Abstract
Apelin and its receptor APJ have pleiotropic effects in mice and humans and play a protective role in cardiovascular diseases at least partially by inhibiting oxidative stress. Our objective was to study the effect of apelin on the progression of kidney disease in mice with established type 1 diabetes. Ove26 mice with type 1 diabetes received daily subcutaneous injections of apelin for 2 or 14 wk. APJ localizes in the glomeruli and blood vessels of kidneys. Renal APJ expression was reduced in diabetic mice but increased after treatment with apelin. Apelin treatment did not affect glycemia, body weight, or blood pressure in diabetic mice. Whole kidney and glomerular hypertrophy, as well as renal inflammation, including monocyte chemoattractant protein 1 and vascular cell adhesion molecule 1 expression, NF-κB activation, and monocyte infiltration, was inhibited after short and long treatment with apelin. Apelin administration significantly reduced albuminuria at 6 mo. Short treatment with apelin was sufficient to reverse the downregulation of the antioxidant enzyme catalase. Expression of angiotensin II and angiotensin type 1 receptor (AT1) in kidneys from diabetic mice treated was not affected by apelin. These findings show for the first time that apelin exerts a protective effect on the diabetic kidney. Short administration is sufficient to reduce kidney and glomerular hypertrophy as well as renal inflammation, but prolonged treatment is required to improve albuminuria. This effect was independent of the activation of the renin angiotensin system but correlated with upregulation of the antioxidant catalase. Apelin may represent a novel tool to treat diabetic nephropathy.
Keywords: diabetes, nephropathy, apelin, inflammation
diabetes is the most common cause of end-stage renal disease (1) and nephropathy affects 20% of patients with type 1 diabetes (60). Early manifestations of diabetic nephropathy include the development of hyperfiltration and hypertrophy, thickening of the glomerular basement membrane, and mesangial matrix expansion. This is followed by increased urinary albumin excretion, glomerulosclerosis, tubulo-interstitial fibrosis, and end-stage renal failure. Current treatment strategies, including tight glycemic control (7a) and inhibition of the renin angiotensin system (32), slow down progression of diabetic nephropathy but do not reverse the course of diabetic nephropathy. The renin-angiotensin-aldosterone system contributes importantly to the pathogenesis of diabetic nephropathy; thus the role played by recently identified novel modulators of angiotensin actions such as apelin would be of significant interest. However, little is known about the effect of apelin in the kidney.
Oxidative stress has been shown to play an important role in the development of diabetic nephropathy (4, 22). Oxidative stress is due to an imbalance between prooxidative pathways and antioxidant pathways. Antioxidant enzymes, such as catalase, are downregulated in the kidney of rodents with type 1 diabetes (27, 28). Induction of type 1 diabetes in catalase knockout mice results in worsened renal injury (23), and therapeutic interventions that improve renal function in diabetic rodents also restore catalase expression. This is the case for inhibitors of the renin-angiotensin system, angiotensin receptor blockers, or angiotensin-converting enzyme inhibitors (28) or ANG-(1–7) (19).
Apelin (APLN) is an endogenous ligand of the G protein-coupled receptor APJ (56). The APLN gene encodes a 77-amino acid precursor, preproapelin, that is cleaved by proteases and is secreted as APLN-36, -17, and -13, corresponding to the number of amino acids (44). These peptides are biologically active and bind to a single receptor APJ with different affinities (44). APLN signaling through APJ mediates a wide range of physiological actions on the vasculature, on immune responses, and on glycolipid metabolism (12, 44). APLN and APJ are expressed in the brain, lungs, heart, and kidneys, and APJ is expressed in neurons, endothelial cells, smooth muscle cells, and cardiomyocytes (44).
Several studies have shown that APLN plays a protective role in cardiovascular disease. APJ, the APLN receptor, is the most significantly upregulated gene among ∼12,000 after mechanical offloading of failing myocardium in patients with heart failure (5). Pretreatment with APLN-13 ameliorated the reduced heart function in rats after ischemia/reperfusion by upregulating antioxidative enzymes (66). Administration of APLN reduces cardiac fibrosis in mice infused with ANG II, by antagonizing ANG II signaling and stimulating production of nitric oxide (51). Several studies conducted in the cardiovascular system have demonstrated a reciprocal antagonism between the ANG II/AT1 and the APLN/APJ axes (3). For instance, activation of APJ by APLN lowers blood pressure while activation of AT1 by ANG II increases blood pressure, and ANG II causes left ventricular hypertrophy and fibrosis, whereas apelin increases cardiac output without causing hypertrophy (3). APLN and APJ levels are maintained in the compensated left ventricular hypertrophy phase in Dahl salt-sensitive rats but decline in heart failure; this decline is mediated by ANG II/AT1 receptor (26).
The regulation and role of the APLN/APJ axis in the normal or the diabetic kidney have not been studied. Plasma APLN concentration is increased in patients with type 1 diabetes (38), but neither regulation of expression of the APLN/APJ system nor its effect on diabetic kidneys has been studied. The current study evaluated whether short- or long-term administration of APLN to mice with established type 1 diabetes attenuates features of diabetic nephropathy. We found for the first time that APLN-13 exerts a protective effect on the diabetic kidney. Short treatment was sufficient to reduced kidney and glomerular hypertrophy as well as renal inflammation, but prolonged treatment was required to improve albuminuria. Apelin also restored the expression of the antioxidant enzyme catalase, which was downregulated in kidneys from diabetic mice. This indicates that the renoprotective effects of APLN-13 could be due to stimulation of antioxidant pathways. Apelin may represent a novel tool in the treatment of diabetic nephropathy.
METHODS
Mouse Model of Type 1 Diabetes
Experiments were carried out in male FVB/Ove26 and their wild-type littermates FVB/NJ (stock no. 005564; Jackson Laboratory), starting at 10 wk of age. Ove26 mice develop hyperglycemia soon after birth (67), and their age corresponds to the duration of diabetes. Control and diabetic mice received daily subcutaneous injections of vehicle (saline) or [pyr1]-APLN-13 (Sigma-Aldrich; 150 μg/kg of body wt) for up to 3 mo. All experiments were approved by the University of Texas Health Science Center, San Antonio, Institutional Animal Care and Use Committee.
Blood Pressure Measurement
Blood pressure was measured in conscious mice using a Hatteras Instruments MC4000MSP tail-cuff blood pressure monitoring system. Mice were trained for 5 days. On the day of recording, they were allowed to rest for 10 min after being placed in the holder before starting the measurement to avoid stress-related changes in blood pressure. Blood pressure was recorded over 30 cuff inflations after 10 training cuff inflations.
Albuminuria and Creatinine Clearance
Mice were placed in metabolic cages before death for 24-h urine collection, and blood was drawn at the time of death. Urine albumin was measured by ELISA using Albuwell M (Exocell, Philadelphia, PA), urine creatinine was measured by ELISA (Cell Biolabs, San Diego, CA), and plasma creatinine was measured by HPLC.
Renal Histochemistry and Morphometric Analysis
Kidneys were fixed in 10% formaldehyde and embedded in paraffin, and 4-μm sections were cut. Sections were stained with periodic acid Schiff (PAS) or Sirius red by the Cancer Therapy and Research Center at the University of Texas Health Science Center, San Antonio and were examined at ×400 in a blinded manner. Images were obtained with an Zeiss Axio Imager A1 microscope. Glomerular tuft area was determined after size calibration using the Image Pro Plus software.
Immunohistochemistry and Immunofluorescence
APJ and WT1 (wilms tumor protein 1) staining.
Frozen sections (4-μm sections) were fixed in cold acetone and quenched in 3% hydrogen peroxide for 6 min. After blocking with Background Sniper (Biocare, Concord, CA) for 20 min, slides were incubated with the rabbit polyclonal anti-WT-1 (1:250; sc-192; Santa Cruz) and anti-APJ (1:100, LifeSpan Biosciences LS-A64) overnight at 4°C in a humidified chamber. After being rinsed, slides were incubated with goat anti-rabbit polymer-horseradish peroxidase (Biocare, Concord, CA) for 20 min at room temperature. Immunoreactivity was visualized with 3-3′-diaminobenzidine (DAB; Biocare).
Cluster of differentiation 68, monocyte chemoattractant protein 1, vascular cell adhesion molecule 1, and megalin staining.
