Abstract
The measurement of the fractional breakdown rate (FBR) of muscle proteins during physiological non-steady state of amino acids (AAs) presents some challenges. Therefore, the goal of the present experiment was to modify the bolus stable isotope tracer injection approach to determine both fractional synthesis rate (FSR) and FBR of leg muscle protein during a physiological non-steady state of AAs. The approach uses the traditional precursor-product principle but is modified with the assumption that inward transport of AAs is proportional to their plasma concentrations. The FBR value calculated from the threonine tracer served as a reference to evaluate the validity of the FBR measurement from the phenylalanine tracer, which was under a non-steady-state condition due to the concomitant injection of unlabeled phenylalanine. Plasma phenylalanine concentration increased more than fourfold after the bolus injection, and thereafter it decreased exponentially, whereas the threonine concentration remained stable. FBR values were similar with the two tracers [0.133 ± 0.003 and 0.148 ± 0.003%/h (means ± SE) for the phenylalanine and threonine tracers, respectively, P > 0.05]. In addition, FSR values for the two tracers were similar (0.069 ± 0.002 and 0.067 ± 0.001%/h for the phenylalanine and threonine tracers, respectively, P > 0.05), indicating that the traditional FSR approach can also be used in the non-steady state. Accordingly, net balance (NB) values were similar (−0.065 ± 0.002 and −0.081 ± 0.002%/h for the phenylalanine and threonine tracers, respectively, P > 0.05). This new method of measuring muscle protein FBR during physiological non-steady state gives reliable results and allows simultaneous measurement of muscle protein FSR and thus a calculation of NB.
Keywords: amino acid kinetics, bolus injection of tracers, gas chromatograph-mass spectrometer, inward transport of amino acids, rabbits
many acute and chronic pathological conditions (e.g., burn injury, peripheral arterial occlusive disease, cancer cachexia, etc.) result in the loss of skeletal muscle mass (4, 13, 15). Aging as a process of physiological change in the human body also results in muscle mass loss, known as sarcopenia. As a result of the loss of muscle mass, patients experience a delay in rehabilitation and the return to normal activity. In elderly people, a loss of muscle mass may lead to loss of strength, which can lead to a loss in function (4, 10, 19) and increased risks of falls and injury (21, 27). Therefore, many studies have been done to evaluate the efficacy of various nutritional and pharmacological interventions with the goal of preserving the muscle mass. For example, ingestion of a balanced amino acid mixture was shown to acutely stimulate muscle protein anabolism (22), and it has been shown that essential amino acids are primarily responsible for this effect (23).
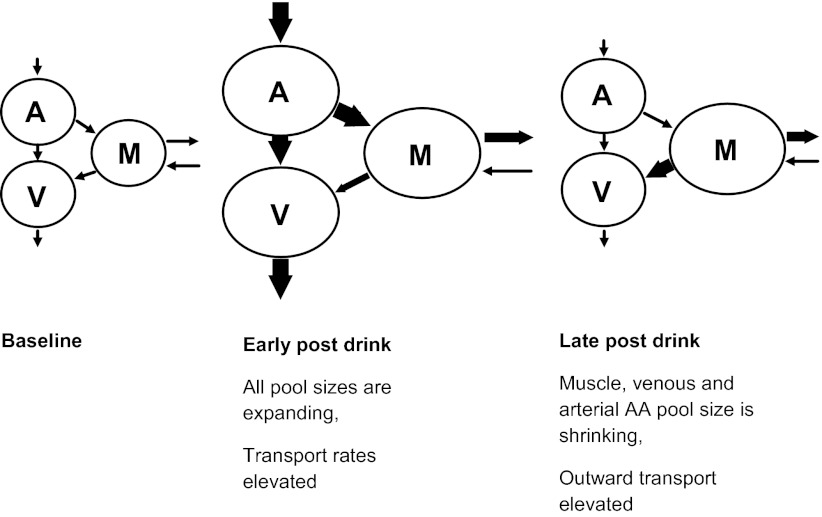
The studies described above used an arteriovenous (A-V) balance approach for measuring muscle protein synthesis and breakdown (2). The A-V balance approach allows one to determine a substrate metabolism across the tissue bed (e.g., muscles in leg or forearm) based on the kinetics of the tracer in the blood from artery and vein that supply and drain, respectively, the tissue of interest and change in concentration of amino acids in the muscle (26). The original description of this approach assumes that a physiological steady state exists, which we know is not the case following an amino acid drink. The schematic illustration shown in Fig. 1 depicts the changes in the free amino acid pool sizes that occur in the circulation and within the muscle following an amino acid drink. Initially, the drink causes an expansion in the arterial pool, which leads to both increased shunting into the venous pool and increased transport of amino acids into muscle and other cells, which leads to elevated muscle free amino acid levels. The plasma amino acid pool sizes will return toward predrink levels in accord with accelerated uptake. This will cause an increased concentration gradient favoring outward transportation of amino acids from the muscle cell to the circulation until muscle amino acid pool sizes also return to predrink levels. These dynamic changes need to be taken into account when the amino acid and protein turnover are measured using tracer kinetic methods. With regard to the A-V model, it is normally assumed that all amino acid uptake and release of a tracer amino acid such as phenylalanine that is neither synthesized nor oxidized in muscle are due to protein synthesis and breakdown. However, altered rates of transmembrane flux in response to changes in amino acid concentrations as described above can raise questions as to the validity of this assumption. We have reported previously that changes in inward and outward fluxes of phenylalanine can be accounted for in the non-steady state resulting from amino acid intake by means of a correction factor applied in conjunction with the A-V model (16). Nonetheless, there is always concern that a correction factor may have validity only in the particular circumstances assessed. Furthermore, there are practical limitations to the application of the A-V model in human subjects. Although the A-V approach has proven useful in determining rates of tissue protein synthesis, breakdown, and net balance, interpretation can be limited by the fact that the unit usually includes several tissues. For example, under normal conditions the leg provides a reasonable representation of muscle metabolism, but in some circumstances the metabolic contribution from nonmuscle tissues (mainly skin) may be significant, such as after a burn injury (3). Also, the method is quite invasive, requiring the catheterization of both femoral artery and vein. Finally, the required measurement of leg blood flow can be problematic in terms of both obtaining indocyanine green and the variability of the measurement.
