Abstract
Increased angiotensin II (ANG II) or adenosine can potentiate each other in the regulation of renal hemodynamics and tubular function. Diabetes is characterized by hyperfiltration, yet the roles of ANG II and adenosine receptors for controlling baseline renal blood flow (RBF) or tubular Na+ handling in diabetes is presently unknown. Accordingly, the changes in their functions were investigated in control and 2-wk streptozotocin-diabetic rats after intrarenal infusion of the ANG II AT1 receptor antagonist candesartan, the adenosine A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), or their combination. Compared with controls, the baseline blood pressure, RBF, and renal vascular resistance (RVR) were similar in diabetics, whereas the glomerular filtration rate (GFR) and filtration fraction (FF) were increased. Candesartan, DPCPX, or the combination increased RBF and decreased RVR similarly in all groups. In controls, the GFR was increased by DPCPX, but in diabetics, it was decreased by candesartan. The FF was decreased by candesartan and DPCPX, independently. DPCPX caused the most pronounced increase in fractional Na+ excretion in both controls and diabetics, whereas candesartan or the combination only affected fractional Li+ excretion in diabetics. These results suggest that RBF, via a unifying mechanism, and tubular function are under strict tonic control of both ANG II and adenosine in both control and diabetic kidneys. Furthermore, increased vascular AT1 receptor activity is a contribution to diabetes-induced hyperfiltration independent of any effect of adenosine A1 receptors.
Keywords: AT1 receptors, A1 receptors, tubular function, Na+ handling, diabetes
adenosine and the renin-angiotensin system (RAS) are involved in the regulation of intrarenal hemodynamics and tubular function (18, 53). It is well-known that activation of either angiotensin II (ANG II) AT1 receptors or adenosine A1 receptors alters kidney function (18, 53). ANG II, acting on AT1 receptors, reduces glomerular filtration rate (GFR) and renal blood flow (RBF) by increasing pre- and postglomerular resistances (8, 44). Indeed, the most commonly used treatment to reduce the progression of proteinuric kidney disease in diabetics includes targeting the RAS (3). Adenosine controls renin release (2, 15, 25, 48), has direct vascular effects (1, 32, 33, 38), and is a fundamental component of the tubuloglomerular feedback (TGF) mechanism (46, 53).
Diabetic nephropathy is a leading cause of morbidity and mortality. Since the initial glomerular hyperfiltration (29) is crucial for the progression of renal injury (24), it is very important to establish and target its cause yet the mechanism remains unclear. Reduced preglomerular vascular resistance has been emphasized (7) and commonly attributed to the combination of a reduced TGF (52) and myogenic response of the afferent arteriole (14). Adenosine, through activation of adenosine A1 receptors, is the main effector of the TGF-induced afferent arteriolar vasoconstriction (46, 54). However, diabetic adenosine A1 receptor knockout mice lack the TGF mechanism and still develop hyperfiltration (45), suggesting that TGF is not the only mechanism involved (39). Also, in early diabetic nephropathy there is increased proximal Na+ reabsorption (13) associated with enhanced expression of adenosine A1 receptors (37), tubular hypertrophy, and increased Na+/glucose cotransport (52), which may also contribute to hyperfiltration (39) due to the decreased pressure in the Bowman's space that enhances the net ultrafiltration pressure by a nonvascular mechanism.
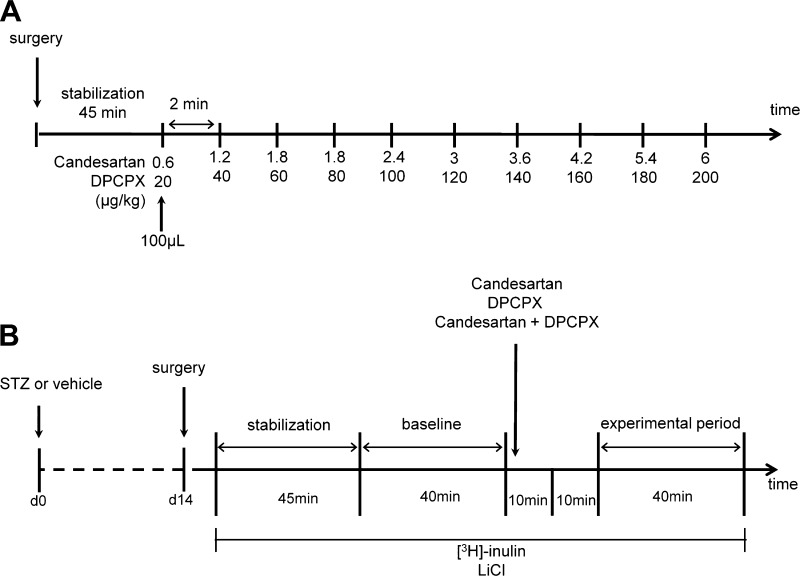
Several studies have addressed the roles of ANG II and adenosine, and their interaction, in controlling vascular tone using either isolated microvessels, in situ perfused kidneys, pathological alterations or systemic manipulation of these vasoactive systems (12, 36, 49, 56). However, the in vivo significance of tonic baseline activation of ANG II and adenosine receptors, and any interaction between the two, are presently unknown, partly due to the systemic effects of blocking these receptors. Furthermore, its relevance for the functional alterations occurring in the diabetic kidney has not previously been demonstrated. Of importance also are the effects of both ANG II and adenosine to enhanced proximal tubule reabsorption via the Na+/H+ exchanger NHE3. These might act in parallel with hyperglycemia-induced Na+-glucose reabsorption to provide a nonvascular site for interaction between the two systems that can only be studied in the intact kidneys of diabetic models. Therefore, the aim of the present study was to elucidate the role of in vivo tonic activation of ANG II and adenosine receptors on renal hemodynamics and tubular function both in control and diabetic rats. For that reason, we developed a protocol (Fig. 1B) in which the drugs were infused directly into the renal artery in a slow step-wise manner, allowing to directly investigate the effects on intrarenal hemodynamics and tubular function without confounding systemic effects. This is important since even small changes in arterial pressure can affect proximal reabsorption and renal hemodynamics.
Fig. 1.
Outline of the two protocols. A: protocol used for determining the dose-response curve for the angiotensin II (ANG II) AT1 receptor antagonist candesartan, or the adenosine A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), or the combination of the two. B: protocol used for investigating the roles of AT1 and A1 receptors on the regulation of intrarenal hemodynamics and tubular Na+ handling. STZ, streptozotocin.
MATERIALS AND METHODS
All chemicals were from Sigma Aldrich (St. Louis, MO) and of highest grade available if not otherwise stated.
Animal model.
