Abstract
Multiple sodium and chloride channels on the apical surface of nasal epithelial cells contribute to periciliary fluid homeostasis, a function that is disrupted in patients with cystic fibrosis (CF). Among these channels is the chloride channel CLCN2, which has been studied as a potential alternative chloride efflux pathway in the absence of CFTR. The object of the present study was to use the nasal potential difference test (NPD) to quantify CLCN2 function in an epithelial-directed TetOn CLCN2 transgenic mouse model (TGN-K18rtTA-hCLCN2) by using the putative CLCN2 pharmacological agonist lubiprostone and peptide inhibitor GaTx2. Lubiprostone significantly increased chloride transport in the CLCN2-overexpressing mice following activation of the transgene by doxycycline. This response to lubiprostone was significantly inhibited by GaTx2 after CLCN2 activation in TGN-CLCN2 mice. Cftr−/− and Clc2−/− mice showed hyperpolarization indicative of chloride efflux in response to lubiprostone, which was fully inhibited by GaTx2 and CFTR inhibitor 172 + GlyH-101, respectively. Our study reveals lubiprostone as a pharmacological activator of both CFTR and CLCN2. Overexpression and activation of CLCN2 leads to improved mouse NPD readings, suggesting it is available as an alternative pathway for epithelial chloride secretion in murine airways. The utilization of CLCN2 as an alternative chloride efflux channel could provide clinical benefit to patients with CF, especially if the pharmacological activator is administered as an aerosol.
Keywords: CFTR, CLCN2, lubiprostone, cystic fibrosis, confocal microscopy, chloride channels, electrophysiology, airway epithelium
airway fluid secretion is necessary for in utero lung growth and expansion. Fetal lung fluid production is driven by a combination of chloride ion transporters including CFTR, CLCN2, TMem16A, and as yet unidentified non-CFTR-dependent conductances (3, 8, 10, 15, 19). Infants with cystic fibrosis (CF) caused by the most common mutation, ΔF508, are born with relatively normal lungs because non-CFTR-related anion transporters are active and sufficient to compensate for lack of a functional CFTR. Moreover, at birth both CFTR and CLCN2 are downregulated and epithelial sodium reabsorption through the epithelial sodium channel (ENaC) helps to reabsorb the excessive fetal lung fluid in preparation for air breathing (25, 29, 37). In adults, CLCN2 and CFTR are important for maintaining periciliary fluid homeostasis and can be detected functionally. We hypothesize that the CLCN2 can be activated in a novel doxycycline-regulated, epithelial-specific CLCN2 mouse model as well as in Cftr−/− mice, and we demonstrate the potential role of an FDA-approved CLCN2/CFTR pharmacological agonist, lubiprostone.
Lubiprostone (Amitiza) is the first in its class of putative CLCN2 channel activators that was recently approved by the FDA for the treatment of idiopathic constipation in adults (33). It is poorly absorbed and therefore targeted to gastrointestinal therapy. Lubiprostone is a bicyclic fatty acid and prostaglandin E1 derivative that has been implicated to activate CLCN2 in a protein kinase A-independent manner (12). There have been six placebo-controlled, double-blind, randomized Phase II or III clinical trials that, in the aggregate of over 1,400 subjects treated twice a day for up to 4 years, demonstrated improvements in constipation (16, 33). Cuppoletti et al. (12) studied the activation of CLCN2 in cell lines and rabbit intestinal epithelia and demonstrated that lubiprostone was a potent activator of CLCN2 and did not regulate CFTR. Other laboratories have extended these studies and detected activation of CFTR at higher doses of lubiprostone (4–6). Sufficient controversy remains concerning the localization of CLCN2 in different tissues and the specific molecular target of lubiprostone. We chose to study the effects of lubiprostone in mice before and after upregulation of hCLCN2 as well as in mice lacking CFTR in their airways to quantify CLCN2-dependent chloride transport. Demonstration of CLCN2 activation by direct delivery to the mucosa in mice lacking CFTR in the airways supports the rationale to develop an aerosol or dry powder delivery vehicle for human CF. Activation of both CLCN2 and CFTR by lubiprostone could eventually be used to augment CFTR-specific rescue therapies under clinical development or in patients with mutations in CFTR that can traffic to the apical cell surface.
METHODS
Animals.
All protocols involving animals were approved by the Johns Hopkins University Animal Care and Use Committee (ACUC). Novel, epithelial-directed, doxycycline-regulated hCLCN2 mice, TGN-k18rtTA-hCLCN2 (TGN-CLCN2), mice were bred according to the following: to confine expression of human CLCN2 (hCLCN2) to epithelial cells, a cytokeratin 18 promoter sequence (gift of James Hu) was inserted prior to the reverse tetracycline-transactivator to control expression of the Tet-On system (Clontech, Mountain View, CA). The resulting K18rtTA construct was 7,532 bp. The human CLCN2 cDNA (gift of Garry Cutting) was placed under the control of the tetracycline response element to produce the pTREhCLCN2 construct (5,933 bp). Both constructs were microinjected into C57BLK6 mouse embryonic stem cells by the Johns Hopkins University transgenic mouse core facility, inserted into donor embryos, and then transplanted into pseudopregnant dams. We obtained two founders containing each construct alone. After several generations were obtained they were back-crossed eight generations in C57Bl6. To avoid any trans-complementation issues, a new colony of TetOnClC2 Bl6 was crossed with Bl6.Cg-Tg(SFTPC-rtTA) strain (Jackson Laboratory, Bar Harbor, ME) to obtain Bl6.rtTATetOnClC2. Pups from TGN-CLCN2 mice were genotyped as described below. Gut corrected, Cftr−/− mice were purchased from Jackson Laboratory [Cftrtm1Unc Tg(FABPCFTR)1Jaw/J] and bred according to their available recommendations. These mice had no alterations to Clcn2 genes. Clcn2−/− mice (30) were bred from a set generously provided by Dr. James Melvin (National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD) and genotyped as described below. These mice had no alterations to Cftr genes. Members of each experimental set were age matched within 4 wk to mice of similar genotype, and all mice were studied at 4–6 mo of age. Both sexes were equally represented in all genotypes.
Genotyping and PCR.
All mice were genotyped by tail clipping, and DNA was extracted by use of the REDExtract-N-Amp Tissue PCR kit (Sigma Aldrich, St. Louis, MO) following the supplied protocol, with the exception that tails were allowed to remain in extraction solution overnight before neutralizing. PCR was performed as directed by the kit, using the 2× REDTaq PCR mix provided, specific primers, and 1–4 μl of extracted DNA solution. Following PCR amplification, samples were run on 1.5% agarose gels containing ethidium bromide (Sigma Aldrich) and visualized by UV illumination. hCLCN2 samples were sometimes run on 2% gels to improve visibility. TGN-CLCN2 mice were genotyped by detecting presence of both the rtTA gene (forward primer: CGC TGT GGG GCA TTT TAC TTT AG, reverse primer: CAT GTC CAG ATC GAA ATC GTC) and human CLCN2 (forward primer: GGC GTG TAC GGT GGG AGG CCT ATA T, reverse primer: TGA GGA TCC CCG GGT ACC GAG CTC) in separate PCR reactions. The hClC2 gene (∼200-bp) was amplified by heating to 95°C for 2 min, followed by 35 cycles of 95°C for 1 min, 75°C for 30 s, and 72°C for 30 s, ending with 72°C for 7 min. The rtTA gene (450-bp) was amplified by heating to 95°C for 3 min, followed by 35 cycles of 94°C for 30 s, 57°C for 1 min, and 72°C for 1 min, ending with 72°C for 2 min.
