Abstract
Liver fibrosis is characterized by excessive deposition of extracellular matrix proteins by myofibroblasts derived from hepatic stellate cells and portal fibroblasts. Activation of these precursors to myofibroblasts requires matrix stiffness, which results in part from increased collagen cross-linking mediated by lysyl oxidase (LOX) family proteins. The aims of this study were to characterize the mechanical changes of early fibrosis, to identify the cells responsible for LOX production in early injury, and to determine which cells in normal liver produce collagens and elastins, which serve as substrates for LOXs early after injury. Hepatocytes and liver nonparenchymal cells were isolated from normal and early-injured liver and examined immediately for expression of LOXs and matrix proteins. We found that stellate cells and portal fibroblasts were the major cellular sources of fibrillar collagens and LOXs in normal liver and early after injury (1 day after bile duct ligation and 2 and 7 days after CCl4 injury). Activity assays using stellate cells and portal fibroblasts in culture demonstrated significant increases in LOX family enzymatic activity as cells became myofibroblastic. LOX family-mediated deoxypyridinoline and pyridinoline cross-links increased after CCl4-mediated injury. There was a significant association between liver stiffness (as quantified by the shear storage modulus G′) and deoxypyridinoline levels; increased deoxypyridinoline levels were also coincident with significantly increased elastic resistance to large strain deformations, consistent with increased cross-linking of the extracellular matrix. These data suggest a model in which the liver is primed to respond quickly to injury, activating potential mechanical feed-forward mechanisms.
Keywords: collagen cross-linking, liver fibrosis, pyridinoline, deoxypyridinoline, extracellular matrix
pathological fibrosis is characterized by excessive accumulation of extracellular matrix (ECM) proteins, most notably fibrillar collagens. The majority of ECM in organ fibrosis is deposited by myofibroblasts, proliferative and motile cells characterized by expression of α-smooth muscle actin (α-SMA), which migrate to the site of injury or differentiate from preexisting cells in the organ. In the liver, the major myofibroblast precursor cells are hepatic stellate cells and portal fibroblasts.
Myofibroblast differentiation from precursor cells requires mechanical tension. This has been shown in vitro for general fibroblast-to-myofibroblast activation (14), as well as for hepatic stellate cell and portal fibroblast activation to myofibroblasts in the liver (19, 36) and suggests that increases in liver stiffness precede myofibroblast activation. We have used ex vivo livers to show that this is true in a rat CCl4 model of liver fibrosis and that the mechanical heterogeneity of the liver also increases early after injury in this model (12, 17).
Increases in liver stiffness are multifactorial and may include increased interstitial pressure from edema, increased ECM protein expression, and collagen stiffening due to cross-linking. Potentially relevant collagen cross-linking agents include tissue transglutaminases, which are increased in residual septae after fibrosis resolution [but have recently been shown to have minimal impact on progression of fibrosis in rodent models (27)], and lysyl oxidase (LOX) family enzymes. The LOXs are secreted copper-dependent amine oxidases that initiate the process of covalent intra- and intermolecular cross-linking of collagens and elastin (34, 35). There are five isoforms: LOX and the LOX-like enzymes LOXL1, LOXL2, LOXL3, and LOXL4, all encoded by separate genes and with overlapping, but distinct, functions and substrate specificity. Multiple studies have shown that LOX-mediated cross-linking significantly increases ECM stiffness (8, 15, 20, 29).
The LOXs play an important role in liver fibrosis. LOX expression in the liver, as determined by immunohistochemistry, is upregulated as early as 24 h after bile duct ligation (BDL) and continues to increase through at least 72 h, preceding increases in α-SMA (7). In a chronic CCl4 model of fibrosis, LOX activity increased 30-fold in rat liver and 15-fold in plasma after 5 wk (32). Treatment of rats with β-aminoproprionitrile (BAPN), an inhibitor of LOX family cross-linking enzymes, reduced early increases in liver stiffness in the CCl4 model (12); we and others have shown that inhibition of LOX family activity also results in decreased numbers of myofibroblasts and decreased fibrosis (9, 10, 12). A recent study showed that treatment with a LOXL2-blocking antibody blunted liver fibrosis in CCl4-treated rats (2).
The LOXs cross-link newly synthesized collagen and elastin fibrils (34, 35). Thus, if the LOXs are important mediators of early fibrosis and the first phases of myofibroblast differentiation (and as part of normal liver matrix homeostasis), there must be nonmyofibroblast sources of fibrillar collagens in the normal and early-injured liver. Our goals were to characterize mechanical changes in the normal liver and the liver very early after injury and to identify the cellular sources of fibrillar collagens and LOXs in an effort to delineate the earliest events in fibrosis.
MATERIALS AND METHODS
Animal models of liver injury.
Liver injury was induced in 250- to 300-g Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) by CCl4 intoxication or BDL. Rats were injected with a sterile-filtered 1:1 mixture of CCl4 (Sigma-Aldrich, St. Louis, MO) in mineral oil (0.2 ml/100 g body wt ip) once (1d and 2d experiments) or twice per week. Cross-linking analyses were also carried out using previously generated samples (12). Mechanical measurements [G′ (shear storage modulus, a measure of elasticity or rigidity) and G′′ (shear loss modulus, a measure of viscosity)] were carried out as previously described (12) and included measurements at varying strains. BDL was carried out according to standard protocols, with the placement of two ligatures around the bile duct. All animal work was approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Primary cell isolation.
All cells were prepared from 500- to 700-g Sprague-Dawley rats. Cells were lysed immediately after isolation for mRNA analysis or cultured.
Hepatocytes were isolated as described elsewhere (33), with minor modifications. Briefly, livers were perfused in situ through the portal vein with 0.05% collagenase type IV (Worthington, Lakewood, NJ). Cells were resuspended in HBSS with Ca2+/Mg2+ (GIBCO, Manassas, VA) containing 0.003% DNase (Sigma-Aldrich), sedimented at 60 g for 3 min at room temperature, and then resuspended in HBSS with Ca2+/Mg2+ and washed again. For separation of hepatocytes from dead and contaminating nonparenchymal cells, the cell suspension was brought to 10% Histodenz (Sigma-Aldrich) and 1% FBS (Gemini Bio-Products, West Sacramento, CA) and layered over 7 ml of 30% Histodenz. After centrifugation at 1,400 g for 15 min, cells were collected from the interface between the two layers.
Hepatic stellate cells were isolated by sequential digestion of the liver with 0.4% pronase (Roche Diagnostics, Indianapolis, IN) and 0.04% type II collagenase (Worthington) followed by density gradient centrifugation over 9% Histodenz (Sigma-Aldrich), as described elsewhere (36).
Portal fibroblasts were isolated after in situ perfusion of livers with 0.3% type II collagenase (Worthington), as described elsewhere (19).
For sinusoidal endothelial cell (SEC) and Kupffer cell isolations (which were carried out separately), livers were perfused and digested in situ with 0.05% type IV collagenase (Worthington). Cell suspensions were separated by density gradient centrifugation using Percoll gradients (11). Kupffer cells were purified by rapid adherence to plastic (15–30 min at 37°C). SEC were purified via centrifugal elutriation using a Beckman-Coulter Avanti J-201 centrifuge with a JE 5.0 elutriator rotor and large chamber. Cells were eluted in HBSS without Ca2+/Mg2+. Centrifugation speed was kept constant (2,500 rpm) with a variable pump flow rate. SEC were collected at a pump speed of 60–80 ml/min (with increases of 2 ml/min). One liter of cell suspension was collected and centrifuged at 1,900 rpm for 7 min.