Fig. 1.
Response of arterial (A), venous (V), and intramuscular free (M) amino acid pools to an amino acid drink. Initially, the drink causes an expansion in the arterial pool, which leads to both increased shunting into the venous pool and increased transport of amino acids into muscle and other cells, which leads to elevated muscle free amino acid levels. The plasma amino acid pool sizes will eventually return to predrink levels, which will cause an increased concentration gradient favoring outward transportation of amino acids from the muscle cell to the circulation until muscle amino acid pool sizes also return to predrink levels. The inward arrow (toward muscle) represents the “rate of release of amino acids from muscle protein and amino acid synthesis,” and the outward arrow (from the muscle) represents the “rate of incorporation of amino acids into muscle protein and irreversible loss” (26). And since phenylalanine and threonine do not metabolize in the muscle so that there is no amino acid synthesis and irreversible loss, thus these arrows represent incorporation and release of amino acids to and from muscle protein for these 2 particular amino acids. These dynamic changes need to be taken into account when measuring the amino acid and protein turnover using tracer kinetic methods.
We have developed a less invasive approach toward measuring both tissue synthesis and breakdown (and thus net balance) that does not require catheterization of femoral vessels or measurement of tissue blood flow (7, 17, 28). This approach involves giving a bolus injection of labeled amino acids and taking peripheral blood samples and muscle biopsies. In addition to being less invasive than arterial and venous catheterization, the new approach also has the advantages that the measurement of blood flow is not required and that it is logistically simpler to give a bolus of tracer rather than a constant infusion of tracer. We have found that the new approach generates results that are comparable with the A-V approach (7, 28) under controlled conditions. Nevertheless, this new approach also assumes the presence of a physiological steady state, which would not be expected to apply if one wants to measure the acute response to perturbations such as ingestion of a bolus of amino acids or protein. Therefore, the goal of the present experiment was to modify the bolus tracer injection approach to determine both fractional sysnthesis rate (FSR) and fractional breakdown rate (FBR) of leg muscle protein during a physiological non-steady state. We have assessed the validity of the new approach by comparing simultaneously the FSR and FBR computed by threonine and phenylalanine tracers when a large unlabeled phenylalanine bolus is given. The rationale was that after the bolus injection there was a non-steady state of phenylalanine kinetics, whereas threonine kinetics remained stable. Thus, the FBR value calculated from the threonine tracer served as a reference to evaluate the validity of the measurement from the phenylalanine tracer, which was under a non-steady-state condition due to the concomitant injection of a large dose of unlabeled phenylalanine.
METHODS
Animals.
We used male New Zealand white rabbits (Myrtle's Rabbitry, Thompson Station, TN) weighing ∼4.5 kg. The rabbits were housed in individual cages and fed Lab Rabbit chow 5326 (Purina Mills, St. Louis, MO) for weight maintenance. This study was approved by the Animal Care and Use Committee of the University of Texas Medical Branch, Galveston, TX.
Unlabeled l-phenylalanine.
l-Phenylalanine was purchased from Sigma-Aldrich (St. Louis, MO).
Isotopes.
l-[ring-13C6]phenylalanine (99% enriched), U-13C9-15N-phenylalanine (99% enriched), l-[U-13C4,15N]threonine (99% enriched), and [15N]threonine (99% enriched) were purchased from Cambridge Isotope Laboratories (Woburn, MA).
Experimental design.
The anesthetic and surgical procedures were described in our previous studies (28). In brief, after an overnight food deprivation with free access to water, the rabbits were anesthetized with ketamine and xylazine. The hair of the skin on the neck and legs was shaved, and catheters were placed in the right jugular vein and left carotid artery. The venous line was used for infusion of anesthetics and saline. The arterial line was used for collection of arterial blood and monitoring of heart rate and mean arterial blood pressure. A second venous line was placed in an ear vein for bolus amino acid injection. A tracheal tube was placed via tracheotomy. We studied five rabbits. Each rabbit underwent two identical tracer studies on the same day, except that the amount of unlabeled phenylalanine was 30 (low dose) and 50 mg/kg (high dose) for the first and the second studies, respectively (Table 1). The doses of l-[ring-13C6]phenylalanine were 6 and 8.3 mg/kg for the first and the second studies, respectively (Table 1). After the first tracer study, the rabbits remained anesthetized for 2 h of the “tracer washout period” before the second study.
