Abstract
Inflammation and antenatal glucocorticoids, the latter given to mothers at risk for preterm birth, affect lung development and may contribute to the development of bronchopulmonary dysplasia (BPD). The effects of the combined exposures on inflammation and antenatal glucocorticoids on transforming growth factor (TGF)-β signaling are unknown. TGF-β and its downstream mediators are implicated in the etiology of BPD. Therefore, we asked whether glucocorticoids altered intra-amniotic lipopolysaccharide (LPS) effects on TGF-β expression, its signaling molecule phosphorylated sma and mothers against decapentaplegic homolog 2 (pSmad2), and the downstream mediators connective tissue growth factor (CTGF) and caveolin-1 (Cav-1). Ovine singleton fetuses were randomized to receive either an intra-amniotic injection of LPS and/or maternal betamethasone (BTM) intramuscularly 7 and/or 14 days before delivery at 120 days gestational age (GA; term = 150 days GA). Saline was used for controls. Protein levels of TGF-β1 and -β2 were measured by ELISA. Smad2 phosphorylation was assessed by immunohistochemistry and Western blot. CTGF and Cav-1 mRNA and protein levels were determined by RT-PCR and Western blot. Free TGF-β1 and -β2 and total TGF-β1 levels were unchanged after LPS and/or BTM exposure, although total TGF-β2 increased in animals exposed to BTM 7 days before LPS. pSmad2 immunostaining increased 7 days after LPS exposure although pSmad2 protein expression did not increase. Similarly, CTGF mRNA and protein levels increased 7 days after LPS exposure as Cav-1 mRNA and protein levels decreased. BTM exposure before LPS prevented CTGF induction and Cav-1 downregulation. This study demonstrated that the intrauterine inflammation-induced TGF-β signaling can be inhibited by antenatal glucocorticoids in fetal lungs.
Keywords: bronchopulmonary dysplasia, prematurity, chorioamnionitis, lung development, intrauterine infection, lipopolysaccharide, transforming growth factor-β
a high percentage of preterm infants is exposed to antenatal inflammation (20, 46), including chorioamnionitis (48), sepsis (38, 41), inflammation resulting from oxidative stress (43), and volutrauma (44). Exposure to inflammation is associated with the development of bronchopulmonary dysplasia (BPD) (5, 10, 44). BPD is characterized by a decrease in alveolarization and vascularization and by the need for supplemental oxygen (19). The etiology of BPD is multifactorial and very complex. For example, in clinical practice, antenatal glucocorticoids are given to women at risk for preterm birth. However, glucocorticoids also inhibit alveolarization (31, 50).
Transforming growth factor-β (TGF-β), which is not only a growth factor but also an anti-inflammatory cytokine, has been linked to the etiology of BPD (21, 26). The relevance of TGF-β in BPD is supported by findings in several animal models (12, 23, 45, 47). Interestingly, glucocorticoids decrease TGF-β production in experimental models with pulmonary cells (32, 49). TGF-β is expressed in three isoforms, TGF-β1, TGF-β2, and TGF-β3, each of which have distinct roles in the developing lung as regulators of cell proliferation, remodeling, repair (3, 30), and as an anti-inflammatory cytokine (3). With activation by cleavage from the latent TGF-β-binding protein, TGF-β binds TGF-β receptor II, which then forms a complex with TGF-β receptor I (TGF-βRI) (3, 30). TGF-βRI then can phosphorylate Smad2 and -3, which in turn complex with Smad4, leading to TGF-β-activated gene transcription (3, 30). TGF-β also has effects through several downstream mediators, including connective tissue growth factor (CTGF) and caveolin-1 (Cav-1). CTGF contributes to tissue remodeling and fibrosis (25) and may contribute to the pathogenesis of BPD through both ventilation-mediated injury and inflammation (1, 8, 23). Cav-1 is a component of caveolae, omega-shaped invaginations of the plasma membrane that facilitate protein trafficking and signal transduction (17, 51). Cav-1 regulates the contractile phenotype of maturing airway smooth muscle cells under influence of TGF-β1 (15). Altered expression of Cav-1 has been implicated in the pathogenesis of several lung diseases (14). Cav−/− mice have thickened alveolar walls and irregular alveolar spaces with increased deposition of extracellular matrix and TGF-β activity, whereas overexpression of Cav-1 aggravates lipopolysaccharide (LPS)-induced inflammation and subsequent lung injury (17, 29).
Previously, we reported that TGF-β1 and phosphorylated sma and mothers against decapentaplegic homolog 2 (pSmad2) were upregulated in fetal lambs exposed to intrauterine LPS and brief postnatal ventilation (22, 23). These increases were associated with downregulation of CTGF and Cav-1 (22, 23). It is, however, unknown how a combined exposure to antenatal inflammation and glucocorticoids, common for many preterm fetuses (6, 13), might affect TGF-β signaling and its downstream mediators. Therefore, we asked whether glucocorticoids alter the effect of LPS on TGF-β expression, the phosphorylation of its transcription factor Smad2, and the expression of its regulated genes CTGF and Cav-1. We hypothesized that maternal betamethasone would modulate LPS-induced activation of the TGF-β signaling pathway and the expression of CTGF and Cav-1.
METHODS
Animal model and sampling protocol.
All studies were approved by the Animal Ethics Committees at The University of Western Australia (animal ethics protocol RA/3/100/830) and Cincinnati Children's Hospital Medical Center. The experimental design and lung maturation outcome of this study was published previously (24). Time-mated ewes with singleton fetuses were randomly assigned to one of six treatment groups to receive an intra-amniotic injection of LPS (10 mg Escherichia coli 055:B5; Sigma Aldrich, St. Louis, MO), and/or an intramuscular injection of betamethasone (BTM) [0.5 mg/kg maternal weight, Celestone Soluspan; Schering-Plough, North Ryde, New South Wales (NSW), Australia], and/or an equivalent injection of saline for control animals at 107 days and/or 114 days gestational age (GA). Because of fetal losses, the animals that had been assigned to a 14-day BTM group were reassigned to other groups, since combined exposures were given a higher priority (24). All ewes in this study received a single intramuscular injection of 150 mg medroxyprogesterone acetate (Depo-Provera; Kenral, NSW, Australia) at 100 days GA to reduce the risk of preterm birth induced by BTM treatment. Medroxyprogesterone is not known to produce any side effects in the developing lung (16). Lambs were surgically delivered at 120 days GA (term = 150 days GA) and killed directly after birth. Lung tissue from the right lower lobe was snap-frozen, and the right upper lobe was inflation-fixed in 10% buffered formalin for 24 h.