The purity of all primary cells was assessed by immunostaining. Cells were seeded on uncoated glass slides (Kupffer cells, hepatic stellate cells, and portal fibroblasts) or on slides coated with collagen I (SEC and hepatocytes; BD Falcon, Bedford, MA). Cells were stained using antibodies against hepatocyte nuclear factor-4α (1:50 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) for hepatocytes, CD68 (1:200 dilution; Abcam, Cambridge, MA) for Kupffer cells, desmin (1:1,000 dilution; Sigma-Aldrich) for hepatic stellate cells, elastin (1:500 dilution; Cedarlane Laboratories, Burlington, NC) for portal fibroblasts, and CD32b (1:100 dilution; Santa Cruz Biotechnology) for SEC. Cell viability was determined by 0.4% trypan blue exclusion. Only cell preparations found to be ≥95% pure and viable were used for experiments.
Quantitative PCR.
Total RNA was isolated from primary cells immediately after isolation, without intervening culture, using the RNeasy Micro kit (Qiagen, Valencia, CA). Two-step real-time PCR (quantitative RT-PCR) was performed using SuperScript III reverse transcriptase and random primers (Invitrogen) and Fast SYBR Green (Applied Biosystems, Carlsbad, CA). A relative calibration curve with 10-fold serial dilutions was built for every target. PCR primers were designed using Vector NTI (Invitrogen) or an online tool from Integrated DNA Technologies (http://www.idtdna.com/Scitools/Applications/RealTimePCR/). All primers were designed to span introns. 12S ribosomal protein mRNA was used for normalization. Sequences are shown in Table 1.
Table 1.
Primer sequences
| Sequences | |
|---|---|
| Collagen I (α2) | |
| Forward | TTCTCTACTGGTGAAACCTGC |
| Reverse | ACCCCTTCTGCGTTGTATTC |
| Collagen III (α1) | |
| Forward | GGCCTTGCGTGTTTGATATTC |
| Reverse | GAAGTCTCTGAAGCTGATGGG |
| Collagen IV (α1) | |
| Forward | AGTTCCCCGCCGTCTCTGTT |
| Reverse | ACAATCACCCTTCGCAGCGG |
| Elastin | |
| Forward | CTTGGAGGCATTGGCGGAGT |
| Reverse | CAATACCACCGACAGCCCCA |
| LOX | |
| Forward | GAGGAAATCGTAGCAGTA |
| Reverse | CTACATCCAGGCATCCACG |
| LOXL1 | |
| Forward | CCCTTGTCTCCCCTTGAAAAG |
| Reverse | GGTCCATGTTCACTCCCTGAG |
| LOXL2 | |
| Forward | GAGTTATCCCTGGCACACTGC |
| Reverse | TCCAGATAGGCGGTCTGTTGT |
| LOXL3 | |
| Forward | CCCAGGAACAGGCCGAATCT |
| Reverse | GGGTACAGTCACTGTTTCCCCAGC |
| LOXL4 | |
| Forward | ACTTCACTCAGGTGGATGGG |
| Reverse | CCACAGGTGGAACCTCATCT |
| RPS12 | |
| Forward | CCTCGATGACATCCTTGG |
| Reverse | GGAAGGCATAGCTGCTGG |
LOX, lysyl oxidase; LOXL, LOXL1, LOXL2, LOXL3, and LOXL4, LOL-like enzymes; RPS12, ribosomal protein S12.
LOX activity assay.
LOX enzymatic activity was determined as described elsewhere (26) with minor modifications using conditioned media collected over 24 h from cultures of primary hepatic stellate cells and portal fibroblasts. Cells from a single animal were used for each individual experiment. At least three animals were used for each cell type, with three technical repeats for every time point. Conditioned media were clarified by centrifugation at 10,000 g for 30 min at 4°C with addition of protease inhibitors without EDTA (Roche) and concentrated 100-fold by ultrafiltration at 15°C for 1.5 h at 3,700 g in Amicon Ultra-15 concentrators (10-kDa cutoff; Millipore, Carrigtwohill, Ireland). After concentration, equal volumes of a solution containing 4 M urea and 20 mM sodium borate (pH 8.0) were added to the samples for cryoprotection, and the samples were frozen at −80°C.
LOX activity was assessed fluorometrically using 30 μM Amplex red reagent (Invitrogen, Carlsbad, CA) in 1 M urea, 0.5 U/ml horseradish peroxidase (Worthington), 10 mM cadaverine (Sigma-Aldrich), and 45 mM sodium borate (pH 8.2). Samples were incubated at 37°C for 1 h, with readings taken every minute for 20 min (excitation at 530 nm and emission at 590 nm; Synergy 2 plate reader with Gen 5 1.10 software). To avoid interference from possible preexisting H2O2 and confounding from other amine oxidases that also use cadaverine as substrate, we calculated activity by subtracting fluorescence with BAPN from fluorescence without BAPN, after normalization to the protein content of the sample. A calibration curve was generated with H2O2 (range 0.125–2.5 μM), and background fluorescence was corrected using a no-H2O2 control.
Biochemical analyses of collagen cross-links.
Liver samples were minced on ice, washed with cold phosphate-buffered saline and distilled water, lyophilized, and weighed. Aliquots of the dried samples were then hydrolyzed with 6 N HCl and subjected to amino acid and cross-link analyses, as described elsewhere (40). Because of the low hydroxyproline contents in liver samples, nonreducible collagen cross-links (pyridinoline and deoxypyridinoline) were expressed as residues per 1,000 total amino acids.
Statistical analysis.
Values are means ± SE and represent at least three independent experiments with three technical repeats per experiment. For cross-link analysis, values are means ± SD. Statistical significance was calculated by t-test (GraphPad Prism), except as noted.
RESULTS
Liver mechanics change early after injury.
