Abstract
Intestinal P-glycoprotein (Pgp/multidrug resistance 1), encoded by the ATP-binding cassette B1 gene, is primarily involved in the transepithelial efflux of toxic metabolites and xenobiotics from the mucosa into the gut lumen. Reduced Pgp function and expression has been shown to be associated with intestinal inflammatory disorders. Keratinocyte growth factor-2 (KGF2) has emerged as a potential target for modulation of intestinal inflammation and maintenance of gut mucosal integrity. Whether KGF2 directly regulates Pgp in the human intestine is not known. Therefore, the present studies were undertaken to determine the modulation of Pgp by KGF2 using Caco-2 cells. Short-term treatment of Caco-2 cells with KGF2 (10 ng/ml, 1 h) increased Pgp activity (∼2-fold, P < 0.05) as measured by verapamil-sensitive [3H]digoxin flux. This increase in Pgp function was associated with an increase in surface Pgp levels. The specific fibroblast growth factor receptor (FGFR) antagonist PD-161570 blocked the KGF2-mediated increase in Pgp activity. Inhibition of the mitogen-activated protein kinase (MAPK) pathway by PD-98059 attenuated the stimulatory effects of KGF2 on Pgp activity. Small-interfering RNA knockdown of Erk1/2 MAPK blocked the increase in surface Pgp levels by KGF2. Long-term treatment with KGF2 (10 ng/ml, 24 h) also significantly increased PgP activity, mRNA, protein expression, and promoter activity. The long-term effects of KGF2 on Pgp promoter activity were also blocked by the FGFR antagonist and mediated by the Erk1/2 MAPK pathway. In conclusion, our findings define the posttranslational and transcriptional mechanisms underlying stimulation of Pgp function and expression by KGF2 that may contribute to the beneficial effects of KGF2 in intestinal inflammatory disorders.
Keywords: multidrug resistance 1, fibroblast growth factor 10, extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase, transcriptional regulation
p-glycoprotein (pgp), a product of multidrug resistance 1 gene (MDR1/ATP-binding cassette B1) is a transmembrane transporter that is predominantly expressed on the apical surface of intestinal epithelial cells (16, 28). This integral membrane protein functions as an energy-dependent efflux pump that actively extrudes drugs/xenobiotics and bacterial toxins from the cell (26). Pgp plays an important role in the protection of intestinal epithelial cells against harmful substances and xenobiotic toxicity (26). In this regard, previous studies have demonstrated that inoculation of Pgp total knockout mice with Helicobacter bilis, an organism known to cause inflammation in other murine inflammatory bowel disease models, accelerated the development of colitis (20) due to a family of cytolethal-distending toxins (33). Furthermore, recent studies by Guner et al. (12) showed that Pgp total knockout neonatal mice are more susceptible to intestinal inflammation caused by the pathogen Cronobacter sakazakii that has been associated with hospital outbreaks of necrotizing enterocolitis. These animal studies further attest to the role of Pgp in the protection of intestinal epithelia from luminal bacterial toxins and from development of inflammation.
Downregulation of Pgp has been implicated in the pathophysiology of inflammatory gut disorders, although the mechanisms involved are not well understood. For example, Pgp levels are abnormally low in the intestine of patients with newly diagnosed or refractory ulcerative colitis (UC) (1, 13) and Crohn's Disease (5). A decrease in function and expression of Pgp has also been shown in an experimental mouse model of dextran sulfate sodium (DSS)-induced (18) colitis and in IL-10 knockout mice that develop spontaneous colitis under conventional conditions (6). Accordingly, mice lacking the mdr1a gene have been shown to develop spontaneous colitis similar to human UC (23). Given that Pgp downregulation contributes to the pathophysiology of inflammatory gut disorders, agents that increase Pgp function and expression could have therapeutic potential in attenuating gut inflammation.
In this regard, keratinocyte growth factor-2 (KGF2) has recently emerged as a potential therapeutic protein in the amelioration of intestinal inflammation and maintenance of gut mucosal integrity (8, 11, 14, 21). KGF2 [also known as fibroblast growth factor (FGF) 10] is a soluble 170-amino-acid polypeptide secreted by fibroblasts and endothelial cells that acts primarily on epithelial cells via the activation of selective high-affinity transmembrane FGF-2 IIIb receptor of the tyrosine kinase family (7, 9). Previous studies have shown that KGF2 exerts protective effects in DSS-induced colitis mice by reducing the mortality, alleviating the weight loss, and lessening the inflammation as judged by the histological score and colonic myeloperoxidase levels (21). Repifermin, a truncated, recombinant form of KGF2, was also shown to provide protection against intestinal injury in a murine model of DSS colitis (11) and to promote healing of indomethacin-induced jejunal ulceration in rats by stimulating epithelial restitution (14). However, the molecular mechanisms underlying the protective effects of KGF2 in intestinal epithelial cells in both the in vitro cell culture and in vivo animal models are lacking. Moreover, no studies are currently available on the effects of KGF2 on Pgp function and expression in the human intestine. Therefore, the present studies were aimed at examining the direct effects of the growth factor KGF2 on Pgp function and expression in intestinal epithelial cells and delineate the mechanisms involved.
MATERIALS AND METHODS
Materials.
Caco-2 cells were obtained from the American Type Culture Collection (Manassas, VA). [3H]digoxin (40 Ci/mmol) was purchased from Perkin-Elmer Life Sciences (Boston, MA). Substrate digoxin and the Pgp/MDR1 inhibitor verapamil were obtained from Sigma (St. Louis, MO). KGF2 was procured from Thermo Scientific (Rockford, IL) as a recombinant protein. Erk1/2 mitogen-activated protein kinase (MAPK) inhibitors PD-98059 and U-0126 were obtained from Biomol (Plymouth Meeting, PA). Fibroblast growth factor receptor (FGFR) inhibitor PD-161570 was procured from EMD Biosciences (San Diego, CA). Mouse monoclonal MDR1 antibody and goat anti-mouse and goat anti-rabbit antibody conjugated to horseradish peroxidase were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Lipofectamine 2000 was procured from Invitrogen (Carlsbad, CA). All other chemicals were of at least reagent grade and were obtained from either Sigma Chemicals or Fisher Scientific (Pittsburgh, PA).
Cell culture and treatment.
Caco-2 cells were grown in T-75 (75 cm2) plastic flasks at 37°C in a 5% CO2 environment. The culture medium consisted of high-glucose MEM, 20% fetal bovine serum, 20 mM HEPES, 100 IU/ml penicillin, and 100 μg/ml streptomycin. Cells used for these studies were between passages 25 and 45, were plated on 12-well Transwell inserts at a density of 1 × 104 cells/well, and were used for experiments at day 21 after plating as described previously (25). Fully differentiated Caco-2 monolayers were treated with KGF2 (10 ng/ml) from the basolateral side for 1- or 24-h time points in the serum-free cell culture medium supplemented with 0.2% BSA.
In separate sets of experiments, cells were pretreated with the specific FGFR antagonist PD-161570 (10 μM) or Erk1/2 MAPK inhibitor PD-98059 (30 μM) for 1 h and then coincubated with KGF2 for an additional 1 h.
Pgp-dependent digoxin flux activity.
Pgp activity was determined by measuring verapamil-sensitive [3H]digoxin flux in Caco-2 cells as previously described (25). Transport studies were performed in triplicates in the apical-to-basolateral (ab) and basolateral-to-apical (ba) directions with flux buffer containing [3H]digoxin (1 μCi/ml supplemented with 1 μM unlabeled digoxin) from either the apical (200 μl) or basolateral (500 μl) compartments at room temperature in the presence or absence of Pgp inhibitor verapamil (10 μM). Samples (100 μl) were taken from receiver compartments (initial flux) at 15 min and then (final flux) at 60 min. The radioactivity of the receiver samples was determined using a Packard Tri-Carb 1600TR Liquid Scintillation analyzer (Packard Instruments; Perkin Elmer). The ab or ba values of the final flux were subtracted from the initial flux, and digoxin flux activity was expressed as a ratio of verapamil-sensitive ba flux to ab flux.
Cell surface biotinylation.
