Abstract
Protein kinase C (PKC) and large conductance Ca2+-activated potassium channels (BK) are downregulated in the detrusor smooth muscle (DSM) in partial bladder outlet obstruction (PBOO). DSM from these bladders display increased spontaneous activity. This study examines the involvement of PKC in the regulation of spontaneous and evoked DSM contractions and whether pharmacologic inhibition of PKC in normal DSM contributes to increased detrusor excitability. Results indicate the PKC inhibitor bisindolylmaleimide 1 (Bim-1) prevented a decline in the amplitude of spontaneous DSM contractions over time in vitro, and these contractions persist in the presence of tetrodotoxin. Bim-1 also reduced the basal DSM tone, and the ability to maintain force in response to electrical field stimulation, but did not affect maximum contraction. The PKC activator phorbol-12,13-dibutyrate (PDBu) significantly reduced the amplitude and increased the frequency of spontaneous contractions at low concentrations (10 nM), while causing an increase in force at higher concentrations (1 μM). Preincubation of DSM strips with iberiotoxin prevented the inhibition of spontaneous contractions by PDBu. The BK channel openers isopimaric acid and NS1619 reduced the Bim-1-induced enhancement of spontaneous contractions in DSM strips. Our data suggest that PKC has a biphasic activation profile in the DSM and that it may play an important role in maintaining the quiescent state of the normal bladder during storage through the effects on BK channel, while helping to maintain force required for bladder emptying. The data also suggest that PKC dysfunction, as seen in PBOO, contributes to detrusor overactivity.
Keywords: protein kinase C, bladder, BK channel, spontaneous contractions
the normal bladder smooth muscle displays relatively little spontaneous and nonvoiding contractions (NVC) compared with animal models of partial bladder outlet obstruction (PBOO) and patients with benign prostatic hyperplasia (BPH)-induced PBOO (5, 15, 16). Detrusor smooth muscle (DSM) strips from PBOO bladders show smooth muscle hypertrophy and remodeling with alteration in the signal transduction pathways that regulate force generation by DSM (5, 17). More recent studies have shown that, along with an increase in mass, changes in regulatory proteins such as protein kinase C (PKC) and large conductance Ca2+-activated potassium channels (BK) are increasingly being linked to this PBOO-associated altered phenotype. Both PKC and BK channels are downregulated in PBOO in rabbits, rats, and patients with BPH (5, 6, 17). The downregulation of BK channels has been linked to higher levels of myosin light chain phosphorylation, which is believed to play a role in detrusor overactivity (DO), and NVC associated with PBOO (5).
There is increasing evidence that BK channels are involved in the regulation of smooth muscle excitability in general and DSM in particular (5, 20, 21). The BK channels limit smooth muscle excitability by the negative feedback regulation of intracellular calcium levels, thus maintaining the DSM in a relatively quiet state under normal resting conditions (5, 9, 10, 14). BK channel knockout mice show increased detrusor contractility, increased bladder pressure, and urine leakage, providing further evidence that loss of BK channel function is involved in DO (25). Furthermore, PBOO in rats (13), rabbits, and patients with BPH are all associated with downregulation of BK channels (5) and increased DO, characterized by an increase in spontaneous and NVC. These studies lend further credence to the idea that BK channels play a vital role in regulating DSM excitability and thus may influence the development of spontaneous and NVC contractions under pathological conditions.
PKC is an 80-kDa protein that is also involved in the regulation of smooth muscle tone and force maintenance (11, 19, 26). Recent evidence suggests that PKC can regulate the sensitivity of BK channels for calcium and other substrates in smooth muscle and nonmuscle cells (3, 28). The ability of PKC to regulate the sensitivity of BK channels could have important implications for bladder function. A recent report indicates that PKC can phosphorylate-specific serine residues on the BK α- and β-subunits. When the serine residues were substituted for alanine, phosphorylation did not occur leading to a loss of function (28). This study provides strong evidence of a PKC-BK channel interaction at the molecular and functional level. A link between PKC and spontaneous contractions has also been suggested in studies by Ozaki et al. (19). These investigators report that low levels of spontaneous oscillatory activity in the pregnant myometrium are associated with increased levels of PKC, compared with the nonpregnant myometrium, which shows much higher levels of spontaneous contractions and lower PKC levels (19). The possibility that PKC may regulate BK channels in DSM suggests that PKC dysfunction, like that of BK channels, may also contribute to DO. PKC also plays an important role in the generation of myogenic tone in rabbit basilar artery (27) and ferret coronary artery (7).
In the present study, we utilized pharmacologic inhibition of PKC in normal DSM strips to determine if modulation of PKC-mediated regulation mimics spontaneous contractions seen in the PBOO-associated alteration in PKC and BK channel function. We examined the hypothesis that PKC may play an important role in DSM excitability by regulating the activity of BK channels. Our results indicate that inhibition of PKC results in a prolonged period of spontaneous contractions and that low level activation of PKC by phorbol-12,13-dibutyrate (PDBu) can suppress spontaneous bladder contractions. Our results also indicate, for the first time, that PKC has a biphasic activation profile in DSM, inhibiting spontaneous contractions at low activation, while increasing basal muscle tone at higher concentrations. Inhibition of PKC resulted in a reduced ability to maintain force in response to electrical field stimulation (EFS). Finally, we demonstrate that inhibition of BK channels by iberiotoxin (IBTX) prevents PDBu-induced inhibition of spontaneous contractions, while the BK channel openers NS1619 and isopimaric acid (ISPA) could inhibit bisindolylmaleimide-1 (Bim-1) prolonged spontaneous contractions in isolated muscle strips. We conclude that PKC may be able to modulate the sensitivity of DSM through its effect on BK channels and that PKC dysfunction due to pharmacologic inhibition in normal DSM strips mimics PBOO-associated PKC and BK channel downregulation seen in PBOO.
MATERIALS AND METHODS
Organ bath studies of detrusor spontaneous contractions.