Cftr−/− mice were genotyped by using a common primer (GAG AAC TGG AAG CTT CAG AGG), a mutant primer (TCC ATC TTG TTC AAT GGC C) to create a 357-bp band, and a wild-type primer (TCC ATG TAG TGG TGT GAA CG) to create a 526-bp band. Heterozygotes showed both bands. Samples containing all three primers were heated to 94°C for 3 min, followed by 35 cycles of 94°C for 30 s, 58°C for 45 s, and 72°C for 45 s, ending with 72°C for 2 min.
Clcn2−/− mice were genotyped using a common reverse primer (ACA CCC AGG TCC CTG CCC AAT CTG G), a mutant forward primer (CCT GGA AGG TGC CAC TCC CAC TGT CC) to create a 300-bp band, and a wild-type forward primer (ATG TAT GGC CGG TAC ACT CAG GAA CTC) to create a 200-bp band. Heterozygotes again showed both bands. Samples containing all three primers were heated to 94°C for 1 min, 72°C for 1 min, and then cycled through 94°C for 10 s, 60°C for 10 s, and 72°C for 30 s 30 times.
Genotype verification by immunoblot.
Mice designated for tissue harvest were euthanized via CO2 inhalation. Tissues from the lung, trachea, and nasal cavity were dissected and quickly placed on wet ice. The harvested tissues were homogenized in TPER (Thermo Fisher Scientific, Pittsburgh, PA) with protease inhibitors (Sigma Aldrich) by use of a tissue homogenizer. Samples were centrifuged and supernatant was obtained. Protein concentration was determined with the Pierce BCA protein assay kit (Thermo Fisher); 40 μg of total protein was loaded into each well for the CFTR and β-actin blots (Fig. 1F). Electrophoresis and Western transfer were performed by standard procedures. The 0.2-μm nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA) was blocked for 1 h using 5% Blotting Grade Blocker (Bio-Rad). The mouse monoclonal CFTR antibody M3A7 (EMD Millipore, Billerica, MA) was added 1:500, followed by ECL anti-mouse IgG, HRP-linked whole antibody from sheep (GE Healthcare). β-Actin was detected with use of HRP-conjugated β-actin (13E5) rabbit monoclonal antibody (Cell Signaling Technology, Beverly, MA). Endogenous CLCN2 expression was undetectable in whole tissue lysate, so immunoprecipitation was performed. The lysates were adjusted to 1 mg/ml each, then precleared with protein G Sepharose 4 Fast Flow beads (GE Healthcare) and the supernatant was obtained. The samples were rotated with rabbit anti-CLCN2 antisera S787 (40) and Sepharose beads, centrifuged, and eluted, then 20 μl eluted protein was loaded for each sample into a 6% SDS polyacrylamide gel. A mouse monoclonal anti-human CLCN2 primary antibody (WH0001181M1, Sigma-Aldrich) was added 1:1,000, followed by ECL anti-native IgG, HRP-linked whole antibody from donkey (GE Healthcare).
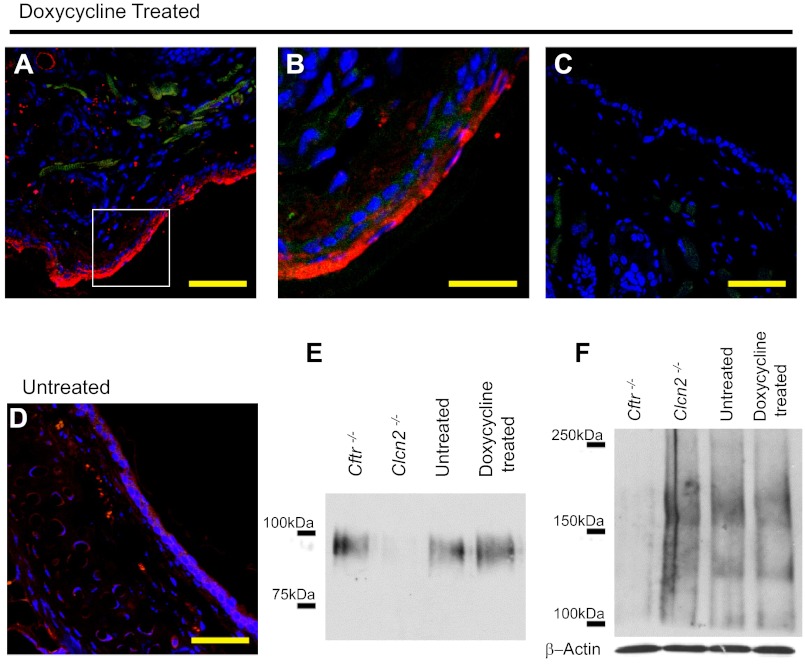
Fig. 1.
Doxycycline-treated TGN-CLCN2 mice show elevated CLCN2 expression. Expression of CLCN2 in doxycycline-treated mouse nasal section (representative of duplicate). Doxycycline-treated or untreated mouse nasal cavities were handled as described in methods. Nasal sections were stained with antibodies to CLCN2 (red), actin (green) to outline cell structure, and DAPI for nuclei (blue). A: immunostained confocal microscopy image shows CLCN2 expression in doxycycline-treated nasal cavity. B: high magnification of image in A shows CLCN2 (arrow) is expressed along the apical region of the epithelia. C: no primary antibody-treated tissue section to demonstrate absence of CLCN2 signal as a negative control. D: native untreated tissue shows reduced CLCN2 expression. Scale bar = 50 μm (A, C, D), 20 μm (B). For E and F, nasal epithelial tissue was excised from Cftr−/−, Clcn2−/−, doxycycline-treated TGN-CLCN2, and untreated TGN-CLCN2 mice. The same samples were equalized for total protein and used to determine both CLCN2 (E) and CFTR (F) expression. E: CLCN2 immunoblot of nasal tissue dissected from Cftr−/−, Clcn2−/−, doxycycline-treated, and untreated mice. CLCN2 was immunoprecipitated and separated on a 6% SDS PAGE gel. After transfer, the membrane was probed for CLCN2 as described in methods. CLCN2 (97 kDa) expression increased following induction, is considerable in the Cftr−/− mouse, and is absent in the Clcn2−/− mouse. F: CFTR immunoblot of nasal whole tissue lysate. CFTR expression (immature B-band ∼135 kDa, mature C-band ∼170 kDa) is detectable in the Clcn2−/−, untreated TGN-CLCN2, and doxycyline-treated TGN-CLCN2 mice, and absent in the Cftr−/− mouse. Equal sample loading in each lane was confirmed by an independent immunoblot for β-actin.
Immunolocalization and confocal microscopy.
Formalin-fixed, paraffin-embedded mouse nasal cavities were sectioned with 5-μm thickness. The tissue sections were dewaxed with xylene and rehydrated successively in 100, 95, and 70% ethanol and water. The sections were blocked with 3% BSA in PBS for 30 min at room temperature. The sections were incubated with primary antibodies against hCLCN2 (Alamone Labs, Jerusalem, Israel) or actin and fluorescent labeled secondary antibodies (Alexa Fluor 488 for actin and Alexa Fluor 568 for CLCN2, Molecular Probes, Life Technologies, Carlsbad, CA). The sections were counterstained with DAPI (4′,6-diamidine-2′-phenylindole dihydrochloride) to identify nuclei. Negative controls were treated similarly but without primary antibody against CLCN2. Slides were viewed and digital photography performed with a Zeiss LSM 510 meta confocal microscope using a ×40/1.3 oil immersion objective.
Reagents and solutions for NPD.
