Abstract
Protein posttranslational processing is a cellular mechanism fundamental to the generation of bioactive peptides, including the anorectic α-melanocyte-stimulating hormone (α-MSH) and thyrotropin-releasing hormone (TRH) peptides produced in the hypothalamic arcuate (ARC) and paraventricular (PVN) nuclei, respectively. Neuropeptide Y (NPY) promotes positive energy balance in part by suppressing α-MSH and TRH. The mechanism by which NPY regulates α-MSH output, however, is not well understood. Our results reveal that NPY inhibited the posttranslational processing of α-MSH's inactive precursor proopiomelanocortin (POMC) by decreasing the prohormone convertase-2 (PC2). We also found that early growth response protein-1 (Egr-1) and NPY-Y1 receptors mediated the NPY-induced decrease in PC2. NPY given intra-PVN also decreased PC2 in PVN samples, suggesting a reduction in PC2-mediated pro-TRH processing. In addition, NPY attenuated the α-MSH-induced increase in TRH production by two mechanisms. First, NPY decreased α-MSH-induced CREB phosphorylation, which normally enhances TRH transcription. Second, NPY decreased the amount of α-MSH in the PVN. Collectively, these results underscore the significance of the interaction between NPY and α-MSH in the central regulation of energy balance and indicate that posttranslational processing is a mechanism that plays a specific role in this interaction.
Keywords: α-melanocyte-stimulating hormone, early growth response protein-1, neuropeptide Y, proopiomelanocortin, thyrotropin-releasing hormone
obesity is a major health and socioeconomic concern that has reached epidemic proportions in developed nations. Despite efforts to abate the problem, cases of obesity continue to rise. Thus, it is imperative to better understand the physiological mechanisms controlling food intake and body weight.
Neuropeptide Y (NPY) is a potent orexigen that has received attention as an antiobesity drug target (69). NPY is produced both centrally and peripherally. Although it is widely distributed throughout the brain, in situ hybridization studies reveal that NPY is most densely localized to the hypothalamic arcuate nucleus (ARC) (19, 41). Hypothalamic NPY plays an important role in energy balance and responds to changes in energy status. For example, NPY increases during food deprivation and returns to baseline levels following refeeding in the hypothalamic ARC and paraventricular nucleus (PVN) (1, 2, 29). Hypothalamic NPY also responds to changes in energy status signals, as it decreases with elevated leptin or insulin levels and increases with elevated ghrelin, growth hormone, or glucocorticoid levels (69). In addition, rodent models of genetic obesity including the fa/fa Zucker rat as well as db/db and ob/ob mice demonstrate increased hypothalamic NPY levels (69). Likewise, NPY mRNA levels are higher in the hypothalamus of rats made obese by eating a high-fat diet for 22 wk compared with their lean controls (24). Moreover, individuals kept on high-fat diet that were resistant to becoming obese had lower NPY mRNA levels than obese individuals, indicating that hypothalamic NPY contributes to the diet-induced obese condition (24). Collectively, these studies demonstrate the significance of hypothalamic NPY in regulating energy homeostasis and body weight.
A recent study revealed the importance of ARC NPY in regulating energy balance, as moderate manipulation of NPY specifically in the ARC altered food intake and promoted positive energy balance (64). NPY neurons within the ARC innervate hypothalamic neurons involved in energy balance control, including cells in the PVN that produce the anorectic thyrotropin-releasing hormone (TRH) as well as cells within the ARC that produce the anorectic α-melanocyte-stimulating hormone (α-MSH) (3, 6, 8, 9). Evidence suggests that NPY inhibits the release of α-MSH from the ARC (8, 18, 57). However, it remains unknown whether NPY regulates the production of α-MSH. To generate α-MSH, its prohormone POMC must undergo a series of proteolytic cleavages catalyzed by the enzymes prohormone convertase-1 [PC1, also referred to as PC3 (63)] and prohormone convertase-2 (PC2), followed by further modification via carboxypeptidase E (CPE) and N-acetyltransferase (11, 44, 67). Because of this maturation process, it is critical to know whether NPY regulates α-MSH peptide content rather than to focus only on changes at the level of POMC transcription.
One way that α-MSH exerts its anorexigenic actions is by binding melanocortin receptors on TRH neurons in the PVN, causing the phosphorylation of cAMP response element-binding protein (CREB), thereby increasing prepro-TRH expression (15, 21, 46, 59). TRH is essential for energy balance because it regulates the hypothalamic-pituitary-thyroid (HPT) axis that controls whole body metabolism (22, 33). There are several mechanisms by which NPY may regulate TRH. For example, NPY is known to decrease prepro-TRH mRNA (14, 15) by inhibiting α-MSH-induced CREB activation (59). It is also possible that NPY indirectly regulates TRH by reducing the synthesis and release of ARC α-MSH. Another possibility is that NPY regulates pro-TRH processing, which occurs by the cleavage of paired basic residues catalyzed primarily by PC1 and to a lesser extent by PC2 (10, 17, 60). CPE/carboxypeptidase D (CPD) then removes the basic residue(s) (23, 47), followed by amidation via peptidylglycine α-amidating monooxygenase (PAM) to produce TRH (12, 50).
The preswent study investigates the regulation of two critical energy-regulating peptides, TRH and α-MSH, by NPY. We focus particularly on the regulation of peptide content by measuring TRH and α-MSH using RIA because it is the level of the active peptide that is most biologically relevant. This is especially important when studying peptide hormones, as most peptide hormones are initially synthesized as large inactive precursors that are posttranslationally processed into smaller bioactive products, stored in secretory vesicles, and released when the cell receives specific stimuli (61). Prohormone processing is an important but understudied mechanism that is essential not only in rodents, but also in humans (44, 45, 65, 67). For example, several landmark studies demonstrate that the abnormal processing of prohormones such as POMC causes obesity in humans. Obesity has been reported in patients with a mutation in PC1 (25) as well as in a patient with a defect in POMC processing (5). Studies from mice show similar results: mutations in PC1 (37) or CPE (42) or the deletion of the PC1 and PC2 transcription factor NhLH2 (28) all cause obesity. Overall, evidence indicates that prohormone processing is important in humans and rodents, especially in terms of energy balance. In addition, studies demonstrate that POMC processing is regulated by leptin and insulin (67), but it is not known whether NPY regulates either POMC or proTRH processing.
We chose the rat model for the present studies for several reasons. This laboratory has extensively characterized the Sprague-Dawley rat model for energy balance studies as well as gene and peptide analyses, including POMC and pro-TRH processing. The rat provides a larger source of material for peptide analysis than mice. Moreover, the POMC sequence in the mouse differs from that of the rat, making it difficult to extrapolate a mouse model to the rat unless POMC processing in the mouse is described. Also, studies from this lab using the Sprague-Dawley rat model have demonstrated that, in the PVN and ARC, leptin regulates the prohormone convertases that play a major role in pro-TRH and POMC processing (52, 58). The current study extends these finding to determine whether NPY also regulates pro-TRH and POMC processing enzymes. The ultimate goal is to uncover mechanisms governing energy balance so that we may in the future target these mechanisms for the production of antiobesity drugs. Therefore, another reason that we use the Sprague-Dawley rat model is that this is considered the best model to represent obesity caused by overeating in humans (7). Rats present many characteristics similar to humans in terms of genetic susceptibility to diet-induced obesity, making them an excellent model for studying the central control of energy regulation (36). In addition, we recently characterized hormonal and physiological characteristics of the Sprague-Dawley rat model of diet-induced obesity (49). Thus, future studies will build off the present study and determine how NPY regulates TRH and α-MSH in the diet-induced obesity state.
Our results demonstrate that NPY reduces the conversion of POMC to α-MSH via attenuation of PC2 mediated by the transcription factor early growth response protein-1 (Egr-1). Furthermore, centrally infused NPY decreased the amount of α-MSH projected/released into the PVN, along with PVN TRH peptide content. We also found that NPY decreased PC2 in the PVN, which may reduce pro-TRH processing. Our results indicate a specific mechanism by which NPY regulates α-MSH, which in turn has implications for TRH regulation.
MATERIALS AND METHODS
Animals.
Male Sprague-Dawley rats (200–235 g) from Harlan Laboratories (Wilmington, MA) were housed in cages under standard environmental conditions with rat chow and water ad libitum. All experimental protocols were approved by the Institutional Animal Care and Use Committee of Rhode Island Hospital/Brown University. Animals were anesthetized with intraperitoneal (ip) injections of 50 mg/kg ketamine and 0.25 mg/kg Domitor. An intracerebroventricular (icv) 21-gauge stainless steel guide cannula (Plastic One, VA) was implanted according to coordinates obtained from the Paxinos and Watson atlas (anteroposterior: −0.8 mm, lateral: −1.2 mm, ventral: −3.6 mm). Guide cannulas were assessed for correct placement by monitoring water intake upon icv administration of angiotensin II (40 ng/rat; Sigma, St. Louis, MO). For intra-PVN cannulations, coordinates were anteroposterior: −1.9 mm, lateral: 0.5 mm, and ventral: −7.3 mm (43). Placement was verified by India ink test. Since ip anesthesia could cause stress and increase hypothalamic pCREB levels, the animals were jugular vein catheterized the day before the experiment to ensure rapid and stress-free anesthesia.