Analysis of TGF-β1 and TGF-β2.
Frozen lung tissue was homogenized (PRO Quick Connect Generators part no. 02–07095; PRO Scientific, Oxford, CT) in ice-cold RIPA buffer (Sigma Aldrich) containing 0.1% protease inhibitors (Sigma Aldrich) and subsequently centrifuged at 12 relative centrifugal force for 5 min at 4°C. Free, bound, and total TGF-β1 and TGF-β2 (referred to by R&D Systems as active, latent, and total TGF-β) were measured with R&D DuoSet ELISA development kits (human TGF-β1: DY240, human TGF-β2: DY302; R&D Systems, Minneapolis, MN) that cross-react with porcine, rodent, and ovine TGF-β1 and -β2, respectively, but not with other isoforms. Experiments were done according to the manufacturer's instructions and as previously described (23, 27). Protein concentrations of TGF-β1 and TGF-β2 were calculated per kilogram body weight.
Immunohistochemistry.
Paraffin-embedded lung sections (4 μm, transverse) were stained for pSmad2 (Ser465/467) (no. 3101; Cell Signaling Technology, Boston, MA) and CTGF (sc-14939; Santa Cruz Biotechnology, Santa Cruz, CA). CTGF staining was performed as described previously (23). For pSmad2, the sections were deparaffinized in an ethanol series, and endogenous peroxidase activity was blocked by incubation with 3% H2O2 in milli-Q. Antigen retrieval was performed by incubating the sections in heated citrate buffer (10 mM, pH 6.0) for 30 min. To block aspecific binding, the slides were incubated with 5% normal goat serum in 1× Tris-buffered saline (pH 7.6) with 0.1% Tween. Sections were incubated overnight at 4°C with the diluted primary antibody (pSmad2, 1:2,000; CTGF, 1:75). After incubation with the appropriate secondary antibody, immunostaining was enhanced with the Vectastain ABC peroxidase Elite kit (PK-6200; Vector Laboratories, Burlingame, CA) and stained with nickel sulfate-diaminobenzidine. Subsequently, the sections were rinsed in Tris/saline and incubated with Tris/cobalt. After counterstaining with 0.1% Nuclear Fast Red, the sections were washed and dehydrated. Evaluation was performed by light microscopy (Axioskop 40; Zeiss) with LeicaQWin Pro version 3.4.0 software (Leica Microsystems). Sections were scored for positive pSmad2 or CTGF with a semiquantitative scoring system by two blinded observers: 1, little staining; 2, some staining; 3, strong staining; 4, very strong staining.
RNA extraction and real-time PCR.
Total RNA was extracted from frozen lung tissue using the SV Total RNA Isolation system (Z3100; Promega, Madison, WI) according to the manufacturer's instructions. Genomic DNA contamination was removed by treatment with RQ1 DNase (M610A; Promega), and the RNA was tested for the presence of genomic GAPDH as described previously. Total RNA was reverse transcribed with the First Strand cDNA synthesis kit (4379012001; Roche-Applied, Mannheim, Germany) according to the manufacturer's instructions using anchored oligo primers. RT-PCR reactions were performed in duplicate with the LightCycler 480 SYBR Green I Master mix (4707516001; Roche-Applied) on a LightCycler 480 Instrument according to the manufacturer's instructions. RT-PCR results were normalized to ovRSP15 (22), a housekeeping gene, and mean fold changes in mRNA expression were calculated by the ΔΔCt method (28). CTGF primers were based on the published CTGF cDNA sequence of Ovis aries (NM_001164714.1). CTGF primer sequences were as follows: forward: 5′- TATAGCTCCAGCGACAGCTC-3′, reverse: 5′- ACGAACTTGACTCAGCCTCA-3′, amplicon size = 64 bp, melting temperature = 62°C. Cav-1 primers have been reported previously (22).
Western blot.
The Western blot for Cav-1 was performed as described previously (22). Western blots were probed with primary antibodies to Cav-1 (sc-894; Santa Cruz Biotechnology), pSmad2 (no. 3101; Cell Signaling Technology), and Smad2 (sc-7910; Santa Cruz Biotechnology) followed by the corresponding horseradish peroxidase-conjugated secondary antibody (32430/32460; Pierce, Bonn, Germany). For normalization of the experiments, membranes were stripped, as recommended by the manufacturer (Pierce Biotechnology), and reprobed with an antibody against β-actin (sc-130301; Santa Cruz Biotechnology). Specific protein bands were visualized using enhanced chemiluminescence (SuperSignal West Dura; Pierce Biotechnology) and detected using the LAS 3000 computer-based luminescent image analyzer (FujiFilm, Tokyo, Japan). Accumulated signals were analyzed using AIDA software (Raytest, Straubenhardt, Germany).
Data analysis.
Results are given as means ± SE. The groups were compared using one-way ANOVA with Tukey's test for post hoc analysis. Statistical analysis was performed by GraphPad Prism version 5.0. Significance was accepted at P < 0.05.
RESULTS
LPS- and BTM-induced expression of TGF-β pathway members.
