Abstract
The present study characterized the TGR5 expression and the signaling pathways coupled to this receptor that mediates the relaxation of gastric smooth muscle. TGR5 was detected in gastric muscle cells by RT-PCR and Western blotting. Treatment of cells with the TGR5-selective ligand oleanolic acid (OA) activated Gαs, but not Gαq, Gαi1, Gαi2, or Gαi3, and increased cAMP levels. OA did not elicit contraction, but caused relaxation of carbachol-induced contraction of gastric muscle cells from wild-type mice, but not tgr5−/− mice. OA, but not a selective exchange protein activated by cAMP (Epac) ligand (8-pCPT-2′-O-Me-cAMP), caused phosphorylation of RhoA and the phosphorylation was blocked by the PKA inhibitor, myristoylated PKI, and by the expression of phosphorylation-deficient mutant RhoA (S188A). Both OA and Epac ligand stimulated Ras-related protein 1 (Rap1) and inhibited carbachol (CCh)-induced Rho kinase activity. Expression of RhoA (S188A) or PKI partly reversed the inhibition of Rho kinase activity by OA but had no effect on inhibition by Epac ligand. However, suppression of Rap1 with siRNA blocked the inhibition of Rho kinase by Epac ligand, and partly reversed the inhibition by OA; the residual inhibition was blocked by PKI. Muscle relaxation in response to OA, but not Epac ligand, was partly reversed by PKI. We conclude that activation of TGR5 causes relaxation of gastric smooth muscle and the relaxation is mediated through inhibition of RhoA/Rho kinase pathway via both cAMP/Epac-dependent stimulation of Rap1 and cAMP/PKA-dependent phosphorylation of RhoA at Ser188. TGR5 receptor activation on smooth muscle reveals a novel mechanism for the regulation of gut motility by bile acids.
Keywords: bile acids, G protein-coupled receptor, oleanolic acid, smooth muscle relaxation, TGR5 receptor
bile acids (BA), synthesized from cholesterol in liver and secreted into duodenum in response to food, play an important role in lipid digestion and absorption. Nearly 95% of BA are reabsorbed in the distal part of the ileum and colon and transported back to the liver via enterohepatic circulation. The primary bile acids, cholic acid (CA) and chenodeoxycholic acid (CDCA), are converted to their respective secondary bile acids deoxycholic acid (DCA) and lithocholic acid (LCA) by bacterial enzymes of the intestine. It is now very well established that BAs, in addition to their classic function in lipid digestion, act as signaling molecules with systemic endocrine functions such as regulation of bile acid, glucose and lipid metabolism, immune response, and cell proliferation and differentiation (5, 6, 32).
The endocrine function of bile acids is mediated through activation of nuclear and plasma membrane receptors, including the nuclear farnesol-X-receptor (FXRα, NR1H4) and plasma membrane-bound G protein-coupled receptor TGR5, also known as GPBAR1 (G protein-coupled bile acid receptor 1) (8, 15, 16, 23, 34). FXR expression is more abundant in liver and gut compared with other tissues and is more selectively activated by CDCA compared with other bile acids. FXRs function as sensors of metabolic signals and play an important role in the regulation of bile acid, glucose, and cholesterol homeostasis by controlling the expression of genes in specific metabolic pathways (15, 29). By downregulating the expression of CYP7α1, a key enzyme in BA biosynthesis, FXR exerts a negative-feedback control on BA biosynthesis (13). Activation of FXR increases glycogen biosynthesis in liver facilitating decrease in blood glucose levels (14, 36).
TGR5 expression is more abundant in liver, adipose tissue, and intestine compared with other tissues and is more selectively activated by LCA compared with other bile acids (8, 16). TGR5 participates in control of insulin release from pancreas, and this effect is mediated indirectly through release of glucagon-like peptide (GLP-1) and glucose-dependent insulinotropic peptide (GIP) from enteroendocrine cells and direct activation of β cells in the pancreas (7, 10). TGR5 is also highly expressed in the epithelium of gallbladder, where activation of TGR5 promotes gallbladder filling with bile (12). This is consistent with the fact that tgr5−/− mice are refractory to the formation of gallstones in response to the high-fat diet (33).
Activation of TGR5 also regulates gut motility. Luminal administration of bile acids inhibits gastric emptying and intestinal transit (25). This effect could be due to release of hormones such as GLP-1 and peptide YY (PYY) from enteroendocrine cells, release of nitric oxide from inhibitory motor neurons, and/or direct effect on smooth muscle of the gut. Studies by Poole et al. (25) demonstrated that the expression of TGR5 in inhibitory motor neurons of the enteric nervous system and addition of DCA to isolated segments of ileum and colon suppressed spontaneous phasic activity. Lavoie et al. (11) demonstrated that the expression of TGR5 in gallbladder smooth muscle and addition of LCA to isolated segments of gallbladder reduced spontaneous Ca2+ fluxes and caused membrane hyperpolarization. However, to date, there are no experimental data on the signal transduction pathways initiated by TGR5 receptors to modulate gastrointestinal smooth muscle function. In the present study, we demonstrated that in gastric smooth muscle, TGR5 receptors are coupled to Gαs/cAMP pathway and activation of these receptors leads to muscle relaxation via both PKA-dependent phosphorylation of RhoA at Ser188, and protein kinase A (PKA)-independent, exchange protein activated by cAMP (Epac)-dependent stimulation of Ras-related protein 1 (Rap1).
MATERIALS AND METHODS
Reagents.
Oleanolic acid (OA) was obtained from Sigma-Aldrich (St. Louis, MO). Antibodies against TGR5, Rap1, and RhoA were obtained from Abcam (Cambridge, MA). [35S]GTPγS was obtained from NEN Life Sciences Products (Boston, MA). Western blotting and chromatography material were obtained from Bio-Rad Laboratories (Hercules, CA). Collagenase and soybean trypsin inhibitor were obtained from Worthington Biochemical (Freehold, NJ). All other reagents were obtained from Sigma (St. Louis, MO).
TGR5 knockout mice.
TGR5 knockout mice (tgr5−/−) were generated in a hybrid (129S3/SvlmJ×C57BL/6) background and were maintained in a temperature-controlled environment with a 12:12-h light/dark cycle and free access to food and water. Age- and sex-matched animals were used between 6 and 12 wk of age (31). Animals were killed by anesthetic overdose and bilateral thoracotomy. All experiments with animals were approved by Institutional Animal Care and Use Committees.
Preparation of dispersed and cultured gastric muscle cells.
Smooth muscle cells were isolated from the circular muscle layer of rabbit stomach by sequential enzymatic digestion, filtration, and centrifugation, as described (20, 21). The cells were resuspended in enzyme-free medium consisting of 120 mM NaCl, 4 mM KCl, 2.6 mM KH2PO4, 2 mM CaCl2, 0.6 mM MgCl2, 25 mM HEPES, 14 mM glucose, and 2.1% Eagles' essential amino acid mixture (pH 7.4). The partly digested tissues were washed with 50 ml of enzyme-free medium, and the muscle cells were allowed to disperse spontaneously for 30 min. The cells were harvested by filtration through 500-μm Nitex and centrifuged twice at 350 g for 10 min to eliminate cell debris. In some experiments, the muscle cells were cultured in DMEM containing 10% FBS until they attained confluence and were used in the first passage.
Detection of TGR5 in rabbit gastric muscle cells by conventional PCR.
Cultured gastric muscle cells were treated with the RNaqueous reagent (Ambion, Austin, TX) for total RNA extraction. The potentially contaminated genomic DNA was removed by treating 10 μg of the RNA sample at 37°C for 30 min with 1 μl of TURBO DNase (Ambion) followed by an extraction with phenol:chloroform:isoamylalcohol (25:24:1). RNA (2 μg) was used to synthesize cDNA using SuperScript II reverse transcriptase (Applied Biosystems, Foster, CA) with random hexanucleotide primers. Conventional PCR was performed on cDNA using the HotMaster Taq DNA polymerase kit (Epicentre Biotechnologies, Madison, WI). The primers for rabbit TGR5 (GenBank Accession no. NM_001082648) were forward 5′-CTG TCA CTG GCC CTG GCA AGC-3′ and reverse 5′-CGC CAC ATA GCG CTC CCC GTG-3′, generating a fragment of 282 bp.
Determination of TGR5 in dispersed smooth muscle by Western blotting.
