Abstract
Our recent studies showed that contents of necrotic renal proximal tubular cells (RPTC) from 2 × 106 cells/ml directly induced death of cultured renal interstitial fibroblasts. However, it remains unknown whether nonlethal number of necrotic RPTC would also alter the fate of renal interstitial fibroblasts. To address this issue, renal interstitial fibroblasts (NRK-49F) were exposed to necrotic RPTC supernatant (RPTC-Sup) obtained from 2 × 104 to 5 × 105 cells/ml. These concentrations of RPTC did not induce cell death, but led to inactivation of renal fibroblasts as indicated by reduced expression of α-smooth muscle actin and fibronectin, two hallmarks of activated fibroblasts. Concurrently, the same doses of necrotic RPTC-Sup suppressed phosphorylation of epidermal growth factor receptor (EGFR) and signal transducers and activators of transcription-3 (STAT3) in a time- and dose-dependent manner, but did not affect phosphorylation of platelet-derived growth factor receptor-β, AKT, and extracellular signal-regulated kinase 1/2. The presence of sodium orthovanadate, a general protein tyrosine phosphatase (PTP) inhibitor or TCS-401 (a selective PTP1B inhibitor), abrogated those effects of RPTC-Sup, whereas coincubation with the EGFR inhibitor (Gefitinib) or silencing of EGFR with siRNA preserved the ability of RPTC-Sup in suppressing renal fibroblast activation and STAT3 phosphorylation. Moreover, RPTC-Sup treatment induced PTP1B phosphorylation and its interaction with EGFR. Collectively, these results indicate that nonlethal necrotic RPTC-Sup can induce inactivation of renal interstitial fibroblasts, which occurs through a mechanism involved in PTP1B-mediated inhibition of EGFR signaling.
Keywords: renal interstitial fibroblasts, renal epithelial cells, EGFR, PTP1B
fibroblasts in the interstitial compartments of the kidney provide the basic skeleton of the tissue. Fibroblasts are also the major source for the constituents of extracellular matrix (ECM) and are important for the maintenance of the renal tissue architecture under physiological conditions (29). Under pathological conditions like acute kidney injury (AKI), renal interstitial fibroblasts undergo myofibroblast transition and produce matrix proteins, which are essential for wound healing and renal repair in the process of renal recovery. In addition, renal interstitial fibroblasts located in the renal cortex and outer medulla are also capable of producing erythropoietin, an important regulator for survival, differentiation, and proliferation of erythroid precursors in the bone marrow (1, 2, 9, 18, 25). Hence, the maintenance of renal fibroblast property and its population is critical for the integrity and structure of renal epithelium, renal repair, and stability of red blood cells.
In response to AKI, a wide variety of tyrosine kinase growth factor receptors such as epidermal growth factor receptor (EGFR) and platelet-derived growth factor receptor-β (PDGFRβ) are activated in the kidney (3, 11, 19, 39). These two receptors are widely expressed in the mammalian kidney, including proximal tubule and interstitial fibroblasts, and they play divergent roles in renal physiological as well as pathological conditions. EGFR can regulate cell proliferation, and differentiation, as well as production of pro- and anti-inflammatory mediators, and regulation of hemodynamics (6, 22, 39). PDGFRβ activation is also required for renal fibroblast activation and proliferation (20, 24). Stimulation of EGFR and PDGFRβ results in activation of multiple intracellular signaling pathways including the extracellular signal-regulated kinase (ERK), the Janus kinase/signal transducers and activators of transcription-3 (JAK/STAT), and the phosphoinositide-3-kinase (PI3K)/Akt (22, 23, 27, 47).
Tyrosine phosphorylation of membrane receptors and intracellular signaling molecules is reversible, and this dynamic process is controlled by the protein tyrosine phosphatases (PTPs). An abnormal increase of receptor tyrosine kinases (RTKs) leads to fatal consequences like cancer, fibrosis, and developmental abnormalities (16, 41). PTPs are a large and structurally diverse family of enzymes, which catalyze the dephosphorylation of tyrosyl-phosphorylated proteins and this process can either antagonize or potentiate protein kinase signaling (16, 41). Although PTPs have a common mechanism for dephosphorylation process, they exhibit fine substrate specificity and regulate specific signal transduction events and physiological processes. Among four subfamilies of PTPs (pTyr-specific PTPs, dual specificity phosphatases, Cdc25 phosphatases, and LMW PTP) (44, 49), PTP1B is a ubiquitously expressed PTP that is localized on intracellular membranes. PTP1B can negatively regulate several RTKs including EGFR, PDGFRβ by dephosphorylation, and subsequently attenuating activation of their downstream signaling pathways (5, 16, 21). PTP1B is also able to directly induce dephosphorylation of some intracellular signaling molecules including STAT3 (15).
Previous studies suggested the possible cross-talk between renal epithelial cells and interstitial fibroblasts in the kidney (13, 42). In most instances, this cellular event is an adaptive mechanism for repair and regeneration. However, under severe AKI, damaged or injured cells may release multiple cytokines and cellular contents into interstitial space, where they impact the growth and survival of interstitial cells like fibroblasts. This concept is supported by our recent in vitro observations that the contents from 2 × 106 cells/ml of necrotic RPTC directly induce death of renal interstitial fibroblasts (34–36). However, the degree of renal injury may vary from mild to severe, depending on the type of insults and exposure time. Currently, it remains unclear whether the contents released from the lower number of damaged RPTC would also affect the biological functions of renal myofibroblasts, in particular, their activation and proliferation, and if so, what mechanisms are involved. In this study, we addressed these issues using in vitro cultured system of renal interstitial fibroblasts and proximal tubular cells.
MATERIALS AND METHODS
Chemicals and antibodies.
TCS401 was obtained from Tocris Bioscience (Bristol, UK). Gefitinib was purchased from LC Laboratories (Woburn, MA). Small interfering RNA (siRNA) specific for rat EGFR and antibodies to fibronectin, EGFR, PTP1B, P2X7, PCNA, and GAPDH were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). α-Smooth muscle actin (α-SMA), α-tubulin, and all other chemicals were purchased from Sigma (St. Louis, MO). Phospho tyrosine (PY20) antibody was obtained from BD Biosciences (San Jose, CA). Secondary antibodies of anti-mouse IgG (Mouse TrueBlot ULTRA) and anti-rabbit IgG (Rabbit TrueBlot) were purchased from eBioscience (San Diego, CA). Transforming growth factor (TGF)-β1 was purchased from R&D Systems (Minneapolis, MN). All other antibodies used in this study were purchased from Cell Signaling Technology (Danvers, MA).
Cell culture.
Rat renal interstitial fibroblasts (NRK-49F) and immortalized mouse renal proximal tubular cells (RPTC) were used in this study. Both of them were cultured in DMEM/Nutrient-F12 (DMEM/F12, Sigma) containing 5% fetal bovine serum (FBS), penicillin, and streptomycin in an atmosphere of 5% CO2-95% air at 37°C. NRK-49F was 60–70% confluent when used for various treatments. When necessary, various inhibitors were directly added to the culture and then incubated for desired time as indicated in the figure legends.
Preparation of necrotic RPTC cell lysate and treatment.