Table 1.
General characteristics
| Parameters | Body Weight, kg | Rectal Temperature, °C | Heart Rate, beats/min | MAP, mmHg | Unlabeled Phe, mg/kg | Phe Tracer, mg/kg | Thr Tracer, mg/kg |
|---|---|---|---|---|---|---|---|
| Baseline | 4.56 ± 0.04 | 37.9 ± 0.2 | 150 ± 7 | 72 ± 1 | |||
| Study 1 | 37.9 ± 0.1 | 148 ± 1 | 68 ± 1 | 30 | 6 | 20 | |
| Study 2 | 38.0 ± 0.2 | 157 ± 2 | 56 ± 1* | 50 | 8.3 | 20 |
Values are means ± SE.
MAP, mean arterial blood pressure; Phe, phenylalanine; Thr, threonine.
Data for rectal temperature, heart rate, and MAP are averages of 3 measurements during the experiments. Unlabeled Phe, Phe tracer, and Thr tracer are the concentrations of unlabeled Phe, Phe, and Thr tracers, respectively (mg/kg), injected during the study periods.
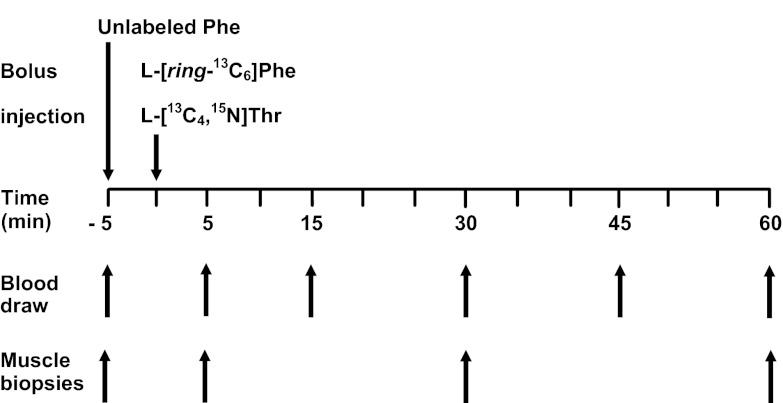
The infusion protocol is presented in Fig. 2. After the baseline blood and muscle samples were obtained, a dose of unlabeled phenylalanine was injected intravenously (30–50 mg/kg) within 30 s (Fig. 2). Five minutes after the injection of unlabeled phenylalanine, l-[ring-13C6]phenylalanine (6–8.3 mg/kg) and l-[U-13C4,15N]threonine (20 mg/kg) were injected within 30 s. Arterial blood was drawn at 5, 15, 30, 45, and 60 min, and muscle samples from vastus lateralis were taken at 5, 30, and 60 min after the tracer injection. Plasma was separated from blood by centrifugation and was stored at −80°C for later analysis. The muscle samples were immediately frozen in liquid nitrogen; afterwards, the samples were stored at −80°C before later analysis.
Fig. 2.
Stable isotope tracer protocol. Unlabeled l-phenylalanine (Phe) was injected at −5 min, and l-[ring-13C6]Phe and l-[U-13C4,15N]threonine (Thr) were injected at 0 min. Muscle biopsies were obtained at baseline and at 5, 30, and 60 min. Blood was drawn at baseline and at 5, 15, 30, 45, and 60 min.
Arterial blood pressure, heart rate, and rectal temperature were maintained stable by adjusting the infusion rates of anesthetics and 0.9% sodium chloride as well as a heating lamp. The vital signs were recorded at baseline before the infusion of unlabeled phenylalanine and thereafter every 30 min for the duration of the study.
Sample analyses.
To 0.5 ml of plasma, [U-13C9-15N]phenylalanine (70 nmol/ml) and [15N]threonine (250 nmol/ml) were added for calculation of phenylalanine and threonine concentrations; the volume of the internal standard solution of phenylalanine added to each plasma (0.5 ml) was 0.05 or 0.1 ml to account for the decline of unlabeled phenylalanine concentration after the bolus injection; the volume of the internal standard solution of threonine was 0.05 ml. The t-butyldimethylsilyl derivatives of amino acids were prepared from the supernatant of plasma (26).
Muscle samples were processed as we have described previously (15, 18). [U-13C9-15N]phenylalanine (25 nmol/ml) and [15N]threonine (65 nmol/ml) were added to the muscle sample at a ratio of 2 and 1.5 μl to 1 mg of wet tissue to measure the concentration of intramuscular free phenylalanine and threonine, respectively, in nmol/mg of wet tissue. The samples were homogenized in 100 g/l perchloric acid. The supernatant was separated for measurement of intracellular free phenylalanine and threonine enrichment and concentration. The protein precipitate was further processed to measure muscle-bound phenylalanine and threonine enrichment and content. To measure muscle phenylalanine and threonine content (nmol/mg dry tissue), [U-13C9-15N]phenylalanine (1.5 nmol/l) and [15N]threonine (7 nmol/l) were added to the tissue sample at a ratio of 2 μl to 1 mg of dry tissue. Afterwards, samples were hydrolyzed with 6 N hydrochloric acid and further processed for t-butyldimethylsilyl derivatives of amino acids.
Calculations.