Proteins (50 μg) were separated by SDS-PAGE and transferred to nitrocellulose membranes (Immobilon-FL, Millipore, Billerica, MA). The membrane was blocked, incubated with antibodies to TGR5 (Abcam, Cambridge, MA), and following washing incubated with secondary antibody conjugated to horseradish peroxidase (Santa Cruz biotechnology, Santa Cruz, CA). Immunolabeled proteins were detected by the ECL Plus enhanced chemiluminescence detection system (Amersham Life Science Buckinghamshire, UK).
Identification of TGR5 receptor-activated G proteins in smooth muscle membranes.
G proteins selectively activated by TGR5 ligands were identified by the method of Okamoto et al. (22). Ten milliliters of muscle cell suspension (2 × 106 cells/ml) was homogenized in 20 mM HEPES medium (pH 7.4) containing 2 mM MgCl2, 1 mM EDTA, and 2 mM dithiothreitol. After centrifugation at 27,000 g for 15 min, the crude membranes were solubilized for 60 min at 4°C in 20 mM HEPES medium (pH 7.4) containing 2 mM EDTA, 240 mM NaCl, and 0.5% solution containing 10 mM HEPES (pH 7.4), 100 μM EDTA, and 10 mM MgCl2 in the presence or absence of OA (10 μM). The reaction was stopped with 10 vol of 100 mM Tris-HCl medium (pH 8.0) containing 10 mM MgCl2, 100 mM NaCl, and 20 μM GTP, and the mixture was placed in wells precoated with specific G protein antibodies. Coating with G protein antibodies (1:1,000) was done after the wells were first coated with anti-rabbit IgG for 2 h on ice. After incubation for 2 h on ice, the wells were washed three times with phosphate buffer solution containing 0.05% Tween 20, and the radioactivity from each cell was counted by liquid scintillation (18).
cAMP generation.
cAMP was measured by ELISA (Arbor Assays, Ann Arbor, MI). OA (10 μM) was added to 0.5 ml of cell suspension (106 cells/ml) together with a threshold concentration of 3-isobutyl-1-methylxanthine (IBMX, 1 μM), and the reaction was terminated after 60 s with 6% cold trichloroacetic acid (vol/vol). The mixture was centrifuged at 2,000 g for 15 min at 4°C; the supernatant was extracted three times with 2 ml diethyl ether, and the samples were lyophilized. The samples were reconstituted for ELISA and acetylated with triethylamine/acetic anhydride (3:1 vol/vol) for 30 min. cAMP was measured in triplicate using 100-μl aliquots, and the measurements were expressed as percent increase above basal level.
cAMP-dependent PKA assay.
One milliliter of cell suspension was incubated in HEPES medium with OA (10 μM) or OA with protein kinase inhibitor, myristoylated PKI (1 μM), for 60 s at 31°C. The reaction was stopped by rapid centrifugation, and the pellet was rinsed with a medium containing (in mM) 50 Tris·HCl (pH 7.4), 10 EDTA, 0.5 IBMX, 10 2-mercaptoethanol, and 100 NaCl and homogenized in 0.5 ml of ice-cold medium. The mixture was centrifuged at 48,000 g for 15 min, and the supernatant was used as a source of protein kinase. The enzyme activity was measured by ELISA (Arbor Assays, Ann Arbor, MI).
Transfection of phosphorylation site-deficient RhoS188A in cultured smooth muscle cells.
RhoA mutant cDNA was subcloned into the multiple cloning sites (EcoRI) of the eukaryotic expression vector (pEXV), and a myc tag was incorporated into the NH2 terminus. Recombinant plasmid cDNAs (2 μg each) were transiently transfected into cultured smooth muscle cells using Lipofectamine plus Reagent. The cells were transfected with 1 μg of pGreen Lantern-1 DNA to monitor expression. Control cells were cotransfected with 2 μg of pEXV and 1 μg of pGreen Lantern-1 DNA. Transfection efficiency was monitored by the expression of green fluorescent protein using FITC filters. Expression was demonstrable in over 80% of the cells (21).
Phosphorylation of RhoA.
Phosphorylation of RhoA was measured from the amount of 32P incorporated after immunoprecipitation with specific RhoA antibody. Smooth muscle cell suspensions (10 ml; 3 × 106 cells/ml) were incubated with [32P]orthophosphate for 4 h at 31°C. One-milliliter samples were then incubated with OA (10 μM) in the presence or absence of myristoylated PKI (1 μM) or the cAMP analog (8-pCPT-2′-O-Me-cAMP, 10 μM) that selectively activates Epac for 10 min. The reaction was terminated with an equal volume of lysis buffer (final concentration in mM: 150 NaCl, 10 MgCl2, 1 PMSF, 10 EDTA, 10 Na2P2O7, 50 NaF, 0.2 Na3VO4, plus 1% Triton X-100, 0.5% SDS, 0.75% deoxycholate, 10 μg/ml leupeptin, and 100 μg/ml aprotinin). The cell lysates were separated by centrifugation at 13,000 g for 15 min at 4°C, and they were incubated with polyclonal RhoA antibody for 2 h at 4°C and with 40 μl of protein A-Sepharose for another 1 h. The immunoprecipitates were washed, extracted with Laemmli buffer, and separated by electrophoresis on 10% SDS-PAGE. After transfer to PVDF membranes, and 32P-labeled RhoA was visualized by autoradiography (21).
Assay for Rho kinase activity.
Rho kinase activity was determined in cell extracts by immunokinase assay as described previously (20). Cultured (after transfection) or freshly dispersed smooth muscle cells were solubilized with lysis buffer containing 50 mM Tris·HCl (pH 7.5), 0.1% SDS, 0.5% sodium deoxycholate, 1% Nonidet P-40, 150 mM NaCl, 1 mM PMSF, 10 μg/ml aprotinin, 10 μg/ml pepstatin A, and 10 μg/ml leupeptin. An equal amount of protein extract was incubated with Rho kinase antibody for 2 h, protein A/G agarose was added, and the samples were incubated at overnight at 4°C. The immunoprecipitates were washed twice in a medium containing 10 mM MgCl2, 40 mM HEPES (pH 7.4) and then incubated for 5 min on ice with 5 μg of myelin basic protein. The assay was initiated by the addition of 10 μCi of [γ-32P]ATP (3,000 Ci/mmol) and 20 μM ATP, followed by incubation for 10 min at 37°C. The 32P-labeled myelin basic protein was absorbed onto phosphocellulose disks, and free radioactivity was removed by repeated washing with 75 mM phosphoric acid. The amount of radioactivity on the disks was measured by liquid scintillation.
Activation of Rap1.
Freshly dispersed gastric smooth muscle cells were treated with OA (10 μM) in the presence or absence of myristoylated PKI (1 μM) for 10 min, and cells were solubilized with lysis buffer containing 50 mM Tris·HCl (pH 7.5), 0.1% SDS, 0.5% sodium deoxycholate, 1% Nonidet P-40, 150 mM NaCl, 1 mM PMSF, 10 μg/ml aprotinin, 10 μg/ml pepstatin A, and 10 μg/ml leupeptin. The lysates were incubated for 1 h with GST-fusion protein containing the Rap1 binding domain of Rap1GDS precoupled with glutathione-Sepharose to isolate activated GTP-bound Rap1. The Sepharose beads were washed with lysis buffer and suspended in Laemmli sample buffer; the samples were run on SDS-PAGE, transferred to nitrocellulose membranes, and blotted with Rap1 polyclonal antibody and the proteins were detected by the ECL Plus enhanced chemiluminescence detection system (Amersham Life Science Buckinghamshire, UK).
Measurement of relaxation in dispersed muscle cells.
Inhibition of contraction (i.e., relaxation) by OA was expressed as the decrease in maximal cell contraction induced by 0.1 μM CCh as described previously (18, 19). A 0.5-ml aliquot of cell suspension (104 cells/ml) was incubated for 10 min with 0.2 ml of medium containing test agents and the reaction terminated with 1% acrolein. In control experiments, 0.2 ml of medium was added without test agents. The length of the first 50 muscle cells encountered in successive fields was measured by scanning micrometry, and relaxation was measured in muscle cells maximally contracted with CCh (0.1 μM); the response was expressed as the decrease in maximal contraction. Basal muscle cell length was similar in different series of experiments and was 94 ± 5 μm.
Preparation of small interfering RNA.
