Abstract
Autoregulation is critical for protecting the kidney against arterial pressure elevation and is compromised in some forms of hypertension. Evidence indicates that activated lymphocytes contribute importantly to cardiovascular injury in hypertension. We hypothesized that activated lymphocytes contribute to renal vascular dysfunction by impairing autoregulation and P2X1 receptor signaling in ANG II-infused hypertensive rats. Male Sprague-Dawley rats receiving ANG II infusion were treated with a lymphocyte proliferation inhibitor, mycophenolate mofetil (MMF) for 2 wk. Autoregulation was assessed in vitro and in vivo using the blood-perfused juxtamedullary nephron preparation and anesthetized rats, respectively. ANG II-treated rats exhibited impaired autoregulation. At the single vessel level, pressure-mediated afferent arteriolar vasoconstriction was significantly blunted (P < 0.05 vs. control rats). At the whole kidney level, renal blood flow passively decreased as renal perfusion pressure was reduced. MMF treatment did not alter the ANG II-induced hypertensive state; however, MMF did preserve autoregulation. The autoregulatory profiles in both in vitro or in vivo settings were similar to the responses from control rats despite persistent hypertension. Autoregulatory responses are linked to P2X1 receptor activation. Accordingly, afferent arteriolar responses to ATP and the P2X1 receptor agonist β,γ-methylene ATP were assessed. ATP- or β,γ-methylene ATP-induced vasoconstriction was significantly attenuated in ANG II-infused hypertensive rats but was normalized by MMF treatment. Moreover, MMF prevented elevation of plasma transforming growth factor-β1 concentration and lymphocyte and macrophage infiltration in ANG II-infused kidneys. These results suggest that anti-inflammatory treatment with MMF prevents lymphocyte infiltration and preserves autoregulation in ANG II-infused hypertensive rats, likely by normalizing P2X1 receptor activation.
Keywords: autoregulation, inflammation, renal blood flow, mycophenolate mofetil, lymphocytes, afferent arteriole
efficient renal autoregulatory function is critical for controlling renal perfusion and for maintaining stable glomerular capillary pressure in the face of arterial pressure (AP) fluctuations. Renal autoregulation is accomplished by precise adjustments of preglomerular resistance. A decline in afferent arteriolar autoregulatory efficiency renders downstream glomerular capillaries vulnerable to glomerular capillary hypertension. Prolonged glomerular hypertension can lead to glomerulosclerosis and chronic renal failure. Studies from both hypertensive human subjects and animal models indicate that reduced autoregulatory efficiency can precede renal structural changes, particularly in African Americans (27). This suggests that early vascular change is a key feature of renal dysfunction in hypertension (2, 27). However, the underlying mechanisms for this critical decline of function remain uncertain.
ATP is an endogenous ligand of P2 purinoceptors and activates members of two P2 receptor subfamilies, P2X and P2Y. Evidence indicates that ATP is released from many renal cell types and acts as an autocrine and paracrine factor in regulating vascular reactivity, autoregulation, and tubular transport (1, 14, 18). ATP can be released from macula densa cells in response to tubuloglomerular feedback stimulation causing afferent arteriolar vasoconstriction (18). ATP concentration in the interstitial fluid is directly correlated with pressure-mediated preglomerular microvascular resistance and the tubuloglomerular feedback activation (14, 25). P2X1 receptors are predominantly expressed in afferent arterioles (4, 34) where they regulate preglomerular microvascular resistance and autoregulatory behavior (14).
Chronic ANG II infusion not only impairs renal autoregulation, but it also attenuates afferent arteriolar responses to ATP and P2X1 receptor activation (17, 34). Recent studies indicate that activated lymphocytes contribute to the development of hypertension and renal injury (5–7, 11, 23). We hypothesized that activated lymphocytes contribute to hypertension-induced renal vascular injury by impairing autoregulation and P2X1 receptor signaling in ANG II-infused hypertensive rats. We found that immunosuppression with mycophenolate mofetil (MMF), a lymphocyte proliferation inhibitor (24), preserved normal autoregulatory behavior in ANG II-infused hypertensive rats in vitro at the single afferent arteriole level and in vivo at a whole kidney level despite persistent hypertension. At the same time, MMF also preserved P2X1 receptor-mediated afferent arteriolar vasoconstriction and significantly reduced albuminuria and proteinuria.
METHODS
Animal model and monitoring.
Male Sprague-Dawley rats (n = 199, Charles River Laboratories, Raleigh, NC) weighing between 225 and 250 g were used for experiments. Rats were fed standard chow (Harlan Teklad, Madison, WI) and had free access to water. All animals were treated according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals using procedures approved by the Institutional Animal Care and Use Committee at Georgia Health Sciences University.
Hypertension was induced using osmotic mini-pumps (Alzet, Cupertino, CA) filled with ANG II (60 ng/min sc for 13–14 days, Phoenix Pharmaceuticals, Belmont, CA) as previously described (10). Four groups were studied: control, control + MMF (CellCept, Roche Laboratories, Nutley, NJ), ANG II alone, and ANG II + MMF. MMF was delivered daily (30 mg·kg body wt−1·day−1) by gavage simultaneously during ANG II infusion to inhibit lymphocyte proliferation (24). MMF treatment began at the same time as the ANG II infusion. Systolic blood pressure was monitored weekly by tail cuff (IITC, Woodland Hills, CA). Additional rats from either ANG II-infused (n = 5) or ANG II + MMF (n = 6) groups were implanted with telemetry transmitters (Data Sciences, St. Paul, MN) as described (3). Limited telemetry measurements were included to confirm that MMF did not produce subtle blood pressure effects that were not detectable by tail-cuff plethysmography.
In vitro blood-perfused juxtamedullary nephron preparation.
Renal autoregulatory and microvascular reactivity studies were performed using the in vitro blood-perfused juxtamedullary nephron (JMN) preparation as described previously (10). Two identically treated rats were needed for each experiment (kidney and blood donors). Briefly, rats were anesthetized with pentobarbital sodium (50 mg/kg ip). The right kidney from the kidney donor was perfused with Tyrode's buffer containing 5.2% bovine serum albumin (Calbiochem, La Jolla, CA) and dissected for videomicrospy experiments. Blood was collected from the kidney donor and the blood donor and processed to remove neutrophils, lymphocytes, macrophages, and platelets. Plasma and washed erythrocytes were reconstituted to achieve a final hematocrit of ∼33%. Upon completion of all dissection procedures, and with the prepared kidney on the stage of a Nikon Eclipse E600FN microscope (Nikon, Tokyo, Japan) fitted with a Nikon water-immersion objective (×40), the kidney perfusate was switched to the reconstituted blood. The inner diameter of afferent arterioles was measured at a single site at 12-s intervals using a calibrated image-shearing monitor (model 908, Vista Electronics, Valencia, CA) and calculated from the average of all measurements obtained during the final 2 min of each 5-min treatment period. The following protocols were conducted:
Autoregulatory behavior of afferent arterioles.
