Abstract
Hypozincemia, with hepatic zinc accumulation at the expense of other organs, occurs in infection, inflammation, and aseptic lung injury. Mechanisms underlying zinc partitioning or its impact on extrahepatic organs are unclear. Here we show that the major zinc-binding protein, metallothionein (MT), is critical for zinc transmigration from lung to liver during hyperoxia and preservation of intrapulmonary zinc during hyperoxia is associated with an injury-resistant phenotype in MT-null mice. Particularly, lung-to-liver zinc ratios decreased in wild-type (WT) and increased significantly in MT-null mice breathing 95% oxygen for 72 h. Compared with female adult WT mice, MT-null mice were significantly protected against hyperoxic lung injury indicated by reduced inflammation and interstitial edema, fewer necrotic changes to distal airway epithelium, and sustained lung function at 72 h hyperoxia. Lungs of MT-null mice showed decreased levels of immunoreactive LC3, an autophagy marker, compared with WT mice. Analysis of superoxide dismutase (SOD) activity in the lungs revealed similar levels of manganese-SOD activity between strains under normoxia and hyperoxia. Lung extracellular SOD activity decreased significantly in both strains at 72 h of hyperoxia, although there was no difference between strains. Copper-zinc-SOD activity was ∼4× higher under normoxic conditions in MT-null compared with WT mice but was not affected in either group by hyperoxia. Collectively the data suggest that genetic deletion of MT-I/II in mice is associated with compensatory increase in copper-zinc-SOD activity, prevention of hyperoxia-induced zinc transmigration from lung to liver, and hyperoxia-resistant phenotype strongly associated with differences in zinc homeostasis during hyperoxic acute lung injury.
Keywords: zinc, metallothionein, hyperoxia, autophagy, CuZn-SOD, acute lung injury
whole body zinc homeostasis is efficiently controlled by a combination of absorption via gastrointestinal tract and excretion of this essential dietary micronutrient (26). This in turn is coupled to precise cellular and molecular control via coordinated activity of a large family of zinc importers, transporters, and the metal-binding protein metallothionein (MT) (13, 31, 35). In the presence of excess plasma zinc secondary to zinc infusion or exposure to dexamethasone or IL-6, metallothionein-mediated zinc transport into the liver occurs as a mechanism for maintaining zinc homeostasis (6). Hepatic zinc accumulation is particularly vital in inflammation and infection, supporting zinc-dependent acute phase reactions and host defense mechanisms, and MT is required for zinc accumulation in the livers of endotoxin-treated mice (47). Insight into the molecular mechanisms in the setting of sepsis (5) and liver-directed aseptic injury (e.g., turpentine) are evolving, and important roles for metallothionein and zinc transporters are becoming more apparent (32, 34). Whole body zinc depletion (secondary to dietary restriction or alcohol exposure) is associated with hypersensitivity to polymicrobial sepsis (24), hyperoxia, or macrophage and alveolar epithelial cell dysfunction (21), and the respective phenotypes can be reversed with dietary repletion of zinc (29, 60). In contrast to dysregulated iron metabolism and anemia of chronic disease, the functional significance of hepatic accumulation of zinc and hypozincemia in sepsis (perhaps host defense by sequestering zinc away from pathogens) or inflammation (supporting zinc-dependent hepatic acute phase reaction and/or altered carbohydrate metabolism) remain unclear (34). Redistribution of whole body zinc may also occur in aseptic, non-liver-targeted injury such as hyperoxia (28). However, neither the contribution of MT to nor the functional impact of hepatic accumulation of zinc in the pathogenesis of hyperoxic acute lung injury is apparent. Therefore we hypothesize that hepatic zinc accumulation is MT dependent and important for maintaining zinc homeostasis during hyperoxia. Here we determined 1) the role of MT in mediating hepatic accumulation of zinc in hyperoxic acute lung injury and 2) the effect of ablating MT-I/II expression on phenotypic response of mice to hyperoxia. The present study demonstrates that there is an MT-dependent hepatic accumulation of zinc in intact mice exposed to hyperoxia and concomitant intrapulmonary zinc losses that are associated with an increased sensitivity to hyperoxia in wild-type (WT) compared with MT-null mice. The resistance of MT-null mice to hyperoxia would not be predicted from experience supporting its antioxidant activity (27) and distinguishes its metal-binding properties from these latter functions (6).
METHODS
All procedures were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Experiments in WT and MT-null mice were performed to test the hypothesis that MT plays a protective role in hyperoxic acute lung injury. All experimental measurements were made at baseline (0 h) and at 24, 48, and 72 h after initiation of the experiments.
Mice and exposure protocol.
Female MT-null mice (129S7/SvEvBrd-Mt1tm1Bri Mt2tm1Bri/J), with their respective matching 129S1/SvImJ wild-type mice (age 6–8 wk), were purchased from the Jackson Laboratory (Bar Harbor, ME). Female mice were chosen because of their increased sensitivity to hyperoxia compared with male mice (50). The mice were randomly assigned to either normoxia (control) or hyperoxia treatment groups. Animals were placed in an airtight chamber and exposed to either room air or 95% oxygen continuously up to 4 days (96 h). Oxygen was supplied to the chamber at a flow rate of 3–4 l/min. Oxygen concentrations were continuously monitored at outflow by use of a Datex capnograph (GE Healthcare, Waukesha, WI).
Bronchoalveolar lavage.
Total bronchoalveolar lavage (BAL) cell and differential leukocyte counts were performed at the end of experiments using 5 × 0.8 ml phosphate-buffered saline (PBS). BAL total protein content as a marker of permeability changes was measured by the modified Bradford assay (Bio-Rad, Hercules, CA).
Measurement of lung physiology.
At 0, 24, 48, and 72 h, mice were anesthetized with an intraperitoneal injection of 60 mg/kg pentobarbital sodium (Ovation Pharmaceuticals, Deerfield, IL). A tracheostomy was performed and mice were attached to the ventilator. Following 5 min of stabilization, animals received deep lung inflation to 30 cmH2O distending pressure to ensure uniform lung recruitment and normalization to the same preassessment volume history. Physiological recordings were performed with a computer-controlled ventilator (flexiVent, Scientific Respiratory Equipment, Montreal, Canada). Mice were ventilated with a tidal volume of 0.2 ml at a respiratory rate of 200 breaths/min. Lung mechanics (tissue damping and elastance H) were measured at specific intervals by triggering the ventilator to deliver a custom input flow wave developed to characterize murine lung impedance over the physiological range of breathing frequencies (20 to 200 breaths/min). Quasi-static pressure-volume (P-V) curves and compliances were generated by calculating the slope of each curve (3).
Western blot analysis.
Expression of metallothionein, β-actin, autophagy markers (LC3-I and -II) and antioxidant enzymes [superoxide dismutase (SOD)] were examined by Western blot. Liver and lungs were harvested immediately after the animals were euthanized and perfused free of blood with PBS. Tissues were homogenized using a Dounce glass homogenizer with protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 mg/ml aprotinin, 1 mg/ml leupeptin) on ice and then centrifuged at 8,500 g for 5 min. The resulting protein supernatants were subjected to 4–12% SDS-PAGE gel electrophoresis and transferred to a nitrocellulose membrane (Invitrogen, Carlsbad, CA). The membrane was probed with antibodies against metallothionein, LC3-I/II, and Mn- and CuZn-SOD (R & D Systems, Minneapolis, MN) or β-actin (Sigma-Aldrich, St. Louis, MO), followed by horseradish peroxidase-coupled detection. We reported details of the extracellular SOD (EC-SOD) antibody (10, 44). The band densities were measured using the NIH ImageJ software.
Histology and immunofluorescence.
We perfused and fixed mouse lung tissues with 2% paraformaldehyde and sectioned at 8 μm by use of a Microm HM 500. For structural imaging of the lung tissues, we stained the sections with hematoxylin and eosin (22). For immunofluorescence imaging, tissue sections were rehydrated with PBS (ThermoFisher Scientific, Waltham, MA), permeabilized with 0.1% Triton X-100 (Sigma-Aldrich) for 20 min, then washed with PBS (1×) and with 0.5% BSA buffer (3×). Tissues were blocked with 2% BSA and then washed once with 0.5% BSA. Staining was accomplished with rabbit anti-LC3 and anti-mouse EC-SOD (1:500) subsequently with secondary Cy3-conjugated goat anti-rabbit antibody (1:1,000) (Jackson ImmunoResearch, West Grove, PA). All tissues were counterstained with Alexa Fluor 647-conjugated phalloidin (1:250) (Invitrogen) and 1% bisbenzimide (ThermoFisher Scientific) to label F-actin and nuclei, respectively. Optical sections (0.4 μm) in z-axis were acquired with Olympus FluoView 1000 confocal microscope (Olympus, Lehigh Valley, PA). Images were obtained with a ×60 optical lens with ×1.0 digital zoom at same exposure parameters.
Transmission electron microscopy.
Specimens were fixed in cold 2.5% glutaraldehyde in 0.1 M PBS, rinsed in PBS, postfixed in 1% osmium tetroxide with 0.1% potassium ferricyanide, dehydrated through a graded series of ethanol, and finally embedded in Epon. Semithin (300 nm) sections were cut on a Reichert Ultracut (Reichert, Depew, NY), stained with 0.5% toluidine blue and examined under the light microscope. Ultrathin sections (65 nm) were stained with uranyl acetate and Reynold's lead citrate and examined on JEOL 1011 transmission electron microscope (JEOL, Tokyo, Japan).