Small interfering RNA (siRNA) oligonucleotides specific for rabbit TGR5 was obtained from Invitrogen (Carlsbad, CA). The sequence of TGR5 siRNA was as follows: forward 5′-CCC AAC UUC UCC UUC CUC UTT-3′ and reverse 5′-AGA GGA AGG AGA AGU UGG GTT-3′. Double-stranded RNA oligoribonucleotide NNGCGCGCUUUGUAGGAUUCA (5′-3′) was used as a control siRNA. Rap1 siRNA was as follows: CCT TTC CAC CAC GGT GAA A (5′-3′). Double-stranded RNA oligoribonucleotide NNGCGCGCUUUGUAGGAUUCA (5′-3′) was used as a negative control. Smooth muscle cells were seeded on 100-mm plates and allowed to adhere overnight. At the time of transfection, smooth muscle cells were 80–85% confluent and the siRNAs were transfected into smooth muscle cells at a final concentration of 20 μM by use of Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Cells were kept for 24–48 h before IBMX and ligand treatment.
Statistical analysis.
The results are expressed as means ± SE of n experiments, and statistical significance was determined using Student's t-test for paired or unpaired values.
RESULTS
Expression of TGR5 in gastric smooth muscle cells.
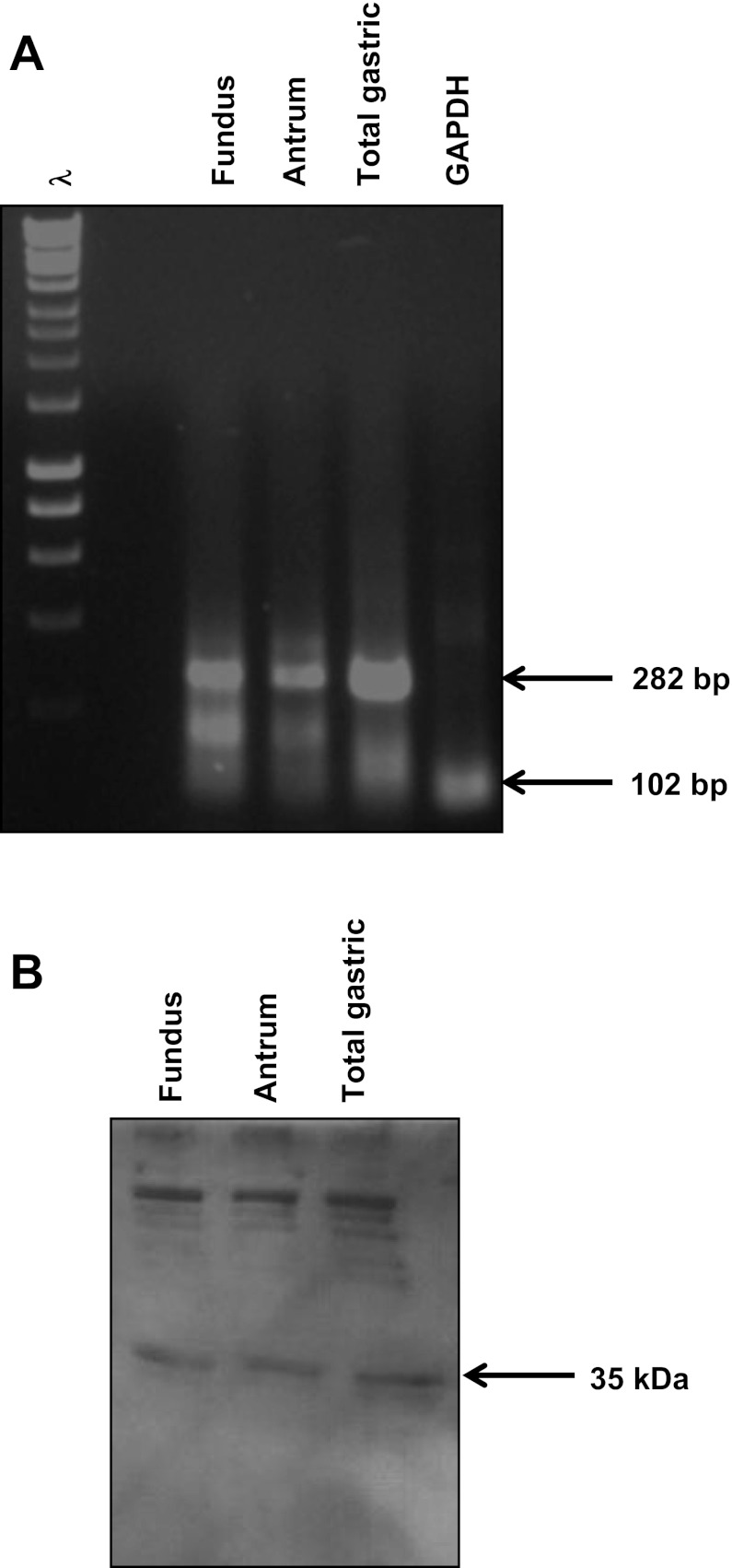
TGR5 receptor was detected by RT-PCR on RNA extracted from cultured muscle cells from antrum and fundus of rabbit stomach in first passage using primers based on sequences of human, mouse, and rat cDNAs. A PCR product of the expected size was obtained using RNA isolated from fundus, antrum, and total gastric tissue (Fig. 1A). Western blot analysis using specific antibody to TGR5 demonstrated the presence of TGR5 receptor (35 kDa) in lysates derived from gastric muscle cells (Fig. 1B).
Fig. 1.
Expression of TGR5 in smooth muscle cells. A: TGR5 expression was measured in cultured gastric muscle cells by RT-PCR. Using mRNA from cultured muscle cells and specific primers for TGR5, a band of predicted size (282 bp) was amplified. B: protein expression was measured in freshly prepared dispersed muscle by Western blot. Using cell lysates prepared from freshly dispersed muscle cells and a specific antibody to TGR5 (1:1,000 dilution) a band of 35 kDa corresponding to TGR5 was detected by chemiluminescence.
TGR5 receptors couple to Gs/cAMP/PKA pathway in gastric smooth muscle.
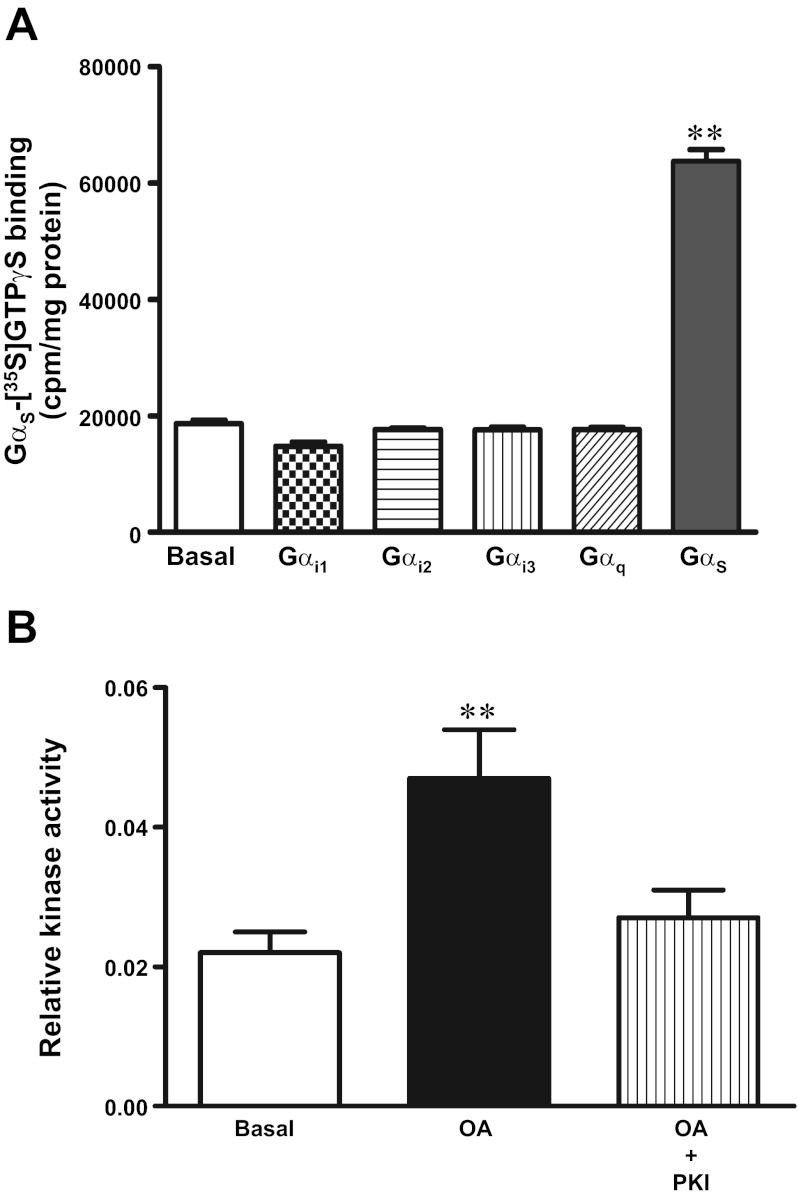
The TGR5 selective ligand, OA (10 μM), activated Gαs causing a significant increase in the binding of [35S]GTPγS to Gαs (259 ± 34% increase), but not to Gαi1 (2 ± 4%), Gαi2 (3 ± 4%), Gαi3 (6 ± 7%), or Gαq (3 ± 6%), in solubilized membranes derived from freshly dispersed smooth muscle cells (Fig. 2A). Consistent with the activation of Gαs, OA (10 μM) caused an increase in cAMP levels (46 ± 7% increase above basal levels) and stimulated cAMP-dependent protein kinase (PKA) (Fig. 2B). The selective PKA inhibitor myristoylated PKI (PKI, 1 μM) blocked the stimulation of PKA activity.
