Abstract
Recent clinical observations demonstrate adiponectin (APN), an adipocytokine with potent cardioprotective actions, is significantly reduced following myocardial ischemia/reperfusion (MI/R). However, mechanisms responsible for MI/R-induced hypoadiponectinemia remain incompletely understood. Adult male mice were subjected to 30-min MI followed by varying reperfusion periods. Adipocyte APN mRNA and protein expression and plasma APN and TNFα concentrations were determined. APN expression/production began to decline 3 h after reperfusion (reaching nadir 12 h after reperfusion), returning to control levels 7 days after reperfusion. Plasma TNFα levels began to increase 1 h after reperfusion, peaking at 3 h and returning to control levels 24 h after reperfusion. TNFα knockout significantly increased plasma APN levels 12 h after reperfusion but failed to improve APN expression/production 72 h after reperfusion. In contrast, TNF receptor-1 (TNFR1) knockout significantly restored APN expression 12 and 72 h after reperfusion, suggesting that other TNFR1 binding cytokines contribute to MI/R-induced APN suppression. Among many cytokines increased after MI/R, lymphotoxin-α (LTα) was the only cytokine remaining elevated 24–72 h after reperfusion. LTα knockout did not augment APN levels 12 h post-reperfusion, but did so by 72 h. Finally, in vitro treatment of adipocytes with TNFα and LTα at concentrations seen in MI/R plasma additively inhibited APN expression/production in TNFR1-dependent fashion. Our study demonstrates for the first time that LTα is a novel suppressor of APN expression and contributes to the sustained hypoadiponectinemia following MI/R. Combining anti-TNFα with anti-LTα strategies may achieve the best effects restoring APN in MI/R patients.
Keywords: adipokines, diabetes, reperfusion injury, tumor necrosis factor
early reperfusion after coronary occlusion remains the most effective means of limiting ischemic myocardial injury. However, evidence from animal studies, as well as clinical observations, demonstrates that reperfusion itself may cause additional cell death, defined as “reperfusion injury” (3). Strong epidemiological evidence suggests type 2 diabetes not only causes coronary vascular injury, thereby increasing ischemic heart disease prevalence, but also exacerbates cardiac injury after ischemia/reperfusion insult in these patients (2, 8, 15). Clarifying the molecular link between type 2 diabetes and cardiovascular injury may therefore help identify novel effective therapeutic interventions attenuating postischemic myocardial injury, reducing myocardial ischemic morbidity, and ultimately decreasing diabetic mortality.
Adiponectin (APN) is a protein hormone primarily produced by adipocytes (18). In contrast to the majority of adipokines (e.g., TNFα), which are significantly increased in diabetic patients and proinflammatory, APN is markedly reduced in diabetic patients and is potently protective of the vasculature (16, 25, 27). Plasma APN levels significantly decrease after tissue injury, such as acute lung injury caused by ovalbumin challenge (26). Numerous epidemiological studies reveal the association between hypoadiponectinemia and increased cardiovascular disease risk in obesity and diabetes (4, 7, 13, 32). Additionally, recent clinical observations demonstrate post-MI plasma APN levels correlate positively with myocardial salvage index and ejection fraction recovery (23). Persistent plasma hypoadiponectinemia post myocardial infarction is predictive of future adverse cardiac events (1). However, the molecular mechanisms responsible for post-MI APN suppression remain unidentified.
TNFα is a well-recognized prooxidative cytokine belonging to the same structural family (C1q/TNF superfamily) as APN. Despite their conformational similarities, these two cytokines exert opposite biological effects and reciprocally regulate each other's expression and production. Clinical observations demonstrate that elevated plasma TNFα is associated with low plasma APN levels (11), and anti-TNFα treatment increases plasma APN (12). Although it is well known that TNFα significantly increases after MI/R, and in vitro experiments demonstrate TNFα inhibits APN mRNA expression (5), whether augmented TNFα post-MI is solely responsible for hypoadiponectinemia has not been previously investigated.
Lymphotoxin-α (LTα, also known as TNFβ) is a member of the TNF family, synthesized primarily by activated T and B lymphocytes (17). Previous studies demonstrate that LTα shares the same membrane receptors as TNFα, exerting its biological effect largely via TNF receptor 1 (TNFR1) and TNFR2 activation (17). However, the effect of LTα on APN expression has never been previously investigated.
Therefore, the aim of the present study was to determine the mechanisms responsible for post-MI hypoadiponectinemia, and to assess the role of TNFα and other cytokines in the regulation of APN after MI.
MATERIALS AND METHODS
Animals.
Male adult wild-type (WT, C57BL/6), TNFα knockout (TNFα−/−), TNFR1 knockout (TNFR1−/−), and LTα knockout (LTα−/−) mice (body weight 25–28 g) were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were confirmed by specific primer genotyping. All experiments were performed in adherence with the National Institutes of Health Guidelines on the Use of Laboratory Animals and were approved by the Thomas Jefferson University Committee on Animal Care.
Experimental protocols.
Mice were anesthetized with 2% isoflurane, and myocardial ischemia reperfusion (MI/R) was produced by temporarily exteriorizing the heart via a left thoracic incision and placing a 6-0 silk suture slipknot around the left anterior descending coronary artery (28). Slipknot release occurred after 30-min MI, and myocardial reperfusion commenced for 1 h for 7 days. Sham-operated control mice (sham MI) underwent the same surgical procedures except that the suture placed under the left coronary artery was not tied. At the specified time point after reperfusion, six animals in each group were euthanized. Blood was drawn for ELISA determination of APN, TNFα, and LTα. The epididymal fat pad was utilized for determination of adipose tissue APN mRNA expression.
Determination of plasma APN, TNFα, and LTα concentrations.
