Abstract
Although neutrophil extracellular traps (NETs) form to prevent dissemination of pathogenic microorganisms, excessive release of DNA and DNA-associated proteins can also perpetuate sterile inflammation. In this study, we found that the danger-associated molecular pattern protein high-mobility group box 1 (HMGB1) can induce NET formation. NET formation was found after exposure of wild-type and receptor for advanced glycation end products-deficient neutrophil to HMGB1, whereas deficiency of Toll-like receptor (TLR)4 diminished the ability of neutrophils to produce NETs. Incubation of neutrophils with HMGB1 significantly increased the amount of DNA and histone 3 released as well as intracellular histone 3 citrullination, a signaling event that precedes chromatin decondensation. In vivo, neutrophils isolated from bronchoalveolar lavages of mice exposed to LPS and HMGB1 showed consistently greater ability to produce NETs compared with pulmonary neutrophils from mice that received LPS alone. In contrast, mice treated with LPS and neutralizing antibody to HMGB1 had decreased amounts of the inflammatory cytokines TNF-α and macrophage inflammatory protein 2, as well as of free DNA and histone 3 in bronchoalveolar lavage fluids. Airway neutrophils from LPS-exposed mice that had been treated with anti-HMGB1 antibodies showed decreased citrullination of histone 3. These results demonstrate that interactions between HMGB1 and TLR4 enhance the formation of NETs and provide a novel mechanism through which HMGB1 may contribute to the severity of neutrophil-associated inflammatory conditions.
Keywords: inflammation, lipopolysaccharide
neutrophil activation, associated with production of antibacterial peptides, reactive oxygen intermediates, cytokines, and other inflammatory mediators, as well as release of DNA into the extracellular milieu, plays a central role in innate host defense and modulation of inflammation. Neutrophil extracellular traps (NETs) are produced through the release of DNA by neutrophils (8, 10, 25) and have been shown to ensnare invading bacteria within capillary beds, thereby preventing microbial dissemination (8, 10, 19, 36). In addition to DNA, NETs contain additional proteins that may facilitate microbial eradication, including histones, elastase, and myeloperoxidase (26, 32, 33, 46). NETs are formed in response to proinflammatory stimuli, including LPS, IL-8, and TNF-α, as well as through enhanced reactive oxygen species (ROS) generation by NADPH oxidase (8, 10, 33, 34, 36).
Although NETs play important roles in host defense through trapping bacteria or other pathogens, extensive formation of NETs with increased amounts of extracellular DNA can contribute to perpetuation of sterile inflammation and tissue damage. For example, NETs were implicated in vascular dysfunction during sepsis (11, 19, 35). Recent studies suggest that extensive NET formation contributes to autoimmune diseases, such as systemic lupus erythematosus and lupus nephritis (11, 16).
Although high-mobility group box 1 protein (HMGB1) was originally described as a nuclear nonhistone DNA-binding protein (1), it has subsequently been shown that HMGB1 can be released from dying cells as well as by activated macrophages and other cellular populations (9, 43, 44). Plasma and tissue levels of HMGB1 are elevated in acute inflammatory conditions, such as during severe infection, burns, hemorrhage, and lung injury (1, 4, 20). Increased circulating concentrations of HMGB1 are also associated with chronic inflammatory disorders, including rheumatoid arthritis and systemic lupus erythematosus. HMGB1 participates in enhancing inflammatory reactions by potentiating the activity of proinflammatory mediators, such as LPS and cytokines, as well as by diminishing phagocytosis of apoptotic cells (15, 37, 39). Therapies directed against HMGB1 have been shown to be beneficial in neutrophil-associated inflammatory conditions, including acute lung injury, sepsis, and ischemia-reperfusion-induced tissue injury (20, 31, 40).
In the present experiments, we investigated the potential role of HMGB1 in contributing to NET formation and found that HMGB1 can induce NETs both in vitro as well as in vivo through a Toll-like receptor (TLR)4-dependent mechanism. These findings highlight a novel pathway by which HMGB1 may contribute to tissue damage and organ dysfunction mediated by neutrophils during acute inflammatory processes.
MATERIALS AND METHODS
Mice.
TLR4-deficient mice (C57BL/10ScNJ Tlr4lps−del), as well as control wild-type mice (C57BL/10ScSnJ), were purchased from The Jackson Laboratory (Bar Harbor, ME). C57BL/6 mice deficient in receptor for advanced glycation end products (RAGE) (RAGE−/−) were a gift from Dr. A. Bierhaus (University of Heidelberg, Heidelberg, Germany) (23). Male C57BL/6 mice and knockout mice, 8–12 wk old, were used for experiments. Mice were housed and studied at the University of Alabama at Birmingham, using protocols approved by the Institutional Animal Care and Use Committee.
Reagents.
RPMI 1640 was purchased from BioWhittaker (Walkersville, MD). FBS and penicillin-streptomycin were obtained from Gemini Bioproducts (Calabasas, CA). Custom antibody mixtures (Abs) and negative selection columns for neutrophil isolation were from StemCell Technologies (Vancouver, British Columbia, Canada). Recombinant HMGB1 and mutant HMGB1 lacking the COOH-terminal tail (ΔC-HMGB1) were prepared as previously described (3, 42). Polyclonal antibodies to neutralize HMGB1 were prepared as previously described (12). Mouse monoclonal antibody to histone 3-FITC was obtained from Abcam (Cambridge, MA). Alexa Fluor 488, Alexa Fluor 555-labeled secondary antibodies, and Sytox Green probe were purchased from Invitrogen (Carlsbad, CA). Phorbol myristate acetate (PMA), diphenyleneiodonium (DPI), Escherichia coli 0111:B4 endotoxin (LPS), IgG, and DNAse I were purchased from Sigma-Aldrich (St. Louis, MO). The Chromogenic LAL kit was obtained from Pierce Biotechnology (Rockford, IL).
Isolation of neutrophils.