Cell surface biotinylation studies were performed in Caco-2 monolayers using Sulfo-NHS-SS-Biotin (1.5 mg/ml; Pierce) in borate buffer (in mM: 154 NaCl, 7.2 KCl, 1.8 CaCl2, and 10 H3BO3, pH 9.0) as described previously (10). Labeling was allowed to proceed at 4°C to prevent endocytosis and internalization of antigens for 60 min. The biotinylated antigens were immunoprecipitated using streptavidin agarose beads, and the biotinylated proteins were released by boiling in Leamli buffer containing 100 μM dithiothreitol. Proteins were subjected to SDS-PAGE followed by immunoblotting with anti-MDR1 (Pgp) antibody (25). The surface Pgp levels were compared with total cell antigen as determined by immunoblotting in solubilized cell extract.
Erk1/2 MAPK small-interfering RNA transfections.
For small RNA interference studies, Caco-2 cells (10 × 104 cells/well) were plated on six-well Transwell inserts 24 h before transfection. After 24 h, Caco-2 cells were transfected with Erk1/2 MAPK-specific small-interfering RNA (siRNA, 100 μM) and scrambled siRNA (100 μM) (Qiagen, Valencia, CA) using Lipofectamine 2000 transfection reagent (Invitrogen) as recommended by the manufacturer. Post-siRNA transfection (72 h), cells were used for biotinylation experiments in the presence or absence of KGF2. In a separate set of experiments, cell lysates were prepared from both scrambled and Erk1/2 MAPK-specific siRNA-transfected cells to assess Erk1/2 MAPK expression.
Western blotting.
KGF2-treated and untreated Caco-2 cells were prepared as described previously (25). Lysates were run on a 7 or 12% gel and then transferred onto a nitrocellulose membrane. Nitrocellulose membranes were then used for immunoblotting with anti-MDR1 (Pgp) antibody (25) or phospho-Erk1/2 (Thr202 and Tyr204 of Erk1; Thr185 and Tyr187 of Erk2) or total Erk1/2 antibody.
Real-time PCR.
RNA was extracted from KGF2-treated and untreated Caco-2 cells as described previously by us (25). Human (h) MDR1 and histone (internal control) was amplified with gene-specific primers as described previously (5, 24). Relative levels of hMDR1 mRNA were expressed as percent of control normalized to histone.
Transfections.
Caco-2 cells were transfected with MDR1 promoter fragment (p-1,073/+703, cloned upstream of the luciferase reporter gene in pGL2-Basic) and β-galactosidase expression vector by electroporation using the Amaxa Nucleofactor System as described previously (25). MDR1 promoter activity was expressed in terms of relative luciferase activity normalized to β-galactosidase activity. Transfected Caco-2 cells were treated with KGF2 (10 ng/ml) from the basolateral side for 8-, 16-, or 24-h time points in 1% serum cell culture medium supplemented with 0.2% BSA. In a separate set of experiments, transfected cells were pretreated with the specific FGFR antagonist PD-161570 (10 μM) or the Erk1/2 MAPK inhibitor U-0126 (10 μM) for 1 h and then coincubated with KGF2 for another 24 h.
Statistical analysis.
Results are expressed as means ± SE of three to five independent experiments. Students t-test or one-way ANOVA with Tukey's test was used for statistical analysis. P ≤ 0.05 was considered statistically significant.
RESULTS
Short-term effects of KGF2 on Pgp function in Caco-2 cells.
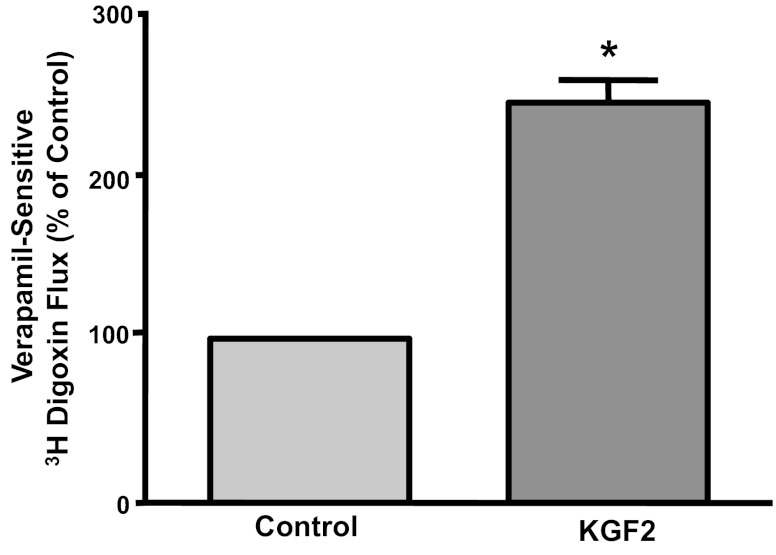
Caco-2 monolayers were treated with KGF2 (10 ng/ml) from the basolateral side for a time period of 1 h, and Pgp function was measured as verapamil-sensitive [3H]digoxin flux. As shown in Fig. 1, verapamil-sensitive [3H]digoxin flux was significantly increased (∼2-fold) by KGF2 compared with untreated controls. However, KGF2 did not show any effect on Pgp activity at 15- or 30-min time points (data not shown). These data indicate that Pgp activity is stimulated by short-term treatment with KGF2 in human intestinal epithelial cells. It should be noted that there was no significant change in ab transport in KGF2-stimulated Caco-2 cells compared with control cells (0.036 ± 0.014 vs. 0.041 ± 0.021 nmol of digoxin/45 min).
Fig. 1.
Short-term effect of keratinocyte growth factor-2 (KGF2) on P-glycoprotein (Pgp) activity in Caco-2 cells. Overnight serum-starved postconfluent Caco-2 cells were treated with KGF2 (10 ng/ml) in serum-free cell culture medium supplemented with 0.2% BSA for 60 min. [3H]digoxin flux (1 μM) was measured as described in materials and methods. Results are expressed as %control and represent means ± SE of 6 separate experiments performed in triplicate. *P < 0.05 compared with untreated control.
KGF2 effects on Pgp function are receptor mediated.
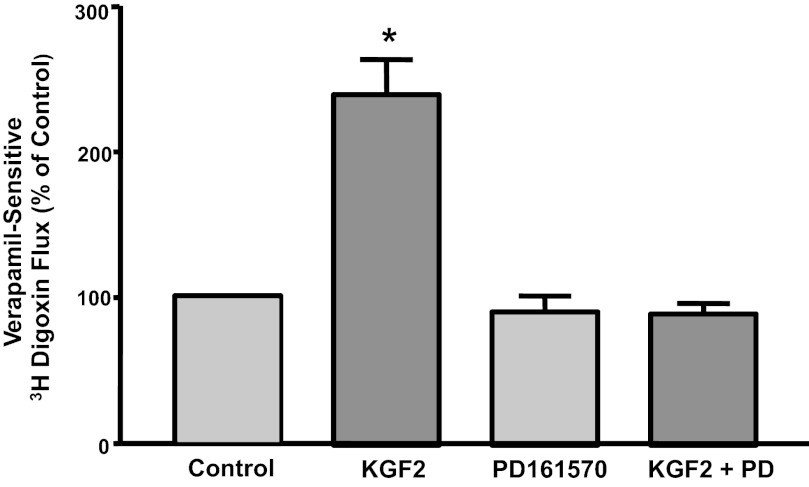
To examine whether the effects of KGF2 (FGF10) are dependent on FGFR, we examined the effects of KGF2 on Pgp activity in the presence of the specific FGFR antagonist PD-161570 (10 μM). The stimulatory effects of KGF2 on Pgp function were abrogated in the presence of the antagonist (Fig. 2). These data indicate that the effects of KGF2 on Pgp function are FGFR signaling dependent.
Fig. 2.
Effects of KGF2 on Pgp activity in Caco-2 cells are receptor mediated. Postconfluent Caco-2 cells were pretreated with specific fibroblast growth factor (FGF) receptor antagonist PD-161570 (10 μM) for 60 min in the serum-free cell culture medium and then coincubated with KGF2 (10 ng/ml) in serum-free cell culture medium supplemented with 0.2% BSA for 60 min. [3H]digoxin flux (1 μM) was measured as described in materials and methods. Results are expressed as %control and represent means ± SE of 4 separate experiments performed in triplicate. *P < 0.05 compared with untreated control.
KGF2 effects on Pgp activity in Caco-2 cells are Erk1/2 MAPK dependent.