RPTC were harvested and washed twice with sterile PBS and then reconstituted to a desired number of cells (2 × 106 or 5 × 105 cells/ml) in complete culture media. These cells were immediately used for cell lysate preparation by repetitive (5 cycles) freezing at −80°C and thawing at 37°C. Then, cell lysates were centrifuged at 15,000 rpm for 20 min to obtain RPTC supernatant (RPTC-Sup). For the experiments, 1/3 volume (330 μl/ml) of medium was removed and 330 μl of RPTC-Sup were added to the culture. When we replaced 330 μl/ml of medium with RPTC-Sup, the final concentration of cell lysate was equal to the RPTC-Sup from ∼1.7 × 105 cells/ml. As a control, the same volume of complete medium (5 times freeze and thaw) was added.
Transfection of siRNA into cells.
siRNA oligonucleotides targeted specifically to rat EGFR or rat P2X7 (750 pmol) were used in this experiment. siRNA (750 pmol) was transfected into NRK-49F (1 × 106 cells) using the nucleofector kit T and the Amaxa Nucleofector device (Gaithersburg, MD) according to the manufacturer's instructions. In parallel, 750 pmol of scrambled siRNA were used to control for off-target changes in NRK-49F. After transfection, cells were cultured in antibiotic-free DMEM/F-12 for 24 h before they were used for the experiments.
Nuclear staining.
After treatment, cells were washed with PBS, fixed in methanol, and then stained with DAPI. Cells with condensed nuclei and/or DNA fragmentation were considered to be apoptotic. Cells in four random fields (×200) of each sample were counted, and three independent experiments were conducted in triplicates.
Immunoblot analysis.
After various treatments, cells were washed once with ice-cold PBS and harvested in a cell lysis buffer. Proteins (25 μg) were separated by SDS-PAGE and transferred to nitrocellulose membranes. After incubation with 5% nonfat milk for 1 h at room temperature, membranes were incubated with a primary antibody for overnight at 4°C and then incubated with appropriate horseradish peroxidase-conjugated secondary antibody for 1 h in room temperature. Bound antibodies were visualized by chemiluminescence detection.
Immunoprecipitation.
Cells were treated with RPTC-Sup for 24 h and they were immediately lysed on ice by using RIPA buffer (Cell Signaling Technology) with protease inhibitor cocktail (Roche Applied Science). After being precleared, 5 μg of anti-PTP1B antibody were added to 750 μg of protein in a total volume of 1,000 μl. After being mixed for 2 h at 4°C, 30 μl of protein G-agarose beads (Santa Cruz Biotechnology) were added to each lysate and gently mixed for overnight at 4°C. Then, beads were rinsed three times with lysis buffer (plus protease inhibitors). Thirty microliters of 2× SDS loading buffer were added to each sample and boiled for 10 min and Western blot analysis was carried out. For phospho-tyrosine (PY20) detection, membranes were blocked with 5% BSA in TBST for 1 h in room temperature. For other molecules, membranes were blocked with 5% milk in TBST. After being blotted with primary antibody overnight, membranes were washed and then incubated with appropriate secondary antibody (mouse TrueBlot or rabbit TrueBlot) prepared in 5% milk.
Densitometry.
The quantitative analysis of different proteins was carried out by using ImageJ software developed at the National Institutes of Health. The quantification is based on the intensity (density) of band, which is calculated by area and pixel value of the band. The quantification data are given as ratio between target protein and loading control (housekeeping protein).
Statistical analysis.
Data are presented as means ± SD and were subjected to one-way ANOVA. Multiple means were compared using Tukey's test, and differences between two groups were determined by Student's t-test. P < 0.05 was considered statistically significant.
RESULTS
Exposure of nonlethal concentrations of necrotic RPTC-Sup does not cause renal interstitial fibroblast cell death.
Recently, we observed that cultured renal fibroblasts die due to both necrosis and apoptosis when they are exposed to the cellular contents from 2 × 106 cells/ml of necrotic RPTC (36). However, it remains unclear whether the nonlethal concentration of necrotic RPTC-Sup would also affect the biological functions of renal interstitial fibroblasts. To address this issue, we first examined the effect of the supernatant from various concentrations of RPTC below 2 × 106 on two hallmarks of cell death [cleavage of poly(ADP-ribose) polymerase (PARP) and caspase-3] in rat renal interstitial fibroblasts (NRK-49F). H2O2-treated cells were used as positive control. As shown in Fig. 1A, exposure of NRK-49F to H2O2 for 3 h resulted in generation of fragments of PARP at 89 kDa and caspase-3 at 17 kDa. However, these fragments were not seen in NRK-49F cells exposed to the supernatant obtained from the number of RPTC ranging from 2 × 104 to 5 × 105 cells/ml. Since it has been reported that cleavage of PARP to 55/45-kDa fragments indicates necrosis (14), and fragmentation of PARP to 89 kDa and caspase-3 into 17 kDa indicates apoptosis (45), these data suggest that the supernatant collected from this range of the number of RPTC does not cause cell death in renal interstitial fibroblasts.
Fig. 1.
Effect of necrotic renal proximal tubular cells (RPTC) on viability of cultured NRK-49F. Normally cultured NRK-49F cells with 5% FBS were treated with indicated concentrations of RPTC supernatant (RPTC-Sup) for 48 h (A). NRK-49F cells treated with 1 mM H2O2 for 3 h were used as positive control to induce cell death (A-C). After treatments, cell lysates were prepared and subjected to immunoblot analysis with antibodies for cleaved caspase-3, cleaved poly(ADP-ribose) polymerase (PARP), or GAPDH. A: representative immunoblots from 3 or more experiments. NRK-49F cells were treated with RPTC-Sup from 5 × 105 cells/ml for 48 h or RPTC-Sup prepared from 2 × 106 cells/ml for 24 h. After treatments, cells were stained with DAPI and photographed with fluorescent microscope to observe nuclei morphology (×200) and shrunken and condensed nuclei were counted (B). Values are means ± SD of 3 independent experiments (C). Bars with different letters (a, b) are significantly different from one another (P < 0.01).
To confirm this observation, we further examined cell death in cultured NRK-49F by using DAPI staining. As shown in Fig. 1, B and C, incubation of NRK-49F with the RPTC-Sup from 2 × 106 but not from 5 × 105 cells/ml induced cell death as shown by nuclear shrinkage and condensation, a feature of apoptosis. The condensed nuclei were also observed in NRK-49F cells exposed to 1 mM H2O2. These data are consistent with the above results and support the concept that the supernatant generated from RPTC below 5 × 105 cells/ml is unable to induce cell death in NRK-49F.
Necrotic RPTC inhibits activation of cultured renal interstitial fibroblasts.