Plasma APN level was determined via commercial ELISA kit (ALPCO Diagnostics, Salem, NH) per manufacturer's instructions. Plasma TNFα and LTα concentrations were determined via mouse TNFα and LTα ELISA kits (BioLegend, San Diego, CA) per manufacturer's instructions.
Quantitative PCR analysis of APN expression in mouse adipose tissues.
Total RNA was isolated from epididymal adipose tissue via RNeasy Lipid Tissue Midi Kit (Qiagen, Valencia, CA). A standard PCR protocol was performed utilizing an Applied Biosystems Prism 7900 Sequence Detection System. cDNA from WT mice's epididymal fat pads were synthesized from 2 μg of total RNA and 200 ng of Oligo(dT) primer via Superscript III RNase H-Reverse Transcriptase protocol (Invitrogen, Carlsbad, CA). For quantitative PCR, samples were analyzed in triplicate in 15-μl reaction volumes (10 ng of cDNA, 450 nmol of primer, 7.5 μl of Master Mix, and water) per standard protocol provided by SyBR Green PCR Master Mix (Applied Biosystems).
Adipocyte cell culture.
3T3-L1 preadipocytes were grown to confluence in DMEM containing 10% FBS and penicillin-streptomycin (100 U/ml) at 37°C in a humidified 5% CO2 atmosphere. Cells were induced to adipocyte differentiation 24 h after confluence by changing the medium to DMEM supplemented with 10% FBS, 5 μg/ml insulin, 0.5 mM 3-isobutylmethylxanthine, and 0.25 mM dexamethasone sodium phosphate for 48 h. Thereafter, cells were grown in medium containing only insulin and refreshed subsequently every 48 h. Cells were treated with TNFα and LTα (100 pg/ml) alone or in combination 9–10 days after differentiation (defined as >90% adipocyte phenotype). The effect of TNFα or LTα on APN mRNA expression (PCR), protein expression (Western), and secretion (APN concentration in culture medium) was determined 3–72 h after treatment. To knock down TNFR1 protein expression, 50 nM TNFR1 siRNA or scramble siRNA (Santa Cruz Biotechnology) was transfected into differentiated 3T3-L1 cells (6 days after differentiation induction) via Lipofectamin 2000 reagent (Invitrogen, Grand Island, NY) per manufacturer's instructions. After transfection (72 h), the efficiency of siRNA-mediated TNFR1 knockdown was determined by Western blot. Cells were then treated with TNFα/LTα as described above.
Western blot analysis.
Proteins were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels, transferred to nitrocellulose membranes, and incubated with primary antibodies (anti-APN and anti-tubulin; Cell Signaling Technology, Danvers, MA) followed by horseradish peroxidase-conjugated secondary antibody (Abcam, Cambridge, MA). The blot was developed with a Supersignal Chemiluminescence detection kit (Pierce, Rockford, IL) and observed with a Kodak Image Station 4000R Pro (Rochester, NY).
Statistical analysis.
All values in the text and figures are presented as means ± SE of n independent experiments. Data (except Western blot density) were subjected to one-way ANOVA followed by Bonferoni correction for post hoc test. Western blot densities were analyzed with the Kruskal-Wallis test followed by Dunn's post hoc test. Probabilities of 0.05 or less were considered statistically significant.
RESULTS
MI/R inhibits APN expression/production.
APN mRNA expression began to decline as early as 3 h after reperfusion, reaching nadir 12 h after reperfusion (72% reduction vs. sham MI/R group). APN mRNA expression slightly increased 24 and 48 h post-reperfusion and then modestly declined again 72 h after reperfusion. After 7 days of reperfusion, APN recovered to levels statistically similar to those of control (Fig. 1A). The plasma APN level trend was similar to adipocyte APN mRNA expression, except that plasma APN protein nadir occurred 24 h after reperfusion (Fig. 1B).
Fig. 1.
Time course of adipocyte adipnectin (APN) mRNA (A) expression and plasma APN concentrations (B) after reperfusion (hours); n = 5 for mRNA expression at each time point, and n = 12 for plasma APN at each time point. **P < 0.01 vs. control (time 0).
Different effect of TNFα and TNFR1 knockout on post-MI/R APN suppression.
It is well recognized that MI/R stimulates TNFα production. Moreover, previous in vitro studies have demonstrated that TNFα inhibits APN expression. To determine the causative role of TNFα in post-MI/R hypoadiponectinemia, we determined the effect of TNFα knockout on post-MI/R APN expression. Adipocyte TNFα expression was slightly increased before MI/R in TNFα knockout mice (Fig. 2A). TNFα knockout almost completely abolished the inhibitory effect of MI/R on APN expression 12 h post-MI/R (P < 0.01). However, TNFα knockout had no effect on APN expression 72 h after MI/R.
Fig. 2.
Effect of TNFα (A) and TNF receptor 1 (TNFR1; B) knockout on adipocyte APN expression after reperfusion; n = 5 at each time point. **P < 0.01 vs. control (time 0).
TNFα achieves its biological function via activation of two cell membrane receptors, TNFR1 and TNFR2. Our recent in vitro experimental results demonstrate the inhibitory effect of TNFα on APN expression is mediated by TNFR1. TNFR1 knockout mice were employed to determine whether TNFR1 knockout might similarly affect in vivo APN expression. TNFR1 knockout had a similar effect on APN expression as TNFα knockout before and 12 h after MI/R (Fig. 2B). Although TNFα knockout failed to restore APN expression 72 h after reperfusion, TNFR1 knockout markedly reversed the inhibitory effect of MI/R on APN expression 72 h after reperfusion. These results suggest that, although TNFα overproduction is largely responsible for the inhibitory effect of MI/R on APN expression at an early time point (i.e., 12 h), other cytokines capable of binding with TNFR1 are responsible for the inhibitory effect of MI/R on APN expression at a late time point (i.e., 72 h).