Measurement of SOD activity.
The lungs were homogenized in 3 ml of 50 mM potassium phosphate with 0.3 M potassium bromide, pH 7.4. SOD specific activity was measured in the EC-SOD as well as the CuZn- and Mn-SOD fractions using a xanthine/xanthine oxidase system to generate superoxide anion and measuring the rate of reduction of acetylated cytochrome c as described (10, 56). SOD activity was determined in a 100-μl assay mixture containing 1 mM acetylated cytochrome c, 1 mM xanthine, and 1 mM EDTA in 50 mM potassium phosphate buffer, pH 7.8, at 25°C. Xanthine oxidase was added to give a rate of reduction of cytochrome c of ∼0.0075 absorbance units/min (±10%). The absorbance was measured at 550 nm with a Spectramax 340 spectrophotometer (Molecular Devices, Sunnyvale, CA). SOD activities were calculated from assays in which there was an inhibition of cytochrome c reduction of between 40 and 50%. Although all samples initially were analyzed at the same volume, the volume of sample was then adjusted repeatedly until the level of inhibition fell within this range. One unit of SOD activity was defined as the amount of SOD that gives 50% inhibition of the rate of cytochrome c reduction. An SOD standard curve was plotted with various dilutions of a CuZn-SOD standard at 40 McCord-Fridovich units/ml. One unit in the assay was equivalent to 0.027 McCord-Fridovich units/ml based on the standard curve. The total SOD (before DDC or cyanide treatment), Mn-SOD (after diethyldithiocarbamate or cyanide treatment), and CuZn-SOD activities (the difference between total SOD and Mn-SOD measurements) were determined (7, 36). EC-SOD was separated from intracellular CuZn- and Mn-SOD by concanavalin A-Sepharose chromatography as described (11, 43). EC-SOD activity analysis was performed on the eluates as described above for other SOD isoforms (9).
Measurement of zinc concentration.
We used zinc-free HBSS to flush lungs and liver free of blood. Electrothermal atomic absorption spectrometry and inductively coupled plasma mass spectrometry (ICP-MS) were used to measure lung and liver zinc content. We initially measured a representative group of lung and liver tissues using atomic absorption, and because of low levels of zinc in the hyperoxic lung tissues we adapted ICP-MS owing to its improved sensitivity (45). The lung-to-liver zinc ratios were similar using both methods. Lung and liver zinc contents are reported from individual animals as matched ratios (Fig. 2).
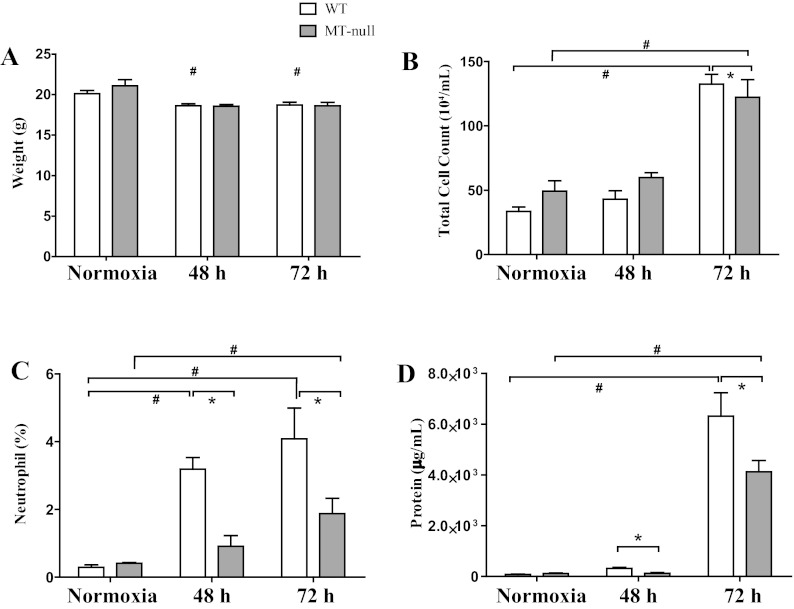
Fig. 2.
Both wild-type (WT) and MT-null mice had similar weight loss following hyperoxia exposure, but the WT mice had more pronounced lung injury. A: both WT and MT-null mice lost weight, and the weight loss was statistically significant as early as 48 h, but there was no difference in the rate of weight loss between WT and MT-null mice (#P < 0.05). At baseline both WT (n = 10) and MT-null (n = 6) mice had similar total cell counts in the bronchoalveolar lavage (B) and percent neutrophils (C). Although the total cell count did not change between the WT (n = 6) and MT-null (n = 6) mice by 48 h of hyperoxia, the differential count of neutrophils was significantly higher in WT mice compared with MT-null mice (*P < 0.05). The WT bronchoalveolar lavage (BAL) neutrophils were also significantly elevated at 48 h of hyperoxia compared with baseline normoxia (#P < 0.05). A similar pattern for total cell counts and percent neutrophils was observed at 72 h of hyperoxia between the WT (n = 6) and MT-null (n = 8) (*P < 0.05) as well as within the WT and MT-null groups between the baseline normoxia and 72-h time points (#P < 0.05). The lung injury was also corroborated by the measurement of BAL protein concentration (D). At 48 and 72 h, the WT had significantly higher BAL protein concentrations than the MT-null mice (*P < 0.05). At 72 h, the BAL protein concentration was also significantly elevated within the groups (#P < 0.05).
Electrothermal atomic absorption spectrometry.
Dried liver and lung tissues (∼0.3 g) were digested in concentrated 67–70% nitric acid (Fisher, Fair Lawn, NJ) in Teflon digestion vessels. Tissue-free blanks were similarly prepared. Samples and blanks were digested (200°C, 800 psi, 15 min) in CEM MarsXpress microwave system (CEM, Matthews, NC). Digested tissues were transferred to polypropylene volumetric flasks and reconstituted to 50 ml with 2% HNO3, and trace metal acid was diluted in twice-deionized H2O. All plasticware was prewashed with 2% HNO3 and dried in HEPA-filtered laminar-flow cabinet configured for trace metal work (AirClean Systems, Raleigh, NC). Zinc was analyzed by graphite-furnace atomic absorption spectroscopy (AAnalyst 800, PerkinElmer, Waltham, MA) employing Zeeman background correction with magnesium nitrate (Sigma-Aldrich) [99.999% Mg(NO3)2·6H2O] as matrix modifier. Calibration curves of zinc (Zn2+) in 2% HNO3 were prepared from 99.999% zinc shot (Sigma-Aldrich) and 1,000 mg/l commercial standard SPEX CertiPrep (Spex, Metuchen, NJ). All experiments were measured in triplicate.
Inductively coupled plasma mass spectrometry.
Tissue samples were dried at 55°C for 48 h prior to analysis. A 250 mg aliquot of dried tissue was digested for 6 h in 30% nitric acid and analyzed for zinc by ICP-MS. All data were normalized on a dry weight basis not to bias the results as a result of different degrees of lung or liver injury and edema. At least 10% of each analytical batch consisted of quality control samples. Analytical accuracy was assessed with reagent blank, initial and continuing calibration verification standards, and standard reference material. Analytical precision was assessed with sample duplicates.
Measurement of ALT specific activity.
Measurement of serum alanine transaminase (ALT) specific activity was performed by monitoring the rate of NADH oxidation in a coupled reaction including lactate dehydrogenase as an indirect marker of liver injury in serum samples. The reaction includes transamination of l-alanine to pyruvate through ALT-mediated catalysis. Pyruvate is then converted to lactate by lactate dehydrogenase, during which NADH is oxidized to NAD+. The oxidation of NADH to NAD+ is accompanied by decreased absorbance at 340 nm. ALT specific activity is rate limiting and therefore oxidation is directly proportional to the ALT specific activity, presented as units per liter (Cayman Chemical, Ann Arbor, MI).
Data analysis.
Data are expressed as means ± SD. A one-way ANOVA and post hoc comparisons were performed. A P value of < 0.05 was considered significant. The values of the P-V loop areas were compared by use of paired two-tailed t-tests.
RESULTS
Effect of hyperoxia on pulmonary and hepatic levels of MT and zinc.