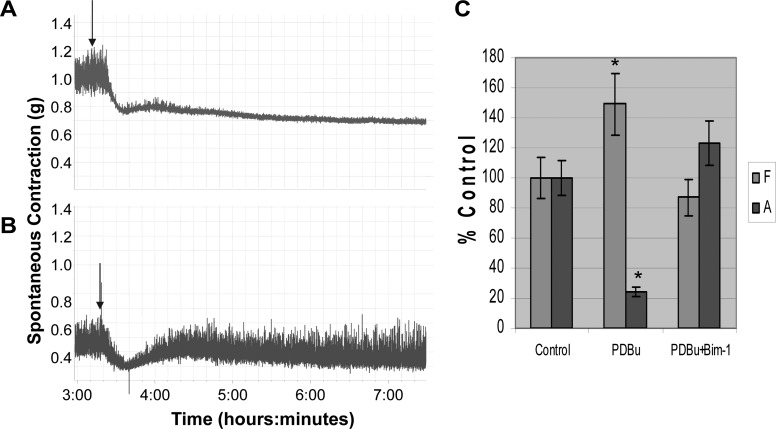
All animal experimental protocols were approved by the University of Pennsylvania Institutional Animal Care Use Committee. Male New Zealand white rabbits were anesthetized with ketamine/xylazine (25 mg/10 mg im). Anesthesia was maintained with pentobarbital (25 mg/kg iv), and the bladders were removed and placed into Tyrode's buffer (124.9 mM NaCl, 2.5 mM KCl, 23.8 mM NaHCO3, 0.5 mM MgCl2, 0.4 mM NaH2PO4, 1.8 mM CaCl2, and 5.5 mM dextrose). Longitudinal DSM strips (∼50 mg) were placed in organ baths containing 15 ml Tyrode's buffer equilibrated with 95% O2-5% CO2. One end of the strip was attached to a glass rod at the bottom of the organ chamber (Radnoti) while the other end was attached to a force displacement transducer connected to an AD Instruments power-lab and computerized system (Colorado Springs, CO). After a 1-h equilibration, the length of optimal force development (L0) was determined by increasing the length of each strip by 1.5-mm increments until maximal contractile force to EFS (80 V; 1 ms; 32 Hz) was achieved (Fig. 1). The muscle strips were allowed to equilibrate a further 30 min, in fresh Tyrode's buffer to stabilize at L0 before recording spontaneous contractions.
Fig. 1.
In vitro organ bath studies showing a representative example of the length-tension curve. A: representative example of a length-tension curve performed before each experiment to optimize muscle length. Detrusor smooth muscle (DSM) strips weighing ∼50 mg (3 × 8 mm) were stretched in small increments (∼1.5 mm) sequentially to avoid muscle damage and stimulated with electrical field stimulation (EFS; 80 V, 32 hz, 1-ms duration) until maximum contraction was achieved. The stretch and stimulus cycles were repeated until there was no further increase in force to EFS at which time the muscle strip was determined to be at its optimal length or optimal length (L0). B: summary data from 9 separate experiments for force generation in response to EFS. The average maximum contraction at L0 was 6.2 ± 0.24 g force.
Drugs.
Once the spontaneous contractions achieved a stable baseline, the muscle strips were treated with several drugs to study their effects on spontaneous activity. Muscle strips were treated with the following drugs acquired from Sigma Chemicals: PDBu, IBTX, tetrodotoxin (TTX), ISPA, and NS1619. Bim-1 was acquired from Calbiochem (St. Louis, MO). Both Bim-1 (14 nM), a specific PKC inhibitor, and IBTX (1 μM), a specific BK channel inhibitor, were added to individual muscle strips to determine their effects on spontaneous contractions, which were recorded over a period of 8–10 h. The PKC activator PDBu (10 nM) was added to untreated muscle strips and to muscle strips pretreated with Bim-1 (14 nM) and IBTX to determine its effect on spontaneous contractions with and without the inhibitors. Muscle strips were treated with Bim-1 and IBTX, inhibitors of PKC, and BK channels, respectively, to determine their effects on spontaneous contractions. At or close to maximal amplitude, the muscle strips were then treated with the BK channel openers ISPA (50 μM), and NS1619 (50 μM), or PDBu to determine their ability to reverse these spontaneous contractions. Untreated muscle strips served as controls. The data from the organ baths were recorded and analyzed on AD Instruments computerized, analysis program.
Average cycle minimum and integral force.
The average cycle minimum (ACM) is the trough or low point between each spontaneous contraction cycle measured in grams of tension. When measured in series over time, the ACM is a measure of the basal smooth muscle tone. The integral force is the area under the time force curve that encompasses the rising and falling phases of the contraction cycle in response to stimulation and is a measure of the ability to maintain force that is required for bladder emptying. We utilized the AD Instruments software analysis tool to calculate both the ACM and the integral force in evaluating changes in basal smooth muscle tone, and force maintenance respectively.
Statistical analysis.
All data are expressed as the means ± SE and analyzed by one-way ANOVA. P < 0.05 was considered to be statistically significant.
RESULTS
Representative length tension curve.
L0 is the optimal length for muscle contraction in smooth muscle at which point maximum contraction is achieved in response to stimulation. Figure 1A shows a representative length-tension curve in which the DSM strip has been stretched to its optimal length, L0, after a series of sequential stretches and stimulations by EFS. This procedure helps to normalize and optimize the length of each muscle strip for maximum contraction and ensures that differences in contraction are not due to random differences in muscle length. It also helps to ensure that muscle strips are not damaged during stretch since the force at L0 is either equal to or slightly greater than the previous contraction. Figure 1B shows the summary data showing the average maximum contraction in response to EFS at L0 was 6.2 ± 0.24 g tension, which is slightly greater than the prior contraction (n = 8). This procedure was performed before all physiological experiments.
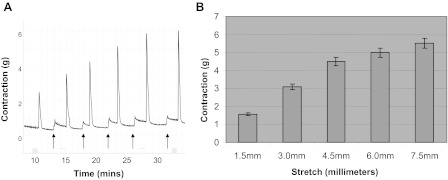
Effect of Bim-1 and TTX in normal muscle strips.
Normal DSM shows spontaneous contractions in vitro. These contractions may play important role in modulating DSM tone during bladder filling in vivo; however, when amplified in frequency and/or magnitude, they may contribute to pathologic changes such as that seen in DO. Figure 2A is a representative untreated control muscle strip showing spontaneous contractions. The amplitude of the contractions increases and then declined significantly over the duration of the recording, while the frequency increased correspondingly compared with time zero. Table 1 (control) shows that at 8 h the frequency of contractions increased to 170% of the value measured at the beginning of the recording (time zero), while the amplitude decreased to 17% of that measured at time zero. To determine whether or not the decline in the amplitude of spontaneous contractions in the control was due to a decline in smooth muscle potency, we measured the contractility at the end of 7 h in response to EFS (Fig. 2E). These muscle strips were able to generate a force of 80 ± 10% compared with the beginning of the recording that was not significant (P = 0.12). Figure 2B shows the effect of Bim-1, and Fig. 2C shows the effect of Bim-1 in the presence of TTX on spontaneous contractions. Note that the amplitude of spontaneous contractions in these recordings is maintained at a high level throughout, compared with that in Fig. 2A, and as shown in the expanded scale (Fig. 2D). Table 1 shows that in the presence of Bim-1 alone, the frequency at 8 h is 106% of that at the beginning (zero time), which was not significant, but the amplitude had increased to 131%, which was significant (P < 0.05). Figure 2C shows the effect of Bim-1 in the presence of TTX. Again, Table 1 reveals no significant change in frequency at 8 h, compared with time zero (109%); however, amplitude increased to 123%, which was barely significant (P = 0.048). The data reveal that at 8 h, in the presence of Bim-1, the amplitude of spontaneous contractions is maintained at a significantly higher level compared with time zero, and compared with control at 8 h, and TTX did not have a significant effect on this response (Table 1). The data also show that there was no significant difference in the frequency at 8 h for Bim-1, and Bim-1 plus TTX compared with time zero (106 and 109%, respectively); however, both of these were significantly different from control (170%) at 8 h.
