-2 BBe cells is associated with decreased claudin-5 expression and localization at cellular junctions.
Fig. 1.
Chymase decreases expression of the tight junction (TJ) protein claudin-5. A: Western blots showing claudin-1, -2, -3, and -5, occludin, and actin expression in Caco-2 BBe cells stimulated with vehicle-PBS (control) or 0.05 U/ml chymase for 0–12 h. B: quantitative densitometric analysis of claudin-5 levels in Caco-2 BBe cells stimulated with vehicle-PBS or 0.05 U/ml chymase for 0–12 h. C: immunofluoresence analysis of claudin-5 and E-cadherin in Caco-2 BBe cells stimulated basolaterally with vehicle-PBS or 0.05 U/ml chymase for 24 h. Green, claudin-5; red, E-cadherin; yellow, colocalization. Magnification ×100. Values are means ± SE. *P < 0.05, **P < 0.01.
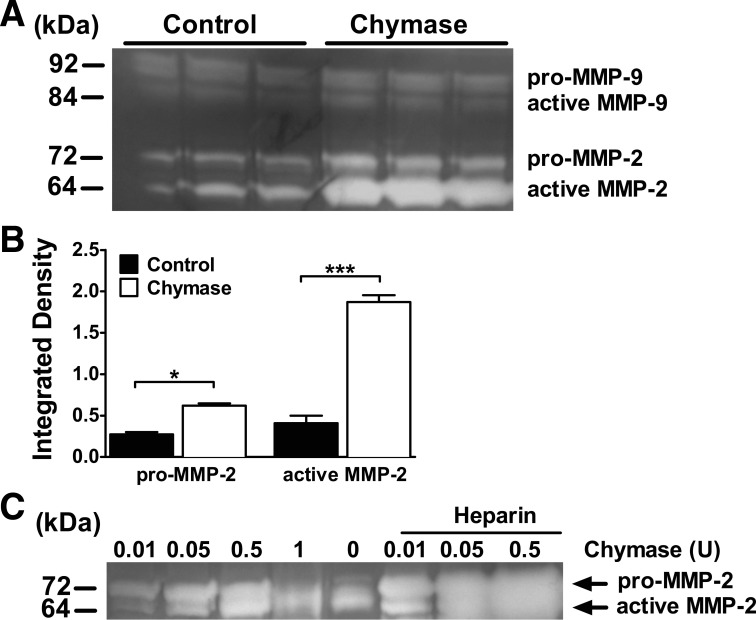
Chymase stimulates intestinal epithelial cell MMP-2 production, secretion, and activation in Caco-2 BBe cells.
Chymase cleaves several physiological substrates important for tissue remodeling and ECM degradation through direct cleavage of ECM proteins, such as fibronectin, vitronectin, and procollagen, and through the activation of ECM-degrading proteases, including MMPs (31, 69, 75). Notably, MMPs have previously been linked to degradation of TJ proteins, including claudin-5 (45). Gelatin zymography analysis revealed that chymase stimulation of Caco-2 BBe cells induced a significant increase in the total MMP-2 protein level (Fig. 2, A and B). Importantly, we observed an increase in the level of pro-MMP-2 and active MMP-2, indicating that chymase also enhanced MMP-2 activation (Fig. 2, A and B). The chymase-mediated induction was specific to MMP-2, as we observed no significant change in expression of the other gelatinase, MMP-9, following chymase stimulation (Fig. 2A). The demonstration of increased active MMP-2 in Caco-2 BBe cells following chymase stimulation suggests that chymase may directly induce MMP-2 activation. Activation of MMP-2 is mediated by multiple pathways, including membrane-bound membrane type 1 (MT1) MMP/tissue inhibitor of MMP (TIMP)-2 (MT1-MMP/TIMP-2) complexes, oxidative stress, sulfhydryl-reactive compounds, conformational changes, and other proteinases, including MMPs (64). To determine whether chymase can directly activate MMP-2, we incubated recombinant chymase with purified pro-MMP-2. We show that chymase directly induced a dose-dependent increase in MMP-2 activation (Fig. 2C). Additionally, the level of chymase-induced MMP-2 activation was increased in the presence of the glycosaminoglycan (GAG) heparin (35), which enhances chymase proteolytic activity (Fig. 2C). Collectively, these data confirm that chymase directly induces MMP-2 activation.
Fig. 2.
Chymase stimulated matrix metalloproteinase (MMP)-2 expression. A: gelatin zymography analysis of pro-MMP-9 and -MMP-2 and active MMP-9 and MMP-2 in basolateral supernatants from Caco-2 BBe cells stimulated basolaterally with vehicle-PBS (control) or 0.05 U/ml chymase for 24 h. B: densitometric quantification of pro-MMP-2 and active MMP-2 in Caco-2 BBe cells. C: MMP-2 (250 ng) was incubated with increasing doses of chymase (0–1 U) in the absence or presence of heparin in a 1:1 mass ratio with chymase. Zymography is representative of 3 independent experiments. Horizontal bar shows presence of heparin. Values are means ± SE. *P < 0.05; ***P < 0.01.
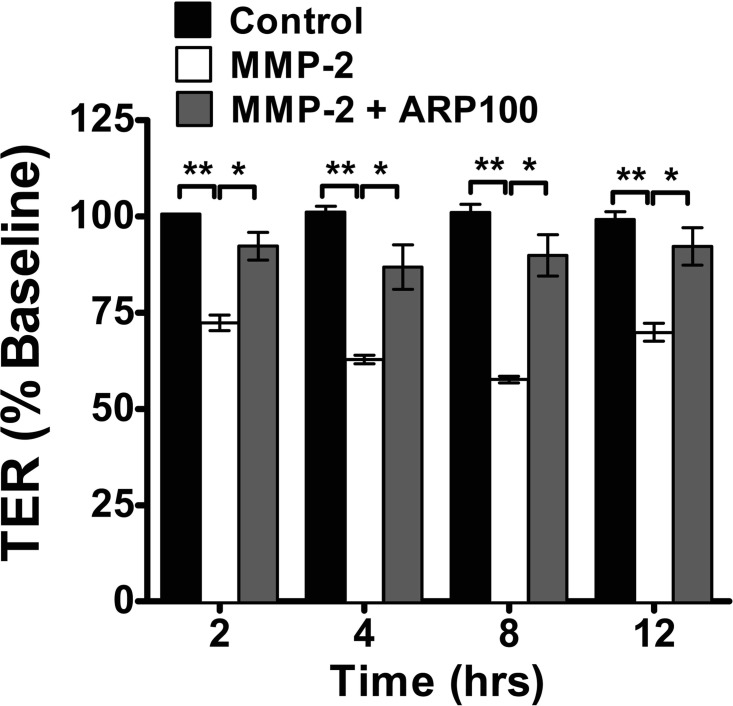
MMP-2 disrupts epithelial barrier function in Caco-2 BBe cells.
Experimental analyses have demonstrated that MMP-2 disrupts endothelial TJs (87) and blood-retinal barrier function (18), which led us to hypothesize that MMP-2 induces intestinal epithelial barrier dysfunction. MMP-2, and not vehicle, stimulation of Caco-2 BBe cells induced a significant time-dependent decrease in TER (Fig. 3). Notably, the kinetics of MMP-2-mediated reduction in TER were comparable to those of chymase (Fig. 1B), demonstrating a reduction in TER by 4 h of stimulation that was sustained over 12 h (Fig. 3) (19). To confirm MMP-2 specificity, we examined MMP-2-induced TER in the presence of the specific MMP-2 inhibitor ARP100 (43, 54, 72) and show that ARP100 significantly blocked MMP-2-induced decreased TER (Fig. 3). These findings demonstrate that MMP-2 is sufficient to disrupt intestinal epithelial barrier function in Caco-2 BBe cells.
Fig. 3.