Male Sprague-Dawley rats (300–350 g; Charles River) with free access to water and standard rat chow were injected with streptozotocin (50 mg/kg) or vehicle into the tail vein. Blood glucose concentration was measured using test reagent strips (MediSense, Bedford, MA). Animals were considered diabetic if blood glucose concentration was and remained >18 mmol/l within 48 h after streptozotocin injection. Experiments were conducted 14 days after induction of diabetes. All experiments were performed in accordance with the National Institutes of Health Guidelines for Use and Care of Laboratory Animals and approved by the local Animal Care and Use Committee.
Dose-response curve for candesartan and 8-cyclopentyl-1,3-dipropylxanthine.
Normoglycemic animals were anesthetized with thiobutabarbital (120 mg/kg ip; Inactin) and surgically prepared as previously described (28). Briefly, animals were placed on a heated operating table at 38°C and tracheostomized. Polyethylene catheters were placed in the left femoral vein for infusion of Ringer solution (5 ml·kg body wt−1·h−1 for controls and 10 ml·kg body wt−1·h−1 for diabetics) and in the left femoral artery for blood pressure measurements. The bladder was catheterized for free urinary drainage of the right kidney. The left kidney was exposed by a left subcostal flank incision, immobilized in a plastic cup, and embedded in pieces of saline-soaked cotton wool, and the surface was covered with paraffin oil (Apoteksbolaget, Gothenburg, Sweden) to prevent evaporation and keep the tissue moist at body temperature. Thereafter, a catheter was advanced ∼1–2 mm into the left renal artery, through a lumbar artery, for kidney-specific delivery of drugs. All infusion solutions administered into the renal artery contained lissamine green to visually verify homogenous intrarenal distribution of the vasoactive substances. The ANG II AT1 receptor antagonist candesartan (AstraZeneca, Mölndal, Sweden) was administered in doses between 0.6 and 6.0 μg/kg and the adenosine A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) at doses between 20 and 200 μg/kg to separate animals. DPCPX was dissolved in saline with a final concentration of 1% DMSO. A third group of animals received a combination of both antagonists. After 45 min of stabilization, each dose was infused every 2 min in a total volume of 100 μl (Fig. 1A).
In vivo intrarenal blockade of ANG II and adenosine receptors.
Control and diabetic animals were anesthetized with thiobutabarbital (Inactin; 120 and 80 mg/kg ip for normoglycemic and hyperglycemic animals, respectively) and surgically prepared as described above. [3H]inulin (185 kBq·kg−1·h−1; ARC, St. Louis, MO) and LiCl (4 mg ip bolus plus continuous infusion 2.1 mg·h−1·rat−1; Ref. 28) were infused in the femoral vein. After 45 min of stabilization, a 40-min period for baseline measurements was followed by an additional 40-min experimental period in which candesartan (4.2 μg/kg), DPCPX (140 μg/kg), or both were infused in the renal artery in a total volume of 700 μl. Since the half-life of candesartan is approximately 9 h (6) and that of DPCPX ∼5 h (17), a single bolus dose of each drug was infused and full receptor blockade assumed for the duration of the experiments. To minimize spill-over to the systemic circulation, the infusion was stepwise during ∼10 min followed by a 10-min delay before the experimental period started (Fig. 1B). Blood samples for measurement of blood gas parameters were collected at the end of each period.
In some animals, the experimental period of candesartan or DPCPX was followed by a second experimental period in which the ANG II AT2 receptor antagonist S-(+)-1-{[4-(dimethylamino)-3-methylphenyl]methyl}-5-(diphenylacetyl)-4,5,6,7-tetrahydro-1H-imidazo[4,5-c]pyridine-6-carboxylic acid di(trifluoroacetate) salt hydrate (PD-123,319; 0.5 mg/kg) or the adenosine A2 receptor antagonist 3,7-dimethyl-1-propargylxanthine (DMPX; 0.5 mg/kg) was administrated, respectively. In these experiments, the blood gas parameters were not assessed due to the large volume of blood required for these measurements.
Measurements of kidney hemodynamics and tubular function.
Mean arterial blood pressure (MAP) was measured using a transducer (model P23dB; Statham Laboratories, Los Angeles, CA) connected to the left femoral artery catheter. Total RBF was measured using an ultrasound probe (Transonic Systems, Ithaca, NY) placed around the left renal artery; care was taken so that the probe remained in the same position throughout the protocol. These parameters were continuously recorded with a Power Lab instrument (AD Instruments, Hastings, UK). GFR was determined by [3H]inulin clearance. 3H activities in urine and plasma were measured by liquid scintillation. Blood gas parameters were measured from samples drawn from the right femoral artery and left renal vein using an iSTAT System (Abbott Laboratories; Ref. 35). The left vein blood sample was carefully collected using an angled pointed needle connected to a preheparinized syringe. Tubular Na+ reabsorption was determined using Li+ clearance (50). Kidney weights were determined at the end of the experiment. Urine volumes were measured gravimetrically, and urinary Na+ and Li+ and plasma Li+ concentrations were quantified by flame spectrophotometry in a multianalyzer (model IL543; Instrumentation Lab, Milan, Italy).
Calculations.
GFR was calculated using the formula GFR = U * V/P, where U and P denote the quantity of [3H]inulin in the urine and plasma samples, respectively, and V denotes the urine flow rate. Renal vascular resistance (RVR) was calculated as MAP divided by total RBF. The filtration fraction (FF) was calculated using the formula FF = GFR/[total RBF * (1 − Hct)]. In vivo renal oxygen consumption was estimated from the arteriovenous difference in O2 content {O2ct = ([Hb] * oxygen saturation * 1.34 + * Po2 0.003) * total RBF}. Tubular Na+ transport (TNa) was calculated as TNa = [PNa] * GFR − [UNa] * urine flow, where [PNa] and [UNa] are plasma and urine Na+ concentration, respectively. Fractional Na+ excretion (FENa) was estimated from [UNa] * [Pinulin]/[PNa] * [Uinulin], and fractional Li+ excretion (FELi) from [ULi] * [Pinulin]/[PLi] * [Uinulin], where [Pinulin] and [Uinulin] represent plasma and urinary inulin concentration, and [PLi] is plasma Li+ concentration.
Statistical evaluation.
Multiple comparisons within the same group were performed using one-way ANOVA for repeated measurements followed by Dunnett's post hoc test in the dose-response curves. Differences between the groups during baseline or in response to a treatment were evaluated by two-way ANOVA followed by Tukey's test. Comparison within the same group before and after the treatment was performed using Student's paired t-test or one-way ANOVA for repeated measurements followed by Tukey's test, as appropriated. Data presented in Table 1 were analyzed using unpaired Student's t-test. Descriptive statistics are presented as means ± SE. For all comparisons, P < 0.05 was considered statistically significant.