Fig. 2.
In vitro organ bath studies showing the effect of bisindolylmaleimide 1 (Bim-1) on spontaneous contractions in rabbit bladder DSM. A: spontaneous contractions in a normal control muscle strip without exogenous stimulation. Under normal conditions, the regulation of these contractions may play an important role in modulating and maintaining a dynamic DSM tone that is consistent with normal bladder functioning, whereas deregulation may lead to bladder dysfunction. Bim-1 (14 nM) was added at 80 min (B) and tetrodotoxin (TTX; C; 1.0 μM) was added at 60 min to show any effect on the frequency and amplitude of spontaneous contractions in the isolated muscle strips. D: expanded representation of the recordings in Fig. 2, A–C, at 7 h. E: summary data for control showing that the control muscle strip (Fig. 2A) in which there is a significant decrease in the amplitude of spontaneous contractions, can still generate an average of 80 ± 10% force in response to EFS at 7 h (P = 0.12; n = 6), which was not significant, compared with the beginning of the recording (1 h).
Table 1.
Quantification of frequency and amplitude of contraction
| 0 h |
2 h |
4 h |
6 h |
8 h |
10 h |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Specimen | F, % | A, % | F, % | A, % | F, % | A, % | F, % | A, % | F, % | A, % | F, % | A, % |
| Control | 100 | 100 | 71 ± 9.6† | 255 ± 28† | 65 ± 3.2† | 239 ± 20† | 104 ± 6.1 | 133 ± 16 | 170 ± 23† | 17 ± 0.9† | 159 ± 19† | 22 ± 0.72† |
| Bim-1 | 100 | 100 | 86 ± 8.4 | 266 ± 22* | 96 ± 5.8 | 189 ± 19* | 100 ± 5.8 | 152 ± 21 | 106 ± 11* | 131 ± 17* | 111 ± 10 | 114 ± 14 |
| Bim-1 + TTX | 100 | 100 | 87 ± 8.1 | 248 ± 27 | 109 ± 8.8* | 190 ± 24* | 110 ± 11 | 175 ± 9.3* | 109 ± 14* | 123 ± 18* | 115 ± 13* | 119 ± 15* |
Values represent means ± SE. Summary of the data for the frequency (F) and amplitude (A), as a percentage of the initial F and A at time 0 (100%), of spontaneous contractions recorded over 10 h for control, and of muscle strips in the presence of bisindolylmaleimide 1 (Bim-1) alone and Bim-1 plus tetrodotoxin (TTX). Note the significant decline in A and increase in F of the control at 8 h compared with Bim-1 and Bim-1 plus TTX. Bim-1 and Bim-1 plus TTX maintained a high A of spontaneous contractions throughout the period of the recording, 131 and 123%, respectively, at 8 h, compared with control of only 17% of that recorded at time 0. Whereas the F increased significantly in control to 170% at 8 h compared with time 0, there was no significant change for Bim-1 and Bim-1 plus TTX (106 and 109%, respectively) compared to time 0. The A of spontaneous contractions for control at 8 h (17%) was also significantly different from Bim-1 (131%) and Bim-1 plus TTX (123%) at 8 h. This was also true for F at 8 h where the control was 170% and Bim-1 and Bim-1 plus TTX were 106 and 109%, respectively. There was no significant change in the F at 8 h for Bim-1 and Bim-1 plus TTX compared to time 0; however, changes in A were just barely significant. Data also indicate that TTX did not affect the ability of Bim-1 to maintain a high level of spontaneous contraction. Data represent the summary of 6 separate experiments (n = 6).
P < 0.05, significantly different from control;
P < 0.05, significantly different from initial measurement at time 0.
In vitro organ bath studies showing the effect of Bim-1, and Bim-1 plus TTX between 5 and 7 h.
Figure 3, A–C, represents an expanded view between 5 and 7 h taken from Fig. 2. To determine whether or not the decline in the amplitude of spontaneous contractions over time in control untreated muscle strips was due to changes in basal tone, we evaluated the ACM which is a measure of basal smooth muscle tone. Figure 3, A (control), B (Bim-1; 14 nM), and C (Bim-1 plus TTX; 1 μM) show expanded representative recordings of isolated muscle strips between 5 and 7 h when the decline in the amplitude of spontaneous contractions in the control (Fig. 3A) is greatest. In these experiments, we calculated the ACM to determine if there were any changes in basal smooth muscle tone during the period from 5 to 7 h. Figure 3D shows the summary data for the ACM measured over the time period. The summary data in Fig. 3D show no significant change in the ACM, in grams of tension, for control (Fig. 3A), Bim-1-treated (Fig. 3B), and Bim-1-treated plus TTX (Fig. 3C) over the 2-h duration of the measurement. This indicates that the basal tone did not change significantly, over this time period, for all three recordings, even as the amplitude of spontaneous contractions declined significantly for the control. However, Bim-1 (Fig. 3B) caused a significant decrease in the ACM to 0.05 ± 0.008 g tension compared with control (Fig. 3A) with an ACM of 1 ± 0.11, while Bim-1 plus TTX (Fig. 3C) reversed that inhibition to 0.31 ± 0.04, which was significantly different from control, and Bim-1-treated muscle strips (n = 6; P < 0.05). The partial recovery by Bim-1 plus TTX suggests possible neuronal involvement in mediating DSM basal tone.
Fig. 3.
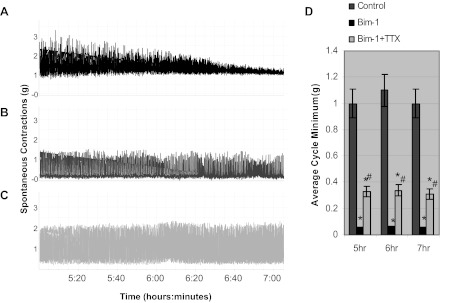
In vitro organ bath studies showing the effect of Bim-1, and Bim-1 plus TTX between 5 and 7 h. A (control), B (Bim-1; 14 nM), and C (Bim-1 plus TTX; 1 μM): representative expanded recordings of isolated muscle strips between 5 and 7 h. These recordings were isolated and expanded directly from Fig. 2 between 5 and 7 h, since the compressed data over many hours tends to distort the amount of shift that is actually occurring in the average cycle minimum (ACM; basal tone). For these recordings, we calculated the ACM from 5 to 7 h to determine if there was a significant shift in basal smooth muscle tone during this period. D: summary data for the ACM measured over time. Summary data in D show no significant change in the ACM in grams of tension from beginning (5 h) to end (7 h) for control (A), Bim-1-treated (B), and Bim-1-treated plus TTX (C) over the 2-h duration of the measurement (n = 6; P = 0.059). However, Bim-1 (B) caused a significant decrease in the ACM to 0.05 ± 0.008 g tension compared with control, 1 ± 0.11 g tension (n = 6; P < 0.05). Bim-1 plus TTX (C) reversed that inhibition to 0.31 ± 0.04, which was significantly different from Bim-1 alone and control (*P < 0.05, significantly different from control; #P < 0.05, significantly different from Bim-1; n = 6).
