Abstract
Recently, we reported that recovery of tissue perfusion in the ischemic hindlimb was reduced, inflammatory response increased, and survival of distal limb tissue compromised in connexin 40 (Cx40)-deficient (Cx40−/−) mice. Here we evaluate whether genotype-specific differences in tissue perfusion, native vascular density, arteriogenesis, blood pressure, and chronic ANG II type 1 receptor (AT1R) activation contribute to poor recovery of ischemic hindlimb tissue in Cx40−/− mice. Hindlimb ischemia was induced in wild-type (WT), Cx40−/−, and losartan-treated Cx40−/− mice by using surgical procedures that either maintained (mild surgery) or compromised (severe surgery) perfusion of major collateral vessels supplying the distal limb. Pre- and postsurgical hindlimb perfusion was evaluated, and tissue survival, microvascular density, and macrophage infiltration were documented during recovery. Hindlimb perfusion was compromised in presurgical Cx40−/− versus WT mice despite comparable native microvascular density. Hindlimb perfusion 24 h postsurgery in Cx40−/− and WT mice was comparable after mild surgery (collateral vessels maintained), but compromised arteriogenesis in Cx40−/− animals nevertheless limited subsequent recovery of tissue perfusion and compromised tissue survival. Prolonged pre- and postsurgical treatment of Cx40−/− mice with losartan (an AT1R antagonist) normalized blood pressure but did not improve tissue perfusion or survival, despite reduced macrophage infiltration. Thus it appears Cx40 is necessary for normal tissue perfusion and for recovery of perfusion, arteriogenesis, and tissue survival in the ischemic hindlimb. Our data suggest that Cx40−/− mice are at significantly greater risk for poor recovery from ischemic insult due to compromised regulation of tissue perfusion, vascular remodeling, and prolonged inflammatory response.
Keywords: gap junction, vascular remodeling, inflammation, collateral blood vessels, losartan, ANG II type 1 receptor, connexin 40 deficient
in addition to forming gap junction channels that mediate electrical and chemical intercellular signaling between neighboring cells, connexins support transmembrane signaling through functional hemichannels and intracellular signaling through interactions with a diverse array of intracellular proteins including growth and disease-activated kinases as well as cytoskeletal and adhesion proteins (6, 23, 38). The complex phenotypes of connexin-deficient animals likely reflect, in part, this diversity of underlying mechanism. The vascular connexins have been shown to contribute to vascular function via each of these mechanisms. Particularly germane to the current study, the vasculature of connexin 40 (Cx40)-deficient (Cx40−/−) mice suffers several functional deficits, including compromised upstream conduction of vasodilatory responses (9), spontaneous vasospasms sufficient in magnitude to transiently interrupt blood flow (10), a probable increase in vascular permeability (42), a proinflammatory phenotype (6), altered expression of multiple genes (e.g., Cx37, CD73, endothelial nitric oxide synthase, and VCAM1) (6, 20, 24, 35), and chronic hypertension due, in part, to overproduction of renin by the kidney (10, 25, 39). Each of these deficits in vascular function, in addition to compromising normal vascular function, could negatively impact recovery of ischemic tissue (5, 16, 21, 30, 38).
Previously, we showed that Cx40−/− mice, subjected to femoral and saphenous artery-vein pair resection (here termed severe surgery), were more likely to suffer irreversible injury than either wild-type (WT) or Cx37−/− mice (15). We concluded from these studies that the larger reduction in distal limb perfusion evident 24 h postsevere surgery in Cx40−/− mice led to a more extensive injury, predisposing the limb to an aggressive inflammatory response that led to distal necrosis and poor recovery of the injured limb. In this previous study, perfusion of the surgical limb was normalized to perfusion of the contralateral nonsurgical limb (to permit the distinction of local from possible systemic effects of ischemic injury). As a consequence of this normalization, potential genotype-specific differences in perfusion before and subsequent to ischemic injury could have been missed. In the current study we evaluated Cx40−/− and WT animals for possible differences in distal-limb perfusion. In addition, to assess the contribution of compromised arteriogenesis in poor recovery of severe surgery Cx40−/− mice, we implemented, in both WT and Cx40−/− animals, a femoral artery ligation model (here termed mild surgery) that preserves the capacity for function of the collateral vessels supplying the distal limb. Finally, because Cx40−/− animals are hypertensive due, in part, to chronic activation of ANG II type 1 receptors (AT1R), and because both hypertension and chronic AT1R activation represent significant risk factors in the ischemic injury setting (25, 27, 33, 40), in the current study we also determine whether chronic AT1R blockade, which others have shown reduces blood pressure in Cx40−/− animals to untreated WT levels (1, 10), might improve recovery from ischemic injury in Cx40−/− mice.
