Abstract
Cystic Fibrosis (CF) is a chronic lung disease characterized by chronic neutrophilic airway inflammation and increased levels of neutrophil elastase (NE) in the airways. We have previously reported that NE treatment triggers cell cycle arrest. Cell cycle arrest can lead to senescence, a complete loss of replicative capacity. Importantly, senescent cells can be proinflammatory and would perpetuate CF chronic inflammation. By immunohistochemistry, we evaluated whether airway sections from CF and control subjects expressed markers of senescence, including p16INK4a (p16), a cyclin-dependent kinase inhibitor, phospho-Histone H2A.X (γH2A.X), and phospho-checkpoint 2 kinase (phospho-Chk2), which are also DNA damage response markers. Compared with airway epithelium from control subjects, CF airway epithelium had increased levels of expression of all three senescence markers. We hypothesized that the high load of NE in the CF airway triggers epithelial senescence by upregulating expression of p16, which inhibits cyclin-dependent kinase 4 (CDK4). Normal human bronchial epithelial (NHBE) cells, cultured in air-liquid interface were treated with NE (0, 200, and 500 nM) to induce visible injury. Total cell lysates were collected and evaluated by Western analysis for p16 protein expression and CDK4 kinase activity. NE significantly increased p16 expression and decreased CDK4 kinase activity in NHBE cells. These results support the concept that NE triggers expression of senescence markers in CF airway epithelial cells.
Keywords: neutrophil elastase, p16ink4a, phospho-histone 2A.X, phospho-Chk2, telomere length
patients with cystic fibrosis (CF) are plagued by recurrent infections and neutrophilic inflammation in their airways that begin at an early age and result in a decline in lung function (17, 18, 25, 29, 42). There are very high concentrations (μM) of neutrophil elastase (NE), a serine protease, in the airways of patients with CF (3, 18, 26). NE, in concert with increased levels of chemokines present in the bronchoalveolar lavage fluid of patients with CF (4), can lead to airway epithelial injury. We have previously reported that NE induces airway epithelial cell cycle arrest or quiescence (15). Quiescence is a reversible process and cells may re-enter the cell cycle at a later time. However, cells may also be committed to apoptosis or senescence (33, 37). Senescence is the complete loss of replicative capacity and is generally considered a permanent cell fate (33). Senescence is associated with a variety of insults including oxidative stress, DNA damage, and telomere dysfunction (10). Senescence is triggered by several mechanisms: 1) a cell cycle-regulated pathway with increased expression of p16INK4a (p16), a cyclin-dependent kinase inhibitor (CKI) that inhibits cyclin-dependent kinase 4 (CDK4) activity and retinoblastoma protein (Rb) phosphorylation (33); 2) a DNA damage response pathway with increased expression of markers such as phospho-histone 2AX (γ-H2A.X) or phospho-checkpoint kinase 2 (p-Chk2) (7); or 3) telomere shortening associated with aging (23) or with recurrent or chronic lung injury diseases, such as chronic obstructive pulmonary disease (COPD) (32, 39).
Inflammation has been reported to initiate senescence in other organs including pancreas, liver, and kidney (2, 21, 24, 30, 34, 35). In addition, senescent cells exhibit an inflammatory phenotype and produce inflammatory mediators including cytokines, chemokines, and metalloproteases (11, 20, 38, 46). These inflammatory mediators in turn trigger a vicious cycle perpetuating the inflammatory response, as well as propagating and maintaining senescence and contributing to the chronicity and severity of disease. In CF, senescence may be a mechanism to sustain or augment inflammation with increased airway epithelial injury and failure of normal repair. We sought to determine whether airway epithelial senescence occurs in the CF lung. We performed immunohistochemical analyses for senescence markers and analysis of DNA for changes in telomere length. Then we tested whether NE activates epithelial senescence pathways in vitro.
MATERIALS AND METHODS
Subjects.
Airway sections from control lung donors and patients with CF were obtained from the Duke Lung transplant program and Department of Pathology with approval from the Duke Institutional Review Board. All samples were deidentified of protected health information. CF genotypes were not available.
Immunohistochemistry and histological quantitative analysis.
Formalin-fixed and paraffin-embedded airway tissue from 9 patients with CF and 12 control subjects were available for immunohistochemistry (43). The following senescence and DNA damage response markers were evaluated: p16 [Clone G175–405 mouse mAb; BD Biosciences no. 550834; 1:100; citrate antigen retrieval; alkaline phosphatase detection, pink color with methyl green counterstain], γ-H2AX [clone JBW301 (SER139) mouse mAb; Millipore no. 05–636; 1:200; 0.25 mM EDTA, pH 8, 95°C antigen retrieval; diaminobenzidine (DAB), brown color with hematoxylin counterstain], and p-Chk2 [Clone C13C1 (Thr68) rabbit mAb; Cell Signaling no. 2197; 1:400, citrate antigen retrieval; DAB, brown color with hematoxylin counterstain]. Airway tissue sections incubated with mouse monoclonal antibody IgG1κ served as the negative control for p16 and γ-H2AX [clone MOPC-31C mouse mAb IgG1κ; BD Biosciences no. 557273] and sections incubated with rabbit monoclonal antibody IgG served as the negative control for p-Chk2 [clone DA1E rabbit mAb IgG XP™; Cell Signaling no. 3900].
Five to seventeen images of large airways were taken at ×40 to cover the full intact epithelium. Areas with wrinkled or overlapped tissue were not included. The tissue around the airways was erased in Photoshop (Adobe) to assess the staining only in the epithelium. The quantitative analysis was carried out using a color thresholding method in ImageJ (version 1.45s, NIH). For each antibody, two macros were created to automatically calculate the area of positive cells to the antibody and the total area of the epithelium. To avoid biased assessment the same macro was run for all the images of the same marker. From each original photomicrograph, two downstream images were automatically generated representing the stained and total area of the tissue. These images were used to confirm correlation between the stained and total area of the tissue and the original image. The antibody expression was calculated as a percentage by the ratio between the stained area and total area of the epithelium (22).
Telomere length evaluation by quantitative real-time PCR.