Fig. 2.
TGR5 receptors are coupled to Gαs/cAMP/PKA pathway in gastric smooth muscle. A: selective activation of Gs by oleanolic acid (OA). Membranes prepared from dispersed smooth muscle cells were incubated with [35S]GTPγS in the presence or absence of OA (10 μM) for 20 min. Aliquots were added to wells precoated with antibodies to Gαs, Gαq, Gαi1, Gαi2, and Gαi3 for 2 h and the bound radioactivity measured. OA caused significant increase in binding of [35S]GTPγS. Gα complexes were coated with Gαs, but not with Gαq, Gαi1, Gαi2, or Gαi3. Values are means ± SE of 4 experiments. **P < 0.001 vs. basal. B: stimulation of protein kinase A (PKA) activity by OA. Dispersed muscle cells were treated with OA (10 μM) in the presence or absence of selective PKA inhibitor, myristoylated PKI (1 μM) for 1 min and stimulation of PKA activity was measured by ELISA. Results are expressed as relative PKA activity. Values are means ± SE of 3 experiments. **P < 0.001 vs. basal.
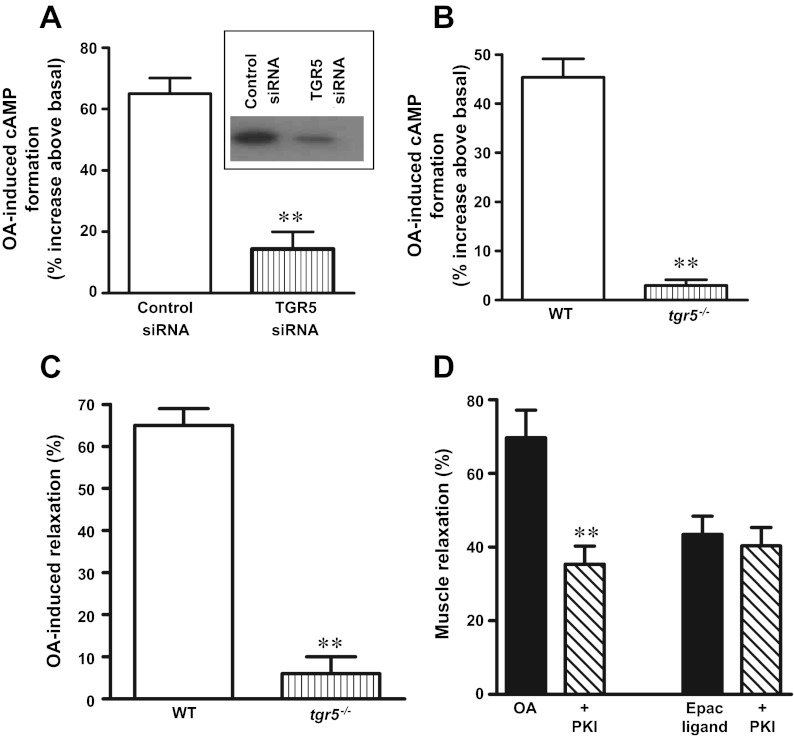
Specific involvement of TGR5 in OA-induced stimulation of cAMP was examined using cultured muscle cells transfected with TGR5-specific siRNA. Suppression of TGR5 expression with TGR5 siRNA abolished the OA (10 μM)-induced increase in cAMP levels (3 ± 4% increase above basal) compared with cells transfected with control siRNA (45 ± 4% increase above basal) (Fig. 3A). Similarly, the OA induced increase in cAMP levels (43 ± 2% increase) was abolished in muscle cells isolated from tgr5−/− mice (3 ± 5% increase) (Fig. 3B). These results provide conclusive evidence that OA-induced cAMP formation is mediated via TGR5 receptors.
Fig. 3.
OA-induced cAMP formation and muscle relaxation via TGR5. A: TGR5-mediated cAMP formation. Cultured muscle cells transfected with control siRNA or TGR5-specific siRNA were treated with OA (10 μM) for 1 min, and cAMP formation was measured by ELISA. Inset: downregulation of TGR5 was determined by Western blot. B: muscle cells isolated from wild-type (WT) and tgr5−/− mice were treated with OA (10 μM) for 1 min, and the cAMP formation was measured by ELISA. Results are expressed as percent increase above basal levels. Values are means ± SE of 3 experiments. **P < 0.001 vs. control siRNA or WT. C: TGR5-mediated muscle relaxation. Dispersed gastric muscle cells from WT and tgr5−/− mice were treated with the contractile agonist carbachol (CCh, 0.1 μM) in the presence or absence of OA (10 μM) for 10 min, and the decrease in muscle cell length was measured by scanning micrometry. Decrease in muscle cell length from the basal length reflected muscle contraction, and inhibition of contractile response reflected muscle relaxation. The effect of CCh is similar in both WT (26 ± 3% decrease from basal cell length of 91 ± 3 μm) and tgr5−/− (25 ± 4% decrease in cell length of 88 ± 5 μm). OA alone had no effect on basal muscle cell length, but significantly inhibited CCh-induced sustained contraction in muscle cells from WT mice, but not in muscle cells from tgr5−/− mice. Results are expressed as % inhibition of contraction (relaxation) by OA. Values are means ± SE of 3 experiments. **P < 0.001 vs WT. D: PKA-dependent and -independent relaxation in response to OA. Dispersed gastric muscle cells were treated with the contractile agonist CCh (0.1 μM) in the presence or absence of OA (10 μM) or cAMP analog that selectively activates Epac (8-pCPT-2′-O-Me-cAMP, 10 μM) for 10 min, and the decrease in muscle cell length was measured by scanning micrometry. In some experiments the effect of OA or 8-pCPT-2′-O-Me-cAMP on CCh-induced contraction was measured in the presence of PKA inhibitor myristoylated PKI (1 μM). Relaxation was expressed as % inhibition of CCh-induced sustained contraction (28 ± 3% decrease in cell length from the basal length of 94 ± 5 μm). Values are means ± SE of 4 experiments. **P < 0.001 vs. response in the absence of myristoylated PKI.
Muscle relaxation in response to TGR5 activation.
As shown previously, treatment of dispersed muscle cells with CCh (0.1 μM) for 10 min caused muscle contraction (26 ± 3% decrease in length from the basal cell length of 91 ± 3 μm). Treatment of cells with OA (10 μM) alone had no significant effect on muscle contraction (3 ± 5% decrease in cell length), but inhibited sustained contraction (i.e., caused muscle relaxation) induced by CCh. OA-induced muscle relaxation was abolished in muscle cell isolated from tgr5−/− mice (5 ± 4% relaxation) compared with muscle cells from wild-type mice (65 ± 4% relaxation), suggesting that OA-induced relaxation is mediated via TGR5 (Fig. 3C).
Treatment of cells with a cAMP analog, 8-pCPT-2′-O-Me-cAMP (10 μM, Epac ligand) that activates the exchange factor, Epac, but not PKA also caused inhibition of sustained contraction induced by CCh (0.1 μM). Relaxation in response to OA (10 μM), but not 8-pCPT-2′-O-Me-cAMP, however, was partly inhibited by myristoylated PKI (1 μM), suggesting that relaxation by OA was partly mediated by PKA (Fig. 3D).
