Abstract
Lipopolysaccharide (LPS)-mediated endothelial activation contributes to lung inflammation and alveolar remodeling seen in premature infants with bronchopulmonary dysplasia (BPD). The mechanisms underlying LPS-mediated oxidative stress and proinflammatory signaling in human pulmonary microvascular endothelial cells (HPMEC) remain unclear. We hypothesized that NADPH oxidase (Nox) mediates LPS-induced endothelial activation in HPMEC by regulating phosphorylation of Toll-like receptor (TLR) pathway proteins. LPS-induced expression of intercellular adhesion molecule 1 (ICAM-1) was associated with increased 2-OH-E+ (marker for superoxide formation) levels and was attenuated by apocynin and the Nox inhibitor, VAS2870. LPS triggered membrane translocation of p67phox, suggesting activation of Nox2. Silencing Nox2, but not Nox4, suppressed LPS-induced ICAM-1 expression in HPMEC. Immunoprecipitation studies showed that inhibitor of κ-B kinase-β (IKK-β) serine phosphorylation induced by LPS was inhibited by Nox2 silencing. We examined whether Nox2-dependent, LPS-mediated IKK-β phosphorylation was regulated by protein phosphatase 2A (PP2A) or TGF-β associated kinase-1 (TAK1) in HPMEC. LPS increased PP2A activity in HPMEC, and inhibition of PP2A did not alter LPS-mediated ICAM-1 expression but attenuated IKK-β phosphorylation. TAK1 inhibition decreased LPS-induced ICAM-1 expression in HPMEC, and Nox2 silencing attenuated LPS-mediated TAK1 phosphorylation (Thr184/187). We demonstrate that Nox2 regulates LPS-mediated endothelial activation in pulmonary endothelial cells by modulating phosphorylation of key kinases in the TLR signaling cascade. Our data support a novel mechanism by which Nox-dependent signaling regulates proinflammatory signaling in pulmonary endothelial cells. Inhibition of vascular Nox may potentially limit lung injury and alveolar remodeling caused by infections in BPD.
Keywords: lipopolysaccharide, lung endothelial cells, NADPH oxidase 2, intercellular adhesion molecule-1, inhibitor of κ-B kinase-β phosphorylation, TGF-β associated kinase-1
bronchopulmonary dysplasia (BPD), a debilitating lung disease affecting premature infants, remains the major cause of pulmonary morbidity and mortality during infancy (14). Bacterial inflammation in the saccular lung contributes to vascular remodeling in BPD, characterized histologically by arrested vascular growth with decreased arborizaton and dysmorphic capillaries (56, 57). Lipopolysaccharide (LPS) derived from bacteria is a potent mediator of endothelial activation; a complex change in the endothelial phenotype is characterized notably by expression of cellular adhesion molecules and cytokines such as intercellular adhesion molecule 1 (ICAM-1), IL-8, and monocyte chemoattractant protein-1, and increased endothelial-leukocyte interactions (3, 58). LPS-mediated endothelial activation in the lung promotes neutrophil influx, increased vascular permeability, inflammation, and alveolar matrix digestion that contribute to vascular remodeling in BPD (6, 11, 45). Although it is known that LPS-mediated endothelial injury is associated with increased reactive oxygen species (ROS) formation, the mechanisms underlying LPS-mediated oxidant formation and endothelial activation in pulmonary endothelial cells remain unknown (2, 8, 19).
NADPH oxidases (Nox) belong to a family of enzymes that generate superoxide (O2−) by one electron reduction of oxygen using NADPH as an electron donor (5, 28). The Nox family comprises seven members, with each isoform differing in mode of activation, tissue distribution, and cellular compartmentalization (5, 7, 28). In endothelial cells, Nox-derived ROS is essential for maintaining endothelial cell phenotype and function, whereas pathological activation of Nox contributes to inflammation and acute lung injury (3, 54). Ligand-mediated Nox activation in the endothelium requires translocation of cytosolic regulatory subunits to the membrane-bound catalytic complex for Nox2 and Nox1, whereas Nox4 is constitutively active and is regulated at the level of expression (5, 7, 28). Nox2, Nox4, and Nox1 have been shown to mediate LPS sensitivity in human neutrophils, aortic endothelial cells, and guinea pig gastric cells, respectively (38). The Nox isoform that mediates LPS-induced endothelial activation in human pulmonary microvascular endothelial cells (HPMEC) remains unknown. Furthermore, the mechanisms by which Nox regulates LPS-induced ICAM-1 expression (a marker of endothelial activation essential for leukocyte transendothelial migration) in HPMEC have yet to be characterized (58).
In the canonical toll-like receptor (TLR) signaling pathway, LPS-mediated activation of the inhibitor of κ-B kinase (IKK) complex facilitates degradation of IκB with subsequent nuclear translocation of NF-κB and induction of the inflammatory transcriptional program (1, 10). IKK-β, a catalytic subunit of the IKK complex is responsible for rapid (min) activation of NF-κB, whereas IKK-α-dependent NF-κB activation requires hours (15, 48). Phosphorylation of serine residues 177 and 181 in the activation loop of IKK-β results in a conformational change, leading to restoration of kinase activity (13, 21). Studies using antioxidants and hydrogen peroxide suggest that IKK-β phosphorylation in response to inflammatory stimuli is amenable to redox regulation (20, 26, 34). However, because of the wide-ranging effects of chemicals on cellular signaling, these results have to be interpreted with caution. We hypothesized that LPS-induced endothelial activation is regulated by Nox through posttranslational modifications in TLR signaling pathway kinases. In this study, we demonstrate that Nox2 regulates LPS-induced ICAM-1 expression in HPMEC by facilitating serine phosphorylation of IKK-β. Furthermore, our data suggests that phosphorylation of TGF-β associated kinase-1 (TAK1), a member of the MAPKKK family (MAP3K7), is also modulated by Nox2.
MATERIALS AND METHODS
Cell culture and reagents.
HPMEC from ScienCell (Carlsbad, CA) were used between passages 3–4 for all experiments. HPMEC were characterized by presence of reactivity to vWF/Factor VIII and PECAM1 (using immunofluorescence) and by uptake of DiI-Ac-LDL. HPMEC were grown in endothelial cell medium (ECM) supplemented with fetal bovine serum, antibiotics, and endothelial cell growth serum as recommended by the manufacturer (ScienCell) in a humidified incubator containing 5% CO2 at 37°C. LPS (100 ng/ml) and Apocynin (Apo, 4′-Hydroxy-3′-methoxyacetophenone), a Nox inhibitor, were both from Sigma (St. Louis, MO). VAS2870 [3-benzyl-7-(2-benzoxazolyl) thio-1,2,3,-triazolo (4,5-d) pyrimidine], a reversible Nox inhibitor, was obtained from Vasopharm (kind gift of Dr. Reinhard Schinzel, Würzburg, Germany). Okadaic acid (OA), a protein phosphatase 2A (PP2A) inhibitor, and (5Z)-7-oxozeaenol (iTAK, antibiotic LL Z1640–2), a TAK1 inhibitor, were both from Santa Cruz Biotechnologies (Santa Cruz, CA). (5Z)-oxozeanol, a compound similar to (5Z)-7-oxozeanol but without kinase activity, was from Upstate Biotechnology (Billerica, MA). For experiments with inhibitors, cells were pretreated with the chemicals for 40 min before the addition of LPS.
Immunoblotting for ICAM-1 expression.
Whole cell lysates prepared from HPMEC after various treatments were used. Cells were washed with PBS twice and then lysed in a modified RIPA buffer (10 mM Tris·HCl, pH 7.4, 100 mM NaCl, 0.5% sodium deoxycholate, 1 mM EDTA, 1 mM NaF, 2 mM Na3VO4, 0.1% SDS, 1% Triton-100, and 10% glycerol) containing commercially available protease inhibitors and phosphatase inhibitors (Sigma). Clarified cell lysates obtained after centrifugation at 13,000 revolution/min for 6 min were used for Western blotting. Protein quantification was done using a BCA protein assay from Thermo Fisher (Rockford, IL) according to the manufacturer's protocol using BSA as a protein standard. Protein (30 μg) was resolved using SDS-PAGE, and immunoblotting was done following standard protocol. Blots were incubated overnight at 4°C with mouse anti-ICAM-1 (Santa Cruz Biotechnology, 1:1,000) and mouse anti-β-Actin (Sigma, 1:5,000). Blots were developed using enhanced chemiluminescence (ECL), and densitometry was performed using ImageJ Software (NIH). β-Actin was used for normalization.
Detection of intracellular superoxide formation.