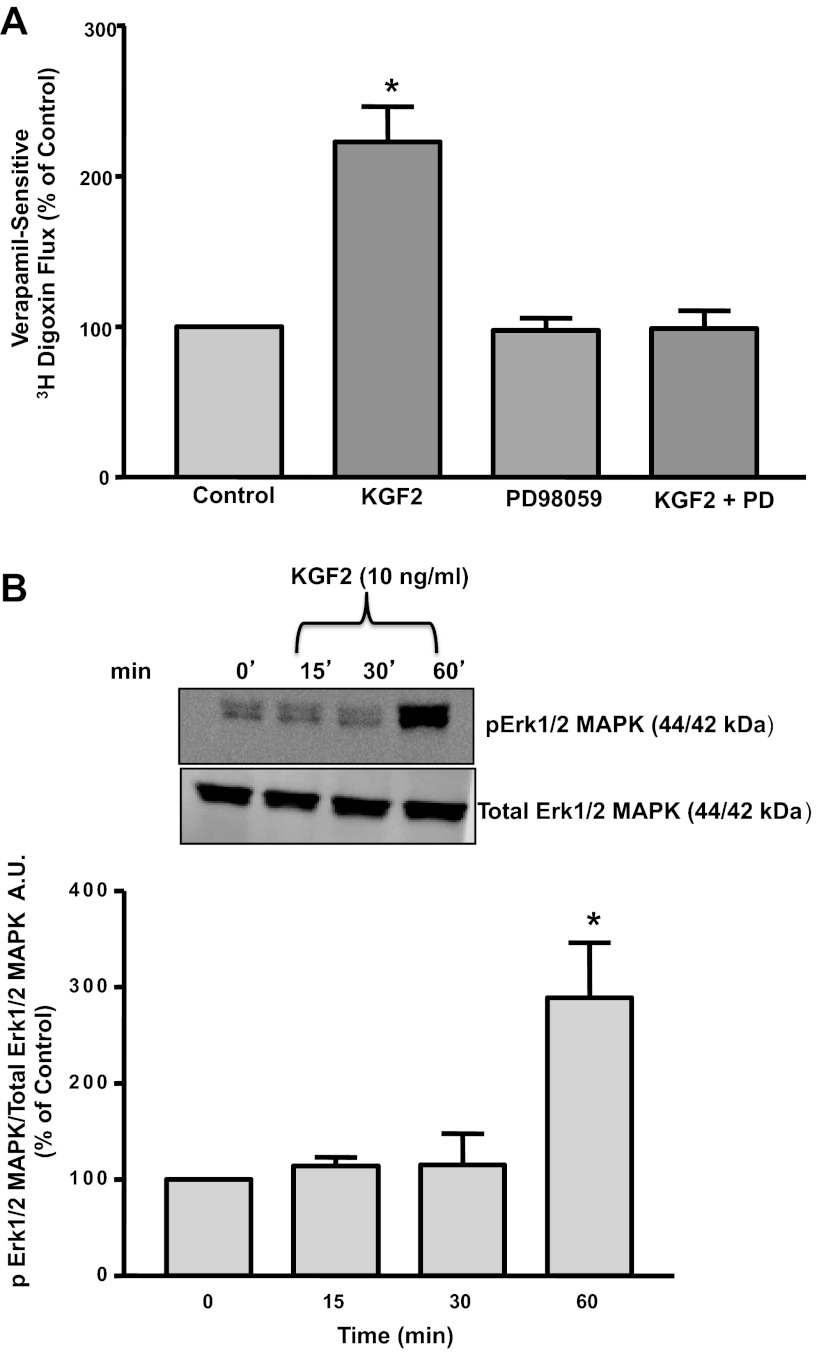
Previous studies demonstrated that KGF2 attenuated H2O2-induced DNA damage in alveolar epithelial cells via the activation of the Erk1/2 MAPK pathway (29). Therefore, we examined whether the effects of KGF2 on Pgp function in intestinal epithelial cells occur via a similar pathway. As shown in Fig. 3A, the specific Erk1/2 MAPK inhibitor PD-98059 (30 μM) blocked the stimulatory effects of KGF2 on Pgp activity in Caco-2 cells, suggesting the involvement of Erk1/2 MAPK in KGF2-mediated effects. We also assessed the phosphorylation levels of Erk1/2 MAPK in Caco-2 cells treated with KGF2, since Erk1/2 MAPK activation is dependent on its phosphorylation. KGF2 significantly increased the phosphorylation of Erk1/2 MAPK by approximately twofold at 60 min but not at 15 or 30 min compared with untreated controls (Fig. 3B).
Fig. 3.
A: effect of Erk1/2 mitogen-activated protein kinase (MAPK) inhibitor on KGF2-induced stimulation of Pgp activity in Caco-2 cells. Postconfluent Caco-2 cells were pretreated with the specific inhibitor of Erk1/2 MAPK PD-98059 (30 μM) for 60 min in the serum-free cell culture medium and then coincubated with KGF2 (10 ng/ml) in serum-free cell culture medium supplemented with 0.2% BSA for 60 min. [3H]digoxin flux (1 μM) was measured as described in materials and methods. Results are expressed as %control and represent means ± SE of 5 separate experiments performed in triplicate. *P < 0.05 compared with untreated control. B: KGF2 induces Erk1/2 MAPK phosphorylation in Caco-2 cells. Caco-2 cells were incubated with KGF2 (10 ng/ml) in serum-free cell culture medium supplemented with 0.2% BSA for different time intervals ranging from 15 to 60 min. After the cells were washed with 1× PBS, extracted proteins (75 μg) were subjected to Western blot analysis on 12% SDS-polyacrylamide gel using phospho-specific Erk1/2 MAPK antibody (pErk1/2 MAPK). The blots were stripped and reprobed with the ERK1/2 MAPK antibody (total Erk1/2 MAPK) to indicate equal loading of protein in each lane. A representative blot of 3 different experiments is shown. The data were quantified by densitometric analysis and expressed as arbitrary units and represent means ± SE of 3 separate experiments. *P < 0.05 compared with untreated control (0 min).
KGF2 increases surface Pgp expression.
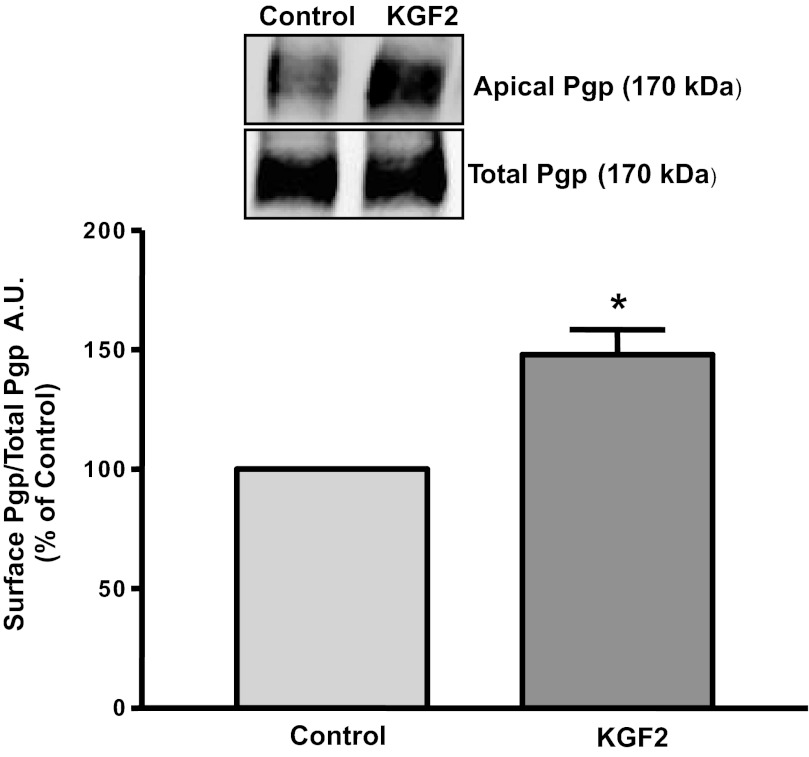
It is well known that membrane transporters under short-term conditions are regulated via altering their levels on plasma membranes (10, 19, 27, 31). We next examined the effects of KGF2 on surface levels of Pgp by cell surface biotinylation studies. Our results showed that KGF2 treatment significantly increased the surface levels of Pgp (170 kDa), whereas the total cellular Pgp levels did not change (Fig. 4). These data are in parallel with an increase in Pgp activity in Caco-2 cells. Densitometric analysis of the protein bands suggested that KGF2 treatment increased surface Pgp levels by 40–50% compared with controls. These results suggest that KGF2 under short-term conditions increases the level of Pgp on the plasma membrane.