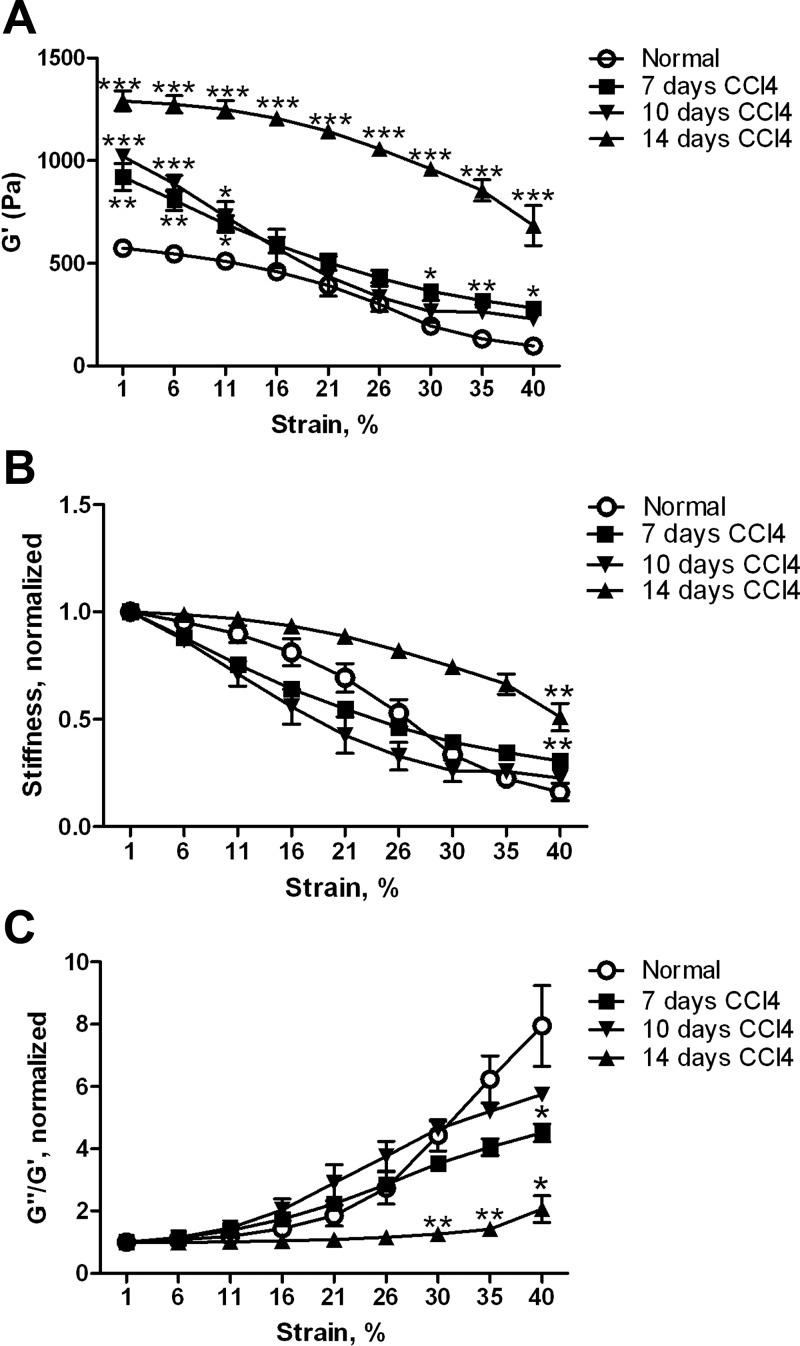
We previously used the rat CCl4 model of liver fibrosis to demonstrate that liver stiffness increased early after injury, before the first major wave of myofibroblast differentiation (12). Treatment of animals with the LOX inhibitor BAPN suggested that LOX family activity was responsible at least in part for this increase (12). We have now carried out more detailed mechanical measurements and shown that they are consistent with increased collagen cross-linking as a cause of increased stiffness. We determined the stiffness (G′) of livers at 0–14 days after initiation of CCl4 intoxication. In all cases, livers were removed 3–4 days after an injection of CCl4, when edema was minimal. As we observed previously (12), the livers of CCl4-treated animals were significantly stiffer than normal livers beginning as early as 7 days after the start of treatment (Fig. 1A). When shear strain was increased, marked strain softening was observed in samples from all time points (Fig. 1A), although the degree of softening was less pronounced at later time points (Fig. 1B). The reduction in strain softening paralleled a decrease in the ratio of the loss modulus to the storage modulus (G″/G′; Fig. 1C) with strain, consistent with formation of a more elastic tissue due to increased collagen cross-linking.
Fig. 1.
Mechanical properties of the liver change in early fibrosis. Livers from normal and CCl4-intoxicated rats at days 7, 10, and 14 were analyzed by shear rheometry, with shear storage and loss moduli G′ and G″, respectively, determined over a range of strains. A: liver stiffness (G′) was increased by day 7. Livers demonstrated marked softening with increased strain. For G′ at minimal strain, all values are significantly different from normal: P < 0.01 at day 7, P < 0.001 at day 10, and P < 0.001 at day 14. B: data in A normalized to 1 at low (1%) strain to demonstrate different shapes of strain curves. For G′ at maximum strain, P < 0.01 for normal vs. day 7 and normal vs. day 14. C: increased elasticity of livers after CCl4, as manifested by differences in curves of G″/G′ vs. strain (normalized to 1 at starting strain); n = 3–6 for each time point. *P < 0.05; **P < 0.01; ***P < 0.001 vs. normal.
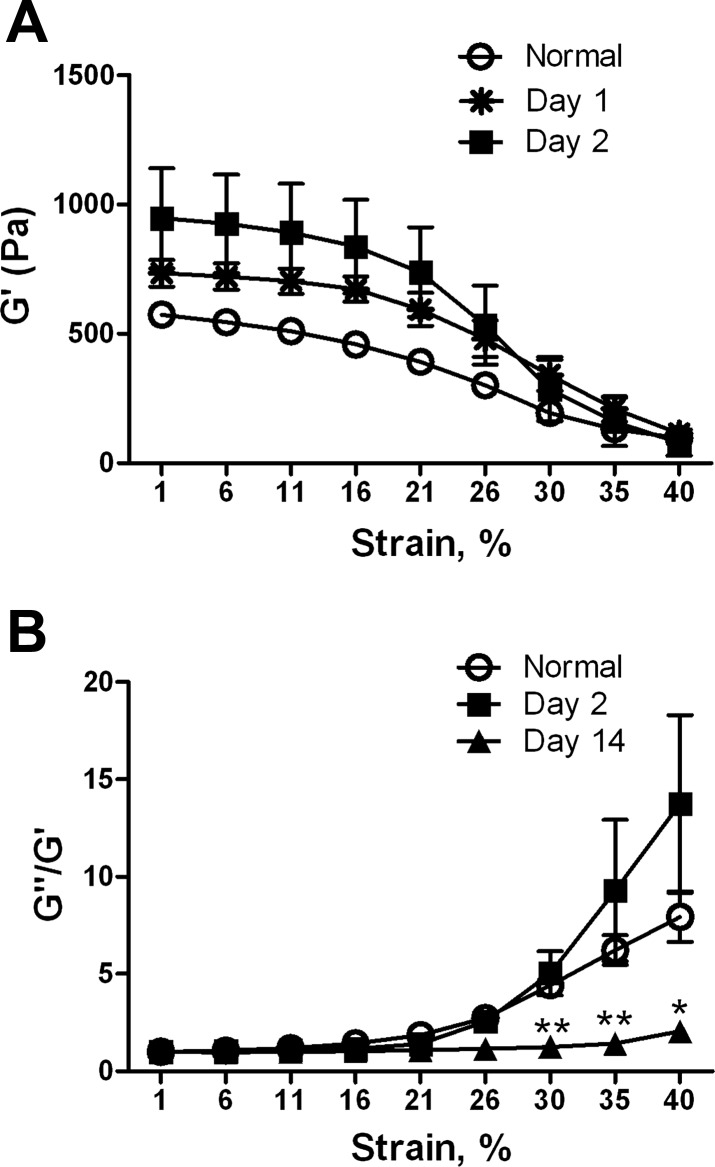
To rule out edema as a cause of the increased stiffness, we examined livers at 1 and 2 days after CCl4 injection, when there is significant edema. G′ at minimal strain was increased, but pronounced strain softening persisted (Fig. 2A), and the shape of the G″/G′ vs. strain curve was similar to that of normal, as opposed to early fibrotic, liver (day 14; Fig. 2B). Thus the strain dependence of the shear modulus and G″/G′ enabled us to differentiate edematous from fibrotic liver and suggested that matrix cross-linking, rather than edema, was the cause of the significant mechanical changes we observed.
Fig. 2.
Mechanical properties of edematous liver. Livers were removed from rats 1 or 2 days after CCl4 injection, when edema is pronounced. A: liver stiffness (G′) is increased compared with normal, although strain softening is unchanged. By 1-way ANOVA there is no difference between the 3 curves. At low strain, days 1 and 2 are significantly different from normal (P < 0.05). B: G″/G′ vs. strain demonstrates that, despite increased stiffness, edematous livers can be distinguished from livers with early fibrosis (day 14). Normalized curves are shown; n = 3–5 for each time point. Similar results were obtained with day 1 livers (not shown). For G″/G′ at 40% strain, normal vs. day 2, no significant difference; normal vs. day 14, significant at strains >30%. *P < 0.05; **P < 0.01.
Increased collagen cross-linking is seen early after injury and correlates with stiffness.