O2− levels were quantified using HPLC analysis of 2-hydroxyethidium (2-OH-E+) formation as described before (60). Control and LPS-treated cells were incubated in the dark for 30 min with 15 μM dihydroethidium from Invitrogen (Carlsbad, CA). Cell pellets obtained by centrifugation were resuspended in 300 μl of 1% Triton X-100 in PBS and then lysed. After n-butanol extraction and drying in 100% N2, sample residues were reconstituted in 100 μl of ice-cold 1 M phosphate buffer (pH 2.6), centrifuged, and supernatants transferred to amber-colored HPLC vials. Samples were injected into the HPLC system (Agilent Technologies, Santa Clara, CA) and hydroethidine, ethidium, and 2-OH-E+ were separated by a linear increase in CH3CN concentration. Elution was monitored by a UV detector at 210 and 350 nm and a fluorescence detector with excitation and emission at 510 and 595 nm, respectively. The area under the 2-OH-E+ fluorescent peaks was measured for each sample and compared with known concentrations of the standard, expressed in pM/mg protein.
Cellular fractions.
Detergent-based fractionation was performed using buffers prepared as described before (17). After two washes with PBS, HPMECs were removed by trypsinization and 500 μl of ice-cold cytoplasmic buffer with protease inhibitors was added. The cell suspension was incubated at 4°C while spinning end over end for 1 h, and then centrifuged at 2,000 g to pellet cells and identify the cytoplasmic fraction (supernatant). A sample (400 μl) of the membrane buffer was added to the cell pellets and vortexed, incubated on ice for 30 min, and then spun at 7,000 g to pellet the remaining cell fragments and to identify the membrane fraction (supernatant). Finally, 400 μl of the nuclear buffer was added to the cell pellets, which were vortexed and then kept on ice for 2 h. The insoluble fraction was pelleted by spinning at 11,000 g, and the nuclear fraction (supernatant) was stored for further analysis. Rabbit anti-Grp94 (Invitrogen, 1:500) and mouse anti-GAPDH (Santa Cruz Biotechnology, 1:1,000) were used to normalize the membrane and whole-cell fractions, respectively, by immunoblotting. Densitometry was performed using ImageJ Software (NIH).
Immunoprecipitation for phosphorylation studies.
Cells grown to the 90% confluence in 100-mm dishes had various treatments, including silencing. A sample (500 μg) of the protein was incubated with the primary antibody overnight at 4°C and for 2 h with protein A sepharose beads. Upon completion, the beads were washed twice with ice-cold TBS, after which 100 μl of TBS and 2× Laemmli buffer was added to each sample, and the beads were boiled for 10 min. Proteins were separated SDS-PAGE gel and blotted for phosphorylated proteins. After the blots were developed using ECL, they were stripped with Restore Plus stripping buffer (Thermo Fisher) and probed with the primary antibody specific to the protein immunoprecipitated. Primary antibodies used for immunoprecipitation were mouse anti-TAK1 (Santa Cruz Biotechnology, 1:500) or mouse anti-IKK-β (Santa Cruz Biotechnology, 1:1,000) for TAK1 and IKK-β, respectively. For experiments with TAK1 phosphorylation, a rabbit anti-p-TAK1 (Cell Signaling, Danvers, MA; 1:500) targeting TAK1 phosphorylated at Thr184/187 was used. When probing for IKK-β phosphorylation, a mouse anti-phosphoSerine (Santa Cruz Biotechnology, 1:500) was used. Densitometry was performed using ImageJ Software (NIH), and changes in phosphorylation were normalized for TAK1 or IKK-β.
siRNA-mediated Nox2 and Nox4 gene silencing.
siRNA sequences targeting Nox2 and Nox4 were purchased from Santa Cruz Biotechnology, and transfections were performed as previously reported (32, 33). For the nonsilenced cells, control siRNA (consisting of scrambled sequence that does not interfere with cellular function) was used (Santa Cruz Biotechnology). Based on the manufacturer's instructions, silencing protocols were modified for 60-mm (ICAM-1 expression) and 100-mm (for phosphorylation studies) culture dishes. Briefly, cells were cultured with antibiotic-free ECM until 60–80% confluent. The media were then aspirated and cells washed twice with the siRNA transfection medium. The plates were then incubated with either the control or siRNA strand (8 μg) in transfection medium and incubated for 16 h. Subsequently, the reagents were aspirated off, and normal ECM was gently put on the plates. The cells were grown for another 48 h and then treated with LPS. Silencing efficiency was determined by PCR (below) and by Western blotting using rabbit anti-Nox 2 (Santa Cruz Biotechnology, 1:500) and rabbit anti-Nox 4 (Santa Cruz Biotechnology, 1:500) antibodies. β-Actin was used for normalization and densitometry done using ImageJ Software.
Quantification of ICAM-1, Nox2, and Nox4 mRNA expression using real-time PCR.
Total RNA was extracted from HPMEC using the RNeasy Mini Kit from Qiagen (Valencia, CA), and cDNA was synthesized from 1 μg of RNA using the iScript cDNA synthesis kit from Bio-Rad (Hercules, CA), according to the manufacturer's instructions. The transcripts were amplified, and the gene expression data were collected with a Bio-Rad IQ5 with SYBR Green Mastermix from Bio-Rad. The primers for Nox2, Nox4, ICAM-1, and GAPDH were obtained from Operon (Huntsville, AL). They consisted of Nox2 (sense CAAGATGCGTGGAAACTACCTAAG, anti-sense TCCCTGCTCCCACTAACATCA), Nox4 (sense CTGCTGACGTTGCATGTTTC, anti-sense TTCTGAGAGCTGGTTCGGTT), ICAM-1 (sense- TATGGCAACGACTCCTTC, anti-sense- CATTCAGCGTCACCTTG), and GAPDH (sense- GGTGAAGGTCGGAGTCAAC, anti-sense- CAAAGTTGTCATGGATGAC). The primers for Nox1 were purchased from Santa Cruz Biotechnology. GAPDH was used as a housekeeping gene. Relative gene expression of Nox1, Nox2, Nox4, and ICAM-1 were calculated by the traditional 2−ΔΔCt method (49).
PP2A activity in HPMEC.
PP2A activity was quantified by measuring the phosphate released from threonine phosphopeptide (KRpTIRR) by PP2A using the PP2A Immunoprecipitation Phosphatase Assay Kit (Upstate Biotechnology). Cells were lysed in modified RIPA buffer containing protease inhibitors, and the protein concentration was quantified using the BCA assay. The manufacturer's protocol was followed with one modification: instead of separate 2-h incubations with protein A agarose beads followed by incubation with the PP2A antibody, we concurrently incubated the cell lysates with both protein A agarose beads and PP2A antibody for 2 h, which resulted in more robust phosphate absorbance values. For experiments that demonstrated inhibition of PP2A activity with OA, OA was added to the beads for 10 min before the addition of the threonine phosphopeptide. PP2A activity was quantified by measuring the absorbance of phosphate released in the reactive system at 650 nm using a microtiter plate reader. Absorbance values for samples were compared with the standard curve, and PP2A activity was expressed in pmol/100 μg of protein.
Statistical analysis.
Statistical analysis was done using STATA 11 (StataCorp LP, Dallas, TX). Data are presented as means ± SD. P < 0.05 was considered significant for experiments. For 2-OH-E+ measurements, log10 transformed data were compared between LPS-treated and control cells using an unpaired t-test. Fold changes in protein levels relative to the untreated, control cells were quantified by densitometry and were compared between various treatments using ANOVA. The Bonferroni test was used in conjunction with ANOVA to perform pairwise comparisons between groups. For mRNA studies, changes in gene expression with various treatments were calculated relative to expression in control cells and compared between different treatment groups using ANOVA. For PP2A activity, mean values for phosphate released were compared using an unpaired t-test.
RESULTS
LPS-mediated superoxide formation and endothelial activation in HPMEC.