Bone marrow neutrophils were purified using a negative selection column purification system, as previously described (42, 50). Briefly, bone marrow cell suspensions were isolated from the femur and tibia of a mouse by flushing with RPMI 1640 medium with 5% FBS. The cell suspension was passed through a glass wool column and collected by subsequent washing with PBS containing 5% FBS. Negative selection to purify neutrophils was performed by incubation of the cell suspension with biotinylated primary antibodies specific for the cell surface markers F4/80, CD4, CD45R, CD5, and TER119 (StemCell Technologies) for 15 min at 4°C followed by incubation with antibiotin tetrameric antibodies (100 μl, StemCell Technologies) for 15 min. The complex of antitetrameric antibodies and cells was then incubated with colloidal magnetic dextran iron particles (60 μl, StemCell Technologies) for an additional 15 min at 4°C. The T cells, B cells, red blood cells, monocytes, and macrophages were captured in a column surrounded by a magnet, allowing the neutrophils to pass through. Neutrophil purity, as determined by Wright-Giemsa-stained cytospin preparations, was consistently greater than 98. Viability of purified bone marrow neutrophils was determined after Trypan blue staining and was consistently greater than 95%. Human peripheral neutrophils were purified using a negative selection purification system using Stem Cells Purification Kit (StemCell Technologies). Neutrophil purity was greater than 96% (Wright-Giemsa-staining) with 98% cell viability (Trypan blue).
Quantification of DNA release from neutrophils.
To follow NET formation, fresh mice neutrophils (2 × 105 cells) were seeded in Costar 96-well black plates (Corning, MA) in the presence of 0.5% fetal bovine serum and Sytox Green (5 μM), a non-cell-permeant DNA binding dye. The cells were incubated with PMA, DPI, LPS, or HMGB1 for indicated time at 37°C, and then released DNA was quantified by reading Sytox Green fluorescence at various time points. In selected experiments, cells were treated with DNAse I (200 U/ml). Sytox Green fluorescence was measured using microplate fluorescence reader (Fluostar OPTIMA spectrophotometer; BMG LABTECH Microplate Readers, Alexandria, VA), at an excitation wavelength of 492 nm and an emission wavelength of 530 nm.
Immunostaining and confocal microscopy.
Neutrophils were cultured on poly-L-lysine-coated glass coverslips and treated as described in figure legends. Cells were then washed with PBS and incubated with paraformaldehyde (4%) for 30 min at room temperature. Cells were then incubated with PBS/BSA (3%) for 30 min at room temperature, followed by inclusion of primary mouse monoclonal antihistone 3 antibody labeled with FITC. After 30 min, cells were washed with PBS, and samples were mounted with emulsion oil solution containing DAPI to visualize nuclear and released DNA. Confocal microscopy was performed as previously described (2) using a confocal laser-scanning microscope (model LSM 710 confocal microscope; Carl Zeiss MicroImaging, Jena, Germany) provided by the High Resolution Imaging Facility at the University of Alabama at Birmingham.
Western blot analysis.
Western blot analysis was performed as previously described (43). Briefly, cell lysates of neutrophils (3.5 × 106/ well) were prepared using lysis buffer containing Tris pH 7.4 (50 mM), NaCl (150 mM), NP-40 (0.5%, vol/vol), EDTA (1 mM), EGTA (1 mM), okadaic acid (1 nM), and protease inhibitors. Lysis buffer was also used to prepare lung homogenates, as described previously (43). Cell lysates or lung homogenates were sonicated and then centrifuged to remove insoluble material at 10,000 g for 15 min at 4°C. Protein concentration in the supernatants was determined using the Bradford reagent (Bio-Rad, Hercules, CA) with BSA as a standard. Samples were mixed with Laemmli sample buffer and boiled for 5 min. Equal amounts of proteins (50 μg/sample) were resolved by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred onto PVDF membranes (Immobilon P; Millipore, Billerica, MA). The membranes were probed with specific antibodies as described in the figure legends followed by detection with horseradish peroxidase-conjugated goat anti-rabbit IgG. Bands were visualized by enhanced chemiluminescence (Super Signal; Pierce Biotechnology) and quantified by AlphaEaseFC software (Alpha Innotech, San Leandro, CA). Each experiment was carried out two or more times using cell populations obtained from separate groups of mice. Of note, to determine amount of released histone 3, cells were incubated with 4 U of DNase I for 30 min before medium collection and Western blot analysis.
Acute lung injury model.
Acute lung injury was induced by intratracheal administration of 1 mg/kg LPS in 50 μl of PBS as previously described (50). With this model, ALI is characterized by neutrophil infiltration into the lung interstitium and airways, development of interstitial edema, and increased pulmonary proinflammatory cytokine production, with the greatest degree of injury being present 24 h after LPS exposure (50, 52). Briefly, mice were anesthetized with isofluorane and then suspended by their upper incisors on a 60° incline board. The tongue was gently extended and LPS or PBS solution deposited into the pharynx (7, 14, 43, 53). In selected experiments, neutralizing antibody to HMGB1 (125 μg in 500 μl PBS) or control antibody was injected (i.p.) to mice 2 h before LPS instillation and 12 h after. Lungs were harvested 24 h after LPS administration and homogenates prepared using buffer containing Tris pH 7.4 (50 mM), NaCl (150 mM), NP-40 (0.5%, vol/vol), EDTA (1 mM), EGTA (1 mM), okadaic acid (1 nM), and protease inhibitors. Lung homogenates were then sonicated on ice for 90 s and centrifuged (16,000 g for 10 min at 4°C), and protein concentration was determined using the Bradford assay. In selected experiments, bronchoalveolar lavages (BALs) (30 μl) were mixed with Laemmli sample buffer and boiled for 5 min followed by Western blot analysis for histone 3 or HMGB1.
Cytokine ELISA.
ELISA were used to measure cytokines in BAL fluid. Levels of TNF-α and macrophage inflammatory protein (MIP)-2 were determined using commercially available ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions and as previously described (51, 54).
Statistical analysis.
Statistical significance was determined by the Wilcoxon rank sum test (independent 2- group Mann-Whitney U-test) as well as Student's t-test for comparisons between two groups. Multigroup comparisons were performed using one-way ANOVA with the Tukey's post hoc test. A value of P < 0.05 was considered significant. Analyses were performed on SPSS version 16.0 for Windows.
RESULTS
HMGB1 induces the formation of neutrophil extracellular traps.