Nasal potential difference (NPD) solution “Ringer” saline solution consists of commercial Ringer Injection (1.48 mM NaCl, 2.25 mM CaCl2·2H2O, 4.05 mM KCl, Baxter Healthcare, Deerfield, IL) + 2.4 mM K2HPO4, 0.4 mM KH2PO4, 1.2 mM MgCl2·6H2O. “Amiloride” is Ringer + 100 μM amiloride (Sigma Aldrich). “Zero Chloride” is a chloride-free gluconate-substituted Ringer solution that consists of double-distilled H2O + 100 μM amiloride + 148 mM Na gluconate + 2.25 mM Ca gluconate + 4.05 mM K gluconate + 2.4 mM K2HPO4, 0.4 mM KH2PO4, 1.2 mM MgSO4·7H2O. “Lubiprostone” is a Zero Chloride solution + 20 μM lubiprostone (Sucampo Pharmaceuticals, Bethesda, MD). “GaTx2” is Lubiprostone solution + GaTx2 at 100, 200, or 400 nm (Gentaur Molecular Products, Kampenhout, Belgium). “CFTRinh-172” is Lubiprostone solution + CFTR inhibitor 172 at 30, 60, or 90 μM (Sigma Aldrich) (90 μM CFTRinh-172 solution also contained 20 μM GlyH-101; EMD Millipore, Temecula, CA). “ATP” is Zero Chloride + 100 μM ATP (Sigma Aldrich). Solutions used for NPD measurements with TGN-CLCN2 mice all contained 30 μM CFTR inhibitor 172 to reduce CFTR contribution. Solutions for Cftr−/− and Clcn2−/− mice did not contain CFTR inhibitor 172 or GlyH-101 unless specified. Lubiprostone, CFTR inhibitor 172, and GlyH-101 were dissolved in DMSO to 1,000× the desired concentration and then added 1:1,000 to NPD solutions, with DMSO never exceeding 1% by volume. We have previously demonstrated that 1% DMSO has no effect on NPD response (27).
NPD.
All NPD measurements were performed under general anesthesia with intraperitoneal (IP) injection of a ketamine-xylazine mixture (0.9% saline + 10% ketamine + 1% xylazine) according to an approved ACUC protocol. To upregulate hCLCN2 expression in TGN-CLCN2 mice, doxycycline (Sigma Aldrich) was administrated 24 h prior to NPD measurement (IP injection 40 mg/kg body wt). Mice were intubated by visualization with an otoscope, followed by a guide wire and a catheter (Jelco winged IV catheter 22G). Throughout the NPD, breathing was supported with an in-house custom-made mouse mechanical ventilation system. Polyethylene tubing (PE-10 of diameter 0.5 mm) was cut at a 45° angle and inserted 3–5 mm into the nare, the other end connected to a high-impedance millivoltmeter (World Precision Instruments, Sarasota, FL), and nasal depth was finely adjusted to achieve maximally negative NPD reading. The potential difference (PD) was measured against a reference subcutaneous ground inserted into the skin of the abdomen. NPD solutions were warmed to 37°C and perfused into the nasal cavity at a rate of 25 μl per min with a syringe pump for a minimum of 3 min or until the voltage reached steady state. The data were recorded by an ADInstruments PowerLab/4SP data acquisition system using the LabChart and Scope software (ADInstruments, version 6 for Windows). After NPD measurements, mice were placed on a heating pad until awake, then returned to cages and allowed minimum 3 days of recovery before repetition of anesthesia and NPD.
Statistical analysis.
Significance was determined by using a paired Student's t-test for TGN-CLCN2 mice to compare their responses before and after doxycycline treatment. All other data were compared by an unpaired Student's t-test. Data are shown as mean of all replicates ± SE.
RESULTS
Immunolocalization of hCLCN2 in nasal epithelia of TGN-K18rtTA-hCLCN2 mice.
Juvenile mice carrying both K18rtTA and hCLCN2 transgenes (verified by genotyping), with and without upregulation of the hCLCN2 with doxycycline for 24 h, were euthanized, and the nasal epithelial fixed in situ following isolation of tissue for immunostaining (one representative of duplicate mice). CLCN2 was detected by immunostaining with Alamone Labs CLCN2 antibody, and confocal microscopy as described in methods. Figure 1 shows composite representative images from the nose of treated and untreated animals. A strong CLCN2 signal is seen in doxycycline-treated animals in the apical membrane regions of nasal epithelial cells (Fig. 1, A and B). This signal is much lighter in untreated animals (Fig. 1D) and absent in the no-primary-antibody control (Fig. 1C). Immunoblotting against human CLCN2 in nasal and lung tissue homogenates that were harvested from naïve mice and mice after upregulation of hCLCN2 with doxycycline yielded a stronger signal at 97–100 kDa in the doxycycline-treated animals (Fig. 1E) than untreated controls. Considerable CLCN2 expression is noted in nasal epithelia of Cftr−/− mice, but not Clcn2−/− mice, consistent with immunolocalization data. CFTR is not expressed in the Cftr−/− mice (Fig. 1F). Differences in CFTR expression appear to be slight in the remaining three mouse types. The Clcn2−/− mice show comparable or slightly increased CFTR expression compared with untreated mice, which theoretically demonstrate wild-type expression levels. The doxycycline-treated mice appear to have comparable or slightly reduced CFTR expression compared with the untreated control.
Functional CLCN2 is upregulated by doxycycline in nasal epithelia of TGN-K18rtTA-hCLCN2 mice.