Intracerebroventricular infusions.
Infusions were performed icv on free-moving animals with a 30-gauge needle that extended 0.5 mm below the guide cannula. The injection tip was connected by polyethylene tubing to a 25-μl Hamilton syringe, which was secured into a microprocessor-controlled infusion pump (Bioanalytical Systems) set to inject 100 μl/min. A dose-response experiment was first performed to determine the optimal NPY dose, wherein we used 0 (control), 1, 5, and 10 μg of human NPY (Sigma, St. Louis, MO; n = 3) in 5 μl of artificial cerebrospinal fluid (aCSF; 140 mM NaCl, 3.35 mM KCl, 1.15 mM MgCl2, 1.26 mM CaCl2, 1.2 mM Na2HPO4, and 0.3 mM NaH2PO4, pH 7.4), and the control group received 5 μl of aCSF alone. After 60 min, ARC/median eminence (ME) and PVN samples were collected. Given that the 5 μg of NPY resulted in the greatest change in peptides (ACTH and α-MSH), further experiments were preformed wherein fed rats received either 5 μg human NPY (Sigma) in 5 μl of aCSF or 5 μl of aCSF as controls. Animals were euthanized 60 min postinfusion, and the ARC/ME and PVN samples were collected for peptide or protein analyses. For the immunohistochemistry (IHC) experiments, three different treatments were used (n = 9). The first group received 5 μl of aCSF, the second group received 5 μg of Melanotan II (MTII, Sigma) in 5 μl of aCSF, and the third group received 5 μg of NPY followed by 5 μg of MTII 10 min later. Whole body perfusions were performed 30 min following the final infusion.
Perfusions for brain fixation.
The perfusion procedure is described in Refs. 39, 49. Briefly, animals were deeply anesthetized with pentobarbital sodium (50 mg/kg) through the jugular catheter 3 min prior to perfusion. A 25-g syringe tip attached by tubing to an infusion pump was inserted into the left ventricle. The circulation was flushed with saline (6 ml/min) for 2 min and then with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 45 min. Following perfusion, the hypothalamic section of the brain was collected and cryoprotected in 20% sucrose solution. The hypothalamic blocks were then frozen in TFM freezing medium (TBS, Durham, NC) and cut into 25-μm-thick coronal sections on a sliding cryostat. The PVN was stored in 0.02% sodium azide containing PBS at 4°C.
Identification of pCREB in TRH neurons by double IHC.
The IHC procedure was performed as described (49). Briefly, pro-TRH-pCREB double IHC staining, PVN tissue was washed with PBS and blocked for 1 h in PBS solution containing 3% normal donkey serum, 0.25% Triton X-100, and 0.02% sodium azide. Sections were incubated for 72 h at 4°C with monoclonal mouse anti-pCREB (anti-P-Ser133, 1:500; Cell Signaling, Beverly, MA) and polyclonal rabbit anti-pro-TRH (anti-PYE17, 1:3,000), developed and characterized in our laboratory (e.g., 51) in blocking solution. The sections were washed with PBS and then incubated for 2 h with a combination of donkey anti-mouse Alexa fluor 488 (green) and donkey anti-rabbit Alexa fluor 594 (red) secondary antibodies (each 1:1,000; Molecular Probes, Eugene, OR). Sections were then washed and mounted onto slides and coverslipped using mounting medium with DAPI (blue; Vector Laboratories, Burlingame, CA). Images were obtained with a Nikon E800 microscope (Nikon, Melville, NY), and a Spot II digital camera (Diagnostic Instruments, Sterling Heights MI) and the software program MagnaFire (Indigo Scientific UK) were used for visualization. Light intensity was adjusted to prevent oversaturation, and photos were taken in monochrome. pCREB and pro-TRH fluorescence photographs were combined using the RGB channels of Adobe Photoshop (Adobe, San Jose, CA) to allow for visualization of double-labeled cells. Confocal images (for pCREB and pro-TRH staining) were acquired with a Nikon PCM 2000 (Nikon) using the argon (488 nm) laser and green helium-neon (543 nm) laser. Serial optical sections were performed with Simple 32 C-imaging computer software (Compix, Tualilatin, OR). Z series sections were collected at 0.7 μm with ×40 Plan Apo lens or ×10 Plan Apo lens. A scan zoom of ×1 was used in the acquisition of images, which were then processed and reconstructed in NIH Image shareware (NIH, Springfield, VT). Adobe Photoshop was used for the image assembly.
Sections doubly labeled for Pro-TRH/pCREB were used for quantitative analysis. Pro-TRH immunostaining (red fluorescence) was confined to perikarya and dendrites, allowing for easy detection of nuclear pCREB (green fluorescence) or the leptin signaling molecule pSTAT3 (brown chromatic) by staining. The percentage of pro-TRH neurons containing a pCREB-labeled nucleus was determined for each treated animal. For each brain section analyzed, the number of doubly labeled neurons (Pro-TRH and pCREB) on one side of the third ventricle was counted. That number was then expressed as a percentage of the total pro-TRH-labeled neurons in that region. The percentages for individual sections from each treatment group were then averaged. All nuclei with positive staining were counted in the pro-TRH neurons of the PVN with visible DAPI-positive nuclei on each side of the third ventricle. That relationship was expressed as a percentage, which represents positive pro-TRH neurons in each section of the PVN compared with the total number of DAPI-positive pro-TRH neurons observed. More than 100 fields per brain section and per animal were counted to ensure statistical relevance to the study.
ARC and PVN sample collection and preparation.
PVN (Bregma −1.3 to −2.3 mm) and ARC (Bregma −2.5 to −3.5 mm) samples were microdissected and frozen immediately in liquid nitrogen and then placed in −80°C (35). Samples were subjected to peptide extraction with 2 N acetic acid supplemented with a protease inhibitor cocktail to measure peptides by specific RIA or protein extraction with RIPA buffer (50 mM Tris·HCl, pH 7.4, 150 mM NaCl, 0.5% sodium deoxycholate, 0.1% SDS, 1% NP-40) supplemented with protease inhibitor cocktail for Western blot analysis. All Western blot analyses and RIA were performed on ARC or PVN samples taken from individual animals. Samples were not pooled. For intra-PVN samples only, the portion of PVN injected was collected and used for analyses.
Radioimmunoassay.
RIA analysis of ACTH and α-MSH is described previously by our laboratory (52). Briefly, the α-MSH RIA was performed using an anti-α-MSH antiserum (1:20,000), developed in-house and 5,000 cpm of 125I desacetyl α-MSH tracer. The sensitivity of the assays was ∼11.5 pg/tube, and the intra- and interassay variabilities were 5–6 and 9–12%, respectively. The α-MSH antibody used in this condition can detect 100% of desacetyl α-MSH but does not cross-react with the free acid nonamidated form of α-MSH, ACTH, or CLIP. The ACTH RIA was performed using our in-house anti-ACTH antiserum (1:30,000) and 5,000 cpm of 125I-ACTH tracer. The sensitivity of the assays was ∼10.0 pg/tube, and the intra- and inter-assay variabilities were ∼5–7 and 10–11%, respectively. The ACTH antibody used in this assay does not cross react with any form of α-MSH. It is important to note that the ACTH antibody does cross-react with the POMC precursor, which was demonstrated using purified POMC (kindly donated by Dr. Anne White from the University of Manchester). However, since the prohormone broke down considerably, we could not determine the percentage of cross reactivity.
Western immunoblotting.
Protein samples were separated on 8% acrylamide gel and transferred onto a PVDF membrane. Membranes were washed in PBS with 0.1% Tween 20 (PBS-T) and then blocked with 5% BSA for 60 min. Membranes were then probed with a primary antibody overnight at 4°C. Primary antibodies included PC1 and PC2 (1:10,000 dilution, a gift from Dr. Nabil Seidah). Other antibodies were from Santa Cruz Biotechnology: Egr-1 (1:500 dilution, sc-110), CPE (1:1,000, sc-136252), prolylcarboxypeptidase (PRCP, 1:500, sc-49272), and β-actin (1:10,000) as a loading control. PC1, PC2, and Egr-1 antibodies have been characterized in previous studies (40, 58). The following day, the membranes were washed in PBS-T (3 times for 5 min) and incubated in the appropriate secondary antibody for 60 min. Membranes were then incubated with enhanced chemiluminescence buffer for 1 min and visualized using the Alpha Innotech imaging system (ProteinSimple). Band density was analyzed using ImageJ (National Institutes of Health).