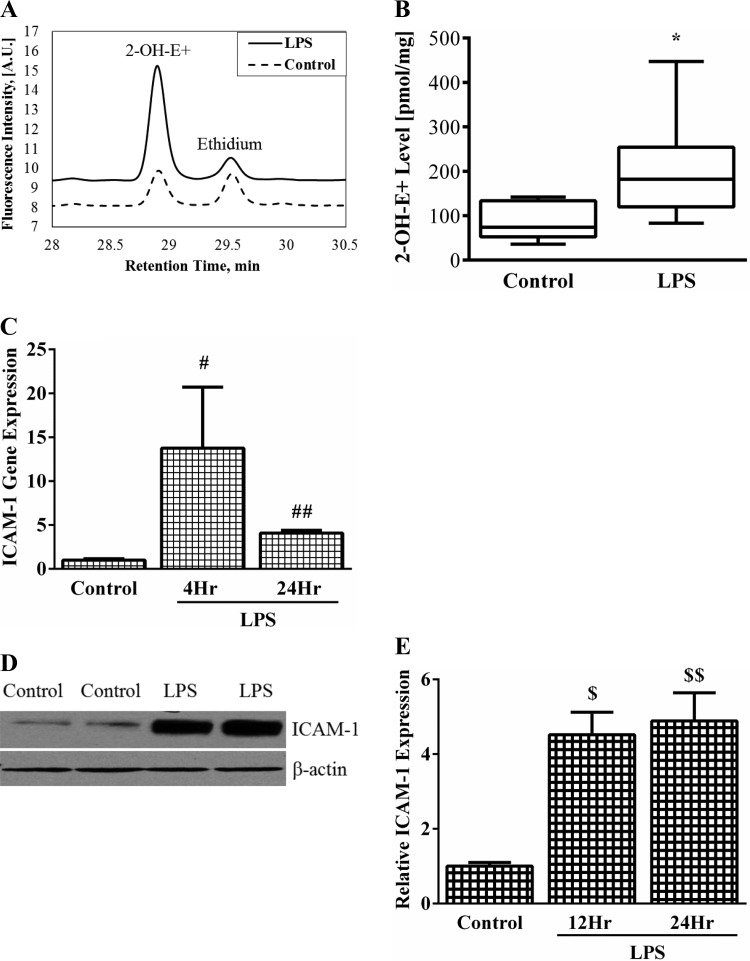
We measured 2-OH-E+ levels (marker for O2− formation) and ICAM-1 expression in HPMEC to determine whether LPS-induced endothelial activation is associated with increased formation of this oxidant. The oxidation of dihydroethidium to 2-OH-E+ was increased by 2.5-fold 1 h after treatment with LPS (Fig. 1, A and B). This is strongly suggestive of an LPS-dependent increase in the rate of superoxide formation. To assess LPS-mediated endothelial stimulation, we quantified the expression of the endothelial cell adhesion molecule ICAM-1 in HPMEC, as increased expression has been shown to be a marker of pulmonary vascular injury in premature infants at risk of BPD (45). LPS induced a 14-fold increase in ICAM-1 mRNA expression at 4 h, which waned to fourfold by 24 h (Fig. 1C). ICAM-1 protein expression was robustly induced greater than fourfold by LPS at 12 h and fivefold by 24 h (Fig. 1, D and E). These data demonstrate that LPS-mediated endothelial activation in HPMEC and an increase in the cellular superoxide level both occur within a few hours of LPS stimulation.
Fig. 1.
The effect of lipopolysaccharide (LPS) on 2-OH-E+ formation and intercellular adhesion molecule (ICAM)-1 expression in human pulmonary microvascular endothelial cells (HPMEC). A: 2-OH-E+ peaks (pM/mg protein) were measured by HPLC to quantify intracellular superoxide formation. A representative chromatogram showing LPS-treated and control cells is shown. B: cumulative data from 3 experiments showing increased 2-OH-E+ formation with LPS treatment at 1 h. *P = 0.006 (control vs. LPS-treated), n = 3. C: ICAM-1 mRNA expression was quantified in whole cell lysates by real-time PCR at 4 and 24 h. #P = 0.03 (control vs. 4 h LPS- treated); ##P = 0.002 (control vs. 24 h LPS-treated), n = 4. D: representative blot showing changes in ICAM-1 expression after LPS treatment for 12 h. E: densitometric quantification of ICAM-1 expression after 12 and 24 h LPS treatment. $P = 0.001 (control vs. 12 h LPS); $$P = 0.001 (control vs. 24 h LPS), n = 4.
Effect of NADPH-oxidase activity manipulation on LPS-mediated endothelial activation.
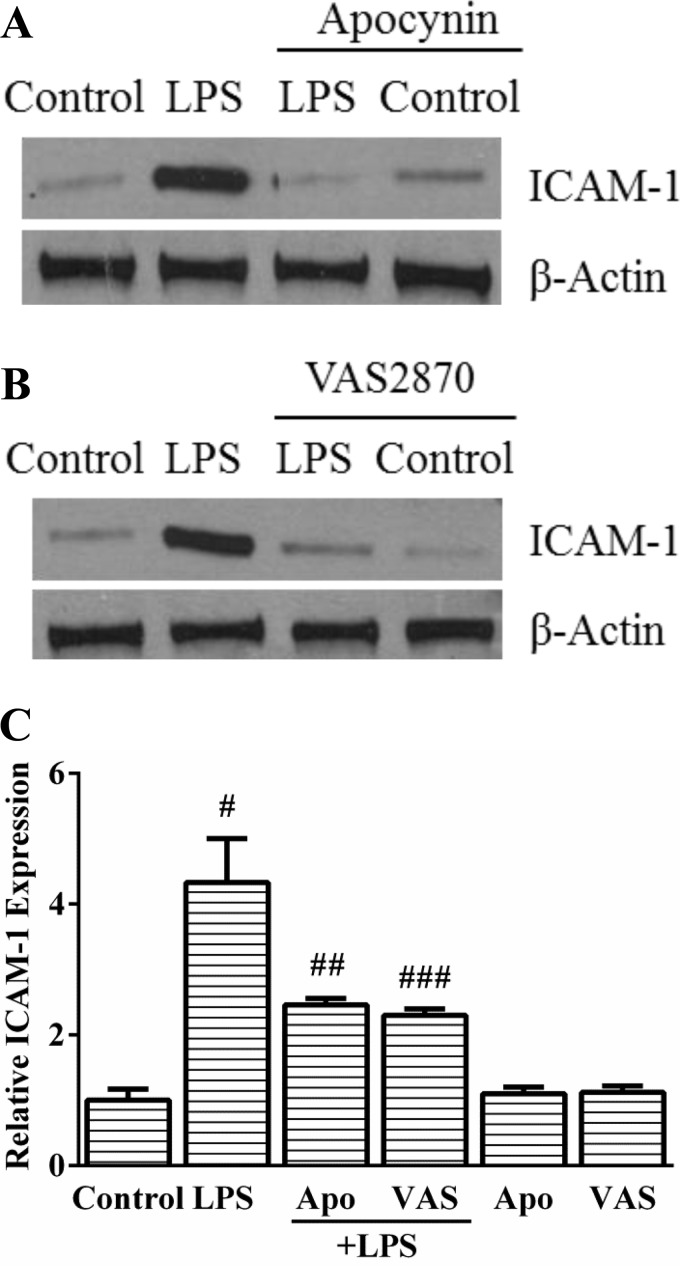
To determine whether LPS-induced endothelial activation is Nox dependent, we used complementary approaches (chemical inhibitors and siRNA). Apocynin, a compound that can quench superoxide or act as a Nox inhibitor, attenuated LPS-mediated ICAM-1 expression by >50% (Fig. 2, A and C) in HPMEC at 12 h. Because of the variable effects of apocynin on Nox inhibition, we assessed the effect of the novel Nox inhibitor VAS2870 on LPS-mediated endothelial activation in HPMEC (16). Pretreatment with VAS2870 inhibited ICAM-1 expression induced by LPS by >50% (Fig. 2, B and C) in HPMEC at 12 h. These experiments suggest that chemical inhibition of Nox activity attenuates LPS-mediated endothelial activation in HPMEC.
Fig. 2.
Apocynin and VAS2870 inhibit LPS-induced ICAM-1 expression at 12 h in HPMEC. A and B: ICAM-1 expression was quantified in whole cell lysates obtained from control, LPS-treated, LPS+Apocynin (500 μM)-treated (A), and VAS2870 (10 μM)-treated cells (B) by immunoblotting. C: changes in LPS-induced ICAM-1 expression with Apocynin and VAS2870 quantified by densitometry. #P = 0.001 (control vs. LPS); ##P = 0.02 (LPS vs. LPS+Apocynin); ###P < 0.001 (LPS vs. LPS+VAS2870), n = 5.
Characterization of Nox isoform that mediates LPS responsiveness in HPMEC.