Signaling mechanisms involved in OA-induced muscle relaxation.
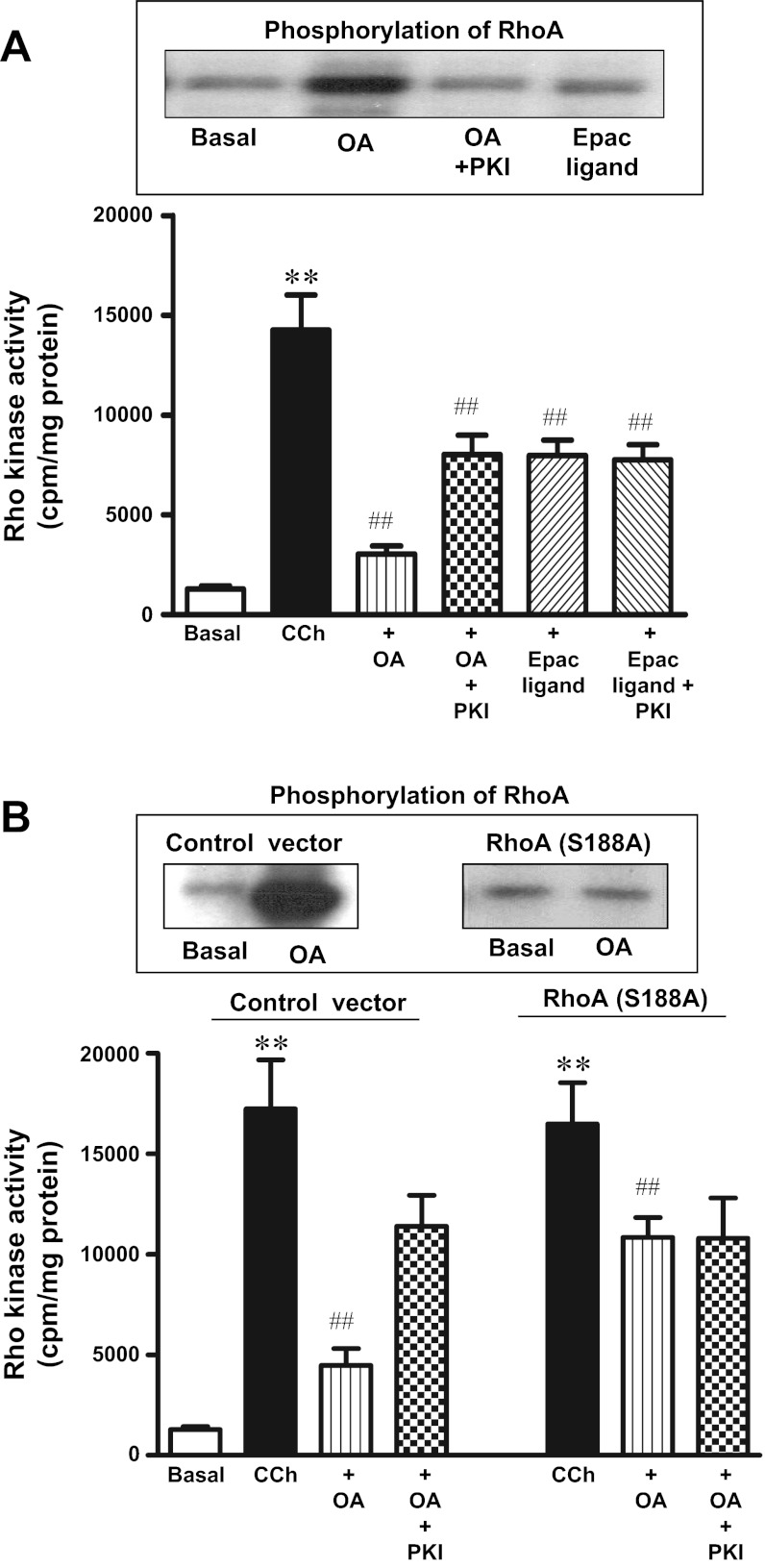
Previous studies have shown that ACh-induced sustained contraction was mediated via activation of RhoA/Rho kinase pathway and relaxation of sustained contraction by agents that stimulate PKA or PKG was mediated by inhibition of Rho kinase activity via phosphorylation of RhoA (21). Consistent with the inhibition of CCh (0.1 μM)-induced sustained contraction, cotreatment of cells with OA (10 μM) inhibited (83 ± 6% inhibition) CCh (0.1 μM)-stimulated Rho kinase activity; the inhibition was partly suppressed (41 ± 5% inhibition) by myristoylated PKI (1 μM) (Fig. 4A). Partial inhibition of Rho kinase activity by PKI is consistent with the partial inhibition of OA-induced muscle relaxation by PKI.
Fig. 4.
OA-induced phosphorylation of RhoA and inhibition of Rho kinase activity. A: RhoA phosphorylation (top). Freshly dispersed gastric smooth muscle cells labeled with 32P were incubated with OA (10 μM) in the presence or absence of myristoylated PKI (1 μM) or the cAMP analog that selectively activates Epac (8-pCPT-2′-O-Me-cAMP, 10 μM) for 10 min. RhoA immunoprecipitates were separated on SDS-PAGE, and RhoA phosphorylation was identified by autoradiography. In some experiments, cells were incubated with myristoylated PKI (1 μM). Rho kinase activity (bottom). Freshly dispersed muscle cells were treated with OA (10 μM) or 8-pCPT-2′-O-Me-cAMP (10 μM) in the presence or absence of myristoylated PKI (1 μM) for 10 min. Rho kinase activity was measured by immunokinase assay as described in materials and methods. Results are expressed as cpm/mg protein. Values are means ± SE of 4 experiments. **P < 0.01 vs. basal, ##P < 0.01 vs. CCh. B: RhoA phosphorylation (top). Cultured muscle transfected with control vector or vector containing phosphorylation-deficient RhoA mutant (S188A) were labeled with 32P and incubated with OA (10 μM) for 10 min. RhoA immunoprecipitates were separated on SDS-PAGE, and RhoA phosphorylation was identified by autoradiography. Rho kinase activity (bottom). Cultured muscle transfected with control vector or vector containing phosphorylation-deficient RhoA (S188A) were treated with CCh (0.1 μM) in the presence or absence of OA (10 μm) for 10 min. In some experiments cells were incubated with myristoylated PKI (1 μM). Rho kinase activity was measured by immunokinase assay. Results are expressed as cpm/mg protein. Values are means ± SE of 4 experiments. **P < 0.01 vs. basal. ##P < 0.01 vs. CCh.
Previous studies showed that inhibition of Rho kinase activity by PKA was mediated via phosphorylation of RhoA at Ser188 (21). Treatment of freshly dispersed smooth muscle cells with OA (10 μM) for 10 min caused phosphorylation of RhoA, and phosphorylation was inhibited by PKI (1 μM) (Fig. 4A). Treatment of cells with 8-pCPT-2′-O-Me-cAMP (10 μM) for 10 min had no effect on RhoA phosphorylation (Fig. 4A).
The role of RhoA in mediating the inhibitory effect of OA on Rho kinase activity was examined in cultured smooth muscle cells expressing RhoA (S188A), which lacks the PKA phosphorylation site. Phosphorylation of RhoA in response to OA (10 μM) was blocked in cells expressing RhoA (S188A) (Fig. 4B). Expression of RhoA (S188A) had no effect on CCh-induced Rho kinase activity, but partly blocked the inhibition of CCh-stimulated Rho kinase activity by OA. These results are consistent with the partial blockade of inhibition of CCh-induced Rho kinase activity by PKI, but in contrast with the blockade of OA-induced phosphorylation of RhoA (Fig. 4B). These results raise the possibility that inhibition of RhoA-mediated Rho kinase activity by OA involves PKA-independent mechanism(s). The possibility of a cAMP-dependent, PKA-independent Epac-mediated pathway was investigated using 8-pCPT-2′-O-Me-cAMP. Although 8-pCPT-2′-O-Me-cAMP had no effect on RhoA phosphorylation, it inhibited CCh-stimulated Rho kinase activity (42 ± 6% inhibition). Inhibition of CCh-stimulated Rho kinase activity by 8-pCPT-2′-O-Me-cAMP was not affected by PKI (39 ± 5% inhibition) (Fig. 4A). These results suggest that inhibition of Rho kinase activity by OA is mediated by a PKA-dependent mechanism via phosphorylation of RhoA and PKA-independent mechanism via activation of Epac.