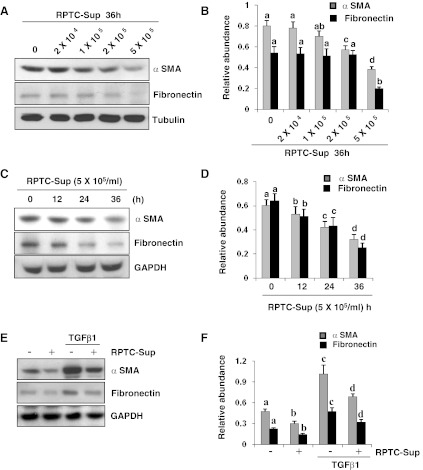
We previously showed that normally cultured NRK-49F cells in the medium containing 5% FBS are activated fibroblasts that express α-SMA and fibronectin, two hallmarks of myofibroblasts (32, 33). To examine whether the nonlethal number of RPTC-Sup would alter the activation state of renal fibroblasts, NRK-49F cells were directly exposed to the RPTC-Sup collected from 2 × 104 to 5 × 105 cells/ml and then we examined expression levels of α-SMA and fibronectin. Figure 2 shows that exposure of renal fibroblasts to RPTC-Sup ranging from 2 × 104 to 5 × 105 cells/ml for 36 h dose dependently reduced the expression of α-SMA and fibronectin (Fig. 2, A and B). At 2 × 105 and 5 × 105 cells/ml, the expression of α-SMA and fibronectin was reduced by ∼50 and 70%, respectively. RPTC-Sup (1 × 105 cells/ml) also significantly reduced the expression of α-SMA, but it did not significantly affect the expression of fibronectin (Fig. 2, A and B). The time course study with 5 × 105 cells/ml further demonstrated that the expression level of α-SMA and fibronectin in renal fibroblasts was slightly decreased within 12 h after exposure to necrotic RPTC-Sup and rapidly reduced at 24 h, and further decreased up to twofold at 36 h (Fig. 2, C and D). We also assessed the effect of nonlethal RPTC-Sup on the proliferation of cultured renal fibroblasts. Surprisingly, treatment of NRK-49F with the RPTC-Sup obtained from 5 × 105 cells/ml, the highest dose used in this study, did not affect cell proliferation as measured by the MTT assay and expression levels of PCNA and cylcin D1 (data not shown). Collectively, our data indicate that necrotic RPTC can inhibit renal fibroblast activation, but not their proliferation.
Fig. 2.
Necrotic RPTC reduce the expression of α-SMA and fibronectin expression in cultured NRK-49F. Normally cultured NRK-49F cells with 5% FBS were treated with indicated concentrations of RPTC-Sup for 36 h (A and B) or cells were treated with necrotic RPTC-Sup prepared from 5 × 105 cells/ml for the indicated time (12, 24, and 36 h; C and D) or cells were starved overnight with DMEM-F12 medium containing 0.5% FBS and then treated with necrotic RPTC-Sup in the presence or absence of TGF-β1 (1 ng/ml) for 24 h (E and F). Cell lysates were prepared and subjected to immunoblot analysis with antibodies for α-SMA, fibronectin, α-tubulin, or GAPDH (A, C, E). Representative immunoblots from 3 experiments are shown. The levels of α-SMA and fibronectin were quantified by densitometry and normalized with α-tubulin or GAPDH (B, D, F). Values are means ± SD of 3 independent experiments. Bars with different letters (a–d) are significantly different from one another (P < 0.01).
It has been reported that TGF-β1 signaling plays a critical role in the activation of renal fibroblasts and development of renal fibrosis. To examine whether necrotic RPTC-Sup would also modulate TGF-β1-induced activation of renal fibroblasts, we treated NRK-49F cells with TGF-β1 in the presence or absence of necrotic RPTC-Sup. The expression of α-SMA and fibronectin was detected in normally cultured NRK-49F and TGF-β1 enhanced expression of these two molecules. Necrotic RPTC-Sup reduced basal level of α-SMA and fibronectin expression, and also largely inhibited TGF-β1-stimulated expression of these two molecules (Fig. 2, E and F). These results suggest that necrotic RPTC-Sup not only suppressed serum (a mixture of growth factors) but also cytokines-induced activation of renal fibroblasts.
Necrotic RPTC inhibits activation of EGFR in cultured renal interstitial fibroblasts.
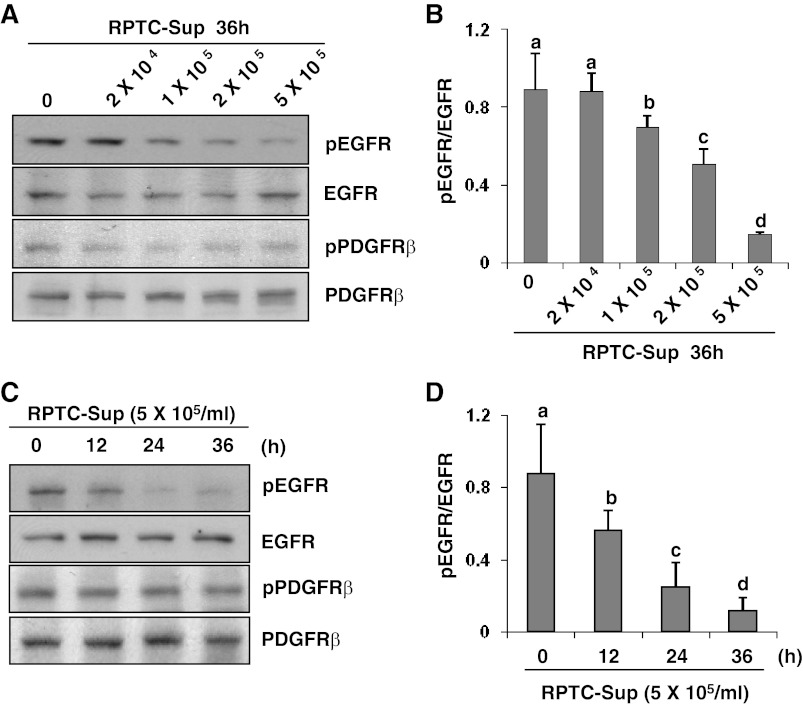
The EGFR and PDGFRβ are the major regulators of fibroblast activation. The phosphorylation of EGFR at Tyr1068 and PDGFRβ at Tyr751 is the major site for the positive modulation of fibroblast activation (3, 22, 24, 39). As such, we examined the effect of RPTC-Sup on the activation of these two receptors. A concentration- and time-dependent decrease of phosphorylation level of EGFR (Tyr1068) without altering total EGFR level was observed in fibroblast cells following exposure to RPTC-Sup (Fig. 3). The phospho-EGFR level was slightly decreased (up to 1-fold) when NRK-49F cells were exposed to lower concentrations of RPTC-Sup (2 × 104 and 2 × 105 cells/ml) and its level further decreased (up to 5-fold) in higher concentration of RPTC-Sup (5 × 105 cells/ml)-exposed cells (Fig. 3, A and B). Moreover, a time course study with 5 × 105 cells/ml showed that phospho-EGFR level significantly decreased (∼30% reduction) within 12 h after necrotic RPTC-Sup treatment and decreased up to 70% at 24 h and further decreased ∼90% at 36 h (Fig. 3, C and D). In contrast, the level of phospho-PDGFRβ and total PDGFRβ was not affected by necrotic RPTC-Sup (Fig. 3, A and C). In addition, we examined the effect of necrotic RPTC-Sup on EGFR phosphorylation within 6 h. EGFR phosphorylation was not changed by necrotic RPTC-Sup at any time points from 0.5 to 6 h during this period (data not shown).
Fig. 3.