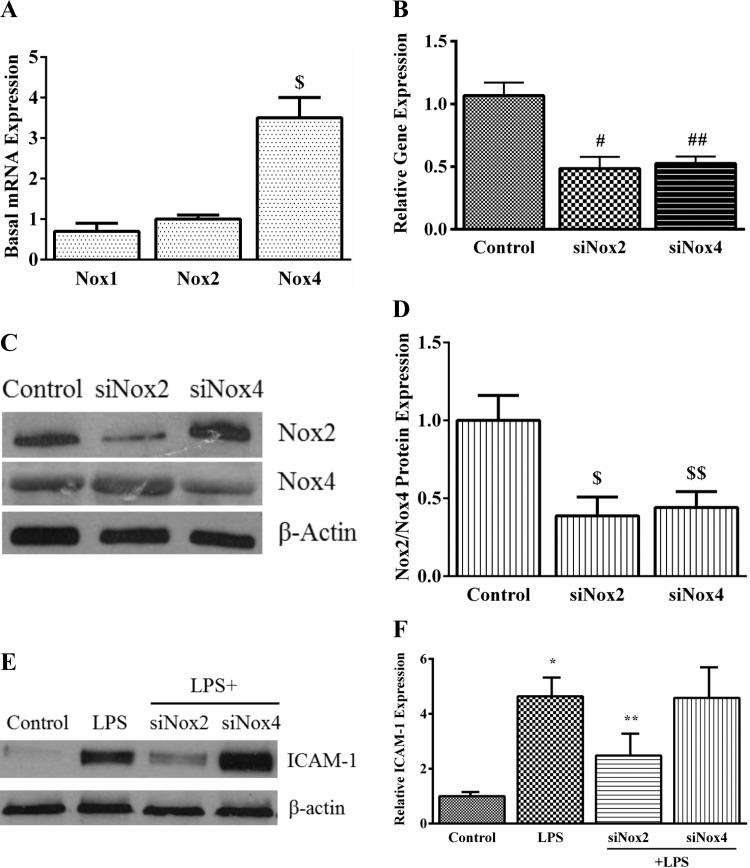
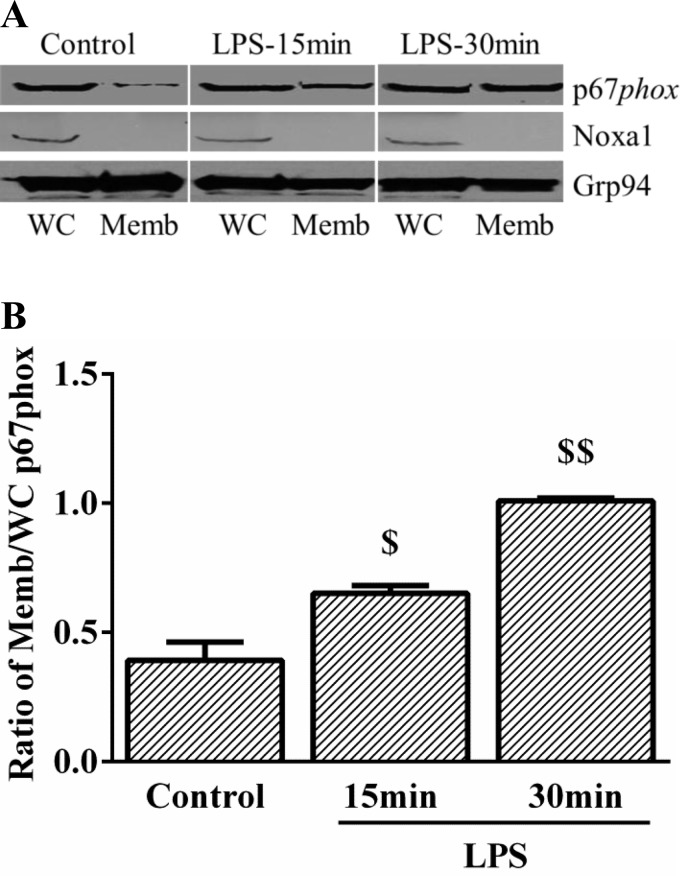
Our initial screen revealed that Nox1, Nox2, and Nox4 were expressed at the transcript level in HPMEC. Nox2 and Nox1 mRNA expression were not significantly different, but Nox4 expression was 3.5-fold higher than Nox2 (Fig. 4A). To identify the specific isoform of Nox activated by LPS in HPMEC, we examined changes in the cellular compartmentalization of Nox2 and Nox1 cytoplasmic subunits after LPS treatment. Nox4 activation does not involve changes in the intracellular distribution of its subunits. We found that LPS treatment resulted in membrane translocation of p67phox (a subunit of Nox2) at 15 and 30 min (Fig. 3, A and B). We did not detect Noxa1, the cytoplasmic subunit of Nox1, in the membrane fraction with or without LPS treatment in HPMEC. To confirm that LPS-mediated endothelial activation in HPMEC is Nox2 dependent, we used siRNA to decrease Nox2 or Nox4 expression before LPS treatment and quantified ICAM-1 expression. We obtained 52% and 54% reduction in mRNA expression of Nox2 and Nox4, respectively, 48 h after silencing with respective oligonucleotides in HPMEC (Fig. 4B). Correspondingly, there was an ∼60% decrease in the Nox2 and Nox4 protein after silencing (Fig. 4, C and D). Inhibition of Nox2 attenuated LPS-induced ICAM-1 expression by ∼50% in HPMEC at 24 h (Fig. 4, E and F). Nox4 silencing did not significantly alter LPS-mediated ICAM-1 expression (Fig. 4, E and F).
Fig. 4.
The effect of Nox2 and Nox4 siRNA on LPS-induced ICAM-1 expression in HPMEC. A: basal mRNA expression of Nox1, Nox2, and Nox4 was quantified by real-time PCR in control HPMEC. GAPDH was used as the housekeeping gene. The graph shows Nox1 and Nox4 mRNA expression relative to Nox2. *P = 0.01 (Nox2 vs. Nox4), n = 3. B: Nox2 and Nox4 mRNA expression was quantified by real-time PCR in whole cell lysates 48 h after siRNA treatment. #P < 0.001 (control vs. siNox2 cells); ##P = 0.002 (control vs. siNox4 cells), n = 5. C and D: Nox2 and Nox4 protein were quantified in whole cell lysates 48 h after silencing with specific siRNA by immunoblotting. Representative blot (C) and cumulative data from 3 experiments are shown (D). $P = 0.001 (control vs. siNox2); $$P = 0.006 (control vs. siNox4), n = 3. E and F: ICAM-1 expression was quantified 24 h after LPS treatment in control, LPS-treated, LPS+siNox2, and LPS+siNox4 cells by Western blotting. Representative blot (E) and cumulative data from 5 experiments are shown (F). *P < 0.001 (control vs. LPS-treated); **P = 0.004 (LPS vs. LPS+siNox2), n = 5.
Fig. 3.
Effect of LPS on p67phox intracellular compartmentalization. A: whole cell (WC) and membrane (Memb) fractions obtained from control and LPS-treated HPMEC by differential buffer extraction were immunoblotted for p67phox and Noxa1. Image represented originally contained nuclear fractions that are not shown. B: p67phox expression normalized to Grp94 was quantified by densitometry to show the increase in p67phox in the membrane fraction with LPS. $P = 0.02 (control vs. 15 min LPS); $$P = 0.004 (control vs. 30 min LPS), n = 4.
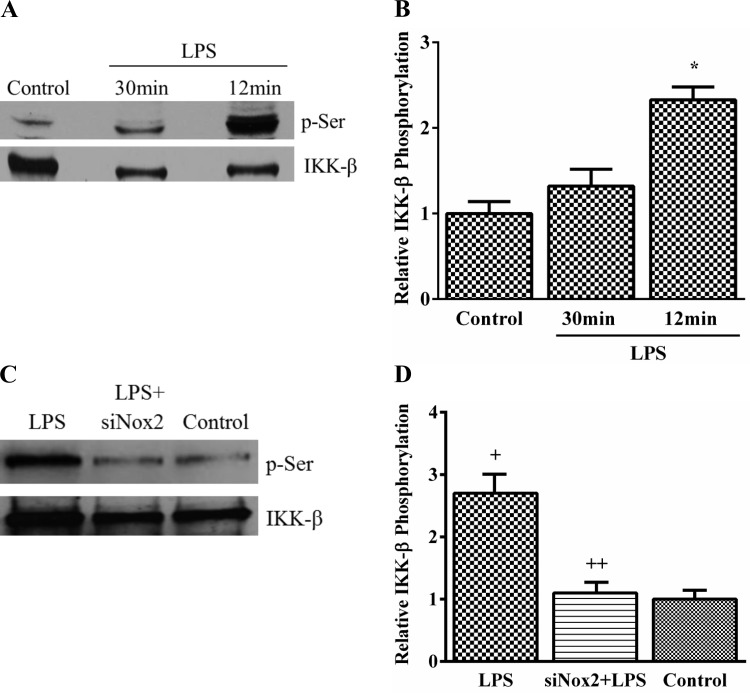
Modulation of IKK-β phosphorylation with Nox2 silencing.
We examined serine phosphorylation of IKK-β with or without LPS treatment in HPMEC by immunoprecipitation. LPS induced a 2.7-fold increase in IKK-β phosphorylation at 12 min with waning of the effect by 30 min (Fig. 5, A and B). To determine whether LPS-induced IKK-β phosphorylation was Nox2 dependent, we assessed the effect of Nox2 silencing on IKK-β phosphorylation. LPS-induced IKK-β phosphorylation was attenuated by >60% with Nox2 inhibition (Fig. 5, C and D). These data demonstrate that Nox2 regulates IKK-β phosphorylation in response to LPS.
Fig. 5.