MMP-2-mediated intestinal epithelial permeability: tranepithelial resistance (TER) of Caco-2 BBe cells stimulated basolaterally with ARP100 (5 μg/ml) in vehicle-PBS (a specific MMP-2 inhibitor, control) or 25 ng/ml MMP-2 in the absence or presence of ARP100 (5 μg/ml). Values (means ± SE) are expressed as percent TER change from time 0; n = 4–8 individual wells per data point. *P < 0.01; **P < 0.001.
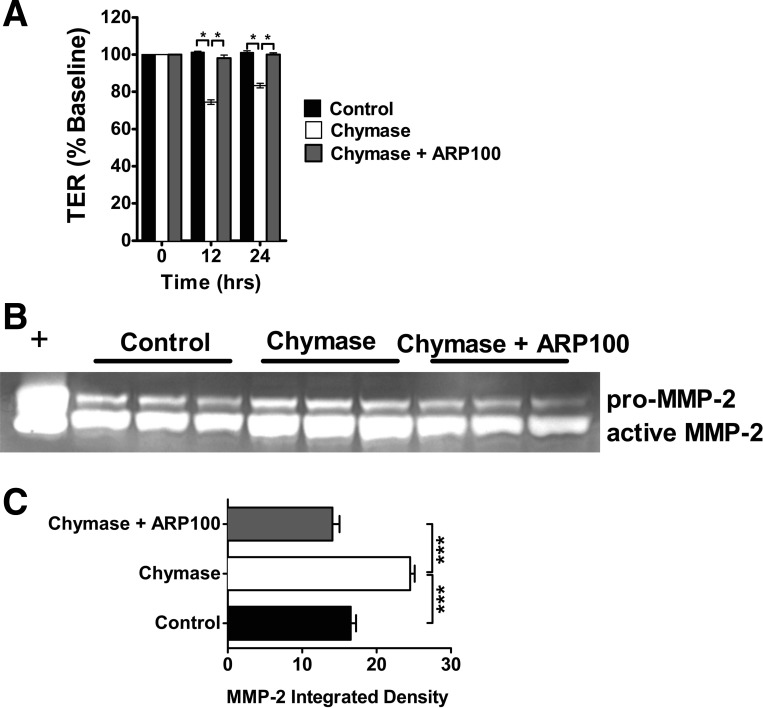
MMP-2 inhibition blocks chymase-induced Caco-2 BBe cell intestinal epithelial permeability.
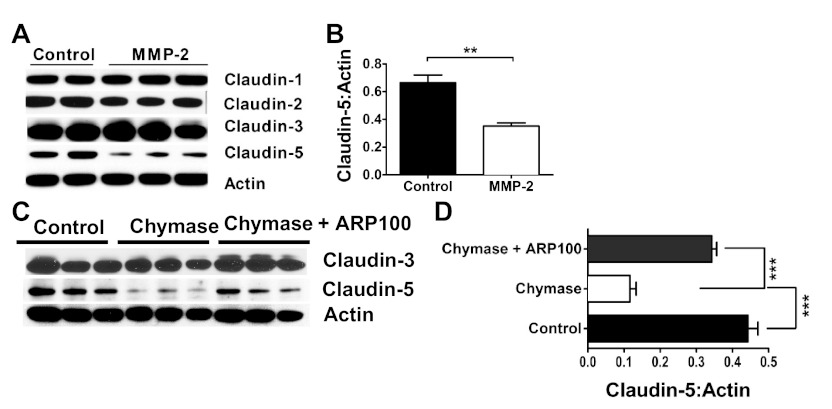
After the demonstration that MMP-2 can regulate intestinal epithelial permeability and that the temporal regulation of chymase-induced MMP-2 production and activation correlated with the TER response (Fig. 3) (19), we hypothesized that chymase-mediated intestinal epithelial permeability in Caco-2 BBe cells is mediated by MMP-2. Chymase stimulation of Caco-2 BBe cells induced a significant decrease in TER compared with control-treated cells (Fig. 4A). Addition of the MMP-2 inhibitor ARP100 attenuated the chymase-induced decrease in TER (Fig. 4A). Moreover, the TER of Caco-2 BBe cells stimulated with chymase for 12 and 24 h in the presence of ARP100 was comparable to that of control-treated cells (Fig. 4A). Gelatin zymography revealed that ARP100 abrogated chymase-induced MMP-2 activation, but not production (Fig. 4, B and C), indicating that inhibition of the chymase-mediated TER response was not due to loss of MMP-2 expression but, rather, level of activation. These data indicate that the chymase-induced decrease in TER is mediated by a MMP-2-dependent mechanism. We next assessed the relative contribution of MMP-2 to the dysregulation of the intestinal epithelial TJ protein claudin-5 (Fig. 5). Stimulation of Caco-2 BBe cells with MMP-2 decreased claudin-5 protein expression (Fig. 5, A and B). Notably, MMP-2 had no effect on claudin-1, -2, and -3 or occludin, indicating MMP-2-specific effects on claudin-5 (Fig. 5, A and B, and results not shown). To determine the contribution of MMP-2 to the chymase-mediated decrease in TJ expression, we assessed claudin-3 and -5 levels in chymase-stimulated Caco-2 BBe cells in the presence of the MMP-2 inhibitor ARP100 (Fig. 5, C and D). Consistent with our previous demonstration, chymase stimulation of Caco-2 BBe cells induced a significant decrease in claudin-5 protein levels (Fig. 5, C and D). Notably, the chymase-mediated decrease in claudin-5 protein levels was significantly blocked in the presence of the MMP-2 inhibitor ARP100 (Fig. 5, C and D). Importantly, the chymase-mediated effects were independent of any modification of claudin-3 levels, confirming chymase specificity (Fig. 5C). These results suggest that chymase-mediated dysregulation of claudin-5 was mediated by MMP-2-dependent pathways.
Fig. 4.
Chymase-mediated intestinal epithelial permeability is attenuated by MMP-2 inhibition. A: TER of Caco-2 BBe cells was stimulated basolaterally with ARP100 (5 μg/ml) in vehicle-PBS (control) or chymase (0.05 U/ml) in the absence or presence of ARP100 (5 μg/ml). Values are expressed as percent TER change from time 0. B and C: gelatin zymography of basolateral supernatant and densitometry of MMP-2 activity of Caco-2 BBe cells stimulated basolaterally with ARP100 (5 μg/ml) in vehicle-PBS or chymase (0.05 U/ml) in the absence or presence of the MMP-2 inhibitor ARP100 (5 mg/ml). Values are means ± SE; n = 4 individual wells per data point. *P < 0.01; ***P < 0.005.
Fig. 5.
MMP-2-mediated expression of the TJ protein claudin-5. A and B: Western blots of claudin-1, -2, -3, and -5 and densitometric analysis of claudin-3 and/or -5 in Caco-2 BBe cells stimulated basolaterally with vehicle-PBS (control) or MMP-2 (25 ng/ml). C and D: Western blots and densitometric analysis of claudin-3 and/or -5 in Caco-2 BBe cells stimulated basolaterally with ARP100 (5 μg/ml) in vehicle-PBS or 0.05 U/ml chymase in the presence or absence of ARP100 (5 μg/ml). Ratio of claudin-5 to actin is expressed as Claudin-5:Actin. Values are means ± SE from duplicate experiments. **P < 0.01; ***P < 0.005.
Chymase-induced intestinal epithelial permeability in Caco-2 BBe cells is dependent on PAR-2.