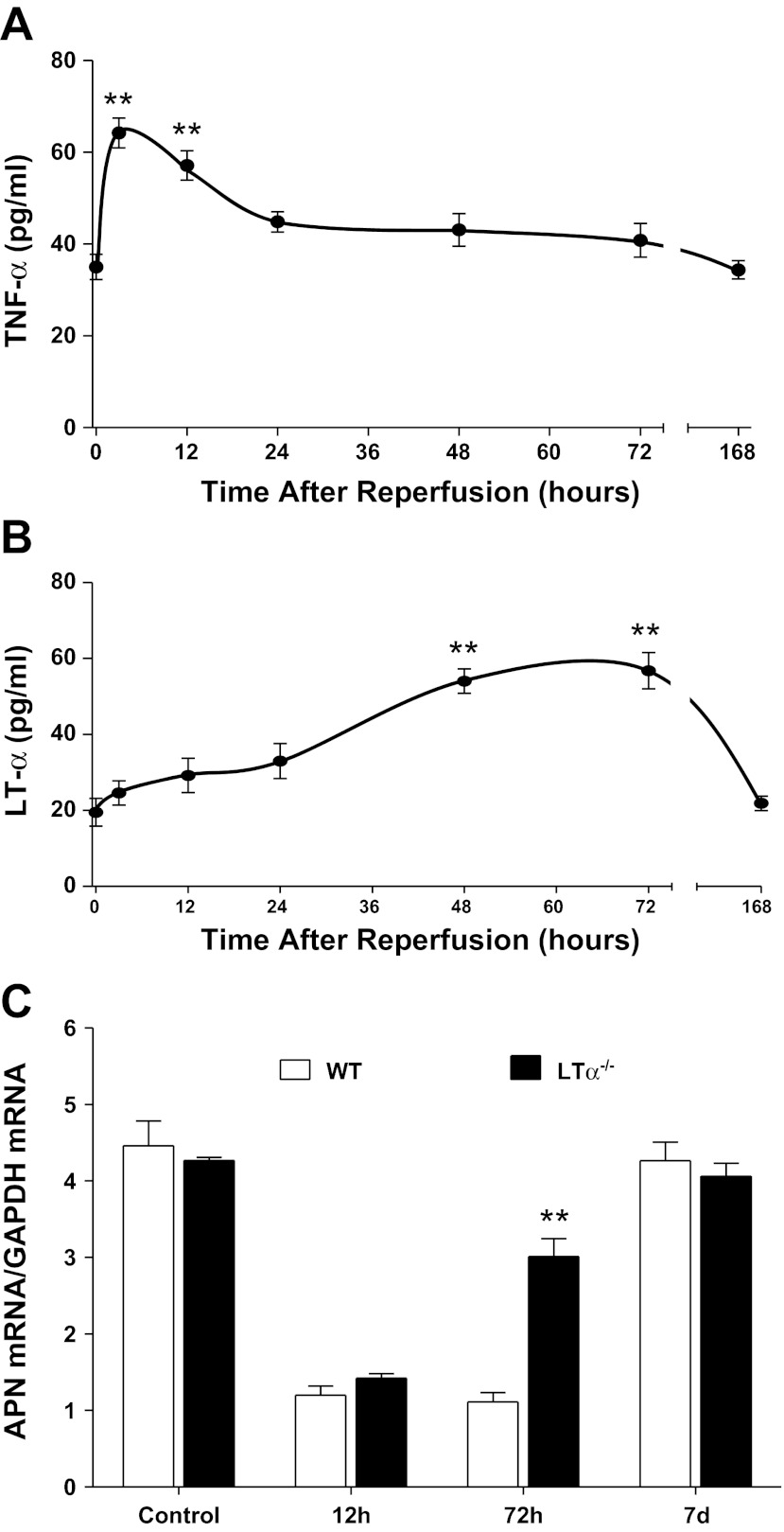
To identify novel molecules inhibiting APN expression, plasma concentrations of TNFα, IL-1β, IL-6, IL-18, TGFβ, and LTα were determined throughout the MI/R period. Among the cytokines investigated, LTα was an attractive candidate for the following three reasons. First, plasma TNFα levels rapidly increase after reperfusion, returning to control levels 24 h after reperfusion (Fig. 3A), suggesting that plasma TNFα may not be responsible for late-phase (i.e., 72 h after reperfusion) APN suppression. Second, although multiple cytokines increased after MI/R (data not shown), LTα was the only protein that remained elevated 48–72 h after reperfusion (Fig. 3B), a time point during the second phase of APN suppression, not reversed by TNFα knockout. Third, previous studies have demonstrated that LTα shares the same receptors with TNFα. To obtain direct evidence supporting the role of LTα in late-phase APN suppression after MI/R, we determined the effect of LTα knockout on APN expression. LTα knockout had no effect on APN expression before MI or 12 h after reperfusion (Fig. 3C). However, APN expression levels were significantly increased 72 h after reperfusion. Taken together, these results suggest that LTα may be responsible for late-phase APN suppression after MI/R.
Fig. 3.
Time course of plasma TNFα (A) and lymphotoxin-α (LTα; B) after reperfusion, and effect of LTα knockout on adipocyte APN expression after reperfusion (C); n = 12 for plasma TNFα/LTα at each time point, and n = 5 for mRNA expression at each time point. **P < 0.01 vs. control (time 0).
Additive effect of TNFα and LTα on adipocyte APN expression.