Antigen retrieval from paraffin-embedded sections (4 μm) was performed in citrate buffer at 100°C for 6 min. Primary antibodies [cluster of differentiation 68 (CD68), Serotec MCA 1957; monocyte chemoattractant protein 1 (MCP1), Abcam ab25124; vascular cell adhesion molecule 1 (VCAM1), Abbiotec 250908; and megalin, LifeSpan Biosciences LS-B105] were incubated overnight at 4°C in a humidified chamber. After being rinsed, slides were incubated with biotinylated secondary antibody for 20 min at room temperature. Streptavidin-peroxidase complex (LASB+ System-AP; DAKO) was used for amplification and immunoreactivity was visualized with Fast (DAKO).
Reverse Transcription and Quantitative RT-PCR
RNA was extracted from kidney cortex (∼50 μg of tissue) using Trizol (Invitrogen) and used for reverse transcription (RT) using iScript RT SuperMix (Bio-Rad). The resulting cDNAs were used for quantitative PCR using the RT2 qPCR Master Mix and the following primers from SABiosciences/Qiagen: VCAM1: cat. no. PPM03208B (RefSeq no. NM-011693); MCP1: cat. no. PPM03151G (RefSeq no. NM-011333); catalase: cat. no. PPM04394C (RefSeq no. NM-009804); and GAPDH: cat. no. PPM02946E (RefSeq no. NM-008084).
The quantitative (q)PCR was run in a MasterCycler RealPlex4 (Eppendorf). Quantitation of the mRNAs was performed using the 2−ΔΔCt method using GAPDH as a housekeeping gene.
Immunoblot Analysis
Immunoblot analysis was performed as described in (7, 13). Briefly, slices of kidney cortex were homogenized in lysis buffer (no. FNN-0071; Invitrogen) supplemented with protease inhibitor mix (P-2714; Sigma), 1 mM PMSF, and 5 mM orthovanadate. Protein concentration was measured and 10–20 μg of whole cell lysates were separated on SDS-PAGE, transferred to nitrocellulose membranes, and probed with anti-APJ (LS-A64; LifeSpan Biosciences), anti-MCP1 (ab25124; Abcam), VCAM1 (250906; Abbiotec), anti-p65 (8242; Cell Signaling Technology), anti-pp65 (3033; Cell Signaling Technology), anti-catalase (ab-16731; Abcam), anti-activated AT1 (905-743-100; Assay Design), anti-AT1 (LS-B4614; LifeSpan Biosciences) and anti-actin antibody (A-5441; Sigma), and IRDye800- or IRDye700-coupled secondary antibodies were used for detection using Odyssey Infrared Imaging System (LiCor Biosciences, Lincoln, NE).
Nuclear Extracts
Nuclear extracts were prepared using NE-PER nuclear and cytoplasmic extraction reagent kit (Thermo Scientific). Briefly, 20–100 mg of kidney tissue were cut into small pieces, washed once with cold PBS, and collected by centrifugation. Tissues were homogenized using a Dounce homogenizer in Cytoplasmic Extraction Reagent I (CER I) and incubated on ice for 10 min. Cytoplasmic Extraction Reagent II (CER II) was added, and samples were centrifuged. Nuclei pellets were resuspended in nuclear extraction reagent and incubated 10 min on ice, repeated for 40 min. After centrifugation, supernatants (nuclear extracts) were collected. Protein concentration was measured, and 20 μg of nuclear extracts were used for immunoblotting.
Chromatin Immunoprecipitation Assay
Chromatin immunoprecipitation (ChIP) assay was performed as described by Nelson et al. (41). Cross-linked chromatin from kidney cortex were sheared by sonication and used for immunoprecipitation using ChIP-validated p65 antibody (8242; Cell Signaling Technology) or control IgG (sc-3888; 1:100; Santa Cruz Biotechnology). DNA was purified from the immunoprecipitates using a 10% Chelex (Bio-Rad) solution supplemented with proteinase K (0.2 mg/ml final), incubated at 55°C for 30 min followed by boiling for 15 min, and used for quantitative PCR using primers amplifying the region spanning 1 kb 5′ of the transcription start site (+1, −1,000) of the VCAM1 promoter [cat. no. GPM1050041(-)01A; SABiosciences/Qiagen].
Renal Content of APLN-13 and ANG II
The renal content of APLN-13 and ANG II was measured by enzyme immunoassay kit from Phoenix Pharmaceuticals, (Burlingame, CA) according to manufacturer's instruction. Equal amounts of renal cortical homogenates (250 μg) were used for each sample.
Statistics
Data were expressed as means ± SE and ANOVA for comparison among multiple groups using the Tukey posttest analysis for comparison, using the GraphPad Prism 5.0 software. Values of P < 0.05 (after multiple comparison adjustment as needed) were considered significant.
RESULTS
Administration of Apelin to Mice
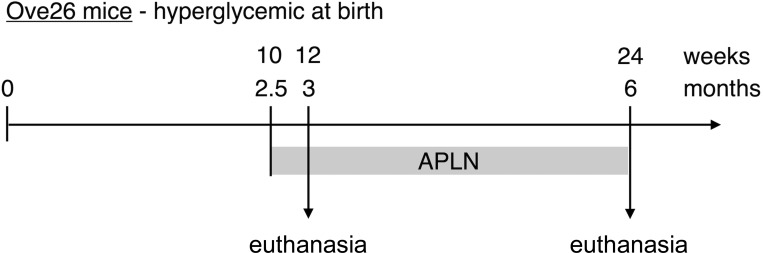
Ove26 mice develop hyperglycemia at birth and have established diabetic nephropathy by 3 mo of age (67). Diabetic Ove26 mice and control littermates received one daily subcutaneous injection of APLN-13 (150 μg·kg−1·day−1), starting at 10 wk (2.5 mo) of age. One group of mice was killed at 12 wk (3 mo) of age and the other group at 24 wk (6 mo) of age.
Clinical Parameters
Diabetic Ove26 mice and nondiabetic littermates were treated with one daily subcutaneous injection of apelin (150 μg·kg−1·day−1) at 2.5 mo of age. One group of mice was killed at 3 mo of age (and 2 wk of apelin treatment) and the group at 6 mo of age (and 3.5 mo of apelin treatment) (Fig. 1). Table 1 shows that apelin treatment did not reduce hyperglycemia in diabetic Ove26 mice at 3 and 6 mo. Diabetic mice lose a significant amount of body weight at 3 and 6 mo, and weight loss was not modified by apelin. Blood pressure was measured in 6-mo-old mice: diabetic Ove26 mice were significantly hypotensive, as reported by Zheng et al. (67), and apelin treatment did not affect blood pressure. Circulating levels of apelin have been reported to be higher in patients with type 1 diabetes (38), but the local contents of apelin have not been measured. Therefore, we measured intrarenal content of APLN-13 in kidney cortex homogenates by ELISA. Renal APLN-13 levels were decreased ∼40% in kidney cortex from diabetic mice at 3 and 6 mo of diabetes and were restored by apelin treatment. These data and the data from Fig. 2 suggest that APLN-13 upregulates its own expression and the expression of its receptor.
Fig. 1.
Experimental design. Diabetic Ove26 mice and control littermates were treated with 1 daily subcutaneous injection of apelin (150 μg·kg−1·day−1) at 10 wk (2.5 mo) of age. Mice were euthanized at 12 wk (3 mo) and 24 wk (6 mo) of age.
Table 1.
Clinical parameters
| 3 mo |
6 mo |
|||||
|---|---|---|---|---|---|---|
| Control | Diabetes | Diabetes + apelin | Control | Diabetes | Diabetes + apelin | |
| Glycemia, mg/dl | 145 ± 8 | 586 ± 14* | 560 ± 40* | 125 ± 7 | 552 ± 13* | 580 ± 10* |
| Body weight, g | 27 ± 1 | 19 ± 1* | 18 ± 1* | 30 ± 1 | 19 ± 1* | 17 ± 1* |
| Systolic blood pressure, mmHg | ND | ND | ND | 106 ± 5 | 86 ± 2* | 89 ± 2* |
| Renal apelin, pg/mg tissue | 27.8 ± 0.4 | 17.0 ± 0.4* | 28.8 ± 0.4† | 22.5 ± 0.4 | 16.2 ± 0.3* | 24.1 ± 0.4† |
Values are means ± SE. Glycemia and body weight were measured at the time of death, and systolic blood pressure was recorded using the tail-cuff method in 6-mo-old the week before death.
P < 0.01 vs. control,
P < 0.01 vs. diabetes by ANOVA.
Fig. 2.