Table 1.
Comparison between control and diabetic rats during baseline
| Control Rats | Diabetic Rats | |
|---|---|---|
| Blood glucose, mM | 6.5 ± 0.3 | 23.7 ± 0.5* |
| Body weight, g | 377 ± 5 | 355 ± 4* |
| Left kidney weight, g | 1.24 ± 0.02 | 1.73 ± 0.03* |
| MAP, mmHg | 108 ± 2 | 107 ± 2 |
| RBF, ml/min | 8.4 ± 0.2 | 8.4 ± 0.2 |
| RVR, mmHg·min−·ml | 13.2 ± 0.4 | 13.2 ± 0.4 |
| GFR, ml/min | 1.6 ± 0.1 | 3.5 ± 0.2* |
| FF, % | 10.9 ± 0.9 | 21.9 ± 1.2* |
| Urine flow, μl/min | 5.4 ± 0.6 | 28.6 ± 1.8* |
| TNa, μmol/min | 226 ± 17 | 492 ± 27* |
| FENa, % | 0.2 ± 0.0 | 0.3 ± 0.0* |
| FELi, % | 40.9 ± 5.6 | 24.5 ± 3.0* |
| Q{↓o2}, μmol/min | 8.8 ± 1.1 | 14.0 ± 1.4* |
Results are expressed as means ± SE for n = 34–44, except for oxygen consumption (Q{↓o2}) where n = 22–23. MAP, mean arterial pressure; RBF; renal blood flow; RVR, renal vascular resistance; GFR, glomerular filtration rate; FF, filtration fraction; TNa, tubular Na+ transport; FENa, fractional Na+ excretion; FELi, fractional Li+ excretion.
P < 0.05.
RESULTS
Dose-response curves for the AT1 receptor antagonist candesartan, A1 receptor antagonist DPCPX or the combination of the two antagonists.
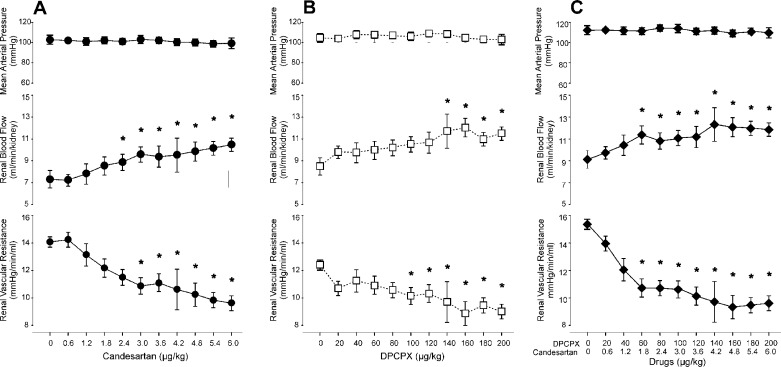
Intrarenal infusions of Candesartan, DPCPX and their combination dose-dependently increased RBF and decreased RVR without affecting MAP (Fig. 2). Maximal effects were obtained with 4.2 μg/kg of candesartan and 140 μg/kg of DPCPX, which were therefore chosen for the subsequent experiments.
Fig. 2.
Dose-response curves for the AT1 receptor antagonist candesartan (A), the A1 receptor antagonist DPCPX (B), or the combination of the two (C) on mean arterial pressure, total renal blood flow and renal vascular resistance. Results expressed as means ± SE for n = 5–9/group. *P < 0.05 vs. time 0.
In vivo intrarenal blockade of ANG II and adenosine receptors: vascular effects.
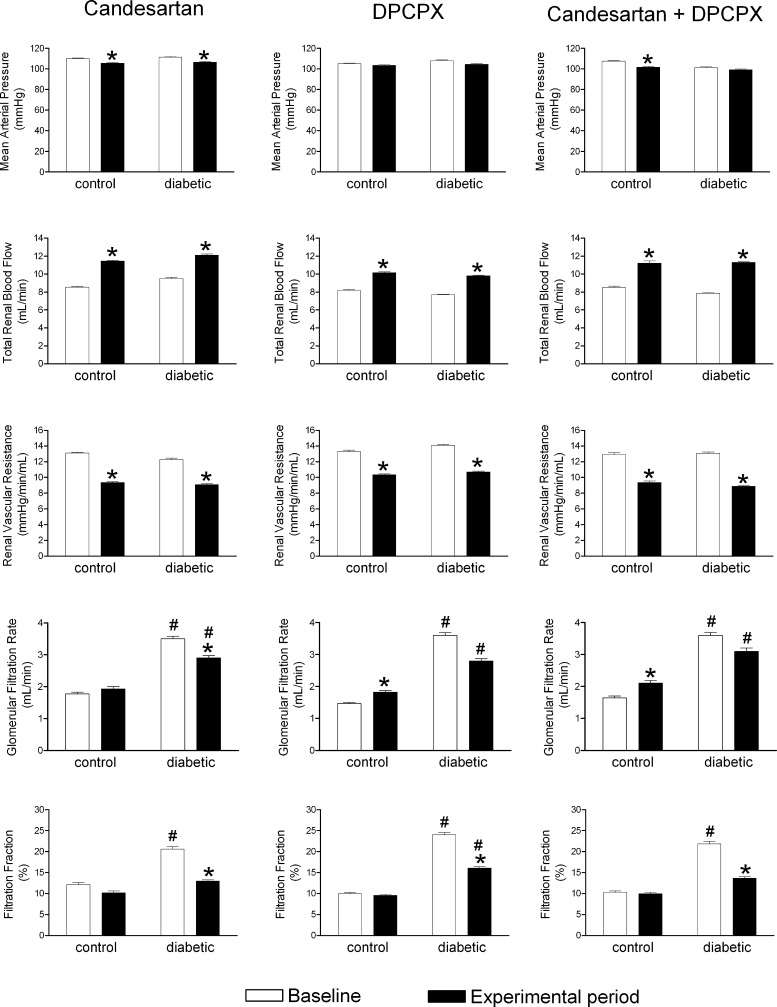
Streptozotocin increased blood glucose, decreased body weight, and increased kidney weight compared with controls (Table 1). Remarkably, although baseline MAP, RBF, and RVR were similar, the GFR of diabetic rats was doubled compared with the controls, resulting in a doubling of the filtration fraction (FF) (Table 1). Candesartan reduced MAP in control (∼4 mmHg) and diabetic (∼5 mmHg) animals, as did the combination of the two antagonists in controls (∼6 mmHg; Fig. 3). DPCPX had no effect on MAP in either group (Fig. 3). Candesartan, DPCPX, or the combination increased RBF by ∼33%, resulting in a reduction of RVR by ∼27% in both controls and diabetics (Fig. 3).