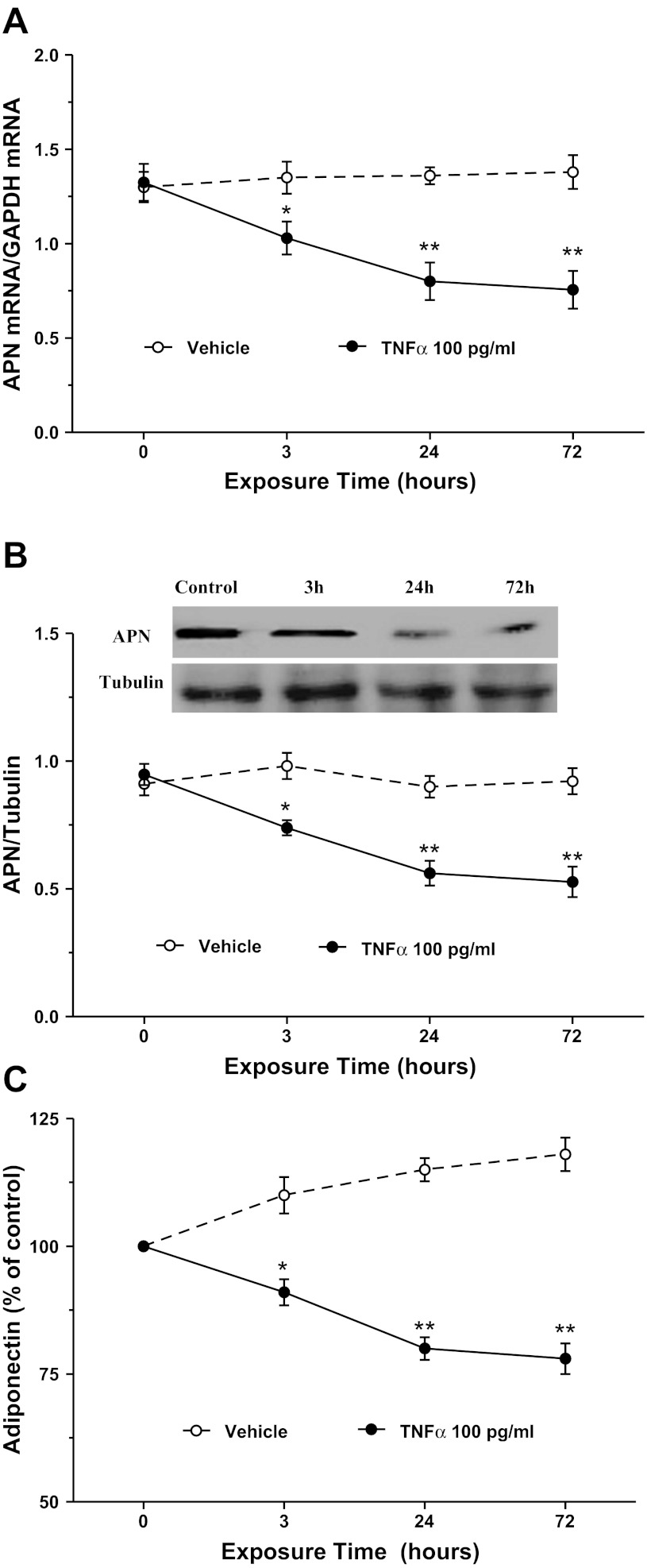
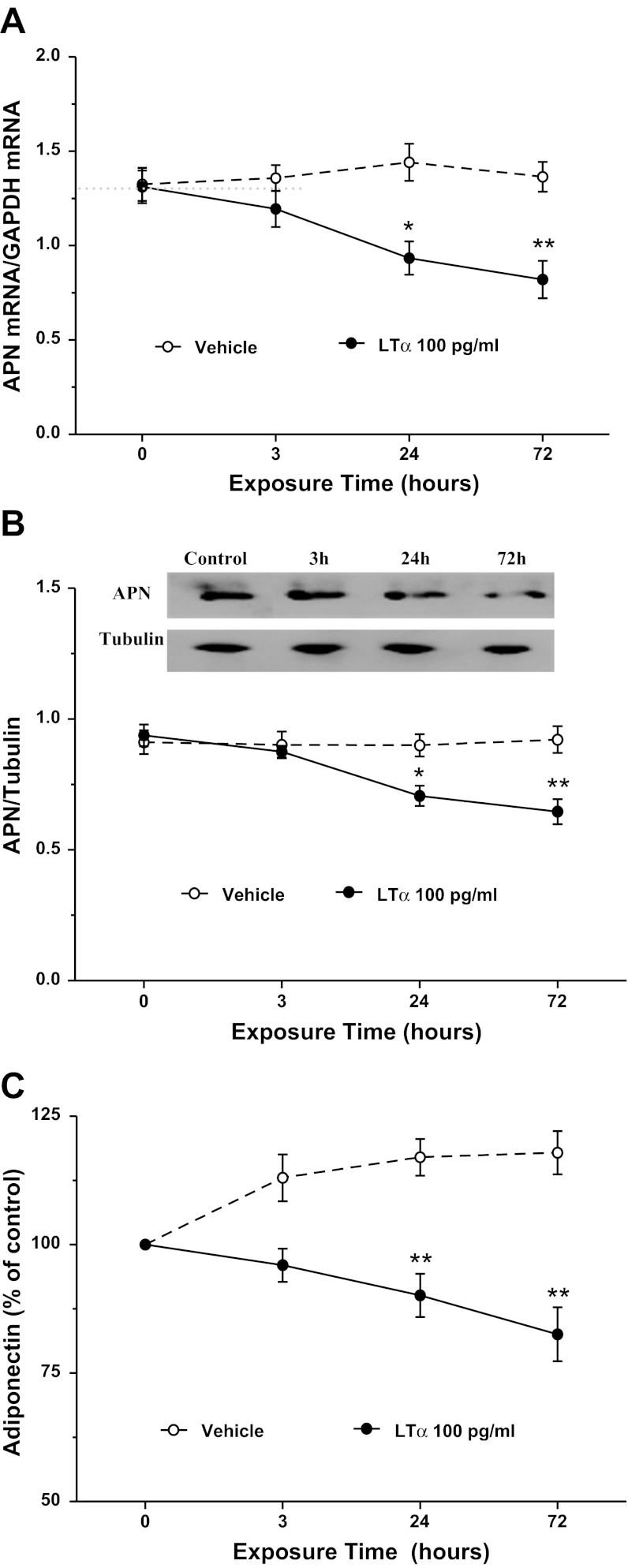
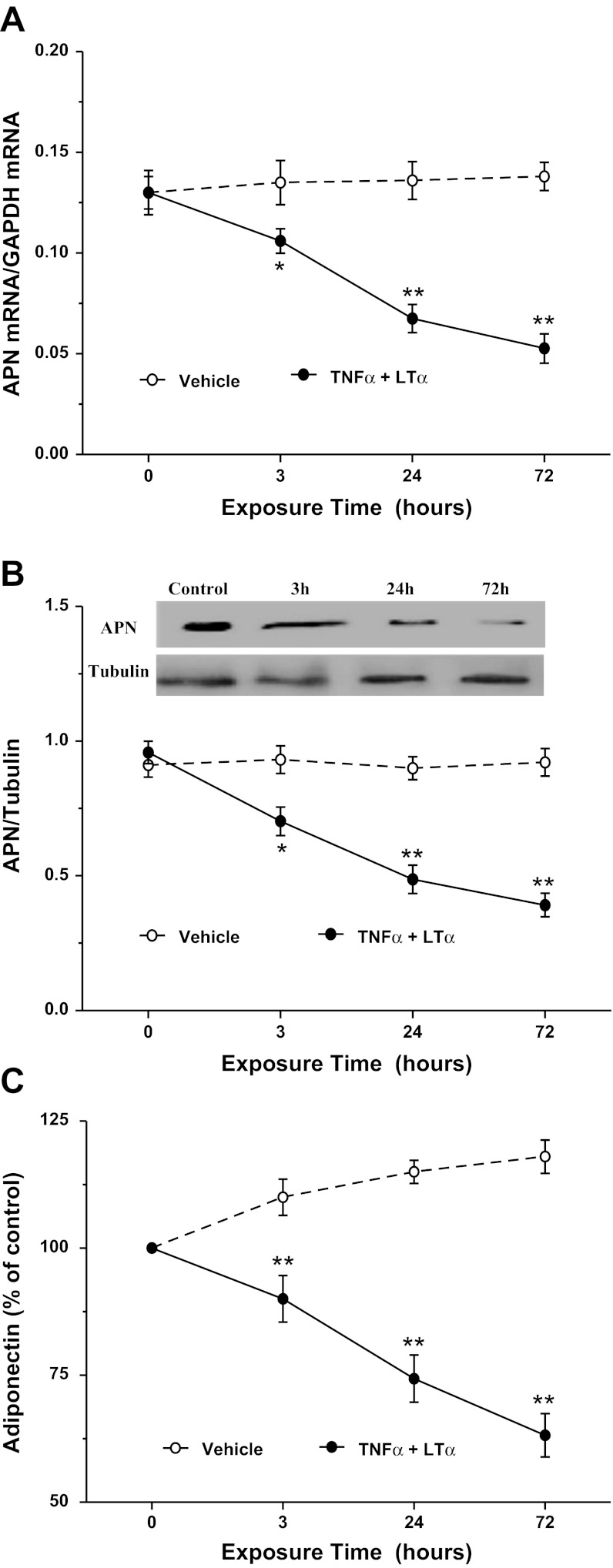
The aforementioned in vivo experimental results strongly suggest that TNFα and LTα are two cytokines inhibiting APN expression after MI/R. We next performed in vitro experiments to determine whether these two cytokines might additively inhibit adipocyte APN expression. Consistent with previous reports, TNFα treatment significantly inhibited APN mRNA expression (Fig. 4A), protein expression (Fig. 4B), and APN secretion (Fig. 4C). Treatment of adipocytes with LTα at concentrations observed in plasma after MI/R significantly inhibited APN mRNA expression (Fig. 5A), protein expression (Fig. 5B), and APN secretion (Fig. 5C). Finally, treatment with TNFα and LTα additively inhibited APN expression and production (Fig. 6, A–C).
Fig. 4.
Effect of TNFα on APN mRNA expression (A), protein expression (B), and secretion (C) in cultured 3T3-L1 cells; n = 8–10 independent experiments at each time point. *P < 0.05, **P < 0.01 vs. control (time 0).
Fig. 5.
Effect of LTα on APN mRNA expression (A), protein expression (B), and secretion (C) in cultured 3T3-L1 cells; n = 8–10 independent experiments at each time point. *P < 0.05, **P < 0.01 vs. control (time 0).
Fig. 6.
Effect of combined TNFα and LTα treatment on APN mRNA expression (A), protein expression (B), and secretion (C) in cultured 3T3-L1 cells; n = 8–10 independent experiments at each time point. *P < 0.05, **P < 0.01 vs. control (time 0).