We show that distal limb perfusion was significantly less in Cx40−/− than WT mice, due largely to a reduced perfusion area. Because microvascular density was not different in Cx40−/− and WT mice, this deficit of perfusion likely reflects a temporal deficit of perfusion arising from compromised regulation of blood flow distribution to the distal limb of Cx40−/− animals. When comparing recovery after mild versus severe surgery in Cx40−/− and WT mice, we further show that this compromised regulation of blood flow distribution along with compromised arteriogenesis (outward remodeling of collateral vessels) contribute to the poor postischemic surgery recovery in Cx40−/− mice. Finally, we show that AT1R receptor blockade (oral losartan) for 3 days or 8 wk before mild surgery did not significantly improve recovery of the postsurgical limb, despite reducing both blood pressure and the muscle area infiltrated by activated macrophages. Together, the data suggest that normalization of blood pressure and reduction of the inflammatory response consequent to AT1R blockade are insufficient to compensate for other deficits of vascular function, in particular compromised regulation of flow distribution and arteriogenesis, in the postischemic limb of Cx40−/− mice.
MATERIALS AND METHODS
Animals.
For this study, 2- to 6-mo-old WT and homozygous Cx40−/− mice (C57Bl/6 strain) of both sexes were used in accordance with protocols approved by the University of Arizona's Institutional Animal Care and Use Committee. Animals were permitted access to food and untreated or drug-treated water ad libitum.
Unilateral hindlimb ischemia and recovery.
Unilateral hindlimb ischemia was induced by one of two surgical strategies: femoral and saphenous artery-vein pair resection, here termed severe surgery, and femoral artery ligation, here termed mild surgery, as previously described (14, 15). For the severe surgery, ligatures were placed around the femoral artery-vein pair upstream of the epigastric and gracilis artery branch-points and around the saphenous artery-vein pair midway between the knee and ankle, and the intervening lengths of vessel were resected. For the mild surgery, ligatures were placed on the femoral artery between the epigastric and popliteal branches and the intervening length of artery was severed. After either procedure, ischemia was documented by laser Doppler perfusion imaging (see below) and mice were scored at multiple postsurgery time points for surgical limb appearance and use as previously described (14, 15). In brief, ischemic limbs were ascribed, by an investigator blinded to genotype and surgical procedure, a nonparametric score based on the following descriptors for appearance (relative to the contralateral control limb): 0 = no difference; −1 = minor discoloration; −2 = moderate discoloration; −3 = severe discoloration and/or mild necrosis; −4 = severe necrosis and/or distal tissue auto-amputation. Any mice receiving a −4 limb appearance score before the end of the scheduled recovery period were euthanized and for subsequent time points limb appearance was set to −4 for that animal; mice never receiving a −4 limb appearance score were sacrificed 2 wk after severe surgery or 3 wk after mild surgery. Similarly, for hindlimb use assessment, while monitoring animal gait, the following nonparametric scores were ascribed based on the following descriptors: 0 = normal use; −1 = plantar flexion but no toe reflex; −2 = no plantar flexion but foot used for balance, and no toe reflex; −3 = no plantar flexion or weight on limb and no toe reflex; −4 = dragging of limb. Animals euthanized before the conclusion of the 2-wk recovery period were not included in the use scores postmortem. For both use and appearance data, median scores are plotted in the corresponding figures.
Laser Doppler perfusion imaging.