Fig. 3.
Vascular effects of the in vivo intrarenal blockade of ANG II and adenosine receptors. Mean arterial pressure, total renal blood flow, renal vascular resistance, glomerular filtration rate, and filtration fraction of control and diabetic animals before and after blockade of AT1 receptors by candesartan (4.2 μg/kg), A1 receptors by DPCPX (140 μg/kg), or the combined blockade of AT1 and A1 receptors. Results expressed as means ± SE for n = 8–16. *P < 0.05 vs. baseline within the same group. #P < 0.05 vs. corresponding control.
In controls, DPCPX and combination of the two antagonists, but not candesartan alone, increased GFR, whereas FF remained unaffected (Fig. 3). In diabetics, candesartan, but neither DPCPX nor the combination, reduced GFR (Fig. 3). Although the absolute decrease in GFR was similar when inhibiting AT1 and A1 receptors in diabetic rats (−0.71 ± 0.29 ml/min; n = 14; P = 0.028 vs. −0.79 ± 0.40 ml/min; n = 13; P = 0.073), only candesartan resulted in a statistical significant decrease due to the more heterogeneous response in the DPCPX group. Furthermore, also the combination of candesartan and DPCPX failed to statistically reduce GFR (−0.53 ± 0.37 ml/min; n = 11; P = 0.178) due to a large variability in the response. However, FF was reduced by all three treatments administered to diabetics but only normalized following candesartan or the combination of candesartan and DPCPX (Fig. 3).
Superimposed infusion of PD-123,319 to that of candesartan or DMPX to that of DPCPX caused no relevant alterations to the effects observed with candesartan or DPCPX alone (Table 2 and 3).
Table 2.
Renal effects of the intrarenal blockade of ANG II AT1 and AT2 receptors in control and diabetic rats
| Control Rats |
Diabetic Rats |
|||||
|---|---|---|---|---|---|---|
| Baseline | Candesartan | Candesartan + PD-123,319 | Baseline | Candesartan | Candesartan + PD-123,319 | |
| MAP, mmHg | 120 ± 4 | 116 ± 5 | 111 ± 5*† | 114 ± 4 | 111 ± 3 | 108 ± 3* |
| RBF, ml/min | 8.8 ± 0.6 | 11.6 ± 0.4* | 11.0 ± 0.4* | 9.9 ± 0.8 | 12.7 ± 1.0* | 12.2 ± 1.0* |
| RVR, mmHg·min−·ml | 13.8 ± 0.8 | 10.0 ± 0.4* | 10.1 ± 0.4* | 12.1 ± 1.2 | 9.3 ± 1.0* | 9.3 ± 0.9* |
| GFR, ml/min | 2.3 ± 0.3 | 2.2 ± 0.3 | 2.1 ± 0.3 | 3.5 ± 0.2‡ | 3.3 ± 0.4‡ | 2.7 ± 0.3 |
| FF, % | 40 ± 4 | 32 ± 3 | 36 ± 7 | 69 ± 8‡ | 46 ± 4* | 41 ± 7* |
| Urine flow, μl/min | 11 ± 2 | 15 ± 2* | 11 ± 2† | 27 ± 5‡ | 21 ± 3 | 22 ± 3‡ |
| TNa, % | 320 ± 47 | 302 ± 35 | 295 ± 35 | 488 ± 35‡ | 486 ± 60‡ | 381 ± 50 |
| FENa, % | 0.5 ± 0.1 | 0.8 ± 0.2* | 0.6 ± 0.1* | 0.6 ± 0.1 | 0.9 ± 0.2 | 1.1 ± 0.2 |
Results are expressed as means ± SE for n = 5–7. Effects during baseline and after blockade of AT2 receptors with PD-123,319 (0.5 mg/kg) superimposed on prior AT1 receptor blockade by candesartan (4.2 μg/kg).
P < 0.05 vs. baseline within the same group.
P < 0.05 vs. candesartan within the same group.
P < 0.05 vs. corresponding control.
Table 3.
Renal effects of the intrarenal blockade of adenosine A1 and A2 receptors in control and diabetic rats
| Control Rats |
Diabetic Rats |
|||||
|---|---|---|---|---|---|---|
| Baseline | DPCPX | DPCPX + DMPX | Baseline | DPCPX | DPCPX + DMPX | |
| MAP, mmHg | 110 ± 4 | 111 ± 3 | 109 ± 3 | 116 ± 3 | 111 ± 4* | 111 ± 4* |
| RBF, ml/min | 7.4 ± 0.3 | 9.7 ± 0.3* | 9.6 ± 0.3* | 7.8 ± 0.2 | 10.1.±0.2* | 9.8 ± 0.3* |
| RVR, mmHg·min−·ml | 14.9 ± 0.7 | 11.4 ± 0.5* | 11.5 ± 0.4* | 14.9 ± 0.5 | 11.0 ± 0.4* | 11.4 ± 0.5* |
| GFR, ml/min | 1.5 ± 0.2 | 2.0 ± 0.3 | 1.7 ± 0.2 | 3.7 ± 0.4‡ | 2.9 ± 0.2‡ | 2.5 ± 0.4‡ |
| FF, % | 38 ± 5 | 37 ± 4 | 33 ± 3 | 72 ± 9‡ | 53 ± 5* | 54 ± 10‡ |
| Urine flow, μl/min | 5 ± 1 | 16 ± 2* | 8 ± 1† | 24 ± 4‡ | 38 ± 7*‡ | 28 ± 4†‡ |
| TNa, % | 200 ± 26 | 264 ± 37 | 228 ± 31 | 507 ± 59‡ | 396 ± 31‡ | 391 ± 61‡ |
| FENa, % | 0.1 ± 0.0 | 1.3 ± 0.2* | 0.7 ± 0.1*† | 0.3 ± 0.1‡ | 1.4 ± 0.3* | 1.3 ± 0.3*‡ |
Results are expressed as means ± SE for n = 6–8. Effects during baseline and after blockade of A2 receptors by 3,7-dimethyl-1-propargylxanthine (DMPX; 0.5 mg/kg) superimposed on prior A1 receptor blockade by 8-cyclopentyl-1,3-dipropylxanthine (DPCPX; 140 μg/kg).
P < 0.05 vs. baseline within the same group.
P < 0.05 vs. DPCPX within the same group.
P < 0.05 vs. corresponding control.
In vivo intrarenal blockade of ANG II and adenosine receptors: tubular effects.