Frozen aliquots of two million passage 1 airway epithelial cells from control (n = 18) and patients with CF (n = 18) were used to collect genomic DNA. DNA was extracted using the PureLink spin-column-based extraction kits according to the manufacturer's instructions (Invitrogen). The genomic DNA was used to assess telomere length by qPCR as described by O'Callaghan and French (27). Briefly, a single copy gene (SCG), 36B4, was used as an internal control and standard curves for telomere DNA (TEL), and the SCG were created as described (27). Telomere qPCR and SCG qPCR were performed in separate 96-well plates. Samples were evaluated in two groups to be able to run all samples in triplicate and have an equivalent number of control and CF samples on each 96-well PCR plate. All samples were run in triplicate on an ABI 7300 Sequence Detection System with the SDS Ver. 1.9 software (Applied Biosystems). Reactions included a nontemplate control and positive control (NIH3T3 for long telomeres and HA1 for short telomeres, kindly provided by Dr. Christopher Counter, Duke University). A 20-μl reaction volume was used that contained 16 μl of TEL or SCG Power SYBR master mix (Applied Biosystems, no. 4367659) and 4 μl of standard oligomer diluted in a solution containing plasmid DNA (pGL3) to maintain a constant 20 ng of total DNA per reaction tube or subject DNA sample (20 ng; CTRL or CF). TEL and SCG values were determined from the respective standard curves, a TEL/SCG ratio determined for each sample and then divided by 92, the total number of telomeres on 23 pairs of normal human chromosomes, to provide the absolute telomere length (aTL). The aTL for each subject was used for the statistical analyses and graphs.
SCG amplification is crucial for the accuracy of the results generated in the qPCR assay; therefore, the amplification of 36B4 was compared with another SCG β-globin. The relative ratio of 36B4/β-globin for all the samples was ∼0.98 ranging from 0.96–1.00, indicating that equal copy numbers of single copy gene per cell were amplified (data not shown).
Normal human bronchial epithelial cell culture.
Primary normal human bronchial epithelial (NHBE) cells were harvested by proteolytic digestion (Protease Type XIV; no. P5147, Sigma) from human tracheobronchial tissues obtained from Lung Transplant Program and the Department of Pathology, Duke University Medical Center. The protocol was approved by the Institutional Review Board for Clinical Investigations, Duke University Medical Center. After protease digest, to avoid fibroblast contamination, NHBE cells were removed by gentle washing of the airway surface without scraping. Initial plating and expansion was performed on PureCol (no. 5005-B, Advanced BioMatrix) -coated 10-cm tissue culture dishes in small airway basal media (Lonza):DMEM-H (1:1) supplemented with seven factors: insulin (4 μg/ml), transferrin (5 μg/ml), epidermal growth factor (0.5 ng/ml), dexamethasone (0.1 μM), cholera toxin (20 ng/ml), bovine pituitary extract (50 mg/500 ml; Gemini Bio-products), and BSA (0.5 mg/ml). After initial harvest and expansion, cells were plated on a six-well Transwell Clear chambers (24-mm diameter, Costar/Corning) and cultured in air-liquid interface (ALI) as previously described (44, 45). Cells were cultured in ALI for 9–10 days before experiments.
Neutrophil elastase treatment and Western analyses.
NHBE cells were treated with NE (0, 200, or 500 nM) or control vehicle, apically and basolaterally, for 2–5 h until visible injury occurred, and then lysates were collected as previously described (15). Cell lysates (60–80 μg) were separated by SDS-PAGE on a 4–20% gradient polyacrylamide gel (Bio-Rad), transferred, and immunoblotted for human p16INK4 (p16) expression as a biomarker of senescence [clone G175–1239 mouse monoclonal antibody (mAb); BD Biosciences no. 554079; 1:1,500]. p16 protein expression was normalized to β-actin expression (Sigma, 1:5,000). Densitometric analysis was performed with Image Quant TL software (GE Healthcare) (15).
Immunoprecipitation and kinase activity assay of CDK4.
p16 inhibits CDK4 activity, resulting in inhibition of cell cycle progression, thus leading to senescence. We performed a CDK4 kinase assay to demonstrate the effect of NE on CDK4 activity. Cell lysates collected for Western analyses were also used for these studies. Lysates (400 μg) were first precleared with magnetic Protein G Dynabeads (Invitrogen) and then incubated with a mouse monoclonal antibody for CDK4 (clone DCS-31; Santa Cruz sc-56277; 2 μg, 4°C overnight). Antigen-antibody complexes were captured using magnetic Protein G Dynabeads (Invitrogen). Beads were washed twice with 1× lysis buffer (Cell Signaling 9803), then twice with 1× kinase buffer (Cell Signaling 9802). Beads bound with CDK4 were incubated with 1.0 μg His-Tagged Rb substrate (Upstate) and 200 μM ATP in 20 μl kinase buffer at 30°C for 30 min. Samples were then boiled for 5 min with SDS-PAGE loading buffer (4×, 12.5 μl) and loaded on a 4–20% gel (Bio-Rad). After transfer, membranes were probed with a phospho-Rb antibody (Ser 795; Cell Signaling 9301; 1:1,000) to assess CDK4-mediated phosphorylation at serine 795. Membranes were reprobed with a polyclonal rabbit CDK4 antibody (Santa Cruz, sc-260, 1:1,000) to demonstrate equivalent CDK4 immunoprecipitation and for normalization.
Statistical analysis.
All NHBE experimental results are expressed as means ± SE, and differences between mean values were analyzed by ANOVA with post hoc analysis by the Wilcoxon Rank sum test (Statistix 8, Analytical Software) (36). P values <0.05 were considered statistically significant.
RESULTS
Increased expression of senescence and DNA damage markers in CF airway sections.