In vitro studies.
For studies determining the mechanisms by which NPY decreases POMC processing, we used the mouse N43-5 hypothalamic POMC-positive cell line (CELLutions Biosystems). These cells were ideal because they contain POMC as well as the melanocortin receptor (MC4R), PC1, PC2, and CPE. We also used a POMC-positive corticotropic AtT20 cell line transfected with PC2 to measure both PC2 and α-MSH release. N43-5 and AtT20 cells were each grown in DMEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 10 μg/ml streptomycin. For protein analyses, cells adhered overnight in six-well plates. Depending on the experiment, cells were transiently transfected with a CMV plasmid containing PC2 cDNA (a gift from Dr. Nabil Seidah), Egr-1 cDNA (Addgene), or NPY-Y1R and NPY-Y5R cDNA (OriGene). All transfections were performed using lipofectamine 2000 (Invitrogen) following the manufacturer's instructions. Briefly, cells were incubated with cDNA (4 μg)-lipofectamine complex for 6 h in serum-free medium. The medium was changed to DMEM for overnight incubation. Dose-response curves indicated that 100 μM NPY yielded the greatest change in both PC2 promoter activity and protein levels. Thus, 24 h after transfection, cells were treated with NPY (100 μM) or vehicle control. For cAMP control experiments, untransfected serum-starved (6 h) N43-5 cells were treated for 90 min with the cAMP activators MTII (50 μM), forskolin (10 μM), or 8-Br-cAMP (100 μM) and compared with a PBS control. For all experiments, cells were collected using HEPES-KOH buffer (20 mM HEPES, 125 mM NaCl, 1 mM EDTA, 20 mM NaF, 0.1% Triton X, 0.1% NP-40).
For luciferase assays, N43-5 cells adhered overnight in 96-well plates. Using lipofectamine 2000, cells were transfected with the human PC2-luciferase promoter construct. Specifically, we used the DNA from the human PC2 promoter (789 bp to −1 bp relative to the translation initiation codon) inserted in the pGL2-Basic vector (27). Cells were incubated with both PC2 promoter (0.15 μg) and a renilla control (pRL-SV40 0.15 μg, Promega) for 6 h in antibiotic-free, serum-free medium. The medium was later changed to complete DMEM for overnight incubation. Cells were then treated with NPY (100 μM) or vehicle control. For cAMP control experiments, cells were treated with PBS control or the cAMP activators MTII (50 μM), forskolin (10 μM), or 8-Br-cAMP (100 μM) for 90 min. Luminescence of luciferase and renilla was determined using the Dual-Glo luciferase assay system (Promega), and luminescence was read with the TopCount NXT microplate luminescence counter (PerkinElmer).
Statistical analysis.
The results for each treatment are presented as mean ± SE. A two-tailed t-test was used to analyze differences between two groups. An analysis of variance (ANOVA), followed by Tukey's HSD post hoc test, was used to analyze differences between more than two groups. A Shapiro-Wilk W goodness-of-fit test was used to test for normal distributions, and Levene's test (two-group comparisons) or Bartlett's test (comparisons between more than two groups) was used to test for homogenous variances in all variables. For variables with nonnormal distributions and unequal variances, a nonparametric Mann Whitney U-test or Kruskal-Wallis test was used. A Welch's correction was used for comparisons with unequal variances but normal distribution of the data. Differences were considered to be significant at P < 0.05. Prizm (version 4.0b; GraphPad Software, La Jolla, CA). Statistics for each comparison are presented in figure legends.
RESULTS
NPY decreases α-MSH maturation and production.
Previous results have demonstrated that NPY diminishes the release of the anorectic α-MSH (3, 6, 8, 9), yet the effect of NPY on α-MSH production remained unknown. We investigated whether NPY altered POMC levels as well as the processing of POMC to α-MSH. We began by infusing NPY icv in rats and measuring POMC by Western blot and α-MSH by RIA. In a dose-response experiment, we found that NPY significantly decreased POMC (Fig. 1A) and α-MSH (Fig. 1B). Using the 5-μg dose that significantly decreased both POMC and α-MSH, we found that 1 h was the optimal time for NPY to reduce the active α-MSH levels (Fig. 1C). Furthermore, rats given 5 μg of NPY icv for 1 h eat significantly more food than vehicle controls (Fig. 1D).
Fig. 1.
NPY given icv reduced ARC POMC and α-MSH levels. Male rats were icv infused with NPY, and ARC samples were collected for Western blot (protein content) or RIA (peptide content) analyses. See text for definitions. A and B: dose-response. A: POMC protein levels (n = 4; ANOVA, P = 0.002). B: α-MSH peptide content (n = 4; Kruskal-Wallis test, P = 0.005). C: time-response. α-MSH peptide content (n = 4; Kruskal-Wallis test, P = 0.04). D: food intake (g) was measured in animals 60 min after icv infusion with NPY (5 μg) or vehicle control, which were the optimal conditions for changes in α-MSH. Food intake 60 min postinfusion was significantly greater in NPY-treated rats (control n = 9 and NPY n = 10; Mann-Whitney U-test, P = 0.0015). Data are means ± SE. Tukey's HSD test was used for ANOVA post hoc analyses, and Dunn's test was used for Kruskal-Wallis post hoc analyses. Data are means ± SE. *P < 0.05, **P < 0.01 vs. control.
To examine the effect of NPY on POMC-derived peptides, we then measured ARC ACTH and α-MSH using specific RIAs of rats given 5 μg of NPY icv for 1 h and found that both ACTH (Fig. 2B) and α-MSH (Fig. 2C) levels were lower than vehicle controls. However, the magnitude of the decrease in α-MSH (P < 0.0096) was greater than the decrease in ACTH (P = 0.037) or POMC (P = 0.034). These data suggest a change in POMC processing (Fig. 2A), specifically a reduction in the conversion of ACTH to α-MSH. Thus, we further analyzed the extent of NPY-induced attenuation in ACTH and α-MSH. We calculated an 18.8% reduction in the molar ratio of α-MSH to ACTH in NPY-treated rats compared with their controls, indicating that the processing of ACTH to α-MSH is diminished with NPY treatment (Table 1).
Fig. 2.
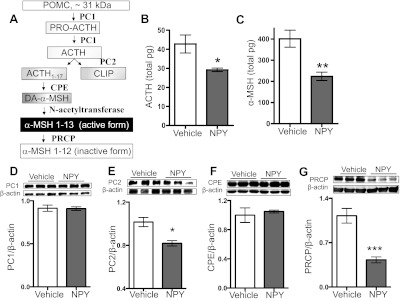
NPY administration reduced POMC-derived peptides and POMC processing enzyme PC2 levels. See text for definitions. A: simplified model of the POMC processing cascade is depicted and has been described in the pituitary and the hypothalamic ARC. B and C: ACTH and α-MSH were measured in the same ARC samples collected 60 min after icv infusion of NPY (5 μg) or vehicle control. B: ACTH peptide content (control n = 6, NPY n = 4; t-test with Welch's correction, P = 0.0369). C: α-MSH peptide content (control n = 6, NPY n = 4; t-test, P = 0.0096). These results were repeated with the same response in a separate experiment, n = 9/group. D: PC1 protein levels (control n = 6, NPY n = 5; t-test, P = 0.92). E: PC2 protein levels (control n = 6, NPY n = 4; t-test, P = 0.034). These results were repeated with the same response in 2 independent experiments, n = 4/group/experiment. F: CPE protein levels (control n = 6, NPY n = 5; t-test, P = 0.60). G: PRCP protein levels (control n = 5, NPY n = 4; t-test, P = 0.0015). Data are means ± SE. *P < 0.05, **P < 0.01 vs. control.
Table 1.
RIA analyses of ACTH and α-MSH from the hypothalamic arcuate nucleus of animals icv infused with NPY
| ACTH | %Change ACTH | α-MSH | %Change α-MSH | Molar Ratio* | %α-MSH:ACTH | |
|---|---|---|---|---|---|---|
| No treatment | 14.0 ± 1.55 (6) | 247.0 ± 24.74 (6) | 17.64 | |||
| NPY | 9.6 ± 0.30 (4) | 31.4 | 137.6 ± 12.05 (4) | 44.3 | 14.33 | |
| %Decrease in pmol/mg ratio | 18.8 |
aData are represented as means ± SE for each peptide as fmol/mg protein.
Molar ratio is the molar ratio between α-MSH and ACTH.
We then measured the protein level of POMC processing enzymes. PC2 protein levels significantly decreased in the ARC of NPY-treated rats (Fig. 2E), whereas the other POMC processing enzymes PC1 and CPE (Fig. 2, D and F) levels were similar to controls'. Interestingly, the α-MSH degrading enzyme PRCP decreased with NPY infusion (Fig. 2G). These results suggest that the NPY attenuation of POMC processing is specific to PC2.