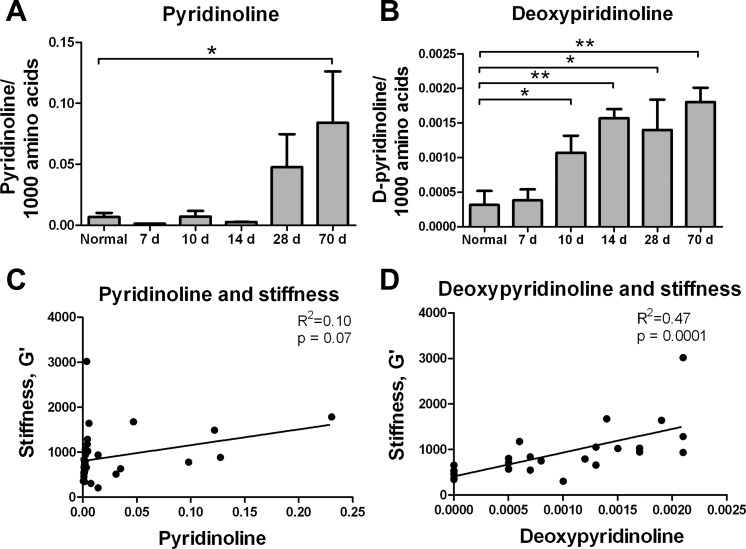
To investigate whether altered mechanics are associated with collagen cross-linking, we measured the LOX-mediated collagen cross-links pyridinoline and deoxypyridinoline directly in livers from rats treated with CCl4 for up to 70 days. Formation of these trivalent, mature, nonreducible cross-links involves two hydroxylysine aldehyde (Hylald) residues in the telopeptides and one Hyl (in the case of pyridinoline) or lysine (deoxypyridinoline) residue in the helical domain of collagen (39). We found that the content of pyridinoline increased significantly at 70 days (Fig. 3A), while the content of deoxypyridinoline was significantly elevated by 10 days and continued to increase with time (Fig. 3B). There was no significant correlation between pyridinoline and whole liver stiffness (Fig. 3C); however, deoxypyridinoline cross-links were significantly correlated with liver stiffness (r2 = 0.47, P = 0.0001; Fig. 3D). Thus, LOX-mediated cross-linking increased early after injury with CCl4, in parallel with mechanical changes suggestive of increased cross-linking.
Fig. 3.
Lysyl oxidase (LOX)-mediated cross-links increase early after CCl4-mediated injury. A and B: mature LOX-mediated collagen cross-links [pyridinoline and deoxypyridinoline (D-pyridinoline)] in livers from rats with CCl4-induced fibrosis and normal, noninjected controls. Deoxypyridinoline cross-links were increased as early as 10 days. Values are means ± SD; n = 3–6 per time point. *P < 0.05; **P < 0.01. C and D: correlation between whole liver shear modulus (G′) and pyridinoline and deoxypyridinoline.
BDL livers also demonstrate increased stiffness at early points.
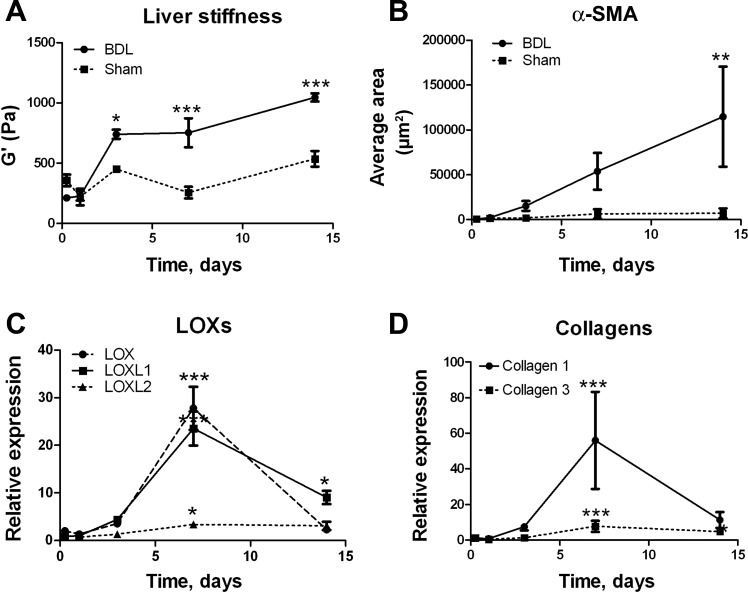
Rat livers after BDL also showed increases in stiffness early after injury, preceding significant increases in collagen expression (Fig. 4, A, B, and D). Although LOX inhibition experiments are difficult in this model because of biliary excretion of BAPN, LOX, LOXL1, and LOXL2 mRNA expression increased significantly after injury (Fig. 4C). Stiffness continues to increase after the peak of LOX mRNA expression, likely reflecting protein expression and maturation of collagen cross-links, as well as (at later points) deposition of new matrix. Thus two models of liver fibrosis in rats show early mechanical changes consistent with increased collagen cross-linking.
Fig. 4.
Liver stiffness and LOX family expression increase rapidly after bile duct ligation (BDL). Rats underwent BDL or sham surgery, and livers were removed at days 0–14. A: G′ was measured by rheometry. B: α-smooth muscle actin (α-SMA) expression was assessed by immunostaining. *P < 0.05; **P < 0.01; ***P < 0.005 vs. sham. C and D: expression of LOXs and collagens in liver lysates determined by real-time PCR; n = 5–6 for all data points. For each individual experiment, values were normalized to 18S expression; values shown in graphs were normalized to those for samples from sham-operated animals from the same time point. *P < 0.05; ***P < 0.005 vs. 6 h after surgery.
LOXs are produced primarily by hepatic stellate cells and portal fibroblasts in healthy and acutely injured liver.
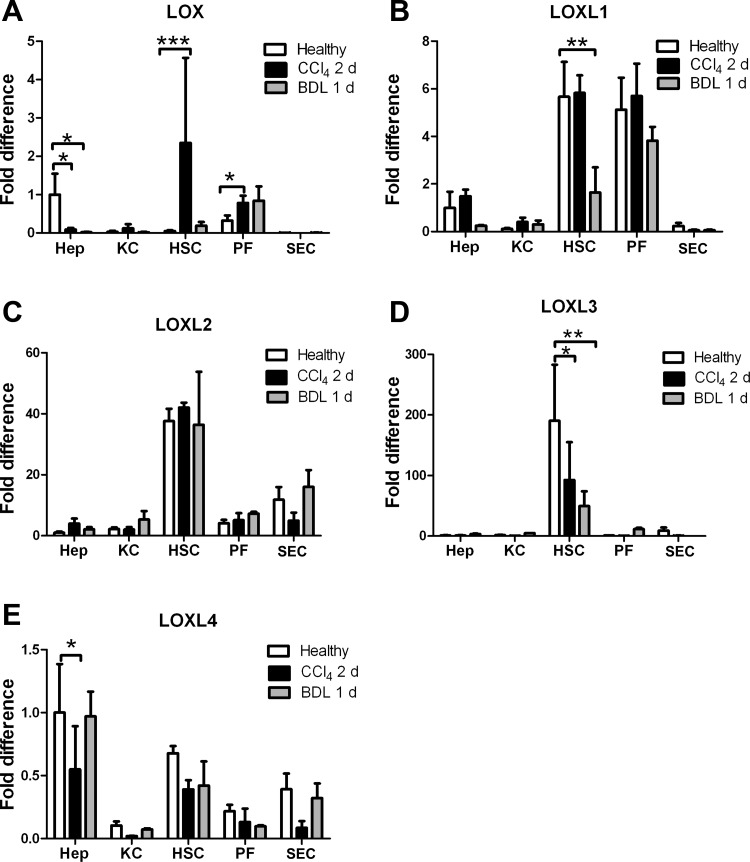
Whole liver analyses have shown that LOX expression increases after liver injury (7, 32). To identify the source of LOXs, we determined ECM expression in five primary cell types from normal livers: portal fibroblasts, hepatic stellate cells, hepatocytes, Kupffer cells, and SEC. We lysed cells immediately after isolation, without intervening culture, thus avoiding culture artifacts. We found that hepatic stellate cells and portal fibroblasts are the major sources of LOXL1, LOXL2, and LOXL3 (Fig. 5, B–D, compare open bars). LOXL4 is widely expressed, and LOX in the normal liver is expressed primarily by hepatocytes and portal fibroblasts (Fig. 5, A and E). Cholangiocytes demonstrated minimal expression of any of the LOX family members compared with normal portal fibroblasts (data not shown).