Fig. 4.
KGF2 increases Pgp surface expression. Overnight serum-starved postconfluent Caco-2 cells were treated with KGF2 (10 ng/ml) in serum-free cell culture medium supplemented with 0.2% BSA for 60 min and subjected to biotinylation at 4°C using sulfo-NHS-SS-biotin. After solubilization, biotinylated proteins were extracted with streptavidin-agarose from equal amounts of total cellular protein. Surface and total cellular fractions were run on 7% SDS-polyacrylamide gel electrophoresis followed by transfer to nitrocellulose membrane. The blot was immunostained with a mouse anti-Pgp/multidrug resistance 1 [MDR1 (Pgp)]. A representative blot of 3 different experiments is shown. The data were quantified by densitometric analysis and expressed as arbitrary units and represent means ± SE of 3 separate experiments.
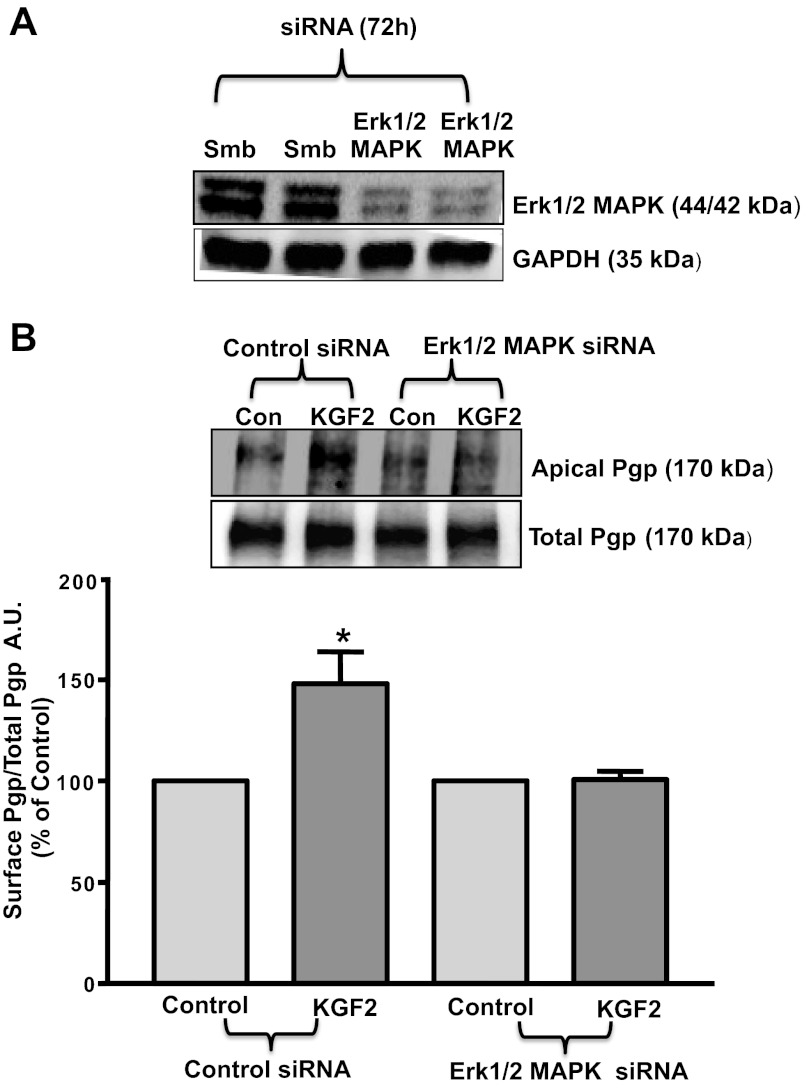
Erk1/2 MAPK inhibition abrogates the KGF2-induced increase in surface Pgp expression.
Because Erk1/2 MAPK was shown to be involved in mediating the effects of KGF2 on Pgp function in Caco-2 cells, we next examined whether the increase in surface Pgp levels by KGF2 was also dependent on the Erk1/2 MAPK pathway. For these studies, we performed siRNA transfections in Caco-2 cells with scrambled siRNA or Erk1/2 MAPK-specific siRNA. As shown in Fig. 5A, Erk1/2 MAPK expression was significantly knocked down in Erk1/2 MAPK-specific siRNA-transfected cells compared with cells transfected with scrambled siRNA. Furthermore, cell surface biotinylation studies showed that KGF2 treatment significantly increased the surface levels of Pgp (170 kDa), whereas the total cellular Pgp levels did not change (Fig. 5B) in scrambled siRNA-transfected cells. However, KGF2 did not show any effect in cells transfected with Erk1/2 MAPK-specific siRNA. Densitometric analysis of the protein bands suggested that KGF2 treatment increased surface Pgp levels by 40–50% compared with control in scrambled but not in Erk1/2 MAPK-specific siRNA-transfected cells. These results further suggest that Erk1/2 MAPK is involved in the KGF2-induced increase in Pgp levels on the plasma membrane.
Fig. 5.
Erk1/2 MAPK knockdown abrogates KGF2-induced increase in Pgp surface expression. A: Erk1/2 MAPK small-interfering RNA (siRNA) causes knockdown of Erk1/2 MAPK expression in Caco-2 cells. Scrambled (Smb) or Erk1/2 MAPK siRNA-transfected Caco-2 cell lysates (75 μg) were subjected to 12% SDS-PAGE followed by transfer to nitrocellulose membrane. The blot was probed with anti-Erk1/2 MAPK antibody (Erk1/2 MAPK) or anti-GAPDH (GAPDH) antibody. B: cell surface biotinylation in siRNA-transfected Caco-2 cells. Overnight serum-starved cells transfected with scrambled siRNA or with Erk1/2 MAPK siRNA were treated with KGF2 (10 ng/ml) in serum-free cell culture medium supplemented with 0.2% BSA for 60 min and subjected to biotinylation at 4°C using sulfo-NHS-SS-biotin. After solubilization, biotinylated proteins were extracted with streptavidin-agarose from equal amounts of total cellular protein. Surface and total cellular fractions were run on 7% SDS-polyacrylamide gel electrophoresis followed by transfer to nitrocellulose membrane. The blot was immunostained with a mouse anti-Pgp/MDR1 (Pgp). A representative blot of 3 different experiments is shown. The data were quantified by densitometric analysis and expressed as arbitrary units and represent means ± SE of 3 separate experiments.