Egr-1 and NPY-Y1R mediate the NPY-induced decrease in PC2.
Because the α-MSH and PC2 response to NPY in the ARC occurred relatively quickly (60 min), we also wanted to explore whether Egr-1 regulated these changes. It has been demonstrated that Egr-1 has CRE elements in its promoter and that α-MSH activates Egr-1 through a cAMP-dependent mechanism (31, 56). Thus, NPY downregulation of cAMP may decrease Egr-1. In addition, PC2 contains two Egr-1 binding sites in its promoter (27, 44), and the human PC2 promoter is activated by Egr-1 (27). This pathway is illustrated in Fig. 7. We began by showing that icv NPY infusion decreased ARC Egr-1 protein levels (Fig. 3A). We then moved to an in vitro system for more mechanistic studies.
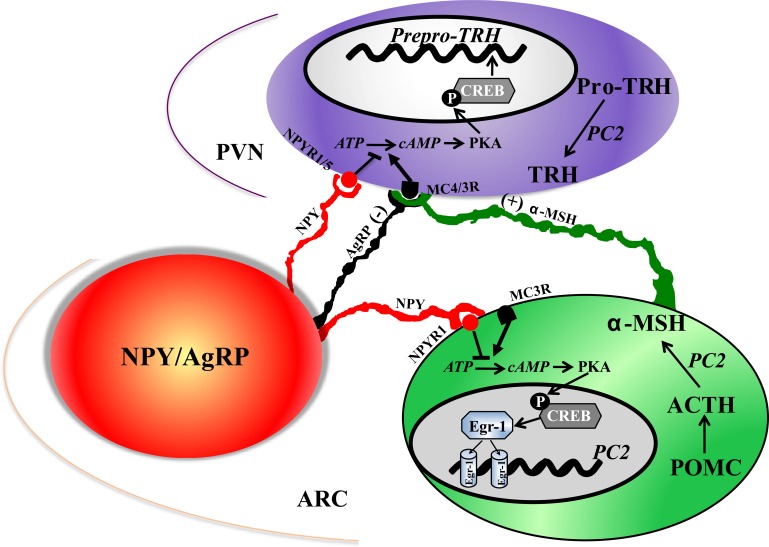
Fig. 7.
Schema of proposed pathways in which NPY regulates α-MSH and TRH. We focus on NPY-producing neurons in the ARC, although NPY derived from other sources could have these effects. This figure summarizes, in a very simplified manner, the various mechanisms that have been suggested to mediate the effects of NPY. Our data support the hypothesis that NPY regulates TRH by direct and indirect pathways. NPY regulates TRH directly by binding to Y1/Y5 receptor(s) on TRH neurons in the PVN causing a decrease in pCREB levels, thereby decreasing pro-TRH transcription, and NPY decreased PC2-mediated pro-TRH processing. In the indirect pathway, NPY reduces α-MSH peptide content by altering its maturation and reducing the amount of α-MSH released into the PVN.
Fig. 3.

NPY decreases PC2 and Egr-1. A: Egr-1 protein in the ARC of control and NPY icv-infused rats (control n = 5, NPY n = 4; t-test, P = 0.0297). B–G: experiments were performed using the hypothalamic POMC-positive N43-5 cell line. B: N43-5 cells were transfected with PC2 promoter-luciferase construct along with pRL-renilla SV40. Twenty-four hours later, cells were treated with vehicle control (V) or cAMP activators forskolin (FK10 μM), MTII (50 μM), or 8-Br-cAMP (100 μM) for 1.5 h and then lysed. Values are shown normalized to renilla (n = 3/group ANOVA, P < 0.05). Results were repeated in 2 independent experiments (n = 3/group/experiment). C: PC2 protein levels with and without overexpression of Egr-1 by cDNA transfection (n = 5/group, t-test, P < 0.0001). Results were repeated in 2 independent experiments (n = 3/group/experiment). D: Egr-1 protein levels with and without NPY (100 μM; n = 6/group, t-test, P < 0.0337). Results were repeated in an independent experiment (n = 3/group). E: PC2 promoter activity (normalized to renilla) in cells treated with 100 μM NPY for 0, 30, 60, and 90 min (n = 6/group, ANOVA, P = 0.0004). Results were repeated in an independent experiment (n = 3/group). F: PC2 protein levels at 0, 30, 60, and 90 min post-NPY treatment (100 μM) in N43-5 cells transfected with PC2, NPY-Y1 receptor (NPY-Y1R), and Egr-1 (n = 4/group, ANOVA, P = 0.0042). Results were repeated in an independent experiment (n = 3/group). G: PC2 protein levels at 0, 30, 60, and 90 min post-NPY treatment (100 μM) in N43-5 cells transfected with PC2 and NPY-Y1R but not Egr-1 (n = 4/group, Kruskal-Wallis test, P = 0.067). Results were repeated in an independent experiment (n = 3/group). Tukey's test was used for all post hoc analyses. See text for definitions. Data are means ± SE. *P < 0.05, **P < 0.01 vs. control.
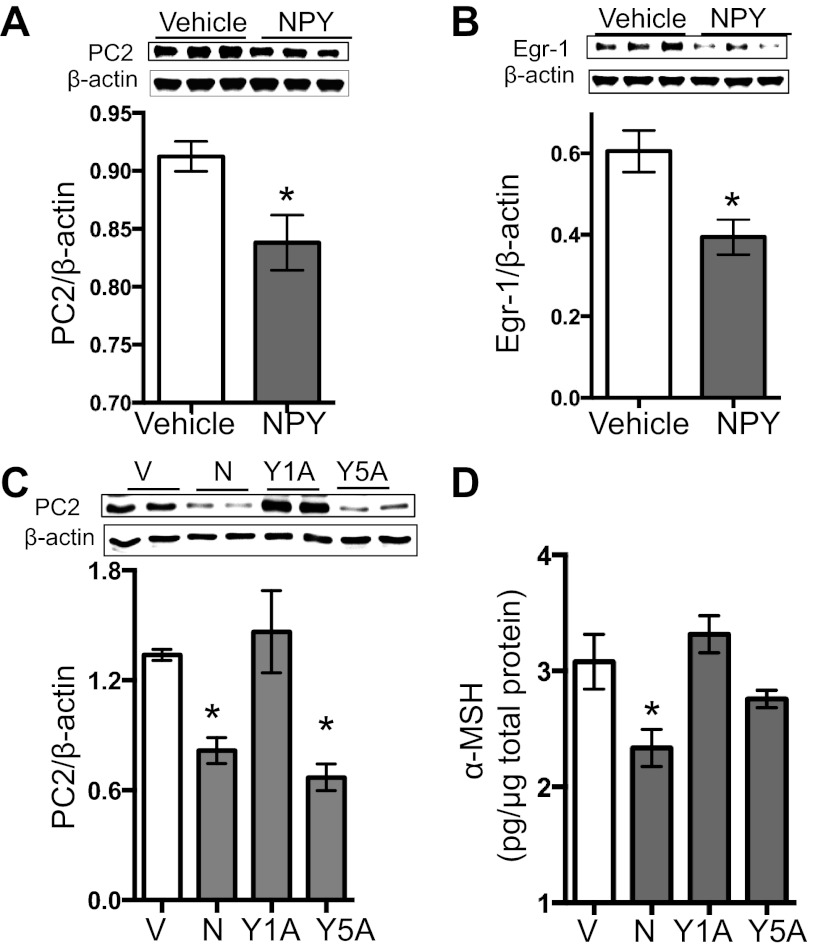
Because ARC samples contain both POMC and NPY/AgRP neurons, each expressing PC2, we wanted to investigate changes in PC2 explicitly in POMC neurons. Therefore, we used N43-5 cells, which are derived from an immortalized POMC neuronal cell line. Western blot analysis of N43-5 cells indicated a band at the expected molecular weight for Egr-1, NPY-Y1, and NPY-Y5 receptors, but expression was low (data not shown). Consequently, we overexpressed Egr-1, NPY-Y1, and NPY-Y5 receptors for our experiments. We first demonstrated that activating cAMP levels with forskolin, MTII, or 8-Br-cAMP elevated PC2 promoter activity (Fig. 3B); thus, the cAMP/PKA pathway regulates PC2. We then supported the finding that Egr-1 regulates PC2 (27), as overexpression of Egr-1 augmented PC2 protein levels (Fig. 3C). We further demonstrated that NPY reduced Egr-1 protein 30 min post-administration (Fig. 3D).