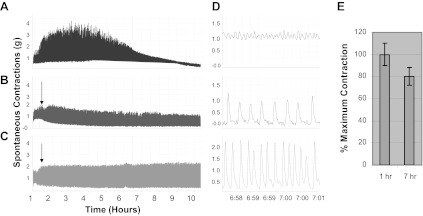
Effect of the PKC inhibitor Bim-1 on EFS-evoked contraction.
While the regulation of spontaneous contractions may play a role in modulating DSM tone during bladder storage, active force generation and the ability to maintain force are required for bladder emptying. We evaluated the effect of Bim-1 on the ability of DSM to generate and maintain force in response to EFS. EFS works by releasing neurotransmitters from nerve terminals that bind receptors on the smooth muscle leading to contraction. Figure 4A shows the maximum response to EFS in the absence of Bim-1, while Fig. 4B shows the maximum response to EFS after 1-h preincubation with Bim-1. The ability of the bladder to empty requires rapid force generation of sufficient magnitude and the ability to maintain that force long enough to ensure complete bladder emptying. The recording in Fig. 4B illustrates that inhibition of PKC by Bim-1 resulted in a significantly reduced ability to maintain force, as measured by the integral force (area under the time force curve), as shown in Fig. 4C, compared with control. Figure 4C shows the summary data revealing that Bim-1 caused a 50 ± 5.5% decrease in the area under the curve compared with control (P < 0.05; n = 6). Maximum force generation in the presence of Bim-1 was 95 ± 8.5%, which was not significantly different from control (P = 0.068; n = 6). The data indicate that Bim-1 had a significant inhibitory effect on the ability to maintain force (integral force) but did not affect peak force generation.
Fig. 4.

In vitro organ bath studies showing the effect of Bim-1 on EFS-evoked contraction and force maintenance in DSM strips. Shown is the effect of EFS (80 V, 32 Hz, 1 ms) on peak contraction and force maintenance (integral force) in isolated muscle strips. A: control muscle strip in the absence of Bim-1. In B the muscle strip was first preincubated with Bim-1 (14 nM) for 1 h before stimulation with EFS. Summary data in C show the integral force (area under the time force curve) as a measure of force maintenance and the peak force generation in the presence and absence of Bim-1. Data reveal that Bim-1 caused a 50 ± 5.5% decrease in the integral compared with control (P < 0.05; n = 6). The maximum force generation in the presence of Bim-1 was 95 ± 8.5%, which was not significantly different from control (P = 0.068).
Concentration-dependent activation of PKC with PDBu.
PKC has been reported to have opposing actions in the intestinal epithelia (23). To evaluate whether or not this phenomenon exists in DSM, we studied PKC activation by PDBu at low (10 nM) and high (1 μM) concentrations. Figure 5 demonstrates that PKC activation by PDBu shows a concentration-dependent, biphasic activation profile in DSM. Figure 5A shows a control muscle strip in the absence of stimulation. Figure 5B shows the effect of 10 nM PDBu on spontaneous contraction, and Fig. 5C shows the effect of 1.0 μM PDBu on basal smooth muscle tone (arrows). PDBu (10 nM) caused a remarkable decrease in spontaneous contractions of the DSM strips (Fig. 5B). Figure 5C shows the effect of a high concentration of PDBu (1.0 μM) on basal tone. The summary data in Fig. 5D revealed that PDBu (10 nM) resulted in a 73 ± 10.9% decrease in amplitude and a 34 ± 8.4% increase in frequency of spontaneous contractions (P < 0.05; n = 6). Figure 5E revealed that at a high concentration PDBu (1.0 μM) caused a 200 ± 34.7% increase in basal tone from baseline, which was highly significant (P = 0.025; n = 9).
Fig. 5.
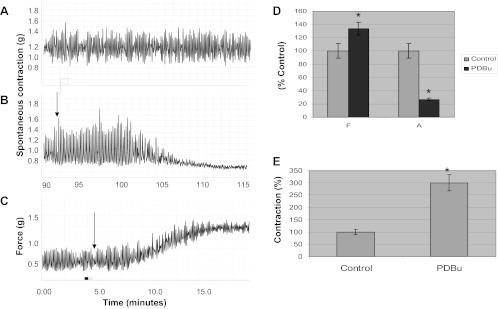
In vitro organ bath studies on isolated muscle strips showing biphasic effect on DSM tone. A: DSM strip showing spontaneous contractions in the absence of any drug stimulation (control). B: effect of a low concentration of phorbol-12,13-dibutyrate (PDBu; 10 nM) on spontaneous contractions. Arrow indicates the point of addition of PDBu. C: effect of a high concentration of PDBu (1.0 μM) on basal smooth muscle tone. D: summarizes the effect of 10 nM PDBu on frequency and amplitude for A and B. Data are calculated as a percentage of control for frequency and amplitude. The low concentration of PDBu (10 nM) resulted in a 73 ± 10.9% decrease in the amplitude of spontaneous contractions (P < 0.05) and a 34 ± 8.4% increase in the frequency (P < 0.05; n = 6). E: summarizes the force generation in response to high PDBu (1 μM) activation. PDBu (1 μM) caused a 200 ± 34.7% increase in force from baseline that was highly significant (*P = 0.025, significantly different from control; n = 9).
Effect of PDBu in the presence of TTX.
PKC has been shown to be able to regulate neuronal activity (1). To determine whether the inhibitory effect of PDBu (10 nM) on spontaneous contractions is influenced by neuronal involvement, isolated muscle strips were preincubated with the neurotoxin, TTX (1.0 μM), for 1 h. Both muscle strips in Fig. 6A and Fig. 6B were preincubated with TTX for 1 h. Figure 6A serves as a control (without PDBu stimulation). Figure 6, A and B, shows that spontaneous contractions persist in the presence of TTX; however, these contractions are decreased upon exposure to PDBu (Fig. 6B, arrow). Figure 6C shows the summary data revealing that PDBu caused a 63.2 ± 7.9% decrease in amplitude and a 37.5 ± 6.1% increase in frequency of spontaneous contractions, indicating that TTX did not affect the ability of PDBu to inhibit spontaneous contractions. Both the change in frequency and amplitude upon addition of PDBu were significantly different from control (Fig. 6A; P = 0.047; n = 6).