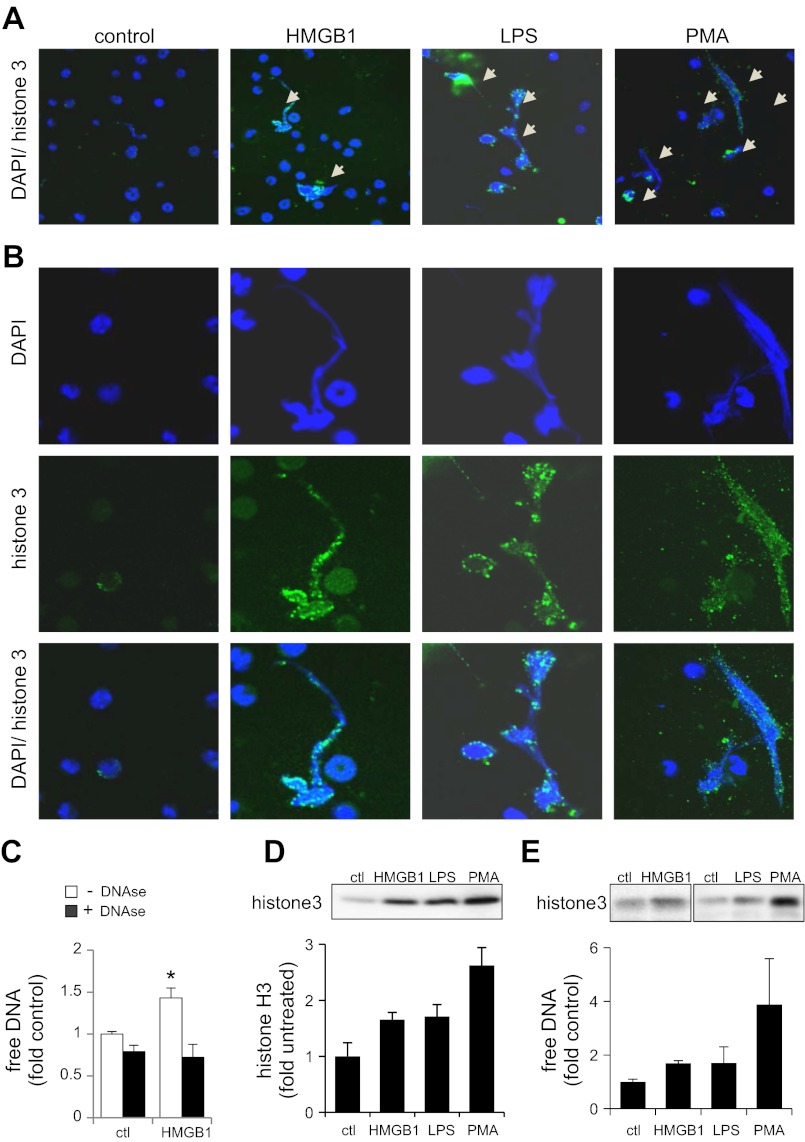
Although HMGB1 has been shown to contribute to neutrophil activation, including the production of proinflammatory cytokines (39), a role for HMGB1 in the formation of neutrophil extracellular traps has not been described. To address this question, neutrophils cultured on Poly-D-Lysine-coated glass coverslips were treated with or without HMGB1, LPS, or PMA and then were stained for DNA with DAPI. Figure 1, A and B, shows a modest increase in extracellular DNA staining after exposure of neutrophils to HMGB1, LPS, and PMA. Confocal microscopy also demonstrated that culture of neutrophils with HMGB1 increased staining for histone 3, a finding previously described in neutrophils after DNA release (27). The ability of HMGB1 to induce NETs was further confirmed by measurement of free DNA in culture medium using the Sytox Green fluorogenic probe as well as Western blot analysis of histone 3 (Fig. 1, C–E). Results obtained from these experiments showed that exposure to LPS or HMGB1 induced increases in free DNA and histone H3 in culture medium from mouse bone marrow or human peripheral neutrophil. Of note, Sytox Green is a cell-impermeable compound and becomes fluorescent only when it interacts directly with DNA. In addition, inclusion of DNase in the neutrophil cultures diminished fluorescence of Sytox Green after cellular exposure to HMGB1 (Fig. 1C).
Fig. 1.
High-mobility group box 1 (HMGB1) induces neutrophil extracellular trap (NET) formation. Bone marrow neutrophils were incubated in 0.5% serum containing HMGB1 (0 or 300 ng/ml), LPS (100 ng/ml), or phorbol myristate acetate (PMA) (10 nM) for 3 h. A: representative images show merged neutrophil DNA (blue) and histone H3 (green) staining. Arrows show NET formation. B: image magnifications obtained from A with indication of NETs and extracellular histone H3. C: neutrophils were treated with HMGB1 (0 or 300 ng/ml) for 3 h followed by inclusion of DNAse (0 or 200 U/ml) for 30 min. Free DNA was then measured using Sytox Green probe. Means ± SD were obtained from 3 independent experiments. D: neutrophils were incubated with HMGB1 (0 or 300 ng/ml), LPS (100 ng/ml), or PMA (10 ng/ml) for 3 h followed by inclusion of DNAse (0 or 200 U/ml) in the cultures for additional 30 min. The culture supernatant was then collected for analysis by Western blot with antibodies to histone H3. Representative Western blots and means ± SD of band optical densitometry obtained from 2 independent experiments are shown. E: human neutrophils were treated as indicated in C followed by measurement of free DNA and Western blot analysis with antihistone 3 antibody. Means ± SD obtained from 2 experiments are shown. *P < 0.05
HMGB1-dependent engagement of TLR4 induces NET formation.
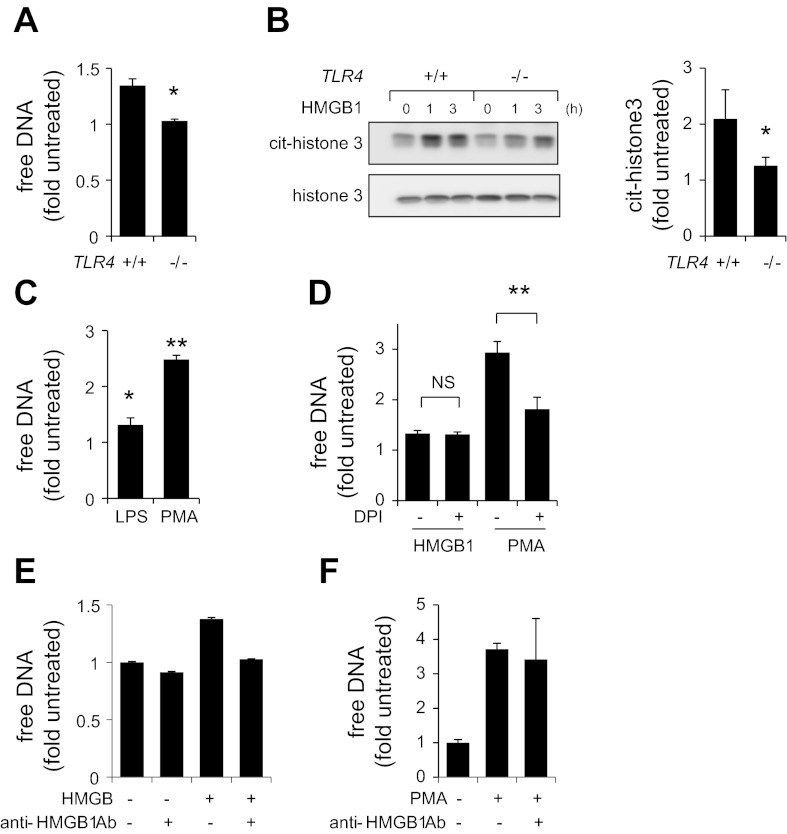
HMGB1 has been previously shown to induce cellular activation through interaction with several receptors, including TLR2, TLR4, and RAGE (1). To determine whether TLR4 is required to induce NET formation, neutrophils isolated from wild-type (TLR4+/+) and TLR4-deficient mice (TLR4−/−) were incubated with HMGB1, and the amount of released DNA was measured using Sytox Green probe. As shown in Fig. 2A, exposure to HMGB1 resulted in DNA release by wild-type neutrophils but had no such effect in TLR4-deficient neutrophils. This finding indicates that TLR4 is required for HMGB1-dependent NET formation.