Following these control experiments, we showed that NPY significantly decreased PC2 promoter activity 30 and 60 min post-NPY administration (P = 0.0004; Fig. 3E). Furthermore, we found that NPY significantly decreased PC2 protein levels 60 and 90 min after treatment (P < 0.0001; Fig. 3F). However, this effect was lost when we did not overexpress Egr-1 (Fig. 3G). Although there was a trend toward reduced PC2 at the 90 min time point, the overall PC2 reduction in response to NPY was not statistically significant when Egr-1 was not transfected (P > 0.05; Fig. 3G). To demonstrate that the effect of NPY on PC2 is not isolated to N43-5 cells, we repeated these experiments in POMC-positive corticotropic AtT20 cells transfected with PC2 and found a parallel decrease in PC2 and Egr-1 protein levels with NPY treatment (Fig. 4, A and B). Taken together, these data indicate that NPY reduces α-MSH, at least in part, by reducing the POMC processing enzyme PC2, which is regulated by Egr-1.
Fig. 4.
NPY-induced decrease in PC2 is also observed in POMC-positive AtT20 cells and is mediated by NPY-Y1R. Experiments were conducted using AtT20 cells transfected with PC2, NPY-Y1R, NPY-Y5R, and Egr-1. A: PC2 protein in AtT20 cells treated with or without NPY (100 μM) for 60 min (n = 4/group, t-test, P = 0.0331). Results were repeated in 2 independent experiments (n = 3/group/experiment). B: Egr-1 protein in AtT20 cells treated with or without NPY (100 μM) for 60 min (n = 3/group, t-test, P = 0.030). Results were repeated in an independent experiment (n = 3/group). C and D: AtT20 cells were pretreated with NPY-Y1 antagonist BIBO3304 in DMSO for 10 min (Y1A) or NPY-Y5 antagonist NTNCB in DMSO (Y5A) for 10 min, after which NPY was administered for 60 min; or cell were pretreated with DMSO control for 10 min followed by NPY (N) or vehicle (V) for 60 min. C: PC2 protein levels (n = 5/group, ANOVA, P = 0.0001). D: α-MSH peptide content in medium (n = 4/group, ANOVA, P = 0.0134). Tukey's test was used for all post hoc analyses. See text for definitions. Data are means ± SE. *P < 0.05, **P < 0.01 vs. control.
To determine whether the NPY-Y1 and or NPY-Y5 receptors play a role in regulating NPY's attenuation of PC2, we used BIBO3304 (NPY-Y1-specific antagonist, 100 nM) or NTNCB (NPY-Y5-specific antagonist, 100 nM), each from TOCRIS Bioscience, for 10 min before NPY administration (68). We found that the NPY-Y1R antagonist blocked the NPY-induced decrease in PC2, but the NPY-Y5R antagonist did not block this effect (Fig. 4C). Since we were ultimately interested in how NPY affects α-MSH release, we investigated the effect of NPY on α-MSH release into the medium of AtT20 cells transfected with PC2. We found that NPY reduced the amount of α-MSH peptide release and that the NPY-Y1-specific antagonist blocked this effect (Fig. 4D). These results demonstrate that NPY-Y1R mediates the effect of NPY on α-MSH and PC2.
NPY decreases TRH levels in the PVN.
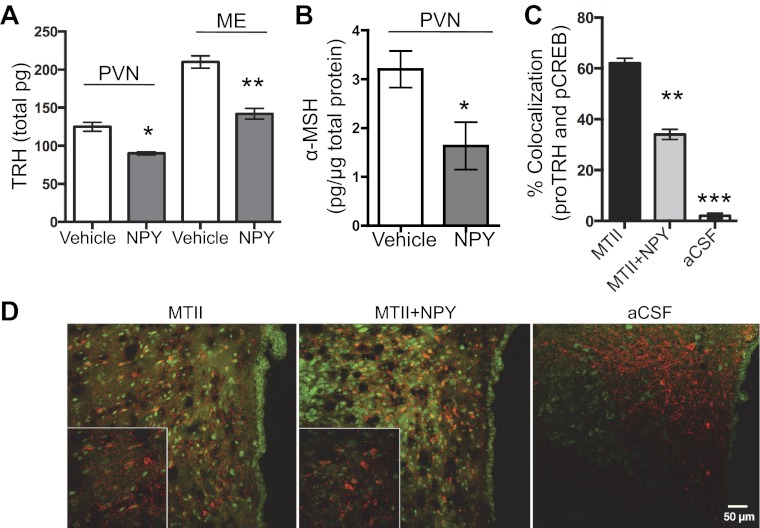
NPY is one of the main inhibitors of prepro-TRH mRNA in the PVN (14, 15). We show here that TRH peptide content in the PVN (30%) and ME (46%) is significantly lower in rats icv-infused with NPY compared with controls (Fig. 5A). One way in which NPY could regulate TRH is by inhibiting the α-MSH-induced increase in TRH production. Our in vivo and in vitro results indicate that NPY significantly reduces α-MSH production and release. Thus, we examined whether NPY affects the amount of α-MSH within the PVN. We observed a significant decrease in α-MSH levels in the PVN of NPY-treated rats (Fig. 5B), suggesting that NPY diminishes the amount of α-MSH available to act on TRH.
Fig. 5.
NPY reduced TRH and α-MSH in the PVN and blocked the MTII increase in pCREB in TRH neurons. A and B: male rats were infused icv with 5 μg NPY, and 60 min later PVN and ME samples were collected for RIA analyses. A: TRH peptide content in the PVN (n = 9/group, t-test, P < 0.05) and ME (n = 9/group, t-test, P < 0.05). Results were repeated for PVN TRH peptide content with the same result (control n = 4, NPY n = 4). B: α-MSH peptide content in the PVN (control n = 8, NPY n = 5, t-test, P = 0.03). C and D: male rats were infused icv with 5 μg NPY and/or 5 μg MTII. Tissue from the PVN was subjected to double IHC for cytoplasmic pro-TRH (red fluorescent) and nuclear pCREB (green fluorescent); 36 fields per section and condition were analyzed. C: colocalization between pro-TRH and pCREB in rats treated with aCSF, MTII, or MTII + NPY (n = 9/group, ANOVA, P < 0.0001). D: representative low and high (bottom left) magnification images are shown for each treatment condition. Rat PVN tissue was treated with aCSF, MTII, or MTII + NPY. Tukey's test was used for all post hoc analyses. See text for definitions. Data are means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control.
Another way in which NPY decreases TRH is by attenuating the α-MSH-induced increase in pCREB (see Fig. 7). Therefore, the percentage of colocalization between pro-TRH and pCREB staining was analyzed following icv injections of the α-MSH analog MTII, NPY + MTII, and aCSF controls. Following icv infusion of MTII, 62 ± 4% of the pro-TRH containing neurons also stained positive for pCREB. Infusion of NPY prior to MTII yielded 34 ± 2% colocalization of pro-TRH/pCREB (Fig. 5, C and D). All together, these results show that NPY effectively counters the MTII activation of the MC4R in TRH neurons, which supports previous findings (59).
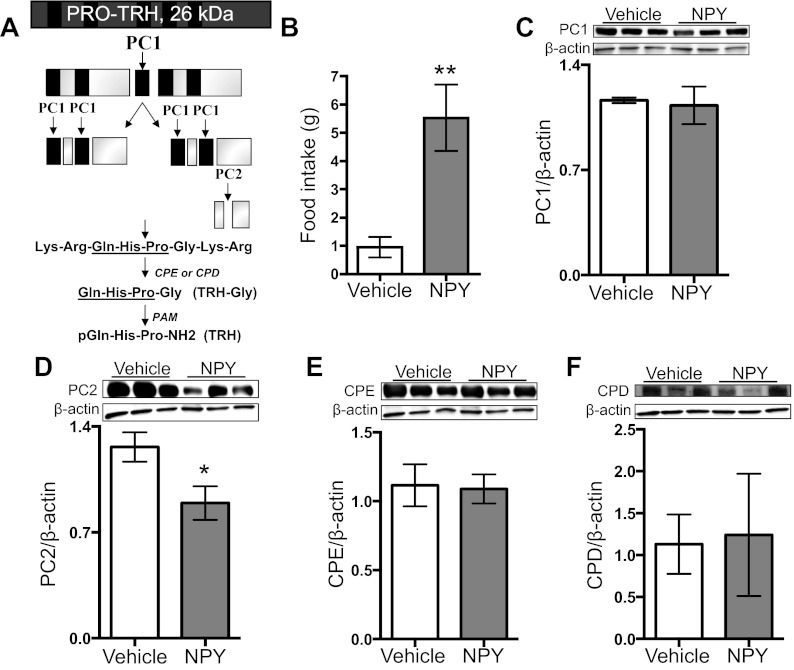
Finally, another way that NPY may reduce TRH is by regulating the processing of its precursor, pro-TRH. Pro-TRH processing has been well characterized by this laboratory and others (reviewed in Ref. 45) and is modeled in Fig. 6A. To address this question, we infused NPY into the PVN and measured pro-TRH processing enzymes. We found that our intra-NPY infusions significantly increased food intake (Fig. 6B). We also found that NPY significantly decreased PC2 in the PVN but did not alter any of the other pro-TRH processing enzymes examined (Fig. 6, C–F).