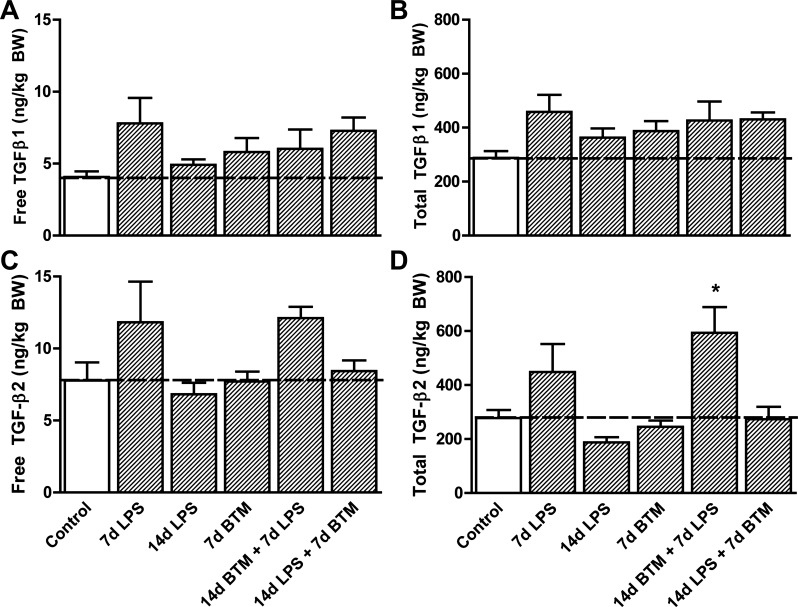
Exposure to LPS and/or BTM did not change the amount of free (Fig. 1A) and total (Fig. 1B) TGF-β1 in the fetal lung tissue compared with controls (Table 1). The amount of free TGF-β2 (Fig. 1C) also remained unchanged. Total TGF-β2 levels increased in the animals that were exposed to BTM 7 days before exposure to LPS (Fig. 1D). There was a nonsignificant trend toward increased TGF-β1 and -β2 levels in the fetal lung 7 days after the LPS exposure.
Fig. 1.
Protein levels of transforming growth factor (TGF)-β1 and -β2 in the fetal lung. A: protein levels of free TGF-β1 in the fetal lung tissue did not change by exposure to intra-amniotic lipopolysaccharide (LPS) and/or maternal betamethasone (BTM). B: total TGF-β1 was unchanged by LPS and/or BTM exposure. C: LPS and/or BTM exposure did not change the protein expression of free TGF-β2. D: total TGF-β2 levels increased in the animals that were exposed to BTM 7 days (d) before exposure to LPS. BW, body weight. *P < 0.05 vs. controls using a one-way ANOVA with Tukey's post hoc test.
Table 1.
Group names and animal numbers
| Group | No. of Animals |
|---|---|
| Control | 5 |
| 7 d LPS | 8 |
| 14 d LPS | 8 |
| 7 d BTM | 7 |
| 14 d BTM + 7 d LPS | 6 |
| 14 d LPS + 7 d BTM | 8 |
LPS, lipopolysaccharide; BTM, betamethasone; d, day.
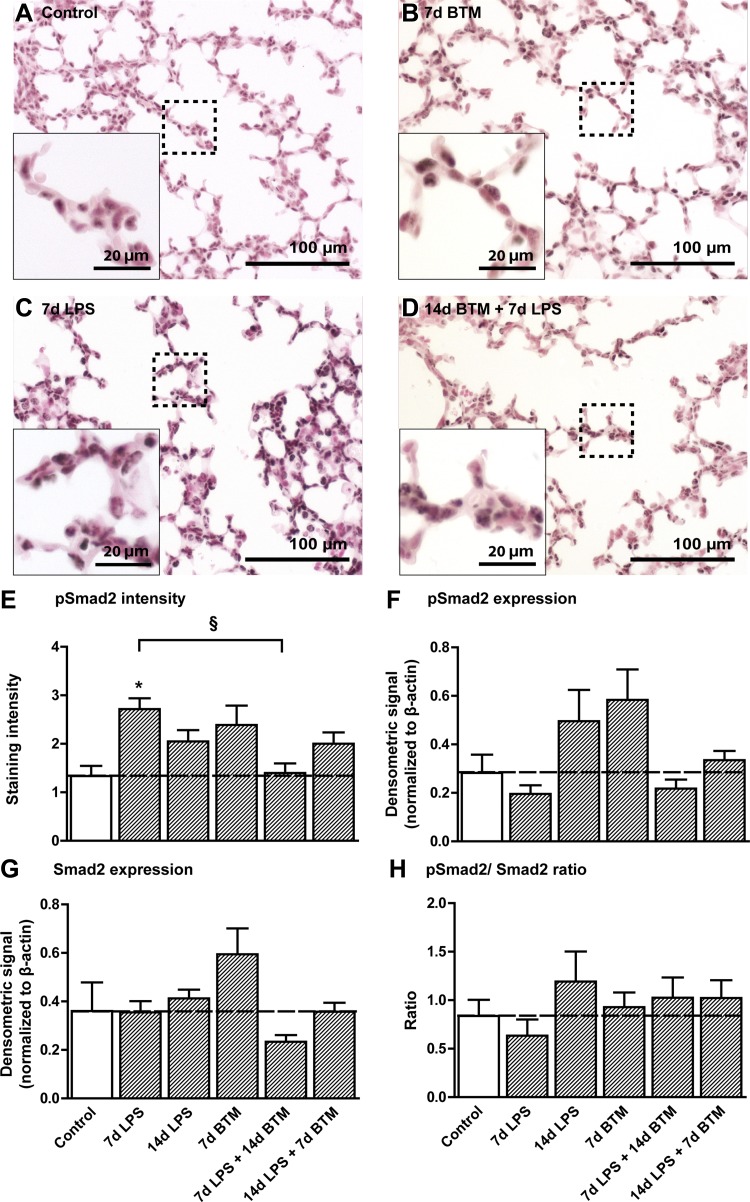
Smad2 phosphorylation was mainly detected in the nuclei of epithelial cells. Representative images are shown for controls (Fig. 2A), 7 day BTM-exposed lungs (Fig. 2B), 7 day LPS-exposed lungs (Fig. 2C), and 14 day BTM followed by LPS-exposed lungs (Fig. 2D). The staining intensity of pSmad2 increased 7 days after the exposure to LPS compared with controls (Fig. 2E). Treatment with BTM 7 days before the exposure to LPS prevented the increase in pSmad2. This increase of Smad2 phosphorylation observed in the nuclei of epithelial cells was not reflected in protein quantifications by Western blot using protein extracts of complete distal lung tissue (Fig. 2F). Similarly, total Smad2 levels and the pSmad2-to-total Smad2 ratio were not statistically different from controls (Fig. 2, G and H).