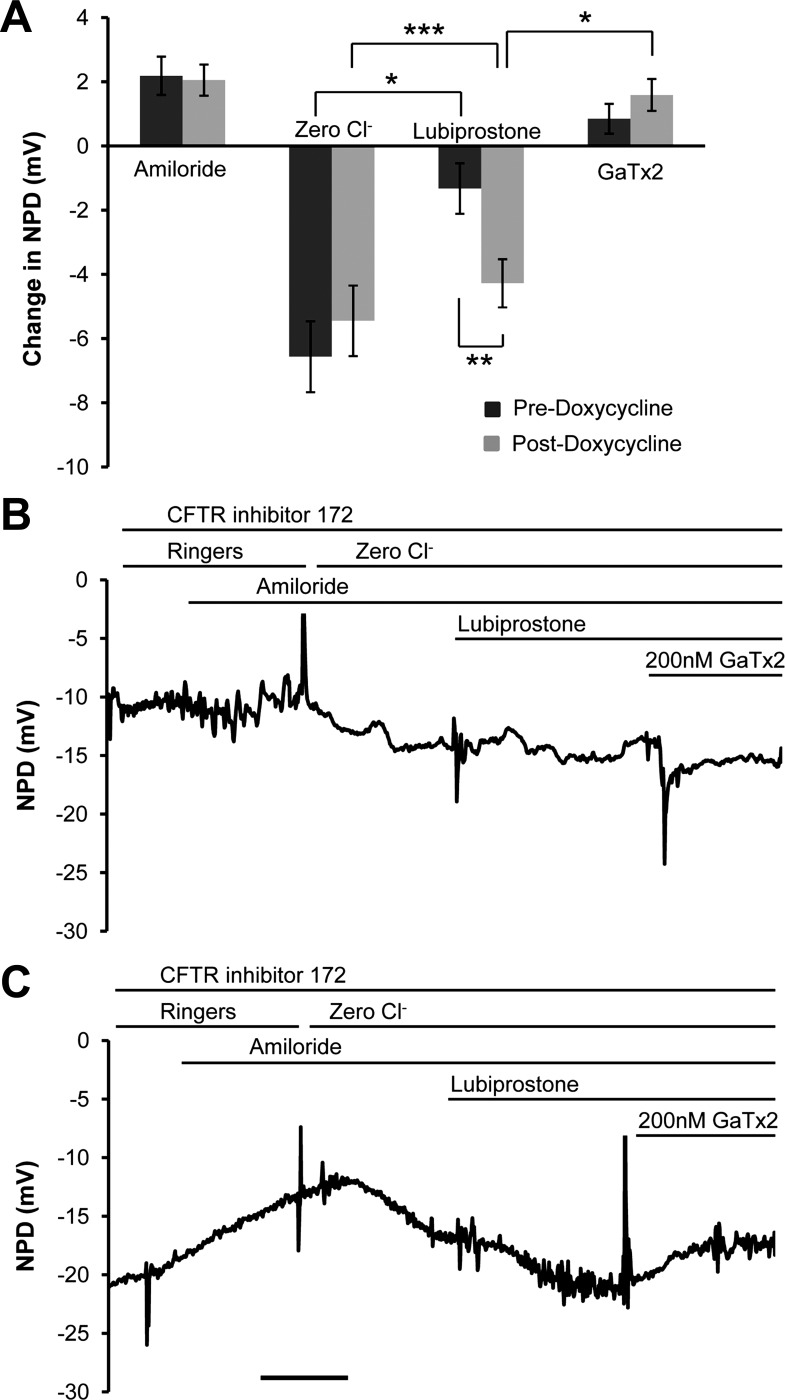
The nasal cavities of 13 anesthetized TGN-CLCN2 mice were superfused with a series of electrolyte solutions, channel blockers, and channel agonists to quantify the contributions of the ENaC, CFTR, and CLCN2 to ion transport, utilizing the nose as a surrogate region for the lower airways where cystic fibrosis is most destructive. This NPD test is entirely analogous to the NPD performed in humans to assess biological activity of CFTR correctors and potentiators and the P2Y2 receptor agonists in clinical trials (2, 27). We modified this protocol to include CFTR inhibitor 172 in all solutions for TGN-CLCN2 mice experiments to reduce efflux by CFTR to quantify changes in CLCN2 activity. Figure 2A compares the changes in PD for each solution before and after upregulation of hCLCN2 with doxycycline. The PD response to a zero chloride perfusion in the presence of amiloride represents anion secretion through any open channels along the chemical concentration gradient from inside the cell to outside the cell. Significant hyperpolarization (also in the negative direction) induced by 20 μM lubiprostone occurred before (−1.32 ± 0.79 mV, P = 0.001) and after (−4.28 ± 0.75 mV, P = 0.00005) doxycycline treatment. We have previously published that, despite the in vitro data from intestinal and kidney cell lines in which lubiprostone is effective at nanomolar concentrations (5, 12), the in vivo NPD chloride channel responses occur at lubiprostone concentrations in the micromolar range. Non-CFTR conductance is reliably activated at 20 μM lubiprostone in the Cftr−/− mouse model (27). After doxycycline treatment, mice responded with a significantly greater PD change (P = 0.005) to lubiprostone perfusion, suggesting that upregulation of the hCLCN2 supplemented the native chloride channels (Fig. 2A). The specific peptide CLCN2 inhibitor GaTx2 (36) significantly antagonized the lubiprostone response only after CLCN2 upregulation by doxycycline (P = 0.01), indicating a shift from basal activation of both CFTR and CLCN2 in the untreated nose to more CLCN2-dependent lubiprostone activity. GaTx2 inhibited 0.85 ± 0.46 mV of the lubiprostone response before doxycycline treatment and 1.59 ± 0.49 mV after treatment. The amiloride-inhibitable PD (ENaC) (predoxycycline = +2.19 ± 0.60 mV and postdoxycycline = +2.05 ± 0.46 mV) and the zero chloride response (predoxycycline = −6.56 ± 1.10 mV and postdoxycycline = −5.45 ± 1.09 mV) were not significantly altered by the brief (24 h) upregulation of CLCN2. Figure 2, B and C, depicts representative experiments from a single mouse before and after treatment with doxycycline. The lubiprostone-mediated CLCN2 activation (in the presence of CFTR inhibitor 172) was significantly inhibited by GaTx2, suggesting an effect on active CLCN2.
Fig. 2.
Induction of hCLCN2 expression for 24 h results in amplification of nasal potential difference (NPD) response to lubiprostone. Mice (n = 13, TGN-CLCN2) were injected with a single intraperitoneal dose of doxycycline as described in methods. NPD was performed under anesthesia before and after treatment. Control mice were studied twice as well. All NPD solutions contained 30 μM CFTRinh-172 to reduce the response by CFTR. A: the change in potential difference (PD) (mean ΔNPD ± SE) in response to each perfusate was calculated and compared in the same mice before and after doxycycline treatment. Treatment with doxycycline significantly amplified the 20 μM lubiprostone response (**P = 0.005). No significant change was observed in response to the amiloride or zero chloride solutions before and after doxycycline treatment. B: NPD tracings of a representative mouse before treatment with doxycycline. C: NPD tracings from the mouse in B after treatment with doxycycline. When the tracing in C is compared with B, the larger response to 20 μM lubiprostone and inhibition by GaTx2 is appreciable after induction of CLCN2. Interestingly, the PD does not depolarize to zero in the presence of inhibitors of ENaC, CFTR, and CLCN2, suggesting incomplete inhibition and/or other types of open ion channels. All scale bars = 3 min. *P < 0.05, **P < 0.01, ***P < 0.001.
Lubiprostone-mediated activation of chloride transport in nasal epithelial of Cftr−/− mice.