Fig. 2.
Toll-like receptor (TLR)4 deficiency diminished DNA release from HMGB1-treated neutrophils. A: free DNA was determined in culture medium collected from wild-type (TLR4+/+) or TLR4-deficient (TLR4−/−) neutrophils that were incubated with or without HMGB1 (300 ng/ml) for 3 h (means ± SD, n = 3; *P < 0.05 compared with untreated samples). B: TLR4+/+ or TLR4−/− neutrophils were treated with HMGB1 (300 ng/ml) for 0, 1, or 3 h. Representative Western blots and optical bend density show level of citrullinated histone 3 (cit-H3) and total amount of histone 3 (H3). Means ± SD were calculated after 3 h of exposure to HMGB1; n = 3; *P < 0.05 compared with untreated cells. C: neutrophils were treated with LPS (100 ng/ml) for 3 h or PMA (10 nM) for 3 h followed by measurement of free DNA (Means ± SD, n = 3; *P < 0.05, **P < 0.01 compared with untreated cells). D: free DNA in culture medium was determined after subsequent exposure of neutrophils to diphenyleneiodonium (DPI) (0 or 20 μM) for 3 min followed by treatment with HMGB1 (300 ng/ml) or PMA (10 nM) for 3 h. (Means ± SD, n = 3; **P < 0.01) NS, not significant. E: HMGB1 (0 or 300 ng) was incubated with anti-HMGB1 (neutralizing) antibody (0 or 10 μg) for 30 min at room temperature. Next, samples were incubated with neutrophils for additional 3 h followed by measurement of free DNA. F: neutrophils were treated with anti-HMGB1 (neutralizing) antibody (0 or 10 μg) or PMA (0 or 10 nM), or PMA and anti-HMGB1 antibody for 3 h. Free DNA was measured using Sytox Green.
The initiation of NET formation is coupled to hypercitrullination of histones by peptidylarginine deiminase 4 (PAD4), followed by chromatin decondensation (22). As shown in Fig. 2B, Western blot analysis demonstrated significant hypercitrullination of histone 3 in HMGB1-treated wild-type, but not in TLR4-deficient, neutrophils. These results provide additional evidence for participation of TLR4 during NET formation, and particularly through mechanisms that involve chromatin decondensation.
Previous studies have found that TLR4 engagement as well as production of ROS by NADPH oxidase can induce NET formation (10, 13, 34). Exposure of neutrophils to PMA resulted in activation of NADPH oxidase and extensive NET formation (Fig. 2C). Incubation of neutrophils with DPI, an inhibitor of NADPH oxidase, diminished DNA release by PMA-stimulated neutrophils. However, DPI had no effect on HMGB1-dependent release of DNA. Thus HMGB1-induced NET formation appears to be independent of NADPH oxidase ROS production.
Results obtained from additional experiments revealed that anti-HMGB1 (neutralizing) antibody diminished the ability of HMGB1 to induce NETs (Fig. 2E). Of note, anti-HMGB1 (neutralizing) antibody had no effect on netosis induced by PMA (Fig. 2F). Because the concentrations of endotoxin in the HMGB1 were less than 0.005 EU/μg, the ability of HMGB1 to enhance NETs was not due to contamination.
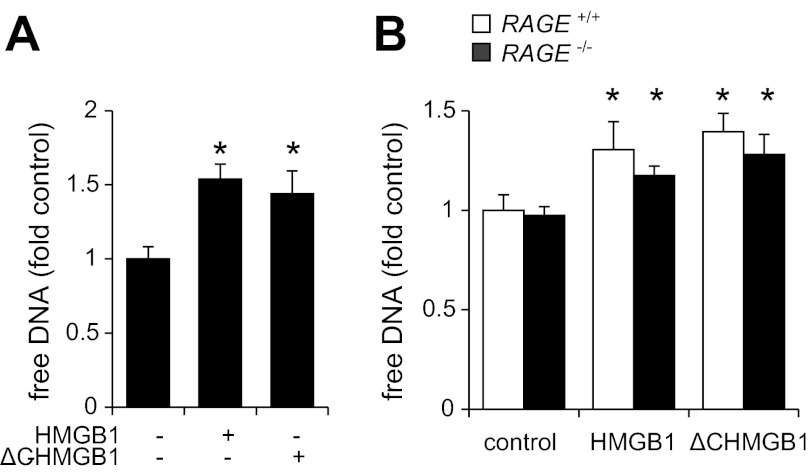
Although HMGB1 can potentially affect NET formation through interaction with RAGE, similar amounts of DNA were released from neutrophils after exposure to HMGB1 or ΔC HMGB1, which is unable to interact with RAGE (Fig. 3A). There also were no apparent differences in the release of DNA from HMGB1-treated RAGE+/+ or RAGE−/− neutrophils, indicating that interactions between RAGE and HMGB1 signaling were not required for NET formation.
Fig. 3.