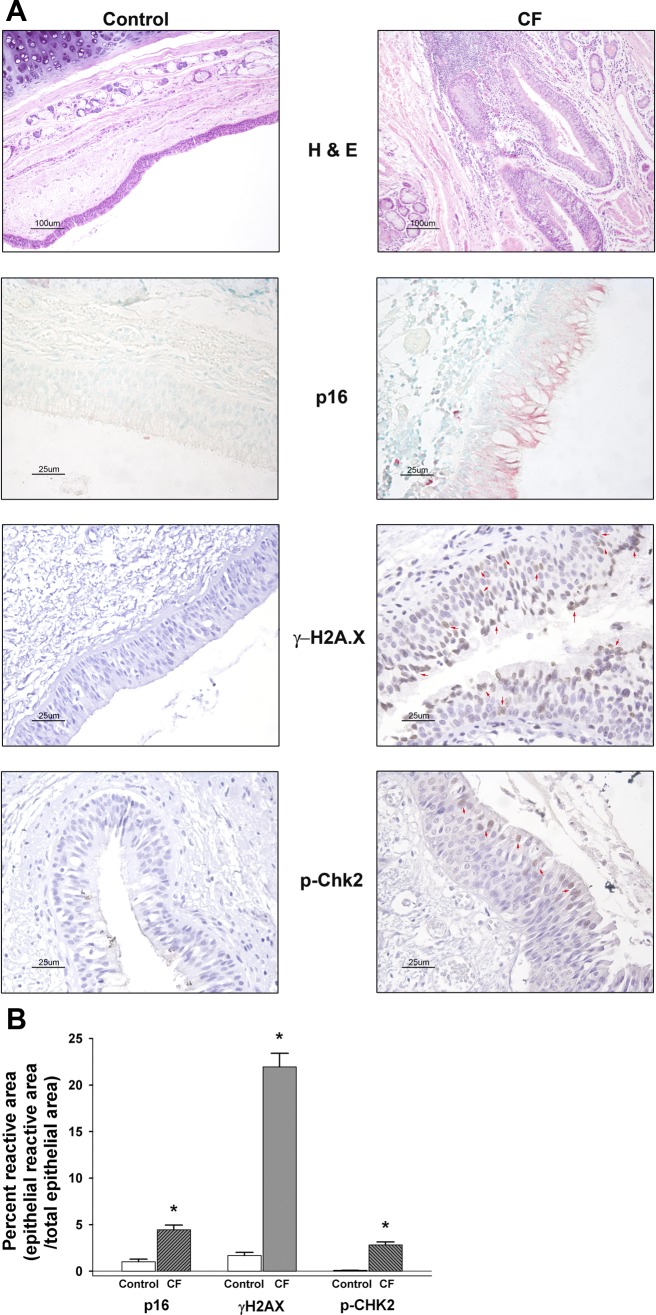
Senescence occurs following airway inflammation or injury as present in the CF lung (46) (see Fig. 1 hematoxylin and eosin image for example of airway inflammation in CF). Senescent cells are proinflammatory (11, 30, 38); therefore, this process is potentially relevant to propagating sustained inflammation in CF. We tested whether CF airway epithelial cells expressed markers consistent with senescence: p16INK4 (p16), a CKI and a biomarker of senescence (19), and DNA damage response markers: phospho-histone 2AX (γ-H2A.X) and phospho-checkpoint kinase 2 (p-Chk2). We performed immunohistochemistry on airway sections from CF lungs from lung transplant recipients, and, as controls, tracheal/bronchial sections from lung transplant donor tissues. We used an unbiased image analysis method to quantitate the expression of each marker in the CF and control airway sections (22). Airway sections from patients with CF compared with control subjects had significantly increased expression of all three markers p16, γ-H2A.X and p-Chk2 (Fig. 1). On the basis of a qualitative assessment, all three markers were predominantly expressed in goblet or ciliated cells, but also some basal cells. Of the three markers, γ-H2A.X showed the most basal cell expression, but it was still less than goblet or ciliated cell marker expression. p-Chk2 was occasionally expressed in basal cells, whereas p16 was only noted in basal cells of one patient. These results suggest that CF airway epithelium was undergoing senescence at greater levels than control individuals.
Fig. 1.
Immunohistochemistry for p16INK4, phospho-Histone H2A.X (γ-H2AX), and phospho-Chk2 (p-Chk2) in control and cystic fibrosis (CF) airway tissue sections. A: representative hematoxylin and eosin (H&E) images (×10 magnification, 100 μm bar) from control (n = 12) and CF (n = 9). Note the increased cellularity of the CF airway epithelium and submucosal area indicative of inflammatory cell infiltration. Control (n = 6) and CF (n = 6) airway tissue sections (5 μm) were immunostained for senescence biomarker cyclin-dependent kinase (CDK) inhibitor, p16INK4 (p16) (alkaline phosphatase detection, pink color with methyl green counterstain), phosphorylated histone 2AX (γ-H2AX; control n = 5, CF n = 7) [diaminobenzidine (DAB), brown color with hematoxylin counterstain], and phospho-Chk2 (p-Chk2; n = 7 for each control and CF) (DAB, brown color with hematoxylin counterstain). Red arrow heads in γ-H2AX and p-Chk2 CF images indicate examples of positively staining nuclei in the epithelium. All senescence marker images were taken at ×40 magnification (25 μm bar) as noted in materials and methods. According to antibody supplier (BD Biosciences), the p16 monoclonal antibody will recognize p16 expression in both the cytoplasm and the nucleus. B: graphic summary of the unbiased quantitative histological analysis for each of the immunostained senescence/DNA damage markers (means ± SE; n = 5–17 images per airway from each control and CF subject). Results are expressed as percentage reactive (stained) area = ratio of epithelial reactive (stained) area to total epithelial area. *CF significantly increased compared with corresponding control subjects stained airways, P < 0.05.
Telomere length assessment.