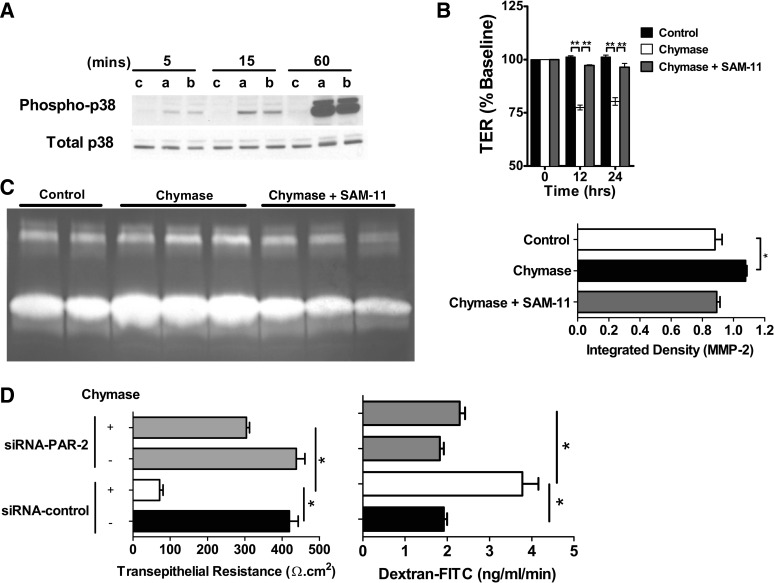
PAR-2 activation in prostate cancer cells leads to MAPK signaling and increased levels of MMP-2 (80). Furthermore, recent studies demonstrated that chymase-induced glomerular albumin permeability is mediated by PAR-2 signaling (62). RT-PCR analysis revealed that Caco-2 BBe cells constitutively express PAR-2 (results not shown). Furthermore, chymase stimulation of Caco-2 BBe cells induced an increase in p38 and p44/42 (ERK1/2) activity at 15 and 60 min (Fig. 6A and results not shown). To assess the relative contribution of PAR-2 to chymase-induced intestinal epithelial permeability, TER was measured in Caco-2 BBe cells stimulated with chymase in the presence of the anti-human PAR-2-neutralizing antibody SAM11 (85). We show that blockade of PAR-2 activation abolished the chymase-induced decrease in TER (Fig. 6B). Notably, loss of PAR-2 activation inhibited chymase-induced MMP-2 activation (Fig. 6C). To confirm the requirement of PAR-2 in chymase-induced intestinal epithelial permeability, we examined TER and dextran-FITC flux in Caco-2 BBe cells transduced with control shRNA or PAR-2 shRNA lentiviral particles. We show that PAR-2 shRNA knockdown of PAR-2 activity abrogated the chymase-induced decrease in TER and dextran-FITC flux (Fig. 6D). These findings confirm a role for PAR-2 in chymase-induced MMP-2 expression and reduced TER and indicate that chymase-induced intestinal epithelial permeability was mediated by PAR-2-dependent pathways.
Fig. 6.
Chymase signals via the protease-activated receptor (PAR)-2 to disrupt intestinal epithelial permeability and increase MMP-2 expression. A: Western blots of total and phosphorylated p38 in Caco-2 BBe cells stimulated apically (a) or basolaterally (b) with 0.05 U/ml chymase or with vehicle control (c) for 0–60 min. B: TER of Caco-2 BBe cells stimulated basolaterally with vehicle-PBS or 0.05 U/ml chymase in the absence or presence of the PAR-2 inhibitor SAM-11 (25 μg/ml). C: densitometric analysis of active MMP-2 in supernatants from Caco-2 BBe cells stimulated basolaterally with vehicle control or 0.05 U/ml chymase in the presence or absence of the PAR-2 inhibitor SAM-11 (25 μg/ml). D: TER and dextran-FITC flux of small interfering RNA (siRNA) control- or siRNA PAR-2-transduced Caco-2 BBe cells following 24 h of stimulation with vehicle or 0.05 U/ml chymase. Values are means ± SE; n = 3–8 individual wells per data point. *P < 0.05; **P < 0.001.
Mast cells mediate epithelial barrier function through chymase secretion.
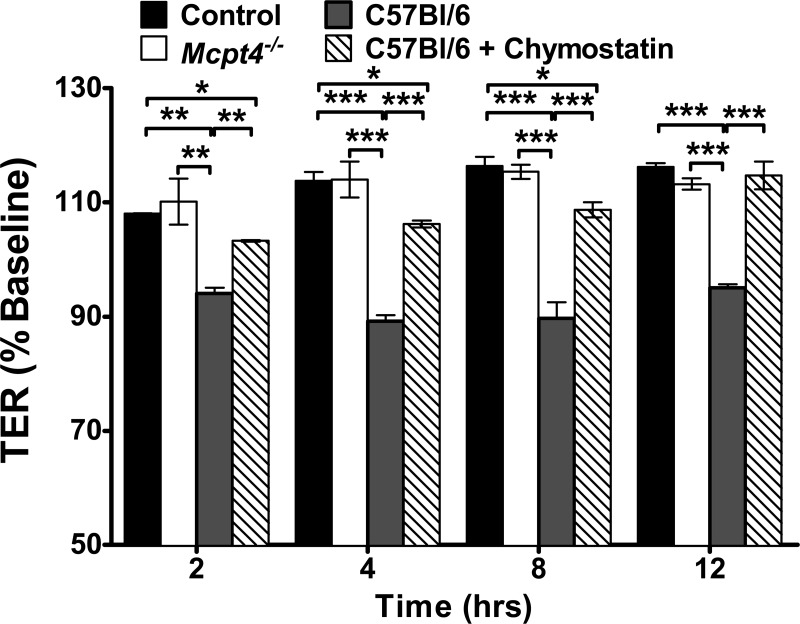
The in vitro experiments described in the present study were performed with purified chymase. To directly assess the role of mast cell-derived chymase in intestinal epithelial barrier function, we developed a BMMC-intestinal epithelial cell culture system. Caco-2 BBe cells were grown as a monolayer on Transwell filters and placed in wells containing unstimulated C57Bl/6 WT or chymase-deficient Mcpt4−/− BMMCs in the basolateral chamber, and TER was monitored. Caco-2 BBe cells maintained a constant TER over 12 h. Addition of unstimulated WT BMMCs induced a time-dependent decrease in intestinal epithelial cell TER that was significant by 2 h and remained for 12 h (Fig. 7). Strikingly, Mcpt4−/− BMMCs did not alter the TER of Caco-2 BBe cell monolayers over the 12-h time course (Fig. 7). Caco-2 BBe cell viability was not significantly different between groups, indicating that the decrease in TER was likely not attributed to an increase in cell death (results not shown). To confirm that the response was chymase-specific, intestinal epithelial cells were incubated with WT BMMCs in the presence of the chymase inhibitor chymostatin. Remarkably, inhibition of chymase proteolytic activity blocked the mast cell-mediated reduction in TER (Fig. 7). Collectively, these results suggest that unstimulated BMMCs constitutively release chymase, which negatively impacts intestinal epithelial barrier function.
Fig. 7.
Murine bone marrow-derived mast cell (BMMC) release of mast cell protease 4 (MCPT-4) mediates intestinal epithelial permeability. TER (0–12 h) was measured in Caco-2 BBe cell monolayers cultured in the absence of BMMCs or cocultured with unstimulated BMMCs from C57Bl/6 wild-type (WT) or chymase-deficient (Mcpt4−/−) mice in the basolateral compartment in the presence or absence of the chymase inhibitor chymostatin (100 mM). Values are means ± SE; n = 4–6 individual wells per group. *P < 0.05; **P < 0.01; ***P < 0.001.