Effects of receptor for advanced glycation end products (RAGE) deficiency on HMGB1-mediated NET formation. Free DNA was determined after exposure of neutrophils to HMGB1 (0 or 300 ng/ml) or ΔC HMGB1 (0 or 300 ng/ml) for 3 h (A), or after treatment of wild-type (RAGE+/+) or RAGE-deficient (RAGE−/−) neutrophils with or without HMGB1 (300 ng/ml) for 3 h (B). Means ± SD, n = 3; *P < 0.05 compared with untreated sample (control).
HMGB1 contributes to NET formation in mice subjected to LPS-induced acute lung injury.
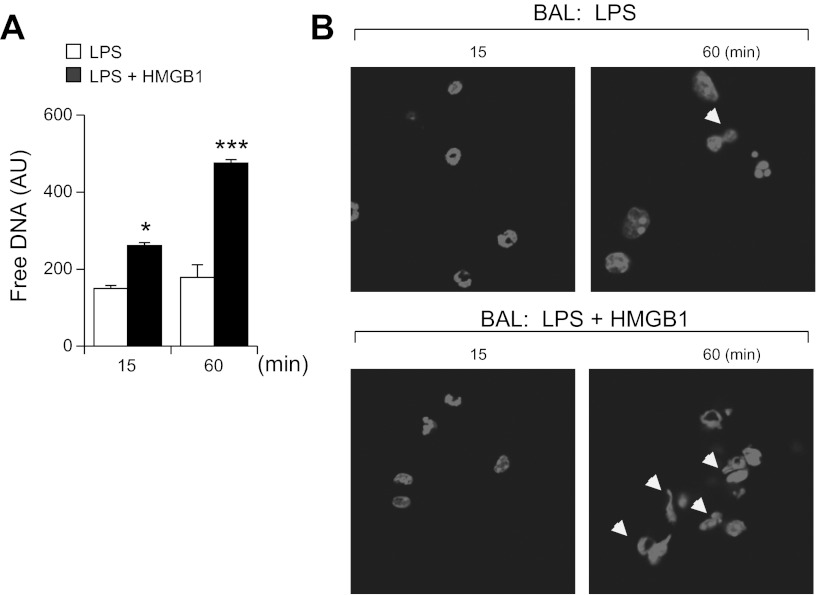
Previous studies have shown that HMGB1 can bind to LPS and that such interactions between LPS and HMGB1 increase the duration and intensity of inflammatory response (41, 49). Therefore, we were interested in determining whether the combination of HMGB1 and LPS is more potent in inducing NET formation than LPS alone. Of note, measurement of the rate and levels of NET formation ex vivo suggests that the free DNA and histone 3 found in BALs of LPS treated mice likely result from enhanced ability of neutrophils to form NETs in vivo. As shown in Fig. 4, A and B, freshly isolated BALs containing neutrophils (e.g., BALs obtained from mice exposed to i.t. LPS or LPS and HMGB1) were cultured for 15 and 60 min followed by detection of free DNA using Sytox Green probes (Fig. 4A). In parallel experiments, DNA was stained with DAPI followed by imaging with microscopy (Fig. 4B). These results suggest that HMGB1 can promote NET formation in vivo.
Fig. 4.
HMGB1 facilitates NET formation ex vivo. A: neutrophils in bronchoalveolar lavages (BALs) were collected 24 h after intratracheal administration of LPS (1 mg/kg) or LPS and HMGB1 (300 ng), and free DNA was determined in BAL neutrophils that were immediately incubated after isolation for 15 or 60 min followed by addition of Sytox probe for 5 min. Means ± SD, n = 3; *P < 0.05 or ***P < 0.001 compared with BALs of mice that were not subjected to HMGB1 administration. B: representative images show NET formation ex vivo (arrows) in BALs obtained from mice treated with LPS or LPS/HMGB1 as indicated in A. BAL neutrophils were incubated immediately after isolation for 15 and 60 min and then DNA stained with DAPI.
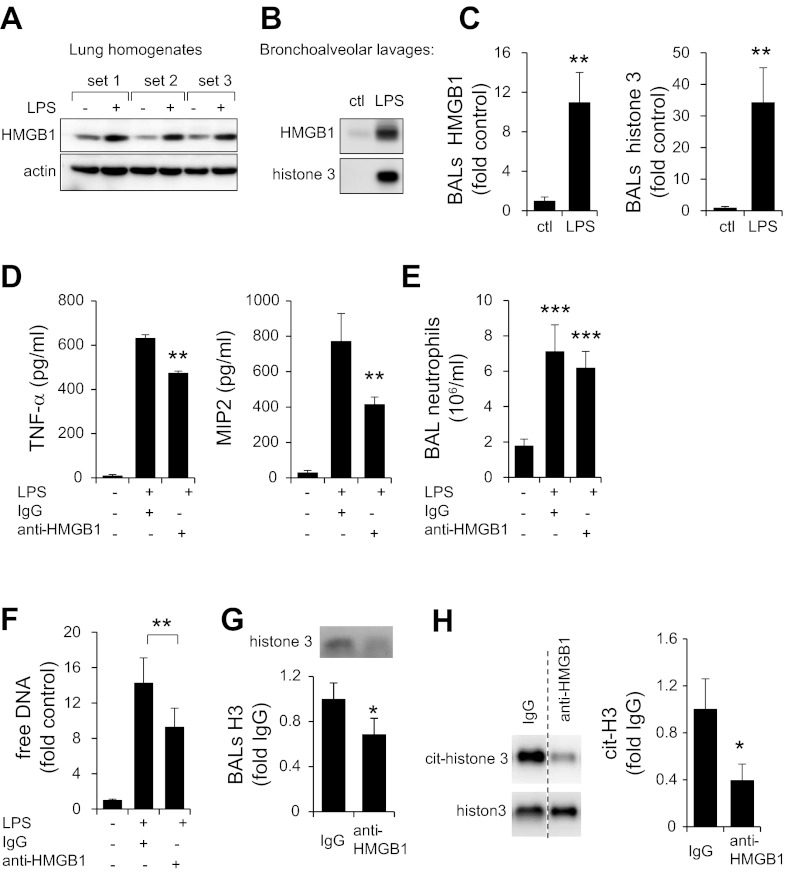
Significant increases in HMGB1 concentrations were present in lung homogenates from LPS-treated mice. Large increases of HMGB1 and histone 3 were also detected in BALs of mice subjected to LPS administration (Fig. 5, A–C). To determine whether endogenous HMGB1 affects NET formation during LPS-induced acute lung injury, mice were treated with LPS and neutralizing antibodies to HMGB1 or isotype-specific control antibodies. As shown on Fig. 5D, administration of anti-HMGB1 antibodies decreased the amounts of TNF-α and MIP-2 in the BAL fluid of LPS-treated mice.