Fig. 6.
NPY does not alter pro-TRH processing enzymes in the PVN. A: simplified model of the pro-TRH processing cascade is depicted and has been described in the rat PVN. One copy of pro-TRH contains 5 copies of TRH. Black bars represent TRH. B–F: male rats received intra-PVN infusion with 5 μg NPY, and 60 min later, food intake was measured and PVN samples collected for Western blot analyses. B: food intake (g; n = 4; t-test, P = 0.0098) C: PC1 protein levels (n = 4; t-test, P = 0.80). D: PC2 protein levels (n = 4; t-test P = 0.047). E: CPE protein levels (n = 4; t-test, P = 0.78). F: CPD protein levels (n = 4; t-test, P = 0.79). Data are means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control.
DISCUSSION
The current study demonstrates that NPY reduces α-MSH peptide content in the ARC, and one mechanism underlying this regulation is that NPY obstructs the posttranslational maturation of α-MSH. We found that NPY specifically reduced the POMC processing enzyme PC2. This is a fairly rapid process mediated by Egr-1 and NPY-Y1R. We further showed that NPY decreased TRH levels in the PVN and ME, supporting previous results that NPY opposes α-MSH-induced phosphorylation of CREB. Intra-PVN NPY administration decreased PC2 levels, which may reduce the processing of pro-TRH. We also demonstrated that NPY decreases the amount of α-MSH in the PVN, suggesting indirect regulation of TRH. These results indicate that NPY affects TRH levels through multiple mechanisms. In terms of POMC and pro-TRH processing, our results suggest that NPY affects PC2 specifically.
NPY exerts its actions on energy balance in part by regulating other energy-mediating peptides. We show for the first time that NPY reduces POMC levels in the ARC. Our studies further reveal that NPY alters the processing of POMC to the bioactive α-MSH. Our in vivo studies demonstrate that both ACTH and α-MSH peptide content are attenuated with NPY treatment. However, the α-MSH:ACTH ratio decreased by 18.8%, indicating that the conversion of ACTH to α-MSH is also impaired by NPY. Central infusion of α-MSH acutely and robustly decreases food intake (e.g., Refs. 4, 13, 54); therefore, the 19% decrease in POMC processing is likely to have biological implications. Indeed, NPY significantly increased food intake over the time course and dose that we observe NPY affects on POMC, α-MSH, and PC2 levels. Furthermore, evidence suggests that defects in POMC processing have metabolic consequences (55). For example, severe obesity was reported in a patient with defective processing specific to POMC (26), which suggests that the NPY-mediated defect in POMC processing may have metabolic costs.
It is possible that NPY differentially affects the transport and/or degradation rates of ACTH and α-MSH, which will affect the ACTH:α-MSH ratio. We attempted to look at α-MSH clearance by measuring PRCP in the ARC of NPY and control rats (66). Interestingly, we observed a decrease in PRCP with NPY icv treatment. This indicates that ARC PRCP likely does not contribute to the NPY-induced decrease in α-MSH:ACTH, but instead the decrease in PRCP may reduce α-MSH clearance when α-MSH levels are low. We do not know whether PRCP levels or activity change at α-MSH target sites with NPY treatment. It is, however, clear from our in vivo and in vitro results that NPY alters POMC processing via reduction in PC2, the enzyme that catalyzes the conversion of ACTH to α-MSH. For example, PC2 protein levels were significantly reduced in NPY-treated rats, and PC2 protein levels decreased with NPY treatment in the POMC-positive N43-5 and AtT20 cell lines. We also found a trend, albeit not statistically significant, for ARC PC2 mRNA levels to decrease with NPY icv infusion (data not shown). The NPY effect on PC2 mRNA within POMC cells may be diluted by PC2 mRNA levels in ARC non-POMC cells like those producing NPY/AgRP as we see a significant difference in PC2 promoter with NPY in POMC-positive cells (Fig. 3E). Since we did observe a significant decrease in PC2 protein with NPY in rat ARC tissue (Fig. 2E), it is possible that NPY may be affecting PC2 protein via other mechanisms such as through the PC2 chaperone 7B2. This will be explored in future studies.
Since NPY did not did not alter the other POMC processing enzymes PC1 or CPE, the affect of NPY on POMC processing appears to be specific to PC2. In addition, we demonstrated that NPY downregulates Egr-1, which is known to increase PC2 promoter activity (27). We also showed that the NPY-induced decrease in PC2 in vitro required overexpression of Egr-1, indicating that Egr-1 mediates NPY regulation of α-MSH maturation.
Previous studies report that NPY neurons synapse with POMC neurons within the ARC and inhibit their activation (8, 9), thereby inhibiting putative α-MSH release. Using frog melanotroph cells, Galas et al. (18) demonstrated that NPY inhibited α-MSH release and cAMP formation by activating a Y5 receptor coupled to a PTX-insensitive G protein. They also showed that NPY suppressed TRH-induced secretion of α-MSH through a Y1 receptor coupled to a PTX-sensitive G protein-coupled receptor. A subsequent study using mice showed that NPY directly inhibited POMC neurons through NPY-Y1 receptor-mediated activation of G protein-coupled inwardly reflecting K+ (GIRK) channels (57). The hyperpolarization of these neurons, mediated by NPY, inhibits action potential firing, which likely inhibits α-MSH release. The present study shows by RIA that α-MSH release is reduced in AtT20 cells treated with NPY. These results, together with our finding that NPY decreases ARC α-MSH production, suggest that NPY may reduce the amount of α-MSH available to produce effects in target neurons. For example, α-MSH is a main regulator of TRH in the PVN (53, 59). We found that NPY-treated rats had significantly less α-MSH in the PVN than controls, which supports the hypothesis that NPY indirectly regulates TRH via attenuated production and release of α-MSH.
There are six NPY receptor subtypes, although NPY-Y6 is not found in the rat (20). NPY-Y1 and -Y5 have received particular attention because of their ability to increase food intake when specifically stimulated (16). The NPY-Y1 receptor appears to be highly important in feeding, as Y1-specific agonists stimulate the appetite phase of food intake. In contrast, Y5-specific agonists influence meal size and duration of food intake (34). Moreover, Y1, but not Y5, antagonists block the effects of NPY on food intake in rats (30), and fasting-induced food intake was reduced in Y1 but not Y5 knockout mice (38). Also, NPY-Y1 receptors are abundant in the ARC and PVN (32, 48, 62). Our results indicate a role for NPY-Y1R in regulating NPY's effects on α-MSH, as blocking these receptors restored the NPY-induced decrease in both PC2 levels and α-MSH release.
In addition to α-MSH, we also observed a significant reduction in TRH peptide content. When we infused NPY directly into the PVN, PC2 levels in PVN tissue decreased similarly to what we observed in ARC tissue with icv NPY infusions. This decrease in PVN PC2 could lead to less TRH, as PC2 plays a role in pro-TRH processing (44). Moreover, we supported previous findings by Sarkar and Lechan (59) that NPY decreases the activation of CREB by α-MSH in TRH neurons.
In conclusion, we propose a model depicting mechanisms by which NPY regulates both α-MSH and TRH (Fig. 7). NPY directly regulates the production and maturation of α-MSH. It also regulates TRH directly and indirectly: directly by opposing α-MSH activation of CREB signaling in TRH neurons and reducing pro-TRH processing by PC2, and indirectly by reducing the production and release of ARC α-MSH. We focus on ARC-derived NPY because NPY is produced most abundantly in the ARC (19, 41); these neurons innervate the PVN (3) and POMC neurons (6, 8, 9), and ARC NPY has well-documented effects on food intake and body weight (64). NPY derived from other neurons could have the same influence on POMC and TRH, which merits future investigation. Overall, our results illustrate the complex interaction among the energy-regulating neuropeptides NPY, α-MSH, and TRH.
GRANTS
These studies were supported in part by National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health Grant R01 DK-085916-01 to E. A. Nillni. Also in part supported by the Initiative for Maximizing Student Diversity (IMSD), NIH to A. M. Toorie.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.E.C., A.M.T., J.S.S., M.M.S., S.H., M.P., and R.S. performed experiments; N.E.C. and E.A.N. analyzed data; N.E.C. and E.A.N. interpreted results of experiments; N.E.C. and E.A.N. prepared figures; N.E.C. and E.A.N. drafted manuscript; N.E.C. and E.A.N. edited and revised manuscript; E.A.N. conception and design of research; E.A.N. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Virginia Hovanesian of Rhode Island Hospital for help in the acquisition and presentation of the confocal images used in this study. We also thank Dr. Gilad Barnea and Mark Johnson for technical advice.
Current Address of M. Perello: Laboratory of Neurophysiology, Multidisciplinary Institute of Cell Biology (IMBICE-CONICET/CICPBA), Calle 526 s/n entre 10 y 11, La Plata, Buenos Aires, Argentina 1900.