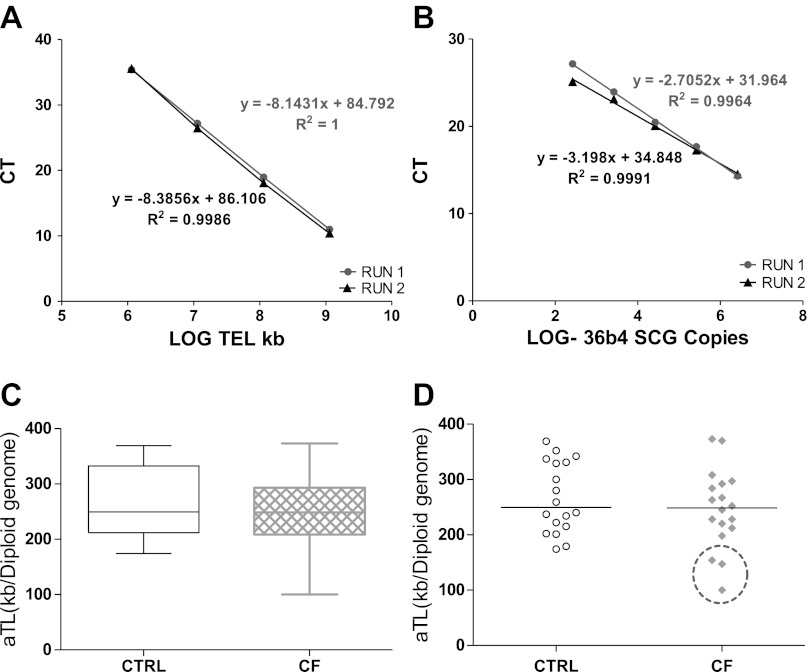
Telomere shortening is one mechanism of senescence. Several studies reported that telomere length is decreased in patients with emphysema and COPD (32, 39), consistent with chronic infection and inflammation, which induce senescence (2, 21, 24, 30, 34, 35). Considering the cellular stress of recurrent exacerbations of infection and inflammation in CF, we evaluated whether CF lung airway epithelial cells also had telomere shortening. The qPCR method utilized to evaluate telomere length was reproducible with very consistent standard curves for both telomere and SCG standards (Fig. 2, A and B). The Ct of telomere ranged from 10–35, and all target samples fell within the standard's linear range. The individual samples with the standard deviation of the triplicate Ct values <1Ct were accepted for further analysis. 98% of the samples analyzed met this criterion. After amplification the telomere DNA kb/reaction was calculated based on the telomere standard curve values as described in materials and methods.
Fig. 2.
Absolute telomere length (aTL) of CF and control airway epithelial cell genomic DNA. DNA was extracted from both control (CTRL) and CF passage 1 airway epithelial cells and analyzed by qPCR for telomere DNA and normalized to single copy gene (SCG) 36B4 (n = 18 per group). On the basis of a standard curve for each telomere and 36B4 DNA, the total telomere length in kb per human diploid genome was calculated. The result was then divided by 92 (total number of telomeres on 23 pairs of human chromosomes) to yield the length per telomere or aTL. A and B: standard curves using oligomer standards for telomere DNA (TEL), 84 bp oligomer, and 36B4 SCG, 75 bp oligomer, were used to calculate the telomere length for each sample. Graphs are plotted with qPCR CT (cycle threshold) as the y-axis. The x-axis is the Log of kb of telomere DNA (TEL) represented by each standard (A) or the Log of 36B4 SCG diploid genome copies represented by each standard (B). These graphs demonstrate the consistent repeatability of the standard curves for this method. C: box-and-whisker plot of CTRL and CF aTL. The line in the middle of each box represents the median. The “whiskers” represent the spread of the data with no outliers noted. D: scatter plot of CTRL and CF aTL. The line represents the median in each group. The three samples circled are those patients with CF whose aTL was below all the control subjects.
Using the real-time PCR (qPCR) method to evaluate telomere length, we determined that there was no significant difference in telomere length between control and CF subjects in this small population (n = 18 per group) (Fig. 2). The CF subjects did have a large spread of telomere length, but no outliers were identified (Fig. 2C). As noted in Fig. 2D, three of the CF subjects (16%) did exhibit telomere shortening compared with all the controls. Because we received all samples deidentified, we do not have any patient information to identify any unique factors about these three patients with CF.