Fig. 6.
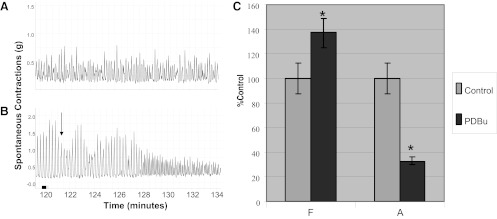
Effect of PDBu on spontaneous contractions in the presence of TTX. In both A and B, muscle strips have been pretreated with 1.0 μM TTX for 1 h. Subsequently, the muscle strip in B was treated with PDBu (10 nM). Arrow indicates the point of addition of PDBu. C: summary data indicating the effect of PDBu on the amplitude and frequency of spontaneous contractions. PDBu caused a 63.2 ± 7.9% decline in the amplitude of phasic contractions compared with the control (*P < 0.05, significantly different from control; n = 6) PDBu also caused a 37.5 ± 6.1% increase in the frequency of spontaneous contractions, which was significant (P = 0.047; n = 6). Note that TTX did not prevent inhibition of spontaneous contractions by PDBu.
Effect of PDBu with and without Bim-1 on spontaneous contractions.
Bim-1 is a competitive inhibitor of PKC and should block the inhibitory effect of low PDBu (10 nM) stimulation on spontaneous contractions. Figure 7A is a control muscle strip without Bim-1 preincubation, while in Fig. 7B the strip has been pretreated with Bim-1(14 nM). Both muscle strips were subsequently treated with PDBu (10 nM) as indicated by the arrows. As can be seen, the control strip without Bim-1 (Fig. 7A) produced a prolonged inhibition in response to PDBu, while the experimental strip (Fig. 7B) with Bim-1 caused a transient inhibition, followed by an extended recovery of the spontaneous contractile activity. Figure 7C shows the summary data indicating that PDBu without Bim-1 caused a 49 ± 14% increase in the frequency of spontaneous contractions and a 76 ± 9.4% decrease in the amplitude, which were both significantly different from control (P < 0.05; n = 6). In the presence of Bim-1 preincubation (Fig. 7B), PDBu caused a transient decrease in tone followed by a net, extended recovery in amplitude over time to 123 ± 9.9% and a decrease in frequency to 87 ± 9.3% of initial levels before addition of PDBu, which were not significantly different (P > 0.05; n = 6). These data indicate that beyond the initial competitive inhibition upon addition of PDBu in Fig. 7B, the inhibitory effect of PDBu (10 nM) is specific and can ultimately be blocked by the specific PKC inhibitor Bim-1.
Fig. 7.
Effect of PDBu with and without Bim-1 in DSM strips. A: effect of 10 nM PDBu on spontaneous contractions in muscle strips without Bim-1 preincubation. Arrows indicate the addition of PDBu. In B, the muscle strips were first preincubated with 14 nM Bim-1 for 1 h and then PDBu (10 nM) was administered (arrow). C: summary data for the effect of PDBu on frequency and amplitude with and without Bim-1, measured over the duration of the recording. The control values for frequency and amplitude represent those values analyzed 30 min before addition of PDBu for each muscle strip and were set at 100%. PDBu without Bim-1 (7A) caused a 49 ± 14% increase in the frequency of spontaneous contractions and a 76 ± 9.4% decrease in the amplitude, which were both significantly different from control (*P < 0.05; n = 6). In the presence of Bim-1 preincubation (B), PDBu caused a transient decrease in tone followed by a net recovery in amplitude over time to 123 ± 9.9% and decrease in frequency to 87 ± 9.3% of initial levels before PDBu, which were not different from control (P > 0.05, not significantly different from control; n = 6). Note the persistence of significant spontaneous activity in the presence of Bim-1 in B, demonstrating the ability of Bim-1 to ultimately block PDBu-induced inhibition of spontaneous contractions.
Effect of preincubation with IBTX on PDBu-induced inhibition of spontaneous contractions.
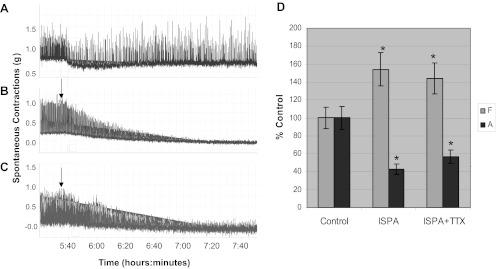
PKC has been reported to be able to regulate BK channels in vascular smooth muscle (28). To investigate whether or not PDBu-induced inhibition of spontaneous contractions was mediated through its effect on BK channels, we preincubated DSM strips with the BK channel inhibitor IBTX (1 μM) for 45 min before addition of PDBu (10 nM). Figure 8A shows the control muscle strip without any stimulation. The muscle strip in Fig. 8B was treated with 10 nM PDBu as indicated by the arrow. In Fig. 8C, the muscle strip was first preincubated with IBTX for 45 min before addition of 10 nM PDBu (arrow). Figure 8D shows the summary data for the effect of PDBu on frequency and amplitude with and without IBTX. PDBu, in the absence of IBTX, resulted an increase in frequency to 148 ± 21.4% (P < 0.05; n = 6) and a decrease in amplitude to 25 ± 3.9% (P < 0.05; n = 6), both of which were significantly different from control in the absence of PDBu stimulation. The PDBu response after preincubation with IBTX (Fig. 8C) was 115 ± 13.6% (P = 0.061; n = 6) for frequency and 84 ± 11.7% (P = 0.067; n = 6), for amplitude, both of which were not significantly different from control in the absence of stimulation. These data suggest that BK channels may be a target for the regulation of DSM tone by PKC.
Fig. 8.

Effect of PDBu on normal DSM strips with and without iberiotoxin (IBTX). A: normal control muscle strip without PDBu or IBTX. B: effect of PDBu (10 nM) on spontaneous contractions in DSM strip. Arrows indicate the addition of PDBu. In C, the muscle strip is first preincubated with 1.0 μM IBTX for 45 min after which 10.0 nM PDBu is added (arrow). D: summary data for the effect of PDBu on frequency and amplitude with and without IBTX. In B, PDBu resulted an increase in frequency to 148 ± 21.4% (P < 0.05; n = 6) and a decrease in amplitude to 25 ± 3.9% (P < 0.05; n = 6), both of which were significantly different (*) from control (A). In C, the PDBu response after preincubation with IBTX was 115 ± 13.6% (P = 0.061; n = 6) for frequency and 84 ± 11.7% (P = 0.067; n = 6) for amplitude, both of which were not significantly different from control (A).
Effect of the BK channel opener ISPA on Bim-1-enhanced spontaneous contractions.