REFERENCES
- 1. Beck B, Jhanwar-Uniyal M, Burlet A, Chapleur-Chateau M, Leibowitz SF, Burlet C. Rapid and localized alterations of neuropeptide Y in discrete hypothalamic nuclei with feeding status. Brain Res 528: 245– 249, 1990 [DOI] [PubMed] [Google Scholar]
- 2. Brady LS, Smith MA, Gold PW, Herkenham M. Altered expression of hypothalamic neuropeptide mRNAs in food-restricted and food-deprived rats. Neuroendocrinology 52: 441– 447, 1990 [DOI] [PubMed] [Google Scholar]
- 3. Broberger C, Visser TJ, Kuhar MJ, Hokfelt T. Neuropeptide Y innervation and neuropeptide-Y-Y1-receptor-expressing neurons in the paraventricular hypothalamic nucleus of the mouse. Neuroendocrinology 70: 295– 305, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Brown KS, Gentry RM, Rowland NE. Central injection in rats of alpha-melanocyte-stimulating hormone analog: effects on food intake and brain Fos. Regul Pept 78: 89– 94, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Challis BG, Pritchard LE, Creemers JW, Delplanque J, Keogh JM, Luan J, Wareham NJ, Yeo GS, Bhattacharyya S, Froguel P, White A, Farooqi IS, O'Rahilly S. A missense mutation disrupting a dibasic prohormone processing site in pro-opiomelanocortin (POMC) increases susceptibility to early-onset obesity through a novel molecular mechanism. Hum Mol Genet 11: 1997– 2004, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci 8: 571– 578, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Cottrell EC, Ozanne SE. Early life programming of obesity and metabolic disease. Physiol Behav 94: 17– 28, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411: 480– 484, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Csiffary A, Gorcs TJ, Palkovits M. Neuropeptide Y innervation of ACTH-immunoreactive neurons in the arcuate nucleus of rats: a correlated light and electron microscopic double immunolabeling study. Brain Res 506: 215– 222, 1990 [DOI] [PubMed] [Google Scholar]
- 10. Cyr NE, Stuart RC, Zhu X, Steiner DF, Nillni EA. Biosynthesis of proTRH-derived peptides in prohormone convertase 1 and 2 knockout mice. Peptides 35: 42– 48, 2012 [DOI] [PubMed] [Google Scholar]
- 11. D'Agostino G, Diano S. Alpha-melanocyte stimulating hormone: production and degradation. J Mol Med (Berl) 88: 1195– 1201, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eipper BA, Bloomquist BT, Husten EJ, Milgram SL, Mains RE. Peptidylglycine alpha-amidating monooxygenase and other processing enzymes in the neurointermediate pituitary, The Melanotropic Peptides. Ann NY Acad Sci 680: 147– 160, 1993a [DOI] [PubMed] [Google Scholar]
- 13. Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385: 165– 168, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Fekete C, Kelly J, Mihaly E, Sarkar S, Rand WM, Legradi G, Emerson CH, Lechan RM. Neuropeptide Y has a central inhibitory action on the hypothalamic-pituitary-thyroid axis. Endocrinology 142: 2606– 2613, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Fekete C, Sarkar S, Rand WM, Harney JW, Emerson CH, Bianco AC, Beck-Sickinger A, Lechan RM. Neuropeptide Y1 and Y5 receptors mediate the effects of neuropeptide Y on the hypothalamic-pituitary-thyroid axis. Endocrinology 143: 4513– 4519, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Fekete C, Sarkar S, Rand WM, Harney JW, Emerson CH, Bianco AC, Lechan RM. Agouti-related protein (AGRP) has a central inhibitory action on the hypothalamic-pituitary-thyroid (HPT) axis; comparisons between the effect of AGRP and neuropeptide Y on energy homeostasis and the HPT axis. Endocrinology 143: 3846– 3853, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Friedman TC, Loh YP, Cawley NX, Birch NP, Huang SS, Jackson IM, Nillni EA. Processing of prothyrotropin-releasing hormone (Pro-TRH) by bovine intermediate lobe secretory vesicle membrane PC1 and PC2 enzymes. Endocrinology 136: 4462– 4472, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Galas L, Tonon MC, Beaujean D, Fredriksson R, Larhammar D, Lihrmann I, Jegou S, Fournier A, Chartrel N, Vaudry H. Neuropeptide Y inhibits spontaneous alpha-melanocyte-stimulating hormone (alpha-MSH) release via a Y(5) receptor and suppresses thyrotropin-releasing hormone-induced alpha-MSH secretion via a Y(1) receptor in frog melanotrope cells. Endocrinology 143: 1686– 1694, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Gehlert DR, Chronwall BM, Schafer MP, O'Donohue TL. Localization of neuropeptide Y messenger ribonucleic acid in rat and mouse brain by in situ hybridization. Synapse 1: 25– 31, 1987 [DOI] [PubMed] [Google Scholar]
- 20. Herzog H, Baumgartner M, Vivero C, Selbie LA, Auer B, Shine J. Genomic organization, localization, and allelic differences in the gene for the human neuropeptide Y Y1 receptor. J Biol Chem 268: 6703– 6707, 1993 [PubMed] [Google Scholar]
- 21. Herzog T, Schlote W. Pituitary adenomas: tumor cell growth by cluster formation. Pattern analysis based on immunohistochemistry. Acta Neuropathol 84: 509– 515, 1992 [DOI] [PubMed] [Google Scholar]
- 22. Hollenberg AN. The role of the thyrotropin-releasing hormone (TRH) neuron as a metabolic sensor. Thyroid 18: 131– 139, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Hook VYH, Loh YP. Carboxypeptidase B-like converting enzyme activity in secretory granules of rat pituitary. Proc Natl Acad Sci USA 81: 2776– 2780, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang XF, Han M, Storlien LH. The level of NPY receptor mRNA expression in diet-induced obese and resistant mice. Brain Res Mol Brain Res 115: 21– 28, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Jackson R, Creemers JWM, Ohagi S, Raffin-Sanson ML, Sanders L, Montague CT, Hutton JC, O'Rahilly S. Obesity and impaired prohormone processing associated with mutations in the human convertase 1 gene. Nat Genet 16: 303– 306, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Jackson RS, O'Rahilly S, Brain C, Nussey SS. Proopiomelanocortin products and human early-onset obesity. J Clin Endocrinol Metab 84: 819– 820, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Jansen E, Ayoubi TA, Meulemans SM, Van De Ven WJ. Regulation of human prohormone convertase 2 promoter activity by the transcription factor EGR-1. Biochem J 328: 69– 74, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jing E, Nillni EA, Sanchez VC, Stuart RC, Good DJ. Deletion of the Nhlh2 transcription factor decreases the levels of the anorexigenic peptides alpha melanocyte-stimulating hormone and thyrotropin-releasing hormone and implicates prohormone convertases I and II in obesity. Endocrinology 145: 1503– 1513, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Kalra SP, Dube MG, Sahu A, Phelps CP, Kalra PS. Neuropeptide Y secretion increases in the paraventricular nucleus in association with increased appetite for food. Proc Natl Acad Sci USA 88: 10931– 10935, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kanatani A, Mashiko S, Murai N, Sugimoto N, Ito J, Fukuroda T, Fukami T, Morin N, MacNeil DJ, Van der Ploeg LH, Saga Y, Nishimura S, Ihara M. Role of the Y1 receptor in the regulation of neuropeptide Y-mediated feeding: comparison of wild-type, Y1 receptor-deficient, and Y5 receptor-deficient mice. Endocrinology 141: 1011– 1016, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Kang JH, Kim MJ, Jang HI, Koh KH, Yum KS, Rhie DJ, Yoon SH, Hahn SJ, Kim MS, Jo YH. Proximal cyclic AMP response element is essential for exendin-4 induction of rat EGR-1 gene. Am J Physiol Endocrinol Metab 292: E215– E222, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Kishi T, Aschkenasi CJ, Choi BJ, Lopez ME, Lee CE, Liu H, Hollenberg AN, Friedman JM, Elmquist JK. Neuropeptide Y Y1 receptor mRNA in rodent brain: distribution and colocalization with melanocortin-4 receptor. J Comp Neurol 482: 217– 243, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Lechan RM, Fekete C. The TRH neuron: a hypothalamic integrator of energy metabolism. Prog Brain Res 153: 209– 235, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Lecklin A, Lundell I, Salmela S, Mannisto PT, Beck-Sickinger AG, Larhammar D. Agonists for neuropeptide Y receptors Y1 and Y5 stimulate different phases of feeding in guinea pigs. Brit J Pharmacol 139: 1433– 1440, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee RG, Rains TM, Tovar-Palacio C, Beverly JL, Shay NF. Zinc deficiency increases hypothalamic neuropeptide Y and neuropeptide Y mRNA levels and does not block neuropeptide Y-induced feeding in rats. J Nutr 128: 1218– 1223, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 273: R725– R730, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Lloyd DJ, Bohan S, Gekakis N. Obesity, hyperphagia and increased metabolic efficiency in Pc1 mutant mice. Hum Mol Genet 15: 1884– 1893, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Marsh DJ, Hollopeter G, Kafer KE, Palmiter RD. Role of the Y5 neuropeptide Y receptor in feeding and obesity. Nat Med 4: 718– 721, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Merchenthaler I, Liposits Z. Mapping of thyrotropin-releasing hormone (TRH) neuronal systems of rat forebrain projecting to the median eminence and the OVLT. Immunocytochemistry combined with retrograde labeling at the light and electron microscopic levels. Acta Biologica Hungarica 45: 361– 374, 1994 [PubMed] [Google Scholar]