Fig. 5.
Effects of HMGB1-neutralizing antibody on NET formation. Representative Western blot and quantitative analysis show amount of HMGB1 and actin determined in whole lung homogenates (A), whereas amounts of HMGB1 and histone 3 detected in BALs obtained from control and mice subjected to LPS induced ALI are also shown (B and C). Lungs were harvested 24 h after saline (control) or LPS (i.t.) administration. D and E: levels of TNF-α and macrophage inflammatory protein (MIP)2 cytokines (D) and numbers of neutrophils (E) were measured in BALs of control mice (saline) or mice that received LPS and IgG or LPS and HMGB1-neutralizing antibody. Means ± SD, n = 4; **P < 0.05, ***P < 0.001. F: free DNA in BAL neutrophils using Sytox Green probe. Means ± SD, n = 3; **P < 0.05. G: representative Western blots and quantitative analysis show amounts of histone 3 found in BALs obtained 24 h after exposure of mice to LPS and treatment with IgG or anti-HMGB1 antibodies. H: Western blot of citrullinated histone 3 (cit-histone 3) and total histone 3 determined in cell extracts that were prepared from BAL neutrophils isolated from mice subjected to LPS (i.t.) and anti-HMGB1 antibody (i.p.) or LPS (i.t.) and IgG (i.p.) administration. Means ± SD, n = 4; *P < 0.05 compared with LPS- and IgG-treated cells.
Additional experiments (Fig. 5, F and G) demonstrated that treatment of mice with anti-HMGB1 antibodies effectively diminished NET formation in vivo, as shown by decreased amounts of free DNA and histone 3 in BALs, compared with that present in control mice that received isotype IgG. In addition, although a similar number of neutrophils was obtained from BALs of LPS-exposed mice treated with control IgG or anti-HMGB1-neutralizing antibodies (Fig. 5E), administration of anti-HMGB1 antibodies diminished intracellular levels of hypercitrullinated histone 3 in BAL neutrophils (Fig. 5H).
DISCUSSION
In this study, we demonstrated that exposure of neutrophils to HMGB1 resulted in enhanced formation of NETs in vitro. The ability of anti-HMGB1 antibodies to diminish NET formation in the airspaces following LPS-induced acute lung injury provides evidence that HMGB1 contributes to NET generation under relevant in vivo conditions. Although the proinflammatory function of HMGB1 in inducing release of cytokines is well established (1, 39), the present studies, by showing that HMGB1 enhances NET formation through TLR4-dependent processes, delineate a novel additional mechanism by which HMGB1 may contribute to inflammatory processes and tissue injury.
The present findings, showing the central role of TLR4-mediating HMGB1-induced NET formation, are consistent with previous studies demonstrating that TLR4 engagement enhances extracellular DNA release and generation of NETs. For example, the absence of TLR4 was associated with diminished DNA release from neutrophils stimulated with H. influenzae (19). The TLR4-MyD88 signaling pathway has been shown to be essential for neutrophils to release DNA (19, 47). However, recent studies have shown that other signaling events, including activation of NADPH oxidase or stimulation of TLR2, can also promote NET formation (13, 45).
Although the mechanisms responsible for NET formation have not been completely delineated, several signaling events are known to precede DNA release, including PAD4-dependent hypercitrullination of histone H3 followed by chromatin decondensation. In the present experiments, extensive H3 citrullination was found in HMGB1-treated neutrophils, whereas significantly decreased citrullination of H3 was found in neutrophils isolated from the BAL fluid of LPS-exposed mice treated with neutralizing antibodies to HMGB1. In addition, we found that exposure of neutrophils to HMGB1 increased interactions between neutrophil elastase and myeloperoxidase, an event that was previously described to occur during NET formation (32). Although the role that myeloperoxidase plays in DNA release has not been delineated, recent studies suggest that myeloperoxidase embedded in NETs may facilitate microbial killing (33).
Previous studies have shown that activation of NADPH oxidase and production of ROS can trigger NET formation (6, 34, 38). Although exposure of neutrophils to the flavoprotein inhibitor DPI diminished NADPH oxidase-mediated NET formation, DPI had no effect on HMGB1-dependent release of DNA into the extracellular space. Such results indicate that the enhancing effect of HMGB1 on NET formation was independent of ROS generated by NADPH oxidase. Similar results, demonstrating NET generation without activation of NADPH oxidase, were previously found in a study examining Staphylococcus aureus-induced NET formation (36). Although previous studies have shown the importance of platelets in NET formation (10), recent findings (27, 29, 30) demonstrated that direct exposure of neutrophils to LPS alone can induce NETs. Our results suggest that HMGB1 promoted NET formation in vivo, as shown by the reduction of histone 3 and free DNA in the BAL fluid of LPS-treated mice that received neutralizing antibodies to HMGB1. However, the present studies do not rule out a contributory role for platelets in NET formation in this setting. Similarly, decreased levels of cytokines in the lungs after administration of anti-HMGB1 antibodies to LPS-treated mice may not necessarily be a direct result of diminished NET formation but could reflect the effects of HMGB1 on other proinflammatory pathways, such as those induced by interactions of HMGB1 with TLR4.
Activated neutrophils play an important role in innate immune responses. Although neutrophils contribute to microbial eradication through direct ingestion, production of inflammatory mediators, and generation of antibacterial peptides, formation of the extracellular traps also has an important contributory role in this process (8, 26). The present experiments, showing that HMGB1 potentiates NET formation under both in vitro and in vivo conditions, demonstrate a novel mechanism by which HMGB1 may contribute to enhancing host defense mechanisms. Of note, recent studies showed that HMGB1 can diminish the ability of neutrophils to kill bacteria through inhibition of NADPH oxidase, a major source of ROS (42). Therefore, the contribution of HMGB1 to microbial eradication by neutrophils would appear to involve enhanced production of NETs that are capable of trapping microorganisms and preventing their dissemination, rather than through directly facilitating neutrophil respiratory burst.
Although clearing infections is an essential component of neutrophil function, extensive formation of NETs may not necessarily be of benefit to the host, especially in the setting of sterile inflammatory conditions (11, 16, 21, 24, 37, 38) where the proinflammatory actions of HMGB1 have been shown to play an important role (18, 20, 39, 45, 48). Several studies have suggested that magnitude of tissue damage and organ dysfunction are associated with the degree of NET formation (17, 19, 28). A direct relationship between enhanced formation of NETs and the severity of lung injury was shown in mice challenged with H1N1 influenza (28). Several mechanisms for the ability of NETs to enhance inflammation have been suggested, including enhanced extracellular concentrations of DNA, myeloperoxidase, and histones (38).