We have shown that Bim-1, a specific PKC inhibitor, can maintain the high amplitude spontaneous contractions in DSM over time compared with control in the absence of Bim-1 (Fig. 2) and that BK channels may be involved in the modulation of spontaneous contractions by PKC. Hence, we wanted to find out whether or not these contractions were reversible by the nonspecific BK opener ISPA. The muscle strips represented in Fig. 9, A–C, were all initially preincubated with Bim-1 (14 nM). Figure 9A served as a control (no ISPA), while Fig. 9C was also pretreated with 1 μM TTX for 1 h. Subsequently, Fig. 9, B and C, was treated with 50 μM ISPA (arrows). Figure 9D shows the summary data for the effect of ISPA on frequency and amplitude with and without TTX. In Fig. 9B, ISPA caused a significant increase in frequency to 154 ± 22.2% and a decrease in amplitude to 43 ± 7.9% of control (P < 0.05; n = 6). In the presence of TTX in Fig. 9C, ISPA caused an increase in frequency to 144 ± 20.7% and a decrease in amplitude to 57 ± 8.9%, which was also significantly different from control (P < 0.05; n = 6). There was no significant difference with and without TTX.
Fig. 9.
Effect of isopimaric acid on spontaneous contractions in normal DSM strips with and without TTX. In A–C, the muscle strips have been initially preincubated with 14.0 nM Bim-1 at 2 h. Subsequently muscle strips represented in C were treated with 1.0 μM TTX for 60 min. Muscle strips in B and C were then treated with 50 μM isopimaric acid (ISPA; arrows). D: summary data for the effect of isopimaric acid on frequency and amplitude with TTX (C) and without TTX (B). In B, isopimaric acid caused a significant increase in frequency to 154 ± 22.2% and a decrease in amplitude to 43 ± 7.9% of the control (P < 0.05; n = 6). In the presence of TTX (C), ISPA acid caused an increase in frequency to 144 ± 20.7% and a decrease in amplitude to 57 ± 8.9%, which was also significantly different from control (*P < 0.05, significantly different from control; n = 6). There was no significant difference in frequency and amplitude with TTX (C) and without TTX (B).
Effect of NS1619 on Bim-1 enhanced spontaneous contractions.
We also evaluated the ability of NS1619, a selective BK channel opener, to reverse Bim-1 enhanced spontaneous contractions with and without TTX. The muscle strips in Fig. 10, A–C, were all treated with Bim-1 (14 nM) initially. Figure 10A served as a control, and Fig. 10C was also treated with TTX (1 μM) for 1 h. Subsequently, the muscle strips in Fig. 10, B and C, were treated with 50 μM NS1619 (arrows). In Fig. 10B without TTX, NS1619 caused a decline in the frequency of spontaneous contractions to 56 ± 7.2% and a decrease in amplitude to 32 ± 4.4% of control value. Both the decline in frequency and amplitude were significant compared with control (P < 0.05; n = 7). In Fig. 10C, with TTX, NS1619 caused a decline in frequency to 72 ± 8.2% and a decrease in amplitude to 29 ± 3.6%, which were both significantly different from control (P < 0.05; n = 7). There was no significant difference in frequency and amplitude with and without TTX.
Fig. 10.

Effects of NS1619 on spontaneous contractions in the presence of Bim-1 and Bim-1 plus TTX. A–C were pretreated with Bim-1 (14 nM) at the beginning of the recording (2 h). C was subsequently treated with 1 μM TTX for 60 min. A served as a control (Bim-1 only), and B and 10C were subsequently treated with NS1619 (50 μM). Arrows indicate addition of NS1619. D: summary data for the effect of NS1619 on spontaneous contractions with TTX (C) and without TTX (B). In B, without TTX, NS1619 caused a decline in the frequency of spontaneous contractions to 56 ± 7.2% and a decrease in amplitude to 32 ± 4.4% of control value (A). Both the decline in frequency and amplitude were significant compared with control (P < 0.05; n = 7). In C, with TTX, NS1619 caused a decline in frequency to 72 ± 8.2% and a decrease in amplitude to 29 ± 3.6%, which were both significantly different from control (P < 0.05; n = 7). There was no significant difference in frequency and amplitude between without TTX (B) and with TTX (C). *Significantly different from control.
DISCUSSION
Several reports indicate that the normal bladder smooth muscle is in a constant state of controlled contraction during the filling stage, when the bladder outlet is closed. These investigators believe that such low level contractions help maintain bladder shape and tone, providing a tonic platform that allows for rapid contraction, and emptying during micturition (4). PBOO is characterized by a loss of bladder control, in which the underlying smooth muscle is associated with an increase in the amplitude and frequency of spontaneous contractions and NVC. Clinically, these changes can manifest as an increase in urinary frequency, urge, and incontinence and are associated with a downregulation of PKC in animal models of obstruction (6). In spite of numerous studies on the physiology and pathophysiology of the bladder smooth muscle, the regulation and underlying mechanism of these contractions remain elusive. In the present study, we evaluated the role of pharmacologic inhibition of PKC by the specific PKC inhibitor Bim-1 in normal bladders, to see if the changes that occur mimics that seen in PBOO, where PKC is downregulated and associated with a significant increase in spontaneous contractile activity (5). We demonstrated that inhibition of PKC by Bim-1 maintains the amplitude of spontaneous contractions, in isolated muscle strips from normal bladders, at a significantly higher level than untreated controls, where the amplitude declined significantly (Fig. 2; Table. 1), and significantly shortens the period of inhibition by PDBu of spontaneous contractions (Fig. 7). We also demonstrate that Bim-1 caused a loss of force maintenance in response to EFS-induced nerve stimulation, as determined by integrating the area under the time force curve (integral force; Fig. 4) and that activation of PKC by the specific PKC activator PDBu can suppress spontaneous contractions.
The ability of the bladder to empty requires the generation of force of sufficient magnitude and the maintenance of that force long enough to facilitate complete bladder emptying. A failure to maintain force due to pharmacologic inhibition of PKC in vitro, as demonstrated in Fig. 4, may reflect PKC dysfunction in vivo associated with PBOO, which could contribute to a failure to empty or incomplete bladder emptying. The downregulation of PKC expression and activity in PBOO bladder (6) smooth muscle, as we have previously shown, is associated with incomplete bladder emptying and a significant increase in spontaneous contractile activity in isolated muscle strips from these bladders. Correspondingly, our present studies show, for the first time, that activation of PKC, at relatively low concentration, in normal DSM strips by the specific PKC activator PDBu suppressed spontaneous contractions in vitro. These studies suggest that PKC dysfunction, due to pathological changes in PBOO and supported by pharmacological inhibition of PKC in normal DSM strips, contributes to detrusor overactivity.