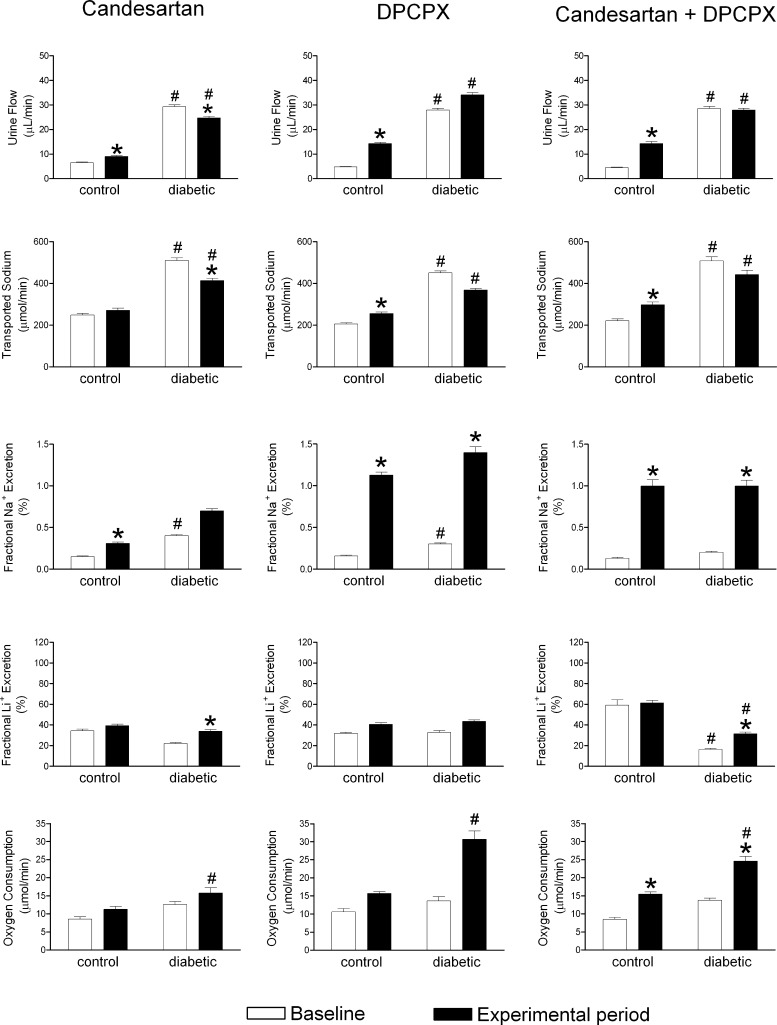
Diabetics had higher baseline urine flow and FENa doubled the rate of net tubular Na+ transport. This remarkable increase in TNa must reflect increased Na+ filtration and enhanced tubular Na+ reabsorption. This can be ascribed predominantly to the proximal tubule since the clearance of lithium (primarily regulated by proximal reabsorption) was reduced significantly in the diabetic rats (Table 1). All three treatments increased urine flow and FENa in controls (Fig. 4). In diabetics, candesartan decreased urine flow and TNa, whereas DPCPX and the combination of the two antagonists had no effect, except for increasing FENa (Fig. 4). Baseline FELi was lower and Qo2 higher in diabetics compared with controls (Table 1), indicating higher proximal tubule reabsorption in diabetics. Candesartan and the combination increased FELi in diabetics (Fig. 4), indicating decreased proximal tubule reabsorption. This was associated with decreased GFR and FF. Neither candesartan nor DPCPX alters oxygen consumption, but the combination of the two increased Qo2 in both controls and diabetics (Fig. 4).
Fig. 4.
Tubular effects of the in vivo intrarenal blockade of ANG II and adenosine receptors. Urine flow, fractional urinary Na+ excretion, fractional urinary Li+ excretion, transported Na+ and total kidney oxygen consumption of control and diabetic animals before and after blockade of AT1 receptors by candesartan (4.2 μg/kg), A1 receptors by DPCPX (140 μg/kg), or the combined blockade of AT1 and A1 receptors. Results expressed as means ± SE for n = 8–16. *P < 0.05 vs. baseline within the same group. #P < 0.05 vs. corresponding control.
Superimposed treatment with PD-123,319 to that of candesartan normalized urine flow in controls (Table 2). Superimposed treatment with DMPX to that of DPCPX normalized urine flow both in control and diabetic animals and attenuated the increase in FENa in controls (Table 3). TNa was not altered by any treatment either in controls or in diabetics (Tables 2 and 3).
DISCUSSION
The main novel finding of the present study is that ANG II, acting on AT1 receptors, and adenosine, acting on A1 receptors, strongly influence renal hemodynamics during baseline conditions in both control and diabetic kidneys via a unifying pathway that requires both receptors to affect vascular tone. The present study also suggests increased AT1 receptor signaling as a crucial mechanism for the elevated FF and glomerular hyperfiltration commonly observed in the diabetic kidney. To the best of our knowledge, this is the first time that the interaction between ANG II AT1 and adenosine A1 receptors in regulating intrarenal hemodynamics is demonstrated in vivo in diabetic animals. Moreover, these data also propose that endogenous ANG II and adenosine have different roles in regulating tubular sodium handling in diabetics compared with controls.
Concomitant blockade of ANG II AT1 and adenosine A1 receptors caused a similar effect to the observed when only adenosine A1 receptors were blocked. Several studies have addressed and demonstrated an interaction between ANG II AT1 and adenosine A1 receptors in the regulation of renal vascular tone (26, 36, 56). Indeed, inhibition of ANG II formation or ANG II AT1 receptor signaling reduces adenosine A1 receptor-mediated vasoconstriction of the renal vasculature (26, 56). Conversely, ANG II AT1 receptor-mediated vasoconstriction is also reduced by adenosine A1 receptor blockade (21). Previous studies have been performed ex vivo or in vivo with systemic administration of the vasoactive substances. Other studies evaluated the responses to increasing doses of exogenous ANG II or adenosine, which may activate other receptor subtypes, counteracting vasoactive mechanisms (22, 27), or nonreceptor-dependent mechanisms (20). However, the present study is, to the best of our knowledge, the first report demonstrating a tonic baseline interaction between these two systems without having any confounding systemic effects or manipulation to increase receptor signaling. Importantly, concomitant blockade of both ANG II AT1 and adenosine A1 receptors resulted in increased RBF, which was of the same magnitude as with either drug alone. These results add further support to a unifying vasodilatory pathway in which both receptor types need to be stimulated to affect vascular tonus.