Fig. 5.
LOX is significantly upregulated in hepatic stellate cells immediately after injury. Primary hepatocytes (Hep), Kupffer cells (KC), hepatic stellate cells (HSC), portal fibroblasts (PF), and sinusoidal endothelial cells (SEC) were isolated from livers of healthy rats, 2 days after CCl4 injection, and 1 day after BDL. Cells were lysed for mRNA isolation immediately, and real-time PCR was carried out to determine expression of all 5 LOX family members: LOX, LOXL1, LOXL2, LOXL3, and LOXL4. All are expressed; however, hepatic stellate cells and, to a lesser extent, portal fibroblasts are the major producers of LOXs in normal liver and shortly after injury. *P < 0.05; **P < 0.01; ***P < 0.005.
To determine whether increases in LOX family expression precede whole liver mechanical changes, we examined primary cells isolated at early time points after liver injury induced by BDL (1 day) or CCl4 intoxication (2 days) (Fig. 5). We observed again that expression was limited to hepatic stellate cells and portal fibroblasts, except for LOXL4, which was expressed by all cells except Kupffer cells (Fig. 5E). The only significant increase in expression at these time points was in LOX expression by hepatic stellate cells after CCl4 injury (Fig. 5A).
LOX is the major isoform upregulated in early CCl4-mediated fibrosis.
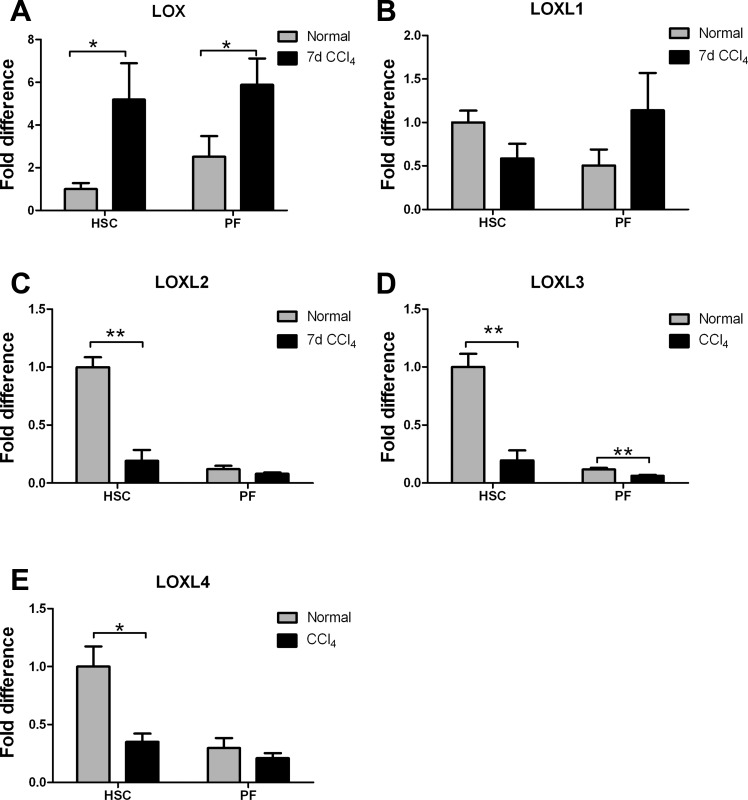
Further evaluation of LOX family expression in hepatic stellate cells and portal fibroblasts freshly isolated from livers 7 days after initiation of CCl4 treatment (2 injections) showed that LOX continues to be the major isoform increased at this time point and that its mRNA is increased in both cell types (Fig. 6A). Other isoforms, with the exception of LOXL1, which increased slightly in portal fibroblasts (P = 0.125), showed decreased mRNA expression (Fig. 6, B–E). Thus, while the liver expresses all five LOX family members even in normal liver, LOX (and potentially LOXL1) is the isoform that increases very early after injury.
Fig. 6.
mRNA expression of LOX, but not other family LOX members, is upregulated in hepatic stellate cells and portal fibroblasts after injury. Cells were isolated from rat livers 7 days after initiation of CCl4 treatment (after a total of 2 injections, on days 1 and 4) and analyzed by real-time PCR for LOX family expression. Only LOX was significantly increased at this time point. *P < 0.05; **P < 0.01.
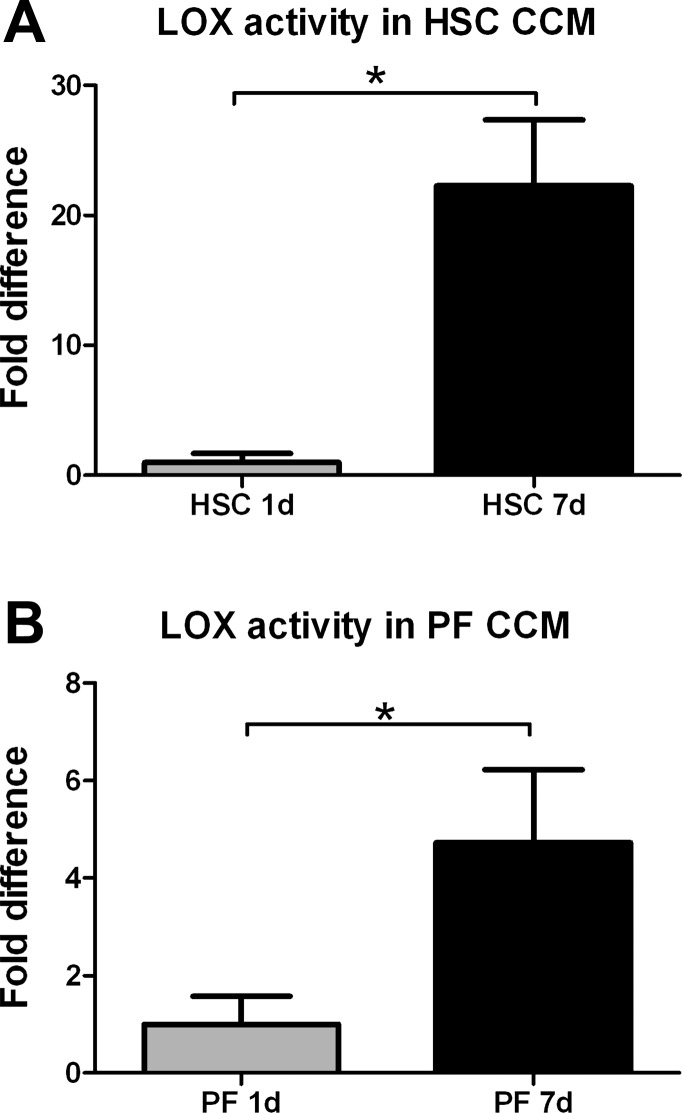
Secretion of active LOXs increases as hepatic stellate cells and portal fibroblasts differentiate in culture.
Hepatic stellate cells and portal fibroblasts undergo differentiation to myofibroblasts in culture. Analysis of LOX family enzymatic activity in these cells demonstrated measurable activity from quiescent cells, with significant increases as the cells became myofibroblasts (Fig. 7). Activity in hepatic stellate cells increased an average of 22.2-fold after 7 days in culture, while activity in portal fibroblasts increased 4.7-fold.