Regulation of APJ in kidneys from diabetic mice. A: renal expression of APJ was studied by immunohistochemistry on sections of paraffin-embedded kidneys from 3-mo-old mice. Arrows point to glomeruli. Top, middle, and bottom: represent 3 different mice. B: APJ protein was measured by immunoblot on kidney cortex homogenates from 3-mo-old mice. Bottom: combined data obtained on kidneys from 5 individual mice for each experimental condition. C: APJ mRNA was measured by RT-quantitative (q)PCR on total RNA extracted from kidney cortex and normalized using GAPDH expression; n = 5 mice per group. Numbers inside brackets represent P values calculated by ANOVA; con, control.
Regulation of APJ in Diabetic Kidneys
Expression and localization of the apelin receptor APJ in mouse kidneys has not been reported. We performed immunohistochemistry on section of paraffin-embedded kidneys from 3-mo-old mice. Figure 2A shows that APJ is expressed in the blood vessels and the glomeruli in control NJ mice. In diabetic Ove26 mice, APJ expression was significantly reduced in both glomeruli and blood vessels and it was restored by a 2-wk treatment with APLN-13. We confirmed these results by performing immunoblot on kidney cortex homogenates using the same antibody. Figure 2B shows that APJ expression is reduced ∼60% in kidneys from 3-mo-old diabetic mice and is restored by a 2-wk treatment with APLN-13. APJ mRNA was measured by RT-qPCR on RNA extracted from kidney cortex from the same mice. Figure 2C shows that APJ mRNA was significantly decreased (∼70%) in diabetic Ove26 mice compared with control and that APJ mRNA expression was restored to control levels by APLN-13 treatment.
Effect of Apelin on Renal Hypertrophy
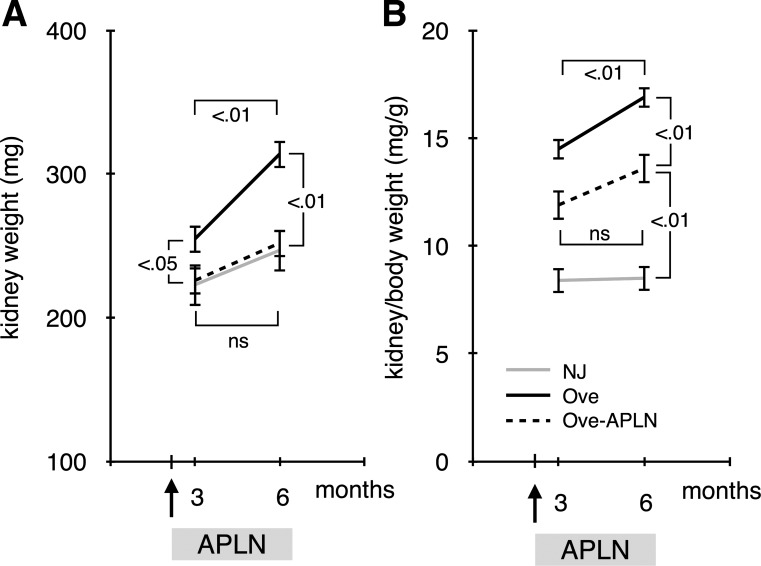
Kidney enlargement is one of the early manifestation of diabetic nephropathy. Figure 3A shows that kidney weight is significantly increased in 3-mo-old diabetic Ove26 mice compared with control NJ mice and that APLN-13 treatment restored kidney weight to control level. At 6 mo of age, kidney weight increased more rapidly in diabetic than in control mice and APLN-13 treatment prevented gain in kidney weight in diabetic Ove26 but not in control NJ mice. Kidney hypertrophy is defined as an increase in kidney-to-body weight ratio. Figure 3B shows that diabetic Ove26 mice developed progressive kidney hypertrophy that was significantly inhibited by APLN treatment. These data show that continuous APLN-13 treatment prevents progression of kidney hypertrophy in diabetic mice.
Fig. 3.
Effect of apelin on renal hypertrophy. A: kidney weight was measured at the time of death. B: renal hypertrophy was defined as increased kidney-to-body weight ratio; n = 5 mice per group. Numbers inside brackets represent P values calculated by ANOVA; ns, not significant.
Effect of Apelin on Glomerular Hypertrophy
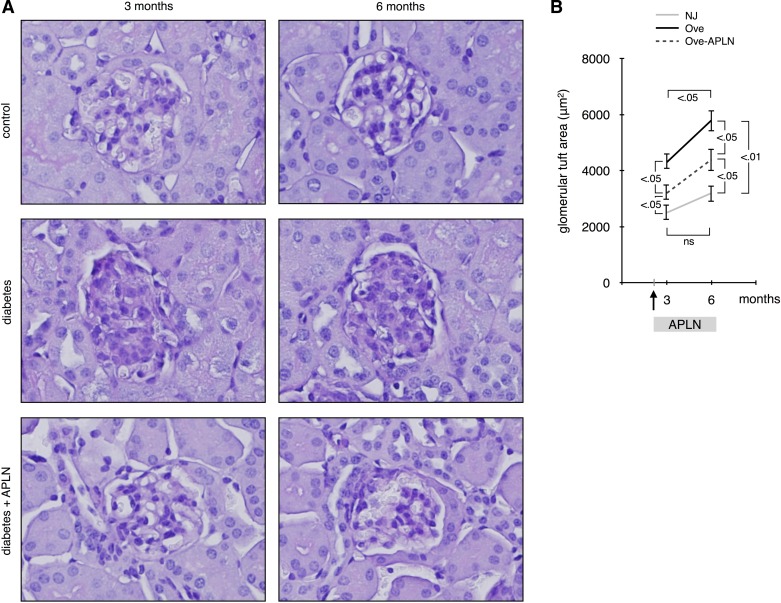
We next assessed glomerular morphology by staining sections of paraffin-embedded kidneys with PAS. Figure 4A shows that glomeruli in diabetic Ove26 mice were enlarged and contained more mesangial matrix, visible as dark purple staining, than control NJ mice at both 3 and 6 mo of age. Treatment with APLN-13 significantly decreased glomerular enlargement and mesangial matrix deposition at 3 and 6 mo. Morphometric analysis of these glomeruli shows that glomerular tuft area in control NJ mice did not increase significantly with age but that in diabetic mice glomerular area doubled at 3 mo and kept becoming larger at 6 mo. APLN-13 treatment significantly reduced glomerular tuft area at both 3 and 6 mo. These data show that continuous APLN-13 treatment reduces progression of glomerular hypertrophy and mesangial matrix deposition in diabetic Ove26 mice.
Fig. 4.
Effect of apelin on glomerular hypertrophy. A: renal histology was studied by periodic acid Schiff staining on section of paraffin-embedded kidneys. B: glomerular tuft area was traced and measured using the Image Pro Plus software. A minimum of 30 glomeruli per mouse were counted; n = 5 mice per group. Numbers inside brackets represent P values calculated by ANOVA; ns, not significant.