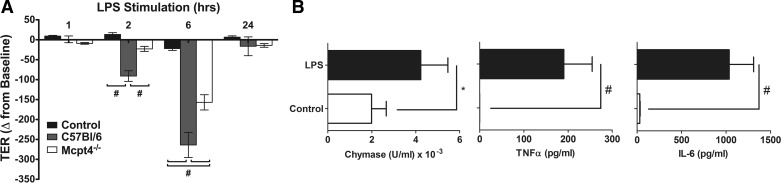
Mast cell activation has been linked to increased intestinal epithelial permeability (20, 88). To determine chymase involvement in activated mast cell regulation of intestinal epithelial permeability, we examined TER in the BMMC-intestinal epithelial cell culture system following LPS stimulation. Addition of LPS-stimulated WT BMMCs induced a time-dependent decrease in intestinal epithelial cell TER that was significant by 2 h and remained for 6 h before returning to baseline by 24 h (Fig. 8A). Similarly, addition of LPS-stimulated Mcpt4−/− BMMCs also reduced TER of Caco-2 BBe cell monolayers over the same time course (Fig. 8A). Notably, the level of reduction of TER was significantly lower in LPS-stimulated Mcpt4−/− BMMCs than WT BMMCs (Fig. 8A). To ascertain potential pathways involved in the regulation of intestinal epithelial TER independent of chymase, we assessed LPS-induced BMMC chymase and cytokine release. We show that LPS stimulation of BMMCs leads to significantly increased chymase, TNF-α, and IL-6 release (Fig. 8B). Collectively, these results suggest that chymase contributes to activated BMMC regulation of intestinal epithelial barrier function; however, pathways independent of chymase can also modify barrier function.
Fig. 8.
LPS-stimulated murine BMMC regulation of intestinal epithelial permeability. TER (0–24 h) was measured in Caco-2 BBe cell monolayers cultured in the absence of BMMCs or cocultured with LPS (1 μg/ml)-stimulated BMMCs from C57Bl/6 WT or chymase-deficient (Mcpt4−/−) mice in the basolateral compartment. Values are means ± SE; n = 4 individual wells per group. *P < 0.05; #P < 0.01.
DISCUSSION
Proteases have long been recognized to contribute to various aspects of epithelial migration, integrity, and barrier function; however, the effects of proteases are complex, as they have been demonstrated to have beneficial and deleterious effects on the epithelium (5, 67). In mice lacking the serine proteases matriptase [membrane-type serine protease-1 (MT-SP1)] and prostatin (CAP1/PRSS8), development of the epidermis is impaired, leading to a defect in skin barrier function and resulting in postnatal lethality (12, 35, 36). Conversely, excessive proteolytic enzymatic activity due to mutations in SPINK-5, which encodes the serine protease inhibitor LEKTI, leads to impaired barrier function and is likely to contribute to the atopic predisposition seen in Netherton syndrome (12). We recently identified a role for the mast cell-derived serine protease chymase in intestinal epithelial permeability (19). We showed increased intestinal epithelial resistance in mice deficient in chymase (Mcpt−/−) (19); however, the molecular basis underlying protease-induced alterations to intestinal epithelial barrier function was not examined. In the present study, we employed an in vitro intestinal epithelial model system to begin to delineate the molecular pathways involved in chymase regulation of intestinal epithelial barrier function. We show 1) that chymase induced an increase in human intestinal epithelial permeability that was dependent on chymotryptic activity and associated with degradation of the TJ protein claudin-5, 2) that chymase stimulates MMP-2 de novo synthesis and activation, 3) that chymase-induced intestinal epithelial permeability is dependent on MMP-2, 4) that chymase stimulated MAPK activation and MMP-2 production and that this was PAR-2 dependent, and 5) that mast cell-derived chymase-induced intestinal epithelial permeability is dependent on PAR-2.
We identified a role for chymase in intestinal epithelium-derived MMP-2 protein synthesis and activation. MMP activation is regulated at multiple levels, including transcription, proteolytic degradation, and inhibition (9, 52). Proteolytic activation of MMP-2 predominantly occurs in an extracellular manner in cooperation with MT1-MMP and TIMP-2 (66). However, the demonstration of proteolytic activation of MMP-2 in MT1-MMP-deficient mice indicates that pathways independent of MT1-MMP can promote MMP-2 activation (56). Several recent studies demonstrated activation of MMPs by plasmin and proteases, including chymase and even other MMPs (6, 14, 40–42, 69). Consistent with this concept, the levels of active MMP-2 in ear, lung, and heart tissue in Mcpt4−/− mice were significantly reduced compared with WT mice, suggesting a role for chymase in the processing and activation of MMP-2 (69). We demonstrate chymase-mediated MMP-2 activation in the absence of cofactors including TIMP-2, MT1-MMP, or proteoglycan (PG)-GAG, indicating that chymase is sufficient to promote proteolytic MMP-2 activation. A role for chymase in MMP-9 processing and activation in animal models and Mcpt4−/− mice has also been reported (25, 27, 47). We did not observe chymase-induced MMP-9 activation in our in vitro analyses. One possible explanation for these differences is that chymase-mediated MMP-9 processing may occur indirectly via chymase-mediated activation of MT1-MMP/TIMP2 complexes or other proteinases, including MMPs (64), that are absent in our in vitro systems. For example, chymase stimulates proteolytic activation of MMP-1 and -3, and MMP-3 is known to activate MMP-9 (34, 57, 71).
Chymase is a serine protease that possesses chymotrypsin-like cleavage specificity; i.e., it cleaves substrates on the COOH-terminal side of aromatic amino acids (8). Experimental analyses have identified (Arg-Glu-Thr-Tyr-x) residues on the amino terminal (P4-P1) side of the site of hydrolysis as the preferred amino acid at each position (53). Assessment of the predicted amino acid sequence of MMP-2 did not reveal the consensus-preferred substrate sequence Arg-Glu-Thr-Tyr (results not shown). Although we do not know the position where chymase cleaves MMP-2 for its activation, it most likely occurs after the prodomain, which contains the cysteine switch that covers the critical zinc-binding active site. Consistent with this, chymase-induced MMP-1 activation occurs through cleavage of the Leu83-Thr84 bond (57), and in dogs, chymase-induced activation of MMP-9 occurs by Phe88-Gln89 and Phe91-Glu92 bond cleavage (14). Importantly, there are several potential target cleavage sites for chymase in the same range of amino acids for MMP-2.
We show that chymase induced de novo synthesis and activation of MMP-2, which coincided with the chymase-induced increase in intestinal epithelial permeability, suggesting that the chymase-induced response was mediated by MMP-2. Indeed, antagonism of MMP-2 function inhibited the chymase-mediated increase in intestinal epithelial permeability. MMPs exhibit differential proteolytic activity against ECM proteins; for example, the gelatinase subfamily, consisting of MMP-2 and -9, is known to degrade type IV collagen, fibronectin, and gelatin (64). However, recent observations indicate that MMP substrates include a host of non-ECM molecules (64, 89). We demonstrate that chymase-mediated intestinal epithelial permeability was associated with a specific decrease in claudin-5 protein levels. Furthermore, we provide data that MMP-2 induced intestinal epithelial claudin-5 degradation and that blockade of MMP-2 activity significantly attenuated the chymase-mediated decrease in claudin-5 protein levels and increase in intestinal epithelial permeability. Collectively, these data suggest that the chymase-induced increase in intestinal epithelial permeability was mediated via MMP-2-induced claudin-5 degradation. Consistent with this concept, MMP-2 has been shown to intimately associate with TJ proteins such as claudin-5 (39) and alter epithelial barrier function (16). Furthermore, MMP-2 was reported to degrade claudin-5 in the blood-brain barrier during ischemia and reperfusion injury (15, 37, 87). While claudin-5 has been classically associated with endothelial TJs, recent studies demonstrated the expression of claudin-5 in intestinal epithelial cells and showed it to be a potent barrier builder (44). Indeed, overexpression of claudin-5 in Caco-2 cells induced a significant increase in the TER and a 50% reduction in permeability (2). While our studies have been restricted to the transformed intestinal epithelial cell line Caco-2 BBe, there is evidence to suggest that these pathways may well be operational in vivo. MMP-2 has been shown to be expressed by multiple cell populations within the gastrointestinal tract, including human colonic primary epithelial cells, under normal healthy conditions (48, 79), and MMP-2 mRNA levels are upregulated during chronic colonic inflammation associated with IBD (77).