LPS mediates IKK-β phosphorylation in HPMEC. A and B: serine phosphorylation of IKK-β was examined by immunoprecipitating IKK-β from cell lysates obtained from control and LPS-treated cells at 30 and 12 min and immunoblotting using a p-Serine antibody. Representative blot (A) and collective data from 5 experiments are shown (B). *P < 0.001 (control vs. 12 min), n = 5. C and D: IKK-β phosphorylation was quantified at 12 min by immunoprecipitating IKK-β from control, LPS-treated, and LPS+siNox2-treated cells and blotting using a p-Serine antibody. Representative blot (C) and data from 4 experiments are shown (D). +P = 0.001 (control vs. LPS); ++P = 0.002 (LPS vs. LPS+siNox2), n = 4.
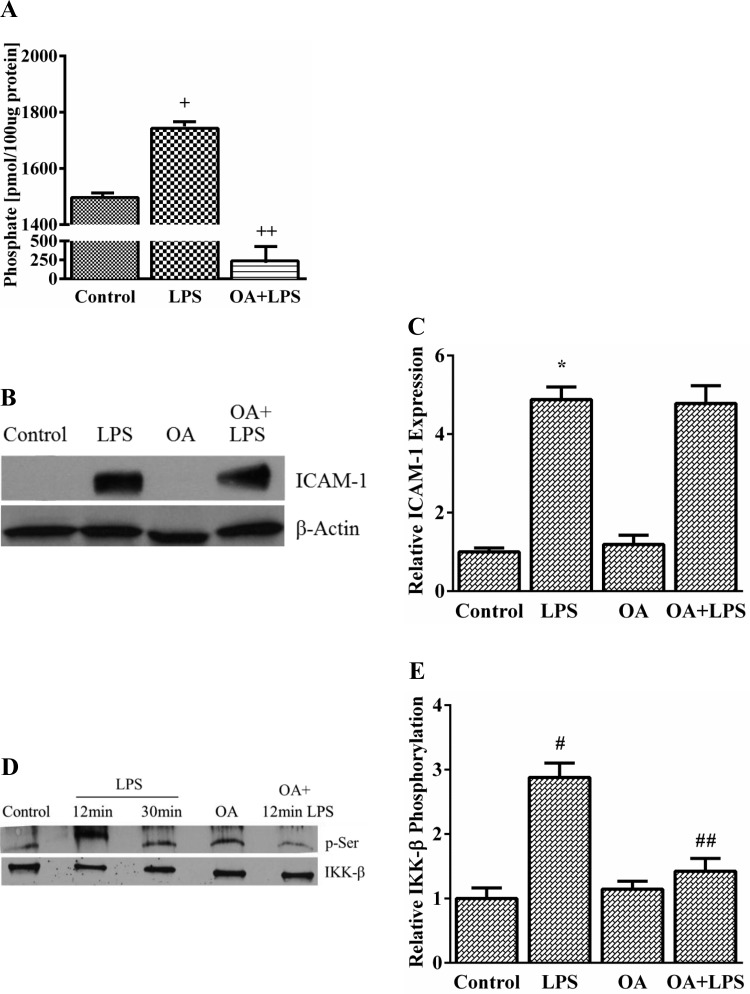
Role of PP2A and TAK1 in LPS-mediated IKK-β phosphorylation.
To determine whether LPS-mediated IKK-β phosphorylation resulted from inhibition of phosphatase activity, we examined PP2A, a serine-threonine phosphatase, which has been reported to regulate IKK-β phosphorylation in other cell types (50). We assessed the effect of LPS and OA (a selective PP2A inhibitor) on PP2A activity in HPMEC. LPS modestly increased PP2A activity by ∼17% in HPMEC, whereas OA strongly suppressed PP2A activity (Fig. 6A). Furthermore, to assess whether LPS-mediated ICAM-1 expression and IKK-β phosphorylation is regulated by PP2A, we used OA to selectively inhibit PP2A activity. Inhibition of PP2A activity did not alter LPS-induced ICAM-1 expression at 24 h (Fig. 6, B and C) but surprisingly decreased LPS-induced IKK-β phosphorylation by ∼50% in HPMEC (Fig. 6, D and E), suggesting that PP2A was not a negative regulator of IKK-β phosphorylation. Taken together, these experiments refute a direct role for PP2A in regulating LPS-dependent IKK-β phosphorylation and ICAM-1 expression in HPMEC.
Fig. 6.
Effect of protein phosphatase 2A (PP2A) inhibition on LPS-induced ICAM-1 expression and IKK-β phosphorylation in HPMEC. A: PP2A activity was quantified in whole cell lysates obtained from control, LPS-treated (12 min), and okadaic acid (OA)-treated (1 nM) cells. +P < 0.001 (control vs. LPS), ++P = 0.01 (control vs. OA-treated cells), n = 3. B and C: ICAM-1 expression after 24-h LPS treatment was examined by immunoblotting lysates from control, LPS-treated, OA-treated (1 nM), and LPS+OA-treated (1 nM) cells. Representative blot (B) and cumulative data from 5 experiments are shown (C). *P = 0.004 (control vs. LPS), n = 5. D and E: IKK-β phosphorylation was quantified by immunoprecipitating IKK-β at 12 and 30 min from control, LPS-treated, OA-treated (1 nM), and LPS+OA-treated (1 nM) whole cell lysates and immunoblotting with the p-Serine antibody. Representative blot (D) and summary data from 3 experiments are shown (E). #P = 0.01 (control vs. 12-min LPS); ##P = 0.009 (LPS vs. LPS+OA), n = 3.
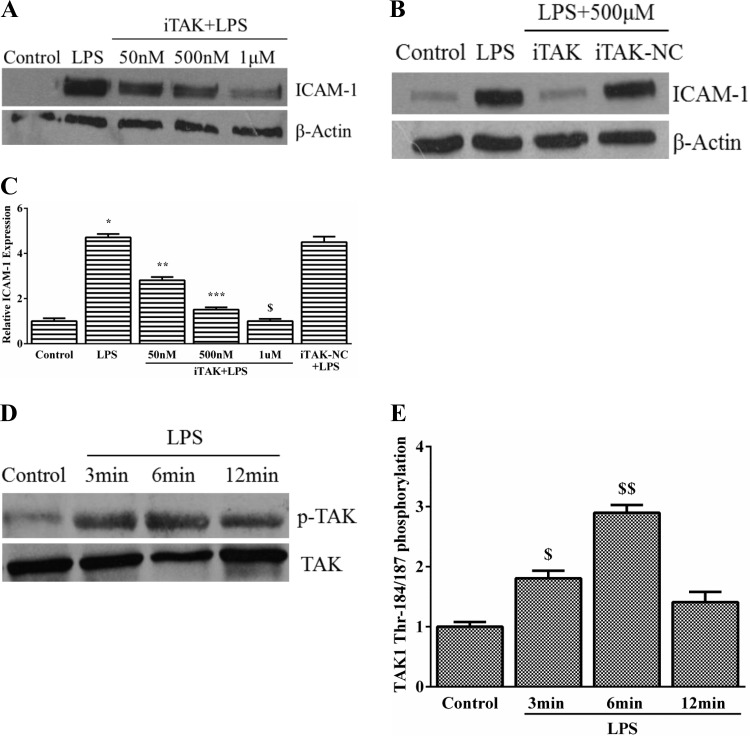
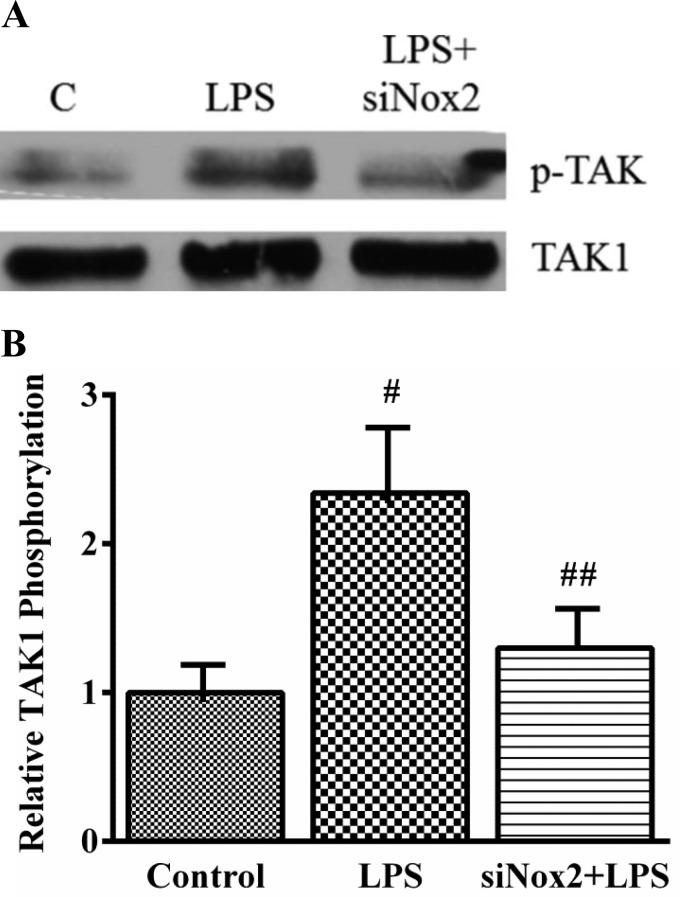
To assess the role of TAK1 in LPS-induced endothelial activation, we examined the effect of the TAK1 inhibitor (5Z)-7-oxozeaenol on LPS-mediated ICAM-1 expression in HPMEC (37, 59). ICAM-1 expression induced by LPS was completely suppressed by TAK1 inhibition in a dose-dependent manner (Fig. 7, A and C). (5Z)-oxozeanol, a compound similar to (5Z)-7-oxozeanol (iTAK) but without kinase activity, did not alter LPS-induced ICAM-1 expression (Fig. 7, B and C). We then examined whether LPS activated TAK1 in HPMEC. Phosphorylation of TAK1 at threonine residues 184/187 in its activation loop is required for kinase activity (51). In HPMEC, LPS treatment resulted in rapid induction of TAK1 threonine phosphorylation, which was evident by 3 min, peaked 2.9-fold by 6 min, and waned by 12 min (Fig. 7, D and E). We then evaluated the effect of Nox2 modulation on LPS-induced TAK1 phosphorylation. Nox2 silencing attenuated TAK1 phosphorylation induced by LPS at 6 min by >60% (Fig. 8, A and B). These data suggest that LPS-induced TAK1 phosphorylation is regulated by Nox2 and is temporally associated with Nox2-dependent IKK-β phosphorylation (Fig. 9).