Fig. 7.
Hepatic stellate cells and portal fibroblasts increase secretion of active LOX family members during myofibroblastic differentiation. Primary cells were placed in culture for up to 7 days. Conditioned media (CCM) were examined by a LOX family activity assay at 1 and 7 days after isolation. *P < 0.05.
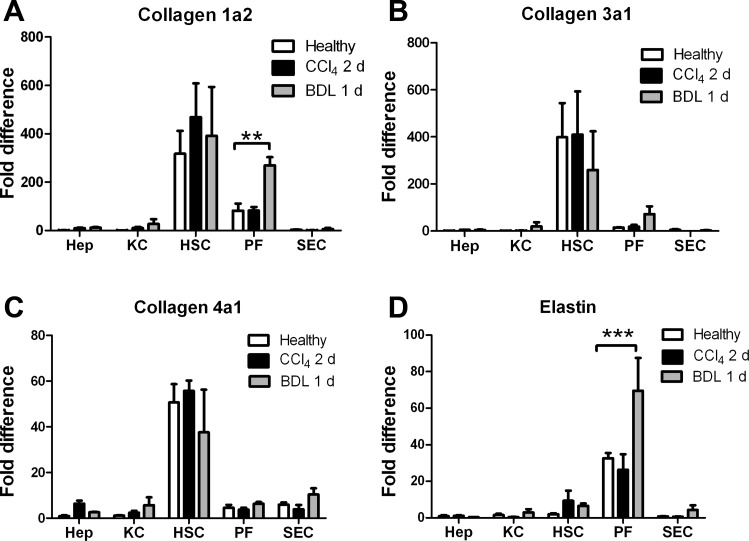
Collagens I, III, and IV are expressed in normal and early-injured liver by hepatic stellate cells and portal fibroblasts.
The LOXs initiate the process of cross-linking, making newly deposited collagens and elastin insoluble. To identify the sources of fibrillar collagen in the liver at baseline, we determined ECM expression in freshly isolated primary cells from normal livers. We found that hepatic stellate cells and, to a lesser extent, portal fibroblasts produce measurable amounts of mRNA for collagens I, III, and IV in normal liver (Fig. 8). Although there is a small amount of collagen IV mRNA expression by hepatocytes, SEC, and Kupffer cells, there is no production of the fibrillar collagens I and III by any cells other than hepatic stellate cells and portal fibroblasts.
Fig. 8.
Hepatic stellate cells and portal fibroblasts produce collagens and elastin in normal and acutely injured liver. mRNA isolated as described in Fig. 5 legend was analyzed by real-time PCR to determine expression of collagen chains (A–C) or elastin (D). Data represent cells from ≥3 independent isolations per cell type, with 3 technical repeats per isolation. **P < 0.01; ***P < 0.005.
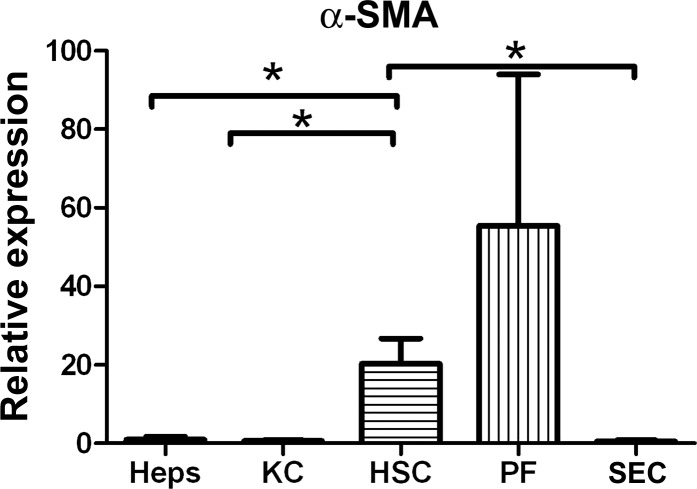
We examined expression of these ECM proteins early after injury in representative models of biliary and parenchymal fibrosis. With the exception of an increase in the α2-chain of collagen I by portal fibroblasts after BDL, collagen mRNA expression remains stable early after CCl4-induced injury (2 days) or BDL (1 day) (Fig. 8, A–C). Elastin, which has been used as a marker of portal fibroblasts in vitro (19), was expressed primarily by portal fibroblasts in normal liver but also, to a small extent, by hepatic stellate cells in injured liver, and its expression by portal fibroblasts was significantly increased after BDL (Fig. 8D). Thus the cells responsible for pathological ECM deposition in fibrosis also express collagens in normal liver and in injured, but nonfibrotic, liver. Interestingly, mRNA expression of the myofibroblast marker α-SMA in hepatic stellate cells and portal fibroblasts was observed at baseline, suggesting that there is a small population of myofibroblasts (or primed myofibroblast precursors) in normal liver (Fig. 9).
Fig. 9.
Hepatic stellate cells and portal fibroblasts from normal liver express α-SMA. mRNA was prepared as described in Fig. 5 legend and analyzed by real-time PCR for expression of α-SMA. *P < 0.05.
DISCUSSION
We demonstrate here that 1) normal livers undergo pronounced strain softening, and this, as well as G″/G′ (reflecting the relationship between viscosity and elasticity in a sample), changes markedly within days of liver injury; 2) LOX family-mediated pyridinoline and deoxypyridinoline collagen cross-links increase with injury, and deoxypyridinoline collagen cross-links, which rise at early time points, are significantly correlated with liver stiffness; 3) LOX and, to a lesser extent, LOXL1 are the major LOX family members with increased expression in early injury; and 4) hepatic stellate cells and portal fibroblasts are the major cellular sources of fibrillar collagens and LOXs in the normal liver and early after injury. These findings have important implications: 1) mechanical changes are due to collagen cross-linking and may mediate major changes in the behavior of cells of the liver at early time points after injury; 2) hepatic stellate cells and portal fibroblasts, which become the major collagen-secreting cells in the fibrotic liver, are “primed” for this function through their matrix-secreting roles in normal liver; and 3) hepatic stellate cells and portal fibroblasts, by virtue of their early expression of LOXs, may have an important role in early injury distinct from their role as matrix-producing cells.
Our findings that liver stiffness increases early after injury in the CCl4 and BDL models (12) (Fig. 4) and that hepatic stellate cells and portal fibroblasts require increased mechanical tension to differentiate to myofibroblasts (19, 25) suggest a model whereby increased stiffness shortly after injury drives the myofibroblastic and then the fibrotic response. Potential causes of increased liver stiffness include changes in the matrix (cross-linking or increased matrix deposition) and tension due to edema or vascular pressure. We demonstrate here that edema can be differentiated from other causes of increased liver stiffness and that the altered mechanics of the liver at day 7 and later are not consistent with edema. The reduction in strain softening, as well as flattening of the G″/G′ vs. strain curves, after injury is most consistent with increased matrix cross-linking. Notably, most studies of stiffness and cell phenotype, including our previous work with stellate cells and portal fibroblasts, have focused on G′ (or the closely related Young's modulus E). The implications of strain softening and G″/G′ on cell behavior are not well understood but may add another layer of complexity to the role of mechanics in disease pathophysiology.
We previously demonstrated that inhibition of LOX family enzymatic activity resulted in blunting of the liver stiffness response early after injury and decreased numbers of myofibroblasts (12); others have also reported decreased fibrosis in animal models after treatment with LOX family inhibitors (2, 9, 10). Critical to our model is that cross-linking occurs before the development of significant fibrosis. We therefore examined LOX family and collagen expression within a small window of time. Our data suggest that small perturbations in expression of LOX itself [which likely occur in response to increased transforming growth factor (TGF)-β] occur at early time points after injury. Surprisingly, given the success of an anti-LOXL2 antibody in preventing CCl4-induced fibrosis in rodents, we did not observe significant changes in LOXL2 at the time points we studied (2); however, it may increase at later time points after injury and play an important role in fibrosis progression, rather than initiation. While early fibrosis represents the process of injury repair and is protective (3), later fibrosis could be considered a pathological process and may involve different subsets of ECM proteins (which undoubtedly contribute to increased stiffness) and ECM-modifying enzymes.