Briefly, each kidney began with a 5-min control period at a perfusion pressure of 100 mmHg. Autoregulatory responses were assessed by measuring the diameter responses when perfusion pressure was reduced to 65 mmHg (5 min) and then increased in 15-mmHg increments to 170 mmHg over 5-min intervals. At the end of the autoregulatory protocol, perfusion pressure was returned to 100 mmHg and norepinephrine (0.1 μM) was applied to determine arteriolar reactivity. Four groups (n = 6–7) were studied: control, control + MMF, ANG II, and ANG II + MMF groups. Since MMF did not change blood pressure or afferent arteriolar autoregulatory behavior in control rats, the control + MMF group was omitted from subsequent experiments.
Afferent arteriolar response to ATP and β,γ-methylene ATP.
Since P2X1 receptors are predominantly expressed in afferent arterioles and are involved in regulating renal autoregulation (14), we directly assessed arteriolar reactivity to endogenous P2 receptor ligand ATP and a P2X1 receptor agonist β,γ-methylene ATP in separate groups using the JMN preparation. Briefly, after a control period (100 mmHg), kidneys from control, ANG II, or ANG II + MMF rats (n = 6–7 for each group) were superfused with increasing ATP or β,γ-methylene ATP concentrations from 0.01 to 100 μM (5-min intervals).
Whole kidney autoregulatory behavior.
To establish that the in vitro observations are consistent with in vivo conditions, experiments were performed in pentobarbital sodium-anesthetized rats in vivo to assess autoregulation of renal blood flow (RBF), as previously described (26). Briefly, rats were anesthetized and the left femoral artery was cannulated to monitor AP. RBF was measured using an ultrasonic flow probe (Transonic Systems, Ithaca, NY) placed around the left renal artery. After a 60-min stabilization period, autoregulatory responses were determined by reducing perfusion pressure from baseline to 70 mmHg in 10-mmHg decrements by adjusting a clamp positioned around the abdominal aorta just proximal to the left renal artery. Perfusion pressure was maintained for 2 min at each step. RBF was recorded using CHART 5 (ADInstruments, Colorado Springs, CO). The protocol was repeated 30–60 min after the ocludder was released. The pressure-RBF relationship was plotted from the average of two repetitions in each rat. Three groups (n = 6–9) were studied in control, ANG II, and ANG II + MMF groups.
For assessment of autoregulatory efficiency, autoregulatory index (AI) was calculated by the change in total RBF over a defined perfusion pressure range from baseline (initial) AP to 100 and 70–90 mmHg, respectively. The equation was follows (26): AI = [(RBF1 − RBF2)/RBF1]/[(AP1 − AP2)/AP1], where subscript 1 is the initial value before pressure changes, and subscript 2 is the value after reducing pressure. When the AI is 0, there is no change in flow in response to the change in pressure, indicating perfect autoregulation. Conversely, when the AI has unit value of 1, changes in pressure cause passive changes in flow, indicating an absence of autoregulation.
Albumin and protein excretion.
Rats were individually housed in metabolic cages on days 0, 6, and 12 of ANG II infusion for a 24-h urine collection. Albumin and protein were measured by ELISA (Bethyl Laboratories, Montgomery, TX) and Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA), respectively.
Plasma TGF-β1 concentration.
Total plasma TGF-β1 concentration was measured using a TGFβ1 Emax ImmunoAssay kit (Promega, Madison, WI), as described previously (10).
Lymphocyte and macrophage infiltration in kidneys.
Kidney sections were prepared for immunohistochemical staining of CD3 and ED-1 as markers of lymphocytes and macrophages, respectively, as described previously (3). Briefly, the right renal artery was cannulated and flushed with Tyrode's buffer to remove erythrocytes, followed by perfusion with 4% paraformaldehyde solution. The kidney was isolated and cut longitudinally into three portions. The middle portion was immersed in 4% paraformaldehyde overnight. Kidney sections (4 μm) were prepared and incubated with primary antibodies to CD3 (Santa Cruz Biotechnology, Santa Cruz, CA) for lymphocytes or ED-1 (CD68; Serotec, Kidlington, UK) for macrophages in humidity chambers at 4°C overnight. The slides were subsequently incubated with secondary antibodies using immunoCruz goat staining kit (Santa Cruz Biotechnology) for CD3 and peroxidase-conjugated goat anti-mouse IgG (Serotec) for ED-1, respectively. Positively stained cells were counted in an area of 500 × 500 μm of renal cortex (×20 magnification) in a blinded fashion. An average of 20 fields per kidney was used to quantify total cellular infiltration.
Statistical analysis.
All values are expressed as group means ± SE. Within-group analysis was conducted using one-way ANOVA for repeated-measures test followed by post hoc analysis (Dunnett's multiple range test). Differences between groups, within each series, were determined by one-way ANOVA and Newman-Keuls multiple range test. P < 0.05 was considered a significant difference.
RESULTS
Effect of MMF on ANG II-induced hypertension.