Although HMGB1 was initially described as a nuclear protein with important regulatory functions associated with gene expression (1), more recent studies have shown that HMGB1 has potent proinflammatory actions and can be classified as a danger-associated molecular pattern mediator. Although therapeutic approaches aimed at blocking cellular release of HMGB1 or inhibiting the actions of extracellular HMGB1 are of benefit in diminishing tissue injury and organ dysfunction in acute and chronic inflammatory conditions, such as hemorrhage, sepsis, and rheumatoid arthritis, there is also reason to believe that HMGB1 plays a beneficial role in microbial eradication, not only through its proinflammatory actions, but also by modulating neutrophil chemotaxis (1, 4, 5, 20, 39). Our present findings, showing that HMGB1 can potentiate NET formation, suggest a novel mechanism by which HMGB1 may enhance host defense to bacterial infection and contribute to inflammatory processes. Future experiments will be necessary to delineate the relative importance of HMGB1-mediated NET formation compared with other pathways by which HMGB1 potentiates inflammatory processes in which neutrophils play important roles.
GRANTS
This work was supported by NIH grants GM87748 and HL107585 to J. W. Zmijewski, GM086416 to J.-F. Pittet, and MONAHAN Foundation (Fulbright Program) and Bettencourt-Schueller Foundation to J.-M. Tadié.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.-M.T., K.J.T., V.J.T., E.A., and J.W.Z. conception and design of research; J.-M.T., H.-B.B., S.J., D.W.P., C.P.B., H.Y., and J.W.Z. performed experiments; J.-M.T., S.J., J.-F.P., E.A., and J.W.Z. analyzed data; J.-M.T., S.J., D.W.P., J.-F.P., E.A., and J.W.Z. interpreted results of experiments; J.-M.T., S.J., and J.W.Z. prepared figures; J.-M.T. and J.W.Z. drafted manuscript; J.-M.T., E.A., and J.W.Z. edited and revised manuscript; J.-M.T., H.-B.B., S.J., D.W.P., C.P.B., H.Y., J.-F.P., K.J.T., V.J.T., E.A., and J.W.Z. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Anna A. Zmijewska for excellent technical assistance.
REFERENCES
- 1.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol 29: 139–162, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bae HB, Zmijewski JW, Deshane JS, Tadie JM, Chaplin DD, Takashima S, Abraham E. AMP-activated protein kinase enhances the phagocytic ability of macrophages and neutrophils. FASEB J 25: 4358–4368, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banerjee S, Friggeri A, Liu G, Abraham E. The C-terminal acidic tail is responsible for the inhibitory effects of HMGB1 on efferocytosis. J Leukoc Biol 88: 973–979, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnay-Verdier S, Fattoum L, Borde C, Kaveri S, Gibot S, Marechal V. Emergence of autoantibodies to HMGB1 is associated with survival in patients with septic shock. Intens Care Med 37: 957–962, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Berthelot F, Fattoum L, Casulli S, Gozlan J, Marechal V, Elbim C. The effect of HMGB1, a damage-associated molecular pattern molecule, on polymorphonuclear neutrophil migration depends on its concentration. J Innate Immun 4: 41–58, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Bianchi M, Hakkim A, Brinkmann V, Siler U, Seger RA, Zychlinsky A, Reichenbach J. Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood 114: 2619–2622, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brass DM, Hollingsworth JW, McElvania-Tekippe E, Garantziotis S, Hossain I, Schwartz DA. CD14 is an essential mediator of LPS-induced airway disease. Am J Physiol Lung Cell Mol Physiol 293: L77–L83, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science 303: 1532–1535, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Chorny A, Anderson P, Gonzalez-Rey E, Delgado M. Ghrelin protects against experimental sepsis by inhibiting high-mobility group box 1 release and by killing bacteria. J Immunol 180: 8369–8377, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med 13: 463–469, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Dorner T. SLE in 2011: Deciphering the role of NETs and networks in SLE. Nat Rev Rheumatol 8: 68–70, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Entezari M, Weiss DJ, Sitapara R, Whittaker L, Wargo MJ, Li J, Wang H, Yang H, Sharma L, Phan BD, Javdan M, Chavan SS, Miller EJ, Tracey KJ, Mantell LL. Inhibition of HMGB1 enhances bacterial clearance and protects against P. aeruginosa pneumonia in cystic fibrosis. Mol Med 18: 477–485, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ermert D, Urban CF, Laube B, Goosmann C, Zychlinsky A, Brinkmann V. Mouse neutrophil extracellular traps in microbial infections. J Innate Immun 1: 181–193, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster WM, Walters DM, Longphre M, Macri K, Miller LM. Methodology for the measurement of mucociliary function in the mouse by scintigraphy. J Appl Physiol 90: 1111–1117, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Friggeri A, Yang Y, Banerjee S, Park YJ, Liu G, Abraham E. HMGB1 inhibits macrophage activity in efferocytosis through binding to the αvβ3-integrin. Am J Physiol Cell Physiol 299: C1267–C1276, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL, Barrat FJ, Banchereau J, Pascual V. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med 3: 73ra20, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, Herrmann M, Voll RE, Zychlinsky A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci USA 107: 9813–9818, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris HE, Andersson U, Pisetsky DS. HMGB1: A multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol 8: 195–202, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Juneau RA, Pang B, Weimer KE, Armbruster CE, Swords WE. Nontypeable Haemophilus influenzae initiates formation of neutrophil extracellular traps. Infect Immun 79: 431–438, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JY, Park JS, Strassheim D, Douglas I, Diaz del Valle F, Asehnoune K, Mitra S, Kwak SH, Yamada S, Maruyama I, Ishizaka A, Abraham E. HMGB1 contributes to the development of acute lung injury after hemorrhage. Am J Physiol Lung Cell Mol Physiol 288: L958–L965, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Leffler J, Martin M, Gullstrand B, Tyden H, Lood C, Truedsson L, Bengtsson AA, Blom AM. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J Immunol 188: 3522–3531, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med 207: 1853–1862, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liliensiek B, Weigand MA, Bierhaus A, Nicklas W, Kasper M, Hofer S, Plachky J, Grone HJ, Kurschus FC, Schmidt AM, Yan SD, Martin E, Schleicher E, Stern DM, Hammerling GG, Nawroth PP, Arnold B. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest 113: 1641–1650, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu CL, Tangsombatvisit S, Rosenberg JM, Mandelbaum G, Gillespie EC, Gozani OP, Alizadeh AA, Utz PJ. Specific post-translational histone modifications of neutrophil extracellular traps as immunogens and potential targets of lupus autoantibodies. Arthritis Res Ther 14: R25, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma AC, Kubes P. Platelets, neutrophils, and neutrophil extracellular traps (NETs) in sepsis. J Thromb Haemost 6: 415–420, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 11: 519–531, 2011 [DOI] [PubMed] [Google Scholar]