Effect of Apelin on Albuminuria
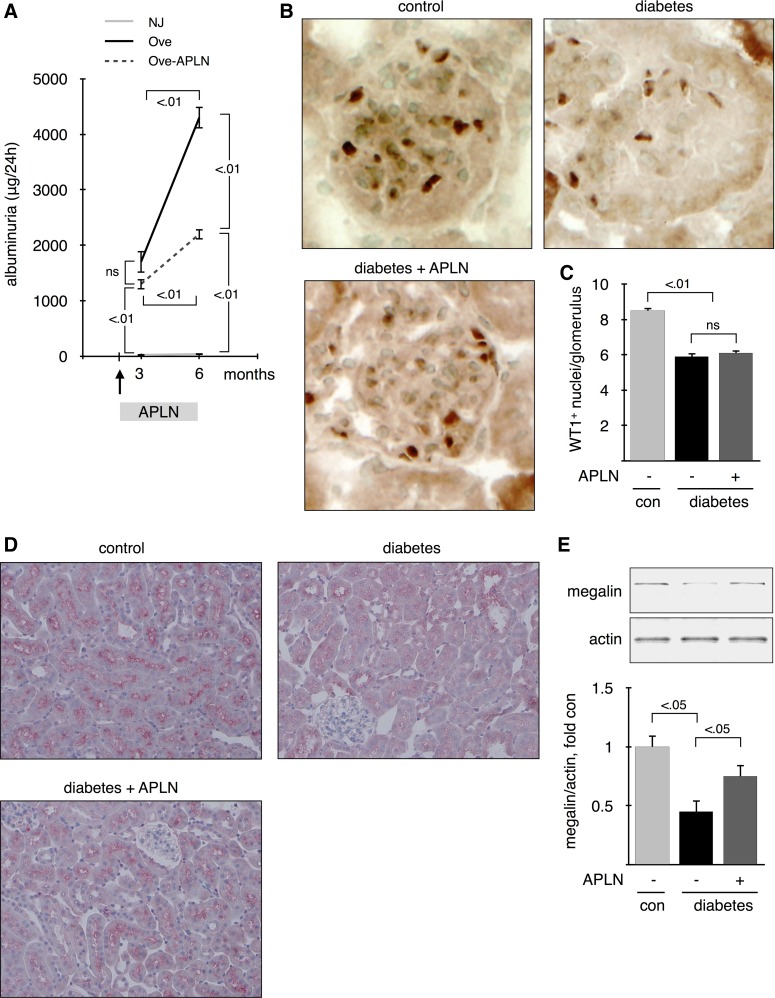
In diabetic patients, albuminuria is associated with increased risk of renal failure and cardiovascular mortality. Figure 5A shows that albuminuria was significantly increased in diabetic Ove26 mice at 3 mo of age and was further increased at 6 mo. APLN-13 treatment did not improve albuminuria at 3 mo but reduced it by ∼50% at 6 mo of age. The podocyte number decreases early in diabetes and further decreases as albuminuria increases. The number of podocytes is inversely related to the degree of albuminuria (36). Podocytes were detected by staining for WT1 (Fig. 5B), and the number of WT1+ nuclei per glomerulus was counted (Fig. 5C). Podocyte number was significantly reduced in 6-mo-old diabetic mice and was not restored by APLN-13 treatment. Albumin reabsorption by the megalin/cubilin complex in the proximal tubules may play a role in maintaining low levels of albuminuria (18). Tubular absorption of albumin has been shown to be significantly reduced in rats with early-stage type 1 diabetes, due to reduced expression of megalin in the brush border of the proximal tubule (59). Figure 5D shows a strong expression of megalin in the brush border of the proximal tubule in control mice that was significantly reduced in mice with 6 mo of diabetes. Treatment with APLN-13 partially restored megalin expression. Megalin expression was quantified by immunoblot. Figure 5E shows that megalin expression was reduced by >50% in kidneys from diabetic mice compared with control mice. APLN-13 treatment partially restores megalin expression to ∼70% of control. These data suggest that APLN-13 reduces albuminuria in diabetic mice by restoring proximal tubular reabsorption of albumin, rather than by reducing podocyte loss.
Fig. 5.
Effect of apelin on albuminuria. A: 24-h urine collection was performed on the eve of death; n = 5 mice per group. B: podocytes were identified as WT1+ nuclei in the glomeruli. WT1 was detected by immunohistochemistry on sections of frozen kidneys from 6-mo-old mice. Top, middle, and bottom: represent 3 different mice. C: WT1+ nuclei were counted by a blinded investigator. Numbers inside brackets represent P values calculated by ANOVA; ns, not significant. Megalin expression was assessed by immunohistochemistry on sections from paraffin-embedded kidneys (D) and by immunoblot on kidney cortex homogenates (E) from 6-mo-old mice; n = 5 mice per group.
Effect of Apelin on Monocyte Infiltration
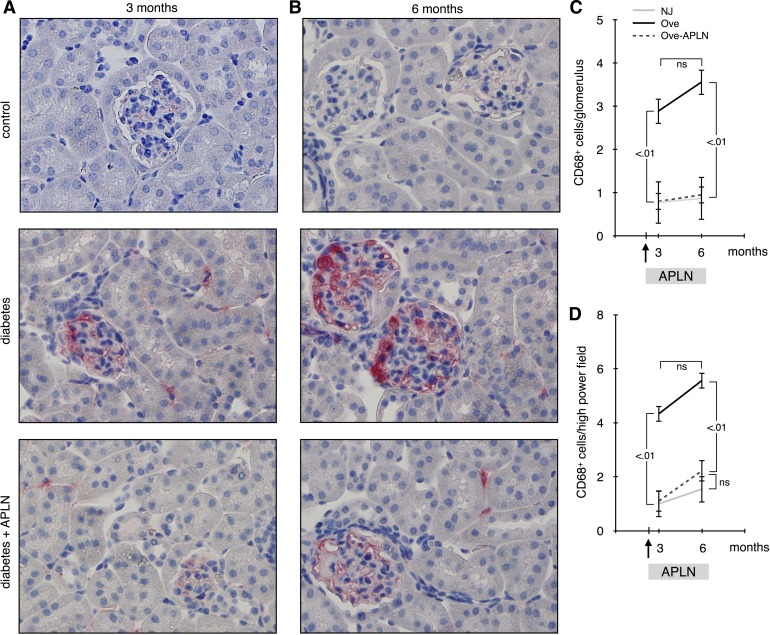
Infiltration of inflammatory cells, including monocytes, in the kidney occurs early in diabetic nephropathy and is involved in the pathogenesis of the disease. Monocytes were detected by staining for CD68 (Fig. 6, A and B), and the number of CD68+ cells in the glomeruli (Fig. 6C) or the tubulointerstitial compartment (Fig. 6D) was counted . The number of monocytes increased significantly in both glomeruli and tubulo-interstitium at 3-mo-old diabetic Ove26 mice and did not further increase at 6 mo. APLN-13 treatment restored monocyte number to control level at 3 and 6 mo. These data show that APLN-13 exerts a profound anti-inflammatory effect on kidneys from diabetic Ove26 mice.
Fig. 6.
Effect of apelin on moncyte infiltration. A: CD68+ cells were detected by immunohistochemistry on sections of paraffin-embedded kidneys from 3 and 6-mo-old mice. CD68+ nuclei were counted by a blinded investigator in glomeruli (C) and the tubulo-interstitial compartment (D). Numbers inside brackets represent P values calculated by ANOVA; ns, not significant.
Effect of Apelin on Mediators of Renal Inflammation
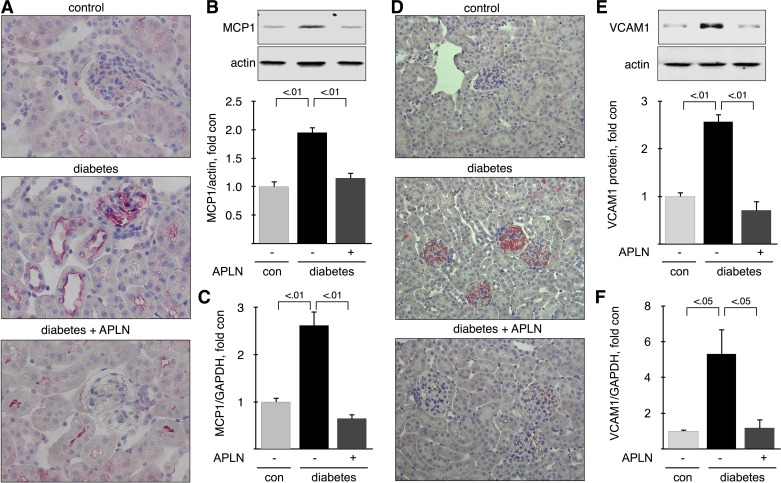
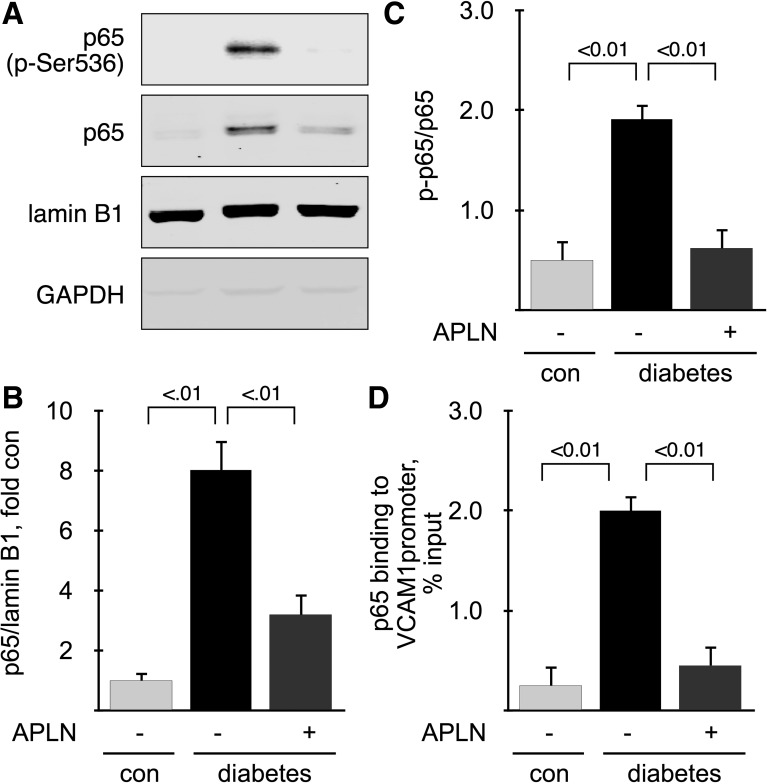
Infiltration of monocytes in the kidney requires coordinated expression of several proinflammatory molecules, such as MCP1, which recruits circulating monocytes to the endothelium and VCAM1 which allows adhesion and infiltration. We detected and localized MCP1 and VCAM1 proteins by immunohistochemistry and immunoblot and mRNAs by RT-qPCR in mice with 6 mo of diabetes. MCP1 was weakly expressed in glomeruli in proximal and distal tubules in control mice; in diabetic mice, MCP1 distribution was not changed but its expression was increased, and treatment with APLN-13 reversed the increase in MCP1 expression (Fig. 7A). Immunoblot analysis showed that MCP1 expression was increased twofold in kidney cortex from diabetic mice and that APLN-13 reversed this increase (Fig. 7B). Renal MCP1 mRNA expression was upregulated by diabetes (∼2.5-fold) and restored by treatment with APLN-13 (Fig. 7C). VCAM1 expression in kidney from control mice was weak; in diabetic mice, VCAM1 staining was strong in glomeruli and appeared in the interstitium. Treatment with APLN-13 reversed the increase in VCAM1 expression (Fig. 7D). Immunoblot analysis showed that VCAM1 expression was increased ∼2.6-fold in kidney cortex from diabetic mice and that APLN-13 reversed this increase (Fig. 7E). Renal VCAM1 mRNA expression was upregulated by diabetes (∼5-fold) and restored by treatment with APLN-13 (Fig. 7F). Expression of proinflammatory molecules, such as MCP1 and VCAM1, is under the control of the NF-κB in diabetic kidneys (15, 65). The most studied dimer is p50:p65, which is activated