NE increased p16 protein expression and decreased CDK4 activity in NHBE cells.
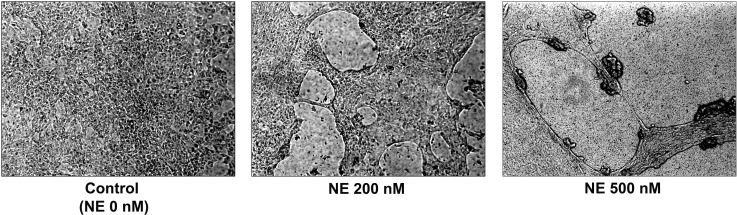
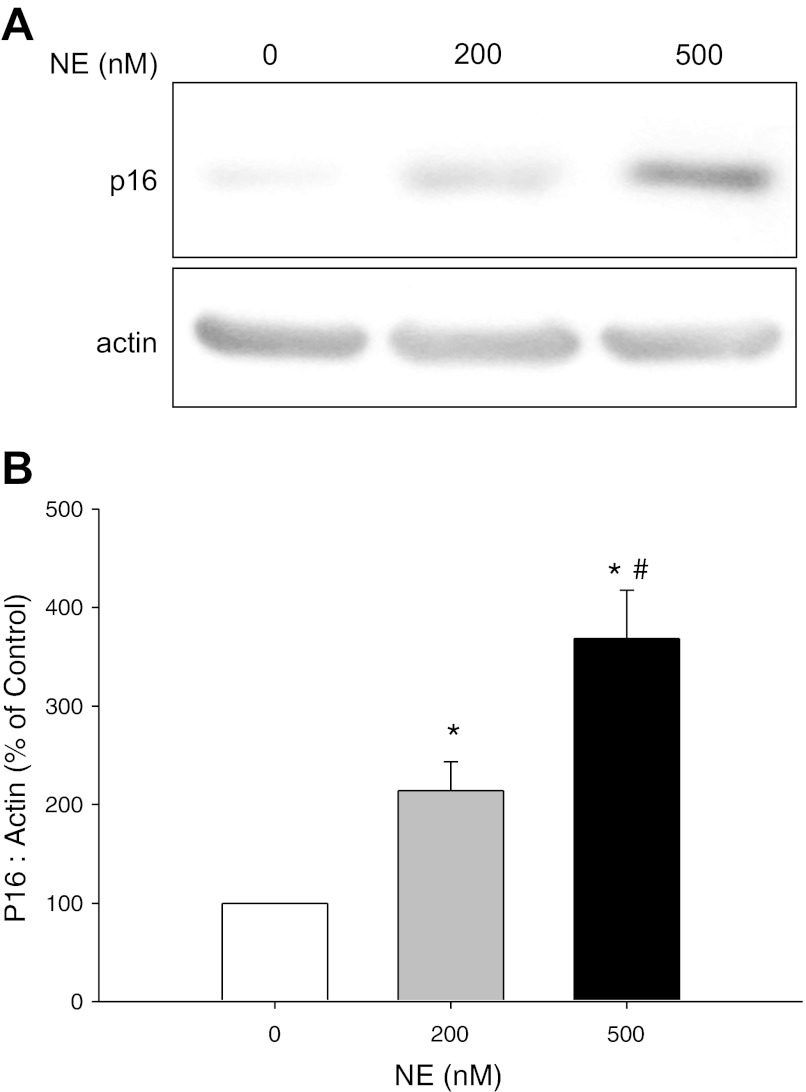
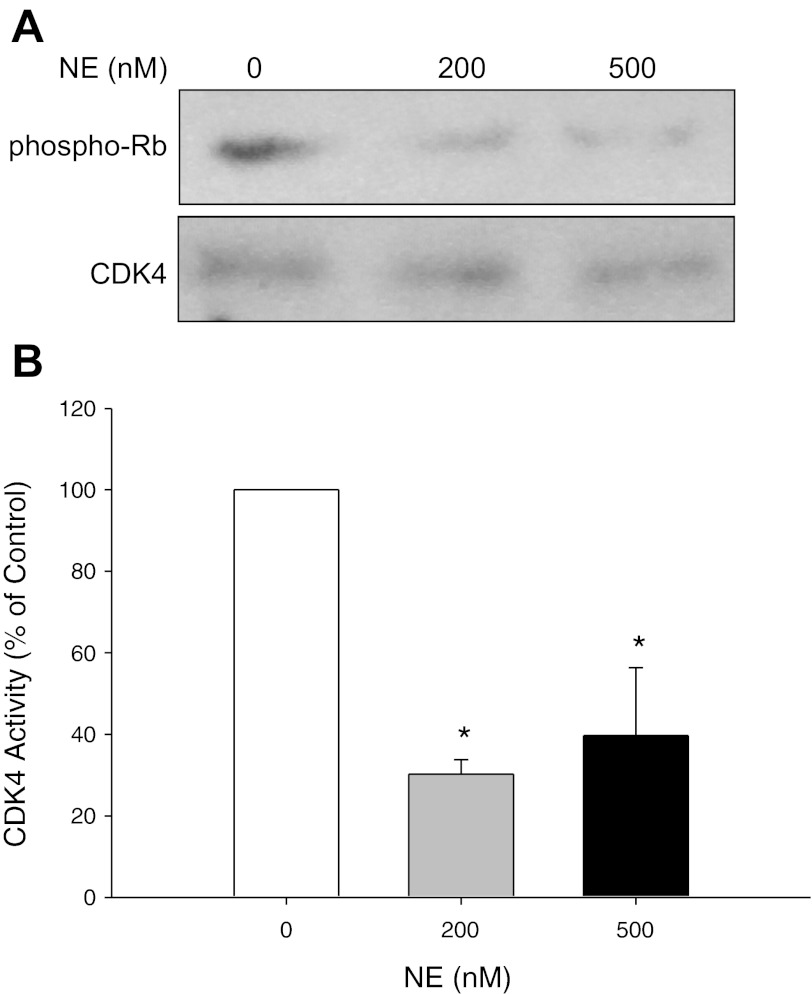
CF is characterized by chronic neutrophilic airway inflammation and persistently high levels of NE in the airways (3, 18, 26). We hypothesized that NE exposure may be one of the senescence triggers in CF airways. We exposed NHBE grown in ALI to varying concentrations of NE or control vehicle. NE treatment injured the epithelial monolayer in a dose-dependent manner (Fig. 3). Western analyses demonstrated a significant increase in p16 protein expression in response to NE treatment (Fig. 4). CDKs, such as CDK4, phosphorylate the Rb at specific phosphorylation sites and enable DNA synthesis to proceed during S phase of the cell cycle. p16 inhibits CDK4 activity, blocking Rb phosphorylation. We performed a kinase assay to evaluate NE-induced alterations of CDK4 activity. Consistent with upregulation of p16 protein expression, NE treatment significantly decreased phosphorylation of Rb, the substrate for CDK4 activity (Fig. 5). Collectively, these results suggest that NE may activate airway epithelial senescence in CF via p16-mediated inhibition of CDK4 and failure of phospho-Rb-triggered DNA synthesis.
Fig. 3.
Neutrophil elastase (NE)-induced injury of normal human bronchial epithelial (NHBE) cells grown in air-liquid interface (ALI) cultures. NHBE were treated with neutrophil elastase (NE; 0, 200, or 500 nM) to induce visible injury. In the absence of NE (0 nM), NHBE form a confluent, differentiated cell monolayer. NE 200 nM exhibits small holes of injury in the cell monolayer. In cells exposed to NE 500 nM, the monolayer is completely disrupted.
Fig. 4.
Western analysis of p16 INK4 protein expression in NHBE cells. NHBE cells grown in ALI culture were treated with control vehicle or NE (0, 200, or 500 nM) for 2–5 h until visible injury occurred. At the end of the treatment period, cell lysates were collected and separated (∼80 μg total protein) on a 4–20% SDS-PAGE. After transfer to nitrocellulose, membrane was probed with a monoclonal antibody for p16INK4 (p16). A monoclonal antibody for β-actin was used to demonstrate equivalent protein loading. A: representative autoradiographs. B: graphic summary of densitometric analysis of autoradiographs from 6 experiments (means ± SE; n = 7–8). Results were expressed as a ratio of p16 protein expression to β-actin protein expression and then expressed as a percentage of the corresponding control. *NE 200 and 500 nM significantly greater than control (NE 0 nM), P < 0.05. #NE 500 nM significantly greater than NE 200 nM, P < 0.05.