Fig. 2.
BTM prevented LPS-mediated induction of phosphorylated sma and mothers against decapentaplegic homolog 2 (pSmad2). pSmad2 staining in alveolar tissue as seen in controls (A), 7 days after BTM treatment (B), 7 days after LPS exposure (C), and after a combination of pretreatment with BTM followed by 7 days LPS (D). E: the staining intensity of pSmad2 increased 7 days after the exposure to LPS compared with controls and was prevented by BTM pretreatment. F: protein quantifications of Smad2 phosphorylation by Western blot did not show any significant changes between groups. G: total Smad2 levels were not statistically different from controls. H: the ratio between phosphorylated and total Smad2 was not significantly different between groups. *P < 0.05 vs. controls and §P < 0.05 between experimental groups using a one-way ANOVA with Tukey's post hoc test.
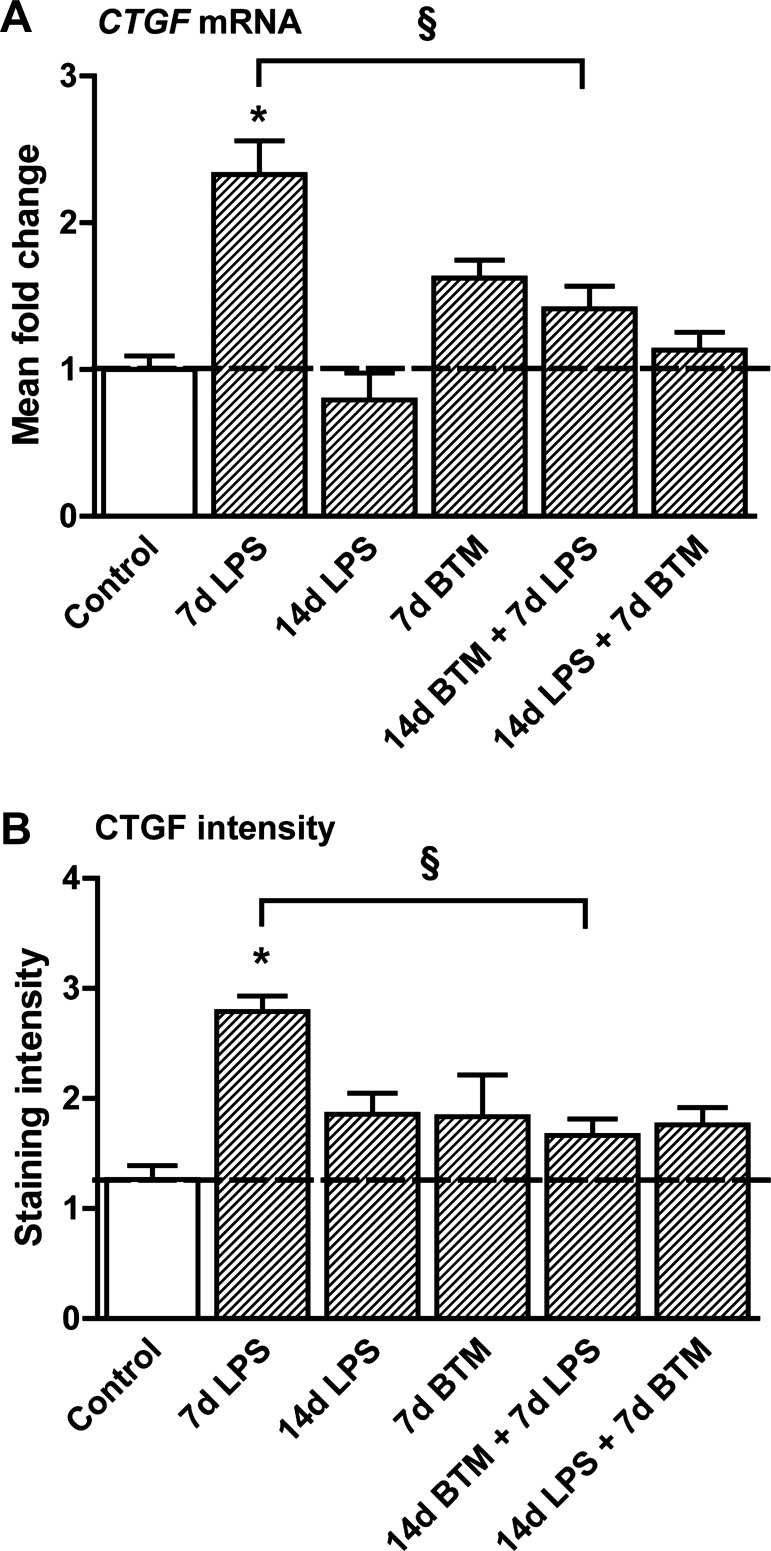
CTGF mRNA levels increased 7 days after the exposure to LPS, and this increase was prevented by BTM treatment 7 days before LPS exposure (Fig. 3A). CTGF staining intensity showed similar changes with increased intensity after LPS exposure (Fig. 3B). Treatment with BTM before the exposure to LPS prevented the increase in CTGF protein levels.
Fig. 3.
BTM prevented LPS-mediated induction of connective tissue growth factor (CTGF) expression. A: CTGF mRNA levels increased 7 days after the exposure to LPS in the fetal lung, which was prevented by BTM pretreatment. B: 7 days after the exposure to LPS CTGF protein levels increased. Pretreatment with BTM before the exposure to LPS prevented the increase in CTGF protein levels. *P < 0.05 vs. controls and §P < 0.05 between experimental groups using a one-way ANOVA with Tukey's post hoc test.
BTM prevented decreased Cav-1 expression after LPS exposure.