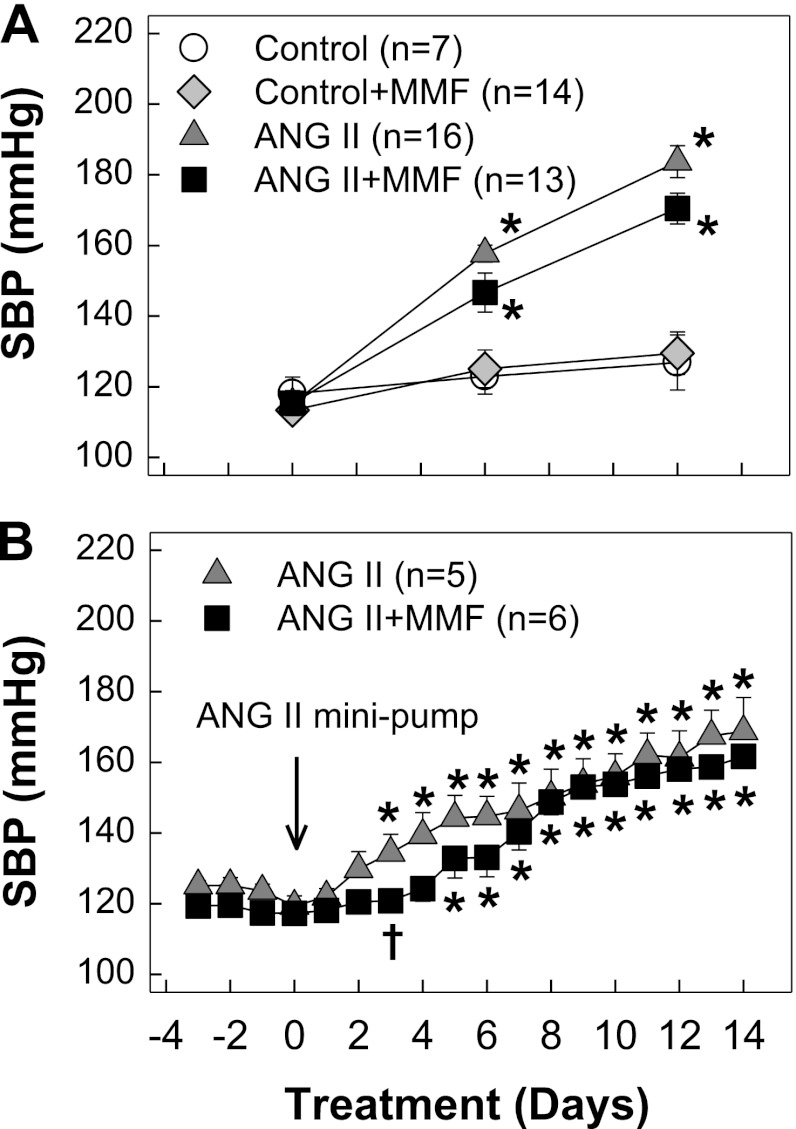
Rats receiving chronic ANG II infusion developed hypertension as monitored by tail cuff (Fig. 1A). The magnitude and progression of hypertension were similar in ANG II alone and ANG II + MMF groups (184 ± 5 vs. 171 ± 4 mmHg, P > 0.05). This result is consistent with telemetry results except the slight lag in the development of hypertension in MMF-treated ANG II-hypertensive rats compared with ANG II infusion alone rats on day 3 (P < 0.05) as shown in Fig. 1B.
Fig. 1.
Systolic blood pressure (SBP). A: SBP was measured by tail-cuff plethysmography in control (white circles), control + mycophenolate mofetil (MMF; 30 mg·kg−1·day−1, gray diamonds), ANG II alone (60 ng/min, gray triangles), and ANG II + MMF (black squares). B: SBP was monitored by telemetry in ANG II alone (gray triangles) and ANG II + MMF (black squares). Values are means ± SE. *P < 0.05, significant difference from day 0. †P < 0.05, significant difference between ANG II and ANG II + MMF groups on the same day.
MMF preserved autoregulatory behavior of afferent arterioles.
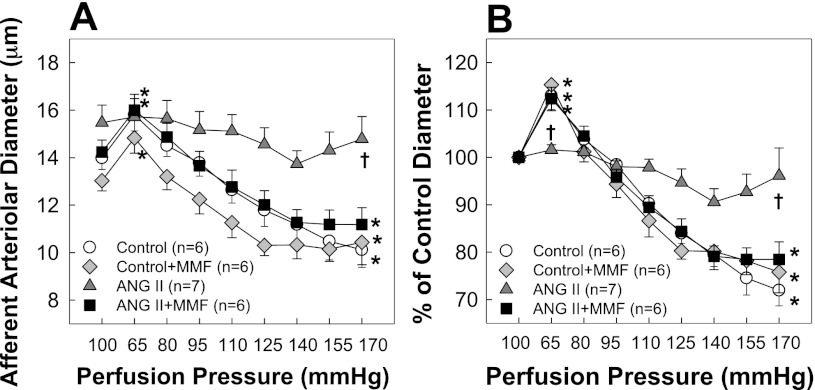
Autoregulatory behavior of afferent arterioles is shown in Fig. 2A (actual diameter) and Fig. 2B (% of control diameter). Baseline diameters of arterioles averaged 14.0 ± 0.5, 12.7 ± 0.4, 15.5 ± 0.7, and 14.2 ± 0.5 μm in control, control + MMF, ANG II, and ANG II + MMF rats, respectively (Fig. 2A). MMF did not change baseline diameter in control or ANG II-infused rats. Arterioles from control rats showed normal pressure-dependent autoregulatory behavior. As shown in Fig. 2B, arteriolar diameter increased to 113 ± 3% of control when perfusion pressure decreased from 100 to 65 mmHg and declined to 72 ± 3% after perfusion pressure was increased to 170 mmHg. Control rats receiving MMF exhibited a nearly identical autoregulatory profile as control rats. The ANG II alone group exhibited markedly blunted pressure-mediated autoregulatory behavior with arteriolar diameter declining to just 96 ± 6% of control at 170 mmHg. Importantly, ANG II + MMF rats exhibited normal autoregulatory behavior despite persistent hypertension. Arteriolar diameter increased to 112 ± 2% of control at 65 mmHg and decreased to 76 ± 5% of control at 170 mmHg. Pressure-mediated autoregulatory responses were preserved (P < 0.05 vs. ANG II alone rats) and indistinguishable from control rats (P > 0.05). Meanwhile, norepinephrine-mediated vasoconstriction was similar across all four groups. Arteriolar diameters decreased to 82 ± 3, 79 ± 3, 88 ± 4, and 80 ± 3% of control in response to 0.1 μM norepinephrine in control, control + MMF, ANG II, and ANG II + MMF rats, respectively (P > 0.05 vs. control rats).
Fig. 2.
Autoregulatory behavior of afferent arterioles. A: effect of renal perfusion pressure changes on afferent arteriolar diameter in control (white circles), control + MMF (30 mg·kg−1·day−1, gray diamonds), ANG II alone (60 ng/min, gray triangles), and ANG II + MMF (black squares) rats. B: data are expressed as percent of control diameter at 100 mmHg during equilibration period. Values are means ± SE. *P < 0.05, significant difference from control diameter. †P < 0.05, significant difference between ANG II and ANG II + MMF groups. Significant differences were obtained at 65-, 110-, 125-, 140-, 155-, and 170-mmHg pressure steps.