Fig. 5.
Western analysis of CDK4 activity in NHBE cells. NHBE cells grown in ALI culture were treated with control vehicle or NE (0, 200, or 500 nM) for 2–5 h until visible injury occurred. At the end of the treatment period, cell lysates were immunoprecipitated (IP) with a mouse monoclonal anti-human antibody to CDK4. Protein complexes were collected with Dynabeads Protein G and then incubated with truncated His-Tagged Retinoblastoma protein (Rb) substrate (1 μg) in kinase reaction buffer. Samples were then separated on a 4–20% SDS-PAGE, transferred to nitrocellulose, and the membrane probed with anti-phospho-pRB (Ser 795) antibody (1:1,000) to measure CDK4 activity phosphorylated at the serine 795 site. An anti-CDK4 polyclonal rabbit antibody (1:1,000) was used to measure total CDK4 protein levels to demonstrate that NE did not change total CDK4 levels and equivalent CDK4 protein was immunoprecipitated. A: representative autoradiographs. B: graphic summary of densitometric analysis of autoradiographs from 3 experiments (means ± SE; n = 4). *NE 200 and 500 nM significantly less than control (NE 0 nM), P < 0.05.
DISCUSSION
CF is a chronic inflammatory lung disease characterized by persistent neutrophil inflammation and bacterial colonization resulting in abnormal repair of the injured airway epithelium (42). In this report, we demonstrated the novel finding that there was increased expression of senescence markers in CF airway epithelium: p16, a CKI that inhibits CDK4 activity and subsequent Rb phosphorylation, and DNA damage markers, phospho-histone 2AX (γ-H2A.X) and phospho-checkpoint kinase 2 (p-Chk2). DNA damage is associated with senescence and can be recognized by a cascade of protein complexes. MRE11, RAD50, and NBS1 form a complex (MRN) that activates the upstream kinases ataxia telangiectasia mutated (ATM), and ATM and Rad-3 related (ATR), which phosphorylate histone H2A.X. Adaptor or DNA damage mediators, such as p53 binding protein-1 (53BP1) and BRCA1, mediate ATM-dependent phosphorylation and activation of the downstream kinase, checkpoint-2 (12). To our knowledge, this is the first report of increased expression of senescence and DNA damage markers in CF airway epithelium.
Telomeres are stretches of repetitive DNA and associated proteins that cap the end of chromosomes. With aging, our telomeres naturally lose 50–200 base pairs of telomeric DNA until the point when they become dysfunctional and trigger a DNA damage response and the cells become senescent. In patients with emphysema and patients with COPD, accelerated or premature telomere shortening has been reported. In a study from Japan comparing lung tissue from emphysema and smoking and nonsmoking control subjects, there is increased expression of p16 in the alveolar epithelial cells of the patients with emphysema (39). These investigators used fluorescent in situ hybridization to demonstrate significant telomere shortening in emphysema subjects and nonemphysematous smokers compared with nonsmokers (39). In a larger study with European subjects, telomere length in circulating leukocytes was evaluated by qPCR, similar to the method we used, and demonstrated significant telomere shortening in patients with COPD compared with either smoker or nonsmoker controls (32). In our small population of patients with CF, there was no difference in the aTL between CF and control epithelial cells; however, three of the patients with CF did demonstrate shortened telomeres compared with all the controls. Results are consistent with the notion that, despite enhanced cell turnover and senescence, in most cases there is sufficient epithelial stem/progenitor cell reserve to maintain normal telomere lengths in the CF airway epithelium. A larger study may be warranted to determine whether there is telomere shortening or alterations in telomerase activity in patients with CF using a well-defined population where results can be correlated with clinical information and disease severity.
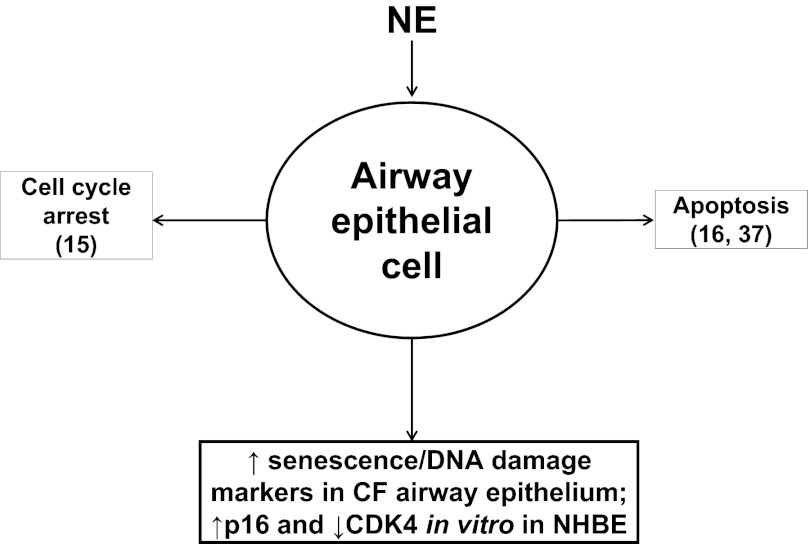
In this report, we demonstrate that NE treatment of differentiated NHBE cells induced a significant increase in p16 protein expression and a corresponding decrease in CDK4 activity as measured by decreased phospho-Rb. These results suggest that NE-induced p16 expression may be one mechanism of causing senescence in CF airways. We and others have reported that NE can trigger reactive oxygen species (ROS) production and oxidative stress in airway epithelial cells (1, 14). NE degrades iron storage or transport proteins, such as ferritin, transferrin, or lactoferrin (5, 6, 13), resulting in the release of catalytically active iron and generating ROS. In addition, CF lungs are exposed to oxidative stress due to systemic glutathione deficiency and mitochondrial oxidative stress (31, 40, 41). Importantly, ROS trigger senescence (8, 9). Thus it is likely that NE exposure contributes to ROS production, oxidative stress, and development of senescence in CF airways. Therefore, NE activates several mechanisms by which it interrupts normal epithelial homeostasis and/or cell fate, namely basal cell hyperplasia and airway epithelial hypertrophy (43), increased epithelial permeability (28), cell cycle arrest (15), apoptosis (16, 37), and senescence (Fig. 6).