We calculated muscle protein FBR using both the traditional FBR formula (Eq. 1) (3, 7) and a new formula (Eq. 2):
| (1) |
where EM(t) is the enrichment of intracellular free amino acids, EA(t) is the enrichment of plasma amino acids at time t, QM/T is the ratio of free intracellular amino acid pool size to protein-bound pool size.
The new formula was derived with the addition of two new assumptions to the traditional FBR approach, namely that inward transport of amino acids is proportional to the plasma amino acid concentration and that protein breakdown is constant over the time period when the measurement is made. If these assumptions hold, the FBR can be calculated by the following formula (see appendix for derivation):
| (2) |
where EM(t) is the enrichment of intracellular free amino acids, EA(t) is the enrichment of plasma amino acids, CA(t) is the concentration of plasma amino acids, and R(t) is the ratio of bound to free intracellular amino acids at time t.
Muscle protein FSR was calculated using the traditional precursor-product formula (26):
| (3) |
where EB(t) is the enrichment of bound amino acids and EM(t) is the enrichment of intracellular free amino acids at time t. This approach also assumes that during the sampling interval the rate of synthesis is constant.
Net protein balance (NB) was calculated using the following formula:
| (4) |
Statistical analysis.
Isotopic enrichments were expressed as tracer/tracee ratio for FBR calculation and as mole percent excess (MPE) for FSR calculation, as required by the methods (26, 28). The differences between the measurements calculated by different methods or tracers were evaluated using Student's t-test. Correlation analyses were performed by examining the Pearson product moment. All data are expressed as means ± SE, and a P value of ≤0.05 was considered statistically significant.
RESULTS
The general characteristics of the animals are presented in Table 1. There were no significant changes in body temperature and heart rate during the tracer studies, and the averages are presented in Table 1. Mean arterial pressure decreased toward the end of study, and the values were significantly lower during the second study period compared with the first and the baseline values (Table 1).
There were no differences in measured values of FBR and FSR between the two study protocols; therefore, we present the combined data from both study periods here.
Enrichments (tracer/trace ratio) and concentrations (nmol/ml) of plasma phenylalanine and threonine during the study are presented in Table 2. Injection of unlabeled and labeled phenylalanine increased the plasma concentration and enrichment of phenylalanine severalfold. Injection of labeled threonine also increased the threonine enrichment. The plasma concentration of threonine increased from baseline initially by 36%. After the initial increase in the concentration and enrichment, all parameters declined exponentially toward the end of the study, except for the plasma threonine concentration, which remained constant for the duration of the study (Table 2). These data, along with the data on intramuscular enrichment (Table 3), presented as tracer/tracee ratio and intramuscular concentration of phenylalanine and threonine (Table 4), and the ratio of free phenylalanine and threonine to protein-bound phenylalanine and threonine (Q/T; Table 4) were used to calculate muscle FBR using Eqs. 1 and 2. All of the measurements of the enrichment in a given sample are measured in duplicate. We calculated the coefficient of variation in a given set of samples (study no. 2), and the coefficient of variation between two measurements within this set of samples varied between 0.005 and 0.09. The protein-bound contents of phenylalanine and threonine, to calculate Q/T, were measured in tissue samples of individual rabbits, and the averages for phenylalanine and threonine were 59.3 ± 2.6 and 66.4 ± 2.4 nmol/mg wet tissue, respectively.
Table 2.
Plasma phenylalanine and threonine concentration and enrichment data
| CA, nmol/ml |
EA (Tracer/Tracee Ratio) |
|||
|---|---|---|---|---|
| Phe | Thr | Phe | Thr | |
| Pre | 89 ± 7 | 112 ± 6 | 0 | 0 |
| 5 Min | 715 ± 96 | 154 ± 6 | 0.2034 ± 0.0096 | 2.5333 ± 0.0802 |
| 15 Min | 451 ± 52 | 150 ± 6 | 0.1320 ± 0.0072 | 1.2410 ± 0.0636 |
| 30 Min | 339 ± 36 | 148 ± 5 | 0.1062 ± 0.0032 | 0.7159 ± 0.0185 |
| 45 Min | 283 ± 28 | 150 ± 4 | 0.0919 ± 0.0021 | 0.5024 ± 0.0146 |
| 60 Min | 259 ± 26 | 150 ± 4 | 0.0794 ± 0.0014 | 0.3842 ± 0.0132 |
Data are means ± SE. Pre, basal/baseline enrichment of the tracers; CA, concentrations of plasma Phe and Thr, expressed in nmol/ml; EA, enrichments of plasma Phe and Thr, expressed as tracer/tracee ratio.
Table 3.
Enrichments of phenylalanine and threonine in the muscle
| Enrichment (Tracer/Tracee Ratio) |
||||
|---|---|---|---|---|
| MIF pool |
Bound pool |
|||
| Phe | Thr | Phe | Thr | |
| Pre | 0 | 0 | 0 | 0 |
| 5 Min | 0.1114 ± 0.0077 | 0.5245 ± 0.0379 | 0.00028 ± 0.00005 | 0.00176 ± 0.00011 |
| 30 Min | 0.1192 ± 0.0071 | 0.6111 ± 0.0185 | 0.00031 ± 0.00004 | 0.00189 ± 0.00010 |
| 60 Min | 0.0997 ± 0.0049 | 0.4598 ± 0.0125 | 0.00032 ± 0.00011 | 0.00198 ± 0.00009 |
Data are means ± SE and expressed as tracer/tracee ratio. MIF pool, enrichment of Phe and Thr in muscle intracellular free (MIF) pool; bound pool, enrichment of the tracer in muscle tissue-bound pool.