Limb perfusion was assessed as previously described (14). In brief, mice were anesthetized with 1.5–5% isoflurane and placed prone on a heating pad to maintain body temperature. Laser Doppler perfusion images (PIM II LDI; Perimed, Stockholm, Sweden) of the mouse's depilated hindquarters were collected in triplicate. A region of interest (ROI) was defined for each limb that encompassed the plantar foot; each ROI contained 138 measured sites (each site was 0.78 × 0.78 mm2) and each site was imaged for 50 ms with a resolution of 256 × 256 pixels (∼10 μm2 pixel resolution). Mean site intensity (corrected for background), a value proportional to blood flow, is calculated as the average across all sites with detected flow and is reported herein as intensity (in volts). Number of sites within the ROI with detected flow is reported as perfused sites. Distal limb perfusion, reported as perfusion, was calculated as the intensity multiplied by perfused sites. For all scans, background was set at 5.6–5.8 V, a value obtained by scanning the hindquarters of a recently euthanized animal wherein blood flow was absent. Mice that were prematurely euthanized after a −4 limb appearance score were ascribed perfused area values of 0 for all postmortem time points.
Blood pressure measurement.
Blood pressure was measured in real time after mice were anesthetized by intraperitoneal injection of 50 mg/kg ketamine (Henry Schein, No. 1049007OK) and 20 mg/kg xylazine (Phoenix Pharmaceuticals, No. NDC57319-362-26). Anesthetized mice were placed supine on a heating pad to maintain body temperature. The left carotid artery was exposed and cannulated with a 27-gauge microcannula mounted on a pressure transducer back-filled with heparanized saline [100 USP units/ml heparin (Henry Schein, No. 2480992OK) in 0.9% saline]. Blood pressure was recorded for at least 5 min (until stability was evident).
Losartan.
An AT1R-specific antagonist, losartan (Sigma, No. 61188; and Tocris Bioscience, No. 3798), was administered orally through the animal's drinking water. The effective dose was determined as follows. Blood pressure was measured as described above before and after injection via a jugular vein cannula of 10, 20, or 30 mg/kg body weight losartan (dissolved in sterile 0.9% saline solution). Systemic blood pressure, calculated as the average pressure across 10 successive beats, was determined 5 min after the start of the record (preinjection blood pressure) and after another 5 min when blood pressure had stabilized after injection of losartan (or vehicle). All three losartan doses were equally effective in reducing blood pressure in WT mice. Based on average mouse weight and assuming consumption of 5 ml water/day, the concentration of losartan required to produce a blood concentration equivalent to that produced by 20 mg/kg intravenous losartan was calculated to be 150 μg/ml. The efficacy of this oral dose was checked in animals provided losartan-containing water for 3 days; blood pressure in Cx40−/− animals dosed in this manner was not different from the blood pressure measured in Cx40−/− animals after intravenous injection of losartan. Thus animals were provided water, ad libitum, with 150 μg/ml losartan potassium for either 3 days (short-term treatment) or 8 wk (long-term treatment) before as well as after surgery, during the recovery period. Water was refreshed every 3–7 days throughout the treatment period. Drug treatment was initiated such that mice would be 4- to 6-mo-old at the time of surgery to ensure they were age-matched with untreated controls. All losartan-treated animals were males, as were the comparison control animals.
Inflammatory response.
To assess the duration of the inflammatory response in the ischemic limb, surgical and control limb gastrocnemius muscles were harvested at the time of euthanasia, typically 2 wk after induction of ischemia by severe surgery. Tissue was whole fixed in 4% paraformaldehyde in divalent cation-free PBS, dehydrated by progressive alcohol wash, and embedded in paraffin. Cross-sections (7 μm thick) of each muscle were mounted on glass slides and probed with anti-F4/80 rat monoclonal antibody (Caltag Laboratories, No. MF48000; 1:100 dilution), which recognizes the F4/80 glycoprotein specifically expressed by activated murine macrophages (2). Antibody binding was visualized by incubation of samples with biotinylated anti-rat secondary antibody (Pierce, No. 31831; 1:100), followed by ready-to-use streptavidin-horse radish peroxidase conjugate (Vector Laboratories, No. B-1105) and DAB Chromogen substrate (DAKO, No. K3466). To quantify the degree of inflammatory response, eight random images were taken of each section of ischemic and nonischemic gastrocnemius. For each image, an investigator blind to genotype and surgical procedure determined a threshold level that excluded background staining in ImageJ and measured the pixel area above threshold. Mean infiltrated area of surgical and nonsurgical limb tissue were determined for each animal, and the fold increase was calculated.