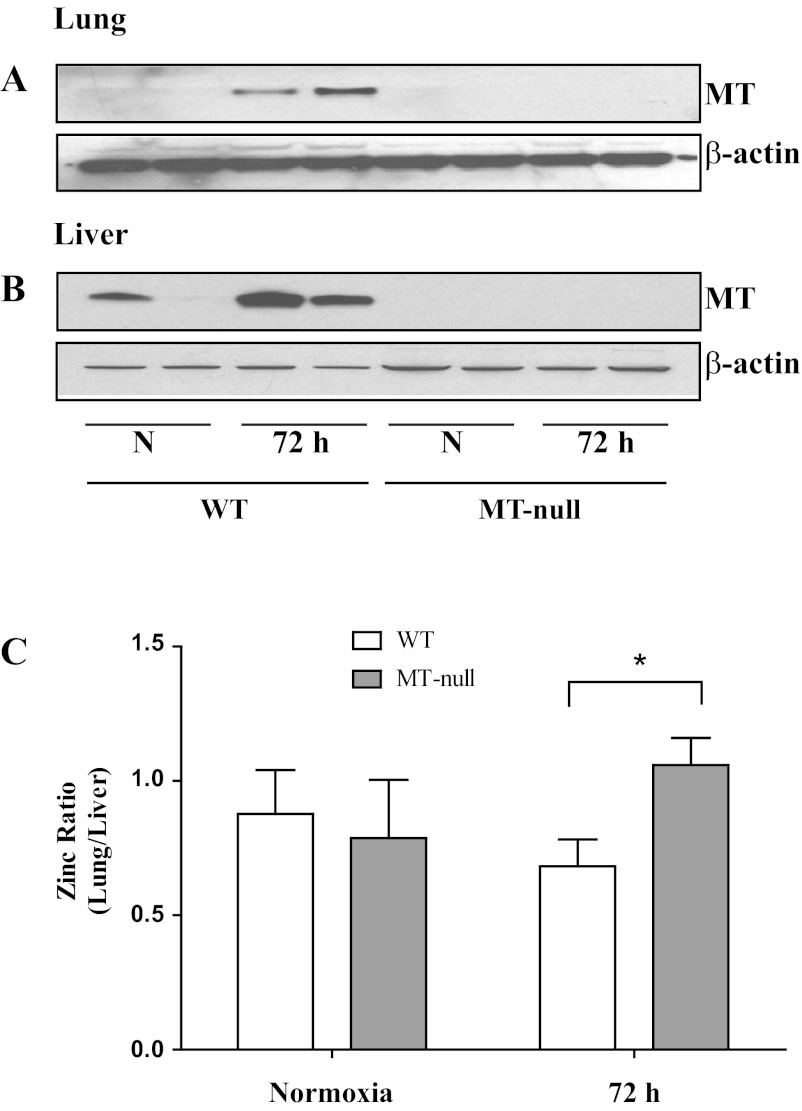
Intrapulmonary immunoreactive MT was detectable at low levels in control WT mice (Fig. 1A) but was readily apparent in liver of one of two mice breathing room air as assessed by Western blot (Fig. 1B). Our results are consistent with published reports demonstrating exposure of WT mice to hyperoxia for 72 h was associated with a notable increase in MT in lung and liver (19, 28, 46, 48, 64). As expected, MT immunoreactivity was not observed in either the lung or liver of MT-null mice, regardless of exposure, confirming that MT-I and -II (and not MT-III or MT-IV) are major pulmonary and hepatic isoforms of MT. We then measured total zinc in lung and liver tissues from normoxia- and hyperoxia-exposed WT and MT-null mice by atomic absorption spectroscopy (n = 4 to 8) and ICP-MS (n = 3 to 4). The absolute data within each organ normalized to dry weight were similar between the two assays. Lung zinc concentrations were normalized to liver for WT and MT-null mice in normoxia and hyperoxia in each individual mouse. WT mice had a significant decrease in lung-to-liver zinc ratio at 72 h of hyperoxia compared with MT-null mice (Fig. 1C). This suggests that MT is required for the transmigration of zinc from lung to liver.
Fig. 1.
Metallothionein (MT) is upregulated in liver and lung tissues after hyperoxia (A and B). Three-day hyperoxia exposure increased lung (A) and liver (B) expression of MT in wild-type mice, whereas the MT-null mice had no expression of MT as expected. The loading control was β-actin, showing that lanes were similarly loaded. Representative protein bands in duplicate are presented (n = 8). In hyperoxia, zinc is partitioned into the liver (C). The lung and liver zinc contents were measured and presented as a ratio of lung-to-liver zinc distribution. At normoxic baseline (N), both wild-type (n = 8) and MT-null (n = 6) mice had similar zinc distribution between the two organs. On the other hand, following 72 h of hyperoxia (72 h), the wild-type mice (n = 11) increased the liver content of MT (as presented in Fig. 1), leading to an increased liver accumulation of zinc with subsequent lowering of lung-to-liver zinc ratio compared with the MT-null mice (n = 8). Values are means ± SD and the difference between the wild-type and MT-null mice was significant at 72 h of hyperoxia (*P = 0.02).
Phenotypic and physiological differences between WT and MT-null mice in response to hyperoxia.
Body weight changes were quantified in early (0–72 h), nonlethal periods of exposure to >95% oxygen. Both WT and MT-null mice lost weight, and the weight loss was statistically significant as early as 48 h, but there was no difference in the rate of weight loss between WT and MT-null mice (Fig. 2A). Total cell count in BAL fluid significantly increased to a similar degree at 72 h in both WT and MT-null mice (Fig. 2B). Differential cell counts indicated a significant influx of polymorphonuclear neutrophils into the lungs of WT compared with MT-null mice at 48 and 72 h hyperoxia (Fig. 2C). Protein concentration in BAL fluid was also higher in WT than MT-null mice at 48 and 72 h of hyperoxia, indicating the development of acute lung injury in the WT mice (Fig. 2D).
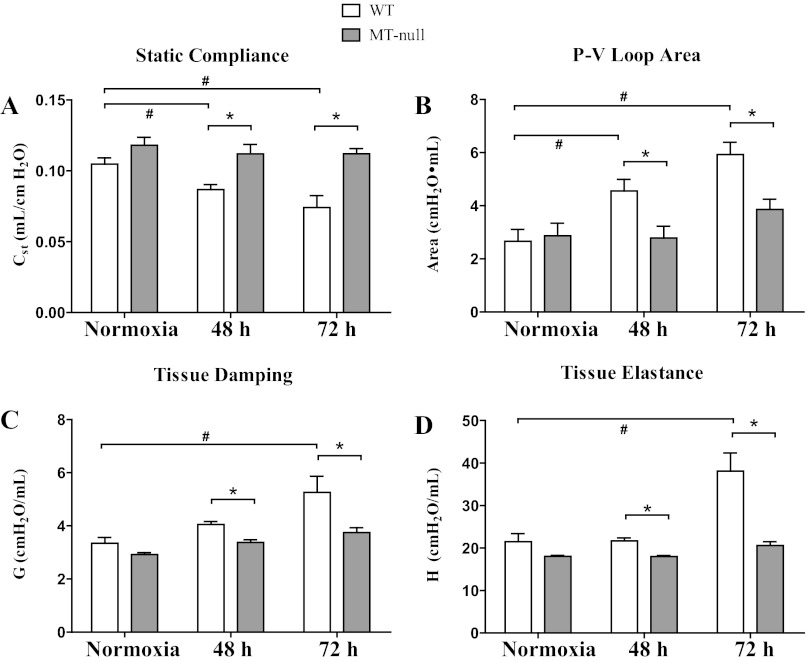
Pulmonary static compliance at 48 and 72 h of hyperoxia significantly decreased in WT but was not affected in MT-null mice (Fig. 3A). Hyperoxia significantly impaired lung hysteresis in WT mice and the increase in P-V loop area was significantly greater in WT than MT-null mice at 48 and 72 h hyperoxia (Fig. 3B) (3). Tissue damping and elastance (Fig. 3C, D) were similarly significantly increased in WT mice during hyperoxia but not affected in MT-null mice. These latter two measurements are closely related to tissue resistance and lung stiffening, respectively. Collectively, changes in lung mechanics are consistent with decreased sensitivity to hyperoxia in MT-null mice.
Fig. 3.
WT had more pronounced lung injury shown with the physiological measurements compared with the MT-null mice. Pulmonary mechanical function was assessed in cohorts of mice at 48 and 72 h of hyperoxia. A: hyperoxia exposure significantly decreased static compliance (Cst) in WT (n = 8, 5, and 10 at 0, 48, and 72 h, respectively) mice but was not affected in MT-null (n = 6, 10, and 9 at 0, 48, and 72 h, respectively) mice (*P < 0.05). Over the course of the experiments, the WT mice also had a loss in static compliance compared with the baseline controls (#P < 0.05). B: hyperoxia significantly impaired lung hysteresis in WT mice and the increase in pressure-volume (P-V) loop area was significantly greater in WT than MT-null mice at 48 and 72 h hyperoxia (*P < 0.05). The WT also had impaired hysteresis over time compared with the baseline controls (#P < 0.05). Tissue damping (G; C) and elastance (H; D) were similarly significantly increased in WT mice during hyperoxia but not affected in MT-null mice. *Statistically significant difference between WT and MT-null mice; #difference over time in the same strain.