- 40. Mora GR, Olivier KR, Mitchell RF, Jr, Jenkins RB, Tindall DJ. Regulation of expression of the early growth response gene-1 (EGR-1) in malignant and benign cells of the prostate. Prostate 63: 198– 207, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Morris BJ. Neuronal localisation of neuropeptide Y gene expression in rat brain. J Comp Neurol 290: 358– 368, 1989 [DOI] [PubMed] [Google Scholar]
- 42. Naggert JK, Fricker LD, Varlamov D, Nishina PM, Rouillie Y, Steiner DF, Carroll RJ, Paigen BJ, Leiter EH. Hyperinsulinemia in obese fat/fat mice associated with a carboxypeptidase E mutation which reduces enzyme activity. Nat Genet 10: 135– 142, 1995 [DOI] [PubMed] [Google Scholar]
- 43. Naleid AM, Grace MK, Chimukangara M, Billington CJ, Levine AS. Paraventricular opioids alter intake of high-fat but not high-sucrose diet depending on diet preference in a binge model of feeding. Am J Physiol Regul Integr Comp Physiol 293: R99– R105, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Nillni EA. Regulation of prohormone convertases in hypothalamic neurons: implications for prothyrotropin-releasing hormone and proopiomelanocortin. Endocrinology 148: 4191– 4200, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Nillni EA. Regulation of the hypothalamic thyrotropin releasing hormone (TRH) neuron by neuronal and peripheral inputs. Front Neuroendocrinol 31: 134– 156, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nillni EA, Vaslet C, Harris M, Hollenberg A, Bjorbak C, Flier JS. Leptin regulates prothyrotropin-releasing hormone biosynthesis. Evidence for direct and indirect pathways. J Biol Chem 275: 36124– 36133, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Nillni EA, Xie W, Mulcahy L, Sanchez VC, Wetsel WC. Deficiencies in pro-thyrotropin-releasing hormone processing and abnormalities in thermoregulation in Cpefat/fat mice. J Biol Chem 277: 48587– 48595, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Parker R, Herzog H. Localization of Y-receptor subtype mRNAs in rat brain by digoxigenin labeled in situ hybridization. Methods Mol Biol 153: 165– 183, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Perello M, Cakir I, Cyr NE, Romero A, Stuart RC, Chiappini F, Hollenberg AN, Nillni EA. Maintenance of the thyroid axis during diet-induced obesity in rodents is controlled at the central level. Am J Physiol Endocrinol Metab 299: E976– E989, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Perello M, Nillni EA. The biosynthesis and processing of neuropeptides: lessons from prothyrotropin releasing hormone (proTRH). Front Biosci 12: 3554– 3565, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Perello M, Stuart R, Nillni EA. Prothyrotropin-releasing hormone targets its processing products to different vesicles of the secretory pathway. J Biol Chem 283: 19936– 19947, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Perello M, Stuart RC, Nillni EA. Differential effects of fasting and leptin on proopiomelanocortin peptides in the arcuate nucleus and in the nucleus of the solitary tract. Am J Physiol Endocrinol Metab 292: E1348– E1357, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Perello M, Stuart RC, Nillni EA. The role of intracerebroventricular administration of leptin in the stimulation of prothyrotropin releasing hormone neurons in the hypothalamic paraventricular nucleus. Endocrinology 147: 3296– 3306, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Poggioli R, Vergoni AV, Bertolini A. ACTH-(1–24) and alpha-MSH antagonize feeding behavior stimulated by kappa opiate agonists. Peptides 7: 843– 848, 1986 [DOI] [PubMed] [Google Scholar]
- 55. Pritchard LE, Turnbull AV, White A. Pro-opiomelanocortin processing in the hypothalamus: impact on melanocortin signalling and obesity. J Endocrinol 172: 411– 421, 2002 [DOI] [PubMed] [Google Scholar]
- 56. Rolli M, Kotlyarov A, Sakamoto KM, Gaestel M, Neininger A. Stress-induced stimulation of early growth response gene-1 by p38/stress-activated protein kinase 2 is mediated by a cAMP-responsive promoter element in a MAPKAP kinase 2-independent manner. J Biol Chem 274: 19559– 19564, 1999 [DOI] [PubMed] [Google Scholar]
- 57. Roseberry AG, Liu H, Jackson AC, Cai X, Friedman JM. Neuropeptide Y-mediated inhibition of proopiomelanocortin neurons in the arcuate nucleus shows enhanced desensitization in ob/ob mice. Neuron 41: 711– 722, 2004 [DOI] [PubMed] [Google Scholar]
- 58. Sanchez VC, Goldstein J, Stuart RC, Hovanesian V, Huo L, Munzberg H, Friedman TC, Bjorbaek C, Nillni EA. Regulation of hypothalamic prohormone convertases 1 and 2 and effects on processing of prothyrotropin-releasing hormone. J Clin Invest 114: 357– 369, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sarkar S, Lechan RM. Central administration of neuropeptide Y reduces alpha-melanocyte-stimulating hormone-induced cyclic adenosine 5′-monophosphate response element binding protein (CREB) phosphorylation in pro-thyrotropin-releasing hormone neurons and increases CREB phosphorylation in corticotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus. Endocrinology 144: 281– 291, 2003 [DOI] [PubMed] [Google Scholar]
- 60. Schaner P, Todd RB, Seidah NG, Nillni EA. Processing of prothyrotropin-releasing hormone by the family of prohormone convertases. J Biol Chem 272: 19958– 19968, 1997 [DOI] [PubMed] [Google Scholar]
- 61. Seidah NG, Chretien M. Eukaryotic protein processing: endoproteolysis of precursor proteins. Curr Opin Biotechnol 8: 602– 607, 1997 [DOI] [PubMed] [Google Scholar]
- 62. Serradeil-Le Gal C, Valette G, Rouby PE, Pellet A, Oury-Donat F, Brossard G, Lespy L, Marty E, Neliat G, de Cointet P, et al. SR 120819A, an orally-active and selective neuropeptide Y Y1 receptor antagonist. FEBS Lett 362: 192– 196, 1995 [DOI] [PubMed] [Google Scholar]
- 63. Smeekens SP, Steiner DF. Identification of a human insulinoma cDNA encoding a novel mammalian protein structurally related to the yeast dibasic processing protease Kex2. J Biol Chem 265: 2997– 3000, 1990 [PubMed] [Google Scholar]
- 64. Sousa-Ferreira L, Garrido M, Nascimento-Ferreira I, Nobrega C, Santos-Carvalho A, Alvaro AR, Rosmaninho-Salgado J, Kaster M, Kugler S, de Almeida LP, Cavadas C. Moderate long-term modulation of neuropeptide Y in hypothalamic arcuate nucleus induces energy balance alterations in adult rats. PLoS ONE 6: e22333, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Steiner DF, Rouille Y, Gong Q, Martin S, Carroll R, Chan SJ. The role of prohormone convertases in insulin biosynthesis: evidence for inherited defects in their action in man and experimental animals. Diabetes Metab 22: 94– 104, 1996 [PubMed] [Google Scholar]
- 66. Wallingford N, Perroud B, Gao Q, Coppola A, Gyengesi E, Liu ZW, Gao XB, Diament A, Haus KA, Shariat-Madar Z, Mahdi F, Wardlaw SL, Schmaier AH, Warden CH, Diano S. Prolylcarboxypeptidase regulates food intake by inactivating alpha-MSH in rodents. J Clin Invest 119: 2291– 2303, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wardlaw SL. Hypothalamic proopiomelanocortin processing and the regulation of energy balance. Eur J Pharmacol 660: 213– 219, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wieland HA, Engel W, Eberlein W, Rudolf K, Doods HN. Subtype selectivity of the novel nonpeptide neuropeptide Y Y1 receptor antagonist BIBO 3304 and its effect on feeding in rodents. Brit J Pharmacol 125: 549– 555, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang L, Bijker MS, Herzog H. The neuropeptide Y system: pathophysiological and therapeutic implications in obesity and cancer. Pharmacol Ther 131: 91– 113, 2011 [DOI] [PubMed] [Google Scholar]