Vascular density.
Microvascular density was visualized and quantified as previously reported (14). In brief, nonischemic gastrocnemius muscle from sham- or severe-surgery mice was harvested, embedded in paraffin, and sectioned as described above. Tissue sections were incubated with biotinylated Griffonia simplifica-1 lectin (Vector Laboratories, No. B-1105; 1:200), which binds to galactosylated surface proteins specifically expressed on vascular endothelium. Lectin-binding was detected by exposure of slides to ready-to-use streptavidin horse radish peroxidase followed by DAB Chromogen solution. As previously described (14, 15), 8–12 random images of each section were taken and microvascular length density was calculated using ImageJ software.
Collateral density was determined in the pial circulation where collaterals are readily identified (vessels connecting the midcerebral, anterior, and posterior cerebral arteries) and visualized (despite their small diameter). Yellow Microfil (Flow-Tech, No. MV-122) was infused via the abdominal aorta in 4-mo-old Cx40−/− and WT mice and, after processing, the number of collateral vessels counted as previously described (14). Isolated brains were immersed in 2.5% cresyl violet counter-stain before imaging to enhance contrast.
Vascular remodeling.
Visualization and quantification of vascular remodeling was performed 3 wk after mild surgery as previously described (14). In brief, the ischemic hindlimb vasculature was maximally vasodilated by perfusion with 10−4 M sodium nitroprusside (Sigma, No. S0501) and 4 mg/l papaverin (Sigma, No. P3510) (in divalent cation-free PBS), perfusion fixed with 1% paraformaldehyde (in divalent cation-free PBS), and filled with radio-opaque Microfil (Flow-Tech, No. MV-122). X-ray angiography (20 kV exposure for 10 s) was used to visualize all vessels with diameters >∼35 μm in the surgical and nonsurgical hindlimbs, as previously described (14). Vessel diameters were measured at their intersection to a line drawn perpendicular to the femur at its midpoint.
Statistics.
Limb appearance and use data are expressed as medians and were tested for significant differences using a standard rank-sum test for nonparametric data. ANOVA and Friedman's test could not be used due to gaps in the data (intrinsic to the dataset). Laser Doppler perfusion imaging data were analyzed using repeated-measures ANOVA and Bonferroni test, unless specified differently. All other data are expressed as means ± SE and were compared using paired Students′ t-test or unpaired Students′ t-test assuming unequal variance (single comparison), as indicated. In all statistical tests, α was set at 0.05.