Table 4.
Concentration of intramuscular free phenylalanine and threonine and Q/T
| Concentration, nmol/mg wet tissue |
||||
|---|---|---|---|---|
| MIF pool |
Q/T |
|||
| Phe | Thr | Phe | Thr | |
| Pre | 0.137 ± 0.035 | 0.189 ± 0.013 | 544 ± 66 | 364 ± 24 |
| 5 Min | 0.481 ± 0.064 | 0.185 ± 0.013 | 142 ± 19 | 374 ± 30 |
| 30 Min | 0.394 ± 0.048 | 0.177 ± 0.013 | 168 ± 22 | 395 ± 34 |
| 60 Min | 0.298 ± 0.029 | 0.179 ± 0.011 | 208 ± 20 | 377 ± 19 |
Data are means ± SE. Q/T, free-to-bound ratio. The Q/T was calculated using protein-bound Phe and Thr content in tissue samples of individual rabbits. The average contents were 59.3 ± 2.6 and 66.4 ± 2.4 nmol/mg wet tissue for Phe and Thr, respectively.
Calculated values of FBR are presented in Table 5. The FBR was calculated using the traditional (Eq. 1) and the modified approaches (Eq. 2) using both phenylalanine and threonine tracers. When the FBR was calculated by the modified approach, the difference between the values derived from the phenylalanine and threonine tracers was ∼11%. The correlation analysis demonstrated that there was a statistically significant relationship between the FBR measurements calculated by the modified model using the two tracers (r2 = 0.707, P < 0.05). When FBR was calculated using the threonine tracer, the difference between the two models was ∼4% (r2 = 0.987, p < 0.05). However, when the phenylalanine tracer was used (when there was a non-steady state of phenylalanine due to injection of unlabeled phenylalanine), the traditional approach gave a value that was 59% lower than the one calculated using the modified approach (r2 = 0.05, P > 0.05). This value was also ∼61% lower than the one calculated by the traditional model using the threonine tracer (r2 = 0.08, P > 0.05; Table 5).
Table 5.
The values of muscle protein FBR measured using the traditional and modified approaches and the values of FSR and NB
| FBR, %/h |
FSR, %/h |
NB, %/h |
||||
|---|---|---|---|---|---|---|
| Phe | Thr | Phe | Thr | Phe | Thr | |
| Traditional model | 0.055 ± 0.003 | 0.142 ± 0.002 | 0.069 ± 0.002 | 0.065 ± 0.001 | 0.014 ± 0.004 | −0.075 ± 0.002 |
| Modified model | 0.133 ± 0.003 | 0.148 ± 0.002 | −0.065 ± 0.002 | −0.081 ± 0.002 | ||
Data are means ± SE.
FBR, fractional breakdown rate; FSR, fractional synthesis rate; NB, net balance.
The values of muscle protein FSR, calculated using the traditional direct incorporation approach (Eq. 3) for both phenylalanine and threonine tracers, are presented in Table 5. The FSR was calculated from data obtained from samples collected 5 and 60 min after the tracer injection, and the data on the intramuscular free and protein-bound enrichment of phenylalanine and threonine are presented in Table 6. The enrichment data are expressed in MPE. There was a 2% difference between the averages of FSR values calculated using phenylalanine and threonine tracers.
Table 6.
Data on muscle Phe and Thr enrichment used for calculation of muscle protein FSR
| MIF, MPE |
Protein Bound, MPE |
|||
|---|---|---|---|---|
| Phe | Thr | Phe | Thr | |
| 5 Min | 0.0957 ± 0.0052 | 0.3415 ± 0.0147 | 0.000275 ± 0.000051 | 0.001760 ± 0.000110 |
| 30 Min | 0.0996 ± 0.0052 | 0.3669 ± 0.0106 | 0.000313 ± 0.000044 | 0.001887 ± 0.000099 |
| 60 Min | 0.0877 ± 0.0033 | 0.3257 ± 0.0105 | 0.000323 ± 0.000048 | 0.001976 ± 0.000087 |
Data are means ± SE.
MPE, mole percent excess. The enrichment values of Phe and Thr in MIF and protein-bound fractions are expressed in MPE.
Using FBR and FSR values, we calculated the leg muscle protein NB using Eq. 4, and the data are presented in Table 5. The average NB calculated by the traditional approach using phenylalanine tracer kinetics was 63% higher (P > 0.05) than the average NB calculated using the modified approach (Table 5). It was also higher by 78 (P > 0.05) and 76% (P > 0.05) compared with the values calculated using threonine tracer by traditional and modified approaches, respectively (Table 5). The difference between the values of NB calculated by the modified approach using phenylalanine and threonine tracers was ∼4% (P > 0.05).