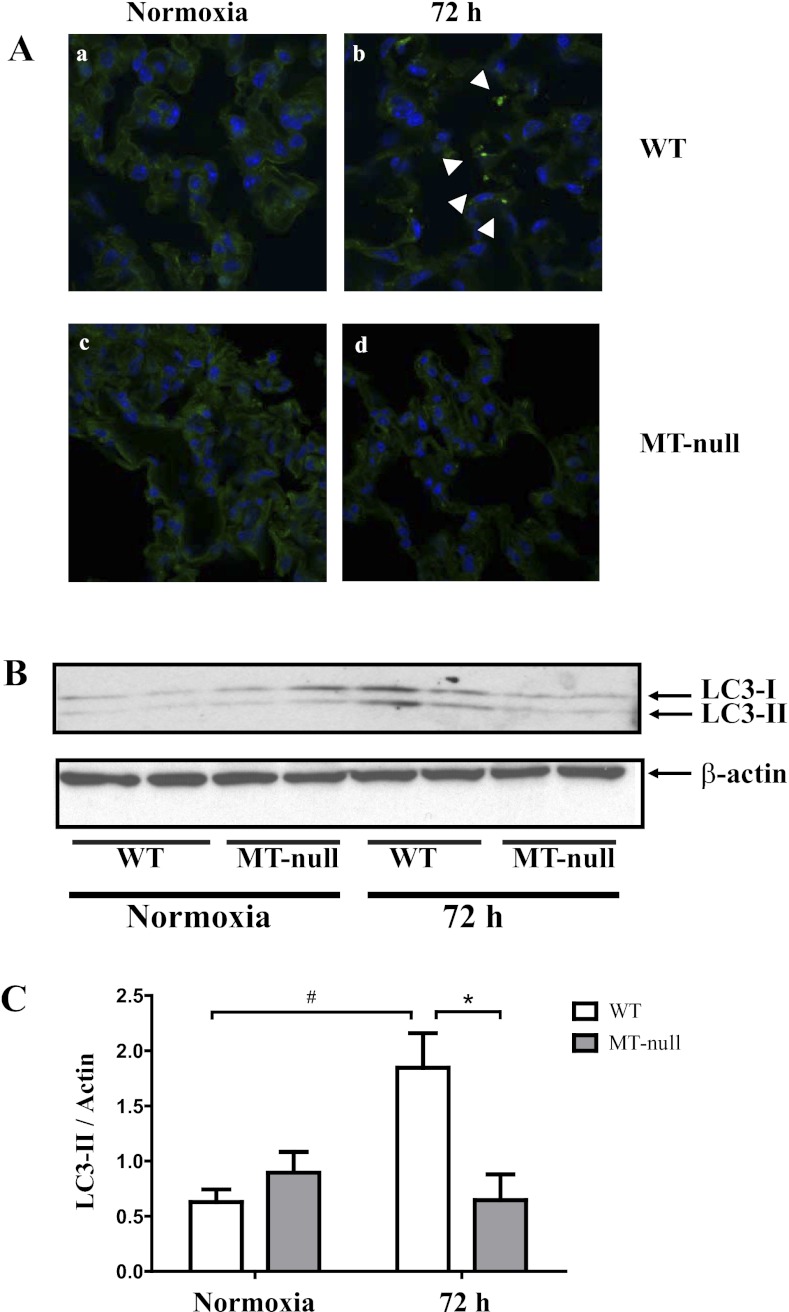
Morphological differences between WT and MT-null mice at 72 h hyperoxia.
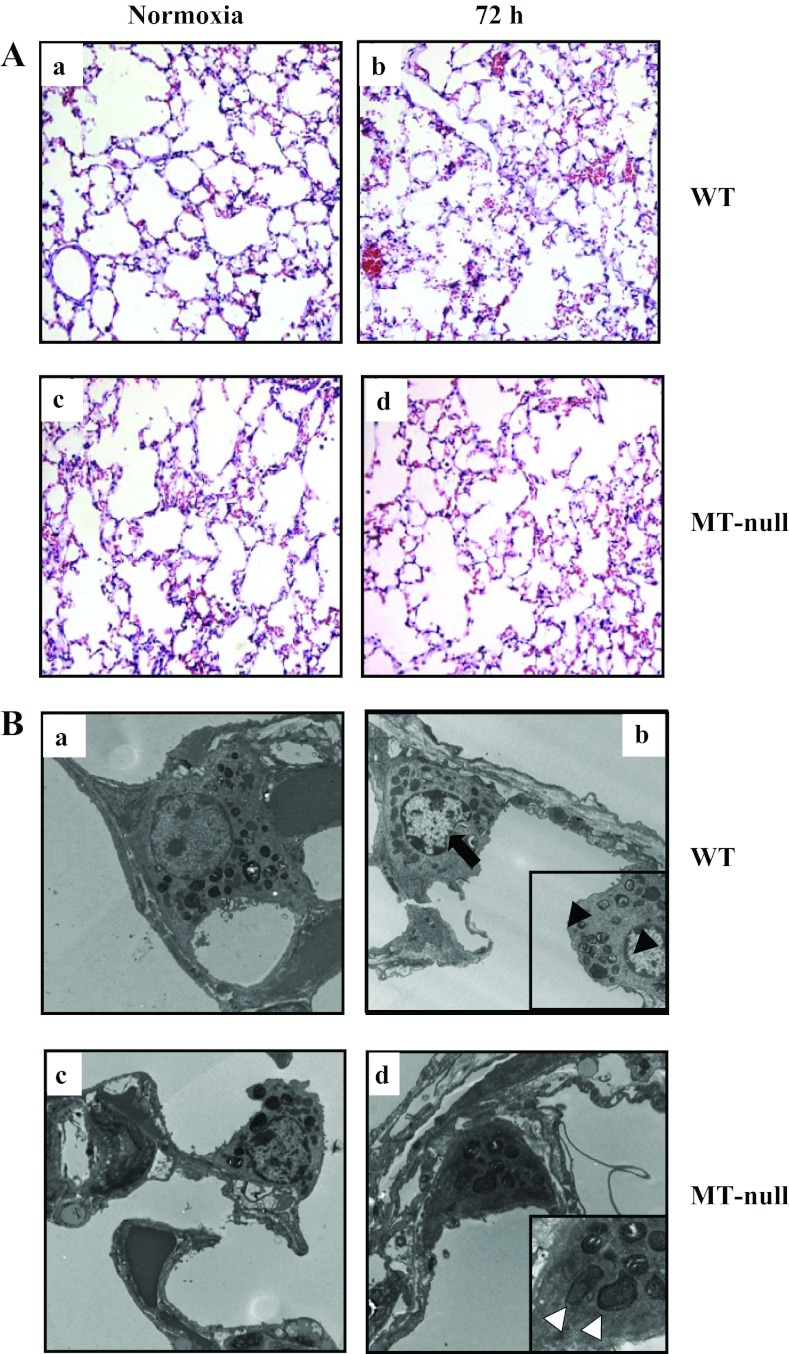
After 72 h of hyperoxia, histopathological changes were apparent in WT but not MT-null mice as determined by light (Fig. 4A) and transmission electron microscopy (Fig. 4B). Lungs of hyperoxic WT mice showed increased inflammatory cells and apparent interstitial edema compared with normoxic controls. Conversely, there was little evidence of increased inflammatory cells or edema in MT-null mice after 72 h of hyperoxia (Fig. 4). At the cellular level, lungs of hyperoxic WT mice showed damage of type I and II epithelial cells including presence of immature autophagosomes and necrotic changes with nuclear disintegration (Fig. 4Bb), whereas ultrastructure of distal epithelium appeared well preserved at 72 h of hyperoxia in MT-null mice (Fig. 4Bd). To confirm our observation of autophagy, lungs were analyzed by immunofluorescence staining for the autophagic protein LC3 (40, 57). LC3 signal showed a diffuse pattern in normoxic WT and MT-null mice (Fig. 5, Aa and Ac). Hyperoxia exposure resulted in a punctate staining pattern for LC3 indicating the induction of autophagy in these cells in WT mice (Fig. 5Ab). However, autophagy was much less pronounced in hyperoxia-treated MT-null mice (Fig. 5Ad). To further confirm our microscopic assessments, lung tissue conversion of LC3-I to LC3-II was quantified in whole lung tissue by Western blot analysis (Fig. 5, B and C). In WT mice, LC3-II protein increased significantly at 72 h of hyperoxia compared with the normoxic state. In contrast, there were no significant changes in LC3-II protein at 72 h of hyperoxia in MT-null mice. Collectively, these data suggest that hyperoxia induces a significantly greater degree of lung injury in WT mice compared with MT-null mice, through autophagic and necrotic cell death pathways.
Fig. 4.
WT had more pronounced lung injury at the tissue and cellular level compared with the MT-null mice. After 72 h of hyperoxia, histopathological changes were apparent in WT mice but not MT-null mice determined by light (Ab vs. Ad) and transmission electron microscopy (Bb vs. Bd). Lung tissue of hyperoxic WT mice (n = 6) showed increased cellularity and apparent interstitial edema compared with normoxic controls as well as MT-null at ×20 magnification (n = 6) mice (Ab). At the cellular level, lungs of hyperoxic WT mice (n = 4) showed damage of both type I and type II epithelial cells including presence of immature autophagosomes and necrotic changes (black arrow) with nuclear disintegration (Bb) and tissue edema (black arrowheads), whereas ultrastructure of distal epithelium appeared well preserved at 72 h of hyperoxia in MT-null mice (n = 4) (Bd) with early autophagosomes (white arrowheads).
Fig. 5.
WT had more pronounced lung injury at the tissue and cellular level compared with the MT-null mice. At baseline, the autophagy marker LC3 staining signal showed a diffuse pattern in both normoxic WT (n = 7) and MT-null mice (n = 6) (Aa and Ac). Hyperoxia exposure resulted in a punctate staining pattern for LC3, indicating the induction of autophagy in WT mice (white arrowheads) (Ab). The LC3 staining intensity was less pronounced in the MT-null mice, suggesting that autophagy was less pronounced in hyperoxia-treated lungs of MT-null mice (Ad). The Western blot analysis supports the findings of the tissue staining findings of autophagy (8). Lung tissue conversion of LC3-I to LC3-II was significantly elevated in the WT mice (n = 6) compared with MT-null mice (n = 6, *P < 0.05) (B and C). In WT mice, LC3-II protein also increased significantly at 72 h of hyperoxia compared with the normoxic state (#P < 0.05). In contrast, there were no significant changes in LC3-II protein at 72 h of hyperoxia in MT-null mice.
Superoxide dismutase in hyperoxic WT and MT-null mice.