MMF preserved afferent arteriolar reactivity to ATP.
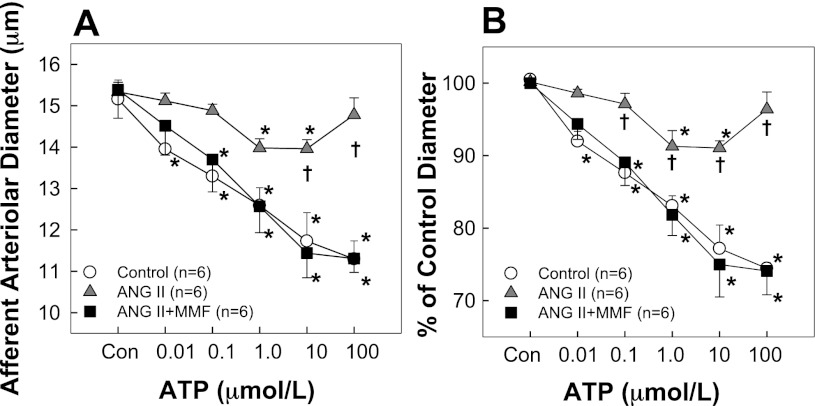
The afferent arteriolar response to ATP is illustrated in Fig. 3. Baseline diameters (Fig. 3A) were similar in all three (15.2 ± 0.5, 15.3 ± 0.2, and 15.4 ± 0.7 μm in control, ANG II, and ANG II + MMF, respectively). Superfusion of ATP caused concentration-dependent vasoconstriction in control rats with diameter decreasing to 74 ± 1% of the control diameter from 15.2 ± 0.5 to 11.3 ± 0.4 μm after exposure to 100 μM ATP. In contrast, ATP-mediated vasoconstriction was significantly blunted in rats receiving ANG II alone. Afferent arteriolar diameter declined to a maximum of just 91 ± 1% of control at concentrations of 1.0 or 10 μM ATP (Fig. 3B). Interestingly, ANG II + MMF rats exhibited normal ATP-mediated vasoconstriction yielding a vasoconstrictor profile that was similar to the response in control rats (P > 0.05) but significantly different from ANG II-infused rats (P < 0.05).
Fig. 3.
Afferent arteriolar responses to ATP. A: effects of ATP on afferent arteriolar diameters in control (white circles), ANG II infusion alone (60 ng/min, gray triangles), and ANG II + MMF (30 mg·kg−1·day−1, black squares) rats, respectively. B: data are expressed as percent of control diameter at 100 mmHg during equilibration period. Values are means ± SE. *P < 0.05, significant difference from control diameter. †P < 0.05, significant difference from ANG II + MMF rats.
MMF preserved afferent arteriolar reactivity to β,γ-methylene ATP.
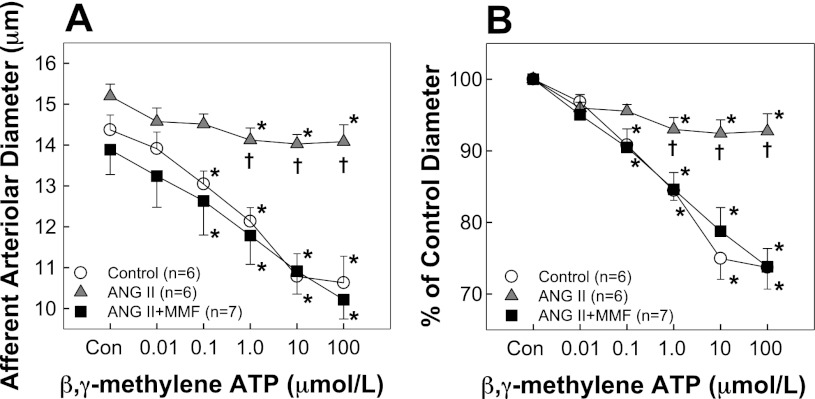
The afferent arteriolar response to the P2X1 receptor agonist β,γ-methylene ATP is depicted in Fig. 4. Baseline diameters (Fig. 4A) averaged 14.8 ± 0.4, 15.2 ± 0.3, and 13.9 ± 0.6 μm (P > 0.05) in control, ANG II, and ANG II + MMF, respectively. Superfusion of β,γ-methylene ATP caused concentration-dependent vasoconstriction in control rats with diameter decreasing from a baseline of 14.8 ± 0.4 μm to 74 ± 3% of control in response to 100 μM β,γ-methylene ATP (Fig. 4B). However, chronic ANG II infusion markedly blunted the vasoconstriction to β,γ-methylene ATP. Diameter decreased from 15.2 ± 0.3 to 14.1 ± 0.4 μm representing 93 ± 2% of the control diameter (Fig. 4B), whereas β,γ-methylene ATP-mediated vasoconstriction was preserved in ANG II + MMF rats.
Fig. 4.
Afferent arteriolar responses to β,γ-methylene ATP. A: effects of β,γ-methylene ATP on afferent arteriolar diameters in control (gray circles), ANG II infusion alone (60 ng/min, gray triangles), and ANG II + MMF (30 mg·kg−1·day−1, gray squares) rats, respectively. B: data are expressed as percent of control diameter at 100 mmHg during equilibration period. Values are means ± SE. *P < 0.05, significant difference from control diameter. †P < 0.05, significant difference from ANG II + MMF rats.
MMF preserved autoregulation of RBF.
We also assessed RBF autoregulation in anesthetized rats in vivo. Figure 5 presents actual (Fig. 5A) and normalized (Fig. 5B) RBF as a function of mean AP (MAP). Baseline MAP averaged 119 ± 1, 153 ± 3, and 152 ± 4 mmHg and RBF (Fig. 5A) averaged 8.7 ± 0.7, 7.7 ± 0.4, and 9.9 ± 0.6 ml·min−1·g kidney wt−1 in control (n = 6), ANG II alone (n = 9), and ANG II + MMF (n = 9) rats, respectively. In control rats, RBF remained constant during stepwise reductions of MAP from 119 to 100 mmHg (Fig. 5), indicating intact RBF autoregulation. In contrast, RBF progressively decreased in ANG II alone rats. RBF declined to 80 ± 3% of the initial RBF after reducing MAP from the baseline MAP to 100 mmHg (Fig. 5B). AI (Fig. 5C) averaged 0.67 ± 0.09 in ANG II rats (P < 0.05 vs. −0.05 ± 0.12 in control rats), indicating impaired autoregulation in ANG II-infused hypertensive rats. Importantly, ANG II + MMF rats exhibited normal autoregulatory behavior. Stepwise decreases in MAP from the baseline to 100 mmHg declined RBF to just 95 ± 2% of the initial RBF (Fig. 5B) yielding an AI of 0.13 ± 0.07 (Fig. 5C), which is similar to control rats and significantly more efficient than rats receiving ANG II alone (P < 0.05).