Recently, we demonstrated that MCPT-4 deficiency in vivo was associated with decreased intestinal permeability and that this phenotype was linked with decreased claudin-3 expression (20). In the present in vitro study using human Caco-2 BBe cells, we revealed that human chymase/MMP-2 stimulated an increase in colonic epithelial barrier function that was linked with loss of claudin-5. On the basis of our in vivo mouse data (20), we would have anticipated that chymase stimulation of Caco-2 BBe cells may enhance epithelial claudin-3 levels; however, we did not observe a change in claudin-3 protein levels. Differential proteolytic degradation of human and murine claudin-3 and -5 is not likely an explanation for this discrepancy, as comparative analyses of these TJ protein sequences did not identify any unique putative chymotryptic or MMP-2 cleavage sites (49). Conversely, our in vitro studies would suggest that MCPT-4 deficiency in vivo would lead to enhanced claudin-5 expression in the small intestine. However, claudin-5 is primarily expressed in endothelial cells and is undetectable or expressed at very low levels in all compartments of the gastrointestinal tract of the mouse (23). It is possible that the differences in chymase-mediated effects on claudin-3 expression between mouse and human may be explained by indirect effects of chymase in vivo. Moreover, while our in vitro studies indicate that chymase acts directly on the intestinal epithelium, we do not know whether the effects of MCPT-4 in vivo can be solely attributed to chymase directly stimulating intestinal epithelial cells. It is possible that chymase in vivo may indirectly alter intestinal epithelial cell permeability and claudin-3 expression. Furthermore, as we have not defined the importance of MMP-2 in chymase-mediated regulation of small intestinal epithelial permeability in the mouse, we cannot exclude the possibility that the in vivo effects of chymase on claudin-3 are independent of MMP-2. Alternatively, claudin-3 dysregulation could be a direct consequence of the increase in intestinal epithelial migration in Mcpt4−/− mice as opposed to a direct consequence of chymase activity. Further studies are ongoing to elucidate the importance of chymase-mediated effects on claudin-3 and -5 in intestinal epithelial permeability and intestinal epithelial migration.
We demonstrate that chymase induced MMP-2 production and intestinal epithelial barrier dysfunction, which was dependent on activation of the protease-activated receptor PAR-2. PAR-2 expression was confirmed by RT-PCR analysis (results not shown), and previous studies demonstrated apical and basolateral expression of PAR-2 on Caco-2 BBe cells (32). The intramolecular activation of PAR-2 leads to G-coupled protein activation and subsequent activation of downstream MAPK pathways (55). Consistent with these observations, we showed that chymase stimulated intestinal epithelial cell MAPK activation (p38 and p44/42). Notably, the p38 MAPK pathway is upstream of activating transcription factor-2 (ATF-2), which is important in the regulation of MMP-2 expression (29, 63). Assessment of ATF-2 activation also revealed increased ATF-2 phosphorylation in Caco-2 BBe cells following chymase stimulation (results not shown), suggesting that chymase induces MMP-2 expression via the p38/ATF-2 pathway. PAR-2 activation has been associated with enhanced vascular permeability (28). We demonstrate that PAR-2 activation was required for chymase-induced MMP-2 expression by intestinal epithelial cells. We postulate that chymase signaling through PAR-2 stimulates MMP-2 production and that MMP-2 induces an increase in intestinal epithelial permeability. An alternative explanation is that PAR-2 activation promoted the redistribution of TJ proteins, leading to altered intestinal epithelial permeability. Consistent with this, basolateral PAR-2 activation of intestinal epithelial cells induced TJ protein redistribution (32). Western blot analysis of total protein from Caco-2 BBe cells revealed loss of claudin-5 protein, suggesting that the phenotype was related to proteolytic loss of claudin-5; however, we cannot rule out a role for PAR-2 signaling in the redistribution of intestinal epithelial TJ proteins and modification of paracellular permeability. These studies warrant further investigation to decipher the specific role of PAR-2 in chymase-mediated epithelial dysfunction.
Mast cells are classically considered to require activation by various stimuli, including bacteria, viruses, and allergens, to undergo degranulation (101). Indeed, we show that LPS stimulation of BMMCs induces TNF-α and IL-6 secretion. The mast cell-derived chymase-induced increase in intestinal epithelial permeability in the absence of external activation (19) (Fig. 8) suggests that mast cells constitutively release chymase independently of exogenous activation (constitutive mast cell degranulation). Mast cells can undergo piecemeal degranulation, i.e., a slow and selective release of mediators in small vesicles that bud from the granules and fuse with the cell membrane, which is postulated to be the most prevalent form of granule loss observed in normal human tissue mast cells in situ yet remains poorly understood. Notably, we also demonstrated increased chymase activity in the presence of heparin. Mast cell granules contain serglycin PG, composed of heparin- or chondroitin sulfate-type GAGs attached to the serglycin core protein. Serglycin PGs interact with mast cell proteases to regulate storage, activity, and processing (49, 65). Through its GAG side chains, serglycin binds chymase with very high affinity, and chymase remains complexed with serglycin PG following mast cell degranulation (17, 61). The interactions between serglycin PG and chymase regulate chymase's substrate specificity and activity (50). It is postulated that heparin interactions with proteins via heparin-binding domains lead to efficient substrate presentation to the chymase-heparin complex, thus facilitating efficient degradation (68). However, heparin-binding properties are not an absolute prerequisite for substrate presentation to chymase, as a number of proteins such as angiotensin I and albumin are non-heparin-binding proteins (49).
Proteases, including tryptase and cathepsin G, have been shown to be constitutively released by mast cells, have the capability to induce PAR and MMP-2 activation, and regulate intestinal epithelial barrier function. However, we demonstrate that chymase primarily drives intestinal epithelial barrier function. The chymase specificity is likely derived from the discrete differences in molecular pathways involved in regulation of protease-mediated PAR, MMP-2 activation, and barrier function. Moreover, while there is evidence of tryptase activation of PAR-2 and MMP-2, tryptase-induced activation of MMP-2 occurs via an PAR-2-independent manner (86). Furthermore, cathepsin G dysregulation of intestinal epithelial barrier function is mediated by PAR-4 on intestinal epithelial cells, and there is little evidence of cathepsin G-induced MMP-2 activation (10, 11). Thus the specificity of chymase over other proteases, such as tryptase and cathepsin G, may be attributable to substrate specificity (PAR-2 vs. PAR-4) within the local intestinal environment.
A number of studies performed by us and others report that, under conditions of mast cell activation (soluble antigen and helminth), altered intestinal epithelial permeability can occur independently of mast cell chymase activity (20, 33). We show that LPS-stimulated BMMCs stimulate an increase in intestinal epithelial permeability that was independent of chymase activity. Activation of mast cells by IgE-dependent and -independent mechanisms leads to the release of mediators, including proteases (tryptases and chymases), autacoid mediators (histamine and serotonin), and cytokines (IL-13, IL-6, and TNF-α), which have been implicated in intestinal epithelial barrier dysfunction via dysregulation of components of trans- and paracellular permeability (30, 73, 88). We show that LPS stimulation of BMMCs induced a decrease in intestinal epithelial permeability, notably that intestinal permeability was mediated by MCPT-4-dependent and -independent mechanisms. These data indicate that, under conditions of immune activation, multiple mast cell-driven pathways may contribute to intestinal epithelial barrier dysfunction independent of MCPT-4.