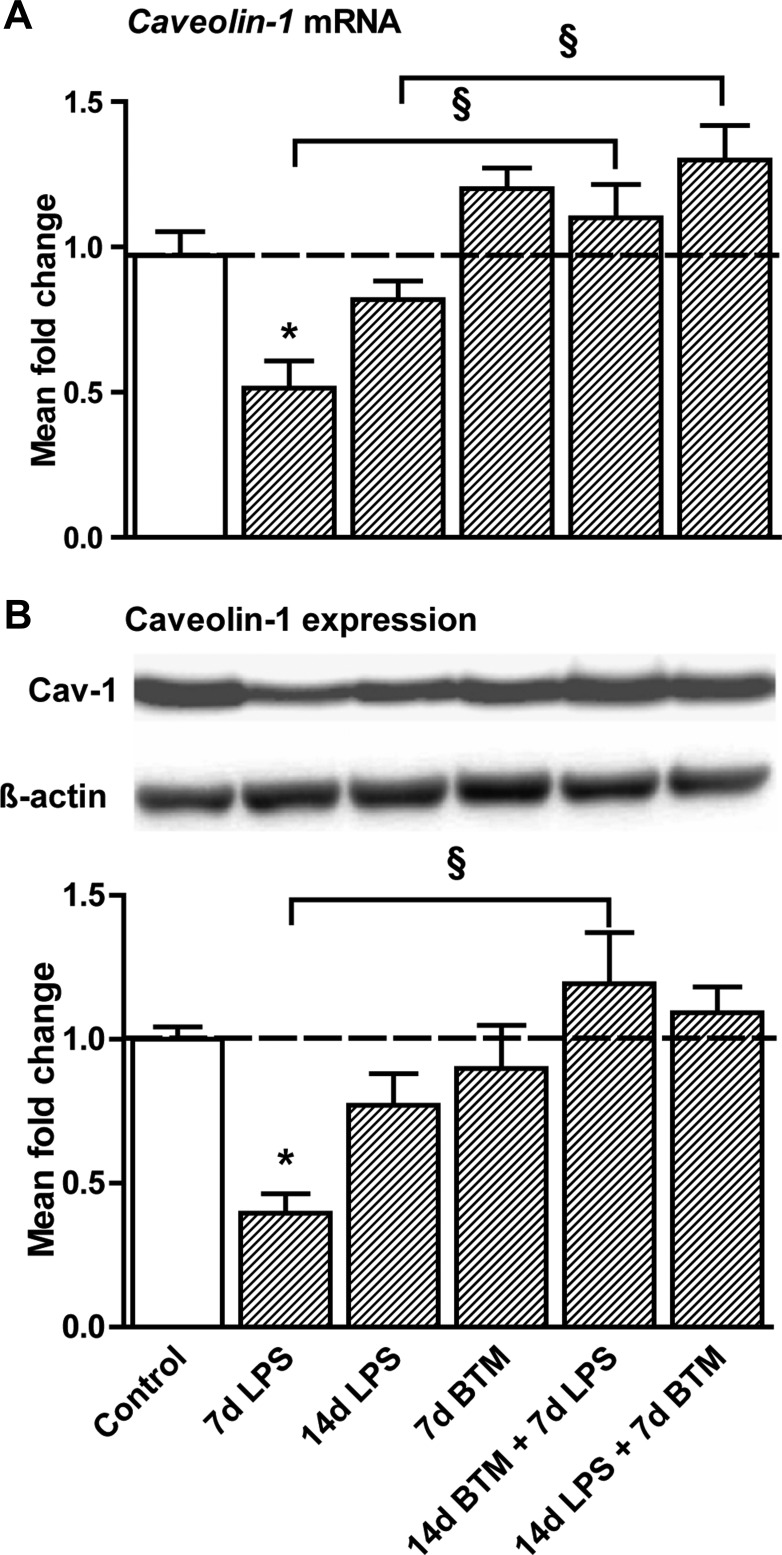
Intra-amniotic LPS decreased Cav-1 mRNA (Fig. 4A) and protein levels (Fig. 4B) 7 days after the exposure by 50% compared with controls. Treatment with BTM before the LPS exposure prevented this decrease. BTM treatment 7 days after the exposure to LPS increased Cav-1 mRNA compared with a single 14-day LPS exposure.
Fig. 4.
LPS-induced decreased caveolin-1 expression prevented by BTM. A: intra-amniotic LPS decreased caveolin-1 mRNA levels 7 days after the exposure by 50% compared with controls. Pretreatment with BTM before the LPS exposure prevented this decrease. BTM treatment 7 days after the exposure to LPS also increased caveolin-1 mRNA compared with animals only exposed to LPS for 14 days. B: caveolin-1 protein levels decreased 7 days after LPS exposure, which was prevented by BTM pretreatment. *P < 0.05 vs. controls and §P < 0.05 between experimental groups using a one-way ANOVA with Tukey's post hoc test.
DISCUSSION
Antenatal exposure to either inflammation or glucocorticoids causes maturational responses in the developing lung but also contributes to the development of BPD (18, 19). A combination of these exposures in preterm neonates is frequent (6, 13), yet it is unclear whether these stimuli create additive or antagonistic effects on signaling pathways that direct lung development.
Recently, we demonstrated in preterm sheep that exposure to intra-amniotic LPS 14 days and maternal BTM 7 days before preterm delivery caused a larger maturational response than either agent alone, as measured by surfactant production and lung compliance (24). A recent meta-analysis by Been and colleagues also concluded that antenatal steroids improved neonatal outcomes after preterm birth associated with chorioamnionitis (4). Surprisingly, the antagonistic combination of inflammation and anti-inflammatory glucocorticoids caused more lung maturation than either stimulus alone. The changes in the fetal lungs have been described as “maturation” because of the improved physiology for gas exchange, but multiple aspects of lung development may be abnormal. It is unknown whether antenatal glucocorticoids and inflammation share common modulatory mechanisms or work in parallel (42).