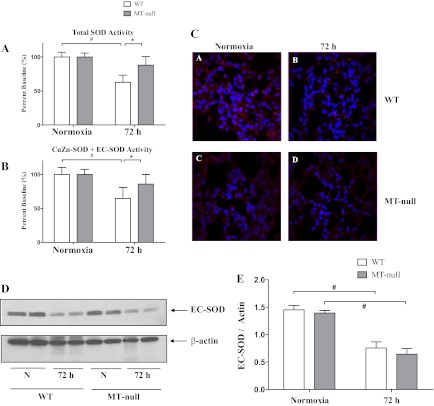
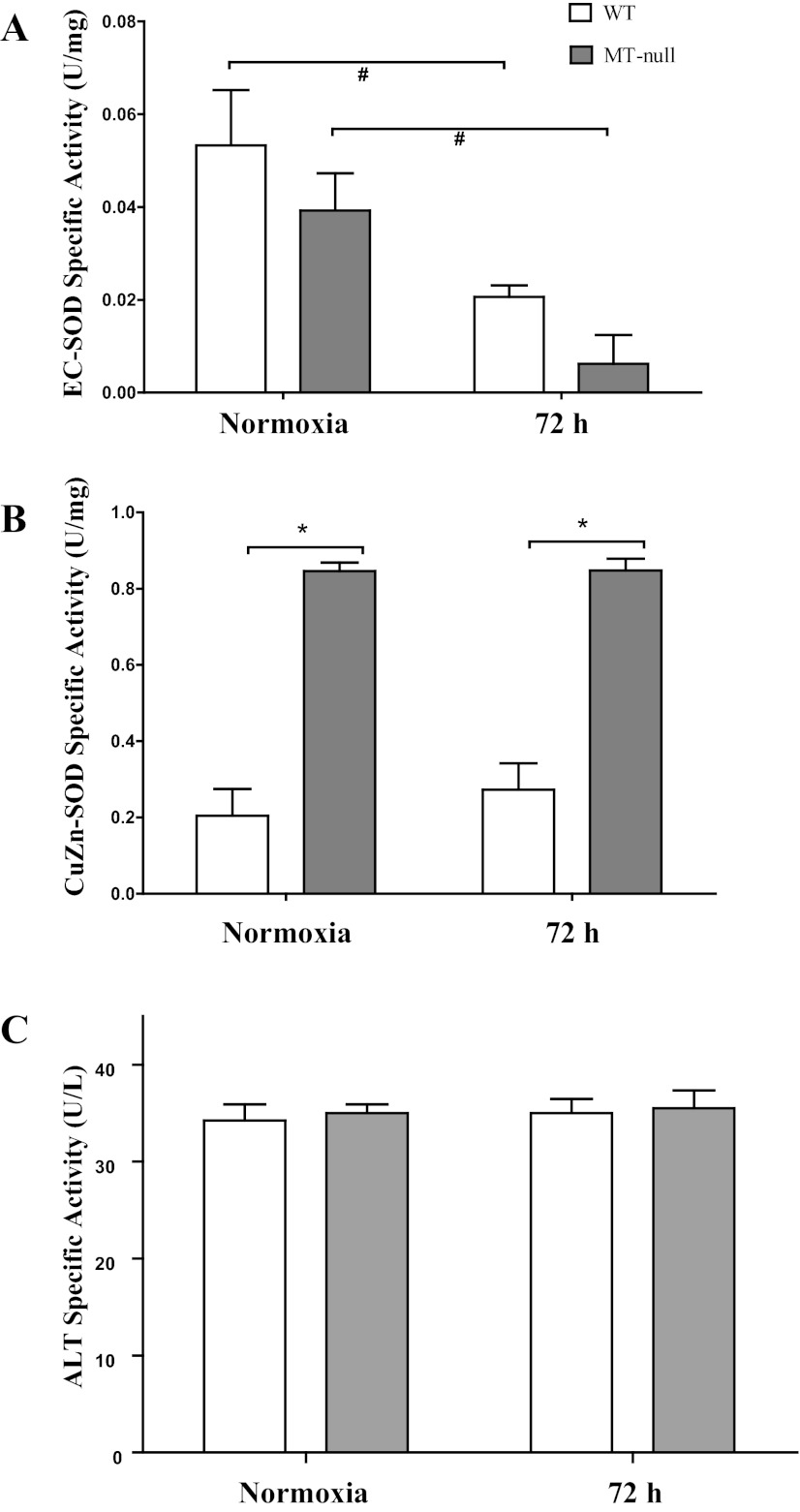
The various forms of the zinc-containing antioxidant enzyme SOD have long been considered important in the context of hyperoxic lung injury (44, 63). Initial measurements of total lung SOD activity were made and a significant decrease in total SOD activity was observed in hyperoxic WT mice only (Fig. 6A). The activity of the non-zinc-binding isoform, Mn-SOD, was similar in both groups and was not significantly affected by hyperoxia in either WT or MT-null mice (data not shown). Zinc-dependent SOD activity was then measured (Fig. 6B) and similar changes of SOD activity were noted to occur in response to hyperoxia (e.g., decrease in WT and no change in MT-null mice) (Fig. 6A). This prompted further investigation of direct measurements of the two forms of zinc-containing SODs: EC-SOD and CuZn-SOD. Hyperoxia caused a decrease in immunoreactive EC-SOD (Fig. 6, C and D) and EC-SOD activity (Fig. 7A) in both WT and MT-null mice. In contrast, immunoreactive CuZn-SOD activity was not affected by hyperoxia in either WT or MT-null mice (Fig. 7B). CuZn-SOD activity, however, was approximately 4× greater in lungs of MT-null mice than WT mice at control and at 72 h of hyperoxia. Collectively these data suggest that EC-SOD is sensitive to hyperoxia regardless of intrapulmonary zinc or MT status consistent with prior studies (44), whereas CuZn-SOD levels are elevated in MT-null mice, perhaps as compensation for effects of MT ablation or alterations in zinc availability.
Fig. 6.
WT had significant decrease in total and CuZn + extracellular superoxide dismutase (EC-SOD) activity compared with the MT-null mice. The total lung antioxidant superoxide dismutase (SOD) capacities of the WT (n = 5) and MT-null mice (n = 5) at baseline were similar, whereas the total SOD activity decreased significantly over time for WT mice (n = 5, #P < 0.05); there was also significant decrease between WT and MT-null mice (n = 5) total SOD activity at 72 h hyperoxia (A). Overall zinc-dependent SOD activity was then measured, and similar changes of SOD activity were noted to occur in response to hyperoxia with a decrease in SOD activity in WT (n = 5) and no change in MT-null mice (n = 5) (#,*P < 0.05) (B). There was similar degree of loss of EC-SOD fluorescence staining following hyperoxia in both WT and MT-null mice (C). This was corroborated with the whole lung Western blot analysis, and the decrease was statistically significant for both WT (n = 5) and MT-null (n = 5) mice (#P < 0.05) (D and E).
Fig. 7.
Both WT and MT-null mice have decreased EC-SOD activity following hyperoxia. Hyperoxia caused a decrease in EC-SOD activity (A) in both WT (n = 5) and MT-null mice (n = 5). CuZn-SOD activity was not affected by hyperoxia in either WT or MT-null mice (B). CuZn-SOD activity, however, was ∼4× greater in lungs of MT-null mice (n = 5) than WT mice (n = 6) at control and at 72 h of hyperoxia (*P < 0.05). Both WT and MT-null mice have similar serum alanine aminotransferase (ALT) specific activity at baseline and following hyperoxia. ALT activity, an indirect marker of liver injury, was not affected by hyperoxia in either WT (n = 4) or MT-null (n = 4) mice (C).
Circulating levels of alanine transaminase in hyperoxic WT and MT-null mice.
The serum ALT specific activity was assessed in normoxic and hyperoxic WT and MT-null mice as a marker of liver injury. The ALT specific activity was similar in between the genotypes in both normoxia and hyperoxia (Fig. 7C).
DISCUSSION
The present study demonstrates for the first time that translocation of zinc from lung to liver in hyperoxic mice is critically dependent on the presence of functional MT (Fig. 1C) (28, 47). Importantly, the lack of zinc translocation in MT-null mice was associated with a resistant phenotype to hyperoxia (Figs. 2–5), underscoring the potential importance of zinc homeostasis in affecting the course of hyperoxic acute lung injury (29). As others have shown, MT is readily induced in lung and liver during hyperoxia (Fig. 1) (28, 64). In contrast to emerging literature suggesting MT as part of cellular defense against partially reduced oxygen and nitrogen species, including our own work in hyperoxic cultured pulmonary endothelium, the role of MT in zinc partitioning seems more critical and detrimental than its antioxidant potential in vivo (6, 35, 49).
Intrapulmonary and intrahepatic MT and zinc in hyperoxia.