In summary, our results demonstrate that mast cell chymase induces a decrease in intestinal epithelial barrier function through the production and activation of MMP-2, signaling through PAR-2, and TJ protein degradation. We speculate that this is one of the mechanisms of barrier dysfunction in mast cell-mediated diseases such as food allergies. Further characterization of PAR-2 signaling, as well as intracellular signaling cascades, may be useful to understand the regulation of intestinal barrier function. The notion that increased intestinal permeability precedes and can predispose patients to chronic inflammatory disease highlights the importance of understanding the mediators and mechanisms that regulate intestinal permeability. Better understanding of these pathways will be useful in the future treatment and prevention of diseases including IBD, food allergies, and diabetes.
GRANTS
This work was supported in part by National Institutes of Health Grants R01-AI-073553 (S. P. Hogan), F30-DK-082113 (K. R. Groschwitz), T32-GM-063483 (K. R. Groschwitz), and T30-DK-078392 (Viral Vector Core of the Digestive Diseases Research Core Center in Cincinnati), a Crohn's Colitis Foundation of America Career Development Award (S. P. Hogan), and an American Heart Association Grant-in-Aid Award (S. P. Hogan).
DISCLOSURES
S. P. Hogan is a consultant with Immune Pharmaceuticals. All other authors have no conflicts of interest, financial or otherwise.
AUTHOR CONTRIBUTIONS
K.R.G. and S.P.H. are responsible for conception and design of the research; K.R.G., D.W., H.O., and R.A. performed the experiments; K.R.G., D.W., H.O., R.A., and S.P.H. analyzed the data; K.R.G., R.A., and S.P.H. interpreted the results of the experiments; K.R.G. and S.P.H. prepared the figures; K.R.G. and S.P.H. drafted the manuscript; K.R.G. and S.P.H. edited and revised the manuscript; K.R.G., D.W., and S.P.H. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank Drs. Gunnar Pejler, Magnus Abrink, Fred Finkelman, Marc Rothenberg, Yui Hsi Wang, and Ariel Munitz for helpful discussions and critical review of the manuscript. We also thank Shawna Hottinger for editorial assistance.
REFERENCES
- 1. Al-Sadi R, Ye D, Dokladny K, Ma T. Mechanism of IL-1β-induced increase in intestinal epithelial tight junction permeability. J Immunol 180: 5653–5661, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amasheh S, Schmidt T, Mahn M, Florian P, Mankertz J, Tavalali S, Gitter A, Schulzke J, Fromm M. Contribution of claudin-5 to barrier properties in tight junctions of epithelial cells. Cell Tissue Res 321: 89–96, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Andersson MK, Enoksson M, Gallwitz M, Hellman L. The extended substrate specificity of the human mast cell chymase reveals a serine protease with well-defined substrate recognition profile. Int Immunol 21: 95–104, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Andersson MK, Karlson U, Hellman L. The extended cleavage specificity of the rodent β-chymases rMCP-1 and mMCP-4 reveal major functional similarities to the human mast cell chymase. Mol Immunol 45: 766–775, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Bacher A, Griebl K, Mackamul S, Mitreiter R, Muckter H, Ben-Shaul Y. protease inhibitors suppress the formation of tight junctions in gastrointestinal cell lines. Exp Cell Res 200: 97–104, 1992 [DOI] [PubMed] [Google Scholar]
- 6. Carmeliet P, Moon L, Lijnen R, Baes M, Lemaitre V, Tipping P, Drew A, Eeckhout Y, Shapiro S, Lupu F, Collen D. Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nat Genet 17: 439–444, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Carratu R, Secondulfo M, de Magistris L, Iafusco D, Urio A, Carbone M, Pontoni G, Carteni M, Prisco F. Altered intestinal permeability to mannitol in diabetes mellitus type I. J Pediatr Gastroenterol Nutr 28: 264–269, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Caughey G, Raymond W, Wolters P. Angiotensin II generation by mast cell α- and β-chymases. Biochim Biophys Acta 1480: 245–257, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases. An overview. Mol Cell Biochem 253: 269–285, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Dabek M, Ferrier L, Annahazi A, Bezirard V, Polizzi A, Cartier C, Leveque M, Roka R, Wittmann T, Theodorou V, Bueno L. Intracolonic infusion of fecal supernatants from ulcerative colitis patients triggers altered permeability and inflammation in mice: role of cathepsin G and protease-activated receptor-4. Inflamm Bowel Dis 17: 1409–1414, 2011 [DOI] [PubMed] [Google Scholar]
- 11. Dabek M, Ferrier L, Roka R, Gecse K, Annahazi A, Moreau J, Escourrou J, Cartier C, Chaumaz G, Leveque M, Ait-Belgnaoui A, Wittmann T, Theodorou V, Bueno L. Luminal cathepsin G and protease-activated receptor 4: a duet involved in alterations of the colonic epithelial barrier in ulcerative colitis. Am J Pathol 175: 207–214, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Descargues P, Deraison C, Bonnart C, Kreft M, Kishibe M, Ishida-Yamamoto A, Elias P, Barrandon Y, Zambruno G, Sonnenberg A, Hovnanian A. Spink5-deficient mice mimic Netherton syndrome through degradation of desmoglein 1 by epidermal protease hyperactivity. Nat Genet 37: 56–65, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Falth-Magnusson K, Kjellman NI, Magnusson KE, Sundqvist T. Intestinal permeability in healthy and allergic children before and after sodium-cromoglycate treatment assessed with different-sized polyethyleneglycols (PEG 400 and PEG 1000). Clin Allergy 14: 277–286, 1984 [DOI] [PubMed] [Google Scholar]
- 14. Fang K, Raymond W, Blount J, Caughey G. Dog mast cell α-chymase activates progelatinase B by cleaving the Phe88-Gln89 and Phe91-Glu92 bonds of the catalytic domain. J Biol Chem 272: 25628–25635, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Feng S, Cen J, Huang Y, Shen H, Yao L, Wang Y, Chen Z. Matrix metalloproteinase-2 and -9 secreted by leukemic cells increase the permeability of blood-brain barrier by disrupting tight junction proteins. PLos One 6: e20599, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garg P, Rojas M, Ravi A, Bockbrader K, Epstein S, Vijay-Kumar M, Gewirtz AT, Merlin D, Sitaraman SV. Selective ablation of matrix metalloproteinase-2 exacerbates experimental colitis: contrasting role of gelatinases in the pathogenesis of colitis. J Immunol 177: 4103–4112, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Gervasoni JE, Jr, Conrad DH, Hugli TE, Schwartz LB, Ruddy S. Degradation of human anaphylatoxin C3a by rat peritoneal mast cells: a role for the secretory granule enzyme chymase and heparin proteoglycan. J Immunol 136: 285–292, 1986 [PubMed] [Google Scholar]
- 18. Giebel S, Menicucci G, McGuire P, Das A. Matrix metalloproteinases in early diabetic retinopathy and their role in alteration of the blood-retinal barrier. Lab Invest 85: 597–607, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Groschwitz K, Ahrens R, Osterfeld H, Gurish M, Han X, Abrink M, Finkelman F, Pejler G, Hogan S. Mast cells regulate homeostatic intestinal epithelial migration and barrier function by a chymase/Mcpt4-dependent mechanism. Proc Natl Acad Sci USA 106: 22381–22386, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Groschwitz K, Hogan S. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol 124: 3–22, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hollander D, Vadheim CM, Brettholz E, Petersen GM, Delahunty T, Rotter JI. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann Intern Med 105: 883–885, 1986 [DOI] [PubMed] [Google Scholar]