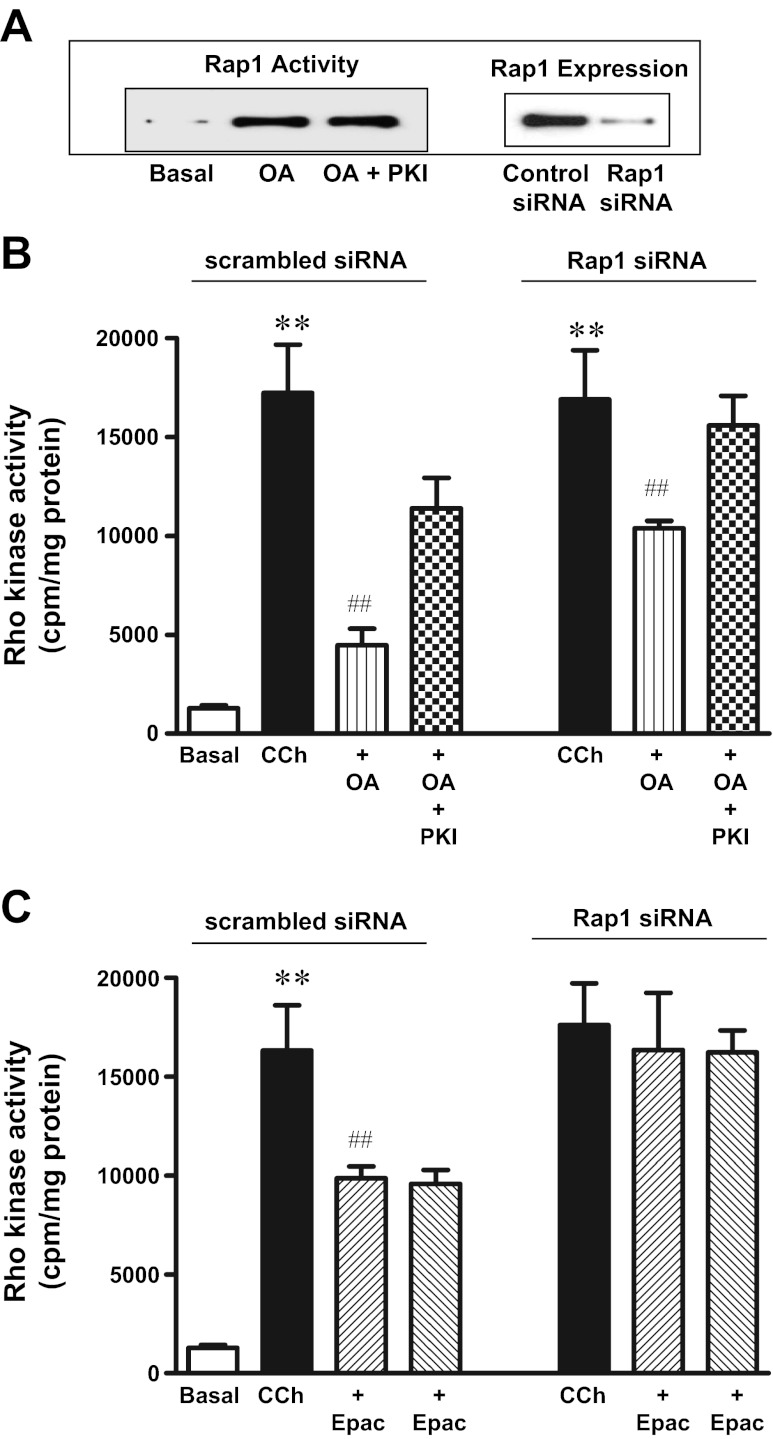
Rap1 is one of the downstream targets of Epac that is shown to regulate Rho kinase activity (38). Treatment of cells with OA (10 μM) for 10 min stimulated Rap1 activity that was not affected by myristoylated PKI (1 μM) (Fig. 5A). In cell transfected with control siRNA, both OA (10 μM) and 8-pCPT-2′-O-Me-cAMP (10 μM) inhibited Rho kinase activity in response to CCh (0.1 μM). Myristoylated PKI partly blocked OA-, but not 8-pCPT-2′-O-Me-cAMP-induced inhibition of Rho kinase activity. Transfection of cells with Rap1 siRNA blocked the inhibition of Rho kinase activity by 8-pCPT-2′-O-Me-cAMP (10 μM), but only partly blocked the inhibition by OA (10 μM), and the residual inhibition was blocked by PKI (Fig. 5, B and C).
Fig. 5.
OA-induced inhibition of Rho kinase activity via Epac/Rap1. A: freshly dispersed gastric smooth muscle cells were treated with OA (10 μM) for 10 min in the presence or absence of myristoylated PKI (1 μM), and Rap1 activity was determined using GST-fusion protein containing the Rap1 binding domain of Rap1GDS to affinity precipitate active Rap1 (GTP-Rap1) as described in methods. Expression of Rap1 in cells transfected with control siRNA or Rap1 siRNA was analyzed by Western blot. B and C: cultured muscle cells transfected with control vector or vector containing Rap1 siRNA were treated with CCh (0.1 μM) in the presence or absence of OA (10 μM) or 8-pCPT-2′-O-Me-cAMP (10 μM) for 10 min. In some experiments cell were incubated with myristoylated PKI (1 μM). Rho kinase activity was measured by immunokinase assay as described in materials and methods. Results are expressed as cpm/mg protein. Values are means ± SE of 4 experiments. **P < 0.01 vs. basal. ##P < 0.01 vs. CCh.
DISCUSSION
The present study presents novel data demonstrating the expression of G protein-coupled bile acid receptor TGR5 in gastric smooth muscle cells, and the signaling pathways coupled to TGR5 to mediate inhibition of agonist-induced muscle contraction (i.e., muscle relaxation). Both mRNA and protein expression of TGR5 was demonstrated in isolated gastric smooth muscle cells. Activation of TGR5 by OA, a potent TGR5 agonist that does not activate the farnesoid X receptor (27), stimulated Gαs activity, cAMP formation and PKA activity leading to muscle relaxation. In this respect, TGR5 receptors conformed to a pattern observed with other Gαs-coupled receptors in gastrointestinal smooth muscle, including vasoactive intestinal peptide/pituitary adenylyl cyclase activating peptide (VPAC2) receptors and β-adrenergic receptors, which stimulate Gαs/cAMP pathway to mediate muscle relaxation (18). Although several physiological functions of BAs are mediated via the nuclear receptor FXR and the membrane receptor TGR5, the suppression of OA-induced cAMP formation and muscle relaxation by TGR5 siRNA or tgr5−/− mice provided evidence for the selective activation of TGR5 in response to OA.