RESULTS
Genotype-dependent differences in hindlimb ischemic response.
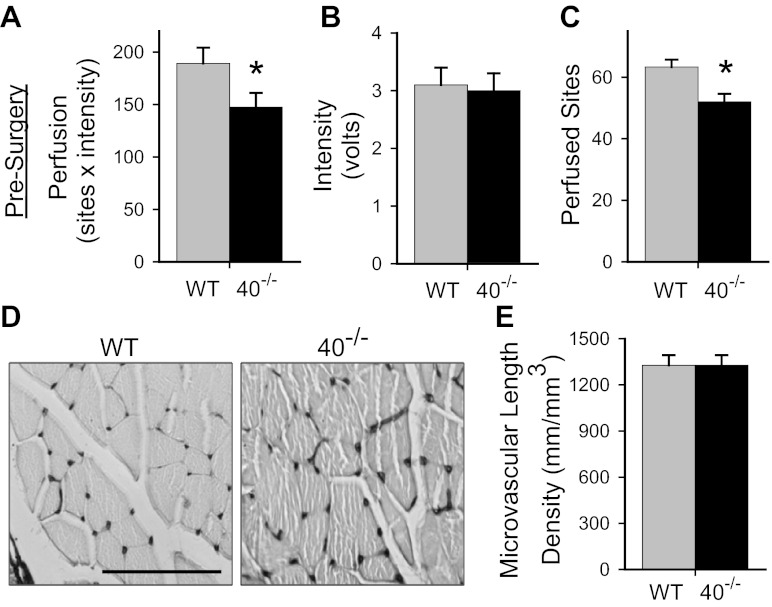
Given the reported adverse effects of Cx40 deficiency on conducted vasodilation (9) and incidence of spontaneous vasospasm (10), we hypothesized that limb perfusion might be compromised in unmanipulated Cx40−/− mice. To test this hypothesis we used laser Doppler perfusion imaging to measure site intensity and number of sites with detectable flow in the distal limbs of Cx40−/− versus WT mice. Mean intensity across sites and the number of detected sites for each limb of each animal were used to calculate limb perfusion (intensity x sites) in the distal limb. Perfusion was significantly reduced in the hindlimbs of Cx40−/− versus WT mice (Fig. 1A). This reduction in perfusion reflected a significant decrease in the number of sites with detectable flow, rather than a reduction in mean intensity across sites (Fig. 1, B and C). To ascertain whether the reduction in sites with flow resulted from a decrease in microvascular density, which might be expected (29, 40) as a consequence of chronic hypertension (rarefaction of vessels), we assessed microvascular density in the gastrocnemius muscle of WT and Cx40−/− mice. The density of microvessels visualized in cross-sections of the gastrocnemius muscle (Fig. 1, D and E) was not different in Cx40−/− versus WT mice. This result suggests that regulation of flow distribution is compromised in Cx40−/− mice.
Fig. 1.
Reduction in perfused area and distal limb perfusion in connexin 40-deficient (Cx40−/−, or 40−/−) mice was not due to reduced microvascular density. Presurgical distal limb perfusion (A) and perfused sites (C) were reduced significantly (*) in Cx40−/− (n = 24, 14 male) compared with wild-type (WT; n = 26, 18 male) mice (P < 0.05 and P < 0.002, respectively). No difference in flow (mean intensity) was detected (B) between WT and Cx40−/− mice. Perfusion and perfused sites also differed in the subset of animals subjected to mild surgery [WT: n = 18 (13 male); Cx40−/−: n = 16 (9 male); P < 0.01 for perfusion; P < 0.003 for perfused sites] but for the subset of animals subjected to severe surgery only trended toward significance [WT: n = 9 (5 male); Cx40−/−: n = 10 (4 male); P = 0.18 for perfusion; P = 0.065 for perfused sites]. D and E: microvasculature was visualized in cross-sections of gastrocnemius muscle (scale bar = 100 μm). No significant differences in microvascular density were observed between Cx40−/− and WT mice [WT n = 15 (6 male); Cx40−/− n = 10, 5 male].