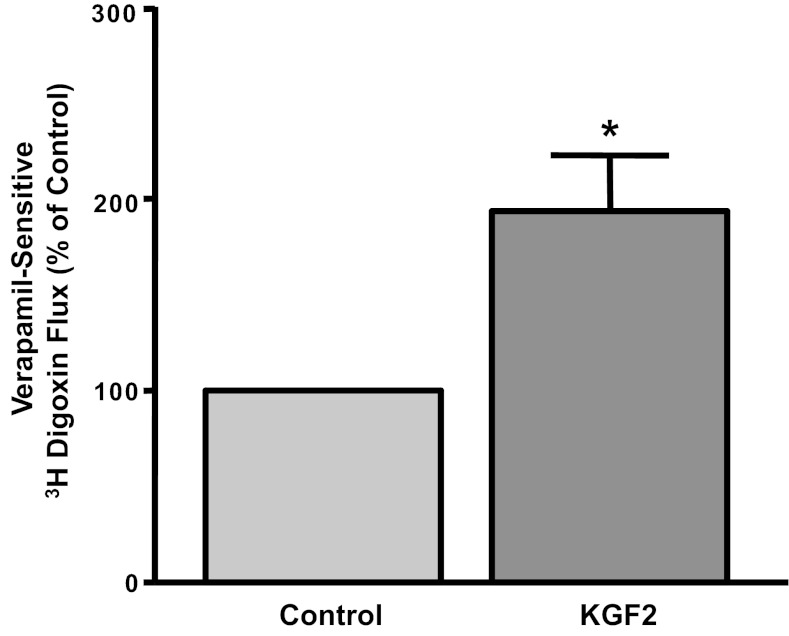
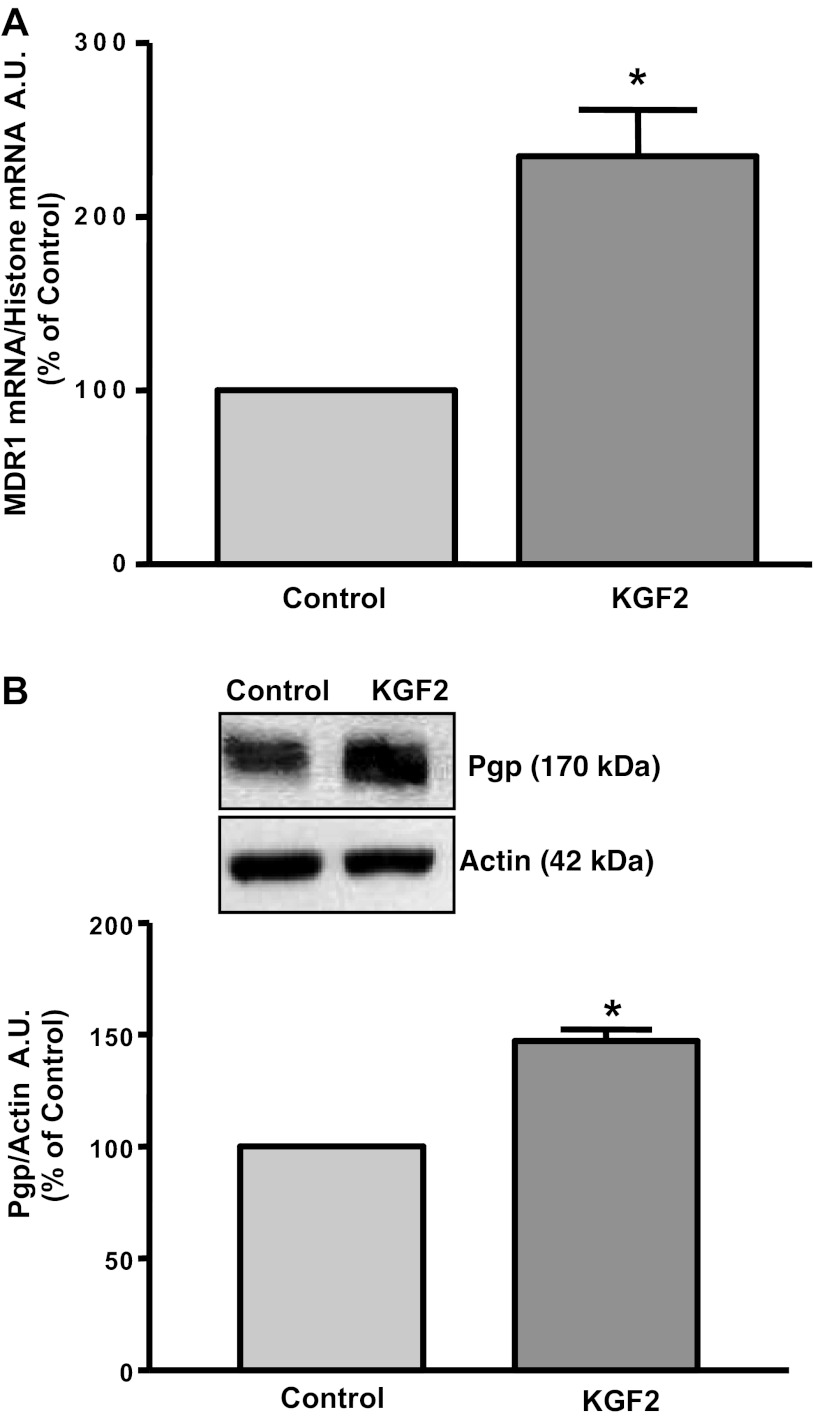
Long-term effects of KGF2 on Pgp function and expression in Caco-2 cells.
KGF2 has been shown to modulate the expression of various genes in different cell types (2, 4, 15, 34). Therefore, we next examined the long-term effects of KGF2 on Pgp function. As shown in Fig. 6, Pgp function was significantly increased in response to 24 h treatment with 10 ng/ml KGF2. To examine whether long-term KGF2 treatment affects Pgp mRNA expression, real-time RT-PCR using hMDR1 (Pgp) and histone (internal control) gene-specific primers were performed. Corresponding with the function, Pgp mRNA levels were significantly increased by about two- to threefold in response to 24 h incubation of Caco-2 monolayers with KGF2 compared with untreated cells (Fig. 7A). Furthermore, Western blot showed that KGF2 also increased expression of Pgp protein (170 kDa). Densitometric analysis revealed that KGF2 significantly enhanced Pgp protein levels in Caco-2 cells by ∼2.5-fold (Fig. 7B). These results indicate that the increase in Pgp activity in Caco-2 cells by KGF2 was found to be consistent with increased MDR1/Pgp mRNA and protein expression.
Fig. 6.
Long-term effect of KGF2 on Pgp activity in Caco-2 cells. Overnight serum-starved postconfluent Caco-2 cells were treated with KGF2 (10 ng/ml) in serum-free cell culture medium supplemented with 0.2% BSA for 24 h. [3H]digoxin flux (1 μM) was measured as described in materials and methods. Results are expressed as %control and represent means ± SE of 5 separate experiments performed in triplicate. *P < 0.05 compared with untreated control.
Fig. 7.
KGF2 induces Pgp mRNA and protein expression in Caco-2 cells. Overnight serum-starved postconfluent Caco-2 cells were treated with KGF2 (10 ng/ml) in serum-free cell culture medium supplemented with 0.2% BSA for 24 h. A: total RNA was then extracted from the cells, and 100 ng were amplified with MDR1 or histone gene-specific primers using 1-step RT-PCR mix containing SYBR Green fluorescence dye for real-time PCR quantitation. The relative abundance of MDR1 mRNA from control and KGF2-treated Caco-2 cells was normalized to histone mRNA (internal control). The data were quantified by densitometric analysis and expressed as %control in arbitrary units. Results represent means ± SE of 3 independent experiments performed in triplicate. *P < 0.05 compared with untreated control. B: control and KGF2-treated cell lysates (75 μg) were subjected to 7% SDS-PAGE followed by transfer to nitrocellulose membrane. The blot was probed with anti-Pgp/MDR1 (Pgp) or anti-β-actin (Actin) antibody. A representative blot of 3 different experiments is shown. The data were quantified by densitometric analysis and expressed as %control in arbitrary units. Results represent means ± SE of 3 independent experiments performed in triplicate. *P < 0.05 compared with untreated control.
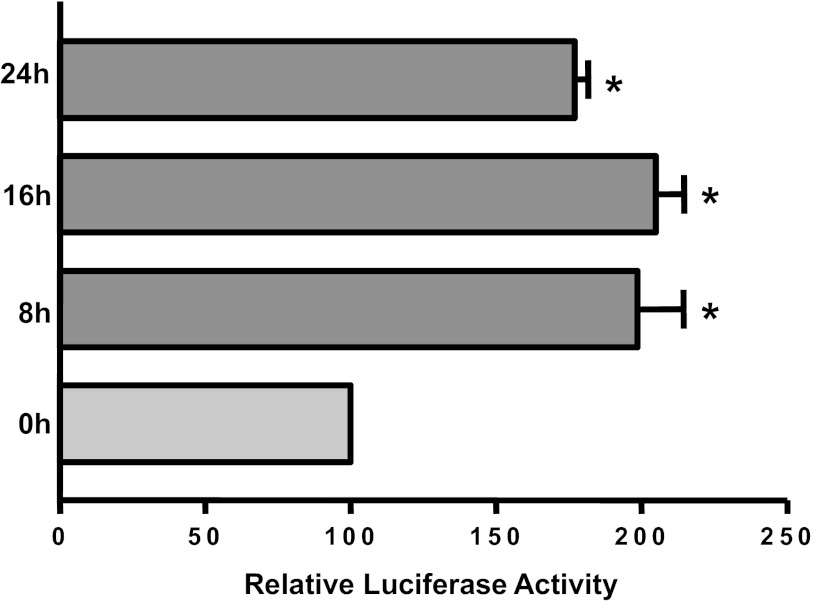
KGF2 increases MDR1 promoter activity.