Our data show that, at early points after the initiation of CCl4 intoxication, pyridinoline and deoxypyridinoline cross-links begin to increase. This is consistent with findings from cirrhotic human livers, when both types of cross-link are increased (4). These are trivalent, stable cross-links derived from the immature divalent cross-links (39). In the current study, because of the limited amounts of material available, we were unable to measure the precursor aldehydes or immature cross-links that would likely have been increased at earlier time points. Liver stiffness is increased by day 7 (Fig. 1A), while deoxypyridinoline does not increase until day 10 (Fig. 3B), an incongruity likely secondary to immature cross-links that we were unable to measure. We found a significant correlation between deoxypyridinoline, but not pyridinoline cross-links and liver stiffness (G′), in the subset of livers for which we had stiffness data. To our knowledge, the relative mechanical impacts of the different forms of cross-links in liver (mature vs. immature and deoxypyridinoline vs. pyridinoline) have not been investigated. Similarly, it is not known what caused a preferential increase in deoxypyridinoline compared with pyridinoline in the early stages of fibrosis. This could be due to the differential expression of specific lysyl hydroxylase isoforms (28, 37). More comprehensive analyses for collagen cross-linking/aldehydes and lysine hydroxylation are warranted to confirm the alteration in cross-linking pattern and to elucidate its molecular mechanisms. We did not evaluate elastin cross-linking, but this may play an important role in liver mechanics and fibrosis, particularly in biliary disease. A better understanding of the gene expression pattern of different LOX and lysyl hydroxylase isoforms and the consequent collagen cross-links and lysine modifications (39) could be important in developing antifibrotic therapies aimed at liver mechanics. The LOXs may also have functions unrelated to collagen and elastin cross-linking, including gene regulation, modification of receptor function (13, 21), and modulation of growth factors such as fibroblast growth factor (18) and TGF-β (1); these functions may be important in hepatocytes and other cell types that demonstrated decreases in LOX family mRNA expression after injury (Fig. 5).
It would be advantageous for the liver to have the ability to mount a rapid response after injury. Recently published work showed that hepatic stellate cells in the normal liver are heterogeneous and that a subpopulation of these cells express myofibroblast markers, apparently primed to initiate repair processes in the context of liver injury (6). We observed α-SMA expression in hepatic stellate cells and portal fibroblasts in normal liver (Fig. 9). It is tempting to speculate that portal fibroblast and hepatic stellate cell subsets expressing α-SMA serve as “rapid responders” and that they are also responsible for the LOX and collagen mRNA expression we observed in the noninjured liver, although this requires further evaluation, since our studies did not involve single cell analyses. All the LOXs are upregulated in response to TGF-β (31) (data not shown), which is one of the earliest growth factors to increase after injury; TGF-β-mediated increases in LOX production in early injury, combined with ongoing deposition of small amounts of collagen, could result in modest regional changes in stiffness, which we have observed in rodent livers as early as 6 days after initiation of CCl4 treatment (16). Recent reports suggest that small increases in collagen stiffness can be amplified by cells, suggesting that they could be significant in driving the progression of fibrosis (38).
Although the cellular source of collagen in fibrosis was at one time the source of significant debate, definitive studies demonstrated that hepatic stellate cells (as opposed to hepatocytes) were the major fibrogenic population (22–24). (These studies were carried out before portal fibroblasts were routinely isolated.) Using RNase protection assays to evaluate mRNA expression in isolated cells, Maher and McGuire (22) found that, at 5 days after BDL or 14 days after initiation of CCl4 treatment, hepatic stellate cells were the major source of collagens I and III, while SECs were a minor source. Our work extends these results by showing that portal fibroblasts have collagen expression patterns similar to those of hepatic stellate cells. Additionally, we have demonstrated that portal fibroblasts and stellate cells are involved in homeostasis of the fibrillar collagens in the normal liver. Unlike Maher and McGuire, we did not observe significant matrix production by SEC, potentially because we examined cells from livers early after CCl4-induced injury and BDL; SEC are likely to be a significant source of at least collagen IV after injury. Although we did not examine cholangiocytes directly, it was recently reported that these cells do not produce collagen I in animal models of fibrosis (5, 30).
In summary, we have identified hepatic stellate cells and portal fibroblasts as sources of matrix proteins and their cross-linking agents in normal liver, and we suggest a model in which the liver is thereby primed to respond quickly to injury, activating potential feed-forward mechanisms. LOX and, potentially, LOXL1 may be targets for antifibrotic therapies, particularly in early disease.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK-058123 and American Recovery and Reinvestment Act supplement to R. G. Wells and National Institutes of Health Grants R21 AR-060978 to M. Yamauchi and R01 GM-083272 to P. A. Janmey. A. Y. Wang was supported by National Institute of Diabetes and Digestive and Kidney Diseases Institutional Training Grant T32 DK-07066.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.P., M.T., A.Y.W., P.C.G., P.A.J., and M.Y. performed the experiments; M.P., M.T., A.Y.W., P.C.G., P.A.J., M.Y., and R.G.W. analyzed the data; M.P. prepared the figures; M.P. and R.G.W. drafted the manuscript; M.P., M.T., A.Y.W., P.C.G., P.A.J., M.Y., and R.G.W. approved the final version of the manuscript; M.T., A.Y.W., P.A.J., M.Y., and R.G.W. interpreted the results of the experiments; R.G.W. is responsible for conception and design of the research; R.G.W. edited and revised the manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge the assistance of the University of Pennsylvania/National Institute of Diabetes and Digestive and Kidney Diseases Center for Molecular Studies in Digestive and Liver Diseases (Grant P30 DK-50306) Molecular Biology Core. We thank Jia-Ji Hui for assistance with BDL and LiKang Chin for assistance with rheometry.