Fig. 6.
Heterogeneity of airway epithelial cell fates in response to NE. NE regulates several alternative airway epithelial cell fates: cell cycle arrest (15), apoptosis (16, 37), and senescence. In this report, we demonstrate that NE upregulated p16 and inhibited CDK4; this pathway induces epithelial senescence.
We propose that NE exposure may lead to senescence by a mechanism dependent on ROS and oxidative stress. It is not known whether NE is an initiator or sustainer of the senescent phenotype, but it is likely a key inflammatory mediator important in regulating overall airway epithelial cell fate in CF. Our findings underscore the need for more robust therapeutic approaches to block inflammation and the protease load in the lungs of patients with CF.
GRANTS
This work supported by NIH grants HL082504 (J. Voynow), ES016836 (J. Voynow), HL081763 (B. Fischer), the Alpha-1 Foundation (B. Fischer) and Duke School of Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: B.M.F. conception and design of research; B.M.F., J.K.W., S.D., A.B.K., S.Z., and P.H. analyzed data; B.M.F., J.K.W., S.D., A.B.K., S.Z., P.H., and J.A.V. interpreted results of experiments; B.M.F., J.K.W., S.D., A.B.K., and P.H. prepared figures; B.M.F., J.K.W., A.B.K., and P.H. drafted manuscript; B.M.F., S.D., and J.A.V. edited and revised manuscript; B.M.F., J.K.W., S.D., A.B.K., S.Z., P.H., and J.A.V. approved final version of manuscript; J.K.W., S.D., A.B.K., S.Z., and P.H. performed experiments.
ACKNOWLEDGMENTS
This work was presented in part at the American Thoracic Society Meeting, Denver, CO, May 2011 and the North American Cystic Fibrosis Conference, Anaheim, CA, October 2011.
REFERENCES
- 1. Aoshiba K, Yasuda K, Yahui S, Tamaoki J, Nagai A. Serine proteases increase oxidative stress in lung cells. Am J Physiol Lung Cell Mol Physiol 281: L556–L564, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Barash H, ERG , Edrei Y, Ella E, Israel A, Cohen I, Corchia N, Ben-Moshe T, Pappo O, Pikarsky E, Goldenberg D, Shiloh Y, Galun E, Abramovitch R. Accelerated carcinogenesis following liver regeneration is associated with chronic inflammation-induced double-strand DNA breaks. Proc Natl Acad Sci USA 107: 2207–2212, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Birrer P, McElvaney NG, Rudeberg A, Sommer CW, Liechti-Gallati S, Kraemer R, Hubbard R, Crystal RG. Protease-antiprotease imbalance in the lungs of children with cystic fibrosis. Am J Respir Crit Care Med 150: 207–213, 1994 [DOI] [PubMed] [Google Scholar]
- 4. Brennan S, Sly PD, Gangell CL, Sturges N, Winfield K, Wikstrom M, Gard S, Upham JW. Alveolar macrophages and CC chemokines are increased in children with cystic fibrosis. Eur Respir J 34: 655–661, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Britigan BE, Edeker BL. Pseudomonas and neutrophil products modify transferrin and lactoferrin to create conditions that favor hydroxyl radical formation. J Clin Invest 88: 1092–1102, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Britigan BE, Hayek MB, Doebbeling BN, Fick RB., Jr Transferrin and lactoferrin undergo proteolytic cleavage in the Pseudomonas aeruginosa-infected lungs of patients with cystic fibrosis. Infect Immun 61: 5049–5055, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8: 729–740, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Chen QM. Replicative senescence and oxidant-induced premature senescence. Beyond the control of cell cycle checkpoints. Ann NY Acad Sci 908: 111–125, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Colavitti R, Finkel T. Reactive oxygen species as mediators of cellular senescence. IUBMB Life 57: 277–281, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Collado M, Serrano M. The power and the promise of oncogene-induced senescence markers. Nat Rev Cancer 6: 472–476, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5: 99–118, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DiTullio RA, Jr, Mochan TA, Venere M, Bartkova J, Sehested M, Bartek J, Halazonetis TD. 53BP1 functions in an ATM-dependent checkpoint pathway that is constitutively activated in human cancer. Nat Cell Biol 4: 998–1002, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Fischer BM, Domowicz DA, Zheng S, Carter JL, McElvaney NG, Taggart C, Lehmann JR, Voynow JA, Ghio AJ. Neutrophil elastase increases airway epithelial nonheme iron levels. Clin Transl Sci 2: 333–339, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fischer BM, Voynow JA. Neutrophil elastase induces MUC5AC gene expression in airway epithelium via a pathway involving reactive oxygen species. Am J Respir Cell Mol Biol 26: 447–452, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Fischer BM, Zheng S, Fan R, Voynow JA. Neutrophil elastase inhibition of cell cycle progression in airway epithelial cells in vitro is mediated by p27kip1. Am J Physiol Lung Cell Mol Physiol 293: L762–L768, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Ginzberg HH, Shannon P, Suzuki T, Hong OVE, Moraes T, Abreu MTH, Cherepanov V, Wang X, Chow CW, Downey GP. Leukocyte elastase induces epithelial apoptosis: role of mitochondrial permeability changes and Akt. Am J Physiol Gastrointest Liver Physiol 287: G286–G298, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Khan TZ, Wagener JS, Bost T, Martinez J, Accurso F, Riches DWH. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med 151: 1075–1082, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Konstan MW, Hilliard KA, Norvell TM, Berger M. Bronchoalveolar lavage findings in cystic fibrosis patients with stable, clinically mild lung disease suggest ongoing infection and inflammation. Am J Respir Crit Care Med 150: 448–454, 1994 [DOI] [PubMed] [Google Scholar]