We next investigated whether the increase in the MDR1 mRNA expression was through a transcriptional mechanism involving increased promoter activity. Caco-2 cells were transfected with reporter construct containing an MDR1 promoter fragment harboring the region between −1,073/+703 of MDR1 gene (+1 represents the transcription initiation site). Posttransfection (24 h), cells were treated with KGF2 basolaterally for an additional 8, 16, and 24 h, and MDR1 promoter activity was assessed. MDR1 promoter activity was markedly increased as early as 8 h incubation time by approximately twofold, which persisted until the 24-h time point (Fig. 8). These results demonstrate that long-term KGF2 treatment increases MDR1 expression through a transcriptional mechanism in human intestinal epithelial cells.
Fig. 8.
Effect of KGF2 on Pgp promoter activity in Caco-2 cells. Caco-2 cells were transiently transfected with MDR1 luciferase promoter construct (p-1,073/+703) along with pCMVβ vector. Transfected cells were treated with KGF2 (10 ng/ml) for different periods of time in media containing 0.2% BSA and 1% FBS after 24 h. Posttransfection (48 h), the promoter activity was measured by luciferase assay. Values were normalized to β-galactosidase activity to correct for transfection efficiency. Results represent means ± SE of 3 separate experiments and are expressed as %control comparing transfected cells treated with KGF2 with untreated cells (control). *P < 0.05 compared with control.
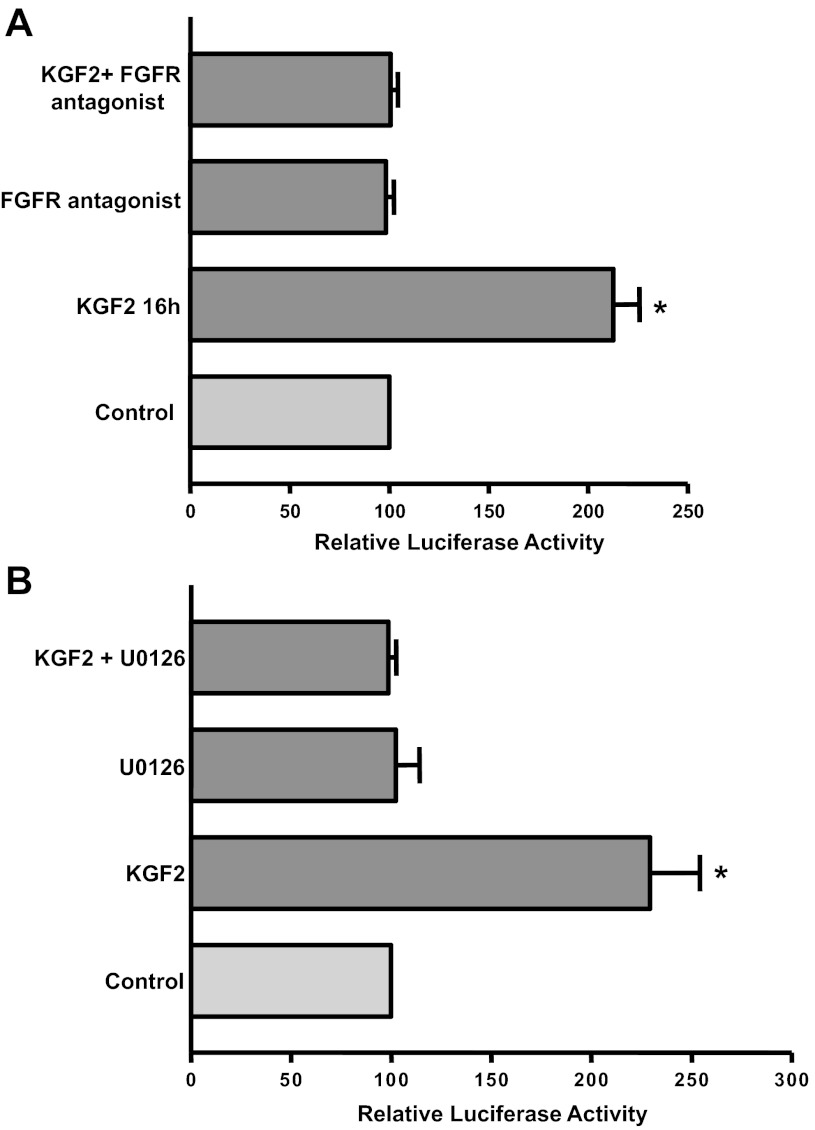
Stimulatory effects of KGF2 on MDR1 promoter activity are FGFR and Erk1/2 MAPK dependent.
The specific FGFR antagonist PD-161570 (10 μM) or the Erk1/2 MAPK inhibitor U-0126 (10 μM) significantly attenuated the stimulatory effects of KGF2 on Pgp promoter activity in Caco-2 cells (Fig. 9, A and B). These data indicate that KGF2 stimulates MDR1 promoter activity via the activation of FGFR and the Erk1/2 MAPK pathway. Furthermore, these results suggest that the KGF2-mediated increase in Pgp promoter activity by Erk1/2 MAPK at the 24-h time point could be because of the early activation of Erk1/2 MAPK signaling within 1 h by KGF2.
Fig. 9.
Effect of FGF receptor antagonist or Erk1/2 MAPK inhibitor on KGF2-induced stimulation of Pgp promoter activity in Caco-2 cells. Caco-2 cells were transiently transfected with MDR1 luciferase promoter construct (p-1,073/+703) along with pCMVβ vector. After 24 h, transiently transfected cells were pretreated with the specific antagonist of FGF receptor PD-161570 (10 μM) (A) or the Erk1/2 MAPK inhibitor U-0126 (10 μM) (B) for 60 min in media containing 0.2% BSA and 1% FBS and then coincubated with KGF2 (10 ng/ml) in media containing 1% FBS for 24 h. Posttransfection (48 h) the promoter activity was measured by luciferase assay. Values were normalized to β-galactosidase activity to correct for transfection efficiency. Results represent means ± SE of 3 separate experiments and are expressed as %control comparing transfected cells treated with KGF2 with untreated cells (control). *P < 0.05 compared with control.
DISCUSSION
KGF2 is involved in a variety of biological processes, including epithelial cell growth, morphogenesis, migration, and restitution (32). Also, KGF2 has been shown previously to be protective in experimental models of intestinal inflammation, indicating a role of KGF2 in mucosal defense, epithelial repair, and treatment of intestinal disorders (8, 11, 14, 21). In the present study, we for the first time demonstrate that the upregulation of Pgp by KGF2 in intestinal epithelial cells occurs by dual mechanisms involving both posttranslational and transcriptional processes.
KGF2 is known to exhibit diverse physiological functions via transmembrane FGF receptor of the tyrosine kinase family (FGFR) (32). Intestinal epithelial Caco-2 cells have been shown to express FGFRs on the basolateral surface (30) and have been used to examine regulation of various physiological processes such as epithelial cell proliferation and restitution in response to KGF2 (17, 30, 34). Our data demonstrated that the selective FGFR antagonist PD-161570 attenuated the stimulatory effects of KGF2 on Pgp function in Caco-2 cells, suggesting that KGF2 effects on intestinal Pgp activity are dependent on FGFR. Furthermore, KGF2 (FGF10) has been shown to exert its effects by binding to the IIIb receptor variant of FGFR2, FGFR-2IIIb (also known as KGFR), in intestinal epithelial cells (7, 32). However, because of the nonavailability of specific antagonists against FGFR-2IIIb, the role of FGFR-2IIIb in mediating the stimulatory effects of KGF2 on Pgp in Caco-2 cells still remains elusive. Further studies are needed to pinpoint the involvement of FGFR-2IIIb in KGF2-mediated effects using siRNAs against FGFR-2IIIb.