Fig. 7.
Role of TGF-β associated kinase-1 (TAK1) in LPS-induced ICAM-1 expression in HPMEC. A: changes in LPS-induced ICAM-1 expression after 24 h with increasing doses of iTAK were quantified by immunoblotting. B: effect of (5Z)-oxozeanol (negative control, iTAK-NC) on ICAM-1 expression after 24-h LPS treatment was examined by immunoblotting. C: changes in LPS-induced ICAM-1 expression with iTAK and iTAK-NC were quantified by densitometry. *P < 0.001 (control vs. LPS); **P = 0.04 (LPS vs. 50 nM iTAK); ***P < 0.001 (LPS vs. 500 nM iTAK); $P = 0.001 (LPS vs. 1 μM iTAK), n = 5. D and E: TAK1 phosphorylation (Thr184/187) was quantified by immunoprecipitating TAK1 from control and LPS-treated cells at 3, 6, and 12 min, and immunoblotting with the anti-phosphoTAK1 antibody. Representative blot (D) and collective data from 4 experiments are shown (E). $P = 0.004 (control vs. 3 min LPS); $$P < 0.001 (control vs. 6 min LPS), n = 4.
Fig. 8.
Effect of Nox2 silencing on LPS-induced TAK1 phosphorylation in HPMEC. TAK1 phosphorylation (Thr184/187) was quantified in by immunoprecipitating TAK1 from whole cell lysates and immunoblotting with the anti-phosphoTAK1 antibody. Representative blot (A) and cumulative data from 5 experiments are shown (B). #P = 0.001 (control vs. LPS-treated); ##P = 0.01 (LPS vs. LPS+siNox2), n = 5.
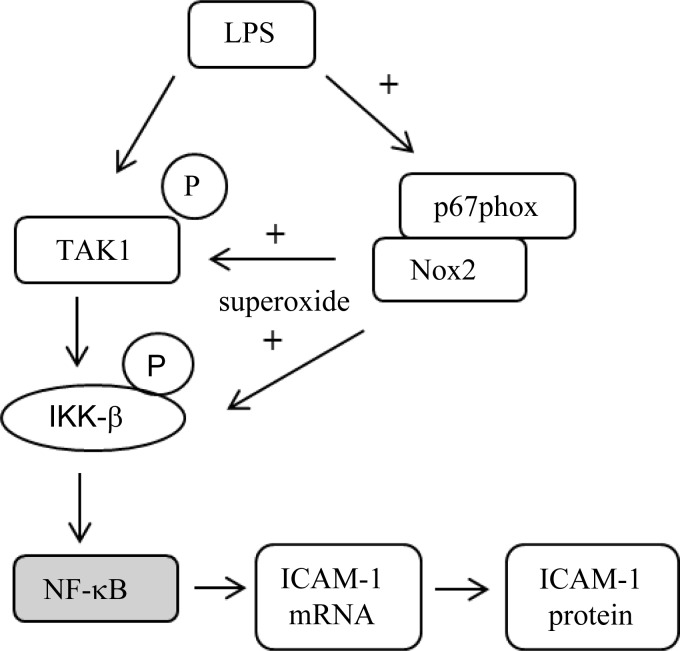
Fig. 9.
Schematic of Nox2-dependent regulation of ICAM-1 expression in LPS-treated HPMEC. NF-κB was not examined in this study.
DISCUSSION
The major finding of this study is the identification of a novel mechanism by which LPS-mediated proinflammatory signaling is regulated by Nox-dependent redox signaling in pulmonary endothelial cells (Fig. 9). We demonstrate that inhibition of Nox alters expression of the endothelial adhesion molecule ICAM-1 in pulmonary microvascular endothelial cells by regulating phosphorylation of TLR pathway proteins (Figs. 5C and 8). We have identified Nox2 as the isoform of Nox that mediates LPS responsiveness and endothelial activation (Figs. 3 and 4E) in HPMEC and show that VAS2870 suppresses LPS-mediated inflammation (Fig. 2B). Furthermore, we demonstrate that LPS-induced IKK-β phosphorylation is regulated by Nox2 and is associated with parallel changes in TAK1 phosphorylation (Figs. 5, 7D, and 8). Elucidating the mechanisms by which Nox regulates bacterial endothelial injury could aid the development of pharmacotherapy to treat sepsis-related lung remodeling in preterm infants.
Our data demonstrating an increase in 2-OH-E+ formation and ICAM-1 (Fig. 1) expression with LPS in HPMEC are consistent with other studies showing a relationship between ROS formation and endothelial inflammation (3, 12). Multiple sources of endothelial ROS (mitochondrial electron-transport chain, Nox, XO, and uncoupled endothelial nitric oxide synthase) could have contributed to LPS-mediated O2− formation in HPMEC (31). We therefore used VAS2870 and apocynin to demonstrate that LPS-induced ICAM-1 expression in HPMEC (Fig. 2) is Nox dependent (43, 52, 55). Although VAS2870 and apocynin may not specifically inhibit only Nox, these results complement our silencing data, indicating that Nox regulates LPS-mediated ICAM-1 expression (16, 53, 55). In HPMEC, LPS treatment induced membrane translocation of the Nox2 subunit p67phox, and Nox2 silencing attenuated LPS-induced ICAM-1 expression (Figs. 3 and 4). Whereas Kim et al. (25) showed that Nox2 mediates LPS-induced matrix metalloproteinase expression in Raw264.7 cells, this is the first report of Nox2 regulating endothelial activation in pulmonary endothelial cells. Our data are in contrast with Park et al. (42), who showed that the carboxyl terminal region of Nox4 directly interacts with TLR4 in HEK293T cells and mediates LPS-induced ROS generation. Because HEK293T cells do not naturally express TLR4 or Nox isoforms other than Nox4, their data establish that TLR receptors can directly interact with Nox proteins. Park et al. (41) also demonstrated that LPS-mediated chemokine expression in human aortic endothelial cells is also Nox4 dependent. However, they did not examine the effect of Nox2 silencing on LPS-induced chemokine expression. In HPMEC, Nox4 silencing did not alter LPS-mediated ICAM-1 expression (Fig. 4E). Although Miyoshi et al. (36) showed that Nox1 plays a role in LPS-induced tissue factor expression in HUVEC cells, their data suggests that this is IL-8 dependent (36). Because Nox isoforms exhibit structural homology and similarities in modes of activation, the specific isoform activated by LPS may depend on the cell- and tissue-specific distribution of Nox and the duration of LPS exposure.