- 19. Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. J Clin Invest 114: 1299–1307, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuilman T, Peeper DS. Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer 9: 81–94, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Lee KE, Bar-Sagi D. Oncogenic KRas suppresses inflammation-associated senescence of pancreatic ductal cells. Cancer Cell 18: 448–458, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin R, Degan S, Theriot BS, Fischer BM, Strachan RT, Liang J, Pierce RA, Sunday ME, Noble PW, Kraft M, Brody AR, Walker JK. Chronic treatment in vivo with beta-adrenoceptor agonists induces dysfunction of airway beta(2) -adrenoceptors and exacerbates lung inflammation in mice. Br J Pharmacol 165: 2365–2377, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lindsey J, McGill NI, Lindsey LA, Green DK, Cooke HJ. In vivo loss of telomeric repeats with age in humans. Mutat Res 256: 45–48, 1991 [DOI] [PubMed] [Google Scholar]
- 24. Melk A, Schmidt BM, Vongwiwatana A, Rayner DC, Halloran PF. Increased expression of senescence-associated cell cycle inhibitor p16INK4a in deteriorating renal transplants and diseased native kidney. Am J Transplant 5: 1375–1382, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Muhlebach MS, Stewart PW, Leigh MW, Noah TL. Quantitation of inflammatory responses to bacteria in young cystic fibrosis and control patients. Am J Respir Crit Care Med 160: 186–191, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Nakamura H, Yoshimura K, McElvaney NG, Crystal RG. Neutrophil elastase in respiratory epithelial lining fluid of individuals with cystic fibrosis induces interleukin-8 gene expression in a human bronchial epithelial cell line. J Clin Invest 89: 1478–1484, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. O'Callaghan NJ, Fenech M. A quantitative PCR method for measuring absolute telomere length. Biol Proced Online 13: 3, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peterson MW, Walter ME, Nygaard SD. Effect of neutrophil mediators on epithelial permeability. Am J Respir Cell Mol Biol 13: 719–727, 1995 [DOI] [PubMed] [Google Scholar]
- 29. Pillarisetti N, Williamson E, Linnane B, Skoric B, Robertson CF, Robinson P, Massie J, Hall GL, Sly P, Stick S, Ranganathan S. Infection, inflammation, and lung function decline in infants with cystic fibrosis. Am J Respir Crit Care Med 184: 75–81, 2011 [DOI] [PubMed] [Google Scholar]
- 30. Ren JL, Pan JS, Lu YP, Sun P, Han J. Inflammatory signaling and cellular senescence. Cell Signal 21: 378–383, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roum JH, Buhl R, McElvaney NG, Borok Z, Crystal RG. Systemic deficiency of glutathione in cystic fibrosis. J Appl Physiol 75: 2419–2424, 1993 [DOI] [PubMed] [Google Scholar]
- 32. Savale L, Chaouat A, Bastuji-Garin S, Marcos E, Boyer L, Maitre B, Sarni M, Housset B, Weitzenblum E, Matrat M, Le Corvoisier P, Rideau D, Boczkowski J, Dubois-Rande JL, Chouaid C, Adnot S. Shortened telomeres in circulating leukocytes of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 179: 566–571, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sharpless NE, DePinho RA. Telomeres, stem cells, senescence, cancer. J Clin Invest 113: 160–168, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, Hainzl A, Schatz S, Qi Y, Schlecht A, Weiss JM, Wlaschek M, Sunderkotter C, Scharffetter-Kochanek K. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest 121: 985–997, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sis B, Tasanarong A, Khoshjou F, Dadras F, Solez K, Halloran PF. Accelerated expression of senescence associated cell cycle inhibitor p16INK4A in kidneys with glomerular disease. Kidney Int 71: 218–226, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Snedecor GW, Cochran WG. Statistical Methods. Ames, IA: Iowa State University Press, 1980, p. 507 [Google Scholar]
- 37. Suzuki T, Moraes TJ, Vachon E, Ginzberg HH, Huang TT, Matthay MA, Hollenberg MD, Marshall J, McCulloch CA, Abreu MT, Chow CW, Downey GP. Proteinase-activated receptor-1 mediates elastase-induced apoptosis of human lung epithelial cells. Am J Respir Cell Mol Biol 33: 231–247, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence exacerbates pulmonary inflammation in patients with chronic obstructive pulmonary disease. Respiration 80: 59–70, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence in patients with pulmonary emphysema. Am J Respir Crit Care Med 174: 886–893, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Velsor LW, Kariya C, Kachadourian R, Day BJ. Mitochondrial oxidative stress in the lungs of cystic fibrosis transmembrane conductance regulator protein mutant mice. Am J Respir Cell Mol Biol 35: 579–586, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Velsor LW, van Heeckeren A, Day BJ. Antioxidant imbalance in the lungs of cystic fibrosis transmembrane conductance regulator protein mutant mice. Am J Physiol Lung Cell Mol Physiol 281: L31–L38, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Voynow JA, Scanlin TF. Cystic Fibrosis. In: Fishman's Pulmonary Diseases and Disorders, edited by Fishman AP, Elias JA, Fishman JA, Grippi MA, Senior RM, Pack AI. New York: McGraw Hill Medical, 2008, p. 863–885 [Google Scholar]
- 43. Voynow JA, Fischer BM, Roberts BC, Proia AD. Basal-like cells constitute the proliferating cell population in cystic fibrosis airways. Am J Respir Crit Care Med 172: 1013–1018, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Voynow JA, Fischer BM, Zheng S, Potts E, Grover A, Jaiswal AK, Ghio AJ, Foster WM. NAD(P)H Quinone Oxidoreductase 1 Is Essential for Ozone-induced Oxidative Stress in Mice and Humans. Am J Respir Cell Mol Biol 41: 107–113, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 45. Zheng S, Byrd AS, Fischer BM, Grover AR, Ghio AJ, Voynow JA. Regulation of MUC5AC expression by NAD(P)H:quinone oxidoreductase 1. Free Radic Biol Med 42: 1398–1408, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhou F, Onizawa S, Nagai A, Aoshiba K. Epithelial cell senescence impairs repair process and exacerbates inflammation after airway injury. Respir Res 12: 78, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]