Binding of KGF2 to KGFR results in receptor dimerization, with subsequent autophosphorylation on tyrosine residues within the intracellular domain and recruitment and phosphorylation of substrate proteins (7). In this regard, previous studies in alveolar epithelial cells have shown KGF2/FGFR signaling to activate Erk1/2 MAPK (29). Our studies also showed that Erk1/2 MAPK is involved in mediating the effects of KGF2, since the specific Erk1/2 MAPK inhibitor PD-98059 blocked KGF2-induced effects on Pgp activity in Caco-2 cells. A number of studies have shown that membrane transport proteins are regulated via recycling events under short-term (acute) conditions between intracellular and apical membrane compartments (vesicular trafficking) (10, 19, 27, 31). However, there are no reports on the role of KGF2 in the regulation of proteins by membrane trafficking in the intestine. With respect to the mechanisms underlying the acute modulation of Pgp activity by KGF2, our results showed that KGF2-mediated stimulation of Pgp occurred via a significant increase in the apical membrane levels of Pgp with no change in the total levels of Pgp in Caco-2 cells. These results suggest that KGF2 induces Pgp in Caco-2 cells via recycling events at the posttranslational level. The increase in the surface expression of Pgp by KGF2 was abrogated in Caco-2 cells by Erk1/2 MAPK siRNA but not by scrambled siRNA. Together, the data indicate that knockdown of Erk1/2 MAPK attenuated KGF2-induced Pgp activity by abolishing the KGF2-induced increase in Pgp membrane levels. Our studies are in agreement with previous studies that also demonstrate that short-term epidermal growth factor treatment of human intestinal epithelial C2bbe cells increased glutamine transport and its surface expression levels via Erk1/2 MAPK- and Rho-dependent pathways (3).
Long-term treatment of Caco-2 cells with KGF2 (24h) also significantly increased Pgp function. The increase in function was consistent with an increase in MDR1/Pgp mRNA and protein levels in Caco-2 cells and occurred via a transcriptional mechanism as KGF2 increased MDR1/Pgp promoter activity. Moreover, KGF2-mediated effects on Pgp promoter activity in Caco-2 cells were found to be receptor dependent, since the specific FGFR antagonist PD-161570 blocked the stimulatory effects of KGF2 on Pgp promoter activity. These results suggest the role of KGF2 in inducing FGFR sensitization and increased ligand (KGF2) receptor binding leading to the transcriptional activation of Pgp by KGF2 in Caco-2 cells. The involvement of ERK1/2 MAPK in mediating the stimulatory effects of KGF2 on Pgp promoter activity further indicated that Erk1/2 MAPK signaling could play an important role in KGF2-induced stimulation of Pgp via both membrane trafficking events (early activation of Erk1/2 MAPK within 1 h) and transcriptional mechanisms. We have previously shown the role of Erk1/2 MAPK in the transcriptional regulation of Pgp by the probiotic bacteria Lactobacillus acidophilus and Lactobacillus rhamnosus in Caco-2 cells (25). Also, our low stringent analysis of Pgp using in silico analysis by Scansite 2.0 (22) identified potential phosphorylation sites for Erk1/2 MAPK. We speculate that these sequence motifs may play an important role in the direct or indirect phosphorylation of Pgp that could result in enhanced Pgp function and expression in response to KGF2. Further studies are needed to address this important issue.
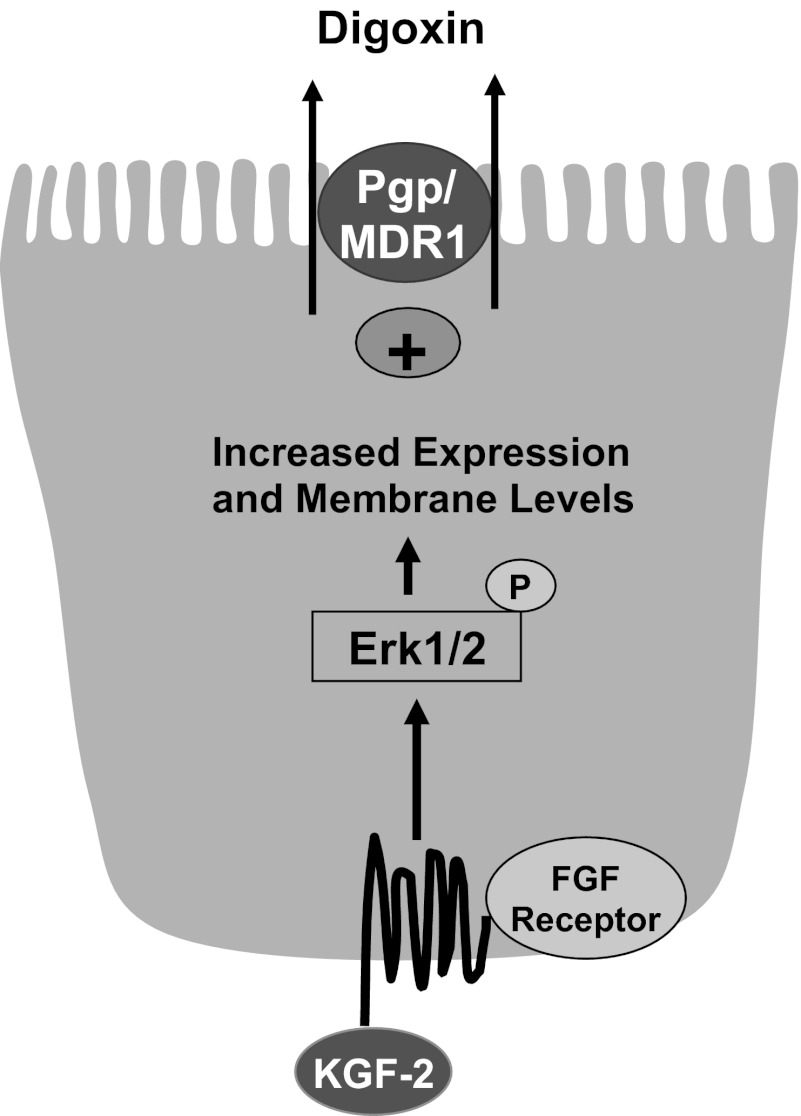
In conclusion, our findings provide novel information on the upregulation of Pgp function and expression by KGF2 via both posttranslational and transcriptional mechanisms (Fig. 10). Our findings showed that KGF2 under short-term conditions stimulated Pgp function in Caco-2 cells via the FGFR and involved an Erk1/2 MAPK-dependent pathway. KGF2-induced stimulation of Pgp-mediated digoxin flux correlated with an increase in the membrane levels of Pgp protein (via recycling events) and was found to be dependent on the Erk1/2 MAPK pathway. Long-term treatment with KGF2 also increased MDR1/Pgp mRNA and protein expression and function in Caco-2 cells. The observed increase in KGF2-induced Pgp expression and function occurred at the transcriptional level, since KGF2 significantly increased Pgp promoter activity via FGFR and the Erk1/2 MAPK pathway (Fig. 10). Because studies in experimental animal models of inflammation have shown that KGF2 decreased inflammation because of its wound-healing and intestinal epithelial restitution effects (8, 11, 14, 21), future studies are needed to investigate whether Pgp upregulation can contribute to beneficial effects of KGF2 and can reverse the decreased Pgp expression observed in inflammatory disorders such as inflammatory bowel disease. In this regard, administration of the anti-inflammatory probiotic L. acidophilus to mice demonstrated an increase in Pgp expression in the ileum and colon and attenuated decreased Pgp expression and inflammation in DSS colitis mice (25). Also, because Pgp/mdr1 total knockout mice develop spontaneous colitis (23), it will be of interest to examine whether Pgp upregulation in intestinal epithelial cells will protect against inflammation/colitis in animal models. The findings may have broader therapeutic implications in treating intestinal inflammatory disorders associated with a decrease in Pgp function and expression and impairment of epithelial integrity.
Fig. 10.
Schematic of the proposed model of KGF2-mediated effects on Pgp.
GRANTS
These studies were supported by the Crohn's and Colitis Foundation of America Grant Ref. No. 1942 and National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-96254 (S. Saksena); DK-54016, DK-81858, DK-92441, and P01 DK-67887 (P. K. Dudeja); DK-71596 (W. Alrefai); and DK-74458 (R. K. Gill). The studies were also supported in part by the Department of Veterans Affairs, Veteran Heath Administration, Office of Research and Development, Biomedical Laboratory Research and Development (P. K. Dudeja and W. A. Alrefai).
AUTHOR CONTRIBUTIONS
Author contributions: S.S., S.P., A.K., M.A. and V.S. performed experiments; S.S., S.P. and A.K. analyzed data; S.S., S.P., A.K., A.N.A and A.A. interpreted results of experiments; S.S. and A.K. prepared figures; S.S drafted manuscript; S.S., R.G., W.A.A. and P.K.D edited and revised manuscript; S.S., R.G., W.A.A. and P.K.D approved final version of manuscript; S.S. and W.A.A. conception and design of research.