MT emerged as a hyperoxia-sensitive gene in salient studies of Veness-Meehan et al. (64) using DNA subtraction hybridization techniques in lungs of hyperoxic rabbits. In situ hybridization localized MT mRNA to chondrocytes, fibroblasts, and type II epithelial cells in hyperoxic mouse lung (48). Microarray analyses confirmed elevations in MT expression during hyperoxia in mouse lungs (46). Hyperoxic-induction of MT mRNA was significantly greater in various sensitive strains of mice, underscoring the utility of this stress gene as a biomarker of lung injury (19). Interestingly, hyperoxia also increased MT gene expression in other tissues such as the retina and liver (28, 71). Of the many zinc-binding proteins in tissues, MT has always assumed an important function as a zinc buffer primarily because of its abundance upon induction by hyperoxia and zinc-binding stoichiometry (7 mol zinc/mol MT) (35). Thus we would predict that under many conditions zinc availability may change with respect to MT levels; this was suggested by Levy et al. (28) and shown more decisively with MT-null mice in the present study (Fig. 1). It is unclear whether our observations regarding hyperoxia-induced changes in intrahepatic zinc and MT in hyperoxia have any corollary to hepatic zinc accumulation and hypozincemia associated with infection or inflammation. In the case of the latter, critical cytokines such as IL-1β (32) and IL-6 (34) affect expression of zinc transporters (e.g., Zip14/Slc39a14), resulting in increased intrahepatic zinc accumulation and thus providing zinc for acute-phase protein synthesis, regulation of gluconeogenesis, and/or control of microbial growth (34). It is noteworthy that MT is required for zinc accumulation in liver after IL-6 infusion (6) and endotoxin-induced inflammation (47). The former observation of MT-dependent hepatic zinc accumulation was not apparent in cultured hepatocytes underscoring the important differences between isolated cells and intact animals (6). Recently, similar conclusions about homeostasis, transmigration, and compartmentalization of zinc from lung to liver were reached by use of an inflammatory liver injury model secondary to HIV-1 transgene expression in intact rats (20).
MT and hyperoxic acute lung injury.
MT maintains metal ion homeostasis and cellular defense against high or toxic levels of essential and nonessential metals (23). The abundance of cysteine residues (∼30 mol%) suggested the ability of MT to quench superoxide anions (61), hydroxyl radicals (1), and partially reduced nitrogen species (70) and set the stage for MT as an antioxidant (27). Support for its antioxidant properties in hyperoxic lung injury emerged from studies by Hart et al. (15), who showed cross-resistance of cadmium-treated rats to heavy metal and hyperoxic toxicity. Further proof awaited the availability of genetically modified mice since cadmium is a promiscuous agent and assigning such cross-resistance to MT alone was ambiguous. Nonetheless, we noted that MT overexpression after either cadmium treatment or direct gene transfer in cultured pulmonary endothelial cells reduced their sensitivity to oxidant injury including hyperoxia (49). With the development of MT-null mice in two laboratories (38, 39), several investigators observed that MT-null mice were sensitive to acute lung injury due to endotoxin (54), ozone (17), and nickel (65). In light of these experiences in which MT-null mice were sensitive to pathophysiological conditions of excess partially reduced oxygen or nitrogen species, we hypothesized that MT-null mice would be sensitive to hyperoxia. Our data clearly show that MT-null mice were resistant to hyperoxia including functional (Figs. 2 and 3) and structural (Fig. 4) outcomes. We pursued early effects (<72 h) of hyperoxia before any lethality was noted in the adult females of either strain and it is unclear whether these phenotypic differences between strains would persist after longer hyperoxic exposure. Although we did not design a priori experiment for mortality, our observation suggests that by 96 h, hyperoxia caused mortality in all of the WT (6/6) and half of the MT-null mice (3/6).
The earliest detectable physiological change in lung mechanics was a decrease in lung compliance by 48 h (Fig. 3A). The mechanism underlying this is unclear but could reflect either changes in tissue forces (Fig. 3D) or surface lining fluid properties (18, 30, 67). Increased cellularity and protein (Fig. 2, B–D) in BAL in hyperoxic WT mice along with interstitial edema (Fig. 4) are consistent with alveolar-capillary barrier dysfunction. Increased neutrophils in BAL (Fig. 2C) and hypercellularity (Fig. 4) reflect enhanced inflammation in WT compared with MT-null mice at 72 h hyperoxia. In hyperoxia-sensitive WT mouse, necrotic injury in distal airway epithelium was apparent with electron microscopy (Fig. 4). Immunoreactive presence of autophagy was present early in WT, but not MT-null mice (Fig. 5). The potential cytoprotective role of autophagy in hyperoxic lung injury was recently described by Tanaka et al. (57). Thus it is possible that, in the hyperoxia-sensitive WT mice in our study, the relatively greater stress than noted in the MT-null mice activated autophagy as revealed by immunohistochemistry (Fig. 5, Aa and Ac) and Western blot analysis of LC3-II (Fig. 5B). As Tanaka et al. (57) suggest, cross talk between autophagy and apoptosis is known to exist, and this cross talk may convert a potentially protective mechanism (e.g., autophagy) under hyperoxia to a contributor of sensitivity toward acute lung injury in WT mouse.
Nachman-Clewner et al. (42) have examined effects of hyperoxia on extrapulmonary tissues for longer periods of time. Similar to what is observed in our model, they noted slight protection in MT-null mice with respect to degeneration of central retinal photoreceptors. These authors did not pursue zinc partitioning during intermittent (3×/wk) exposure (3 h) to 3 atmospheres of 100% oxygen for up to 5 wk. Nonetheless, it is apparent that the role of MT in hyperoxic injury in intact animals, including target (lung, retina) and nontarget (liver), is complex. The observed important differences between cell culture and intact animals (49), considerations about compensatory and/or coexisting pathways, and interactions of MT and essential metals may preclude definitive conclusions on mechanisms and pathways involved in MT as an antioxidant.
SOD, MT, and zinc in hyperoxic acute lung injury.
In light of these latter possibilities and in the case of hyperoxic lung injury, classical oxidative stress model, various forms of SODs that are 1) important in response to hyperoxia (62, 63) and 2) zinc dependent (CuZn- and EC-SOD) may be indirectly or directly affected by alterations in intrapulmonary zinc homeostasis (8, 16, 51, 58). Zinc-dependent SOD activity was decreased after 72 h hyperoxia (Fig. 6) in WT but not MT-null mice. There were no significant differences between strains nor effects of hyperoxia on Mn-SOD. Additional studies were performed to evaluate the effects of hyperoxia and zinc on EC-SOD and CuZn-SOD activities. EC-SOD expression and activity (Figs. 6, 7) were significantly decreased at 72 h of hyperoxia in WT and MT-null mice consistent with previous studies (44). Overexpression of EC-SOD (2, 12) or aerosolized administration of recombinant EC-SOD protein (69) protects mouse lungs against hyperoxia, conversely targeted or inducible ablation of EC-SOD sensitizes mice to hyperoxia (4). Nonetheless, observation that EC-SOD was decreased in both sensitive and resistant strains of mice suggests that EC-SOD per se is sensitive to hyperoxic oxidative damage but is not sufficient to account for the observed phenotypic differences. CuZn-SOD activity was much more abundant than EC-SOD activity in both WT and MT-null mice and importantly there was a 3–4× increase in CuZn-SOD activity in both normoxia and hyperoxia in MT-null mice vs. WT mice (Fig. 7), whereas CuZn-SOD activity was not affected by hyperoxia in either strain. The difference in baseline CuZn-SOD activity between the two strains suggests that MT-null mice compensate, in part, by an elevation in this enzyme. Interestingly, Ghoshal et al. (14) noted compensatory increases in MT-I and -II expression in liver of CuZn-SOD-null mice. The ability of MT to substitute for CuZn-SOD in yeast deleted in the latter underscores the ability of these two proteins to functionally compensate for each other (55). Previous reports using cells from different strains of mice in which MT was genetically ablated did not show baseline differences of this magnitude (27), suggesting important contributions of genetic strain differences in CuZn-SOD activity and its impact on response to hyperoxia. We (33) and others (25) have examined the subtleties of metal ion deliver to CuZn-SOD and apo-SOD and MT can indeed deliver metals (Cu or zinc) to increase activity of CuZn-SOD or apo-CuZn-SOD, in vitro. It is tempting to attribute the resistance of MT-null mice to hyperoxia due to a compensatory increase in CuZn-SOD. However, forced overexpression of CuZn-SOD had very little protective effect in mature mice at normal altitude (66). Thus an alternative explanation for elevated CuZn-SOD activity is that without MT-dependent partitioning of zinc to liver in hyperoxia (Figs. 1 and 2) there is an increased availability of zinc to maintain its activity (52) or that zinc per se (or other zinc-dependent pathways) contributed to hyperoxia-resistant phenotype of MT-null mice.
Hyperoxia and liver injury.
The literature suggests lung-liver communication under oxidative stress. Hyperoxia increases liver injury initiated by agents undergoing oxidative biotransformation and decreases injury due to galactosamine, LPS, and others undergoing reduction (37). In addition to reactive oxygen species, hyperoxia increased liver 5-lipoxygenase and cyclooxygenase-2 levels in newborn mice after 14 days, possibly playing a role in lipid mediators of acute lung injury (53). Liver redox state, glutathione reductase, and cytochrome P-450 1A1/1A2 during hyperoxia modulates lung injury. Under hyperoxic conditions, hepatic cytochrome P-450 1A1/1A2 peak at 48 h and decrease back to baseline by 60 h (41, 68). The decline by 60 h could be due to downregulation or degradation of these enzymes. In another model of reactive oxygen species (ROS)-mediated alcoholic liver injury, murine liver injury was prevented with zinc supplementation in both WT and MT-null mice through inhibition of accumulation of ROS and restore alveolar epithelial and macrophage function (21). Although the above studies suggest a link between lungs and liver under hyperoxic condition and a favorable role for zinc in the prevention of liver injury due to various stressors, the models usually took weeks to months to establish compared with our model of days. This may explain the lack of liver injury in our acute experiments. In this regard, the lack of changes in liver enzymes during hyperoxia in either genotype are reminiscent of the lack of histological changes of either inflammation, fibrosis, or cholestasis in livers of newborn mice at 72 h of hyperoxia (53).