by the classical pathway and promotes gene expression (25, 43). Inactive NF-κB dimers are sequestered in the cytoplasm, and their activation results in nuclear translocation and activating phosphorylation of Ser536 in the case of p65 (61). We therefore assessed activation of the p65 subunit by measuring its nuclear translocation and phosphorylation on Ser536. Nuclear fractions were prepared from kidney cortex from mice with 6 mo of diabetes. Figure 8A shows that lamin B1 was strongly expressed and that GAPDH was almost absent from the nuclear fractions, indicating that the nuclear fractions were devoid of cytosolic contamination. Figure 8A also shows that nuclear content of both total p65 and p-p65 were significantly increased by diabetes and reversed by treatment with APLN-13. Nuclear translocation of p65 was quantified in Fig. 8B. Figure 8C shows quantitation of the phosphorylation of nuclear p65 on Ser536. It shows that nuclear translocation of p65 was accompanied its phosphorylation. These data indicate that p65 was activated in kidney cortex from diabetic mice and inactivated by APLN-13 treatment but does not prove that p65 was involved in upregulation of proinflammatory molecules. Therefore, we measure binding of p65 to VCAM1 promoter by ChIP assay, as described in methods. Because p65 binds to the proximal region of the VCAM1 promoter (two sites at −77 and −63) (24), we chose primers that amplify the region spanning from +1 to −1,000 bases relative to the transcription start site. ChIP assay shows that binding of p65 to VCAM1 promoter was increased 10-fold in kidney cortex from diabetic mice compared with control mice, and that treatment with APLN-13 reversed p65 binding to control levels (Fig. 8D). Together, these data show that APLN-13 reverses activation of NF-κB and its binding to VCAM1 promoter.
Fig. 7.
Effect of apelin on mediators of renal inflammation. Monocyte attractant protein 1 (MCP1; A) and vascular cell adhesion molecule 1 (VCAM1; D) were detected by immunohistochemistry on sections of paraffin-embedded kidneys from 6-mo-old mice. Top, middle, and bottom: represent 3 different mice. MCP1 protein (B) and VCAM1 protein (E) was measured by immunoblot on kidney cortex homogenates from 6-mo-old mice. Bottom: combined data obtained on kidneys from 5 individual mice for each experimental condition. MCP1 mRNA (C) and VCAM1 mRNA (F) were measured by RT-qPCR on total RNA extracted from kidney cortex and normalized using GAPDH expression; n = 5 mice per group. Numbers inside brackets represent P values calculated by ANOVA.
Fig. 8.
Effect of apelin on NF-κB activation. A: nuclear translocation of the p65 subunit of NF-κB was assessed by immunoblot on nuclear fractions purified from kidney cortex from 6-mo-old mice. Loading was assessed by measuring expression of lamin B1. B: ratio of p65/lamin B1 in nuclear extracts. C: ratio of p-p65/p65 in nuclear extracts. D: binding of p65 to VCAM1 promoter by ChIP; n = 5 mice per group. Numbers inside brackets represent P values calculated by ANOVA.
Effect of Apelin on Catalase Expression
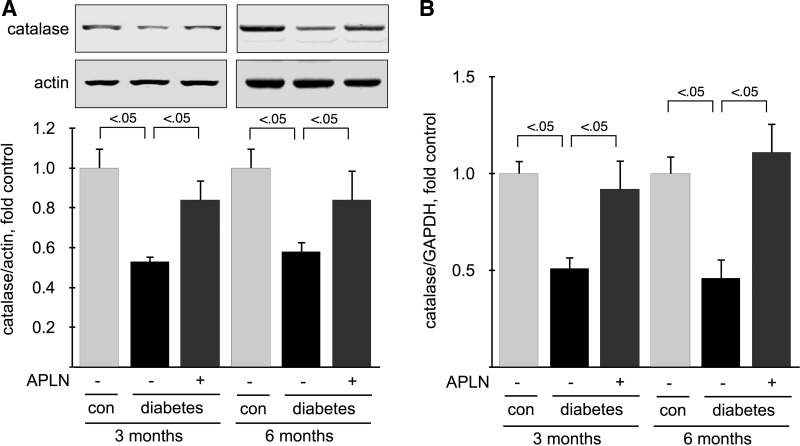
Oxidative stress has been shown to play an important role in the development of diabetic nephropathy, and APLN has been shown to stimulate antioxidant enzymes, including catalase (66). Oxidative stress occurs after an imbalance between pathways that produce oxygen radicals (prooxidants) and pathways that degrade or buffer oxygen radicals (antioxidants). We studied the expression of an antioxidant enzyme, catalase, which degrades hydrogen peroxide to water and oxygen. We measured catalase protein by immunoblot and mRNA by RT-qPCR in mice at 3 and 6 mo of age. Catalase protein (Fig. 9A) and mRNA expression (Fig. 9B) was significantly reduced (∼50%) in kidney cortex from diabetic Ove26 mice at 3 and 6 mo. APLN-13 treatment restored catalase expression to control level at both 3 and 6 mo. These data show that APLN-13 restores the expression of a major antioxidant enzyme, catalase, in kidneys from diabetic Ove26 mice.
Fig. 9.
Effect of apelin on catalase expression. A: catalase protein was detected by immunoblotting on kidney cortex homogenates from 6-mo-old mice. Bottom: densitometric analysis of catalase expression, normalized for loading using actin. B: catalase mRNA was measured by RT-qPCR on total RNA extracted from kidney cortex and normalized using GAPDH expression; n = 5 mice per group. Numbers inside brackets represent P values calculated by ANOVA.
Effect of Apelin on Activation of ANG II/AT1 Receptor
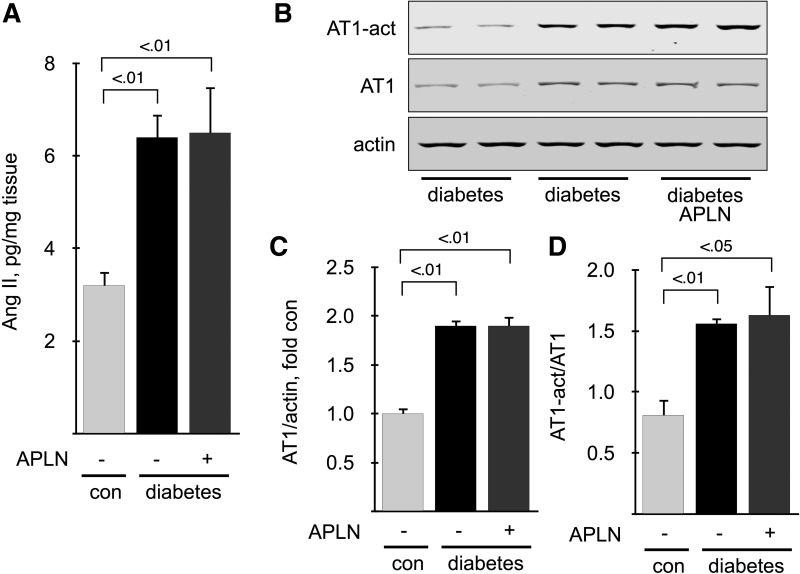
Several studies conducted in the cardiovascular system have demonstrated a reciprocal antagonism between ANG II/AT1 receptor and APLN/APJ (3). Therefore, we measured activation of the renal ANG II/AT1 axis in 6-mo-old mice. The renal cortical content of ANG II (Fig. 10A), as well as AT1 expression (Fig. 10, B and C) and activation, measured by immunoblot using a conformation-specific antibody that recognizes only the activated form of the receptor (7) (Fig. 10, B–D), was significantly increased in diabetic mice, and were not affected by treatment with APLN-13 (Fig. 10A). These data show that treatment with APLN does not antagonize activation of renal cortical ANG II/AT1 receptor in diabetic mice.