REFERENCES
- 1. Atsawasuwan P, Mochida Y, Katafuchi M, Kaku M, Fong KS, Csiszar K, Yamauchi M. Lysyl oxidase binds transforming growth factor-β and regulates its signaling via amine oxidase activity. J Biol Chem 283: 34229–34240, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, Mikels A, Vaysberg M, Ghermazien H, Wai C, Garcia CA, Velayo AC, Jorgensen B, Biermann D, Tsai D, Green J, Zaffryar-Eilot S, Holzer A, Ogg S, Thai D, Neufeld G, Van Vlasselaer P, Smith V. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med 16: 1009–1017, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Bourbonnais E, Raymond VA, Ethier C, Nguyen BN, El-Leil MS, Meloche S, Bilodeau M. Liver fibrosis protects mice from acute hepatocellular injury. Gastroenterology 142: 130–139, 2012 [DOI] [PubMed] [Google Scholar]
- 4. Brenner DA, Waterboer T, Choi SK, Lindquist JN, Stefanovic B, Burchardt E, Yamauchi M, Gillan A, Rippe RA. New aspects of hepatic fibrosis. J Hepatol 32 Suppl: 32–38, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Chu AS, Diaz R, Hui JJ, Yanger K, Zong Y, Alpini G, Stanger BZ, Wells RG. Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology 53: 1685–1695, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. D'Ambrosio DN, Walewski JL, Clugston RD, Berk PD, Rippe RA, Blaner WS. Distinct populations of hepatic stellate cells in the mouse liver have different capacities for retinoid and lipid storage. PLos One 6: e24993, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Desmouliere A, Darby I, Costa AM, Raccurt M, Tuchweber B, Sommer P, Gabbiani G. Extracellular matrix deposition, lysyl oxidase expression, and myofibroblastic differentiation during the initial stages of cholestatic fibrosis in the rat. Lab Invest 76: 765–778, 1997 [PubMed] [Google Scholar]
- 8. Elbjeirami WM, Yonter EO, Starcher BC, West JL. Enhancing mechanical properties of tissue-engineered constructs via lysyl oxidase crosslinking activity. J Biomed Mater Res A 66: 513–521, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Fiume L. Inhibition by aminoacetonitrile of early lesions induced in the liver of rats by carbon tetrachloride. J Pathol Bacteriol 83: 291–293, 1962 [DOI] [PubMed] [Google Scholar]
- 10. Fiume L, Favilli G. Inhibition of experimental cirrhosis by carbon tetrachloride following treatment with aminoacetonitrile. Nature 189: 71–72, 1961 [DOI] [PubMed] [Google Scholar]
- 11. Froh M, Thurman RG, Wheeler MD. Molecular evidence for a glycine-gated chloride channel in macrophages and leukocytes. Am J Physiol Gastrointest Liver Physiol 283: G856–G863, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Georges PC, Hui JJ, Gombos Z, McCormick ME, Wang AY, Uemura M, Mick R, Janmey PA, Furth EE, Wells RG. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol 293: G1147–G1154, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Giampuzzi M, Botti G, Di Duca M, Arata L, Ghiggeri G, Gusmano R, Ravazzolo R, Di Donato A. Lysyl oxidase activates the transcription activity of human collagene III promoter. Possible involvement of Ku antigen. J Biol Chem 275: 36341–36349, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Hinz B, Gabbiani G. Cell-matrix and cell-cell contacts of myofibroblasts: role in connective tissue remodeling. Thromb Haemost 90: 993–1002, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Huang D, Chang TR, Aggarwal A, Lee RC, Ehrlich HP. Mechanisms and dynamics of mechanical strengthening in ligament-equivalent fibroblast-populated collagen matrices. Ann Biomed Eng 21: 289–305, 1993 [DOI] [PubMed] [Google Scholar]
- 16. Levental I, Levental KR, Klein EA, Assoian R, Miller RT, Wells RG, Janmey PA. A simple indentation device for measuring micrometer-scale tissue stiffness. J Phys Condens Matter 22: 194120, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139: 891–906, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li W, Nugent MA, Zhao Y, Chau AN, Li SJ, Chou IN, Liu G, Kagan HM. Lysyl oxidase oxidizes basic fibroblast growth factor and inactivates its mitogenic potential. J Cell Biochem 88: 152–164, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Li Z, Dranoff JA, Chan EP, Uemura M, Sevigny J, Wells RG. Transforming growth factor-β and substrate stiffness regulate portal fibroblast activation in culture. Hepatology 46: 1246–1256, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Lopez B, Gonzalez A, Hermida N, Valencia F, de Teresa E, Diez J. Role of lysyl oxidase in myocardial fibrosis: from basic science to clinical aspects. Am J Physiol Heart Circ Physiol 299: H1–H19, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Lucero HA, Ravid K, Grimsby JL, Rich CB, DiCamillo SJ, Maki JM, Myllyharju J, Kagan HM. Lysyl oxidase oxidizes cell membrane proteins and enhances the chemotactic response of vascular smooth muscle cells. J Biol Chem 283: 24103–24117, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maher JJ, McGuire RF. Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. J Clin Invest 86: 1641–1648, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Milani S, Herbst H, Schuppan D, Hahn EG, Stein H. In situ hybridization for procollagen types I, III and IV mRNA in normal and fibrotic rat liver: evidence for predominant expression in nonparenchymal liver cells. Hepatology 10: 84–92, 1989 [DOI] [PubMed] [Google Scholar]
- 24. Nakatsukasa H, Nagy P, Evarts RP, Hsia CC, Marsden E, Thorgeirsson SS. Cellular distribution of transforming growth factor-β1 and procollagen types I, III, and IV transcripts in carbon tetrachloride-induced rat liver fibrosis. J Clin Invest 85: 1833–1843, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Olsen AL, Bloomer SA, Chan EP, Gaca MD, Georges PC, Sackey B, Uemura M, Janmey PA, Wells RG. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol Gastrointest Liver Physiol 301: G110–G118, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Palamakumbura AH, Trackman PC. A fluorometric assay for detection of lysyl oxidase enzyme activity in biological samples. Anal Biochem 300: 245–251, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Popov Y, Sverdlov DY, Sharma AK, Bhaskar KR, Li S, Freitag TL, Lee J, Dieterich W, Melino G, Schuppan D. Tissue transglutaminase does not affect fibrotic matrix stability or regression of liver fibrosis in mice. Gastroenterology 140: 1642–1652, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pornprasertsuk S, Duarte WR, Mochida Y, Yamauchi M. Lysyl hydroxylase-2b directs collagen cross-linking pathways in MC3T3-E1 cells. J Bone Miner Res 19: 1349–1355, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Rosenbloom J, Abrams WR, Mecham R. Extracellular matrix 4: the elastic fiber. FASEB J 7: 1208–1218, 1993 [PubMed] [Google Scholar]
- 30. Scholten D, Osterreicher CH, Scholten A, Iwaisako K, Gu G, Brenner DA, Kisseleva T. Genetic labeling does not detect epithelial-to-mesenchymal transition of cholangiocytes in liver fibrosis in mice. Gastroenterology 139: 987–998, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sethi A, Mao W, Wordinger RJ, Clark AF. Transforming growth factor-β induces extracellular matrix protein cross-linking lysyl oxidase (LOX) genes in human trabecular meshwork cells. Invest Ophthalmol Vis Sci 52: 5240–5250, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Siegel RC, Chen KH, Greenspan JS, Aguiar JM. Biochemical and immunochemical study of lysyl oxidase in experimental hepatic fibrosis in the rat. Proc Natl Acad Sci USA 75: 2945–2949, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smedsrod B, Pertoft H. Preparation of pure hepatocytes and reticuloendothelial cells in high yield from a single rat liver by means of Percoll centrifugation and selective adherence. J Leukoc Biol 38: 213–230, 1985 [DOI] [PubMed] [Google Scholar]
- 34. Smith-Mungo LI, Kagan HM. Lysyl oxidase: properties, regulation and multiple functions in biology. Matrix Biol 16: 387–398, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Thomassin L, Werneck CC, Broekelmann TJ, Gleyzal C, Hornstra IK, Mecham RP, Sommer P. The Pro-regions of lysyl oxidase and lysyl oxidase-like 1 are required for deposition onto elastic fibers. J Biol Chem 280: 42848–42855, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Uemura M, Swenson ES, Gaca MD, Giordano FJ, Reiss M, Wells RG. Smad2 and Smad3 play different roles in rat hepatic stellate cell function and α-smooth muscle actin organization. Mol Biol Cell 16: 4214–4224, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Uzawa K, Yeowell HN, Yamamoto K, Mochida Y, Tanzawa H, Yamauchi M. Lysine hydroxylation of collagen in a fibroblast cell culture system. Biochem Biophys Res Commun 305: 484–487, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Winer JP, Oake S, Janmey PA. Non-linear elasticity of extracellular matrices enables contractile cells to communicate local position and orientation. PLos One 4: e6382, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yamauchi M, Sricholpech M. Lysine post-translational modifications of collagen. Essays Biochem 52: 113–133, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yamauchi M, Shiiba M. Lysine hydroxylation and cross-linking of collagen. Methods Mol Biol 446: 95–108, 2008 [DOI] [PubMed] [Google Scholar]