Fig. 5.
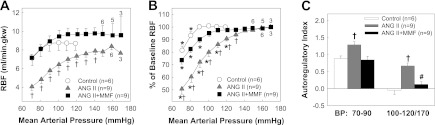
Autoregulation of renal blood flow (RBF) in vivo. Autoregulation of RBF in anesthetized rats from control (white circles), ANG II alone (60 ng/min, gray triangles), and ANG II + MMF (30 mg·kg−1·day−1, black squares). A: actual RBF against mean arterial pressure (MAP). B: normalized RBF as percent of baseline RBF against MAP. Numbers above or below each symbol indicate the animal number studied at each level of MAP. Each point represents the means ± SE. *P < 0.05 vs. baseline RBF in same group. †P < 0.05, significant difference between ANG II and ANG II + MMF groups. C: autoregulatory index over the range from initial arterial pressure to 100 and 70–90 mmHg, respectively. †P < 0.05 vs. control rats. #P < 0.05 vs. ANG II alone.
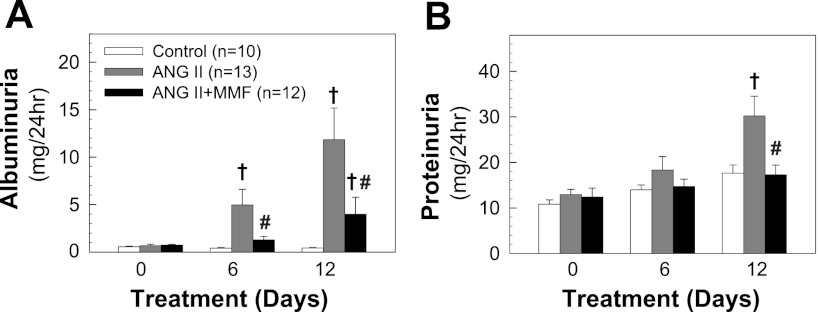
MMF reduced urinary excretion of albumin and protein.
Urinary albumin and protein excretion were depicted in Fig. 6, A and B, respectively. As shown in Fig. 6A, baseline urinary albumin excretion was similar among groups. ANG II infusion markedly increased albuminuria by day 6 (5.0 ± 1.6 mg/day) and continued to increase to 11.8 ± 3.3 mg/day by day 12 (P < 0.05 vs. control rats). In contrast, MMF treatment markedly reduced albuminuria by 3.8-fold (1.3 ± 0.4 mg/day) on day 6 and 3-fold (4.0 ± 1.8 mg/day) on day 12 (P < 0.05 vs. ANG II). ANG II infusion alone also increased urinary protein excretion on day 12 (Fig. 6B, 30.2 ± 4.3 vs. 17.6 ± 1.8 mg/day in control rats, P < 0.05), but not when simultaneously treated with MMF (17.3 ± 2.1 mg/day, P < 0.05 vs. ANG II alone).
Fig. 6.
Urinary albumin and protein excretion. Urine (24 h) collected on days 0, 6, and 12. A: albumin excretion. B: protein excretion. Values are means ± SE. †P < 0.05 vs. control rats. #P < 0.05 vs. ANG II alone rats in the same period of urine collection.
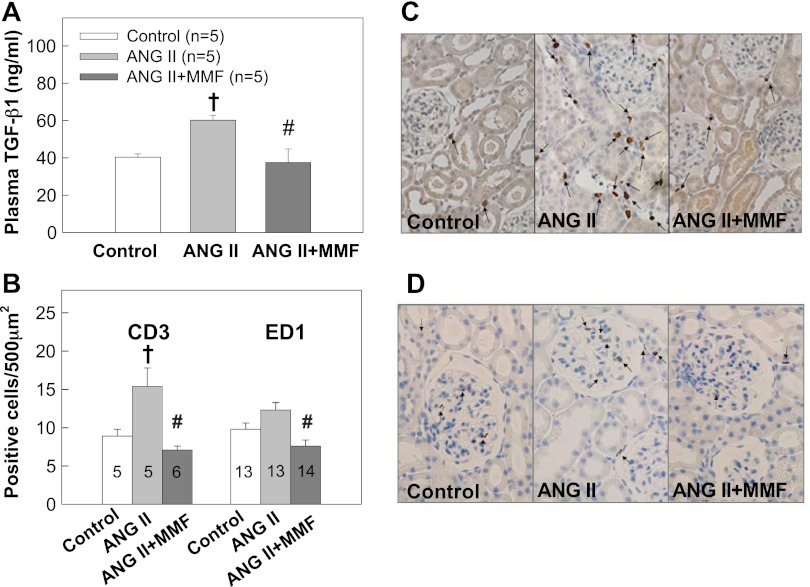
MMF prevented elevation of plasma TGF-β1 concentration.
Plasma TGF-β1 concentration (Fig. 7A) increased significantly in ANG II-infused rats (60 ± 3 vs. 41 ± 2 ng/ml in controls, P < 0.05), but was normalized by MMF treatment (38 ± 7 ng/ml, P < 0.05 vs. ANG II).
Fig. 7.
Plasma transforming growth factor (TGF)-β1 concentration and lymphocyte and macrophage infiltration in renal cortex. A: total plasma TGF-β1 concentration. B: representative graphs of CD3 (left columns)- and ED-1 (right columns)-positive cells, markers for lymphocytes and macrophages, respectively, in renal cortex. Numbers inside each column indicate the numbers of rats from each group. A total of 20 fields per kidney was counted at ×40 magnification. C and D: representative histological images of CD3- and ED-1-positive cells for lymphocytes and macrophages, respectively, in renal cortex. Values are expressed as means ± SE. †P < 0.05 vs. control rats. #P < 0.05 vs. ANG II alone rats.