REFERENCES
- 1. Annese V, Valvano MR, Palmieri O, Latiano A, Bossa F, Andriulli A. Multidrug resistance 1 gene in inflammatory bowel disease: a meta-analysis. World J Gastroenterol 12: 3636–3644, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Asaki T, Konishi M, Miyake A, Kato S, Tomizawa M, Itoh N. Roles of fibroblast growth factor 10 (Fgf10) in adipogenesis in vivo. Mol Cell Endocrinol 218: 119–128, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Avissar NE, Sax HC, Toia L. In human entrocytes, GLN transport and ASCT2 surface expression induced by short-term EGF are MAPK, PI3K, and Rho-dependent. Dig Dis Sci 53: 2113–2125, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Blaisdell CJ, Pellettieri JP, Loughlin CE, Chu S, Zeitlin PL. Keratinocyte growth factor stimulates CLC-2 expression in primary fetal rat distal lung epithelial cells. Am J Respir Cell Mol Biol 20: 842–847, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Blokzijl H, Vander Borght S, Bok LI, Libbrecht L, Geuken M, van den Heuvel FA, Dijkstra G, Roskams TA, Moshage H, Jansen PL, Faber KN. Decreased P-glycoprotein (P-gp/MDR1) expression in inflamed human intestinal epithelium is independent of PXR protein levels. Inflamm Bowel Dis 13: 710–720, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Buyse M, Radeva G, Bado A, Farinotti R. Intestinal inflammation induces adaptation of P-glycoprotein expression and activity. Biochem Pharmacol 69: 1745–1754, 2005 [DOI] [PubMed] [Google Scholar]
- 7. de Giorgi V, Sestini S, Massi D, Ghersetich I, Lotti T. Keratinocyte growth factor receptors. Dermatol Clin 25: 477–485, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Dieckgraefe BK, Korzenik JR, Anant S. Growth factors as treatment options for intestinal inflammation. Ann NY Acad Sci 1072: 300–306, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Finch PW, Rubin JS, Miki T, Ron D, Aaronson SA. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science 245: 752–755, 1989 [DOI] [PubMed] [Google Scholar]
- 10. Gill RK, Borthakur A, Hodges K, Turner JR, Clayburgh DR, Saksena S, Zaheer A, Ramaswamy K, Hecht G, Dudeja PK. Mechanism underlying inhibition of intestinal apical Cl/OH exchange following infection with enteropathogenic E. coli J Clin Invest 117: 428–437, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Greenwood-Van Meerveld B, Venkova K, Connolly K. Efficacy of repifermin (keratinocyte growth factor-2) against abnormalities in gastrointestinal mucosal transport in a murine model of colitis. J Pharm Pharmacol 55: 67–75, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Guner YS, Franklin AL, Chokshi NK, Castle SL, Pontarelli E, Wang J, Wang L, Prasadarao NV, Upperman JS, Grishin AV, Ford HR. P-glycoprotein induction by breast milk attenuates intestinal inflammation in experimental necrotizing enterocolitis. Lab Invest 91: 1668–1679, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gutmann H, Hruz P, Zimmermann C, Straumann A, Terracciano L, Hammann F, Lehmann F, Beglinger C, Drewe J. Breast cancer resistance protein and P-glycoprotein expression in patients with newly diagnosed and therapy-refractory ulcerative colitis compared with healthy controls. Digestion 78: 154–162, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Han DS, Li F, Holt L, Connolly K, Hubert M, Miceli R, Okoye Z, Santiago G, Windle K, Wong E, Sartor RB. Keratinocyte growth factor-2 (FGF-10) promotes healing of experimental small intestinal ulceration in rats. Am J Physiol Gastrointest Liver Physiol 279: G1011–G1022, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Hashimoto S, Nakano H, Suguta Y, Irie S, Jianhua L, Katyal SL. Exogenous fibroblast growth factor-10 induces cystic lung development with altered target gene expression in the presence of heparin in cultures of embryonic rat lung. Pathobiology 79: 127–143, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ho GT, Moodie FM, Satsangi J. Multidrug resistance 1 gene (P-glycoprotein 170): an important determinant in gastrointestinal disease? Gut 52: 759–766, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hughes SE. Differential expression of the fibroblast growth factor receptor (FGFR) multigene family in normal human adult tissues. J Histochem Cytochem 45: 1005–1019, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Iizasa H, Genda N, Kitano T, Tomita M, Nishihara K, Hayashi M, Nakamura K, Kobayashi S, Nakashima E. Altered expression and function of P-glycoprotein in dextran sodium sulfate-induced colitis in mice. J Pharm Sci 92: 569–576, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Janecki AJ, Janecki M, Akhter S, Donowitz M. Basic fibroblast growth factor stimulates surface expression and activity of Na(+)/H(+) exchanger NHE3 via mechanism involving phosphatidylinositol 3-kinase. J Biol Chem 275: 8133–8142, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Maggio-Price L, Shows D, Waggie K, Burich A, Zeng W, Escobar S, Morrissey P, Viney JL. Helicobacter bilis infection accelerates and H. hepaticus infection delays the development of colitis in multiple drug resistance-deficient (mdr1a−/−) mice. Am J Pathol 160: 739–751, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miceli R, Hubert M, Santiago G, Yao DL, Coleman TA, Huddleston KA, Connolly K. Efficacy of keratinocyte growth factor-2 in dextran sulfate sodium-induced murine colitis. J Pharmacol Exp Ther 290: 464–471, 1999 [PubMed] [Google Scholar]
- 22. Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res 31: 3635–3641, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Panwala CM, Jones JC, Viney JL. A novel model of inflammatory bowel disease: mice deficient for the multiple drug resistance gene, mdr1a, spontaneously develop colitis. J Immunol 161: 5733–5744, 1998 [PubMed] [Google Scholar]
- 24. Raheja G, Singh V, Ma K, Boumendjel R, Borthakur A, Gill RK, Saksena S, Alrefai WA, Ramaswamy K, Dudeja PK. Lactobacillus acidophilus stimulates the expression of SLC26A3 via a transcriptional mechanism. Am J Physiol Gastrointest Liver Physiol 298: G395–G401, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saksena S, Goyal S, Raheja G, Singh V, Akhtar M, Nazir TM, Alrefai WA, Gill RK, Dudeja PK. Upregulation of P-glycoprotein by probiotics in intestinal epithelial cells and in the dextran sulfate sodium model of colitis in mice. Am J Physiol Gastrointest Liver Physiol 300: G1115–G1123, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schinkel AH. The physiological function of drug-transporting P-glycoproteins. Semin Cancer Biol 8: 161–170, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Shepherd EJ, Helliwell PA, Mace OJ, Morgan EL, Patel N, Kellett GL. Stress and glucocorticoid inhibit apical GLUT2-trafficking and intestinal glucose absorption in rat small intestine. J Physiol 560: 281–290, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA 84: 7735–7738, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Upadhyay D, Bundesmann M, Panduri V, Correa-Meyer E, Kamp DW. Fibroblast growth factor-10 attenuates H2O2-induced alveolar epithelial cell DNA damage: role of MAPK activation and DNA repair. Am J Respir Cell Mol Biol 31: 107–113, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Visco V, Belleudi F, Marchese C, Leone L, Aimati L, Cardinali G, Kovacs D, Frati L, Torrisi MR. Differential response to keratinocyte growth factor receptor and epidermal growth factor receptor ligands of proliferating and differentiating intestinal epithelial cells. J Cell Physiol 200: 31–44, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Watson RT, Pessin JE. Intracellular organization of insulin signaling and GLUT4 translocation. Recent Prog Horm Res 56: 175–193, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Werner S. Keratinocyte growth factor: a unique player in epithelial repair processes. Cytokine Growth Factor Rev 9: 153–165, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Young VB, Chien CC, Knox KA, Taylor NS, Schauer DB, Fox JG. Cytolethal distending toxin in avian and human isolates of Helicobacter pullorum. J Infect Dis 182: 620–623, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Zhou J, Wu K, Fernandes CL, Cheng AL, Finch PW. Keratinocyte growth factor down-regulates expression of the sucrase-isomaltase gene in Caco-2 intestinal epithelial cells. J Biol Chem 273: 33367–33373, 1998 [DOI] [PubMed] [Google Scholar]