IKK-β phosphorylation in response to inflammatory stimuli has been shown to be modulated by the extracellular application of antioxidants, hydrogen peroxide, or peroxynitrite, suggesting that it is amenable to redox regulation (26, 30). However, the mechanisms by which ROS modulate inflammatory signaling in response to cytokines and LPS are not fully understood. In HPMEC, silencing Nox2 attenuated LPS-mediated IKK-β phosphorylation (Fig. 5). Our data suggest that Nox2-dependent ROS facilitate IKK-β phosphorylation in contrast to Reynaert et al. (46), who showed that hydrogen peroxide inhibits IKK-β phosphorylation. They postulated that oxidation of cysteine-179 in IKK-β by hydrogen peroxide interferes with IKK-β serine 177/181 phosphorylation or IKK-β binding to substrate or accessory proteins (46, 47). There are potentially many reasons for the differences between our results and their data. The dose of hydrogen peroxide (200 μM) used in their study would far exceed the amount of ROS produced by Nox in cells, possibly resulting in contrary effects on IKK activity. Nox-mediated redox signaling is likely to be compartmentalized unlike the more pervasive effects of extracellular application of oxidants. Loukili et al. (34) demonstrated that oxidants increase IKK-β phosphorylation in A549 cells if applied after TNF-α stimulation, and this is consistent with our data. Our data reveal a novel mechanism of crosstalk between an enzyme that mediates redox signaling and a pivotal kinase in the TLR pathway (Fig. 9).
IKK-β phosphorylation can be regulated by kinases like TAK1, phosphatases like PP2A, or may be autoregulatory (15, 48). We examined the hypothesis that LPS-mediated Nox2-dependent IKK-β phosphorylation in HPMEC resulted from decreased PP2A activity. LPS increased PP2A activity in HPMEC, and inhibition of PP2A attenuated LPS-induced IKK-β phosphorylation without altering ICAM-1 expression (Fig. 6). These data negate a direct role for PP2A in LPS-induced IKK-β phosphorylation in HPMEC. PP2A has been previously shown to either facilitate or inhibit IKK-β phosphorylation (4, 27, 50). Barisic et al. (4) showed that inhibition of PP2A resulted in persistent IL-1-mediated IKK-β phosphorylation, supporting negative regulation of IKK-β phosphorylation by PP2A (4). However, Kray et al. (27) demonstrated that TNF-α- induced degradation of IκB was attenuated by OA, which supports positive regulation of IKK-β by PP2A and is consistent with our observation that inhibition of PP2A by OA decreases LPS-induced IKK-β phosphorylation (27).
We examined whether LPS-induced IKK-β phosphorylation was associated with Nox2-dependent activation of TAK1 activation, a member of the mitogen-activated protein kinase family (24, 29). LPS-mediated ICAM-1 expression was attenuated by TAK1 inhibition in a dose-dependent manner in HPMEC (Fig. 7A). Although 5Z-7-oxozeaenol may not specifically inhibit only TAK1, it has been shown to decrease TAK1 activity (37, 59). Phosphorylation of TAK1 at Thr-187 within its activation loop is required for kinase activity (39, 51). In HPMEC, LPS-induced TAK1 phosphorylation (Thr184/187) was attenuated by Nox2 silencing (Fig. 8). To our best knowledge, this is the first report suggesting that TAK1 phosphorylation may be redox regulated (Fig. 9). TAK1 phosphorylation and activation involve association with TAK1-binding proteins, TAB2/TAB3 in response to cytokines and TAB1 in response to osmotic stress (18, 39, 40, 44). However, whether ROS regulate ligand-mediated TAK1 phosphorylation has not been examined. Interestingly, in the intestinal epithelium, TAK1 deficiency results in ROS accumulation, suggesting the possible presence of a feedback loop (22). We have not examined the mechanisms by which Nox2 modulates TAK1 phosphorylation. Possible mechanisms include control of binding between TAB proteins and TAK1, regulation of TAK1 autophosphorylation, and alterations in the balance between kinases-phosphatases that govern TAK1 activity.
In conclusion, we demonstrate that Nox2 regulates LPS-mediated IKK-β phosphorylation and endothelial activation in HPMEC. Although we present novel data on Nox-dependent regulation of LPS-mediated inflammatory signaling, the mechanisms by which Nox-derived ROS modulate IKK-β or TAK1 phosphorylation remain to be clarified. Regulation of IKK-β or TAK1 phosphorylation through Nox can potentially serve as a rapid and reversible fine-tuning mechanism by which the cellular oxidative status modulates the magnitude of proinflammatory responses to bacterial ligands. Our data were derived using primary cells in vitro, and as such the clinical significance of these results to lung injury in BPD needs to be determined. Pulmonary endothelial activation has been shown to be a critical event in the pathogenesis of sepsis-induced lung injury in animal models and can contribute to the vascular injury and remodeling seen in BPD (23, 35). Pharmacological modulation of Nox activity may find use as adjunct therapy to limit lung injury and vascular remodeling in BPD (9).
GRANTS
This work was supported by 1R03HD062693-01A1 (V. Sampath).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: H.M. and E.T. performed experiments; H.M. and V.S. analyzed data; H.M., E.T., N.H., and V.S. interpreted results of experiments; H.M., E.T., and V.S. prepared figures; H.M. and V.S. drafted manuscript; H.M., E.T., N.H., and V.S. approved final version of manuscript; E.T., N.H., and V.S. edited and revised manuscript; N.H. and V.S. conception and design of research.
ACKNOWLEDGMENTS
We thank Drs. Jacek Zielonka with the Department of Biophysics and Free Radical Research Center, Medical College of Wisconsin, Ganesh Samant with the Department of Vascular Developmental Biology, Medical College of Wisconsin, Girija Ganesh Konduri with the Department of Pediatrics-Neonatology, Medical College of Wisconsin, and Ronald Hines with the Department of Pharmacology and Toxicology, Medical College of Wisconsin, for guidance.
REFERENCES
- 1. Akira S. TLR Signaling. Curr Top Microbiol Immunol 311: 1–16, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Al Ghouleh I, Magder S. Nicotinamide adenine dinucleotide phosphate (reduced form) oxidase is important for LPS-induced endothelial cell activation. Shock 29: 553–559, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Alom-Ruiz SP, Anilkumar N, Shah AM. Reactive oxygen species and endothelial activation. Antioxid Redox Signal 10: 1089–1100, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Barisic S, Strozyk E, Peters N, Walczak H, Kulms D. Identification of PP2A as a crucial regulator of the NF-kappaB feedback loop: its inhibition by UVB turns NF-kappaB into a pro-apoptotic factor. Cell Death Differ 15: 1681–1690, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Bose CL, Laughon MM, Dammann CE. Bronchopulmonary dysplasia and inflammatory biomarkers in the premature neonate. Arch Dis Child Fetal Neonatal Ed 93: F455–F461, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Brandes RP, Schroder K. Composition and functions of vascular nicotinamide adenine dinucleotide phosphate oxidases. Trends Cardiovasc Med 18: 15–19, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Brigham KL, Meyrick B, Berry LC, Jr, Repine JE. Antioxidants protect cultured bovine lung endothelial cells from injury by endotoxin. J Appl Physiol 63: 840–850, 1987 [DOI] [PubMed] [Google Scholar]
- 9. Carnesecchi S, Pache JC, Barazzone-Argiroffo C. NOX enzymes: potential target for the treatment of acute lung injury. Cell Mol Life Sci 69: 2373–2385, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carpenter S, O'Neill LA. Recent insights into the structure of Toll-like receptors and post-translational modifications of their associated signalling proteins. Biochem J 422: 1–10, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Chen YH, Lin SJ, Chen YL, Liu PL, Chen JW. Anti-inflammatory effects of different drugs/agents with antioxidant property on endothelial expression of adhesion molecules. Cardiovasc Hematol Disord Drug Targets 6: 279–304, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Dada LA, Sznajder JI. Mitochondrial Ca(2)+ and ROS take center stage to orchestrate TNF-alpha-mediated inflammatory responses. J Clin Invest 121: 1683–1685, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Delhase M, Hayakawa M, Chen Y, Karin M. Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Science 284: 309–313, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, Bauer CR, Donovan EF, Korones SB, Laptook AR, Lemons JA, Oh W, Papile LA, Shankaran S, Stevenson DK, Tyson JE, Poole WK. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol 196: 147 e141–e148, 2007. [DOI] [PubMed] [Google Scholar]
- 15. Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE 2006: re13, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Holden P, Horton WA. Crude subcellular fractionation of cultured mammalian cell lines. BMC Res Notes 2: 243, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Inagaki M, Omori E, Kim JY, Komatsu Y, Scott G, Ray MK, Yamada G, Matsumoto K, Mishina Y, Ninomiya-Tsuji J. TAK1-binding protein 1, TAB1, mediates osmotic stress-induced TAK1 activation but is dispensable for TAK1-mediated cytokine signaling. J Biol Chem 283: 33080–33086, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Irani K. Oxidant signaling in vascular cell growth, death, and survival: a review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ Res 87: 179–183, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Jaspers I, Zhang W, Fraser A, Samet JM, Reed W. Hydrogen peroxide has opposing effects on IKK activity and IkappaBalpha breakdown in airway epithelial cells. Am J Respir Cell Mol Biol 24: 769–777, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Johnson LN, Noble ME, Owen DJ. Active and inactive protein kinases: structural basis for regulation. Cell 85: 149–158, 1996 [DOI] [PubMed] [Google Scholar]