MMF reduced lymphocyte and macrophage infiltration.
The numbers of CD3- or ED-1-positive cells in renal cortical tissue sections were counted and the data are presented in Fig. 7B. The number of CD3-positive cells increased significantly in ANG II rats (P < 0.05 vs. control), but not in ANG II + MMF rats (P < 0.05 vs. ANG II), indicating an increased lymphocyte infiltration in ANG II alone rats. Macrophage infiltration also tended to increase in ANG II rats but did not reach significance compared with control rats. However, MMF treatment significantly reduced the number of macrophages in ANG II rats (P < 0.05 vs. ANG II alone rats). Figure 7, C and D, represents histological images of CD3- and ED-1-positive cells in cortical tissue, respectively.
DISCUSSION
The current study demonstrates that treatment with the immunosuppressor MMF, a potent inhibitor of lymphocyte proliferation, preserves autoregulatory behavior in ANG II-infused hypertensive rats in vitro at the single afferent arteriole level and in vivo at the whole kidney level. MMF treatment also retains afferent arteriolar responsiveness to P2X1 receptor stimulation, despite the fact the degree of high blood pressure was comparable between ANG II and ANG II + MMF groups. Furthermore, MMF treatment protects glomeruli against hypertension-associated injury as indicated by significant decreases in albuminuria and proteinuria. We conclude that reduced glomerular injury reflects improved autoregulatory function, inhibition of lymphocyte, and macrophage infiltration in the kidney and/or by preventing elevation of plasma TGF-β1 concentration.
Growing evidence implicates inflammation, especially lymphocytes, in the progression of hypertensive cardiovascular and renal disease (13, 30). In the present study, we applied MMF, an inhibitor of lymphocyte proliferation, in chronic ANG II-infused hypertensive rats and directly assessed renal autoregulatory behavior at the afferent arteriole and at the whole kidney levels. Consistent with previous in vitro studies (10, 17, 34), afferent arteriolar autoregulatory behavior is attenuated in ANG II-hypertensive rats. Importantly, treatment with MMF preserved normal afferent arteriolar autoregulatory behavior such that it was indistinguishable from control arterioles despite having a similar degree of hypertension development and severity as ANG II alone rats.
We also assessed whole kidney autoregulation in vivo. Consistent with in vitro findings, ANG II-hypertensive rats exhibited a passive pressure/flow relationship indicating impaired autoregulation. In contrast, in rats receiving ANG II infusion + MMF treatment, RBF remained essentially constant when renal perfusion pressure was manipulated within the autoregulatory range. As indicated by the AI, autoregulatory efficiency was essentially normal in ANG II + MMF rats, whereas it was significantly impaired in ANG II alone rats. Interestingly, RBF at baseline AP tended to be higher in MMF-treated ANG II rats compared with ANG II alone rats despite similar blood pressures. Although in the current study we did not assess endothelial function of afferent arterioles, it is well-established that chronic ANG II infusion leads to endothelial dysfunction, enhanced renal superoxide production, and increased renal vascular resistance (19, 33). Furthermore, Krotz et al. (20) reported that mycophenolic acid, the active form of MMF, reduces superoxide generation by inhibiting endothelial Nox2 activity. Therefore, it is possible that increased RBF in ANG II + MMF rats may reflect a beneficial effect of MMF by reducing superoxide production and/or preventing a lymphocyte-mediated inflammatory cascade on renal microvessels. Overall, these data support the idea that inflammatory processes contribute to hypertensive renal injury by impairing renal microvascular function and autoregulation.
ATP is the endogenous ligand for P2 receptor activation and is a postulated mediator of afferent arteriolar autoregulatory signaling (14, 18). P2X1 receptors are mainly present in preglomerular vessels (4, 34) and are involved in pressure-mediated vasoconstriction of afferent arterioles (14, 18). β,γ-Methylene ATP is a selective agonist for P2X1 and P2X3 receptors. Since P2X3 receptors are barely detected in preglomerular microvessels (12, 21), the effect of β,γ-methylene ATP in afferent arterioles is mostly attributed to activation of P2X1 receptors. In the current study, chronic ANG II infusion markedly blunted afferent arteriolar responses to ATP or β,γ-methylene ATP while the vasoconstriction to norepinephrine was similar to control rats. This indicates that the contractile apparatus of afferent arterioles is intact in ANG II-infused hypertensive rats, but the response to P2X1 receptor activation is impaired. Interestingly, MMF treatment preserved afferent arteriolar vasoconstriction to both ATP and β,γ-methylene ATP. These data provide compelling evidence that inflammatory factors contribute to renal microvascular dysfunction by reducing P2X1 receptor reactivity, thereby leading to autoregulatory impairment.
The mechanisms responsible for autoregulatory impairment in ANG II-infused hypertensive rats remain unclear. Impaired autoregulatory behavior and reduced microvascular reactivity to P2X1 receptor activation are consistently observed in ANG II-hypertensive rats (10, 34). Normalization of autoregulation and P2X1 reactivity by MMF implicate lymphocyte-dependent inflammatory mechanisms in compromising afferent arteriolar reactivity to autoregulation and P2X1 receptor activation. Since total P2X1 receptor protein expression is unchanged in preglomerular vessels from ANG II-infused hypertensive rats fed either normal or 8% salt diets (15, 34), the reduction in P2X1 receptor reactivity in ANG II hypertension cannot be explained by reduced P2X1 receptor protein expression. Importantly, ATP and P2X1 receptor-stimulated intracellular Ca2+ signaling is significantly attenuated in preglomerular smooth muscle cells of ANG II-infused hypertensive rats (15, 34), suggesting that trafficking of P2X1 receptors to the sarcolemma or alterations in P2X1 receptor signaling pathways may be compromised in ANG II-hypertensive rats. Attenuated P2X1-mediated signaling could arise from chronic increases in renal interstitial ATP concentration in ANG II-infused rats leading to receptor desensitization (8, 9), which may in turn blunt the downstream signaling and reduce autoregulatory efficiency.