Lung development is orchestrated by multiple pathways, one of which is the TGF-β pathway. A balanced and timed expression of TGF-β is crucial for both embryonic and fetal lung development, since inhibition of TGF-β signaling is essential for lung bud formation (7), whereas overexpression of TGF-β later in lung development inhibits branching morphogenesis (39) and alveolarization (11). We asked how TGF-β, the phosphorylation of its transcription factor Smad2, and its regulated genes CTGF and Cav-1 would be affected by sequential exposure to both LPS-induced inflammation and antenatal glucocorticoids in a large mammalian model relevant to the preterm infant. Exposure of fetal lambs to only LPS increased TGF-β1 and -β2 levels in the lung after 7 days, although not significantly. The moderate increase in TGF-β levels can potentially explain the increased alveolar pSmad2 7 days after LPS exposure. Downstream of TGF-β signaling CTGF increased, which is consistent with the general understanding of this pathway (23, 34). In another study, however, in which lambs received an intra-amniotic injection of LPS plus subsequent short-time mechanical ventilation at a later time point in gestation, a decrease in CTGF was reported despite increased TGF-β1 and pSmad2 levels (23). This decrease in CTGF levels can most likely be explained by the additional damage induced by the short-term mechanical ventilation, which overrules the initial effect of inflammation. An increase in TGF-β signaling is also supported by decreased Cav-1, a structural component of caveolae that facilitates the degradation of TGF-β receptors (9, 37). This is consistent with our previous study in which intrauterine LPS exposure was also associated with decreased Cav-1 and increased pSmad2 levels in the fetal sheep lung after 2 and 7 days of LPS exposure (22).
The anti-inflammatory effects of maternal glucocorticoids include inhibition of TGF-β transcription and production (49). In BPD patients, prenatal glucocorticoid exposure decreased TGF-β levels in endotracheal aspirate fluid (26). In contrast to LPS-exposed lambs, the levels of both pSmad2 and CTGF were at control levels in the lungs of fetal lambs that had been exposed to antenatal BTM in addition to LPS. The same was true for Cav-1 expression, as a negative regulator of TGF-β signaling. Moreover, we even found a tendency for increased Smad2 phosphorylation in lambs exposed to BTM alone for 7 days. To our knowledge, no direct effects of glucocorticoids on Smad2 phosphorylation in lung have been described in literature. One study, however, reported a glucocorticoid-induced increase in Smad2 phosphorylation in the gastric mucosa of suckling rats that was associated with increased cell differentiation (35). Although we found no direct inhibitory effect of prenatal glucocorticoids on TGF-β, maternal glucocorticoids counteracted LPS-induced TGF-β pathway activation. The anti-inflammatory capabilities of glucocorticoids are often attributed to the antagonistic interactions of the glucocorticoid receptor (GR) with several subunits of NF-κB, a transcriptional activator of proinflammatory genes (33, 40). However, glucocorticoids can maintain or even increase LPS-induced inflammation, implying that activation of NF-κB is not the only inducer of proinflammatory gene transcription. Activation of both NF-κB and the GR can have very different effects on signaling pathways and can lead to either upregulation or silencing of different clusters of genes (36). TGF-β signaling has been implicated in inflammation-induced maturation (11, 21, 23). Our results in fetal sheep do not support a role for TGF-β signaling in corticosteroid-induced maturation, since they exhibited a maturational response (24) without elevated levels of the TGF-β pathway members studied here. GR activation by synthetic glucocorticoids like BTM can stimulate Cav-1 expression and caveolae formation selectively in alveolar epithelial cells (2), supporting our observation of the counteractive effect of BTM exposure for LPS-induced TGF-β signaling. It is unclear why TGF-β2 is specifically upregulated after a sequential exposure to LPS and BTM, but not to a single exposure to either stimulus. Perhaps this could be a side effect of coactivation of NF-κB and the GR as described by Rao and colleagues (36).
Our results support the involvement of TGF-β, CTGF, and Cav-1 in the pulmonary response to LPS-induced inflammation, which in turn may be involved in the maturational response seen in the same animals (24). TGF-β pathway components, such as CTGF, have promise as therapeutic targets for the treatment of BPD, particularly after ventilation-induced injury (1). Although our study has identified interactions of LPS, glucocorticoids, and TGF-β in a time-dependent manner, a detailed exploration using this large animal model with multiple time points of assessment is impractical. Because of the nature of the animal model and a lack of specific reagents, it was unfortunately not possible to do more quantitative assessments of pSmad2 and CTGF protein levels. Furthermore, some observed changes did not reach statistical significance, since our group numbers were quite small. We have, however, demonstrated interferences between LPS and corticosteroid-mediated molecular effects in the developing lung. These interactions will pose a challenge for the future development of treatment strategies, since perinatal inflammation and ante- or postnatal administration of glucocorticoids are very common.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grant HD-57869 (S. G. Kallapur), the National Health and Medical Research Council of Australia, the Women and Infants Research Foundation, Western Australia, Veni BWK 016.096.141 from the Dutch Scientific Research Organization and the Research School for Oncology and Developmental Biology, Maastricht University Interdisciplinary Center for Clinical Research, University of Würzburg, Grant IZKF A-58.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.J.C., S.K., E.K., M.W.K., J.P.N., S.G.K., A.H.J., and B.W.K. conception and design of research; J.J.C., S.K., E.K., M.W.K., J.P.N., S.G.K., A.H.J., and B.W.K. performed experiments; J.J.C., S.K., and E.K. analyzed data; J.J.C., S.K., E.K., and B.W.K. interpreted results of experiments; J.J.C., S.K., and E.K. prepared figures; J.J.C., S.K., and E.K. drafted manuscript; J.J.C., S.K., E.K., M.W.K., C.P.S., J.P.N., S.G.K., A.H.J., and B.W.K. edited and revised manuscript; J.J.C., S.K., E.K., M.W.K., C.P.S., J.P.N., S.G.K., A.H.J., and B.W.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Daniele Herbst, Barbera Ottensmeier, Nico Kloosterboer, Richard Dalton, Joe Derwort, Masatoshi Saito, Clare Berry, Carryn McLean, Shaofu Li, and Jennifer Henderson for excellent technical support.