Necrotic RPTC reduce epidermal growth factor receptor (EGFR) phosphorylaton in NRK-49F. NRK-49F cells were treated for 36 h with RPTC-Sup prepared from an indicated number of cells (A and B) or treated with RPTC-Sup from 5 × 105 cells/ml for the indicated time (C and D). The cell lysate was prepared after treatment and subjected to immunoblot analysis for phospho-EGFR (Tyr1068), phospho-platelet-derived growth factor receptor-β (PDGFRβ; Tyr751), EGFR, or PDGFRβ (A and C). Representative immunoblots from 3 experiments are shown. The phosphorylated and total levels of EGFR and PDGFRβ were quantified by densitometry and phosphorylated protein levels were normalized to total protein levels (B and D). Values are means ± SD of 3 independent experiments. Bars with different letters (a–d) are significantly different from one another (P < 0.01).
Effect of necrotic RPTC-Sup on the expression of P2X7 receptor in cultured renal interstitial fibroblasts and the role of P2X7 in renal fibroblast activation.
Since a lethal concentration of necrotic RPTC-Sup (2 × 106 cells/ml) can induce the expression of P2X7 receptor (36), we further examined whether nonlethal concentrations of necrotic RPTC-Sup would also induce the expression of P2X7 receptor in renal fibroblasts and whether it would have any effect on their activation. As shown in Fig. 4, A and B, necrotic RPTC-Sup from 5 × 105 cells/ml induced the expression of P2X7 receptor but it was significantly lower than that induced by a cell death-inducing dose of necrotic RPTC-Sup at 2 × 106 cells/ml. However, knockdown of P2X7 with its specific siRNA did not affect the necrotic RPTC-Sup-induced reduction of α-SMA and fibronectin expression (Fig. 4, C and D).
Fig. 4.

Inhibition of P2X7 receptor does not affect the necrotic RPTC-Sup-induced reduction of α-SMA and fibronectin expression. Normally cultured NRK-49F cells with 5% FBS were treated with RPTC-Sup from an indicated number of cells (2 × 104 to 5 × 105 cells/ml) for 36 h or RPTC-Sup from 2 × 106 cells/ml for 24 h (A and B). After treatments, cell lysates were prepared and subjected to immunoblot analysis with antibodies for P2X7 or α-tubulin (A). NRK-49F cells were transfected with rat-specific small interfering RNA (siRNA) targeting P2X7 or scrambled siRNA. At 24 h after transfection, cells were treated with necrotic RPTC for 36 h (C and D). Cell lysates were prepared and subjected to immunoblot analysis with specific antibodies against P2X7, α-SMA, fibronectin, or α-tubulin (C). Representative immunoblots from 3 experiments are shown. The levels of P2X7, α-SMA, and fibronectin were quantified by densitometry and normalized with α-tubulin (B and D). Values are means ± SD of 3 independent experiments. Bars with different letters (a–d) are significantly different from one another (P < 0.01).
Collectively, our results indicate that necrotic RPTC-Sup at concentrations of RPTC-Sup that suppress renal fibroblast activation inhibits phosphorylation of EGFR. But it induces expression of P2X7; however, P2X7 expression is not associated with inactivation of renal fibroblasts. Thus, we suggest that necrotic RPTC-Sup induces renal fibroblast death and inactivation through independent mechanisms.
Necrotic RPTC inhibits activation of STAT3 in cultured renal interstitial fibroblasts.
It is well-known that STAT3, AKT, and ERK 1/2 are the downstream signaling molecules of multiple receptor tyrosine kinases including EGFR. We next examined the phosphorylation status of STAT3, AKT, and ERK 1/2 in necrotic RPTC-Sup-treated NRK-49F cells. Phosphorylation status of STAT3, AKT, and ERK 1/2 was clearly observed in cultured NRK-49F cells. Necrotic RPTC-Sup exposure resulted in decreased STAT3 phosphorylation, which occurred in a concentration-dependent manner with a dramatic decrease in NRK-49F treated with the supernatant from 5 × 105 cells/ml (Fig. 5, A and C). The time course study with this concentration also showed a reduction in phospho-STAT3 level at 12 h after necrotic RPTC-Sup exposure and more than 70% reduction at 36 h (Fig. 5, B and D), whereas exposure of cultured NRK-49F cells to the same concentration of necrotic RPTC-Sup for 0.5 to 6 h did not alter the level of phospho-STAT3 (data not shown). It should be noted that the time- and dose-dependent decrease of phospho-STAT3 level was parallel with that of phospho-EGFR level. In contrast, necrotic RPTC-Sup did not influence the phosphorylation of AKT and ERK and it did also not alter the expression levels of total STAT3, AKT, and ERK1/2 (Fig. 5, A and B). These data suggest that STAT3 may act as downstream of EGFR to mediate renal interstitial fibroblast activation.
Fig. 5.
Necrotic RPTC decrease STAT3 phosphorylation in NRK-49F. NRK-49F cells were treated for 36 h with RPTC-Sup prepared from an indicated number of cells (A) or treated with RPTC-Sup from 5 × 105 cells/ml for the indicated time (C). The cell lysate was prepared after treatments and then subjected to immunoblot analysis for phospho-STAT3 (Tyr705), STAT3, phospho- AKT, AKT, phospho-ERK 1/2, or ERK 1/2 (A and C). Representative immunoblots from 3 experiments are shown. The phosphorylated and total levels of STAT3, AKT, and ERK were quantified by densitometry and phosphorylated protein levels were normalized to total protein levels (B and D). Values are means ± SD of 3 independent experiments. Bars with different letters (a–d) are significantly different from one another (P < 0.01).
Necrotic RPTC-induced dephosphorylation of EGFR and inactivation of renal fibroblasts are mediated by PTP.
As necrotic RPTC-Sup reduces EGFR phosphorylation without altering the level of total EGFR, it would be expected that PTP(s) might be responsible for RPTC-Sup-mediated diminishing of phospho-EGFR. To test this hypothesis, NRK-49F cells were treated with RPTC-Sup in the absence or presence of Na3VO4, a broad-spectrum PTP inhibitor, and fibroblast activation markers and phosphorylation levels of STAT3 and EGFR were analyzed by Western blot analysis. As expected, necrotic RPTC-Sup-induced reduction of phospho-EGFR and phospho-STAT3 levels was replenished by Na3VO4 (Fig. 6, C and D). Application of Na3VO4 also blocked necrotic RPTC-Sup-induced reduction of expression of α-SMA and fibronectin (Fig. 6, A and B). It should be noted that Na3VO4 alone did not change the levels of any of these molecules. These results indicate that necrotic RPTC-Sup exposure induces activation of PTP(s), which leads to EGFR dephosphorylation and renal fibroblast inactivation.
Fig. 6.
Effect of phosphatase inhibitors on necrotic RPTC-induced fibroblast inactivation. NRK-49F cells were pretreated with different concentrations of sodium orthovanadate (Na3VO4) for 1 h and then exposed to the necrotic RPTC-Sup prepared from 5 × 105 cells/ml for an additional 36 h. Cell lysates were prepared and subjected to immunoblot analysis with specific antibodies against α-SMA, fibronectin, α-tubulin, phospho-EGFR, EGFR, phospho-STAT3, or STAT3 (A and C). Representative immunoblots from 3 experiments are shown. The levels of α-SMA and fibronectin were quantified by densitometry and normalized with α-tubulin (B). The phosphorylated and total levels of STAT3 and EGFR were quantified by densitometry and phosphorylated protein levels were normalized to total protein levels (D). Values are means ± SD of 3 independent experiments. Bars with different letters (a–d) are significantly different from one another (P < 0.01).