DISCUSSION
Our goal was to develop a stable isotope tracer method to measure muscle protein FBR during physiological non-steady state. We used a bolus injection of unlabeled phenylalanine to develop the physiological non-steady state of phenylalanine and labeled phenylalanine and threonine to assess muscle protein FBR. We performed measurements using both a previously described approach (traditional) (26, 28), which does not account for the physiological non-steady state, and the new (modified) approach, which accounts for the non-steady state. The results obtained from phenylalanine and threonine tracers using the new approach were significantly correlated (r2 = 0.707, p < 0.05), which supports the notion that we have properly accounted for the non-steady state in the free phenylalanine pools. Thus, the new approach gives reliable results and satisfactorily achieves our goals.
The main difference between the new and the traditional approaches is based on two assumptions, namely that the inward transport of amino acids into the tissue is proportional to the plasma amino acid concentration and that the tissue protein breakdown is constant over the time period when the measurement is made. The first assumption is reasonable following the amino acid supplementation and is supported by previous reports. Volpi et al. (24) studied phenylalanine kinetics (utilizing an A-V balance model) during the basal or postabsorptive period and after the oral administration of mixed amino acids. They showed that ingestion of amino acids increased both the plasma concentration of phenylalanine and leg muscle phenylalanine inward transport to about the same extent. These observations are consistent with a more detailed assessment of the regulation of in vivo amino acid transport rates that we performed in pigs (17). The assumption that muscle breakdown is constant over the time of study is probably reasonable as well, since the shifts in rates of protein synthesis and breakdown are not as rapid as the shifts in amino acid transport, and the time between sample collection is reasonably short (55 min). In addition, if a large dose of phenylalanine inhibited muscle protein breakdown, it would equally affect FBR measured by both phenylalanine and threonine tracers, so it does not affect the comparison or validation. And we also did not detect any differences between the FBR values measured using both tracers when low and high doses of unlabeled phenylalanine were used. For example, the FBRs calculated using phenylalanine tracer and the modified formula were 0.14 vs. 0.13%/h, when 30 and 50 mg/kg of unlabeled phenylalanine, respectively, were given (by Student's t-test, 2-tailed paired analysis, P = 0.95). No assumption is required regarding the rate that unlabeled and labeled amino acids are transported out of muscle (see appendix for details).
In the present study, threonine was used as an independent amino acid tracer to evaluate the validity of the phenylalanine tracer in determining muscle protein FBR. Phenylalanine and threonine are not metabolized to any substantial extent in the muscle (5, 9, 11, 18, 22), but they are incorporated into muscle protein at different amounts due to the composition of muscle protein (8); thus we used a higher dose of threonine, which resulted in higher values of threonine enrichment (Table 6). However, the relative A-V kinetics (e.g., ratio of phenylalanine NB to threonine NB) should remain constant over time irrespective of any changes in the absolute amounts of phenylalanine and threonine retained in muscle proteins. This allows prediction of the phenylalanine kinetics on the basis of the threonine kinetics when measured during a steady state in the free threonine concentrations and irrespective of any changes in free phenylalanine concentrations at the same time. Our current results, which demonstrate similar results obtained using phenylalanine and threonine tracers and the modified approach, support the validity of using threonine as an independent “control” tracer.
The averaged FBR value calculated by the traditional approach using phenylalanine tracer was about 61% lower than the one calculated using threonine. In the traditional approach, we assume that the transport doesn't change, which is apparently not correct since one would expect the transport change with the change in concentration. And the current results, obtained using the modified approach, demonstrate the validity of the new approach. Interestingly, the FSR measurement was the same for both tracers, indicating that although the same assumption of steady-state conditions applies for that calculation as for the FBR, violation of this assumption is less important in the case of the calculation of FSR. Additionally, if there are changes in both the precursor enrichment and the total rate of incorporation of amino acids into protein, then the traditional FSR formula will generally not be accurate without modifications (26). In this study, the precursor enrichment was changing, but the total rate of incorporation of amino acids into protein was likely constant. Stated another way, the amount of phenylalanine given apparently did not stimulate muscle protein synthesis (e.g., FSR calculated using phenylalanine tracer did not differ between the studies conducted using low and high doses, 30 and 50 mg/kg, of unlabeled phenylalanine). The P value was 0.6 when a two-tailed paired Student t-test analysis was performed. Smith et al. (20), but not others (12, 14), demonstrated that an injection of a flooding dose of phenylalanine increased incorporation of tracer into human skeletal muscle protein. Thus there is still a controversy over whether phenylalanine by itself can stimulate muscle protein synthesis, but in our study, apparently no stimulation occurred (16). The modified FBR calculation presented here does not require protein synthesis to be constant over the time period of the measurement. If this study is conducted under conditions in which protein synthesis is changing, then the FSR calculation (but not the FBR calculation) will need further correction. Additionally, the current study was conducted using a bolus injection of unlabeled phenylalanine, and thus the effect of a meal has not been validated. This can be a subject of further independent studies in both animal models and humans.
We observed an increase in plasma threonine concentration 5 min after injection of tracers and unlabeled phenylalanine. This increase in plasma threonine is unexpected, and we do not have an explanation as to why this happened. However, the formula only accounts for the change in plasma amino acid concentrations between the 5- and 60-min time points, and since the concentration of plasma threonine remained at steady state between these time points, the initial increase in threonine does not affect the calculations.