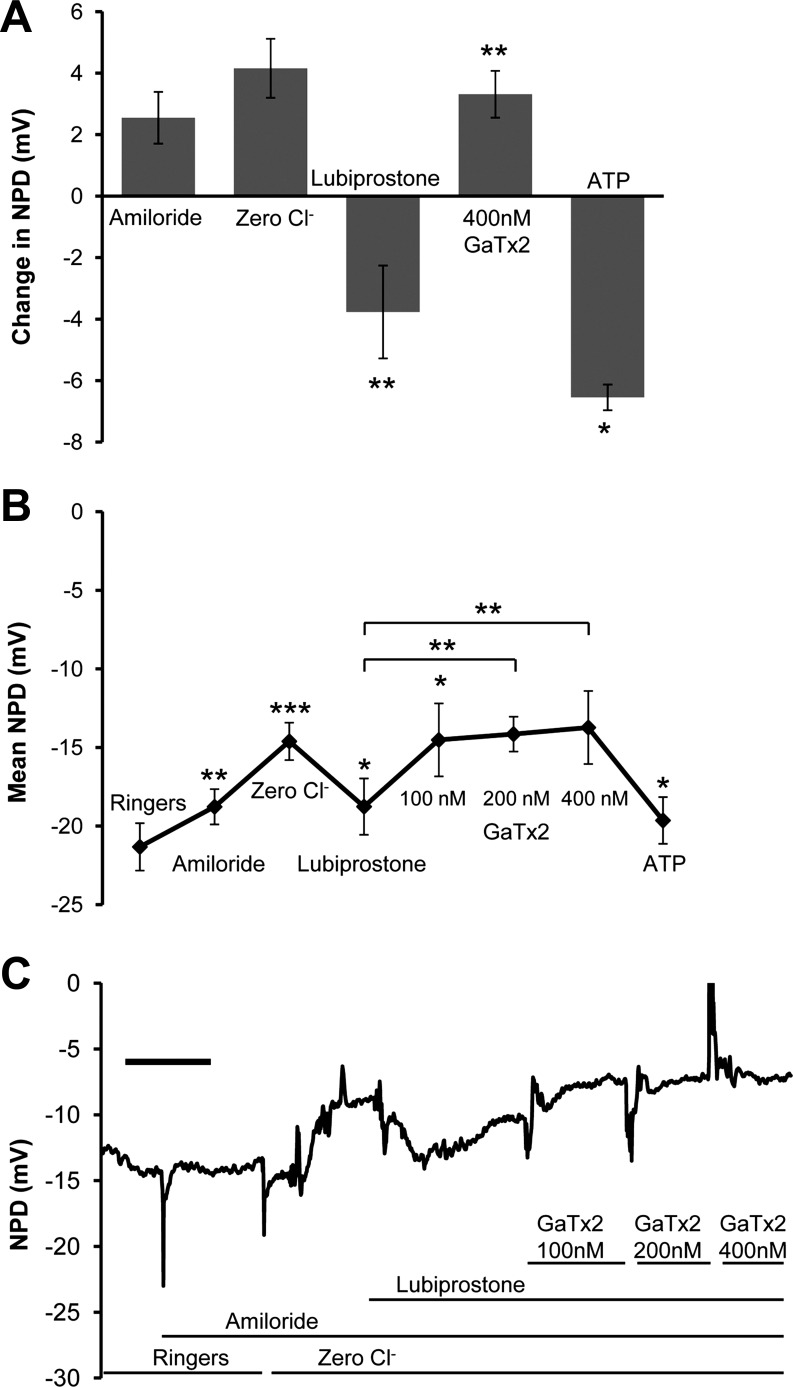
Given the controversy regarding the mechanism of lubiprostone action in humans, we tested the ability of lubiprostone to increase chloride secretion in nasal epithelia of 12 gut-corrected Cftr−/− mice (4–6, 9, 11–13). These NPDs did not include CFTR inhibitor 172. Figure 3A quantifies the amiloride, zero chloride, lubiprostone, and GaTx2 responses in the Cftr−/− model. Importantly, both amiloride (+2.55 ± 0.84 mV) and amiloride in low-chloride solutions (+4.15 ± 0.96 mV) led to a continuous depolarization of the PD consistent with ongoing inhibition of sodium reabsorption by ENaC. The lack of a zero chloride repolarization is consistent with lack of open chloride channels of any type; 20 μM lubiprostone significantly stimulated a chloride secretory response (−3.77 ± 0.49 mV, P = 0.001) that was completely inhibited by GaTx2 (+3.32 ± 0.87 mV, P = 0.005), meaning the absolute value of the magnitude of change did not significantly differ. This demonstrates that lubiprostone can restore chloride secretion to cystic fibrosis airway epithelia by utilizing CLCN2. Figure 3B is a composite of the final recorded values of the PD (in mV) at the end of each perfusion of 12 Cftr−/− mice. Note that in this set of experiments a dose escalation was imposed with the GaTx2 in three sequential solutions. Increasing doses of GaTx2 induced further depolarization, presumably antagonizing the CLCN2. Figure 3C is a representative NPD tracing from a single Cftr−/− mouse from this set of experiments. The tracing demonstrates the overall responses of the aggregate cohort.
Fig. 3.
Cftr−/− mice express a lubiprostone-stimulated PD. Mice (n = 12, wtCFTR gut-corrected Cftr−/− mice) underwent NPD as described in methods. In addition to the standard NPD protocol, 400 nM GaTx2 was perfused in the presence of amiloride to block both EnaC- and CLCN2-stimulated currents. ATP was then added to confirm that GaTx2 did not inhibit the calcium-activated chloride conductances. A: the change in PD (mean ΔNPD ± SE) in response to each perfusate was compared. B: the mean PD over 30 s at the end of each perfusion is graphed. As expected, baseline PD is much more negative in these CF mice than the TGN-CLCN2 mice in Fig. 2. The zero chloride response continues to depolarize from the absence of CFTR, and 20 μM lubiprostone reverses the depolarization to more negative values consistent with chloride secretion. GaTx2 blocks and reverses the lubiprostone response, suggesting that there is a CLCN2 conductance at the apical membranes of murine nasal epithelia. The ATP responses are robust, as would be expected for calcium-activated chloride transport. Values were significantly different between zero chloride and lubiprostone (P < 0.02), lubiprostone and 100 nM GaTx2 (P < 0.02), and lubiprostone and 400 nM GaTx2 (P < 0.01) solutions. Increasing concentrations of GaTx2 return the NPD reading to its value prior to lubiprostone. C: NPD tracing from a representative mouse. Scale bar = 3 min. *P < 0.05, **P < 0.01, ***P < 0.001.
Lubiprostone-mediated induction of CFTR-mediated chloride transport in Clcn2−/− mice.
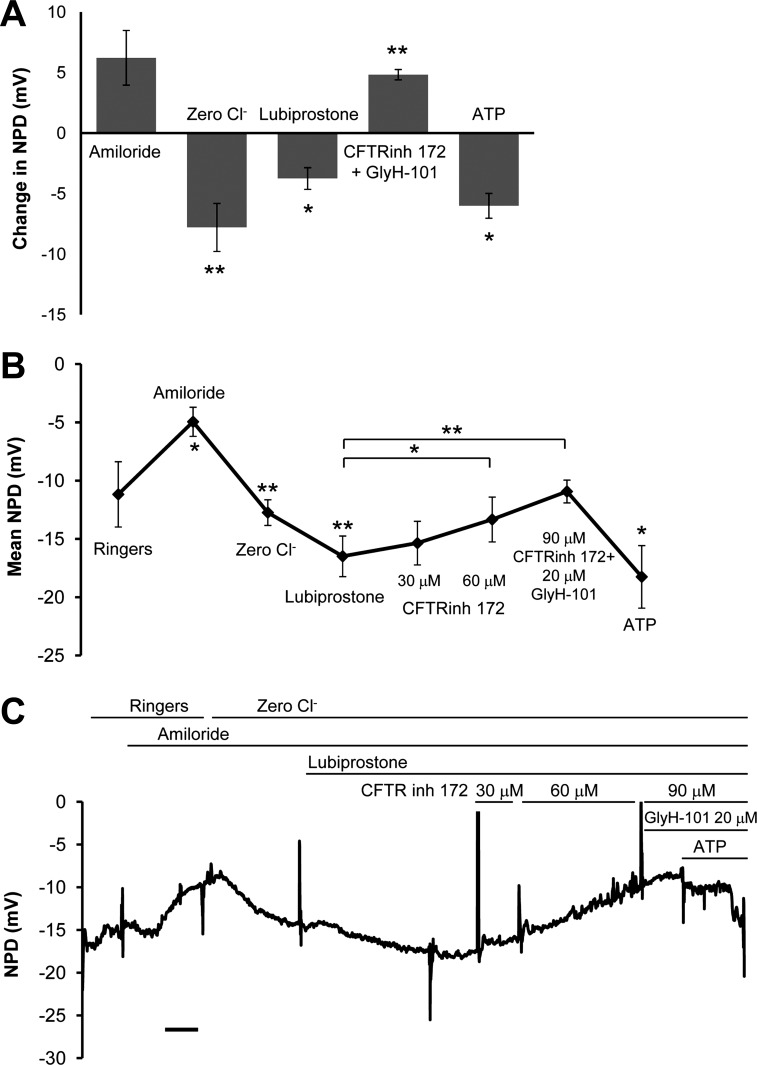
The preponderance of laboratory evidence that lubiprostone is not selective for CLCN2 (4, 6, 9, 13) led us to measure the NPD response to lubiprostone in seven Clcn2−/− mice. Figure 4A quantifies the amiloride, low-chloride, lubiprostone, CFTR inhibitor, and ATP responses in seven Clcn2−/− mice. In contrast to Cftr−/− mice, there was a significant response to low chloride (−7.79 ± 1.98 mV, P = 0.002), suggesting some open CFTR and possibly TMEM16a channels, as well as a significant induction of lubiprostone-mediated chloride transport (−3.75 ± 0.89 mV, P = 0.01). This hyperpolarization was inhibited by the combination of the CFTR inhibitors CFTR inhibitor 172 at 90 μM and GlyH-101 at 20 μM (+4.83 ± 1.24 mV, P = 0.009), which were added only after the 20 μM lubiprostone solution. The ATP response is considerable and confirms independence of calcium-activated chloride conductance from CLCN2 and CFTR conductance (−6.01 ± 0.1.60 mV, P = 0.01). Therefore, in murine nasal epithelia, at 20 μM, lubiprostone appears to act as both a CLCN2 and CFTR chloride channel agonist. Figure 4B displays the aggregate PD (in mV) for each solution and includes each CFTR inhibitor independently. As expected, in Clcn2−/− mice, the addition of high concentrations of two classes of CFTR inhibitor returns the average NPD value to the Ringer baseline level. Figure 4C is a representative NPD that demonstrates the additive effects of the two different classes of CFTR inhibitors in a single mouse.