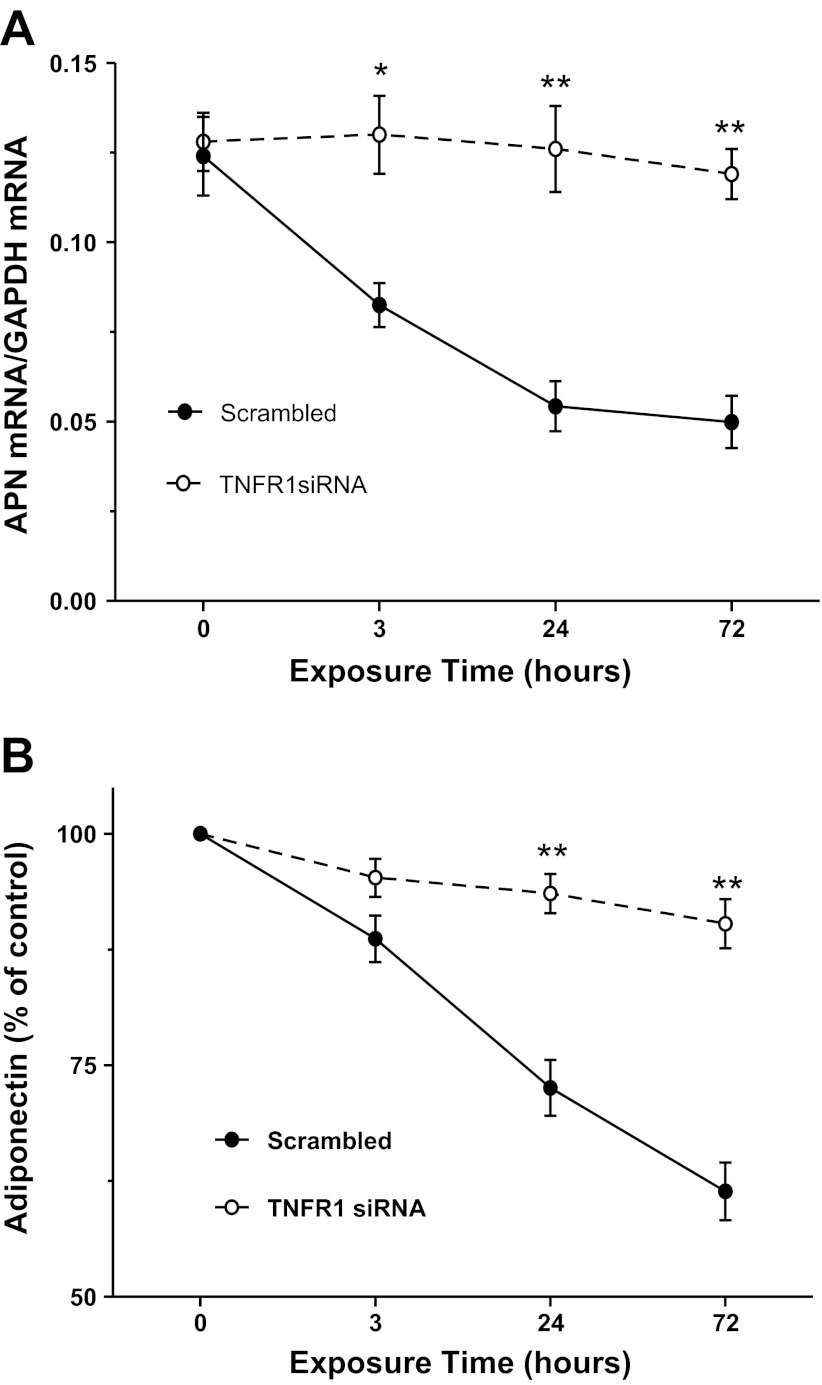
TNFR1 knockout significantly attenuated TNFα/LTα inhibition of APN expression.
In a final attempt to determine the regulatory role of LTα in adipocyte APN expression, we determined the effect of siRNA-mediated TNFR1 expression knockdown on TNFα/LTα-mediated inhibition of APN expression. siRNA against TNFR1 effectively inhibited adipocyte TNFR1 expression (79 ± 4.9%). More importantly, TNFR1 downregulation markedly attenuated the inhibitory effect of TNFα/LTα on adipocyte APN expression (Fig. 7A) and production (Fig. 7B).
Fig. 7.
Effect of TNFR1 knockout on TNFα/LTα suppression of APN mRNA expression (A) and production (B) in cultured 3T3-L1 cells; n = 8–10 independent experiments at each time point. *P < 0.05, **P < 0.01 vs. control (time 0).
DISCUSSION
Substantial evidence supports reciprocal inhibitory regulation between TNFα and APN (14). Clinical observations demonstrate that elevated plasma TNFα is associated with low plasma APN levels (11) and that anti-TNFα treatment increases plasma APN (12). In vitro experiments demonstrate that TNFα markedly inhibits APN mRNA expression and secretion in both dose- and time-dependent manners (5). TNFα also decreases rosiglitazone-stimulated APN promoter activity in adipocytes (31). Several mechanisms have been proposed to explain the inhibitory effect of TNFα on APN expression (14). First, TNFα decreases APN production by suppressing the expression of several factors promoting APN specifically, such as PPARγ, C/EBP, SREBP, DsbA-L, and retinoid X receptor-α. Second, TNFα induces inhibitory transcription factor IGFBP3, which suppresses APN transcription and induces insulin resistance. Finally, TNFα inhibits APN expression by activating JNK and NF-κB systems. Conversely, APN significantly inhibits TNFα expression/production (19). Early studies demonstrate significantly increased TNFα levels in APN knockout mice (30). Recent studies demonstrate that APN inhibits TNFα production via multiple mechanisms, including inhibiting NF-κB activation, promoting anti-inflammatory cytokine production, and activating the cyclooxygenase-2/prostaglandin E2 pathway (6, 24). Utilizing a TNFα knockout approach, we provide the first direct in vivo evidence that TNFα overproduction is responsible for hypoadiponectinemia occurring early after reperfusion. Although other proinflammatory cytokines (such as IL-6 and IL-18) have been previously reported to inhibit APN expression in cultured adipocytes (14), the current study does not support the major role of such cytokines in post-MI/R hypoadiponectinemia, as TNFα knockout recovered greater than 80% APN expression 12 h after reperfusion. A likely explanation for the discrepancy between our current in vivo experimental results and previously reported in vitro results is that plasma IL-6 and IL-18 levels were only slightly elevated and transient after in vivo reperfusion, whereas prolonged high concentrations of IL-6 and IL-18 were utilized in previous culture cell studies (14). This possibility is supported by our data demonstrating that the inhibitory effect of MI/R on APN expression/production is less than previously reported from in vitro cell culture studies by TNFα (91–97% reduction in APN mRNA expression after 1–3 days of TNFα incubation) (5). Most in vitro cell culture studies employed high TNFα concentrations (in the ng/ml range), with nearly complete inhibition of resultant APN expression. In our study, although in vivo MI/R markedly increased plasma TNFα levels compared with controls, such TNFα levels in MI/R animals (at the very maximum) remained in the picogram per milliliter range.