Unaltered GFR in conjunction with decreased FF suggests that these two systems regulate both afferent and efferent arterioles. However, blockade of ANG II AT1 receptors reduced the diabetes-induced glomerular hyperfiltration, indicating increased AT1 receptor-mediated efferent arteriolar vasoconstriction. Augmented GFR in early diabetic nephropathy has been attributed to afferent arteriole dilation and an associated increase in RBF (7). However, we observed no differences in RBF between control and diabetic animals. This divergence of results has previously been attributed to insulin replacement (7, 42). Unaltered RBF has been reported not only in insulin-treated diabetic animals but also in response to salt load (10, 23). Diabetic hyperfiltration is associated with increased Na+/glucose cotransport (52). In the present study, diabetics had increased TNa as reported previously (55), which is associated with increased Qo2 (19). Interestingly, adenosine A1 receptor blockade had the same effect observed in controls, which also suggests a more distal regulation of TNa in diabetics. As opposed to controls, where ANG II seems to influence TNa in different parts of the nephron, in diabetics it mainly increased Na+ transport in more proximal segments of the tubule, since FELi increased after infusion of candesartan or the combination of both antagonists. Further analyses are needed to accurately determine the major site of ANG II AT1 receptor-mediated TNa in diabetics, although it seems that in diabetics it is shifted to the proximal tubule or/and Loop of Henle. It might be speculated that increased proximal tubular reabsorption in diabetes, via enhanced Na+/glucose contransport and enhanced peritubular capillary uptake force due to the increased FF, may reduce the hydrostatic pressure in the Bowman's capsule. This would directly increase the net filtration pressure and thus favor glomerular hyperfiltration (39). If the objective of therapy in early type 1 diabetes is to combat hyperfiltration, and to reduce arterial pressure and oxidative stress, the use of an adenosine A1 receptor antagonist alone, or in combination with ANG II AT1 receptor blockade, does not add anything to the standard-of-care therapy with an ANG II AT1 receptor blockade because only candesartan reduced GFR and this was not augmented by adding DCPCX. The present study also demonstrates that tonic activation of ANG II AT2 or adenosine A2 receptors has no major roles in diabetes.
Hyperglycemia has been shown to increase intrarenal ANG II levels in rats (31), and ANG II AT1 receptor activation is a key mediator of the increased oxidative stress in the diabetic kidney by direct induction of superoxide radical production by the NADPH oxidase (30). Nitric oxide (NO) bioavailability is decreased already in early stages of diabetic nephropathy (4, 34) and ANG II AT1 receptor blockade restores NO levels in the diabetic kidney (4). It is therefore possible that the observed vasodilatation after ANG II AT1 receptor blockade is at least partly due to increased NO bioavailability. On the other hand, in control animals, the renal vasculature is more sensitive to adenosine-mediated vasoconstriction when NO production is inhibited (5). Similarly, the streptozotocin-diabetic rat kidney is more prone to adenosine A1 receptor-mediated vasoconstriction (41), which has been ascribed to decreased NO-dependent vasodilatation of the afferent arteriole (40).
AT1 receptor blockade increased urine flow and FENa but did not alter FELi and TNa in controls. In fact, activation of ANG II AT1 receptors increases TNa in both proximal and distal tubular segments and in collecting ducts (18). ANG II AT2 receptors have been shown to contribute to proximal tubule Na+ reabsorption (11), and the superimposed antagonism of ANG II AT2 receptors to that of ANG II AT1 receptors resulted in unaffected FENa and TNa, which suggests that ANG II-mediated tubular Na+ transport might occur in distal parts of the nephron. Blockade of adenosine A1 receptors in controls increased urine flow, FENa, and TNa, which is in good agreement with published results (53). Surprisingly, FELi was not significantly elevated by DPCPX (57). Although Li+ has been widely used as an indicator of proximal tubule reabsorption in cases of low FENa, as observed in the present report, Li+ may also be reabsorbed in more distal parts of the nephron (16), possibly in the loop of Henle (9, 47). Moreover, most of the previous studies investigating tubular function utilize state of the art micropuncture techniques (26, 43, 57). The nature of this technique only allows investigation of single superficial nephrons (51). Although further studies are needed to fully explain the discrepancies, our results suggest that tonic baseline activation of adenosine A1 receptors regulates TNa in distal parts of the nephron. Superimposed adenosine A2 receptor antagonism to that of adenosine A1 receptor blockade reduced urine flow and decreased FENa. This finding supports a role for in vivo tonic activation of adenosine A2 receptors in mediating diuresis and natriuresis (58), at least when adenosine A1 receptor function is reduced. Simultaneous blockade of ANG II AT1 and adenosine A1 receptors in controls also increased urine flow and FENa, although TNa and Qo2 increased. The most likely explanation for these seemingly contradictory results is that increased tubular Na+ load is due to the increased GFR. Increased Na+ in the macula densa activates TGF by stimulating adenosine production, which activates A1 receptors in the afferent arteriole causing vasoconstriction and normalization of single nephron GFR (46, 53). In the present study, A1 receptor blockade elicited a greater increase in FENa compared with that after blockade of ANG II AT1 receptors. The concomitant increase in GFR together with increased FENa after adenosine A1 receptor blockade implies TGF inactivation.
In conclusion, both ANG II AT1 receptors and adenosine A1 receptors are tonically active in control and diabetic animals during baseline and significantly influence intrarenal hemodynamics and tubular function. The hemodynamic effects mediated by simultaneous blockade of both receptors are not additive, indicating that ANG II and adenosine regulate intrarenal hemodynamics via a unifying pathway that requires activation of both receptor types. However, this study demonstrates that increased AT1 receptor signaling, independent of adenosine A1 receptors, is a mechanistic explanation for diabetes-induced glomerular hyperfiltration. Finally, both ANG II AT1 receptors and adenosine A1 receptors tonically influence tubular Na+ reabsorption in both control and diabetic animals, but the ANG II-mediated effects seem to be altered in the diabetic kidney. The clinical relevance derives from the finding that adenosine A1 receptor blockade alone, or in combination with ANG II AT1 receptor blockade, does not add anything to the standard-of-care therapy with an ANG II AT1 receptor blockade if the objective is to reduce glomerular hyperfiltration and arterial pressure in early type 1 diabetes.
GRANTS
This study was supported by the Swedish Medical Research Council (9940, 72XD-15043, 10840, 14X-2553, and K2003-04X-03522-32), Swedish Society for Medical Research, National Institute of Diabetes and Digestive and Kidney Diseases K99/R00 Grant (DK-077858 to F. Pinho), and FCT, FEDER, and POFC-COMPETE (PTDC/SAU-FCF/67764/2006 and SFRH/BD/43187/2008).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D. Patinha, M.M., and F.P. conception and design of research; D. Patinha and A.F. performed experiments; D. Patinha, A.F., D. Pinho, M.M., and F.P. analyzed data; D. Patinha, M.M., and F.P. interpreted results of experiments; D. Patinha prepared figures; D. Patinha drafted manuscript; D. Patinha, A.A.-T., M.M., and F.P. edited and revised manuscript; D. Patinha, A.F., D. Pinho, A.A.-T., M.M., and F.P. approved final version of manuscript.