EGFR inhibition abolishes the effect of PTP inhibitors on activation of renal fibroblasts.
Although Na3VO4 inhibits EGFR dephosphorylation, its effect on the reversal of α-SMA and fibronectin expression may occur through its nonspecific effect. To rule out this possibility, we examined the effect of Na3VO4 on RPTC-Sup-induced suppression of phosphorylation of EGFR and STAT3 as well as inactivation of renal fibroblasts in the presence of geftinib, a selective EGFR tyrosine kinase inhibitor (6). As shown in Fig. 7A, Na3VO4 inhibited RPTC-Sup-induced reduction of expression of α-SMA and fibronectin, and it also prevented RPTC-Sup-induced diminishing of phospho-EGFR and phospho-STAT3 levels. However, the effects of Na3VO4 on RPTC-Sup-induced alterations were completely abolished in the presence of gefitinib (Fig. 7A). Similar results were also obtained when EGFR was knocked down by a siRNA specifically targeting EGFR (Fig. 7B). These results suggest that PTPs might regulate necrotic RPTC-induced inactivation of renal fibroblasts through the EGFR signaling.
Fig. 7.
EGFR inhibition blocks the effect of Na3VO4 on necrotic RPTC-induced alterations in NRK-49F. NRK-49F cells were pretreated with gefitinib (2 μM) for 15 min and exposed to Na3VO4 (10 μM) for 1 h and then incubated with necrotic RPTC for an additional 36 h (A). NRK-49F cells were transfected with siRNA targeting EGFR or scrambled siRNA for 24 h. After transfection, cells were treated with necrotic RPTC for 36 h in the presence or absence of Na3VO4 (10 μM; B). Cell lysates were prepared and subjected to immunoblot analysis with specific antibodies against α-SMA, fibronectin, α-tubulin, phospho-EGFR, EGFR, phospho-STAT3, or STAT3 (A and B). Representative immunoblots from 3 experiments are shown.
Inhibition of PTP1B reduces RPTC-Sup-induced EGFR dephosphorylation.
Among the members of PTP family, PTP1B is highly expressed in renal tissue (38). The expression or the activity of PTP1B is modulated in response to various stimuli such as metabolic stresses and growth factor (4). Several studies indicate that PTP1B is phosphorylated during interaction with EGFR, and subsequently leading to EGFR dephosphorylation (8, 21). As such, we hypothesized that PTP1B may be the phosphatase that mediates inactivation of EGFR signaling in NRK-49F exposed to necrotic RPTC. To test this hypothesis, we examined whether inhibition of PTP1B would affect the RPTC-Sup-induced inactivation of renal fibroblasts. NRK-49F were pretreated with TCS-401, a PTP1B-specific inhibitor, for 1 h and then exposed to RPTC-Sup for another 36 h. As shown in Fig. 8A, TCS-401 dose dependently inhibited the RPTC-Sup-induced reduction of fibronectin and α-SMA. At 1 μM, TCS-401 reversed the levels of fibronectin and α-SMA about onefold and at a dose of 2 μM, TCS-401 brought back fibronectin and α-SMA expression to near normal levels. Correspondingly, the RPTC-Sup-induced reduction of phospho-EGFR and phospho-STAT3 was returned to the control levels in NRK-49F treated with 1 and 2 μM TCS-401 (Fig. 8B). To confirm whether TCS-401-induced reversal of fibronectin and α-SMA level is through inhibition of EGFR dephosphorylation, NRK-49F cells were treated with RPTC-Sup and TCS-401 in the presence of gefitinib. As shown in Fig. 8, C and D, gefitinib totally blocked the effect of the PTP1B inhibitor on reversal of expression of fibronectin, α-SMA, and phosphorylation of EGFR and STAT3. Collectively, these data indicate that PTP1B plays a critical role in mediating necrotic RPTC-induced inactivation of renal interstitial fibroblasts, which may occur through dephosphorylation of EGFR and subsequent blockage of its downstream signaling pathway.
Fig. 8.

Inhibition of protein tyrosine phosphatase (PTP)1B blocks necrotic RPTC-induced fibroblast inactivation. NRK-49F cells were pretreated with different concentrations of TCS-401 as indicated for 1 h and then incubated with necrotic RPTC-Sup for an additional 36 h. Cell lysates were prepared and subjected to immunoblot analysis with specific antibodies against α-SMA, fibronectin, α-tubulin, phospho-EGFR, EGFR, phospho-STAT3, or STAT3 (A and B). NRK-49F cells were pretreated with gefitinib (2 μM) for 15 min and then incubated with 2 μM TCS-401 for 1 h. After that, RPTC-Sup was added and incubated for an additional 36 h. Cell lysates were prepared and subjected to immunoblot analysis with specific antibodies against α-SMA, fibronectin, α-tubulin, phospho-EGFR, EGFR, phospho-STAT3, or STAT3 (C and D). Representative immunoblots from 3 experiments are shown.
PTP1B interacts with EGFR in necrotic RPTC-treated renal fibroblasts.
As mentioned above, PTP1B acts through direct interaction with its targets. Given that the PTP1B inhibitor is able to inhibit the EGFR signaling in NRK-49F cells exposed to necrotic RPTC, we further examined whether necrotic RPTC promotes the interaction of PTP1B with EGFR. As phospho-EGFR levels were reduced to more than 50% at 24 h (Fig. 3C), we used a 24-h time point to examine the possible interaction of EGFR with PTP1B in renal fibroblasts (Fig. 9B). At 24 h after exposure to necrotic RPTC, NRK-49F cells were collected and cell lysates were immunoprecipitated with an antibody for PTP1B. Immunocomplexes were blotted with an antibody against EGFR or PY20 (a specific tyrosine kinase antibody). As shown in Fig. 9A, NRK-49F cells normally expressed PTP1B and its level was not altered by RPTC-Sup treatment. Although total PTP1B levels were the same in NRK-49F cells treated with/without necrotic RPTC-Sup, tyrosine phosphorylation level of PTP1B was increased in cells treated with RPTC-Sup (Fig. 9B). In addition, an increased EGFR level was detected in PTP1B immunoprecipitates from cells treated with necrotic RPTC-Sup. These data suggest that necrotic RPTC-Sup exposure induces PTP1B tyrosine phosphorylation and its interaction with EGFR, which in turn leads to EGFR dephosphorylation.
Fig. 9.
Necrotic RPTC induce PTP1B tyrosine phosphorylaton and its interaction with EGFR. NRK-49F cells were treated with RPTC-Sup for 36 h and then cell lysates were subjected to immunoblotting for PTP1B and GAPDH (A). Normally cultured NRK-49F cells were treated with necrotic RPTC-Sup for 24 h and cells were harvested. Equal concentrations of protein from each sample were immunoprecipitated with PTP1B antibody and then subjected to Western blot analysis for PTP1B, PY20, or EGFR (B). Representative immunoblots from 3 experiments are shown.