Pharmacologic inhibition of PKC by Bim-1 in normal DSM strips mimics the PKC deficient, hyperactive state of the PBOO bladder and strongly favors a role for PKC in the regulation of spontaneous contractions in DSM. The mechanism by which PKC can promote spontaneous activity is not entirely clear. Our data show that inhibition of PKC by Bim-1 can promote spontaneous activity and activation of PKC by PDBu can suppress spontaneous contractions. Our data also show that the specific BK channel inhibitor IBTX can prevent PDBu-induced inhibition of spontaneous contractions (Fig. 8). This suggests that BK channels may be involved in PKC signaling with regard to the inhibition of spontaneous contractions and that PKC may actually activate BK channels in DSM as reported in other tissues (29). Hence, inhibition of PKC by Bim-1 may be responsible for reducing the inhibitory regulation of BK channels on spontaneous contractions thus resulting in a prolonged period of spontaneous contractions as seen in our studies. Both the stimulatory effect by Bim-1 inhibition and the inhibitory effect of PKC activation by PDBu on spontaneous activity are novel findings, and to our knowledge, have not been reported before in DSM.
Our studies also demonstrate that PKC displays a biphasic activation profile in the DSM, inhibiting spontaneous contractions at low concentrations and generating increased force at higher concentrations (Fig. 5). The ability of PKC to modulate DSM tone and sensitivity by regulating the frequency and amplitude of spontaneous contractions could play an important role in the storage function of the bladder, since uncontrolled spontaneous activity, due to PKC dysfunction, would be counterproductive to bladder storage. The ability of PKC, on the other hand, to generate force at high levels of activation may be important physiologically as the bladder increases in volume by providing a countervailing and stabilizing force (increased wall tension) in response to the increasing mass as the bladder increases in volume. It is also worth noting that this concentration-dependent separation between high and low PKC functions may be important physiologically, since it would allow for the regulation of spontaneous activity without significantly affecting DSM tone during the storage phase of the micturition cycle. The precise mechanism of how the bladder would transition from low to high PKC activity during storage, filling, and emptying remains to be determined.
One possibility is the progressive activation of a stretch-sensitive calcium-dependent PKC, such as PKC-α, as the bladder increases in volume. Studies show that PKC-α, a calcium-dependent PKC, is present within the DSM (6) and that stretch can result in the release calcium from individual myocytes (12). This line of reasoning would suggest that during the early storage phase when stretch is low calcium release would be low and thus PKC activity. However, as the bladder increases in volume and stretch increases, this would result in greater release of calcium and higher levels of activation of PKC leading to an increase in wall tension as the bladder approaches capacity. It is also possible that PKC activity itself does not change significantly. Under this scenario wall tension is multiplied at constant PKC activity as the muscle fibers increase in length, and as they approach L0 (optimal length), in response to bladder filling. Interestingly, inhibition of PKC in the in vitro whole bladder and urethra model significantly reduces bladder emptying (unpublished observation).
Other reports also indicate that PKC regulates the sensitivity of smooth muscle activation and contractility (2, 3, 22). One such study reports that PKCε can regulate contractile function in airway smooth muscle by regulating the release of calcium sparks from the sarcoplasmic reticulum. The authors report that inhibition and deletion of the PKCε gene expression resulted in higher levels of force, while activation by phorbol 12-myristate 13-acetate, a PKC activator, produced less force (18). Furthermore, studies suggest that PKC can regulate the sensitivity of smooth muscle by modulating the coupling strength between calcium and BK channels and also by phosphorylating the BK channels (3, 27, 28). Our own studies indicate that the PKC activator PDBu at relatively low concentrations is a potent inhibitor of spontaneous contractions in DSM strips (Figs. 5, 6, and 7) and that the mechanism of action may involve activation of BK-channels by PKC resulting in an increase in the BK-channel current density and hyperpolarization of the cell membrane.
Our data also suggest that PKC may be constitutively active in DSM. This activity may play an important role during the storage phase of the micturition cycle by modulating the sensitivity of the DSM thus suppressing excessive spontaneous and NVC. This is indicated by the fact that pharmacological inhibition of PKC caused a prolonged high amplitude of spontaneous contractions in vitro compared with control (as shown in Fig. 2). This may be due to the fact that PKC dysfunction reduces the threshold for activation (high sensitivity) of the DSM by local calcium signals that are not normally sufficient to augment contraction. Indeed, studies in the rabbit bladder by Su et al. (24) report that PBOO bladder muscle strips display an elevated sensitivity to carbachol and KCl. These findings may also help to clarify the relationship between PKC dysfunction associated with PBOO, as has been reported, and spontaneous and nonvoiding contractions in these bladders. Other studies report a similar correlation between PKC expression and spontaneous oscillations in other smooth muscle as well (19).
Next, we addressed the possible mechanism by which PKC can regulate spontaneous and NVC in DSM. Our studies demonstrate that the PKC activator PDBu can inhibit spontaneous contractions and the PKC inhibitor Bim-1 can increase the duration of high amplitude spontaneous contractions in isolated muscle strips from normal bladders. Our data also show that the specific BK-channel inhibitor IBTX can block PDBu-induced inhibition of spontaneous contractions (Fig. 8). Interestingly, spontaneous contractions enhanced by Bim-1 (PKC inhibitor) were significantly reduced by the BK channel openers ISPA and NS1619 (Figs. 9 and 10). This suggests a common mechanism of action for inhibition of these contractions, possibly, through activation of BK-channels, since both NS1619 and ISPA have been shown to increase the open probability of BK channels (8). The ability of IBTX, a specific BK inhibitor, to prevent PDBu-induced inhibition of spontaneous contractions gives further credence to the idea that BK channels are involved in PKC signaling in DSM.
In conclusion, our data reveal that PKC may play an important role during the storage phase of the micturition cycle by helping to maintain the DSM in a quiescent state, thus mitigating against uncontrolled spontaneous and NVC. Indirect evidence suggests that this may be accomplished through its ability to activate BK channels and increase the BK-channel current, thus increasing the threshold for activation (low sensitivity) of the DSM during storage. Our data also suggest that PKC may play a role in bladder emptying by helping to maintain force at a high level, which is required for complete bladder emptying. Thus PKC may play a critical role in integrating overall bladder function by helping to harmonize the filling, storage and emptying phases of the micturition cycle.
GRANTS
This study was supported by the O'Brien Urology Research Grant P50-DK-052620 from National Institute of Diabetes and Digestive and Kidney Diseases.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.A.H., Q.L., S. Chang, S.A.Z., S.B., and A.P.M. performed experiments; J.A.H., Q.L., S. Chang, S.A.Z., S.B., A.P.M., and S. Chacko analyzed data; J.A.H., Q.L., S. Chang, A.J.W., A.P.M., and S. Chacko interpreted results of experiments; J.A.H., A.P.M., and S. Chacko prepared figures; J.A.H. and S. Chacko drafted manuscript; J.A.H., S.A.Z., A.J.W., A.P.M., and S. Chacko edited and revised manuscript; J.A.H., S.A.Z., A.J.W., A.P.M., and S. Chacko approved final version of manuscript; S. Chacko conception and design of research.