REFERENCES
- 1. Alapati D, Rong M, Chen S, Hehre D, Rodriguez MM, Lipson KE, Wu S. Connective tissue growth factor antibody therapy attenuates hyperoxia-induced lung injury in neonatal rats. Am J Respir Cell Mol Biol 45: 1169–1177, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Barar J, Campbell L, Hollins AJ, Thomas NP, Smith MW, Morris CJ, Gumbleton M. Cell selective glucocorticoid induction of caveolin-1 and caveolae in differentiating pulmonary alveolar epithelial cell cultures. Biochem Biophys Res Commun 359: 360–366, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Bartram U, Speer CP. The role of transforming growth factor beta in lung development and disease. Chest 125: 754–765, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Been J, Degraeuwe P, Kramer B, Zimmermann L. Antenatal steroids and neonatal outcome after chorioamnionitis: a meta-analysis. Br J Gastroenterol 118: 113–122, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Beeton ML, Maxwell NC, Davies PL, Nuttall D, McGreal E, Chakraborty M, Spiller OB, Kotecha S. Role of pulmonary infection in the development of chronic lung disease of prematurity. Eur Respir J 37: 1424–1430, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Carlo WA, McDonald SA, Fanaroff AA, Vohr BR, Stoll BJ, Ehrenkranz RA, Andrews WW, Wallace D, Das A, Bell EF, Walsh MC, Laptook AR, Shankaran S, Poindexter BB, Hale EC, Newman NS, Davis AS, Schibler K, Kennedy KA, Sanchez PJ, Van Meurs KP, Goldberg RN, Watterberg KL, Faix RG, Frantz ID, 3rd, Higgins RD. Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22 to 25 weeks' gestation. J Am Med Assoc 306: 2348–2358, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen F, Desai TJ, Qian J, Niederreither K, Lu J, Cardoso WV. Inhibition of Tgf beta signaling by endogenous retinoic acid is essential for primary lung bud induction. Development 134: 2969–2979, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Chen S, Rong M, Platteau A, Hehre D, Smith H, Ruiz P, Whitsett J, Bancalari E, Wu S. CTGF disrupts alveolarization and induces pulmonary hypertension in neonatal mice: implication in the pathogenesis of severe bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 300: L330–L340, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol 5: 410–421, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Gantert M, Been JV, Gavilanes AW, Garnier Y, Zimmermann LJ, Kramer BW. Chorioamnionitis: a multiorgan disease of the fetus? J Perinatol Suppl 30: S21–S30, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Gauldie J, Galt T, Bonniaud P, Robbins C, Kelly M, Warburton D. Transfer of the active form of transforming growth factor-beta 1 gene to newborn rat lung induces changes consistent with bronchopulmonary dysplasia. Am J Pathol 163: 2575–2584, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gauldie J, Galt T, Bonniaud P, Robbins C, Kelly M, Warburton D. Transfer of the active form of transforming growth factor-beta 1 gene to newborn rat lung induces changes consistent with bronchopulmonary dysplasia. Am J Pathol 163: 2575–2584, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goldenberg RL, Andrews WW, Faye-Petersen OM, Cliver SP, Goepfert AR, Hauth JC. The Alabama preterm birth study: corticosteroids and neonatal outcomes in 23- to 32-week newborns with various markers of intrauterine infection. Am J Obstet Gynecol 195: 1020–1024, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Gosens R, Mutawe M, Martin S, Basu S, Bos ST, Tran T, Halayko AJ. Caveolae and caveolins in the respiratory system. Curr Mol Med 8: 741–753, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Gosens R, Stelmack GL, Bos ST, Dueck G, Mutawe MM, Schaafsma D, Unruh H, Gerthoffer WT, Zaagsma J, Meurs H, Halayko AJ. Caveolin-1 is required for contractile phenotype expression by airway smooth muscle cells. J Cell Mol Med 15: 2430–2442, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ikegami M, Jobe AH, Newnham J, Polk DH, Willet KE, Sly P. Repetitive prenatal glucocorticoids improve lung function and decrease growth in preterm lambs. Am J Respir Crit Care Med 156: 178–184, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Jin Y, Lee SJ, Minshall RD, Choi AM. Caveolin-1: a critical regulator of lung injury. Am J Physiol Lung Cell Mol Physiol 300: L151–L160, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jobe AH. Glucocorticoids, inflammation and the perinatal lung. Semin Neonatol 6: 331–342, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 163: 1723–1729, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Knox IC, Jr, Hoerner JK. The role of infection in premature rupture of the membranes. Am J Obstet Gynecol 59: 190–194, 1950 [DOI] [PubMed] [Google Scholar]
- 21. Kotecha S, Wangoo A, Silverman M, Shaw RJ. Increase in the concentration of transforming growth factor beta-1 in bronchoalveolar lavage fluid before development of chronic lung disease of prematurity. J Pediatr 128: 464–469, 1996 [DOI] [PubMed] [Google Scholar]
- 22. Kunzmann S, Collins JJ, Yang Y, Uhlig S, Kallapur SG, Speer CP, Jobe AH, Kramer BW. Antenatal inflammation reduces expression of caveolin-1 and influences multiple signaling pathways in preterm fetal lungs. Am J Respir Cell Mol Biol 45: 969–976, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kunzmann S, Speer CP, Jobe AH, Kramer BW. Antenatal inflammation induced TGF-β1 but suppressed CTGF in preterm lungs. Am J Physiol Lung Cell Mol Physiol 292: L223–L231, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Kuypers E, Collins JJ, Kramer BW, Ofman G, Nitsos I, Pillow JJ, Polglase GR, Kemp MW, Newnham JP, Gavilanes AW, Nowacki R, Ikegami M, Jobe AH, Kallapur SG. Intra-amniotic LPS and antenatal betamethasone: inflammation and maturation in preterm lamb lungs. Am J Physiol Lung Cell Mol Physiol 302: L380–L389, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci 119: 4803–4810, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Lecart C, Cayabyab R, Buckley S, Morrison J, Kwong KY, Warburton D, Ramanathan R, Jones CA, Minoo P. Bioactive transforming growth factor-beta in the lungs of extremely low birthweight neonates predicts the need for home oxygen supplementation. Biol Neonate 77: 217–223, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Lee AJ, Lambermont VA, Pillow JJ, Polglase GR, Nitsos I, Newnham JP, Beilharz MW, Kallapur SG, Jobe AH, Kramer BW. Fetal responses to lipopolysaccharide-induced chorioamnionitis alter immune and airway responses in 7-week-old sheep. Am J Obstet Gynecol 204: 364 e317–e324, 2011. [DOI] [PubMed] [Google Scholar]