REFERENCES
- 1. Aki Y, Tomohiro A, Nishiyama A, Kiyomoto K, Kimura S, Abe Y. Effects of KW-3902, a selective and potent adenosine A1 receptor antagonist, on renal hemodynamics and urine formation in anesthetized dogs. Pharmacology 55: 193–201, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Albino-Teixeira A, Matias A, Polonia J, Azevedo I. Blockade of adenosine receptors causes hypertension and cardiovascular structural changes in the rat. J Hypertens Suppl 9: S196–197, 1991 [PubMed] [Google Scholar]
- 3. American Diabetes Association Standards of medical care for patients with diabetes mellitus. Diabetes Care 26, Suppl 1: S33–50, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Awad AS, Webb RL, Carey RM, Siragy HM. Renal nitric oxide production is decreased in diabetic rats and improved by AT1 receptor blockade. J Hypertens 22: 1571–1577, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Barrett RJ, Droppleman DA. Interactions of adenosine A1 receptor-mediated renal vasoconstriction with endogenous nitric oxide and ANG II. Am J Physiol Renal Fluid Electrolyte Physiol 265: F651–F659, 1993 [DOI] [PubMed] [Google Scholar]
- 6. Burnier M, Brunner HR. Angiotensin II receptor antagonists in hypertension. Kidney Int Suppl 68: S107–111, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Carmines PK. The renal vascular response to diabetes. Curr Opin Nephrol Hypertens 19: 85–90, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carmines PK, Morrison TK, Navar LG. Angiotensin II effects on microvascular diameters of in vitro blood-perfused juxtamedullary nephrons. Am J Physiol Renal Fluid Electrolyte Physiol 251: F610–F618, 1986 [DOI] [PubMed] [Google Scholar]
- 9. Fransen R, Boer WH, Boer P, Dorhout Mees EJ, Koomans HA. Effects of furosemide or acetazolamide infusion on renal handling of lithium: a micropuncture study in rats. Am J Physiol Regul Integr Comp Physiol 264: R129–R134, 1993 [DOI] [PubMed] [Google Scholar]
- 10. Griffin KA, Abu-Naser M, Abu-Amarah I, Picken M, Williamson GA, Bidani AK. Dynamic blood pressure load and nephropathy in the ZSF1 (fa/fa cp) model of type 2 diabetes. Am J Physiol Renal Physiol 293: F1605–F1613, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Hakam AC, Hussain T. Angiotensin II AT2 receptors inhibit proximal tubular Na+-K+-ATPase activity via a NO/cGMP-dependent pathway. Am J Physiol Renal Physiol 290: F1430–F1436, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Hall JE, Granger JP, Hester RL. Interactions between adenosine and angiotensin II in controlling glomerular filtration. Am J Physiol Renal Fluid Electrolyte Physiol 248: F340–F346, 1985 [DOI] [PubMed] [Google Scholar]
- 13. Hannedouche TP, Delgado AG, Gnionsahe DA, Boitard C, Lacour B, Grunfeld JP. Renal hemodynamics and segmental tubular reabsorption in early type 1 diabetes. Kidney Int 37: 1126–1133, 1990 [DOI] [PubMed] [Google Scholar]
- 14. Hayashi K, Epstein M, Loutzenhiser R, Forster H. Impaired myogenic responsiveness of the afferent arteriole in streptozotocin-induced diabetic rats: role of eicosanoid derangements. J Am Soc Nephrol 2: 1578–1586, 1992 [DOI] [PubMed] [Google Scholar]
- 15. Jackson EK. Adenosine: a physiological brake on renin release. Annu Rev Pharmacol Toxicol 31: 1–35, 1991 [DOI] [PubMed] [Google Scholar]
- 16. Kirchner KA. Lithium as a marker for proximal tubular delivery during low salt intake and diuretic infusion. Am J Physiol Renal Fluid Electrolyte Physiol 253: F188–F196, 1987 [DOI] [PubMed] [Google Scholar]
- 17. Knight RJ, Collis MG, Yates MS, Bowmer CJ. Amelioration of cisplatin-induced acute renal failure with 8-cyclopentyl-1,3-dipropylxanthine. Br J Pharmacol 104: 1062–1068, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Korner A, Eklof AC, Celsi G, Aperia A. Increased renal metabolism in diabetes. Mechanism and functional implications. Diabetes 43: 629–633, 1994 [DOI] [PubMed] [Google Scholar]
- 20. Lai EY, Martinka P, Fahling M, Mrowka R, Steege A, Gericke A, Sendeski M, Persson PB, Persson AE, Patzak A. Adenosine restores angiotensin II-induced contractions by receptor-independent enhancement of calcium sensitivity in renal arterioles. Circ Res 99: 1117–1124, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Lai EY, Patzak A, Persson AE, Carlström M. Angiotensin II enhances the afferent arteriolar response to adenosine through increases in cytosolic calcium. Acta Physiol (Oxf) 196: 435–445, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Lai EY, Patzak A, Steege A, Mrowka R, Brown R, Spielmann N, Persson PB, Fredholm BB, Persson AE. Contribution of adenosine receptors in the control of arteriolar tone and adenosine-angiotensin II interaction. Kidney Int 70: 690–698, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Lau C, Sudbury I, Thomson M, Howard PL, Magil AB, Cupples WA. Salt-resistant blood pressure and salt-sensitive renal autoregulation in chronic streptozotocin diabetes. Am J Physiol Regul Integr Comp Physiol 296: R1761–R1770, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia 52: 691–697, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Morato M, Sousa T, Guimarães S, Moura D, Albino-Teixeira A. The role of angiotensin II in hypertension due to adenosine receptors blockade. Eur J Pharmacol 455: 135–141, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Munger KA, Jackson EK. Effects of selective A1 receptor blockade on glomerular hemodynamics: involvement of renin-angiotensin system. Am J Physiol Renal Fluid Electrolyte Physiol 267: F783–F790, 1994 [DOI] [PubMed] [Google Scholar]
- 27. Nishiyama A, Inscho EW, Navar LG. Interactions of adenosine A1 and A2a receptors on renal microvascular reactivity. Am J Physiol Renal Physiol 280: F406–F414, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Nordquist L, Brown R, Fasching A, Persson P, Palm F. Proinsulin C-peptide reduces diabetes-induced glomerular hyperfiltration via efferent arteriole dilation and inhibition of tubular sodium reabsorption. Am J Physiol Renal Physiol 297: F1265–F1272, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O'Donnell MP, Kasiske BL, Keane WF. Glomerular hemodynamic and structural alterations in experimental diabetes mellitus. FASEB J 2: 2339–2347, 1988 [DOI] [PubMed] [Google Scholar]