ACKNOWLEDGMENTS
We thank Jocelyn McCabe for assistance in preparing the manuscript.
REFERENCES
- 1. Astman N, Gutnick MJ, Fleidervish IA. Activation of protein kinase C increases neuronal excitability by regulating persistent Na+ current in mouse neocortical slices. J Neurophysiol 80: 1547–1551, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Barman SA, Zhu S, White RE. PKC activates BKCa channels in rat pulmonary arterial smooth muscle via cGMP-dependent protein kinase. Am J Physiol Lung Cell Mol Physiol 286: L1275–L1281, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Bayguinov O, Hagen B, Kenyon JL, Sanders KM. Coupling strength between localized Ca2+ transients and K+ channels is regulated by protein kinase C. Am J Physiol Cell Physiol 281: C1512–C1523, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Brading AF. Spontaneous activity of lower urinary tract smooth muscles: correlation between ion channels and tissue function. J Physiol 570: 13–22, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang S, Gomes CM, Hypolite JA, Marx J, Alanzi J, Zderic SA, Malkowicz B, Wein AJ, Chacko S. Detrusor overactivity is associated with downregulation of large-conductance calcium- and voltage-activated potassium channel protein. Am J Physiol Renal Physiol 298: F1416–F1423, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chang S, Hypolite JA, Mohanan S, Zderic SA, Wein AJ, Chacko S. Alteration of the PKC-mediated signaling pathway for smooth muscle contraction in obstruction-induced hypertrophy of the urinary bladder. Lab Invest 89: 823–832, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dessy C, Matsuda N, Hulvershorn J, Sougnez CL, Sellke FW, Morgan KG. Evidence for involvement of the PKC-α isoform in myogenic contractions of the coronary microcirculation. Am J Physiol Heart Circ Physiol 279: H916–H923, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Gessner G, Cui YM, Otani Y, Ohwada T, Soom M, Hoshi T, Heinemann SH. Molecular mechanism of pharmacological activation of BK channels. Proc Natl Acad Sci USA 109: 3552–3557, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heppner TJ, Bonev AD, Nelson MT. Ca2+-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol Cell Physiol 273: C110–C117, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Herrera GM, Heppner TJ, Nelson MT. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am J Physiol Regul Integr Comp Physiol 279: R60–R68, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Hypolite JA, Chang S, LaBelle E, Babu GJ, Periasamy M, Wein AJ, Chacko S. Deletion of SM-B, the high ATPase isoform of myosin, upregulates the PKC-mediated signal transduction pathway in murine urinary bladder smooth muscle. Am J Physiol Renal Physiol 296: F658–F665, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ji G, Barsotti RJ, Feldman ME, Kotlikoff MI. Stretch-induced calcium release in smooth muscle. J Gen Physiol 119: 533–544, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kita M, Yunoki T, Takimoto K, Miyazato M, Kita K, de Groat WC, Kakizaki H, Yoshimura N. Effects of bladder outlet obstruction on properties of Ca2+-activated K+ channels in rat bladder. Am J Physiol Regul Integr Comp Physiol 298: R1310–R1319, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Layne JJ, Nausch B, Olesen SP, Nelson MT. BK channel activation by NS11021 decreases excitability and contractility of urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol 298: R378–R384, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Levin RM, Haugaard N, O'Connor L, Buttyan R, Das A, Dixon JS, Gosling JA. Obstructive response of human bladder to BPH vs. rabbit bladder response to partial outlet obstruction: a direct comparison. Neurourol Urodyn 19: 609–629, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Levin RM, Longhurst PA, Monson FC, Kato K, Wein AJ. Effect of bladder outlet obstruction on the morphology, physiology, and pharmacology of the bladder. Prostate Suppl 3: 9–26, 1990 [DOI] [PubMed] [Google Scholar]
- 17. Li L, Jiang C, Song B, Yan J, Pan J. Altered expression of calcium-activated K and Cl channels in detrusor overactivity of rats with partial bladder outlet obstruction. BJU Int 101: 1588–1594, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Liu QH, Zheng YM, Korde AS, Li XQ, Ma J, Takeshima H, Wang YX. Protein kinase C-epsilon regulates local calcium signaling in airway smooth muscle cells. Am J Respir Cell Mol Biol 40: 663–671, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ozaki H, Yasuda K, Kim YS, Egawa M, Kanzaki H, Nakazawa H, Hori M, Seto M, Karaki H. Possible role of the protein kinase C/CPI-17 pathway in the augmented contraction of human myometrium after gestation. Br J Pharmacol 140: 1303–1312, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Petkov GV. Role of potassium ion channels in detrusor smooth muscle function and dysfunction. Nat Rev Urol 9: 30–40, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Petkov GV, Nelson MT. Differential regulation of Ca2+-activated K+ channels by β-adrenoceptors in guinea pig urinary bladder smooth muscle. Am J Physiol Cell Physiol 288: C1255–C1263, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Phillippe M. Protein kinase C, an inhibitor of oxytocin-stimulated phasic myometrial contractions. Biol Reprod 50: 855–859, 1994 [DOI] [PubMed] [Google Scholar]
- 23. Song JC, Rangachari PK, Matthews JB. Opposing effects of PKCα and PKCε on basolateral membrane dynamics in intestinal epithelia. Am J Physiol Cell Physiol 283: C1548–C1556, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Su X, Stein R, Stanton MC, Zderic S, Moreland RS. Effect of partial outlet obstruction on rabbit urinary bladder smooth muscle function. Am J Physiol Renal Physiol 284: F644–F652, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Thorneloe KS, Meredith AL, Knorn AM, Aldrich RW, Nelson MT. Urodynamic properties and neurotransmitter dependence of urinary bladder contractility in the BK channel deletion model of overactive bladder. Am J Physiol Renal Physiol 289: F604–F610, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Wang T, Kendig DM, Smolock EM, Moreland RS. Carbachol-induced rabbit bladder smooth muscle contraction: roles of protein kinase C and Rho kinase. Am J Physiol Renal Physiol 297: F1534–F1542, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yeon DS, Kim JS, Ahn DS, Kwon SC, Kang BS, Morgan KG, Lee YH. Role of protein kinase C- or RhoA-induced Ca(2+) sensitization in stretch-induced myogenic tone. Cardiovasc Res 53: 431–438, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Zhou XB, Arntz C, Kamm S, Motejlek K, Sausbier U, Wang GX, Ruth P, Korth M. A molecular switch for specific stimulation of the BKCa channel by cGMP and cAMP kinase. J Biol Chem 276: 43239–43245, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Zhou XB, Wulfsen I, Utku E, Sausbier U, Sausbier M, Wieland T, Ruth P, Korth M. Dual role of protein kinase C on BK channel regulation. Proc Natl Acad Sci USA 107: 8005–8010, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]