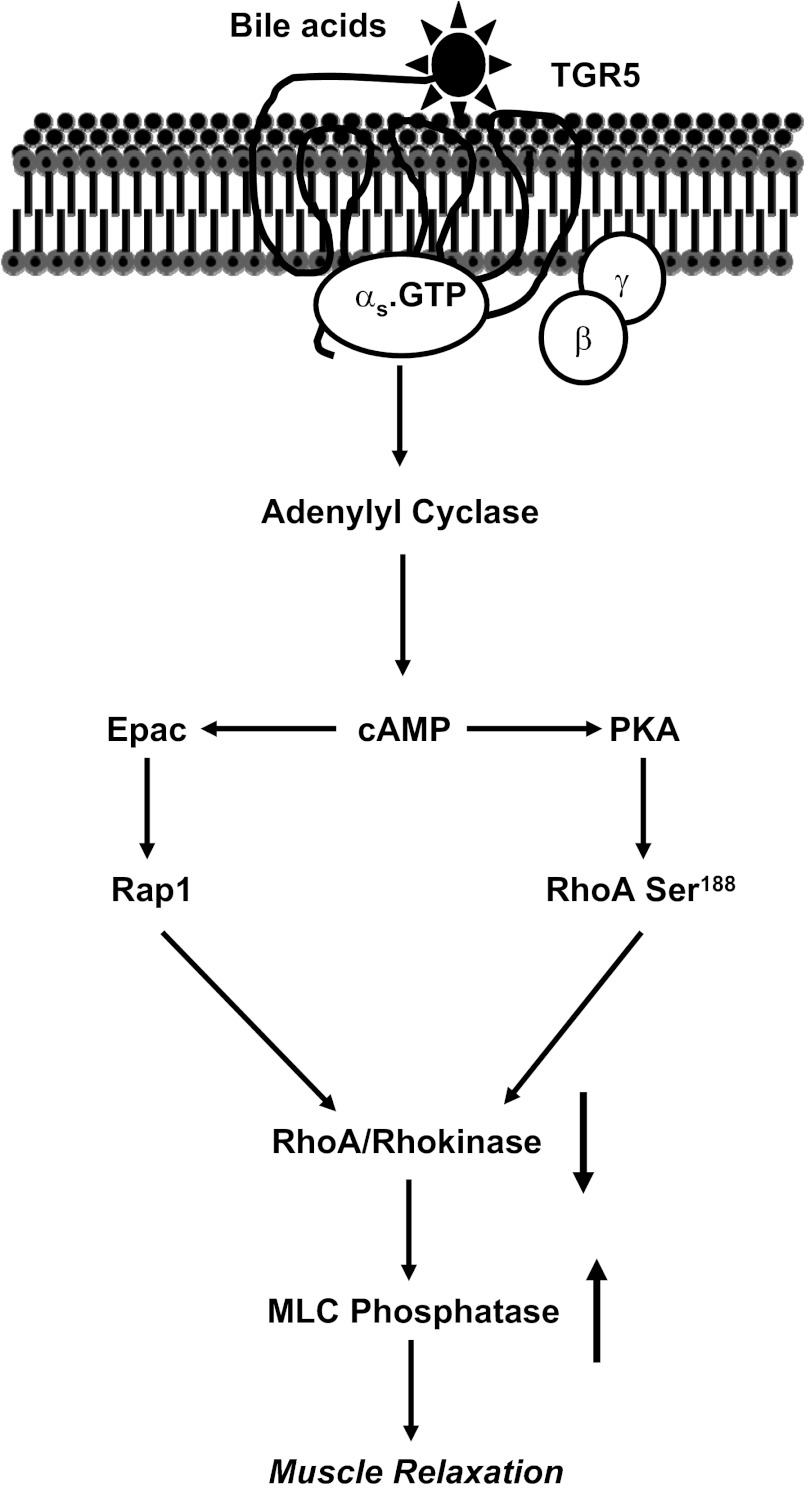
The present study also provided a detailed analysis of the signaling pathways initiated by TGR5 receptors to mediate relaxation of sustained contraction in smooth muscle. A model depicting these pathways is shown in Fig. 6. In gastrointestinal smooth muscle, Ca2+-independent sustained contraction by several contractile agonists is mediated via activation of Gq/G13 and the monomeric G protein, RhoA. Activation of RhoA leads to phosphorylation of MYPT1, a regulatory subunit of MLC phosphatase (MLCP), via Rho kinase, and CPI-17, an endogenous inhibitor of MLCP, via protein kinase C, resulting in the inhibition of MLCP activity and increase in MLC20 phosphorylation (9, 17, 21, 30). Agonists that stimulate the formation of cAMP and cGMP induce muscle relaxation by activating cAMP- and cGMP-dependent protein kinase, respectively. Both kinases stimulate MLCP activity via phosphorylation of RhoA at Ser188 leading to inhibition of RhoA and RhoA-dependent pathways (21). In permeabilized vascular muscle cells, contraction in response to geranylgeranylated RhoA, but not phosphorylation-deficient RhoA mutant (S188A), was inhibited by exogenous PKG, suggesting that phosphorylation of RhoA by PKG plays an important role in mediating muscle relaxation (28).
Fig. 6.
Mechanism of TGR5-mediated muscle relaxation. In gastric smooth muscle TGR5 receptors are coupled to stimulation of Gs and cAMP formation. Activation of TGR5 receptors leads to inhibition of RhoA/Rho kinase-mediated sustained contraction. The mechanism of inhibition involves PKA-dependent phosphorylation of RhoA at Ser188 and PKA-independent, cAMP/Epac-dependent activation of Rap1. Both phosphorylation of RhoA and activation of Rap1 causes inhibition of Rho kinase activity leading to stimulation of MLCP activity and muscle relaxation.
In gastric smooth muscle cells both PKA and PKG phosphorylate RhoA, stimulate its translocation back to the cytosol, and inhibit its activity and the activity of RhoA-dependent Rho kinase at the same time as they inhibit ACh-stimulated sustained MLC20 phosphorylation and sustained muscle contraction (21). Treatment of cells with OA also caused phosphorylation of RhoA, and phosphorylation was blocked in cells expressing RhoA (S188A) or by PKI. However, inhibition of CCh-induced Rho kinase activity by OA was only partly reversed in cells expressing RhoA (S188A) or by PKI, suggesting that PKA-independent mechanisms that do not involve RhoA phosphorylation in the suppression of Rho kinase activity. Our results showing inhibition of Rho kinase activity by the cAMP analog that selectively activate Epac or partial reversal of OA-induced inhibition by suppression of Rap1 suggest that inhibition of Rho kinase activity by OA involves both a PKA-dependent mechanism involving phosphorylation of RhoA and a PKA-independent mechanism involving activation of Epac/Rap1 pathway. Evidence in support of the latter pathway include the following: 1) OA-stimulated Rap1 activity was not affected by myristoylated PKI; 2) the selective Epac ligand inhibited CCh-stimulated Rho kinase activity and muscle contraction, and the effect of Epac ligand is PKA-independent as myristoylated PKI had no effect on inhibition of Rho kinase activity and muscle contraction in response to Epac ligand; and 3) Rap1 siRNA blocked the inhibition of Rho kinase activity in response to Epac ligand, and only partly suppressed the inhibition of CCh-stimulated Rho kinase activity in response to OA, and the residual inhibition was blocked by PKI.
cAMP, in addition to activating PKA, activates Epac, which belongs to a family of guanine nucleotide exchange factors (GEFs). Epacs are multidomain proteins with an NH2-terminal domain containing conserved cyclic nucleotide binding domain (CNBD) and a COOH-terminal catalytic domain. In the absence of cAMP, the regulatory domain interacts with the catalytic domain and inhibits the GEF activity, and this autoinhibition is relieved following the binding of cAMP to the CNBD and conformational change within the protein (2). The effects of PKA and Epac are antagonistic in cardiac fibroblasts with Epac being promigratory and PKA being antimigratory (37). Epac and PKA may act independently or in concert. The effects of Epac and PKA in airway smooth muscle, however, are additive. Both Epac and PKA induce muscle relaxation (26, 38). Understanding the mechanisms involved in PKA- vs. and Epac-mediated relaxation has revealed that one of the targets for Epac is Rap1.
Rap1 is a member of the Ras family of small GTPases, which upon activation serves as an important signaling molecule in several cellular responses (4). Studies in endothelial cells and neurons showed that Rap1 activation via cAMP leads to decrease in RhoA activation (3, 35). The GTPase-activating protein RhoGAP was identified as one of the targets of Rap1, and thus rapid inactivation of GTP-bound RhoA by RhoGAP could lead to inhibition of Rho kinase activity (35). These results suggest that inhibition of CCh-stimulated Rho kinase activity by OA could be due to inhibition of the upstream effector of RhoA in smooth muscle as well. The exact mechanism by which Rap1 inhibits RhoA/Rho kinase pathway in smooth muscle awaits further investigation.
BAs are released into the small intestine to facilitate lipid digestion and absorption. Active reabsorption in the terminal ileum by the apical sodium-dependent bile acid transporter (ASBT) is the main route for circulating bile acids (24). In addition to the systemic effect, more hydrophobic bile acids such as DCA and CDCA can also readily cross the apical and basolateral bilayers to reach the target cell and mediate muscle relaxation. The expression of TGR5 and inhibition of agonist-induced contraction by bile acids in smooth muscle cells has important functional implications. Increase in systemic BA concentration can regulate proximal gut motility by more than one mechanism. They can directly act on TGR5 receptors on smooth muscle to induce relaxation or act on inhibitory motor neurons to release VIP or NO, the main relaxant neurotransmitters in the gut (25). Additional mechanisms could involve membrane hyperpolarization via suppression of spontaneous Ca2+ fluxes (11). Recent work in our laboratory and by others has established that TGR5 receptors are expressed in enteroendocrine cells and activation of these receptors causes the release of GLP-1 and PYY (1, 7). Both GLP-1 and PYY are involved in the inhibition of proximal gut motility. Luminal administration of DCA also inhibited gastric emptying and intestinal transit in mice in vivo (25). Whether the inhibition of gastric function is mediated via muscle, neural, or enteroendocrine mediators requires further research, but identifying the physiological significance of TGR5 receptors in the muscle may prove significant. The results of the present study attain a greater significance in the light of evidence that ASBT expression is altered in motility disorders such as inflammatory bowel diseases.