Fig. 4.
Clcn2−/− mice express a lubiprostone-activated chloride PD that is completely reversible with CFTR inhibitor. Clcn2−/− mice (N = 7) were exposed to increasing concentrations of CFTR inhibitors during the NPD to demonstrate dose-dependent inhibition of lubiprostone (20 μM) mediated conductance. A: the change in PD was calculated during each perfusion. Clcn2−/− mice have baseline PD intermediate between CF (more negative) and CLCN2 overexpressing (less negative) mice. This might reflect the contribution of chloride channels in tempering the tendency to absorb sodium through ENaC. The magnitude of lubiprostone response and subsequent inhibition by a solution containing 90 μM CFTRinh-172 and 20 μM GlyH-101 are not significantly different. B: the last 30 s of each perfusion was averaged. C: a single tracing from a representative mouse shows the dose-dependent inhibition of zero chloride and lubiprostone polarization by CFTR inhibitors. The ATP response by calcium-activated chloride conductance confirms viability of epithelium and presence of ion channels resistant to ENaC and CFTR inhibition. Scale bar = 3 min. *P < 0.05, **P < 0.01, ***P < 0.001.
DISCUSSION
Lubiprostone was initially identified as a CLCN2 agonist during product development (12). More recently, it has been shown to activate both CFTR and CLCN2 in the airway (5) CFTR alone in the airway (13), and it has been argued to require the presence of CFTR in the intestine for activity (6). We have demonstrated that TGN-CLCN2 mice, before overexpression of the human CLCN2 transgene by a single dose of doxycycline treatment, exhibit a basal level of chloride secretion in response to lubiprostone, which we hypothesize is from endogenous murine CLCN2. The endogenous CLCN2 level in nasal epithelia is seen in Fig. 1E. It remains a more remote possibility that there is residual CFTR activation despite the presence of CFTR channel blocker (Fig. 2). CFTR inhibitor 172, which was included in the NPD solutions to reduce the likelihood of CFTR activation, has been shown to completely inhibit forskolin-induced hyperpolarization by CFTR at this concentration (30 μM), but not zero chloride polarization in wild-type mice (34). The zero chloride response in wild-type mice may occur from any type of open chloride channel and is not pathognomonic of CFTR. We also found that higher concentrations of CFTR inhibitors were required to fully block lubiprostone-induced hyperpolarization in the Clcn2−/− mice that contain native CFTR, possibly because of physiological changes to CFTR expression or localization as a result of the Clcn2 knockout. Therefore CFTR inhibitor concentrations used in the TGN-CLCN2 mice at least partially, and at most fully, inhibit the CFTR activation from lubiprostone. Once CLCN2 expression is elevated by doxycycline treatment in these mice, the lubiprostone response is greatly increased, even in the presence of CFTR inhibitors. Significant inhibition by the specific CLCN2 inhibitor GaTx2 after doxycycline treatment confirms that CLCN2 is responsible for the increase in lubiprostone response after overexpression (Fig. 2). This suggests that, in nasal epithelia, lubiprostone is able to activate both channels. However, the use of a CFTR inhibitor does not specifically address the ability of lubiprostone to activate CLCN2 in CF nasal epithelia as it may inhibit but not fully simulate airway epithelial cell function in the absence of CFTR expression. By analyzing Cftr−/− mice, we were able to determine the authentic lubiprostone response for epithelia lacking CFTR and displaying a normal level of CLCN2 ion transport activity. This response was similar to that of TGN-CLCN2 mice after doxycycline-induced overexpression (and in the presence of a CFTR inhibitor) and was inhibited by GaTx2 in a dose-dependent manner, indicating that in nasal epithelia CFTR is not required for lubiprostone-mediated chloride efflux through CLCN2 (Fig. 3). A higher level of response over untreated TGN-CLCN2 mice is expected considering TGN-CLCN2 NPDs were performed with CFTR inhibitors on CFTR-expressing epithelia, where much of the apical chloride conductance is likely CFTR-dependent. Given the sizeable CLCN2 band detected in Cftr−/− murine nasal epithelial homogenates in Fig. 1, the relative abundance, or possibly membrane localization, of CLCN2 may change in the absence of CFTR. In our case, the Cftr−/− nasal cells in vivo responded nearly as well as the nasal cells after doxycycline injection in the CLCN2-overexpressing mice. To test the inverse, whether CLCN2 is required for the CFTR response to prostone, we also analyzed Clcn2−/− mice and clearly showed that lubiprostone can activate chloride secretion in the absence of CLCN2. This response was sensitive to CFTR inhibitors, albeit at high concentrations (Fig. 4), and therefore seems to be CFTR dependent. In these Clcn2−/− mice, where CFTR inhibitors were not added until after lubiprostone challenge, a much stronger response is noted than untreated TGN-CLCN2 mice, suggesting that regardless of CLCN2 expression CFTR responds to the addition of lubiprostone. The expression of CFTR seen in Clcn2−/− mice (Fig. 1F) supports the finding, as CFTR is expressed and available at wild-type levels or greater in this mouse model. Clcn2−/− mice showed baseline PD values less negative than Cftr−/− mice, but more negative than CLCN2 overexpressing TGN-CLCN2 mice after doxycycline treatment, indicating that CLCN2 may play a role in maintaining baseline cell polarization, though a reduced one compared with CFTR. It may do this through chloride conductance and/or by tempering the tendency of ENaC to absorb sodium ions, as CFTR does (5, 22). A molecular mechanism for lubiprostone action on both channels has not been proposed but, however, is feasible in the light of recent insights from several laboratories. CFTR is known to be activated through a cAMP-PKA-dependent signaling pathway, and the importance of the EP4 receptor in increasing intracellular cAMP in response to lubiprostone in sheep airways, leading to CFTR activation, has been demonstrated (13). Concurrently, PKA-independent alternative pathways to chloride secretion are well documented, including the Epac pathway, which can activate protein kinase B (PKB) (17–18). Lubiprostone may activate CLCN2 through a similar PKA-independent pathway; recent data suggests CLCN2 conductance is upregulated by a serum and glucocorticoid inducible kinase, SGK1, and PKB (31). Important to the discussion regarding past analysis of CLCN2's response to lubiprostone is the use of different putative inhibitors of CLCN2; in the absence of a potent and specific CLCN2 inhibitor, CdCl2 (6), glibenclamide (5) and methadone (11) have all been used to argue for or against CLCN2 efflux contribution. The availability of a more potent and specific peptide inhibitor of CLCN2 (36) may improve the ability to confidently determine CLCN2 contribution over less specific inhibitors. GaTx2 is a peptide isolated from the venom of the scorpion Leiurus quinquestriatus hebraeus (Lqh) and shows no inhibitory affect on ClC-1, ClC-3, ClC-4, CFTR, GABAc, Ca2+-dependent Cl− channels, or Shaker ShB-IR K+ channels (36). It inhibits CLCN2 at concentrations as low as 100 pM in vitro but shows its highest rates of inhibition at 10 nM (36). As expected, we found that in vivo somewhat higher concentrations were required to show pronounced inhibition (100–400 nM, Figs. 2 and 3), likely owing to mucosal defenses and the continuous movement of perfused solutions.