DISCUSSION
During the course of AKI, the injured or dying renal tubular cells release their contents to the interstitium where several cell types are situated. Renal interstitial fibroblasts are a cell type adjacent to the damaged tubular epithelium and may be directly exposed to molecules released by injured epithelial cells. To mimic this situation, we recently initiated a series of experiments to study the cross-talk between renal epithelial cells and interstitial fibroblasts in an in vitro cultured system. Our results revealed that exposure of cultured renal interstitial fibroblasts to the supernatant from 2 × 106 of necrotic RPTC resulted in cell death of fibroblasts (34–36). However, a fundamental unanswered question in the renal epithelial-fibroblast cross-talk is whether the necrotic RPTC-Sup at its nonlethal doses would also alter the biological functions of renal interstitial fibroblasts. In the present study, we made an interesting discovery that nonlethal doses of necrotic RPTC-Sup reduced activation of renal fibroblasts and induced dephosphorylation of the EGFR-STAT3, a key signaling pathway responsible for renal fibroblast activation. Furthermore, inhibition of PTP1B protected against necrotic RPTC-Sup-induced responses. Therefore, we suggest that the nonlethal dose of necrotic RPTC-Sup can induce inactivation of renal fibroblasts through PTP1B-mediated suppression of the EGFR-STAT3 signaling pathway (Fig. 10).
Fig. 10.
Mechanisms of necrotic RPTC-Sup-induced inactivation of renal interstitial fibroblasts. Under normal culture condition, EGFR and STAT3 are activated, which is required for activation of renal interstitial fibroblasts. When cells are exposed to necrotic RPTC-Sup, PTP1B is activated, leading to EGFR dephosphorylation and subsequent inactivation of STAT3 and renal interstitial fibroblasts.
To our knowledge, this is the first study demonstrating that nonlethal doses of necrotic RPTC-Sup have an ability to suppress activation of renal interstitial fibroblasts. Our and other studies previously showed that cultured NRK-49F cells are activated fibroblasts with expression of abundances of α-SMA and fibronectin (22, 28, 32, 33). The current study revealed that necrotic RPTC-Sup at nonlethal doses ranging from 2 × 104 to 5 × 105 cells/ml reduced the expression of fibronectin and α-SMA in a time- and dose-dependent manner. This is in contrast to our previous observation that exposure of renal fibroblasts to the supernatant from necrotic RPTC at 2 × 106 caused both apoptosis and necrosis. As the amount of cellular contents released from tubular cells depends on the severity of injury and number of tubular cells died, these studies suggest that cellular contents of RPTC released to the interstitium have divergent impacts on renal myofibroblasts. However, no matter whether renal fibroblasts die or inactivation in response to necrotic RPTC, the net result is that the renal fibroblasts would lose or reduce their ability to produce the matrix proteins in the early phase of AKI.
Fibroblast activation after injury is, in essence, a wound-healing response by which the injured kidney attempts to repair and recover from the injury. Following AKI, the repair of renal epithelium requires repopulation of renal tubular cells, but this cannot occur unless there is remodeling of ECM to reconstitute the original tissue structure since tubular cells need collagen framework along which to grow. In this respect, activated interstitial fibroblasts not only produce ECM components, but they also provide support for renal tubular cells to migrate and replicate (18, 29). In addition, activated fibroblasts also promote efficient closure of denuded area through their contractile properties (29). On the other hand, renal fibroblasts can produce renotropic growth factors and cytokines such as fibroblast growth factor-1 and -7, which may act as paracrine mitogens to stimulate renal tubular cell proliferation. On this basis, the transient activation of renal interstitial fibroblasts surrounding damaged tubules might be beneficial to renal repair and functional recovery in the early phase of AKI, whereas inhibition of renal fibroblast activation by necrotic RPTC might be one of the mechanisms that lead to maladaptive renal repair in the case of severe AKI.
Nevertheless, the persistent and uncontrolled activation of renal fibroblasts will result in excessive production and deposition of ECM and development of renal fibrosis. This has been demonstrated by numerous studies and is associated with production of a large amount of profibrogenetic factors, in particular TGF-β1. TGF-β1 is a potent stimulus for renal fibroblast activation and can be released after a variety of insults. When kidney injury is persistent or repeated, increasing numbers of injured epithelial cells stall between the G2 and M phases of the cell cycle, which results in an ongoing production of TGF-β (46). Overproduction of TGF-β1 in response to severe kidney injury may override the effect of necrotic tubular cells on its stimulation of renal fibroblast inactivation, thereby rendering renal fibroblasts to produce excessive amounts of ECM proteins, and leading to fibrogenesis in the damaged kidney. This hypothesis is supported by our experimental result that necrotic RPTC-Sup was unable to completely block expression of α-SMA and fibronectin in cultured NRK-49F exposed to TGF-β1 and other observations note that severe AKI is often accompanied by aberrant renal repair and fibrogenesis (46).
The mechanism by which RPTC-Sup inactivates renal fibroblasts remains poorly understood. Although we observed that sublethal doses of RPTC-Sup induced the expression of P2X7 receptor, knockdown of this receptor did not affect the ability of necrotic RPTC-Sup to suppress expression of α-SMA and fibronectin in NRK-49F cells, suggesting that P2X7 receptor does not mediate necrotic RPTC-Sup-induced inactivation of renal fibroblasts. As EGFR and PDGFRβ have been shown to play a role in mediating renal fibroblast activation (20, 22, 24), we further examined the effect of necrotic RPTC-Sup on the activation of these two receptors and their downstream signaling pathways. Our results indicated that exposure of NRK-49F to necrotic RPTC-Sup resulted in dephosphorylation of EGFR and STAT3, but not that of phosphorylation of PDGFRβ, AKT, and ERK1/2. These results together with our previous observations show that blockade of EGFR inhibited dephosphorylation of STAT3 in the fibrotic kidney (22). It suggests that necrotic RPTC may reduce activation of renal interstitial fibroblasts through inhibition of the EGFR-STAT3 signaling pathway. In support of this finding, we also showed that inhibition of EGFR phosphorylation with gefitinib or silencing of EGFR with siRNA abolished the effect of necrotic RPTC-Sup-induced inactivation of renal fibroblasts, whereas restoration of EGFR phosphorylation levels with PTP inhibitors (sodium orthovanadate and TCS401) brought back the expression of α-SMA and fibronectin and phosphorylation of STAT3 to the control levels. Nevertheless, the EGFR-STAT3 signaling pathway may not be the sole target of necrotic RPTC since inactivation of this pathway reduced α-SMA and fibronectin expression, but was unable to abolish their expression in cultured NRK-49F cells.