Fig. 10.
Effect of apelin on ANG II and AT1 receptor activation. A: ANG II content was measured by ELISA on kidney cortex homogenates from 6-mo-old mice. B: AT1 activation was measured by immunoblot using a conformation-specific antibody under nondenaturating conditions. membranes were stripped and reprobed with antibodies directed against AT1. C: densitometric analysis of AT1 expression, corrected for actin. D: densitometric analysis of AT1 activation, corrected for AT1 expression; n = 5 mice per group.
DISCUSSION
This study shows that APJ expression is significantly reduced in kidneys from mice with established type 1 diabetes, suggesting that downregulation of APJ could play a role in the development of diabetic nephropathy. Because the intrarenal renin-angiotensin system plays an important role in the development of complications of hyperglycemia (2, 40), and the apelin/APJ axis has been shown to oppose the effect of the renin-angiotensin system in cardiovascular diseases (44), it is possible that reduction in APJ in the kidney leaves the activated local renin-angiotensin system unopposed and thus contributes to the development of diabetic nephropathy.
This study shows that a short administration of [pyr1]apelin-13 (APLN-13) was sufficient to reduced kidney and glomerular hypertrophy as well as renal inflammation, but a prolonged treatment was required to improve albuminuria. APLN-13 also restored the expression of the antioxidant enzyme catalase, which was downregulated in kidneys from diabetic mice. This indicates that the renoprotective effects of APLN-13 could be due to stimulation of antioxidant pathways. The effects of APLN-13 appear to be direct, since it did not affect glycemia, blood pressure, or activation of renal cortical ANG II/AT1 receptor in diabetic mice.
Our observation that renal apelin and its receptor APJ are downregulated in kidneys from diabetic mice suggests that apelin could play a role in maintaining renal function/structure. After apelin treatment, amelioration of renal injury is accompanied by upregulation of renal apelin and APJ. However, APJ knockout mice do not show signs of renal injury (46), suggesting that apelin/APJ plays a protective role during kidney injury. It is interesting that apelin upregulates its own receptor. There is no report of a direct upregulation of APJ by apelin in the literature, but many growth factors/hormones upregulate their own receptor. This is the case for growth factors involved in diabetic nephropathy, such as transforming growth factor-β (37), IL-6 (57), ANG II (63), and VEGF (8).
In diabetic patients, progressive albuminuria significantly contributes to its development and progression (58). The mechanisms of albuminuria are complex and involve several structures in the nephron. Albumin has to cross the endothelial glycocalyx, the glomerular basement membrane, and the slit diaphragm between the foot processes of the podocytes, which are normally not very permeable. After filtration, the albumin is reabsorbed by the megalin/cubilin system expressed on the brush border of the proximal tubule (18). It is likely that development of persistent albuminuria involves dysfunction of several cell types, including the glomerular endothelial cells, podocytes, or proximal tubule epithelial cells (58, 59). Our finding that APLN-13 improves albuminuria without increasing the number of podocytes in glomeruli from diabetic mice indicates that APLN-13 does not protect podocytes but could act on proximal tubule epithelial cells, as suggested by the fact that APLN-13 increases the expression of megalin in the brush border of proximal tubules in kidneys from diabetic mice. Since the apelin receptor APJ is not detectable in this renal structure, the effect of APLN-13 may be indirect. Megalin mRNA expression in the proximal tubules is stimulated by various factors, including vitamin A and vitamin D (33). The mechanism by which Apelin regulates megalin expression remains to be determined.
Early and persistent hypertrophy of the glomeruli and the kidney is a hallmark of diabetic nephropathy in type 1 diabetes (14, 42, 48). Renal hypertrophy is largely due to tubular cell hypertrophy (49, 50), and glomerular hypertrophy is due to an enlargement of capillary lumina and an increase of capillary cell mass (21) with minimal hyperplasia of either compartment (45). Cell hypertrophy in diabetic rats is due to increased protein synthesis in both the tubular and glomerular compartments (45, 55). The absence of APJ expression in the tubules suggests that its role in the reduction of kidney hypertrophy is indirect. Such an indirect link between apelin and cell hypertrophy has been established in the cardiovascular system. Myocardial expression of apelin and APJ decreases during left ventricular hypertrophy in rats subjected to suprarenal aortic banding (10) and in Dahl salt-sensitive rats fed a high-salt diet (26). Administration of apelin to rats with monocrotaline-induced pulmonary hypertension delayed the progression of right ventricle hypertophy (11). Together, these studies suggest that the apelin system plays a protective role against cardiac hypertrophy. The antihypertrophic effect of apelin seems to be linked to its reduction of oxidative stress by upregulation of antioxidant enzymes (16).
Oxidative stress has long been recognized as an important factor in the pathogenesis of diabetic nephropathy (4, 22, 53). Oxidative stress results of an imbalance between pathways that produce oxygen radicals (prooxidants) and pathways that degrade or buffer oxygen radicals (antioxidants), such as catalase. Deletion of catalase in mice worsened renal injury in mice with streptozocin-induced type 1 diabetes (23), indicating that catalase plays a protective role in diabetic nephropathy. We found that even a short treatment with APLN-13 restored catalase levels in kidney cortex from diabetic mice, indicating that APLN-13 exerts a strong antioxidant effect in the diabetic kidney and suggesting that its renoprotective effects could be due to upregulation of antioxidant enzymes.
Clinical studies have established an association between a rise in inflammatory markers and diabetic nephropathy, and renal inflammation could play a direct role in the initiation of renal injury during diabetes (34). Renal NF-κB, a master regulator of inflammation, has been shown to be activated in diabetic mice (54) and humans (39, 47), and strategies that inhibit NF-κB signaling in diabetic mice improve renal function (64). There is a growing body of evidence that apelin exerts anti-inflammatory properties systemically and inside various organs. Circulating levels of apelin are inversely correlated with inflammation markers, among which are IL-6, TNF-α, and hsCRP, in hemodialyzed patients (9) and in kidney allografts recipients (35). Subcutaneous administration of apelin, at doses similar to the dose used in this study, resulted in decreased infiltration of inflammatory cells such as macrophages; decreased expression of proinflammatory cytokines, including TNF-α and IL-6; and decreased cardiac fibrosis in salt-loaded Dahl salt-sensitive rats (17), in lungs from rats exposed to hyperoxia (62), and in aortas from mice subjected to elastase infusion (31). It is possible that the anti-inflammatory effects of apelin are linked to its beneficial effect on vasculature integrity, since apelin induces the formation of enlarged, nonleaky blood vessels (29) that would be less permeable to circulating inflammatory cells. Interestingly, intraperitoneal administration of TNF-α in mice leads to increased expression of apelin in the adipose tissue and the circulation (6). Therefore, it is possible that upregulation of apelin by TNF-α can serve as a compensatory mechanism to limit tissue inflammation.
Studies using inhibitors of the renin-angiotensin system have demonstrated that activation of ANG II/AT1 receptor plays a major role in the pathogenesis of diabetic nephropathy (30, 32). Since a reciprocal antagonism between the ANG II/AT1 and the APLN/APJ axes has been demonstrated in the cardiovascular system (3), it is possible that apelin reduces kidney injury by antagonizing the ANG II/AT1 receptor. Our data show that apelin does not prevent activation of the renal cortical ANG II/AT1 receptor. However, since the AT1 receptor is also expressed in the renal medulla, it is possible that apelin prevents its activation in this compartment. It is possible that apelin directly inhibits AT1 signaling by forming an heterodimer, as suggested by Siddiquee et al. (52). However, this study was performed in Chinese hamster ovary cell, in which both receptors were artificially overexpressed, and might not reflect physiologic conditions. An inhibition of AT1 downstream signaling by apelin is possible and is currently under investigation.