The model allows the use of only one bolus, not two or three boluses, of different isotopes to conduct the study. If there is a steady state of amino acids, then the decay of enrichment of boluses will be the same regardless of when the bolus was given. Because of this, it is possible to extrapolate the decay of enrichment of one bolus to another. However, in the non-steady state (e.g., the current design), the decay of the enrichment of the boluses given at different times will be different. Therefore, it is impossible to use the decay curve of one bolus to estimate the decay curve of another bolus given at a different time; consequently, two or three boluses of tracers cannot be used with this non-steady-state method.
In conclusion, the new approach to calculate the muscle protein FBR during physiological non-steady state after amino acid ingestion gives reliable results. This new approach can increase the reliability of the measurement of the response of muscle protein metabolism to anabolic stimuli such as amino acid intake.
GRANTS
This work was supported by National Institutes of Health Grants P30-AG-024832 and DK-034817 and Shriners Grants 84090 and 85600.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.T., D.L.C., and X.-J.Z. contributed to the conception and design of the research; D.T., D.L.C., and X.-J.Z. performed the experiments; D.T., D.L.C., and X.-J.Z. analyzed the data; D.T., D.L.C., D.N.H., X.-J.Z., and R.R.W. interpreted the results of the experiments; D.T. prepared the figures; D.T., D.L.C., and X.-J.Z. drafted the manuscript; D.T., D.L.C., D.N.H., X.-J.Z., and R.R.W. edited and revised the manuscript; D.T., D.L.C., D.N.H., X.-J.Z., and R.R.W. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Yunxia Lin, Cindy Locklin, Carrie Barone, and Matthew Cotter for excellent technical assistance, Guy Jones and Jariwala Guarang for performing gas chromatography-mass spectrometry analyses, and Steve Schuenke for excellent technical assistance in the preparation of this manuscript.
Please contact the corresponding author directly via e-mail to obtain copies of Excel and Word files to use with the current model.
APPENDIX
Derivation of FBR formula in the non-steady state.
The following is the formula for calculating the FBR of muscle protein:
| (A1) |
where EM(t) is the enrichment of intracellular free amino acids, EA(t) is the enrichment of arterial amino acids, CA(t) is the concentration of arterial amino acids, and R(t) is the ratio of bound to free intracellular amino acids at time t.
The purpose of this appendix is to derive Eq. A1. We will assume that the intracellular free amino acid pool acts like a single pool and that the intracellular free amino acid pool size [denoted as QM(t)] is changing at rate QM′(t). We will assume that the rate at which the intracellular free amino acid pool size is changing is equal to the rate at which unlabeled amino acids enter the intracellular free pool via protein breakdown (denoted as PB) and inward transport (denoted as FM,A) minus the rate that unlabeled amino acids exit the intracellular free pool via protein synthesis (denoted as PS) and outward transport (denoted FV,M), as expressed by the following equation:
| (A2) |
We assume here that there is no other source of amino acids. We will define the variable p(t) by the formula
| (A3) |
Equation A3 can be rearranged to give
| (A4) |
so that Eq. A2 can be written as
| (A5) |
or
| (A6) |
We will assume that the tracer enters the intracellular space only from blood. Under most circumstances, the enrichment of bound protein will be a thousandfold lower than the blood enrichment, so this is a reasonable assumption. The rate that tracer enters into the intracellular space at a given time, t, will thus be equal to the rate that tracee is transported into the cell times the tracer/tracee ratio of blood amino acids, i.e., EA(t) × FM,A(t). Likewise, the rate at which the tracer leaves the intracellular space (via both transport and incorporation into protein) at a given time, t, will be equal to the rate at which the tracee leaves the intracellular space times the tracer/tracee ratio of intracellular amino acids, i.e., EM(t) × PS(t) + FV,M(t).
The rate that the intracellular tracer pool size is changing [denoted as qM′(t)] will be equal to the rate that the tracer enters the intracellular pool minus the rate that the tracer leaves the intracellular pool, i.e.,
| (A7) |
Now, since the intracellular enrichment is by definition equal to the ratio of the tracer/tracee intracellular pool sizes [i.e., EM(t) = qM(t)/QM], we can conclude that
| (A8) |
Differentiating both sides of Eq. A8 with respect to time gives
| (A9) |
So combining Eqs. A7 and A9 gives
| (A10) |
If we substitute Eqs. A4 and A6 into Eq. A10, then
| (A11) |
If we subtract EM(t) × QM(t) from both sides of the above equation and then divide both sides of Eq. A11 by QM(t), we get
| (A12) |
To proceed further, we will assume that protein breakdown is constant and that the inward transport is proportional to the arterial concentration [denoted as CA(t)]. Since by definition p(t) = FM,A (t)/PB(t), p(t) must be proportional to the arterial concentration, so we will define the proportionality constant (denoted as b) to satisfy the relationship:
| (A13) |
Therefore, Eq. A12 can be written as
| (A14) |
If we integrate both sides of Eq. A14 from time t1 to time t2, we get
| (A15) |
which can be rearranged to get
| (A16) |
The above equation applies for any two time points, so it also applies for time points t2 and t3:
| (A17) |
If we solve Eq. A17 for b, we obtain
| (A18) |
If we substitute Eq. A18 into Eq. A16, we get
| (A19) |
Solving for PB gives
| (A20) |
By definition, FBR = PB/T, where T is the pool size of amino acids in bound protein. If T/QM(t) is defined as R(t), then
| (A21) |
which is the desired formula.