In summary, the results of this study identify the expression of TGR5 in gastric muscle and a mechanism of TGR5-mediated smooth muscle relaxation. Our findings demonstrate that activation of Gαs-coupled TGR5 receptor causes gastric muscle relaxation via inhibition of RhoA/Rho kinase pathway leading to stimulation of MLCP activity and MLC20 dephosphorylation. Inhibition of Rho kinase activity was mediated by both PKA-dependent phosphorylation of RhoA at Ser188 and PKA-independent mechanism involving stimulation of Rap1 via Epac.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants (DK-28300, K. S. Murthy; DK-39957, N. W. Bunnett), the National Health and Medical Research Council (63303, 103188, N. W. Bunnett), and the Northern California Institute for Research and Education, Veterans Health Administration (C. U. Corvera).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.R., J.R.G., and K.S.M. conception and design of research; S.R. performed experiments; S.R. and K.S.M. analyzed data; S.R., J.R.G., and K.S.M. interpreted results of experiments; S.R. prepared figures; S.R. and K.S.M. drafted manuscript; S.R., J.R.G., and K.S.M. edited and revised manuscript; S.R., D.P.K., S.M., S.B., R.Z., C.C., N.W.B., J.R.G., and K.S.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Johan Auwerx and Kristina Schoonjans, Univ. of Ecole Polytechnique Federale de Lausanne, Switzerland, for providing the tgr5−/− mice.
REFERENCES
- 1. Bala V, Mahavadi S, Rajagopal S, Zhou R, Kuemmerle FJ, Sanyal JA, Murthy KS. Bile acid-induced stimulation of ERK1/2 activity, GLP-1 and PYY release in enteroendocrine cells are mediated by the activation of Epac/PLC-ε signaling pathway via GS-coupled TGR5. Gastroenterology 140: S147, 2011 [Google Scholar]
- 2. Breckler M, Berthouze M, Laurent AC, Crozatier B, Morel E, Lezoualc'h F. Rap-linked cAMP signaling Epac proteins: compartmentation, functioning and disease implications. Cell Signal 23: 1257–1266, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Cullere X, Shaw SK, Anderson K, Hirahashi J, Luscinskas FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood 105: 1950–1955, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Emmanuelle C. Cellular functions of the Rap1 GTP-binding protein: a pattern emerges. J Cell Sci 116: 435–440, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Fiorucci S, Cipriani S, Mencarelli A, Renga B, Distrutti E, Baldelli F. Counter regulatory role of bile acid activated receptors in immunity and inflammation. Curr Mol Med 10: 579–595, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Houten S, Watanabe M, Auwerx J. Endocrine functions of bile acids. EMBO J 25: 1419–1425, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun 329: 386–390, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S, Fujisawa Y, Fujino M. A G protein-coupled receptor responsive to bile acids. J Biol Chem 278: 9435–9440, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Kitazawa T, Eto M, Woodsome TP, Khalequzzaman M. Phosphorylation of the myosin phosphatase targeting subunit and CPI-17 during Ca2+ sensitization in rabbit smooth muscle. J Physiol 546: 879–889, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knop FK. Bile-induced secretion of glucagon-like peptide-1: pathophysiological implications in type 2 diabetes? Am J Physiol Endocrinol Metab 299: E10–E13, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Lavoie B, Balemba OB, Godfrey C, Watson CA, Vassileva G, Corvera CU, Nelson MT, Mawe G. Hydrophobic bile salts inhibit gallbladder smooth muscle function via stimulation of GPBAR1 reporters and activation of KATP channels. J Physiol 588: 3295–3305, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li T, Holmstrom SR, Kir S, Umetani M, Schimidt DR, Kliewer SA, Mangelsdorf DJ. The G protein-coupled bile acid receptor, TGR5 stimulates gallbladder filling. Mol Endocrinol 25: 1066–1071, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell Biol 6: 507–515, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Ma K, Saha P, Chan L, Moore DD. Farnesoid X receptor is essential for normal glucose homestasis. J Clin Invest 116: 1102–1109, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Manglesdorf DJ, Shan B. Identification of nuclear receptors for bile acids. Science 284: 1362–1365, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, Nakamura T, Itadani H, Tanaka K. Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun 298: 714–719, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol 68: 345–374, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Murthy KS, Jin JG, Grider JR, Makhlouf GM. Characterization of PACAP receptors and signaling pathways in rabbit gastric smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 272: G1391–G1399, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Murthy KS, Makhlouf GM. Differential coupling of muscarinic m2 and m3 receptors to adenylyl cyclases V/VI in smooth muscle. J Biol Chem 272: 21317–21324, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Murthy KS, Zhou H, Grider JR, Brautigan D, Eto M, Makhlouf GM. Differential signaling by muscarinic receptors in smooth muscle: m2 mediated inactivation of myosin light chain kinase via Gi3, cdc42/Rac1 and p21-activated kinase pathway, and m3-mediated MLC20 phosphorylation via Rho-associated kinase/myosin phosphatase targeting subunit I and protein kinase C/CPI 17 pathway. Biochem J 374: 145–155, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murthy KS, Zhou H, Grider JR, Makhlouf GM. Inhibition of sustained smooth muscle contraction by PKA and PKG preferentially mediated by phosphorylation of RhoA. Am J Physiol Gastrointest Liver Physiol 284: G1006–G1016, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Okamoto T, Murayama Y, Hayashi Y, Inagaki M, Ogata E, Nishimoto I. Identification of a Gs activator region of the β2-adrenergic receptor that is autoregulated via protein kinase A-dependent phosphorylation. Cell 67: 723–730, 1991 [DOI] [PubMed] [Google Scholar]
- 23. Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Morre DD, Lehmann JM. Bile acids: Natural ligands for an orphan nuclear receptor. Science 284: 1365–1368, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Paul A, Tian L, Rao A. Bile acid transporters. J Lipid Res 50: 2340–2357, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Poole DP, Godfrey C, Cattaruzza F, Cottrell GS, Kirkland JG, Pelayo JC, Bunnett NW, Corvera CU. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil 22: 814–825, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roscioni SS, Maarsingh H, Elzinga CR, Schuur J, Menzen M, Halayko AJ, Meurs H, Schmidt M. Epac as a novel effector of airway smooth muscle relaxation. J Cell Mol Med 15: 1551–1563, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sato H, Genet C, Strehle A, Thomas C, Lobstein A, Wagner A, Mioskowski C, Auwerx J, Saladin R. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem Biophys Res Commun 362: 793–798, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Smolenski A, Lohmann S, Bertoglio J, Chardin P, Pacaud P, Gervaise L. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J Biol Chem 275: 21722–21729, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102: 731–744, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. TGR5-mediated bile acid sensing controls glucose homestasis. Cell Metabol 10: 167–177, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signaling for metabolic diseases. Nat Rev Drug Dis 7: 678–693, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Vassileva G, Golovko A, Markowitz L, Abbondanzo SJ, Zeng M, Yang S, Hoos L, Tetzloff G, Levitan D, Murgolo NJ, Keane K, Davis HJ, Hedrick J, Gustafson EL. Targeted deletion of Gpbar1 protects mice from cholesterol gallstone formation. Biochem J 398: 423–430, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell Biol 3: 543–553, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Yamada T, Sakisaka T, Hisata S, Baba T, Takai Y. RA-RhoGAP, Rap-activated Rho GTPase-activating protein implicated in neurite outgrowth through Rho. J Biol Chem 280: 33026–33034, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Yanqiao Z, Florence Y, Gabriel B, Hans L, Charisse V, Frank JG, Timothy MW, Peter AE. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci USA 103: 1006–1011, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yokoyama U, Patel HH, Lai NC, Aroonsakool N, Roth DM, Insel PA. The cyclic AMP effector Epac integrates pro- and anti-fibrotic signals. Proc Natl Acad Sci USA 105: 6386–6391, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zieba BJ, Artamonov MV, Jin L, Momotani K, Ho R, Franke AS, Neppl RL, Stevenson AS, Khromov AS, Wodnicka MC, Somlyo AV. The cAMP-responsive Rap1 guanine nucleotide exchange factor, Epac, induces smooth muscle relaxation by down-regulation of RhoA activity. J Biol Chem 286: 16681–16692, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]