- 30. Onozato ML, Tojo A, Goto A, Fujita T, Wilcox CS. Oxidative stress and nitric oxide synthase in rat diabetic nephropathy: effects of ACEI and ARB. Kidney Int 61: 186–194, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Onozato ML, Tojo A, Leiper J, Fujita T, Palm F, Wilcox CS. Expression of NG,NG-dimethylarginine dimethylaminohydrolase and protein arginine N-methyltransferase isoforms in diabetic rat kidney: effects of angiotensin II receptor blockers. Diabetes 57: 172–180, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Osswald H. Renal effects of adenosine and their inhibition by theophylline in dogs. Naunyn Schmiedebergs Arch Pharmacol 288: 79–86, 1975 [DOI] [PubMed] [Google Scholar]
- 33. Osswald H, Spielman WS, Knox FG. Mechanism of adenosine-mediated decreases in glomerular filtration rate in dogs. Circ Res 43: 465–469, 1978 [DOI] [PubMed] [Google Scholar]
- 34. Palm F, Buerk DG, Carlsson PO, Hansell P, Liss P. Reduced nitric oxide concentration in the renal cortex of streptozotocin-induced diabetic rats: effects on renal oxygenation and microcirculation. Diabetes 54: 3282–3287, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Palm F, Fasching A, Hansell P, Kallskog O. Nitric oxide originating from NOS1 controls oxygen utilization and electrolyte transport efficiency in the diabetic kidney. Am J Physiol Renal Physiol 298: F416–F420, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Patzak A, Lai EY, Fahling M, Sendeski M, Martinka P, Persson PB, Persson AE. Adenosine enhances long term the contractile response to angiotensin II in afferent arterioles. Am J Physiol Regul Integr Comp Physiol 293: R2232–R2242, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Pawelczyk T, Grden M, Rzepko R, Sakowicz M, Szutowicz A. Region-specific alterations of adenosine receptors expression level in kidney of diabetic rat. Am J Pathol 167: 315–325, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pawlowska D, Granger JP, Knox FG. Effects of adenosine infusion into renal interstitium on renal hemodynamics. Am J Physiol Renal Fluid Electrolyte Physiol 252: F678–F682, 1987 [DOI] [PubMed] [Google Scholar]
- 39. Persson P, Hansell P, Palm F. Tubular reabsorption and diabetes-induced glomerular hyperfiltration. Acta Physiol (Oxf) 200: 3–10, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pflueger AC, Osswald H, Knox FG. Adenosine-induced renal vasoconstriction in diabetes mellitus rats: role of nitric oxide. Am J Physiol Renal Physiol 276: F340–F346, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Pflueger AC, Schenk F, Osswald H. Increased sensitivity of the renal vasculature to adenosine in streptozotocin-induced diabetes mellitus rats. Am J Physiol Renal Fluid Electrolyte Physiol 269: F529–F535, 1995 [DOI] [PubMed] [Google Scholar]
- 42. Pihl L, Persson P, Fasching A, Hansell P, DiBona GF, Palm F. Insulin induces the correlation between renal blood flow and glomerular filtration rate in diabetes: implications for mechanisms causing hyperfiltration. Am J Physiol Regul Integr Comp Physiol 303: R39–R47, 2012 [DOI] [PubMed] [Google Scholar]
- 43. Quan A, Baum M. Endogenous production of angiotensin II modulates rat proximal tubule transport. J Clin Invest 97: 2878–2882, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rosivall L, Navar LG. Effects on renal hemodynamics of intra-arterial infusions of angiotensins I and II. Am J Physiol Renal Fluid Electrolyte Physiol 245: F181–F187, 1983 [DOI] [PubMed] [Google Scholar]
- 45. Sallstrom J, Carlsson PO, Fredholm BB, Larsson E, Persson AE, Palm F. Diabetes-induced hyperfiltration in adenosine A(1)-receptor deficient mice lacking the tubuloglomerular feedback mechanism. Acta Physiol (Oxf) 190: 253–259, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Schnermann J. Adenosine mediates tubuloglomerular feedback. Am J Physiol Regul Integr Comp Physiol 283: R276–R279, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Shirley DG, Walter SJ, Sampson B. A micropuncture study of renal lithium reabsorption: effects of amiloride and furosemide. Am J Physiol Renal Fluid Electrolyte Physiol 263: F1128–F1133, 1992 [DOI] [PubMed] [Google Scholar]
- 48. Sousa T, Morato M, Albino-Teixeira A. Angiotensin converting enzyme inhibition prevents trophic and hypertensive effects of an antagonist of adenosine receptors. Eur J Pharmacol 441: 99–104, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Spielman WS, Osswald H. Blockade of postocclusive renal vasoconstriction by an angiotensin II antagonists: evidence for an angiotensin-adenosine interaction. Am J Physiol Renal Fluid Electrolyte Physiol 237: F463–F467, 1979 [DOI] [PubMed] [Google Scholar]
- 50. Thomsen K, Schou M, Steiness I, Hansen HE. Lithium as an indicator of proximal sodium reabsorption. Pflügers Arch 308: 1969 [DOI] [PubMed] [Google Scholar]
- 51. Vallon V. Micropuncturing the nephron. Pflügers Arch 458: 189–201, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vallon V. The proximal tubule in the pathophysiology of the diabetic kidney. Am J Physiol Regul Integr Comp Physiol 300: R1009–R1022, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vallon V, Muhlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev 86: 901–940, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Vallon V, Osswald H. Adenosine receptors and the kidney. Handb Exp Pharmacol 443–470, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vallon V, Richter K, Blantz RC, Thomson S, Osswald H. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol 10: 2569–2576, 1999 [DOI] [PubMed] [Google Scholar]
- 56. Weihprecht H, Lorenz JN, Briggs JP, Schnermann J. Synergistic effects of angiotensin and adenosine in the renal microvasculature. Am J Physiol Renal Fluid Electrolyte Physiol 266: F227–F239, 1994 [DOI] [PubMed] [Google Scholar]
- 57. Wilcox CS, Welch WJ, Schreiner GF, Belardinelli L. Natriuretic and diuretic actions of a highly selective adenosine A1 receptor antagonist. J Am Soc Nephrol 10: 714–720, 1999 [DOI] [PubMed] [Google Scholar]
- 58. Zou AP, Nithipatikom K, Li PL, Cowley AW., Jr Role of renal medullary adenosine in the control of blood flow and sodium excretion. Am J Physiol Regul Integr Comp Physiol 276: R790–R798, 1999 [DOI] [PubMed] [Google Scholar]