In conclusion, this study shows that zinc partitioning from lung to liver during hyperoxia is dependent on MT. In contrast to WT mice, MT-null mice increase intrapulmonary zinc levels during hyperoxia, and this is associated with a resistant phenotype to hyperoxia. The protective mechanisms are unclear but may involve either compensated elevations in CuZn-SOD in MT-null mice or activation of other zinc-dependent pathways that inhibit early aspects of hyperoxic acute lung injury. Recently, several groups have noted that zinc deficiency sensitizes experimental animals to acute lung injury (21, 24, 59). Although these models all involved carefully calibrated zinc deficiencies (and successful reversal with zinc repletion), it does open the possibility that zinc supplementation can be rationally used as a therapeutic modality for acute lung injury.
GRANTS
This work was supported by National Institute of Environmental Health Sciences Grant RO1ES016000 (C. L. Fattman), Parker B. Francis Foundation and the Children's Hospital of Pittsburgh Research Advisory Committee Start Up Funds (J. F. Alcorn), National Institute of Health Grants KL2-TR-000146-07 (O. Peck-Palmer), HL-65697 (B. R. Pitt) and K08-HL-086671 (A. M. Kaynar).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.M.L., J.N.M., D.R.F., L.Z., K.J.W., I.K., L.L.P., J.P., J.L., J.D.L., O.M.P.P., D.B.S., C.L.F., T.D.O., and A.M.K. performed experiments; S.M.L., J.N.M., D.R.F., L.Z., K.T., K.J.W., I.K., L.L.P., J.P., J.L., J.D.L., O.M.P.P., D.B.S., C.L.F., T.D.O., and A.M.K. analyzed data; S.M.L., J.N.M., D.R.F., L.Z., K.T., K.J.W., I.K., J.P., J.D.L., O.M.P.P., D.B.S., C.L.F., J.F.A., T.D.O., D.C.A., B.R.P., and A.M.K. interpreted results of experiments; S.M.L., J.F.A., D.C.A., B.R.P., and A.M.K. drafted manuscript; S.M.L., K.J.W., C.L.F., J.F.A., T.D.O., D.C.A., B.R.P., and A.M.K. edited and revised manuscript; K.J.W. and A.M.K. prepared figures; A.M.K. conception and design of research; A.M.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Megan Lang from the Center for Biological Imaging at the University of Pittsburgh for technical assistance with imaging studies and to Dr. Steven McGeehan from University of Idaho for tissue zinc measurements.
REFERENCES
- 1.Abel J, de Ruiter N. Inhibition of hydroxyl-radical-generated DNA degradation by metallothionein. Toxicol Lett 47: 191–196, 1989 [DOI] [PubMed] [Google Scholar]
- 2.Auten RL, O'Reilly MA, Oury TD, Nozik-Grayck E, Whorton MH. Transgenic extracellular superoxide dismutase protects postnatal alveolar epithelial proliferation and development during hyperoxia. Am J Physiol Lung Cell Mol Physiol 290: L32–L40, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bates JH, Irvin CG. Time dependence of recruitment and derecruitment in the lung: a theoretical model. J Appl Physiol 93: 705–713, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Carlsson LM, Jonsson J, Edlund T, Marklund SL. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc Natl Acad Sci USA 92: 6264–6268, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. J Biol Chem 281: 24085–24089, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Coyle P, Philcox JC, Rofe AM. Hepatic zinc in metallothionein-null mice following zinc challenge: in vivo and in vitro studies. Biochem J 309: 25–31, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crapo JD, McCord JM, Fridovich I. Preparation and assay of superoxide dismutases. Methods Enzymol 53: 382–393, 1978 [DOI] [PubMed] [Google Scholar]
- 8.Estevez AG, Crow JP, Sampson JB, Reiter C, Zhuang Y, Richardson GJ, Tarpey MM, Barbeito L, Beckman JS. Induction of nitric oxide-dependent apoptosis in motor neurons by zinc-deficient superoxide dismutase. Science 286: 2498–2500, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Fattman CL, Chang LY, Termin TA, Petersen L, Enghild JJ, Oury TD. Enhanced bleomycin-induced pulmonary damage in mice lacking extracellular superoxide dismutase. Free Radic Biol Med 35: 763–771, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Fattman CL, Chu CT, Kulich SM, Enghild JJ, Oury TD. Altered expression of extracellular superoxide dismutase in mouse lung after bleomycin treatment. Free Radic Biol Med 31: 1198–1207, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Fattman CL, Enghild JJ, Crapo JD, Schaefer LM, Valnickova Z, Oury TD. Purification and characterization of extracellular superoxide dismutase in mouse lung. Biochem Biophys Res Commun 275: 542–548, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Folz RJ, Abushamaa AM, Suliman HB. Extracellular superoxide dismutase in the airways of transgenic mice reduces inflammation and attenuates lung toxicity following hyperoxia. J Clin Invest 103: 1055–1066, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukada T, Yamasaki S, Nishida K, Murakami M, Hirano T. Zinc homeostasis and signaling in health and diseases: Zinc signaling. J Biol Inorg Chem 16: 1123–1134, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghoshal K, Majumder S, Li Z, Bray TM, Jacob ST. Transcriptional induction of metallothionein-I and -II genes in the livers of Cu,Zn-superoxide dismutase knockout mice. Biochem Biophys Res Commun 264: 735–742, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Hart BA, Voss GW, Shatos MA, Doherty J. Cross-tolerance to hyperoxia following cadmium aerosol pretreatment. Toxicol Appl Pharmacol 103: 255–270, 1990 [DOI] [PubMed] [Google Scholar]
- 16.Hartman JR, Geller T, Yavin Z, Bartfeld D, Kanner D, Aviv H, Gorecki M. High-level expression of enzymatically active human Cu/Zn superoxide dismutase in Escherichia coli. Proc Natl Acad Sci USA 83: 7142–7146, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoue K, Takano H, Kaewamatawong T, Shimada A, Suzuki J, Yanagisawa R, Tasaka S, Ishizaka A, Satoh M. Role of metallothionein in lung inflammation induced by ozone exposure in mice. Free Radic Biol Med 45: 1714–1722, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Jain D, Atochina-Vasserman EN, Tomer Y, Kadire H, Beers MF. Surfactant protein D protects against acute hyperoxic lung injury. Am J Respir Crit Care Med 178: 805–813, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnston CJ, Oberdorster G, Finkelstein JN. Recovery from oxidant-mediated lung injury: response of metallothionein, MIP-2, and MCP-1 to nitrogen dioxide, oxygen, and ozone exposures. Inhal Toxicol 13: 689–702, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Joshi PC, Guidot DM. HIV-1 transgene expression in rats induces differential expression of tumor necrosis factor alpha and zinc transporters in the liver and the lung. AIDS Res Ther 8: 36, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joshi PC, Mehta A, Jabber WS, Fan X, Guidot DM. Zinc deficiency mediates alcohol-induced alveolar epithelial and macrophage dysfunction in rats. Am J Respir Cell Mol Biol 41: 207–216, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaynar AM, Houghton AM, Lum EH, Pitt BR, Shapiro SD. Neutrophil elastase is needed for neutrophil emigration into lungs in ventilator-induced lung injury. Am J Respir Cell Mol Biol 39: 53–60, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klaassen CD, Liu J, Choudhuri S. Metallothionein: an intracellular protein to protect against cadmium toxicity. Annu Rev Pharmacol Toxicol 39: 267–294, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Knoell DL, Julian MW, Bao S, Besecker B, Macre JE, Leikauf GD, DiSilvestro RA, Crouser ED. Zinc deficiency increases organ damage and mortality in a murine model of polymicrobial sepsis. Crit Care Med 37: 1380–1388, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koh M, Kim H. The effect of metallothionein on the activity of enzymes involved in removal of reactive oxygen species. Bull Korean Chem Soc 22: 362–366, 2001 [Google Scholar]
- 26.Krebs NF. Overview of zinc absorption and excretion in the human gastrointestinal tract. J Nutr 130: 1374S–1377S, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Lazo JS, Kondo Y, Dellapiazza D, Michalska AE, Choo KH, Pitt BR. Enhanced sensitivity to oxidative stress in cultured embryonic cells from transgenic mice deficient in metallothionein I and II genes. J Biol Chem 270: 5506–5510, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Levy MA, Tsai YH, Reaume A, Bray TM. Cellular response of antioxidant metalloproteins in Cu/Zn SOD transgenic mice exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol 281: L172–L182, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Li H, Cao R, Wasserloos KJ, Bernal P, Liu ZQ, Pitt BR, St Croix CM. Nitric oxide and zinc homeostasis in pulmonary endothelium. Ann NY Acad Sci 1203: 73–78, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Lian X, Qin Y, Hossain SA, Yang L, White A, Xu H, Shipley JM, Li T, Senior RM, Du H, Yan C. Overexpression of Stat3C in pulmonary epithelium protects against hyperoxic lung injury. J Immunol 174: 7250–7256, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiological regulation. Annu Rev Nutr 29: 153–176, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Lichten LA, Liuzzi JP, Cousins RJ. Interleukin-1β contributes via nitric oxide to the upregulation and functional activity of the zinc transporter Zip14 (Slc39a14) in murine hepatocytes. Am J Physiol Gastrointest Liver Physiol 296: G860–G867, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu SX, Fabisiak JP, Tyurin VA, Borisenko GG, Pitt BR, Lazo JS, Kagan VE. Reconstitution of apo-superoxide dismutase by nitric oxide-induced copper transfer from metallothioneins. Chem Res Toxicol 13: 922–931, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Liuzzi JP, Lichten LA, Rivera S, Blanchard RK, Aydemir TB, Knutson MD, Ganz T, Cousins RJ. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci USA 102: 6843–6848, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maret W, Li Y. Coordination dynamics of zinc in proteins. Chem Rev 109: 4682–4707, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Marklund SL. Analysis of extracellular superoxide dismutase in tissue homogenates and extracellular fluids. Methods Enzymol 186: 260–265, 1990 [DOI] [PubMed] [Google Scholar]