- 27.McInturff AM, Cody MJ, Elliott EA, Glenn JW, Rowley JW, Rondina MT, Yost CC. Mammalian target of rapamycin regulates neutrophil extracellular trap formation via induction of hypoxia-inducible factor 1 alpha. Blood 120: 3118–3125, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew AA, Phoon MC, van Rooijen N, Chow VT. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol 179: 199–210, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neeli I, Dwivedi N, Khan S, Radic M. Regulation of extracellular chromatin release from neutrophils. J Innate Immun 1: 194–201, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neeli I, Khan SN, Radic M. Histone deimination as a response to inflammatory stimuli in neutrophils. J Immunol 180: 1895–1902, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Ogawa EN, Ishizaka A, Tasaka S, Koh H, Ueno H, Amaya F, Ebina M, Yamada S, Funakoshi Y, Soejima J, Moriyama K, Kotani T, Hashimoto S, Morisaki H, Abraham E, Takeda J. Contribution of high-mobility group box-1 to the development of ventilator-induced lung injury. Am J Respir Crit Care Med 174: 400–407, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol 191: 677–691, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parker H, Albrett AM, Kettle AJ, Winterbourn CC. Myeloperoxidase associated with neutrophil extracellular traps is active and mediates bacterial killing in the presence of hydrogen peroxide. J Leukoc Biol 91: 369–376, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Patel S, Kumar S, Jyoti A, Srinag BS, Keshari RS, Saluja R, Verma A, Mitra K, Barthwal MK, Krishnamurthy H, Bajpai VK, Dikshit M. Nitric oxide donors release extracellular traps from human neutrophils by augmenting free radical generation. Nitric Oxide 22: 226–234, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Phillipson M, Kubes P. The neutrophil in vascular inflammation. Nat Med 17: 1381–1390, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, Sibley CD, Robbins SM, Green FH, Surette MG, Sugai M, Bowden MG, Hussain M, Zhang K, Kubes P. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol 185: 7413–7425, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Rouhiainen A, Tumova S, Valmu L, Kalkkinen N, Rauvala H. Pivotal advance: analysis of proinflammatory activity of highly purified eukaryotic recombinant HMGB1 (amphoterin). J Leukoc Biol 81: 49–58, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, Lohmeyer J, Preissner KT. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One 7: e32366, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sha Y, Zmijewski J, Xu Z, Abraham E. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol 180: 2531–2537, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Shimazaki J, Matsumoto N, Ogura H, Muroya T, Kuwagata Y, Nakagawa J, Yamakawa K, Hosotsubo H, Imamura Y, Shimazu T. Systemic involvement of HMGB1 and therapeutic effect of anti-HMGB1 antibody in a rat model of crush injury. Shock 37: 634–638, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol 28: 367–388, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Tadie JM, Bae HB, Banerjee S, Zmijewski JW, Abraham E. Differential activation of RAGE by HMGB1 modulates neutrophil-associated NADPH oxidase activity and bacterial killing. Am J Physiol Cell Physiol 302: C249–C256, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tadie JM, Bae HB, Deshane JS, Bell CP, Lazarowski ER, Chaplin DD, Thannickal VJ, Abraham E, Zmijewski JW. TLR4 engagement inhibits AMPK activation through a HMGB1 dependent mechanism. Mol Med 18: 659–668, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, Stolz DB, Geller DA, Rosengart MR, Billiar TR. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med 204: 2913–2923, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Urbonaviciute V, Voll RE. High-mobility group box 1 represents a potential marker of disease activity and novel therapeutic target in systemic lupus erythematosus. J Intern Med 270: 309–318, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, Hayama R, Leonelli L, Han H, Grigoryev SA, Allis CD, Coonrod SA. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol 184: 205–213, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wieland CW, Florquin S, Maris NA, Hoebe K, Beutler B, Takeda K, Akira S, van der Poll T. The MyD88-dependent, but not the MyD88-independent, pathway of TLR4 signaling is important in clearing nontypeable haemophilus influenzae from the mouse lung. J Immunol 175: 6042–6049, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y, Akira S, Bierhaus A, Erlandsson-Harris H, Andersson U, Tracey KJ. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci USA 107: 11942–11947, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Youn JH, Kwak MS, Wu J, Kim ES, Ji Y, Min HJ, Yoo JH, Choi JE, Cho HS, Shin JS. Identification of lipopolysaccharide-binding peptide regions within HMGB1 and their effects on subclinical endotoxemia in a mouse model. Eur J Immunol 41: 2753–2762, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao X, Zmijewski JW, Lorne E, Liu G, Park YJ, Tsuruta Y, Abraham E. Activation of AMPK attenuates neutrophil proinflammatory activity and decreases the severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295: L497–L504, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zmijewski JW, Lorne E, Banerjee S, Abraham E. Participation of mitochondrial respiratory complex III in neutrophil activation and lung injury. Am J Physiol Lung Cell Mol Physiol 296: L624–L634, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zmijewski JW, Lorne E, Zhao X, Tsuruta Y, Sha Y, Liu G, Abraham E. Antiinflammatory effects of hydrogen peroxide in neutrophil activation and acute lung injury. Am J Respir Crit Care Med 179: 694–704, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zmijewski JW, Lorne E, Zhao X, Tsuruta Y, Sha Y, Liu G, Siegal GP, Abraham E. Mitochondrial respiratory complex I regulates neutrophil activation and severity of lung injury. Am J Respir Crit Care Med 178: 168–179, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zmijewski JW, Zhao X, Xu Z, Abraham E. Exposure to hydrogen peroxide diminishes NF-κB activation, IκB-α degradation, and proteasome activity in neutrophils. Am J Physiol Cell Physiol 293: C255–C266, 2007 [DOI] [PubMed] [Google Scholar]