- 28. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Lv XJ, Li YY, Zhang YJ, Mao M, Qian GS. Over-expression of caveolin-1 aggravate LPS-induced inflammatory response in AT-1 cells via up-regulation of cPLA2/p38 MAPK. Inflamm Res 59: 531–541, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Massague J. TGF-beta signal transduction. Annu Rev Biochem 67: 753–791, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Massaro GD, Massaro D. Formation of alveoli in rats: postnatal effect of prenatal dexamethasone. Am J Physiol Lung Cell Mol Physiol 263: L37–L41, 1992 [DOI] [PubMed] [Google Scholar]
- 32. McDevitt TM, Gonzales LW, Savani RC, Ballard PL. Role of endogenous TGF-β in glucocorticoid-induced lung type II cell differentiation. Am J Physiol Lung Cell Mol Physiol 292: L249–L257, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Murphy SH, Suzuki K, Downes M, Welch GL, De Jesus P, Miraglia LJ, Orth AP, Chanda SK, Evans RM, Verma IM. Tumor suppressor protein (p)53, is a regulator of NF-kappaB repression by the glucocorticoid receptor. Proc Natl Acad Sci USA 108: 17117–17122, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nishioka M, Ogawa E, Kinose D, Haruna A, Ohara T, Ito I, Hoshino Y, Ito Y, Matsumoto H, Niimi A, Mio T, Chin K, Hirai T, Muro S, Mishima M. Lipopolysaccharide induced connective tissue growth factor gene expression in human bronchial epithelial cells. Respirology 15: 669–676, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Ogias D, Bitencourt B, Alvares EP, Gama P. Corticosteroids induce the differential expression of TGFbeta isoforms, receptors and signaling in the gastric mucosa of suckling rats. Regul Pept 135: 17–22, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Rao NA, McCalman MT, Moulos P, Francoijs KJ, Chatziioannou A, Kolisis FN, Alexis MN, Mitsiou DJ, Stunnenberg HG. Coactivation of GR and NFKB alters the repertoire of their binding sites and target genes. Genome Res 21: 1404–1416, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Razani B, Zhang XL, Bitzer M, von Gersdorff G, Bottinger EP, Lisanti MP. Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor. J Biol Chem 276: 6727–6738, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Ronnestad A, Abrahamsen TG, Medbo S, Reigstad H, Lossius K, Kaaresen PI, Engelund IE, Irgens LM, Markestad T. Septicemia in the first week of life in a Norwegian national cohort of extremely premature infants. Pediatrics 115: e262–e268, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Serra R, Pelton RW, Moses HL. TGF beta 1 inhibits branching morphogenesis and N-myc expression in lung bud organ cultures. Development 120: 2153–2161, 1994 [DOI] [PubMed] [Google Scholar]
- 40. Smoak KA, Cidlowski JA. Mechanisms of glucocorticoid receptor signaling during inflammation. Mech Ageing Dev 125: 697–706, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, Lemons JA, Donovan EF, Stark AR, Tyson JE, Oh W, Bauer CR, Korones SB, Shankaran S, Laptook AR, Stevenson DK, Papile LA, Poole WK. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 110: 285–291, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Sweet DG, Huggett MT, Warner JA, Moss TJ, Kloosterboer N, Halliday HL, Newnham JP, Kallapur SG, Jobe AH, Kramer BW. Maternal betamethasone and chorioamnionitis induce different collagenases during lung maturation in fetal sheep. Neonatology 94: 79–86, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Syrkina O, Jafari B, Hales CA, Quinn DA. Oxidant stress mediates inflammation and apoptosis in ventilator-induced lung injury. Respirology 13: 333–340, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Van Marter LJ, Dammann O, Allred EN, Leviton A, Pagano M, Moore M, Martin C. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. J Pediatr 140: 171–176, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Vicencio AG, Lee CG, Cho SJ, Eickelberg O, Chuu Y, Haddad GG, Elias JA. Conditional overexpression of bioactive transforming growth factor-beta1 in neonatal mouse lung: a new model for bronchopulmonary dysplasia? Am J Respir Cell Mol Biol 31: 650–656, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Viscardi RM. Perinatal inflammation and lung injury. Semin Fetal Neonat Med 17: 30–35, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Viscardi RM, Atamas SP, Luzina IG, Hasday JD, He JR, Sime PJ, Coalson JJ, Yoder BA. Antenatal Ureaplasma urealyticum respiratory tract infection stimulates proinflammatory, profibrotic responses in the preterm baboon lung. Pediatr Res 60: 141–146, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics 97: 210–215, 1996 [PubMed] [Google Scholar]
- 49. Wen FQ, Kohyama T, Skold CM, Zhu YK, Liu X, Romberger DJ, Stoner J, Rennard SI. Glucocorticoids modulate TGF-beta production. Inflammation 26: 279–290, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Willet KE, Jobe AH, Ikegami M, Kovar J, Sly PD. Lung morphometry after repetitive antenatal glucocorticoid treatment in preterm sheep. Am J Respir Crit Care Med 163: 1437–1443, 2001 [DOI] [PubMed] [Google Scholar]
- 51. Williams TM, Lisanti MP. The Caveolin genes: from cell biology to medicine. Ann Med 36: 584–595, 2004 [DOI] [PubMed] [Google Scholar]