- 37.Marzella L, Muhvich K, Myers RA. Effect of hyperoxia on liver necrosis induced by hepatotoxins. Virchows Arch B Cell Pathol Incl Mol Pathol 51: 497–507, 1986 [DOI] [PubMed] [Google Scholar]
- 38.Masters BA, Kelly EJ, Quaife CJ, Brinster RL, Palmiter RD. Targeted disruption of metallothionein I and II genes increases sensitivity to cadmium. Proc Natl Acad Sci USA 91: 584–588, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michalska AE, Choo KH. Targeting and germ-line transmission of a null mutation at the metallothionein I and II loci in mouse. Proc Natl Acad Sci USA 90: 8088–8092, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy 3: 542–545, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Moorthy B, Nguyen UT, Gupta S, Stewart KD, Welty SE, Smith CV. Induction and decline of hepatic cytochromes P4501A1 and 1A2 in rats exposed to hyperoxia are not paralleled by changes in glutathione S-transferase-alpha. Toxicol Lett 90: 67–75, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Nachman-Clewner M, Giblin FJ, Dorey CK, Blanks RH, Dang L, Dougherty CJ, Blanks JC. Selective degeneration of central photoreceptors after hyperbaric oxygen in normal and metallothionein-knockout mice. Invest Ophthalmol Vis Sci 49: 3207–3215, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oury TD, Ho YS, Piantadosi CA, Crapo JD. Extracellular superoxide dismutase, nitric oxide, and central nervous system O2 toxicity. Proc Natl Acad Sci USA 89: 9715–9719, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oury TD, Schaefer LM, Fattman CL, Choi A, Weck KE, Watkins SC. Depletion of pulmonary EC-SOD after exposure to hyperoxia. Am J Physiol Lung Cell Mol Physiol 283: L777–L784, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Panayi AE, Spyrou NM, Ubertalli LC, White MA, Part P. Determination of trace elements in porcine brain by inductively coupled plasma-mass spectrometry, electrothermal atomic absorption spectrometry, and instrumental neutron activation analysis. Biol Trace Elem Res 71–72: 529–540, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Perkowski S, Sun J, Singhal S, Santiago J, Leikauf GD, Albelda SM. Gene expression profiling of the early pulmonary response to hyperoxia in mice. Am J Respir Cell Mol Biol 28: 682–696, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Philcox JC, Coyle P, Michalska A, Choo KH, Rofe AM. Endotoxin-induced inflammation does not cause hepatic zinc accumulation in mice lacking metallothionein gene expression. Biochem J 308: 543–546, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piedboeuf B, Johnston CJ, Watkins RH, Hudak BB, Lazo JS, Cherian MG, Horowitz S. Increased expression of tissue inhibitor of metalloproteinases (TIMP-I) and metallothionein in murine lungs after hyperoxic exposure. Am J Respir Cell Mol Biol 10: 123–132, 1994 [DOI] [PubMed] [Google Scholar]
- 49.Pitt BR, Schwarz M, Woo ES, Yee E, Wasserloos K, Tran S, Weng W, Mannix RJ, Watkins SA, Tyurina YY, Tyurin VA, Kagan VE, Lazo JS. Overexpression of metallothionein decreases sensitivity of pulmonary endothelial cells to oxidant injury. Am J Physiol Lung Cell Mol Physiol 273: L856–L865, 1997 [DOI] [PubMed] [Google Scholar]
- 50.Prows DR, Hafertepen AP, Gibbons WJ, Jr, Winterberg AV, Nick TG. A genetic mouse model to investigate hyperoxic acute lung injury survival. Physiol Genomics 30: 262–270, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Roberts BR, Tainer JA, Getzoff ED, Malencik DA, Anderson SR, Bomben VC, Meyers KR, Karplus PA, Beckman JS. Structural characterization of zinc-deficient human superoxide dismutase and implications for ALS. J Mol Biol 373: 877–890, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roe JA, Peoples R, Scholler DM, Valentine JS. Silver-binding properties of bovine cuprozinc superoxide-dismutase and the overall stability of selected metal-ion derivatives. J Am Chem Soc 112: 1538–1545, 1990 [Google Scholar]
- 53.Rogers LK, Tipple TE, Britt RD, Welty SE. Hyperoxia exposure alters hepatic eicosanoid metabolism in newborn mice. Pediatr Res 67: 144–149, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takano H, Inoue K, Yanagisawa R, Sato M, Shimada A, Morita T, Sawada M, Nakamura K, Sanbongi C, Yoshikawa T. Protective role of metallothionein in acute lung injury induced by bacterial endotoxin. Thorax 59: 1057–1062, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tamai KT, Gralla EB, Ellerby LM, Valentine JS, Thiele DJ. Yeast and mammalian metallothioneins functionally substitute for yeast copper-zinc superoxide dismutase. Proc Natl Acad Sci USA 90: 8013–8017, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tan RJ, Fattman CL, Watkins SC, Oury TD. Redistribution of pulmonary EC-SOD after exposure to asbestos. J Appl Physiol 97: 2006–2013, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Tanaka A, Jin Y, Lee SJ, Zhang M, Kim HP, Stolz DB, Ryter SW, Choi AM. Hyperoxia-induced LC3B interacts with the Fas apoptotic pathway in epithelial cell death. Am J Respir Cell Mol Biol 46: 507–514, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor CG, Bettger WJ, Bray TM. Effect of dietary zinc or copper deficiency on the primary free radical defense system in rats. J Nutr 118: 613–621, 1988 [DOI] [PubMed] [Google Scholar]
- 59.Taylor CG, Bray TM. Effect of hyperoxia on oxygen free radical defense enzymes in the lung of zinc-deficient rats. J Nutr 121: 460–466, 1991 [DOI] [PubMed] [Google Scholar]
- 60.Thambiayya K, Wasserloos KJ, Kagan VE, Stoyanovsky D, Pitt BR. A critical role for increased labile zinc in reducing sensitivity of cultured sheep pulmonary artery endothelial cells to LPS-induced apoptosis. Am J Physiol Lung Cell Mol Physiol 302: (12) L1287–L1295, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thornalley PJ, Vasak M. Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim Biophys Acta 827: 36–44, 1985 [DOI] [PubMed] [Google Scholar]
- 62.Tsan MF. Superoxide dismutase and pulmonary oxygen toxicity. Proc Soc Exp Biol Med 214: 107–113, 1997 [DOI] [PubMed] [Google Scholar]
- 63.Tsan MF, Tacy NJ, Lindau-Shepard BA, White JE. Protection of rats against oxygen toxicity by tracheal administration of plasmid DNA: role of endogenous tumor necrosis factor. Proc Assoc Am Physicians 109: 409–419, 1997 [PubMed] [Google Scholar]
- 64.Veness-Meehan KA, Cheng ER, Mercier CE, Blixt SL, Johnston CJ, Watkins RH, Horowitz S. Cell-specific alterations in expression of hyperoxia-induced mRNAs of lung. Am J Respir Cell Mol Biol 5: 516–521, 1991 [DOI] [PubMed] [Google Scholar]
- 65.Wesselkamper SC, McDowell SA, Medvedovic M, Dalton TP, Deshmukh HS, Sartor MA, Case LM, Henning LN, Borchers MT, Tomlinson CR, Prows DR, Leikauf GD. The role of metallothionein in the pathogenesis of acute lung injury. Am J Respir Cell Mol Biol 34: 73–82, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.White CW, Avraham KB, Shanley PF, Groner Y. Transgenic mice with expression of elevated levels of copper-zinc superoxide dismutase in the lungs are resistant to pulmonary oxygen toxicity. J Clin Invest 87: 2162–2168, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wikenheiser KA, Wert SE, Wispe JR, Stahlman M, D'Amore-Bruno M, Singh G, Katyal SL, Whitsett JA. Distinct effects of oxygen on surfactant protein B expression in bronchiolar and alveolar epithelium. Am J Physiol Lung Cell Mol Physiol 262: L32–L39, 1992 [DOI] [PubMed] [Google Scholar]
- 68.Wong YL, Smith CV, McMicken HW, Rogers LK, Welty SE. Mitochondrial thiol status in the liver is altered by exposure to hyperoxia. Toxicol Lett 123: 179–193, 2001 [DOI] [PubMed] [Google Scholar]
- 69.Yen CC, Lai YW, Chen HL, Lai CW, Lin CY, Chen W, Kuan YP, Hsu WH, Chen CM. Aerosolized human extracellular superoxide dismutase prevents hyperoxia-induced lung injury. PLoS One 6: e26870, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zangger K, Oz G, Haslinger E, Kunert O, Armitage IM. Nitric oxide selectively releases metals from the amino-terminal domain of metallothioneins: potential role at inflammatory sites. FASEB J 15: 1303–1305, 2001 [DOI] [PubMed] [Google Scholar]
- 71.Zhu Y, Natoli R, Valter K, Stone J. Differential gene expression in mouse retina related to regional differences in vulnerability to hyperoxia. Mol Vis 16: 740–755, 2010 [PMC free article] [PubMed] [Google Scholar]