Inflammatory factors are linked to development of hypertension and hypertension-associated renal injury (3, 6, 13, 30). ANG II is well-known as a proinflammatory stimulator. Previous work showed that the broad-spectrum, anti-inflammatory agent pentosan polysulfate (PPS) normalized P2X1 receptor reactivity and autoregulation in ANG II-induced hypertensive rats (10). Those data revealed a strong association between increased inflammatory status in ANG II-induced hypertension and renal microvascular dysfunction but they did not allow for identification of the cause of the inflammatory signal. To advance the inflammation hypothesis, in this project we focused on the role of lymphocyte activation and infiltration in kidneys by application of MMF, which is rapidly converted to its active form, mycophenolic acid in vivo. Mycophenolic acid is a potent inhibitor of inosine monophosphate dehydrogenase, a key enzyme for lymphocyte proliferation (24). Notably, suppression of lymphocyte activation and proliferation with MMF significantly reduced lymphocyte infiltration in cortical tissue and preserved both pressure- and P2X1 receptor-mediated vasoconstriction in ANG II-hypertensive rats. The progression and magnitude of hypertension induced by ANG II infusion, however, were not significantly altered by MMF, consistent with observations in the 5/6 nephrectomy, l-NAME- and ANG II-induced hypertensive models in rats or mice (5, 7, 22, 28) but different from what has been reported for the DOCA-salt and Dahl salt-sensitive hypertensive models (3, 6). Overall, the current results suggest that activated lymphocytes are associated with renal microvascular dysfunction in ANG II-hypertensive rats.
Furthermore, MMF also prevented elevation of plasma TGF-β1 concentration while preserving P2X1 receptor reactivity and autoregulatory behavior. Thus, our studies demonstrate a close association between elevated TGF-β1 and impaired renal autoregulation. Recent clinical studies indicate that plasma TGF-β1 levels are significantly higher in normotensive or mildly hypertensive African Americans compared with Caucasians (32). Hypertensive African Americans also exhibit less efficient autoregulation and increased prevalence toward renal disease compared with hypertensive Caucasians (27). Elevated plasma TGF-β1 in African Americans may predispose this population to renal disease by impairing renal autoregulation. The source of increased TGF-β is not clear. Roson and colleagues (29) showed that acute ANG II infusion for 2 h increased intrarenal expression of TGF-β1. Crowley et al. (5) reported that renal TGF-β mRNA expression increased significantly in ANG II-infused mice, but this was markedly attenuated by MMF treatment. Finally, acute exposure to TGF-β1 but not platelet-derived growth factor and hepatocyte growth factor attenuates afferent arteriolar autoregulatory behavior in normal rats (31). Collectively, these studies suggest that inflammatory conditions possibly involving TGF-β1 may contribute to autoregulatory impairment in hypertension.
Increased albuminuria and proteinuria are considered risk factors for cardiovascular and renal disease. In contrast to our previous study (10) where PPS failed to prevent albuminuria in ANG II-infused rats, MMF treatment significantly reduced urinary albumin and protein excretion. These different outcomes suggest that PPS, a semisynthetic glycosaminoglycan, might prevent renal vascular dysfunction through direct effects on vascular function per se, whereas MMF directly inhibits lymphocytes and macrophage infiltration and prevents inflammatory cascades from occurring in hypertensive kidneys, thereby preventing functional impairment and injury. In addition, there appeared to be a brief lag in hypertension initiation in ANG II-infused rats receiving MMF treatment, which could potentially contribute to the reduction of albumin excretion in that group.
As we found in our previous study (10), in this hypertensive rat model where ANG II was infused at a low dose with normal-salt diet, we did not find any significant histological changes in renal structure using conventional morphological assessment by light microscopy. However, rats receiving chronic ANG II infusion exhibited marked impairment of renal autoregulation even as early as day 6 after ANG II infusion (16, 34), highlighting the fact that functional abnormalities can be detected before structural abnormalities in the ANG II-infused hypertensive model. Reduced autoregulatory efficiency can accelerate glomerular injury in hypertensive subjects. The current study extends our previous work by demonstrating in vitro and in vivo that selective inhibition of lymphocyte proliferation with MMF ameliorates hypertension-associated renal microvascular dysfunction in ANG II-infused hypertensive rats by normalizing renal autoregulatory behavior and P2X1 receptor-mediated microvascular reactivity and suppressing TGF-β1 accumulation. These results suggest that inflammatory factors linked to lymphocyte activation may promote progressive renal injury in hypertensive populations by impairing renal microvascular function and autoregulatory efficacy. Minimizing renal inflammatory processes may provide a therapeutic intervention that will preserve renal hemodynamics and thereby prevent progressive glomerular injury in hypertensive populations.
GRANTS
This study was supported by grants from the National Institutes of Health (DK044628, HL074167, HL098135, and HL095499 for E. W. Inscho, HL095499 for J. S. Pollock, HL69999 and HL95499 for D. M. Pollock) and by a postdoctoral fellowship (0825465E for Z. Guan) from the Greater Southeast Affiliate of the American Heart Association.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Z.G. and E.W.I. conception and design of research; Z.G., M.I.G., D.A.O., A.K.C., J.L.H., and S.Z. performed experiments; Z.G., D.A.O., and T.Y. analyzed data; Z.G., J.S.P., D.M.P., and E.W.I. interpreted results of experiments; Z.G. prepared figures; Z.G. drafted manuscript; Z.G., D.M.P., and E.W.I. edited and revised manuscript; Z.G., M.I.G., D.A.O., A.K.C., J.L.H., S.Z., T.Y., J.S.P., D.M.P., and E.W.I. approved final version of manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge H. Ocasio for telemetry implantation support.