- 22. Kajino-Sakamoto R, Omori E, Nighot PK, Blikslager AT, Matsumoto K, Ninomiya-Tsuji J. TGF-beta-activated kinase 1 signaling maintains intestinal integrity by preventing accumulation of reactive oxygen species in the intestinal epithelium. J Immunol 185: 4729–4737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kandasamy K, Sahu G, Parthasarathi K. Real-time imaging reveals endothelium-mediated leukocyte retention in LPS-treated lung microvessels. Microvasc Res 83: 323–331, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim SI, Kwak JH, Na HJ, Kim JK, Ding Y, Choi ME. Transforming growth factor-beta (TGF-beta1) activates TAK1 via TAB1-mediated autophosphorylation, independent of TGF-beta receptor kinase activity in mesangial cells. J Biol Chem 284: 22285–22296, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim SY, Lee JG, Cho WS, Cho KH, Sakong J, Kim JR, Chin BR, Baek SH. Role of NADPH oxidase-2 in lipopolysaccharide-induced matrix metalloproteinase expression and cell migration. Immunol Cell Biol 88: 197–204 [DOI] [PubMed] [Google Scholar]
- 26. Korn SH, Wouters EF, Vos N, Janssen-Heininger YM. Cytokine-induced activation of nuclear factor-kappa B is inhibited by hydrogen peroxide through oxidative inactivation of IkappaB kinase. J Biol Chem 276: 35693–35700, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Kray AE, Carter RS, Pennington KN, Gomez RJ, Sanders LE, Llanes JM, Khan WN, Ballard DW, Wadzinski BE. Positive regulation of IkappaB kinase signaling by protein serine/threonine phosphatase 2A. J Biol Chem 280: 35974–35982, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 285: R277–R297, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Lee J, Mira-Arbibe L, Ulevitch RJ. TAK1 regulates multiple protein kinase cascades activated by bacterial lipopolysaccharide. J Leukoc Biol 68: 909–915, 2000 [PubMed] [Google Scholar]
- 30. Levrand S, Pesse B, Feihl F, Waeber B, Pacher P, Rolli J, Schaller MD, Liaudet L. Peroxynitrite is a potent inhibitor of NF-[kappa]B activation triggered by inflammatory stimuli in cardiac and endothelial cell lines. J Biol Chem 280: 34878–34887, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li JM, Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol 287: R1014–R1030, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Li Q, Harraz MM, Zhou W, Zhang LN, Ding W, Zhang Y, Eggleston T, Yeaman C, Banfi B, Engelhardt JF. Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol Cell Biol 26: 140–154, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li Q, Spencer NY, Oakley FD, Buettner GR, Engelhardt J. Endosomal Nox2 facilitates redox-dependent induction of NFκB by TNFalpha. Antiox Redox Signal 11: 1249–1263, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Loukili N, Rosenblatt-Velin N, Rolli J, Levrand S, Feihl F, Waeber B, Pacher P, Liaudet L. Oxidants positively or negatively regulate nuclear factor kappaB in a context-dependent manner. J Biol Chem 285: 15746–15752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295: L379–L399, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miyoshi T, Yamashita K, Arai T, Yamamoto K, Mizugishi K, Uchiyama T. The role of endothelial interleukin-8/NADPH oxidase 1 axis in sepsis. Immunology 131: 331–339, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ninomiya-Tsuji J, Kajino T, Ono K, Ohtomo T, Matsumoto M, Shiina M, Mihara M, Tsuchiya M, Matsumoto K. A resorcylic acid lactone, 5Z-7-oxozeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase kinase. J Biol Chem 278: 18485–18490, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Ogier-Denis E, Mkaddem SB, Vandewalle A. NOX enzymes and Toll-like receptor signaling. Semin Immunopathol 30: 291–300, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Omori E, Inagaki M, Mishina Y, Matsumoto K, Ninomiya-Tsuji J. Epithelial transforming growth factor beta-activated kinase 1 (TAK1) is activated through two independent mechanisms and regulates reactive oxygen species. Proc Natl Acad Sci USA 109: 3365–3370, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Omori E, Morioka S, Matsumoto K, Ninomiya-Tsuji J. TAK1 regulates reactive oxygen species and cell death in keratinocytes, which is essential for skin integrity. J Biol Chem 283: 26161–26168, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Park HS, Chun JN, Jung HY, Choi C, Bae YS. Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovasc Res 72: 447–455, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol 173: 3589–3593, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Paterniti I, Galuppo M, Mazzon E, Impellizzeri D, Esposito E, Bramanti P, Cuzzocrea S. Protective effects of apocynin, an inhibitor of NADPH oxidase activity, in splanchnic artery occlusion and reperfusion. J Leukoc Biol 88: 993–1003, 2010. [DOI] [PubMed] [Google Scholar]
- 44. Prickett TD, Ninomiya-Tsuji J, Broglie P, Muratore-Schroeder TL, Shabanowitz J, Hunt DF, Brautigan DL. TAB4 stimulates TAK1-TAB1 phosphorylation and binds polyubiquitin to direct signaling to NF-kappaB. J Biol Chem 283: 19245–19254, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ramsay PL, O'Brian Smith E, Hegemier S, Welty SE. Early clinical markers for the development of bronchopulmonary dysplasia: soluble E-Selectin and ICAM-1. Pediatrics 102: 927–932, 1998 [DOI] [PubMed] [Google Scholar]
- 46. Reynaert NL, van der Vliet A, Guala AS, McGovern T, Hristova M, Pantano C, Heintz NH, Heim J, Ho YS, Matthews DE, Wouters EF, Janssen-Heininger YM. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc Natl Acad Sci USA 103: 13086–13091, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rossi A, Kapahi P, Natoli G, Takahashi T, Chen Y, Karin M, Santoro MG. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature 403: 103–108, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Schmid JA, Birbach A. IkappaB kinase beta (IKKbeta/IKK2/IKBKB)–a key molecule in signaling to the transcription factor NF-kappaB. Cytokine Growth Factor Rev 19: 157–165, 2008 [DOI] [PubMed] [Google Scholar]
- 49. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protocol 3: 1101–1108, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Shanley TP, Vasi N, Denenberg A, Wong HR. The serine/threonine phosphatase, PP2A: endogenous regulator of inflammatory cell signaling. J Immunol 166: 966–972, 2001 [DOI] [PubMed] [Google Scholar]
- 51. Singhirunnusorn P, Suzuki S, Kawasaki N, Saiki I, Sakurai H. Critical roles of threonine 187 phosphorylation in cellular stress-induced rapid and transient activation of transforming growth factor-beta-activated kinase 1 (TAK1) in a signaling complex containing TAK1-binding protein TAB1 and TAB2. J Biol Chem 280: 7359–7368, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Stielow C, Catar RA, Muller G, Wingler K, Scheurer P, Schmidt HH, Morawietz H. Novel Nox inhibitor of oxLDL-induced reactive oxygen species formation in human endothelial cells. Biochem Biophys Res Commun 344: 200–205, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Sun QA, Hess DT, Wang B, Miyagi M, Stamler JS. Off-target thiol alkylation by the NADPH oxidase inhibitor 3-benzyl-7-(2-benzoxazolyl)thio-1,2,3-triazolo[4,5-d]pyrimidine (VAS2870). Free Radic Biol Med 52: 1897–1902, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Takac I, Schroder K, Brandes RP. The Nox family of NADPH oxidases: friend or foe of the vascular system? Curr Hyperten Rep 14: 70–78, 2012 [DOI] [PubMed] [Google Scholar]
- 55. ten Freyhaus H, Huntgeburth M, Wingler K, Schnitker J, Baumer AT, Vantler M, Bekhite MM, Wartenberg M, Sauer H, Rosenkranz S. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc Res 71: 331–341, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Thebaud B, Abman SH. Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am J Respir Crit Care Med 175: 978–985, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang W, Wei W, Ning Q, Luo XP. [Effect of intra-amniotic endotoxin priming plus hyperoxic exposure on the expression of vascular endothelial growth factor and its receptors in lungs of preterm newborn rats]. Zhonghua Ke Za Zhi 45: 533–538, 2007 [PubMed] [Google Scholar]
- 58. Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood 106: 584–592, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yao J, Kim TW, Qin J, Jiang Z, Qian Y, Xiao H, Lu Y, Qian W, Gulen MF, Sizemore N, DiDonato J, Sato S, Akira S, Su B, Li X. Interleukin-1 (IL-1)-induced TAK1-dependent Versus MEKK3-dependent NFkappaB activation pathways bifurcate at IL-1 receptor-associated kinase modification. J Biol Chem 282: 6075–6089, 2007 [DOI] [PubMed] [Google Scholar]
- 60. Zhao H, Joseph J, Fales HM, Sokoloski EA, Levine RL, Vasquez-Vivar J, Kalyanaraman B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc Natl Acad Sci USA 102: 5727–5732, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]