- 24. Holmes JL, Van Itallie CM, Rasmussen JE, Anderson JM. Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expr Patterns 6: 581–588, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Ishida K, Takai S, Murano M, Nishikawa T, Inoue T, Murano N, Inoue N, Jin D, Umegaki E, Higuchi K, Miyazaki M. Role of chymase-dependent matrix metalloproteinase-9 activation in mice with dextran sodium sulfate-induced colitis. J Pharmacol Exp Ther 324: 422–426, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Jackson PG, Lessof MH, Baker RW, Ferrett J, MacDonald DM. Intestinal permeability in patients with eczema and food allergy. Lancet 1: 1285–1286, 1981 [DOI] [PubMed] [Google Scholar]
- 27. Kakimoto K, Takai S, Murano M, Ishida K, Yoda Y, Inoue T, Jin D, Umegaki E, Higuchi K. Significance of chymase-dependent matrix metalloproteinase-9 activation on indomethacin-induced small intestinal damages in rats. J Pharmacol Exp Ther 332: 684–689, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Kawabata A, Kuroda R, Minami T, Kataoka K, Taneda M. Increased vascular permeability by a specific agonist of protease-activated receptor-2 in rat hindpaw. Br J Pharmacol 125: 419–422, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim ES, Sohn YW, Moon A. TGF-β-induced transcriptional activation of MMP-2 is mediated by activating transcription factor (ATF)2 in human breast epithelial cells. Cancer Lett 252: 147–156, 2007 [DOI] [PubMed] [Google Scholar]
- 30. King SJ, Miller HR. Anaphylactic release of mucosal mast cell protease and its relationship to gut permeability in Nippostrongylus-primed rats. Immunology 51: 653–660, 1984 [PMC free article] [PubMed] [Google Scholar]
- 31. Kofford M, Schwartz L, Schechter N, Yager D, Diegelmann R, Graham M. Cleavage of type I procollagen by human mast cell chymase initiates collagen fibril formation and generates a unique carboxyl-terminal propeptide. J Biol Chem 272: 7127–7131, 1997 [DOI] [PubMed] [Google Scholar]
- 32. Lau C, Lytle C, Straus DS, DeFea KA. Apical and basolateral pools of proteinase-activated receptor-2 direct distinct signaling events in the intestinal epithelium. Am J Physiol Cell Physiol 300: C113–C123, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lawrence CE, Paterson YY, Wright SH, Knight PA, Miller HR. Mouse mast cell protease-1 is required for the enteropathy induced by gastrointestinal helminth infection in the mouse. Gastroenterology 127: 155–165, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Lees M, Taylor DJ, Woolley DE. Mast cell proteinases activate precursor forms of collagenase and stromelysin, but not of gelatinases A and B. Eur J Biochem 223: 171–177, 1994 [DOI] [PubMed] [Google Scholar]
- 35. Leyvraz C, Charles RP, Rubera I, Guitard M, Rotman S, Breiden B, Sandhoff K, Hummler E. The epidermal barrier function is dependent on the serine protease CAP1/Prss8. J Cell Biol 170: 487–496, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. List K, Szabo R, Wertz PW, Segre J, Haudenschild CC, Kim SY, Bugge TH. Loss of proteolytically processed filaggrin caused by epidermal deletion of matriptase/MT-SP1. J Cell Biol 163: 901–910, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu J, Jin X, Liu KJ, Liu W. Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. J Neurosci 32: 3044–3057, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annu Rev Pathol 5: 119–144, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Miyamori H, Takino T, Kobayashi Y, Tokai H, Itoh Y, Seiki M, Sato H. Claudin promotes activation of pro-matrix metalloproteinase-2 mediated by membrane-type matrix metalloproteinases. J Biol Chem 276: 28204–28211, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Monea S, Lehti K, Keski-Oja J, Mignatti P. Plasmin activates pro-matrix metalloproteinase-2 with a membrane-type 1 matrix metalloproteinase-dependent mechanism. J Cell Physiol 192: 160–170, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem 378: 151–160, 1997 [PubMed] [Google Scholar]
- 42. Nagase H, Suzuki K, Cawston TE, Brew K. Involvement of a region near valine-69 of tissue inhibitor of metalloproteinases (TIMP)-1 in the interaction with matrix metalloproteinase 3 (stromelysin 1). Biochem J 325: 163–167, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem 274: 21491–21494, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 161: 653–660, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Noe V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W, Bruyneel E, Matrisian LM, Mareel M. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci 114: 111–118, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Nusrat A, Turner JR, Madara JL. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am J Physiol Gastrointest Liver Physiol 279: G851–G857, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Oyamada S, Bianchi C, Takai S, Chu LM, Sellke FW. Chymase inhibition reduces infarction and matrix metalloproteinase-9 activation and attenuates inflammation and fibrosis after acute myocardial ischemia/reperfusion. J Pharmacol Exp Ther 339: 143–151, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pedersen G, Saermark T, Kirkegaard T, Brynskov J. Spontaneous and cytokine induced expression and activity of matrix metalloproteinases in human colonic epithelium. Clin Exp Immunol 155: 257–265, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pejler G, Abrink M, Ringvall M, Wernersson S. Mast cell proteases. Adv Immunol 95: 167–255, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Pejler G, Karlstrom A. Thrombin is inactivated by mast cell secretory granule chymase. J Biol Chem 268: 11817–11822, 1993 [PubMed] [Google Scholar]
- 51. Prudova A, auf dem Keller U, Butler GS, Overall CM. Multiplex N-terminome analysis of MMP-2 and MMP-9 substrate degradomes by iTRAQ-TAILS quantitative proteomics. Mol Cell Proteomics 9: 894–911, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ra H, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biol 26: 587–596, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Raymond MA, Mollica L, Vigneault N, Desormeaux A, Chan JS, Filep JG, Hebert MJ. Blockade of the apoptotic machinery by cyclosporin A redirects cell death toward necrosis in arterial endothelial cells: regulation by reactive oxygen species and cathepsin D. FASEB J 17: 515–517, 2003 [DOI] [PubMed] [Google Scholar]
- 54. Rossello A, Nuti E, Orlandini E, Carelli P, Rapposelli S, Macchia M, Minutolo F, Carbonaro L, Albini A, Benelli R, Cercignani G, Murphy G, Balsamo A. New N-arylsulfonyl-N-alkoxyaminoacetohydroxamic acids as selective inhibitors of gelatinase A (MMP-2). Bioorg Med Chem 12: 2441–2450, 2004 [DOI] [PubMed] [Google Scholar]
- 55. Rothmeier AS, Ruf W. Protease-activated receptor 2 signaling in inflammation. Semin Immunopathol 34: 133–149, 2012 [DOI] [PubMed] [Google Scholar]
- 56. Ruangpanit N, Price JT, Holmbeck K, Birkedal-Hansen H, Guenzler V, Huang X, Chan D, Bateman JF, Thompsen EW. MT1-MMP-dependent and -independent regulation of gelatinase A activation in long-term ascorbate-treated fibroblast cultures: regulation by fibrillar collagen. Exp Cell Res 272: 109–118, 2002 [DOI] [PubMed] [Google Scholar]
- 57. Saarinen J, Kalkkinen N, Welgus H, Kovanen P. Activation of human interstitial procollagenase through direct cleavage of the Leu83-Thr84 bond by mast cell chymase. J Biol Chem 269: 18134–18140, 1994 [PubMed] [Google Scholar]