LTα, a member of the TNF family, is synthesized primarily by activated T and B lymphocytes (17). It is expressed in either a secreted or a membrane-bound form exhibiting different affinity for various receptors. The secreted soluble homotrimeric form binds to the TNFR1 and TNFR2 receptors with high affinity, whereas the transmembrane heterotrimeric form (one LTα plus two LTβ) binds with high specificity to the LTβ receptor (LTβR). LTα mediates a large variety of inflammatory, immunostimulatory, and antiviral responses, affects cell death or differentiation, and provides an important communicative link between lymphocytes and stromal cells (17). Several genetic and clinical studies have demonstrated that variations in the gene encoding LTα affecting its expression and biological function contribute to the risk of coronary artery disease, myocardial infarction, aortic aneurysm formation, and cerebral infarction (10, 22). Recent clinical studies demonstrate that LTα gene variability is also associated with features of the metabolic syndrome, including increased level of C-reactive protein, hyperinsulinemia, and dyslipidemia (9).
Considerable indirect evidence suggests the interplay between LTα and APN. LTα is a potent proinflammatory factor, whereas APN is a well-recognized anti-inflammatory molecule. Whereas APN knockout increases atherosclerosis formation (20), loss of LTα reduces lesion size by 62% (21). Moreover, LTα gene variability is associated with hypoadiponectinemia (9). Finally, a recent study demonstrates that APN inhibits lymphotoxin-induced adhesion molecule expression in human umbilical vein endothelial cells via inhibition of NF-κB activation (29). Utilizing LTα knockout mice and combining both in vivo and in vitro approaches, we provide the first direct evidence that LTα is a novel APN expression suppressor and contributes to hypoadiponectinemia occurring late after reperfusion. These results also suggest that the previously reported proatherosclerotic role of LTα may be mediated by APN inhibition. Experiments obtaining direct evidence supporting this interesting hypothesis are currently in progress.
In summary, our current study has provided direct evidence that TNFα overproduction immediately after MI/R inhibits cardioprotective APN expression/production and is responsible for the hypoadiponectinemia during the early phase of reperfusion. In contrast, increased LTα production occurring after 48-h reperfusion is a novel suppressor of APN expression/production during the late phase of reperfusion. Moreover, TNFR1 knockout blocked the suppressive effect of both TNFα and LTα on APN. These experimental results suggest that simultaneous inhibition of TNFα and LTα, or targeted inhibition of TNFR1 function, may be novel approaches restoring APN expression/production in MI/R patients.
GRANTS
This research was supported by grants from the American Diabetes Association (1-11-JF56) and the Natural Science Foundation of China (30900592 and 81170199 to Y.-J. Wang and 81270185 to J.-L. Zhao), and National Institutes of Health (HL-63828, HL-096686) and American Diabetes Association (7-11-BS-93) (to X.-L. Ma).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author (s).
AUTHOR CONTRIBUTIONS
Author contributions: W.B.L. and B.L. analyzed data; W.B.L. drafted manuscript; Y.Z., J.Z., X.-L.W., Y.-X.Y., E.G., and Y.-J.W. performed experiments; B.L., W.J.K., X.-L.M., and Y.-J.W. conception and design of research; T.A.C., B.L.L., and W.J.K. approved final version of manuscript.