CLCN2 may be expressed on either the apical or basolateral membranes of epithelial tissues, which may further explain conflicting reports on lubiprostone's molecular target. In mice, CLCN2 prefers the basolateral membranes of intestinal epithelia (32). Reports on lung expression have accumulated from observation of immunodetectable CLCN2 confined to tight junctions alone, to submembrane vesicles alone, to apical plasma membrane residence (7, 26). Given the rapid response of murine nasal epithelia to superfusion of lubiprostone, we suspect a significant portion of CLCN2 resides on or near the apical membrane for recruitment, especially in the absence of CFTR. Immunolocalization confirms a predominant apical membrane expression in our mouse model (Fig. 1). In humans, we have measured a pH-activated chloride PD consistent with CLCN2 by NPD testing, similar to our results in mice (38). CLCN2 expression is most abundant in infants and young adults (28), especially in the epithelia lining the airway (24), which makes it a rational drug target for CF patients. Although a debate exists over lubiprostone's primary target in intestinal epithelial cells, with some laboratories favoring CLCN2 (5, 11–12) and others CFTR (4, 6, 9, 13), cellular localization and expression of CLCN2 seem more favorable in airways, and this could explain the CLCN2 activation seen in Cftr−/− mice (Fig. 3).
In a clinical setting, direct activation of both CLCN2 and a partially functional CFTR mutant with lubiprostone or similar compound would presumably be additive with respect to chloride secretion. Approximately 10–15% of people with CF have at least one mutation that is partially functional at the plasma membrane (14). CFTR corrector molecules in clinical development also restore some CFTR to the cell surface in patients with Class II mutations, including the most common mutation, ΔF508 (14), and Class I, early termination mutations. Examples in current development include VX-809, from Vertex Pharmaceuticals, and Ataluren, from PTC Therapeutics, which have shown preliminary promise in increasing apical CFTR in patients with ΔF508 and Class I mutations, respectively (20). In these cases, restoration of CFTR to the plasma membrane may only provide limited clinical benefit and could be augmented by a drug that could increase efflux not only through partially functional CFTR but through CLCN2 as well.
Lubiprostone is poorly absorbed in the intestine (23), making its use valuable for the treatment of intestinal dysfunction but not those of other body systems when taken by mouth. Therefore formulation of lubiprostone for delivery via aerosolization or dry powder may be the optimal method to achieve localized higher doses without side effects from systemic administration. It is also known that CLCN2 is abundantly expressed in the brain and is activated by hyperpolarization, leading to strict inward rectification, as well as activation by intracellular chloride, cell swelling, and extracellular acidification (39), providing another rationale for an airway-specific delivery method. Aerosol and dry powder delivery methods have been shown to be effective in preliminary trials for agonists of other chloride channels, including denufosol, a P2Y2 agonist that increases intracellular calcium and stimulates calcium-activated chloride conductance (41). Inhaled denufosol tetrasodium reached Phase III clinical trials, with patients seeing a 2.5% improvement in lung function over 24 wk of treatment (21, 35) but ultimately failed in a multinational, multicenter trial (1). A similar delivery method could be used for lubiprostone delivery.
Our data suggest that, in airway epithelia, lubiprostone is functional and capable of activation of CFTR and CLCN2 channels independently; activation of one channel does not require the presence of the other. CLCN2 activation could clinically benefit CF patients with no luminal functional CFTR, including those with Class I and II mutations. CFTR activation may provide additional benefit to those patients with some apically available CFTR, including those with Class III and IV mutations, as well as Class I and II patients being treated with one of the new correctors or read-through drugs currently under investigation.
GRANTS
P. Zeitlin and N. Vij were supported by National Heart, Lung, and Blood Institute Grant R01-HL-59410.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
E.S., S.L., and P.L.Z. conception and design of research; E.S., P.W.K., D.W., and S.L. performed experiments; E.S., P.W.K., and P.L.Z. analyzed data; E.S., S.L., and P.L.Z. prepared figures; E.S. and P.L.Z. drafted manuscript; E.S., N.V., O.K., P.W.K., S.L., and P.L.Z. edited and revised manuscript; E.S., N.V., O.K., P.W.K., D.W., S.L., and P.L.Z. approved final version of manuscript; N.V., O.K., and P.L.Z. interpreted results of experiments.
ACKNOWLEDGMENTS
Lubiprostone was provided by Sucampo Pharmaceuticals (Bethesda, MD) under a Materials Transfer Agreement with Johns Hopkins University.
REFERENCES
- 1.Cystic Fibrosis Foundation Inspire announces disappointing results for CF therapy denufosol (Online). http://www.cff.org/aboutCFFoundation/NewsEvents/2011NewsArchive/01-03-Inspire-Announces-Denufosol-Results.cfm [2011].