In conclusion, our study shows for the first time that APLN-13 exerts a protective effect on the diabetic kidney. A short treatment was sufficient to reduced kidney and glomerular hypertrophy as well as renal inflammation, but a prolonged treatment was required to improve albuminuria. Apelin also restored the expression of the antioxidant enzyme catalase, which was downregulated in kidneys from diabetic mice. This indicates that the renoprotective effects of APLN-13 could be due to stimulation of antioxidant pathways. The results of this study suggest that activation of the apelinergic system may be used as a novel therapeutic intervention to complement current treatments for diabetic kidney disease.
GRANTS
This study was supported by JDRF Research Grant 1-2010-141 (to D. Feliers). The PAS and Sirius red staining were performed by the Cancer Therapy and Research Center at the University of Texas Health Science Center, San Antonio, TX, through National Cancer Insitute Cancer Center Support Grant 2 P30 CA-054174-17.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.T.D. and R.C.C. performed experiments; R.T.D., R.C.C., and D.F. analyzed data; R.T.D. and R.C.C. edited and revised manuscript; D.F. conception and design of research; D.F. interpreted results of experiments; D.F. prepared figures; D.F. drafted manuscript; D.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. B. S. Kasinath and H. E. Abboud (Division of Renal Diseases, University of Texas Health Science Center, San Antonio) for critical reading of the manuscript.
REFERENCES
- 1. American Diabetes Association Position statement: diabetic nephropathy. Diabetes Care 24: S69– S72, 2001 [PubMed] [Google Scholar]
- 2. Andersen S, Tarnow L, Rossing P, Hansen BV, Parving HH. Renoprotective effects of angiotensin II receptor blockade in type 1 diabetic patients with diabetic nephropathy. Kidney Int 57: 601– 606, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Ashley E, Chun HJ, Quertermous T. Opposing cardiovascular roles for the angiotensin and apelin signaling pathways. J Mol Cell Cardiol 41: 778– 781, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes 40: 405– 412, 1991 [DOI] [PubMed] [Google Scholar]
- 5. Chen MM, Ashley EA, Deng DX, Tsalenko A, Deng A, Tabibiazar R, Ben-Dor A, Fenster B, Yang E, King JY, Fowler M, Robbins R, Johnson FL, Bruhn L, McDonagh T, Dargie H, Yakhini Z, Tsao PS, Quertermous T. Novel role for the potent endogenous inotrope apelin in human cardiac dysfunction. Circulation 108: 1432– 1439, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Daviaud D, Boucher J, Gesta S, Dray C, Guigne C, Quilliot D, Ayav A, Ziegler O, Carpene C, Saulnier-Blache JS, Valet P, Castan-Laurell I. TNFα upregulates apelin expression in human and mouse adipose tissue. FASEB J 20: 1528– 1530, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Day RT, Cavaglieri Rde C, Tabatabaimir H, Mantravadi V, Lee MJ, Barnes JL, Kasinath BS, Feliers D. Acute hyperglycemia rapidly stimulates VEGF mRNA translation in the kidney. Role of angiotensin type 2 receptor (AT2). Cell Signal 22: 1849– 1857, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a. Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 329: 977– 986, 1993 [DOI] [PubMed] [Google Scholar]
- 8. Domingues I, Rino J, Demmers JA, de Lanerolle P, Santos SC. VEGFR2 translocates to the nucleus to regulate its own transcription. PLos One 6: e25668, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. El-Shehaby AM, El-Khatib MM, Battah AA, Roshdy AR. Apelin: a potential link between inflammation and cardiovascular disease in end stage renal disease patients. Scand J Clin Lab Invest 70: 421– 427, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Falcao-Pires I, Goncalves N, Gavina C, Pinho S, Teixeira T, Moura C, Amorim MJ, Pinho P, Areias JC, Leite-Moreira A. Correlation between plasma levels of apelin and myocardial hypertrophy in rats and humans: possible target for treatment? Expert Opin Ther Targets 14: 231– 241, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Falcao-Pires I, Goncalves N, Henriques-Coelho T, Moreira-Goncalves D, Roncon-Albuquerque R, Jr, Leite-Moreira AF. Apelin decreases myocardial injury and improves right ventricular function in monocrotaline-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol 296: H2007– H2014, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Falcao-Pires I, Ladeiras-Lopes R, Leite-Moreira AF. The apelinergic system: a promising therapeutic target. Expert Opin Ther Targets 14: 633– 645, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Feliers D, Kasinath BS. Mechanism of VEGF expression by high glucose in proximal tubule epithelial cells. Mol Cell Endocrinol 314: 136– 142, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flyvbjerg A, Mogensen CE, Osterby R, Orskov H. Renal hypertrophy in experimental diabetes. J Diabetes Complications 5: 62– 64, 1991 [DOI] [PubMed] [Google Scholar]
- 15. Fornoni A, Ijaz A, Tejada T, Lenz O. Role of inflammation in diabetic nephropathy. Curr Diabetes Rev 4: 10– 17, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Foussal C, Lairez O, Calise D, Pathak A, Guilbeau-Frugier C, Valet P, Parini A, Kunduzova O. Activation of catalase by apelin prevents oxidative stress-linked cardiac hypertrophy. FEBS Lett 584: 2363– 2370, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Fukushima H, Kobayashi N, Takeshima H, Koguchi W, Ishimitsu T. Effects of olmesartan on apelin/APJ and Akt/endothelial nitric oxide synthase pathway in Dahl rats with end-stage heart failure. J Cardiovasc Pharmacol 55: 83– 88, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Gekle M. Renal tubule albumin transport. Annu Rev Physiol 67: 573– 594, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Giani JF, Burghi V, Veiras LC, Tomat A, Munoz MC, Cao G, Turyn D, Toblli JE, Dominici FP. Angiotensin-(1–7) attenuates diabetic nephropathy in Zucker diabetic fatty rats. Am J Physiol Renal Physiol 302: F1606– F1615, 2012 [DOI] [PubMed] [Google Scholar]
- 21. Gundersen HJ, Mogensen CE, Seyer-Hansen K, Osterby R, Lundbaek K. Early and late changes in the diabetic kidney. Adv Nephrol Necker Hosp 8: 43– 62, 1979 [PubMed] [Google Scholar]
- 22. Ha H, Kim KH. Role of oxidative stress in the development of diabetic nephropathy. Kidney Int Suppl 51: S18– 21, 1995 [PubMed] [Google Scholar]
- 23. Hwang I, Lee J, Huh JY, Park J, Lee HB, Ho YS, Ha H. Catalase deficiency accelerates diabetic renal injury through peroxisomal dysfunction. Diabetes 61: 728– 738, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iademarco MF, McQuillan JJ, Rosen GD, Dean DC. Characterization of the promoter for vascular cell adhesion molecule-1 (VCAM-1). J Biol Chem 267: 16323– 16329, 1992 [PubMed] [Google Scholar]
- 25. Israel A. The IKK complex: an integrator of all signals that activate NF-κB? Trends Cell Biol 10: 129– 133, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Iwanaga Y, Kihara Y, Takenaka H, Kita T. Downregulation of cardiac apelin system in hypertrophied and failing hearts: Possible role of angiotensin II-angiotensin type 1 receptor system. J Mol Cell Cardiol 41: 798– 806, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Kakkar R, Mantha SV, Radhi J, Prasad K, Kalra J. Antioxidant defense system in diabetic kidney: a time course study. Life Sci 60: 667– 679, 1997 [DOI] [PubMed] [Google Scholar]
- 28. Kedziora-Kornatowska K. Effect of angiotensin convertase inhibitors and AT1 angiotensin receptor antagonists on the development of oxidative stress in the kidney of diabetic rats. Clin Chim Acta 287: 19– 27, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Kidoya H, Naito H, Takakura N. Apelin induces enlarged and nonleaky blood vessels for functional recovery from ischemia. Blood 115: 3166– 3174, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Kohzuki M, Yasujima M, Kanazawa M, Yoshida K, Fu LP, Obara K, Saito T, Abe K. Antihypertensive and renal-protective effects of losartan in streptozotocin diabetic rats. J Hypertens 13: 97– 103, 1995 [PubMed] [Google Scholar]