REFERENCES
- 1.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147: 755–763, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Biolo G, Chinkes D, Zhang XJ, Wolfe RR. Harry M. Vars Research Award. A new model to determine in vivo the relationship between amino acid transmembrane transport and protein kinetics in muscle. JPEN J Parenter Enteral Nutr 16: 305–315, 1992 [DOI] [PubMed] [Google Scholar]
- 3.Biolo G, Gastaldelli A, Zhang XJ, Wolfe RR. Protein synthesis and breakdown in skin and muscle: a leg model of amino acid kinetics. Am J Physiol Endocrinol Metab 267: E467–E474, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Burchart K, Beca S, Urban RJ, Sheffield-Moore M. Pathogenesis of muscle wasting in cancer cachexia: targeted anabolic and anticatabolic therapies. Curr Opin Clin Nutr Metab Care 13: 410–416, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castagné V, Moënnoz D, Finot PA, Maire JC. Effects of diet-induced hyperthreoninemia. I. Amino acid levels in the central nervous system and peripheral tissues. Life Sci 53: 1803–1810, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Chinkes DL. Methods for measuring tissue protein breakdown in vivo. Curr Opin Clin Nutr Metab Care 8: 534–537, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Chinkes DL, Zhang XJ, Aarsland A, Norbury WB, Ferrando AA, Wolfe RR, Herndon DN. Comparison of A-V and precursor-product methods for measuring muscle protein breakdown in burned patients (Abstract). FASEB J 22: 869.29, 2008 [Google Scholar]
- 8.Clowes GH, Jr, Randall HT, Cha CJ. Amino acid and energy metabolism in septic and traumatized patients. JPEN J Parenter Enteral Nutr 4: 195–205, 1980 [DOI] [PubMed] [Google Scholar]
- 9.Darling PB, Grunow J, Rafii M, Brookes S, Ball RO, Pencharz PB. Threonine dehydrogenase is a minor degradative pathway of threonine catabolism in adult humans. Am J Physiol Endocrinol Metab 278: E877–E884, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Evans WJ. What is sarcopenia? J Gerontol A Biol Sci Med Sci 50 Spec No: 5–8, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Edgar AJ. Molecular cloning and tissue distribution of mammalian l-threonine 3-dehydrogenases. BMC Biochem 3: 19, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garlick PJ, McNurlan MA, Preedy VR. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J 192: 719–723, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hedberg B, Angquist KA, Henriksson-Larsen K, Sjöström M. Fibre loss and distribution in skeletal muscle from patients with severe peripheral arterial insufficiency. Eur J Vasc Surg 3: 315–322, 1989 [DOI] [PubMed] [Google Scholar]
- 14.Jefferson LS, Rannels DE, Munger BL, Morgan HE. Insulin in the regulation of protein turnover in heart and skeletal muscle. Fed Proc 33: 1098–1104, 1974 [PubMed] [Google Scholar]
- 15.Jeschke MG, Chinkes DL, Finnerty CC, Kulp GA, Suman OE, Norbury WB, Branski LK, Gauglitz GG, Mlcak RP, Herndon DN. Pathophysiological response to severe burn injury. Ann Surg 248: 387–401, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katsanos CS, Chinkes DL, Sheffield-Moore M, Aarsland A, Kobayashi H, Wolfe RR. Method for the determination of the arteriovenous muscle protein balance during non-steady-state blood and muscle amino acid concentrations. Am J Physiol Endocrinol Metab 289: E1064–E1070, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Miller SL, Chinkes DL, MacLean DA, Gore D, Wolfe RR. In vivo muscle amino acid transport involves two distinct processes. Am J Physiol Endocrinol Metab 287: E136–E141, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg LE, Scrivre CR. Disorders of amino acid metabolism. In: Metabolic Control and Disease, edited by Bondy PK, Rosenberg LE. Philadelphia, PA: Sauners, 1980, p. 707–710 [Google Scholar]
- 19.Roubenoff R, Castaneda C. Sarcopenia—understanding the dynamics of aging muscle. JAMA 286: 1230–1231, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Smith K, Reynolds N, Downie S, Patel A, Rennie MJ. Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am J Physiol Endocrinol Metab 275: E73–E78, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. N Engl J Med 337: 1279–1284, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol Endocrinol Metab 277: E513–E520, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Volpi E, Kobayashi H, Mittendorfer B, Sheffield-Moore M, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr 78: 250–258, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol Endocrinol Metab 277: E513–E520, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Williams IH, Sugden PH, Morgan HE. Use of aromatic amino acids as monitors of protein turnover. Am J Physiol Endocrinol Metab 240: E677–E681, 1981 [DOI] [PubMed] [Google Scholar]
- 26.Wolfe RR, Chinkes DL. Isotopic Tracers in Metabolic Research. Principles and Practice of Kinetic Analysis. Hoboken, NJ: Wiley-Liss, 2005 [Google Scholar]
- 27.Wolfson L, Judge J, Whipple R, King M. Strength is a major factor in balance, gait, and the occurrence of falls. J Gerontol A Biol Sci Med Sci 50 Spec No: 64–67, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Zhang XJ, Chinkes DL, Wolfe RR. Measurement of muscle protein fractional synthesis and breakdown rates from a pulse tracer injection. Am J Physiol Endocrinol Metab 283: E753–E764, 2002 [DOI] [PubMed] [Google Scholar]