It is well-known that various protein receptor tyrosine kinase phosphatase(s) are involved in regulation of EGFR dephosphorylation, depending on the cell type and stimuli (21). Our current study indicated that PTP1B is responsible for the RPTC-Sup-induced dephosphorylation of EGFR and inactivation of renal fibroblasts. This is evidenced by several observations: 1) the PTP1B-specific inhibitor (TCS401) abolished RPTC-Sup-induced reduction of phospho-EGFR and expression of fibrotic markers; 2) blockage of EGFR diminished the effect of PTP1B inhibitor on RPTC-Sup-induced inhibition of renal fibroblast activation and STAT-3 phosphorylation; and 3) RPTC-Sup induced PTP1B phosphorylation and its interaction with EGFR. Currently, the initial events triggered by necrotic RPTC-Sup for the activation of PTP1B in renal fibroblasts remain unclear. Several studies demonstrate that renal tubular epithelial cells can produce various cytokines (12, 40, 43) such as IL-6, IL-1β, TNF-α (5, 7, 26) and that metabolic stress can also upregulate the transcription of inflammatory cytokines including TNF-α and IL-6 in cultured human renal cortical tubular cells (12). Given the fact that TNF-α, IL-6, and IL-1β are capable of modulating the activity and/or expression of PTP1B (17, 30, 31, 48), it is possible that the production of these cytokines by necrotic RPTC might stimulate PTP1B activity in renal fibroblasts and subsequently alters phosphorylation levels of some tyrosine kinases including EGFR in the damaged kidney. Further in vivo studies are required to address this issue.
Myofibroblast proliferation is considered to occur secondary to its activation. However, our data indicated that necrotic RPTC suppressed renal fibroblast activation without affecting their proliferation. This is indicated by our observations that necrotic RPTC-Sup reduced the expression of α-SMA and fibronectin, but did not affect the growth rate of fibroblasts and the expression of PCNA, a cell proliferation marker, and cyclin D, a nuclear protein that is involved in promoting cell cycle progression. On this basis, we suggest that renal fibroblast activation and proliferation may be controlled by different mechanisms. In this context, we demonstrated that necrotic RPTC-Sup inhibits phosphorylation of STAT3, but not that of AKT and ERK1/2 in cultured NRK-49F. As phosphorylation of these three molecules represents activation of individual signaling pathways, and AKT and ERK1/2 play a critical role in regulating cell proliferation (10, 37), distinct activation of these signaling pathways may account for the different effects of necrotic RPTC on renal fibroblasts.
In summary, this is the first report showing that the contents released from injured epithelial cells can inactivate renal fibroblasts by dephosphorylation of EGFR through a mechanism involved in PTP1B activation. As the peritubular fibroblasts play a vital role in maintaining the functional and structural integrity of renal tissue and contribute to renal epithelium repair, inactivation of renal fibroblasts by damaged tubular cells may reduce or delay the reparative process of renal epithelium after AKI. Therefore, maintenance of certain number of renal interstitial fibroblasts after AKI may be essential for renal tubular repair and regeneration, and renal functional recovery.
GRANTS
This work was supported by grants from the National Institutes of Health (DK-085065 to S. Zhuang) and the National Nature Science Foundation of China (81270778 to S. Zhuang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.P. and S.Z. conception and design of research; M.P. and L.M. performed experiments; M.P. and L.M. analyzed data; M.P. and S.Z. interpreted results of experiments; M.P. prepared figures; S.Z. drafted manuscript; S.Z. edited and revised manuscript; S.Z. approved final version of manuscript.
REFERENCES
- 1. Asada N, Takase M, Nakamura J, Oguchi A, Asada M, Suzuki N, Yamamura K, Nagoshi N, Shibata S, Rao TN, Fehling HJ, Fukatsu A, Minegishi N, Kita T, Kimura T, Okano H, Yamamoto M, Yanagita M. Dysfunction of fibroblasts of extrarenal origin underlies renal fibrosis and renal anemia in mice. J Clin Invest 121: 3981–3990, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bachmann S, Le Hir M, Eckardt KU. Co-localization of erythropoietin mRNA and ecto-5′-nucleotidase immunoreactivity in peritubular cells of rat renal cortex indicates that fibroblasts produce erythropoietin. J Histochem Cytochem 41: 335–341, 1993 [DOI] [PubMed] [Google Scholar]
- 3. Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev 15: 255–273, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Bourdeau A, Dube N, Tremblay ML. Cytoplasmic protein tyrosine phosphatases, regulation and function: the roles of PTP1B and TC-PTP. Curr Opin Cell Biol 17: 203–209, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Chang Y, Ceacareanu B, Zhuang D, Zhang C, Pu Q, Ceacareanu AC, Hassid A. Counterregulatory function of protein tyrosine phosphatase 1B in platelet-derived growth factor- or fibroblast growth factor-induced motility and proliferation of cultured smooth muscle cells and in neointima formation. Arterioscler Thromb Vasc Biol 26: 501–507, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Chen SC, Guh JY, Lin TD, Chiou SJ, Hwang CC, Ko YM, Chuang LY. Gefitinib attenuates transforming growth factor-beta1-activated mitogen-activated protein kinases and mitogenesis in NRK-49F cells. Transl Res 158: 214–224, 2011 [DOI] [PubMed] [Google Scholar]
- 7. Docherty NG, O'Sullivan OE, Healy DA, Murphy M, O'Neill AJ, Fitzpatrick JM, Watson RW. TGF-β1-induced EMT can occur independently of its proapoptotic effects and is aided by EGF receptor activation. Am J Physiol Renal Physiol 290: F1202–F1212, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Eden ER, White IJ, Tsapara A, Futter CE. Membrane contacts between endosomes and ER provide sites for PTP1B-epidermal growth factor receptor interaction. Nat Cell Biol 12: 267–272, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Erslev AJ, Besarab A. Erythropoietin in the pathogenesis and treatment of the anemia of chronic renal failure. Kidney Int 51: 622–630, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Fagone E, Conte E, Gili E, Fruciano M, Pistorio MP, Lo Furno D, Giuffrida R, Crimi N, Vancheri C. Resveratrol inhibits transforming growth factor-beta-induced proliferation and differentiation of ex vivo human lung fibroblasts into myofibroblasts through ERK/Akt inhibition and PTEN restoration. Exp Lung Res 37: 162–174, 2011 [DOI] [PubMed] [Google Scholar]
- 11. Floege J, Eitner F, Alpers CE. A new look at platelet-derived growth factor in renal disease. J Am Soc Nephrol 19: 12–23, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Fougeray S, Bouvier N, Beaune P, Legendre C, Anglicheau D, Thervet E, Pallet N. Metabolic stress promotes renal tubular inflammation by triggering the unfolded protein response. Cell Death Dis 2: e143, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fujigaki Y, Muranaka Y, Sun D, Goto T, Zhou H, Sakakima M, Fukasawa H, Yonemura K, Yamamoto T, Hishida A. Transient myofibroblast differentiation of interstitial fibroblastic cells relevant to tubular dilatation in uranyl acetate-induced acute renal failure in rats. Virchows Arch 446: 164–176, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Gobeil S, Boucher CC, Nadeau D, Poirier GG. Characterization of the necrotic cleavage of poly(ADP-ribose) polymerase (PARP-1): implication of lysosomal proteases. Cell Death Differ 8: 588–594, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Gu F, Dube N, Kim JW, Cheng A, Ibarra-Sanchez MJ, Tremblay ML, Boisclair YR. Protein tyrosine phosphatase 1B attenuates growth hormone-mediated JAK2-STAT signaling. Mol Cell Biol 23: 3753–3762, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haj FG, Markova B, Klaman LD, Bohmer FD, Neel BG. Regulation of receptor tyrosine kinase signaling by protein tyrosine phosphatase-1B. J Biol Chem 278: 739–744, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Ito Y, Banno R, Hagimoto S, Ozawa Y, Arima H, Oiso Y. TNFalpha increases hypothalamic PTP1B activity via the NFkappaB pathway in rat hypothalamic organotypic cultures. Regul Pept 174: 58–64, 2012 [DOI] [PubMed] [Google Scholar]