- 2.Ahrens RC, Standaert TA, Launspach J, Han SH, Teresi ME, Aitken ML, Kelley TJ, Hilliard KA, Milgram LJ, Konstan MW, Weatherly MR, McCarty NA. Use of nasal potential difference and sweat chloride as outcome measures in multicenter clinical trials in subjects with cystic fibrosis. Pediatr Pulmonol 33: 142–150, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Anderson MP, Gregory RJ, Thompson S, Souza DW, Paul S, Mulligan RC, Smith AE, Welsh MJ. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science 253: 202–205, 1991 [DOI] [PubMed] [Google Scholar]
- 4.Ao M, Venkatasubramanian J, Boonkaewwan C, Ganesan N, Syed A, Benya RV, Rao MC. Lubiprostone activates Cl− secretion via cAMP signaling and increases membrane CFTR in the human colon carcinoma cell line, T84. Dig Dis Sci 56: 339–351, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Bao HF, Liu L, Self J, Duke BJ, Ueno R, Eaton DC. A synthetic prostone activates apical chloride channels in A6 epithelial cells. Am J Physiol Gastrointest Liver Physiol 295: (2) G234–G251, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bijvelds MJC, Bot AGM, Escher JC, de Jonge HR. Activation of intestinal Cl− secretion by lubiprostone requires the cystic fibrosis transmembrane conductance regulator. Gastroenterology 137: 976–985, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Blaisdell CJ, Edmonds RD, Wang XT, Guggino S, Zeitlin PL. pH-regulated chloride secretion in fetal lung epithelia. Am J Physiol Lung Cell Mol Physiol 278: L1248–L1255, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Boucher RC, Cheng EH, Paradiso AM, Stutts MJ, Knowles MR, Earp HS. Chloride secretory response of cystic fibrosis human airway epithelia. Preservation of calcium but not protein kinase C- and A-dependent mechanisms. J Clin Invest 84: 1424–1431, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catalán MA, Flores CA, González-Begne M, Zhang Y, Sepúlveda FV, Melvin JE. Severe defects in absorptive ion transport in distal colons of mice that lack ClC-2 channels. Gastroenterology 142: 346–354, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham SA, Awayda MS, Bubien JK, Ismailov II, Arrate MP, Berdiev BK, Benos DJ, Fuller CM. Cloning of an epithelial chloride channel from bovine trachea. J Biol Chem 270: 31016–31026, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Cuppoletti J, Blikslager AT, Chakrabarti J, Nighot PK, Malinowska DH. Contrasting effects of linaclotide and lubiprostone on restitution of epithelial cell barrier properties and cellular homeostasis after exposure to cell stressors. BMC Pharmacol 12: 3, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuppoletti J, Malinowska DH, Tewari KP, Li QJ, Sherry AM, Patchen ML, Ueno R. SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents. Am J Physiol Cell Physiol 287: C1173–C1183, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Cuthbert A. Lubiprostone targets prostanoid EP4 receptors in ovine airways. Br J Pharmacol 162: 508–520, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drumm ML, Ziady AG, Davis PB. Genetic variation and clinical heterogeneity in cystic fibrosis. Annu Rev Pathol 7: 267–282, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egan ME, Schweibert EM, Guggino WB. Differential expression of ORCC and CFTR induced by low temperature in CF airway epithelial cells. Am J Physiol Cell Physiol 268: C243–C251, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Fukudo S, Hongo M, Kaneko H, Ueno R. Efficacy and safety of oral lubiprostone in constipated patients with or without irritable bowel syndrome: a randomized, placebo-controlled and dose-finding study. Neurogastroenterol Motil 23: 544.e205, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Halm ST, Zhang J, Halm DR. β-Adrenergic activation of electrogenic K+ and Cl− secretion in guinea pig distal colonic epithelium proceeds via separate cAMP signaling pathways. Am J Physiol Gastrointest Liver Physiol 299: G81–G95, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoque KM, Woodward OM, Van Rossum DB, Zachos NC, Chen L, Leung GPH, Guggino WB, Guggino SE, Tse CM. Epac1 mediates protein kinase A-independent mechanism of forskolin-activated intestinal chloride secretion. J Gen Physiol 135: 43–58, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jentsch TJ, Gunther W, Pusch M, Schwappach B. Properties of voltage-gated chloride channels of the ClC gene family. J Physiol 482: 19S–25S, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones AM, Helm JM. Emerging treatments in cystic fibrosis. Drugs 69: 1903–1910, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Kellerman D, Rossi Mospan A, Engels J, Schaberg A, Gorden JA, Smiley L. Denufosol: a review of studies with inhaled P2Y2 agonists that led to Phase 3. Pulm Pharmacol Ther 21: 600–607, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Konstas AA, Koch JP, Korbmacher C. cAMP-dependent activation of CFTR inhibits the epithelial sodium channel (ENaC) without affecting its surface expression. Pflügers Arch 445: 513–521, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Lacy BE, Levy LC. Lubiprostone: a chloride channel activator. J Clin Gastroenterol 41: 345–351, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Lamb FS, Graeff RW, Clayton GH, Smith RL, Schutte BC, McCray PB., Jr Ontogeny of CLCN3 chloride channel gene expression in human pulmonary epithelium. Am J Respir Cell Mol Biol 24: 376–381, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Li T, Koshy S, Folkesson HG. Involvement of αENaC and Nedd4–2 in the conversion from lung fluid secretion to fluid absorption at birth in the rat as assayed by RNA interference analysis. Am J Physiol Lung Cell Mol Physiol 293: L1069–L1078, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Lipecka J, Bali M, Thomas A, Fanen P, Edelman A, Fritsch J. Distribution of ClC-2 chloride channel in rat and human epithelial tissues. Am J Physiol Cell Physiol 282: C805–C816, 2002 [DOI] [PubMed] [Google Scholar]
- 27.MacDonald KD, McKenzie KR, Henderson MJ, Hawkins CE, Vij N, Zeitlin PL. Lubiprostone activates non-CFTR-dependent respiratory epithelial chloride secretion in cystic fibrosis mice. Am J Physiol Lung Cell Mol Physiol 295: L933–L940, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray C, Chu S, Zeitlin P. Gestational and tissue-specific regulation of C1C-2 chloride channel expression. Am J Physiol Lung Cell Mol Physiol 271: L829–L837, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Murray CB, Morales MM, Flotte TR, McGrath-Morrow SA, Guggino WB, Zeitlin PL. CIC-2: a developmentally dependent chloride channel expressed in the fetal lung and downregulated after birth. Am J Respir Cell Mol Biol 12: 597–604, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Nehrke K, Arreola J, Nguyen HV, Pilato J, Richardson L, Okunade G, Baggs R, Shull GE, Melvin JE. Loss of hyperpolarization-activated Cl− current in salivary acinar cells from Clcn2 knockout mice. J Biol Chem 277: 23604–23611, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Palmada M, Dieter M, Boehmer C, Waldegger S, Lang F. Serum and glucocorticoid inducible kinases functionally regulate ClC-2 channels. Biochem Biophys Res Commun 321: 1001–1006, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Pena-Munzenmayer G, Catalan M, Cornejo I, Figueroa CD, Melvin JE, Niemeyer MI, Cid LP, Sepulveda FV. Basolateral localization of native ClC-2 chloride channels in absorptive intestinal epithelial cells and basolateral sorting encoded by a CBS-2 domain di-leucine motif. J Cell Sci 118: 4243–4252, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Rivkin A, Chagan L. Lubiprostone: chloride channel activator for chronic constipation. Clin Ther 28: 2008–2021, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Salinas DB, Pedemonte N, Muanprasat C, Finkbeiner WF, Nielson DW, Verkman AS. CFTR involvement in nasal potential differences in mice and pigs studied using a thiazolidinone CFTR inhibitor. Am J Physiol Lung Cell Mol Physiol 287: L936–L943, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Storey S, Wald G. Novel agents in cystic fibrosis. Nat Rev Drug Discov 7: 555–556, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Thompson CH, Olivetti PR, Fuller MD, Freeman CS, McMaster D, French RJ, Pohl J, Kubanek J, McCarty NA. Isolation and characterization of a high affinity peptide inhibitor of ClC-2 chloride channels. J Biol Chem 284: 26051–26062, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tizzano EF, Chitayat D, Buchwald M. Cell-specific localization of CFTR mRNA shows developmentally regulated expression in human fetal tissues. Hum Mol Genet 2: 219–224, 1993 [DOI] [PubMed] [Google Scholar]
- 38.Uwaifo O, Bamford P, Zeitlin PL, Blaisdell CJ. Acidic pH hyperpolarizes nasal potential difference. Pediatr Pulmonol 41: 151–157, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Verkman AS, Galietta LJV. Chloride channels as drug targets. Nat Rev Drug Discov 8: 153–171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vij N, Zeitlin PL. Regulation of the ClC-2 lung epithelial chloride channel by glycosylation of SP1. Am J Respir Cell Mol Biol 34: 754–759, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yerxa BR, Sabater JR, Davis CW, Stutts MJ, Lang-Furr M, Picher M, Jones AC, Cowlen M, Dougherty R, Boyer J, Abraham WM, Boucher RC. Pharmacology of INS37217 [P(1)-(uridine 5′)-P(4)-(2′-deoxycytidine 5′)tetraphosphate, tetrasodium salt], a next-generation P2Y(2) receptor agonist for the treatment of cystic fibrosis. J Pharmacol Exp Ther 302: 871–880, 2002 [DOI] [PubMed] [Google Scholar]