REFERENCES
- 1. Behrends M, Schulz R, Post H, Alexandrov A, Belosjorow S, Michel MC, Heusch G. Inconsistent relation of MAPK activation to infarct size reduction by ischemic preconditioning in pigs. Am J Physiol Heart Circ Physiol 279: H1111–H1119, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Forrat R, Sebbag L, Wiernsperger N, Guidollet J, Renaud S, De Lorgeril M. Acute myocardial infarction in dogs with experimental diabetes. Cardiovasc Res 27: 1908–1912, 1993 [DOI] [PubMed] [Google Scholar]
- 3. Garcia-Dorado D. Myocardial reperfusion injury: a new view. Cardiovasc Res 61: 363–364, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Goldstein BJ, Scalia R. Adipokines and vascular disease in diabetes. Curr Diab Rep 7: 25–33, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Hector J, Schwarzloh B, Goehring J, Strate TG, Hess UF, Deuretzbacher G, Hansen-Algenstaedt N, Beil FU, Algenstaedt P. TNF-alpha alters visfatin and adiponectin levels in human fat. Horm Metab Res 39: 250–255, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Higuchi A, Ohashi K, Kihara S, Walsh K, Ouchi N. Adiponectin suppresses pathological microvessel formation in retina through modulation of tumor necrosis factor- (alpha) expression. Circ Res 104: 1058–1065, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 20: 1595–1599, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Jagasia D, McNulty PH. Diabetes mellitus and heart failure. Congest Heart Fail 9: 133–139, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Jang Y, Kim HJ, Koh SJ, Hyun YJ, Chae JS, Cho H, Ha JW, Kim BK, Kim JS, Lee JH. Lymphotoxin-alpha gene 252A>G and metabolic syndrome features in Korean men with coronary artery disease. Clin Chim Acta 384: 124–128, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Jang Y, Koh SJ, Kim OY, Kim BK, Choi D, Hyun YJ, Kim HJ, Chae JS, Lee JH. Effect of the 252A>G polymorphism of the lymphotoxin-alpha gene on inflammatory markers of response to cigarette smoking in Korean healthy men. Clin Chim Acta 377: 221–227, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes 52: 1779–1785, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Komai N, Morita Y, Sakuta T, Kuwabara A, Kashihara N. Anti-tumor necrosis factor therapy increases serum adiponectin levels with the improvement of endothelial dysfunction in patients with rheumatoid arthritis. Mod Rheumatol 17: 385–390, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, Arita Y, Okamoto Y, Shimomura I, Hiraoka H, Nakamura T, Funahashi T, Matsuzawa Y. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol 23: 85–89, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Liu M, Liu F. Transcriptional and post-translational regulation of adiponectin. Biochem J 425: 41–52, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Marfella R, D'Amico M, Di Filippo C, Piegari E, Nappo F, Esposito K, Berrino L, Rossi F, Giugliano D. Myocardial infarction in diabetic rats: role of hyperglycaemia on infarct size and early expression of hypoxia-inducible factor 1. Diabetologia 45: 1172–1181, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Maruyoshi H, Kojima S, Otsuka F, Funahashi T, Kaikita K, Sugiyama S, Sakamoto T, Yoshimura M, Shimomura I, Ogawa H. Hypoadiponectinemia is associated with coronary artery spasm in men. Circ J 69: 1154–1156, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Naoum JJ, Chai H, Lin PH, Lumsden AB, Yao Q, Chen C. Lymphotoxin-alpha and cardiovascular disease: clinical association and pathogenic mechanisms. Med Sci Monit 12: RA121–RA124, 2006 [PubMed] [Google Scholar]
- 18. Ouchi N, Shibata R, Walsh K. Cardioprotection by adiponectin. Trends Cardiovasc Med 16: 141–146, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta 380: 24–30, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pchejetski D, Kunduzova O, Dayon A, Calise D, Seguelas MH, Leducq N, Seif I, Parini A, Cuvillier O. Oxidative stress-dependent sphingosine kinase-1 inhibition mediates monoamine oxidase A-associated cardiac cell apoptosis. Circ Res 100: 41–49, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Schreyer SA, Vick CM, LeBoeuf RC. Loss of lymphotoxin-alpha but not tumor necrosis factor-alpha reduces atherosclerosis in mice. J Biol Chem 277: 12364–12368, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Schulz S, Schagdarsurengin U, Rehfeld D, Suss T, Werdan K, Muller-Werdan U, Glaser C. Genetic impact of TNF-beta on risk factors for coronary atherosclerosis. Eur Cytokine Netw 17: 148–154, 2006 [PubMed] [Google Scholar]
- 23. Shibata R, Numaguchi Y, Matsushita K, Sone T, Kubota R, Ohashi T, Ishii M, Kihara S, Walsh K, Ouchi N, Murohara T. Usefulness of adiponectin to predict myocardial salvage following successful reperfusion in patients with acute myocardial infarction. Am J Cardiol 101: 1712–1715, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med 11: 1096–1103, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shimabukuro M, Higa N, Asahi T, Oshiro Y, Takasu N, Tagawa T, Ueda S, Shimomura I, Funahashi T, Matsuzawa Y. Hypoadiponectinemia is closely linked to endothelial dysfunction in man. J Clin Endocrinol Metab 88: 3236–3240, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol 118: 389–395, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Tan KCB, Xu A, Chow WS, Lam MCW, Ai VHG, Tam SCF, Lam KSL. Hypoadiponectinemia is associated with impaired endothelium-dependent vasodilation. J Clin Endocrinol Metab 89: 765–769, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Wang Y, Gao E, Tao L, Lau WB, Yuan Y, Goldstein BJ, Lopez BL, Christopher TA, Tian R, Koch W, Ma XL. AMP-activated protein kinase deficiency enhances myocardial ischemia/reperfusion injury but has minimal effect on the antioxidant/antinitrative protection of adiponectin. Circulation 119: 835–844, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu Y, Zhang C, Wang N, Ling F, Li P, Gao Y, Hua W. Adiponectin inhibits lymphotoxin-beta receptor-mediated NF-kappaB signaling in human umbilical vein endothelial cells. Biochem Biophys Res Commun 404: 1060–1064, 2011 [DOI] [PubMed] [Google Scholar]
- 30. Yamauchi T, Hara K, Kubota N, Terauchi Y, Tobe K, Froguel P, Nagai R, Kadowaki T. Dual roles of adiponectin/Acrp30 in vivo as an anti-diabetic and anti-atherogenic adipokine. Curr Drug Targets Immune Endocr Metabol Disord 3: 243–254, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Zappala G, Rechler MM. IGFBP-3, hypoxia and TNF-alpha inhibit adiponectin transcription. Biochem Biophys Res Commun 382: 785–789, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhu M, Miura J, Lu LX, Bernier M, DeCabo R, Lane MA, Roth GS, Ingram DK. Circulating adiponectin levels increase in rats on caloric restriction: the potential for insulin sensitization. Exp Gerontol 39: 1049–1059, 2004 [DOI] [PubMed] [Google Scholar]