- 18. Kaissling B, Le Hir M. The renal cortical interstitium: morphological and functional aspects. Histochem Cell Biol 130: 247–262, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lautrette A, Li S, Alili R, Sunnarborg SW, Burtin M, Lee DC, Friedlander G, Terzi F. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat Med 11: 867–874, 2005 [DOI] [PubMed] [Google Scholar]
- 20. LeBleu VS, Kalluri R. Blockade of PDGF receptor signaling reduces myofibroblast number and attenuates renal fibrosis. Kidney Int 80: 1119–1121, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu F, Chernoff J. Protein tyrosine phosphatase 1B interacts with and is tyrosine phosphorylated by the epidermal growth factor receptor. Biochem J 327: 139–145, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu N, Guo JK, Pang M, Tolbert E, Ponnusamy M, Gong R, Bayliss G, Dworkin LD, Yan H, Zhuang S. Genetic or pharmacologic blockade of EGFR inhibits renal fibrosis. J Am Soc Nephrol 23: 854–867, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lo HW, Hsu SC, Xia W, Cao X, Shih JY, Wei Y, Abbruzzese JL, Hortobagyi GN, Hung MC. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via upregulation of TWIST gene expression. Cancer Res 67: 9066–9076, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ludewig D, Kosmehl H, Sommer M, Bohmer FD, Stein G. PDGF receptor kinase blocker AG1295 attenuates interstitial fibrosis in rat kidney after unilateral obstruction. Cell Tissue Res 299: 97–103, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Maxwell PH, Ferguson DJ, Nicholls LG, Johnson MH, Ratcliffe PJ. The interstitial response to renal injury: fibroblast-like cells show phenotypic changes and have reduced potential for erythropoietin gene expression. Kidney Int 52: 715–724, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Meldrum KK, Meldrum DR, Hile KL, Yerkes EB, Ayala A, Cain MP, Rink RC, Casale AJ, Kaefer MA. p38 MAPK mediates renal tubular cell TNF-α production and TNF-α-dependent apoptosis during simulated ischemia. Am J Physiol Cell Physiol 281: C563–C570, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Melenhorst WB, Mulder GM, Xi Q, Hoenderop JG, Kimura K, Eguchi S, van Goor H. Epidermal growth factor receptor signaling in the kidney: key roles in physiology and disease. Hypertension 52: 987–993, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Meng LQ, Tang JW, Wang Y, Zhao JR, Shang MY, Zhang M, Liu SY, Qu L, Cai SQ, Li XM. Astragaloside IV synergizes with ferulic acid to inhibit renal tubulointerstitial fibrosis in rats with obstructive nephropathy. Br J Pharmacol 162: 1805–1818, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meran S, Steadman R. Fibroblasts and myofibroblasts in renal fibrosis. Int J Exp Pathol 92: 158–167, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nieto-Vazquez I, Fernandez-Veledo S, de Alvaro C, Lorenzo M. Dual role of interleukin-6 in regulating insulin sensitivity in murine skeletal muscle. Diabetes 57: 3211–3221, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31. Nieto-Vazquez I, Fernandez-Veledo S, de Alvaro C, Rondinone CM, Valverde AM, Lorenzo M. Protein-tyrosine phosphatase 1B-deficient myocytes show increased insulin sensitivity and protection against tumor necrosis factor-alpha-induced insulin resistance. Diabetes 56: 404–413, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Pang M, Kothapally J, Mao H, Tolbert E, Ponnusamy M, Chin YE, Zhuang S. Inhibition of histone deacetylase activity attenuates renal fibroblast activation and interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol 297: F996–F1005, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pang M, Ma L, Gong R, Tolbert E, Mao H, Ponnusamy M, Chin YE, Yan H, Dworkin LD, Zhuang S. A novel STAT3 inhibitor, S3I-201, attenuates renal interstitial fibroblast activation and interstitial fibrosis in obstructive nephropathy. Kidney Int 78: 257–268, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Ponnusamy M, Liu N, Gong R, Yan H, Zhuang S. ERK pathway mediates P2X7 expression and cell death in renal interstitial fibroblasts exposed to necrotic renal epithelial cells. Am J Physiol Renal Physiol 301: F650–F659, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ponnusamy M, Liu N, Sellamuthu R, Zhao TC, Mao H, Zhuang S. Autophagy protects against necrotic renal epithelial cell-induced death of renal interstitial fibroblasts. Am J Physiol Renal Physiol 303: F83–F91, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ponnusamy M, Ma L, Gong R, Pang M, Chin YE, Zhuang S. P2X7 receptors mediate deleterious renal epithelial-fibroblast cross talk. Am J Physiol Renal Physiol 300: F62–F70, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Qu X, Zhang X, Yao J, Song J, Nikolic-Paterson DJ, Li J. Resolvins E1 and D1 inhibit interstitial fibrosis in the obstructed kidney via inhibition of local fibroblast proliferation. J Pathol 228: 506– 519, 2012 [DOI] [PubMed] [Google Scholar]
- 38. Sorenson CM, Sheibani N. Altered regulation of SHP-2 and PTP 1B tyrosine phosphatases in cystic kidneys from bcl-2 −/− mice. Am J Physiol Renal Physiol 282: F442–F450, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Terzi F, Burtin M, Hekmati M, Federici P, Grimber G, Briand P, Friedlander G. Targeted expression of a dominant-negative EGF-R in the kidney reduces tubulo-interstitial lesions after renal injury. J Clin Invest 106: 225–234, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thurman JM. Triggers of inflammation after renal ischemia/reperfusion. Clin Immunol 123: 7–13, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tiganis T. Protein tyrosine phosphatases: dephosphorylating the epidermal growth factor receptor. IUBMB Life 53: 3–14, 2002 [DOI] [PubMed] [Google Scholar]
- 42. van Kooten C, Daha MR. Cytokine cross-talk between tubular epithelial cells and interstitial immunocompetent cells. Curr Opin Nephrol Hypertens 10: 55–59, 2001 [DOI] [PubMed] [Google Scholar]
- 43. van Kooten C, Daha MR, van Es LA. Tubular epithelial cells: a critical cell type in the regulation of renal inflammatory processes. Exp Nephrol 7: 429–437, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Wang WQ, Sun JP, Zhang ZY. An overview of the protein tyrosine phosphatase superfamily. Curr Top Med Chem 3: 739–748, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Wen LT, Caldwell CC, Knowles AF. Poly(ADP-ribose) polymerase activation and changes in Bax protein expression associated with extracellular ATP-mediated apoptosis in human embryonic kidney 293-P2X7 cells. Mol Pharmacol 63: 706–713, 2003 [DOI] [PubMed] [Google Scholar]
- 46. Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med 16: 535–543, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2: 127–137, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Zabolotny JM, Kim YB, Welsh LA, Kershaw EE, Neel BG, Kahn BB. Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. J Biol Chem 283: 14230–14241, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang ZY. Mechanistic studies on protein tyrosine phosphatases. Prog Nucleic Acid Res Mol Biol 73: 171–220, 2003 [DOI] [PubMed] [Google Scholar]