REFERENCES
- 1. Bailey MA, Shirley DG. Effects of extracellular nucleotides on renal tubular solute transport. Purinergic Signal 5: 473– 480, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bidani AK, Griffin KA, Williamson G, Wang X, Loutzenhiser R. Protective importance of the myogenic response in the renal circulation. Hypertension 54: 393– 398, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boesen EI, Williams DL, Pollock JS, Pollock DM. Immunosuppression with mycophenolate mofetil attenuates the development of hypertension and albuminuria in deoxycorticosterone acetate-salt hypertensive rats. Clin Exp Pharmacol Physiol 37: 1016– 1022, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan CM, Unwin RJ, Bardini M, Oglesby IB, Ford AP, Townsend-Nicholson A, Burnstock G. Localization of P2X1 purinoceptors by autoradiography and immunohistochemistry in rat kidneys. Am J Physiol Renal Physiol 274: F799– F804, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Crowley SD, Frey CW, Gould SK, Griffiths R, Ruiz P, Burchette JL, Howell DN, Makhanova N, Yan M, Kim HS, Tharaux PL, Coffman TM. Stimulation of lymphocyte responses by angiotensin II promotes kidney injury in hypertension. Am J Physiol Renal Physiol 295: F515– F524, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Miguel C, Das S, Lund H, Mattson DL. T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 298: R1136– R1142, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Franco M, Martinez F, Rodriguez-Iturbe B, Johnson RJ, Santamaria J, Montoya A, Nepomuceno T, Bautista R, Tapia E, Herrera-Acosta J. Angiotensin II, interstitial inflammation, and the pathogenesis of salt-sensitive hypertension. Am J Physiol Renal Physiol 291: F1281– F1287, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Gever JR, Cockayne DA, Dillon MP, Burnstock G, Ford AP. Pharmacology of P2X channels. Pflügers Arch 452: 513– 537, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Graciano ML, Nishiyama A, Jackson K, Seth DM, Ortiz RM, Prieto-Carrasquero MC, Kobori H, Navar LG. Purinergic receptors contribute to early mesangial cell transformation and renal vessel hypertrophy during angiotensin II-induced hypertension. Am J Physiol Renal Physiol 294: F161– F169, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guan Z, Fuller BS, Yamamoto T, Cook AK, Pollock JS, Inscho EW. Pentosan polysulfate treatment preserves renal autoregulation in ANG II-Infused hypertensive rats via normalization of P2X1 receptor activation. Am J Physiol Renal Physiol 298: F1276– F1284, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449– 2460, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harhun MI, Povstyan OV, Gordienko DV. Purinoreceptor-mediated current in myocytes from renal resistance arteries. Br J Pharmacol 160: 987– 997, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, hypertension. Hypertension 57: 132– 140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Inscho EW. ATP, P2 receptors and the renal microcirculation. Purinergic Signal 5: 447– 460, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Inscho EW, Cook AK, Clarke A, Zhang S, Guan Z. P2X1 receptor-mediated vasoconstriction of afferent arterioles in ANG II-infused hypertensive rats fed a high salt diet. Hypertension 57: 780– 787, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Inscho EW, Cook AK, Murzynowski JB, Imig JD. Elevated arterial pressure impairs autoregulation independently of AT(1) receptor activation. J Hypertens 22: 811– 818, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Inscho EW, Imig JD, Deichmann PC, Cook AK. Candesartan cilexetil protects against loss of autoregulatory efficiency in angiotensin II-infused rats. J Am Soc Nephrol 10, Suppl 11: S178–S183, 1999 [PubMed] [Google Scholar]
- 18. Komlosi P, Bell PD, Zhang ZR. Tubuloglomerular feedback mechanisms in nephron segments beyond the macula densa. Curr Opin Nephrol Hypertens 18: 57– 62, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Kopkan L, Castillo A, Navar LG, Majid DS. Enhanced superoxide generation modulates renal function in ANG II-induced hypertensive rats. Am J Physiol Renal Physiol 290: F80– F86, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Krotz F, Keller M, Derflinger S, Schmid H, Gloe T, Bassermann F, Duyster J, Cohen CD, Schuhmann C, Klauss V, Pohl U, Stempfle HU, Sohn HY. Mycophenolate acid inhibits endothelial NAD(P)H oxidase activity and superoxide formation by a Rac1-dependent mechanism. Hypertension 49: 201– 208, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Lewis CJ, Evans RJ. P2X receptor immunoreactivity in different arteries from the femoral, pulmonary, cerebral, coronary and renal circulations. J Vasc Res 38: 332– 340, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Mattar AL, Machado FG, Fujihara CK, Malheiros DM, Zatz R. Persistent hypertension and progressive renal injury induced by salt overload after short term nitric oxide inhibition. Clinics (Sao Paulo) 62: 749– 756, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Muller DN, Shagdarsuren E, Park JK, Dechend R, Mervaala E, Hampich F, Fiebeler A, Ju X, Finckenberg P, Theuer J, Viedt C, Kreuzer J, Heidecke H, Haller H, Zenke M, Luft FC. Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol 161: 1679– 1693, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nelson PH, Eugui E, Wang CC, Allison AC. Synthesis and immunosuppressive activity of some side-chain variants of mycophenolic acid. J Med Chem 33: 833– 838, 1990 [DOI] [PubMed] [Google Scholar]
- 25. Nishiyama A, Rahman M, Inscho EW. Role of interstitial ATP and adenosine in the regulation of renal hemodynamics and microvascular function. Hypertens Res 27: 791– 804, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Osmond DA, Inscho EW. P2X(1) receptor blockade inhibits whole kidney autoregulation of renal blood flow in vivo. Am J Physiol Renal Physiol 298: F1360– F1368, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Palmer BF. Disturbances in renal autoregulation and the susceptibility to hypertension-induced chronic kidney disease. Am J Med Sci 328: 330– 343, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Quiroz Y, Pons H, Gordon KL, Rincon J, Chavez M, Parra G, Herrera-Acosta J, Gomez-Garre D, Largo R, Egido J, Johnson RJ, Rodriguez-Iturbe B. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from nitric oxide synthesis inhibition. Am J Physiol Renal Physiol 281: F38– F47, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Roson MI, Cao G, Della Penna S, Gorzalczany S, Pandolfo M, Toblli JE, Fernandez BE. Angiotensin II increases intrarenal transforming growth factor-beta1 in rats submitted to sodium overload independently of blood pressure. Hypertens Res 31: 707– 715, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Schiffrin EL. T lymphocytes: a role in hypertension? Curr Opin Nephrol Hypertens 19: 181– 186, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Sharma K, Cook A, Smith M, Valancius C, Inscho EW. TGF-β impairs renal autoregulation via generation of ROS. Am J Physiol Renal Physiol 288: F1069– F1077, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Suthanthiran M, Gerber LM, Schwartz JE, Sharma VK, Medeiros M, Marion R, Pickering TG, August P. Circulating transforming growth factor-beta1 levels and the risk for kidney disease in African Americans. Kidney Int 76: 72– 80, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wilcox CS. Redox regulation of the afferent arteriole and tubuloglomerular feedback. Acta Physiol Scand 179: 217– 223, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Zhao X, Cook AK, Field M, Edwards B, Zhang S, Zhang Z, Pollock JS, Imig JD, Inscho EW. Impaired Ca2+ signaling attenuates P2X receptor-mediated vasoconstriction of afferent arterioles in angiotensin II hypertension. Hypertension 46: 562– 568, 2005 [DOI] [PubMed] [Google Scholar]