- 31. Leeper NJ, Tedesco MM, Kojima Y, Schultz GM, Kundu RK, Ashley EA, Tsao PS, Dalman RL, Quertermous T. Apelin prevents aortic aneurysm formation by inhibiting macrophage inflammation. Am J Physiol Heart Circ Physiol 296: H1329– H1335, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 329: 1456– 1462, 1993 [DOI] [PubMed] [Google Scholar]
- 33. Liu W, Yu WR, Carling T, Juhlin C, Rastad J, Ridefelt P, Akerstrom G, Hellman P. Regulation of gp330/megalin expression by vitamins A and D. Eur J Clin Invest 28: 100– 107, 1998 [DOI] [PubMed] [Google Scholar]
- 34. Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 7: 684– 696, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Malyszko J, Malyszko JS, Pawlak K, Wolczynski S, Mysliwiec M. Apelin, a novel adipocytokine, in relation to endothelial function and inflammation in kidney allograft recipients. Transplant Proc 40: 3466– 3469, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Marshall SM. The podocyte: a major player in the development of diabetic nephropathy? Hormone and metabolic research. Hormon Stoffwechselforschung 37, Suppl 1: 9–16, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Menke A, Geerling I, Giehl K, Vogelmann R, Reinshagen M, Adler G. Transforming growth factor-β-induced upregulation of transforming growth factor-β receptor expression in pancreatic regeneration. Biochim Biophys Acta 1449: 178– 185, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Meral C, Tascilar E, Karademir F, Tanju IA, Cekmez F, Ipcioglu OM, Ercin CN, Gocmen I, Dogru T. Elevated plasma levels of apelin in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab 23: 497– 502, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Mezzano S, Aros C, Droguett A, Burgos ME, Ardiles L, Flores C, Schneider H, Ruiz-Ortega M, Egido J. NF-κB activation and overexpression of regulated genes in human diabetic nephropathy. Nephrol Dial Transplant 19: 2505– 2512, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Miller JA. Impact of hyperglycemia on the renin angiotensin system in early human type 1 diabetes mellitus. J Am Soc Nephrol 10: 1778– 1785, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Nelson J, Denisenko O, Bomsztyk K. The fast chromatin immunoprecipitation method. Methods Mol Biol 567: 45– 57, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Osterby R. Structural changes in the diabetic kidney. Clin Endocrinol Metab 15: 733– 751, 1986 [PubMed] [Google Scholar]
- 43. Perkins ND. The Rel/NF-κ B family: friend and foe. Trends Biochem Sci 25: 434– 440, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Pitkin SL, Maguire JJ, Bonner TI, Davenport AP. International Union of Basic and Clinical Pharmacology. LXXIV. Apelin receptor nomenclature, distribution, pharmacology, and function. Pharmacol Rev 62: 331– 342, 2010 [DOI] [PubMed] [Google Scholar]
- 45. Rasch R, Norgaard JO. Renal enlargement: comparative autoradiographic studies of 3H-thymidine uptake in diabetic and uninephrectomized rats. Diabetologia 25: 280– 287, 1983 [DOI] [PubMed] [Google Scholar]
- 46. Roberts E, Newson M, Pope G, Landgraf R, Lolait S, O'Carroll AM. Abnormal fluid homeostasis in apelin receptor knockout mice. J Endocrinol 202: 453– 462, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schmid H, Boucherot A, Yasuda Y, Henger A, Brunner B, Eichinger F, Nitsche A, Kiss E, Bleich M, Grone HJ, Nelson PJ, Schlondorff D, Cohen CD, Kretzler M. Modular activation of nuclear factor-kappaB transcriptional programs in human diabetic nephropathy. Diabetes 55: 2993– 3003, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Schwieger J, Fine LG. Renal hypertrophy, growth factors, and nephropathy in diabetes mellitus. Semin Nephrol 10: 242– 253, 1990 [PubMed] [Google Scholar]
- 49. Seyer-Hansen K. Renal hypertrophy in experimental diabetes mellitus. Kidney Int 23: 643– 646, 1983 [DOI] [PubMed] [Google Scholar]
- 50. Seyer-Hansen K. Renal hypertrophy in streptozotocin-diabetic rats. Clin Sci Mol Med Suppl 51: 551– 555, 1976 [DOI] [PubMed] [Google Scholar]
- 51. Siddiquee K, Hampton J, Khan S, Zadory D, Gleaves L, Vaughan DE, Smith LH. Apelin protects against angiotensin II-induced cardiovascular fibrosis and decreases plasminogen activator inhibitor type-1 production. J Hypertens 29: 724–731, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Siddiquee K, Hampton J, McAnally D, May LT, Smith LH. The apelin receptor inhibits the angiotensin II type 1 receptor via allosteric trans-inhibition. Br J Pharmacol 2012. August 31 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stanton RC. Oxidative stress and diabetic kidney disease. Curr Diab Rep 11: 330– 336, 2011 [DOI] [PubMed] [Google Scholar]
- 54. Starkey JM, Haidacher SJ, Lejeune WS, Zhang X, Tieu BC, Choudhary S, Brasier AR, Denner LA, Tilton RG. Diabetes-induced activation of canonical and noncanonical nuclear factor-κb pathways in renal cortex. Diabetes 55: 1252– 1259, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Takano Y, Minami H, Misake A, Nakabou Y, Hagihira H. A possible role for ribonuclease in the regulation of protein synthesis in relation to kidney growth of diabetic rat. Tokushima J Exp Med 26: 5– 12, 1979 [PubMed] [Google Scholar]
- 56. Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, Kurokawa T, Onda H, Fujino M. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun 251: 471– 476, 1998 [DOI] [PubMed] [Google Scholar]
- 57. Thabard W, Collette M, Mellerin MP, Puthier D, Barille S, Bataille R, Amiot M. IL-6 upregulates its own receptor on some human myeloma cell lines. Cytokine 14: 352– 356, 2001 [DOI] [PubMed] [Google Scholar]
- 58. Thomas MC. Pathogenesis and progression of proteinuria. Contrib Nephrol 170: 48– 56, 2011 [DOI] [PubMed] [Google Scholar]
- 59. Tojo A, Onozato ML, Ha H, Kurihara H, Sakai T, Goto A, Fujita T, Endou H. Reduced albumin reabsorption in the proximal tubule of early-stage diabetic rats. Histochem Cell Biol 116: 269– 276, 2001 [DOI] [PubMed] [Google Scholar]
- 60. Tuomilehto J, Borch-Johnsen K, Molarius A, Jormanainen V, Lounamaa R, Gronhagen-Riska C, Reunanen A, Sarti C. The unchanging incidence of hospitalization for diabetic nephropathy in a population-based cohort of IDDM patients in Finland. Diabetes Care 20: 1081– 1086, 1997 [DOI] [PubMed] [Google Scholar]
- 61. Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-κB and IκB proteins: implications in cancer and inflammation. Trends Biochem Sci 30: 43– 52, 2005 [DOI] [PubMed] [Google Scholar]
- 62. Visser YP, Walther FJ, Laghmani el H, Laarse A, Wagenaar GT. Apelin attenuates hyperoxic lung and heart injury in neonatal rats. Am J Respir Crit Care Med 182: 1239– 1250, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wei SG, Yu Y, Zhang ZH, Felder RB. Angiotensin II upregulates hypothalamic AT1 receptor expression in rats via the mitogen-activated protein kinase pathway. Am J Physiol Heart Circ Physiol 296: H1425– H1433, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wu J, Guan TJ, Zheng S, Grosjean F, Liu W, Xiong H, Gordon R, Vlassara H, Striker GE, Zheng F. Inhibition of inflammation by pentosan polysulfate impedes the development and progression of severe diabetic nephropathy in aging C57B6 mice. Lab Invest 91: 1459– 1471, 2011 [DOI] [PubMed] [Google Scholar]
- 65. Yang L, Brozovic S, Xu J, Long Y, Kralik PM, Waigel S, Zacharias W, Zheng S, Epstein PN. Inflammatory gene expression in OVE26 diabetic kidney during the development of nephropathy. Nephron Exp Nephrol 119: e8– e20, 2011 [DOI] [PubMed] [Google Scholar]
- 66. Zeng XJ, Zhang LK, Wang HX, Lu LQ, Ma LQ, Tang CS. Apelin protects heart against ischemia/reperfusion injury in rat. Peptides 30: 1144– 1152, 2009 [DOI] [PubMed] [Google Scholar]
- 67. Zheng S, Noonan WT, Metreveli NS, Coventry S, Kralik PM, Carlson EC, Epstein PN. Development of late-stage diabetic nephropathy in OVE26 diabetic mice. Diabetes 53: 3248– 3257, 2004 [DOI] [PubMed] [Google Scholar]