- 59. Sapone A, de Magistris L, Pietzak M, Clemente M, Tripathi A, Cucca F, Lampis R, Kryszak D, Carteni M, Generoso M, Iafusco D, Prisco F, Laghi F, Riegler G, Carratu R, Counts D, Fasano A. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes 55: 1443–1449, 2006 [DOI] [PubMed] [Google Scholar]
- 60. Sears C. Molecular physiology and pathophysiology of tight junctions. V. Assault of the tight junction by enteric pathogens. Am J Physiol Gastrointest Liver Physiol 279: G1129–G1134, 2000 [DOI] [PubMed] [Google Scholar]
- 61. Serafin WE, Katz HR, Austen KF, Stevens RL. Complexes of heparin proteoglycans, chondroitin sulfate E proteoglycans, and [3H]diisopropyl fluorophosphate-binding proteins are exocytosed from activated mouse bone marrow-derived mast cells. J Biol Chem 261: 15017–15021, 1986 [PubMed] [Google Scholar]
- 62. Sharma R, Prasad V, McCarthy E, Savin V, Dileepan K, Stechschulte D, Lianos E, Wiegmann T, Sharma M. Chymase increases glomerular albumin permeability via protease-activated receptor-2. Mol Cell Biochem 297: 161–169, 2007 [DOI] [PubMed] [Google Scholar]
- 63. Song H, Ki SH, Kim SG, Moon A. Activating transcription factor 2 mediates matrix metalloproteinase-2 transcriptional activation induced by p38 in breast epithelial cells. Cancer Res 66: 10487–10496, 2006 [DOI] [PubMed] [Google Scholar]
- 64. Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol 200: 448–464, 2003 [DOI] [PubMed] [Google Scholar]
- 65. Stevens RL, Adachi R. Protease-proteoglycan complexes of mouse and human mast cells and importance of their β-tryptase-heparin complexes in inflammation and innate immunity. Immunol Rev 217: 155–167, 2007 [DOI] [PubMed] [Google Scholar]
- 66. Strongin A, Collier I, Bannikov G, Marmer B, Grant G, Goldberg G. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem 270: 5331–5338, 1995 [DOI] [PubMed] [Google Scholar]
- 67. Swystun VA, Renaux B, Moreau F, Wen S, Peplowski MA, Hollenberg MD, MacNaughton WK. Serine proteases decrease intestinal epithelial ion permeability by activation of protein kinase Cζ. Am J Physiol Gastrointest Liver Physiol 297: G60–G70, 2009 [DOI] [PubMed] [Google Scholar]
- 68. Tchougounova E, Forsberg E, Angelborg G, Kjellen L, Pejler G. Altered processing of fibronectin in mice lacking heparin. A role for heparin-dependent mast cell chymase in fibronectin degradation. J Biol Chem 276: 3772–3777, 2001 [DOI] [PubMed] [Google Scholar]
- 69. Tchougounova E, Lundequist A, Fajardo I, Winberg J, Abrink M, Pejler G. A key role for mast cell chymase in the activation of pro-matrix metalloprotease-9 and pro-matrix metalloprotease-2. J Biol Chem 280: 9291–9296, 2005 [DOI] [PubMed] [Google Scholar]
- 70. Tchougounova E, Pejler G, Abrink M. The chymase, mouse mast cell protease 4, constitutes the major chymotrypsin-like activity in peritoneum and ear tissue. A role for mouse mast cell protease 4 in thrombin regulation and fibronectin turnover. J Exp Med 198: 423–431, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Toth M, Chvyrkova I, Bernardo MM, Hernandez-Barrantes S, Fridman R. Pro-MMP-9 activation by the MT1-MMP/MMP-2 axis and MMP-3: role of TIMP-2 and plasma membranes. Biochem Biophys Res Commun 308: 386–395, 2003 [DOI] [PubMed] [Google Scholar]
- 72. Tuccinardi T, Martinelli A, Nuti E, Carelli P, Balzano F, Uccello-Barretta G, Murphy G, Rossello A. Amber force field implementation, molecular modelling study, synthesis and MMP-1/MMP-2 inhibition profile of (R)- and (S)-N-hydroxy-2-(N-isopropoxybiphenyl-4-ylsulfonamido)-3-methylbutanamides. Bioorg Med Chem 14: 4260–4276, 2006 [DOI] [PubMed] [Google Scholar]
- 73. Turner MW, Boulton P, Shields JG, Strobel S, Gibson S, Miller HR, Levinsky RJ. Intestinal hypersensitivity reactions in the rat. I. Uptake of intact protein, permeability to sugars and their correlation with mucosal mast-cell activation. Immunology 63: 119–124, 1988 [PMC free article] [PubMed] [Google Scholar]
- 74. van Elburg RM, Uil JJ, Mulder CJ, Heymans HS. Intestinal permeability in patients with coeliac disease and relatives of patients with coeliac disease. Gut 34: 354–357, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Vartio T, Seppa H, Vaheri A. Susceptibility of soluble and matrix fibronectins to degradation by tissue proteinases, mast cell chymase and cathepsin G. J Biol Chem 256: 471–477, 1981 [PubMed] [Google Scholar]
- 76. Ventura M, Polimeno L, Amoruso A, Gatti F, Annoscia E, Marinaro M, Di Leo E, Matino M, Buquicchio R, Bonini S, Tursi A, Francavilla A. Intestinal permeability in patients with adverse reactions to food. Dig Liver Dis 38: 732–736, 2006 [DOI] [PubMed] [Google Scholar]
- 77. von Lampe B, Barthel B, Coupland SE, Riecken EO, Rosewicz S. Differential expression of matrix metalloproteinases and their tissue inhibitors in colon mucosa of patients with inflammatory bowel disease. Gut 47: 63–73, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-γ and tumor necrosis factor-α synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol 166: 409–419, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Warnaar N, Hofker HS, Maathuis MH, Niesing J, Bruggink AH, Dijkstra G, Ploeg RJ, Schuurs TA. Matrix metalloproteinases as profibrotic factors in terminal ileum in Crohn's disease. Inflamm Bowel Dis 12: 863–869, 2006 [DOI] [PubMed] [Google Scholar]
- 80. Wilson S, Gallagher S, Warpeha K, Hawthorne S. Amplification of MMP-2 and MMP-9 production by prostate cancer cell lines via activation of protease-activated receptors. Prostate 60: 168–174, 2004 [DOI] [PubMed] [Google Scholar]
- 81. Wisner DM, Harris LR, 3rd, Green CL, Poritz LS. Opposing regulation of the tight junction protein claudin-2 by interferon-γ and interleukin-4. J Surg Res 144: 1–7, 2008 [DOI] [PubMed] [Google Scholar]
- 82. Wu D, Ahrens R, Osterfeld H, Noah TK, Groschwitz K, Foster PS, Steinbrecher KA, Rothenberg ME, Shroyer NF, Matthaei KI, Finkelman FD, Hogan SP. Interleukin-13 (IL-13)/IL-13 receptor α1 (IL-13Rα1) signaling regulates intestinal epithelial cystic fibrosis transmembrane conductance regulator channel-dependent Cl− secretion. J Biol Chem 286: 13357–13369, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wyatt J, Vogelsang H, Hubl W, Waldhoer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet 341: 1437–1439, 1993 [DOI] [PubMed] [Google Scholar]
- 84. Yacyshyn B, Meddings J, Sadowski D, Bowen-Yacyshyn MB. Multiple sclerosis patients have peripheral blood CD45RO+ B cells and increased intestinal permeability. Dig Dis Sci 41: 2493–2498, 1996 [DOI] [PubMed] [Google Scholar]
- 85. Yada K, Shibata K, Matsumoto T, Ohta M, Yokoyama S, Kitano S. Protease-activated receptor-2 regulates cell proliferation and enhances cyclooxygenase-2 mRNA expression in human pancreatic cancer cells. J Surg Oncol 89: 79–85, 2005 [DOI] [PubMed] [Google Scholar]
- 86. Yamamoto K, Kumagai N, Fukuda K, Fujitsu Y, Nishida T. Activation of corneal fibroblast-derived matrix metalloproteinase-2 by tryptase. Curr Eye Res 31: 313–317, 2006 [DOI] [PubMed] [Google Scholar]
- 87. Yang Y, Estrada E, Thompson J, Liu W, Rosenberg G. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab 27: 697–709, 2007 [DOI] [PubMed] [Google Scholar]
- 88. Yu LC, Perdue MH. Role of mast cells in intestinal mucosal function: studies in models of hypersensitivity and stress. Immunol Rev 179: 61–73, 2001 [DOI] [PubMed] [Google Scholar]
- 89. Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGFβ and promotes tumor invasion and angiogenesis. Genes Dev 14: 163–176, 2000 [PMC free article] [PubMed] [Google Scholar]