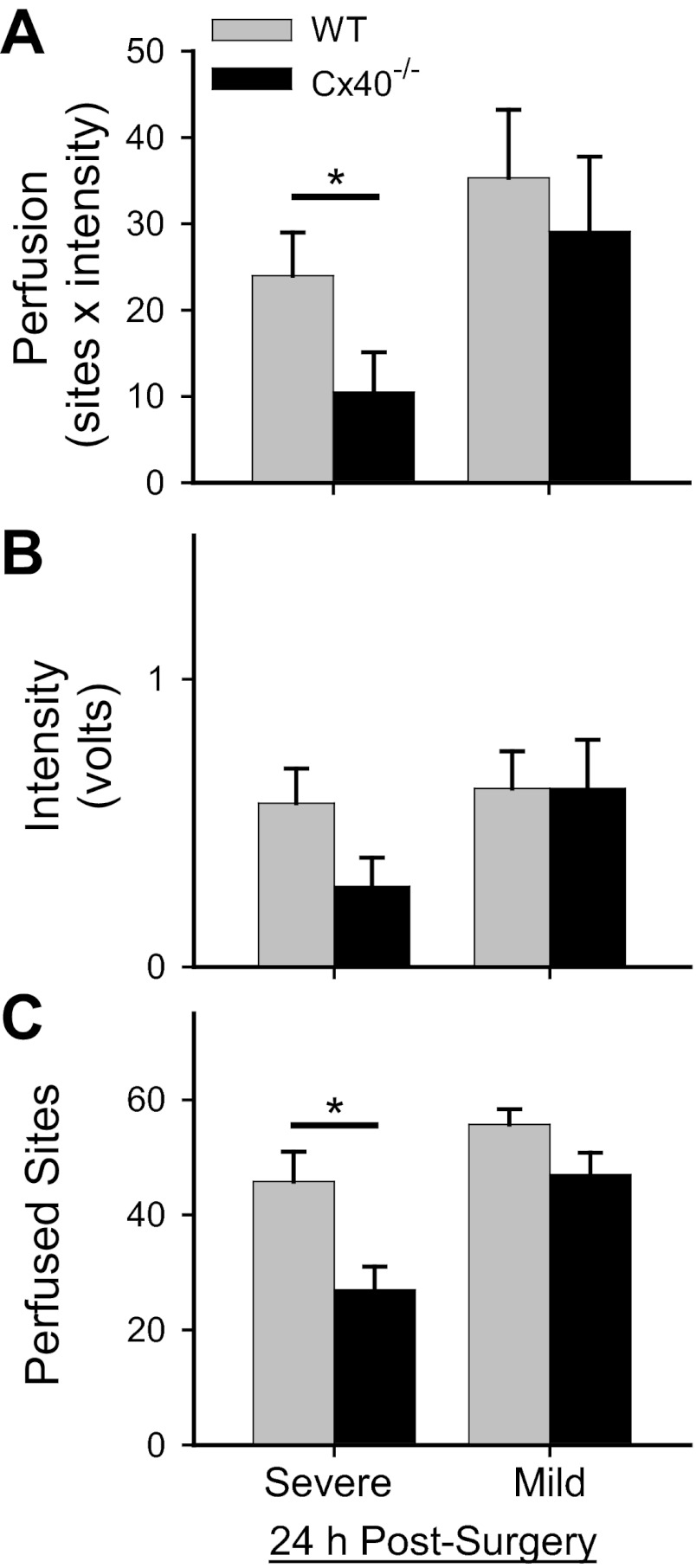
To determine whether impaired regulation of flow distribution (and other potential deficits of vascular function observed in Cx40−/− mice) might adversely affect distribution of blood flow in the postischemic limb, we next examined distal limb perfusion 24 h after inducing hindlimb ischemia (severe or mild surgery). When compared to presurgery values for the same genotype (i.e., vs. data shown in Fig. 1), distal limb perfusion was significantly reduced by ∼91–95% and 76–84% 24 h after, respectively, severe or mild surgery [severe surgery: WT P < 0.001 (n = 9), Cx40−/− P < 0.001 (n = 10); mild surgery: WT P < 0.001 (n = 18), Cx40−/− P < 0.001 (n = 16)]. As shown in Fig. 2A, distal limb perfusion 24 h after severe surgery was significantly less in Cx40−/− versus WT mice. This genotype-specific postsurgical difference in distal limb perfusion could be attributed largely to a genotype-specific significant reduction in perfused area (detected sites) rather than flow (Fig. 2, B and C). In contrast, 24 h after the mild surgery no genotype-specific differences were observed for distal limb perfusion, flow (intensity), or perfused area (sites). These surgical procedures differ in several ways, but critical to this early postsurgery time point the major collateral vessels connecting the gracilis and saphenous arteries are not available to acutely restore blood flow in the severe surgery model but are available in the mild surgery model.
Fig. 2.
Genotype-specific differences in postsurgical (at 24 h) distal limb perfusion and perfused sites were detected only after severe, not mild, surgery. A: distal limb perfusion in Cx40−/− vs. WT differed (*) 24 h after severe surgery [P < 0.02; WT: n = 9 (5 male); Cx40−/−: n = 10 (4 male)] but not mild surgery [P = 0.58; WT: n = 18 (13 male); Cx40−/−: n = 16 (9 male)]. B: flow (intensity) 24 h after both surgery types did not differ in a genotype-specific manner (severe: P = 0.44; mild: P = 0.98). C: number of sites with detectable flow was significantly (*) less in Cx40−/− than WT mice after severe surgery (P < 0.005) but not after mild surgery (P = 0.072).
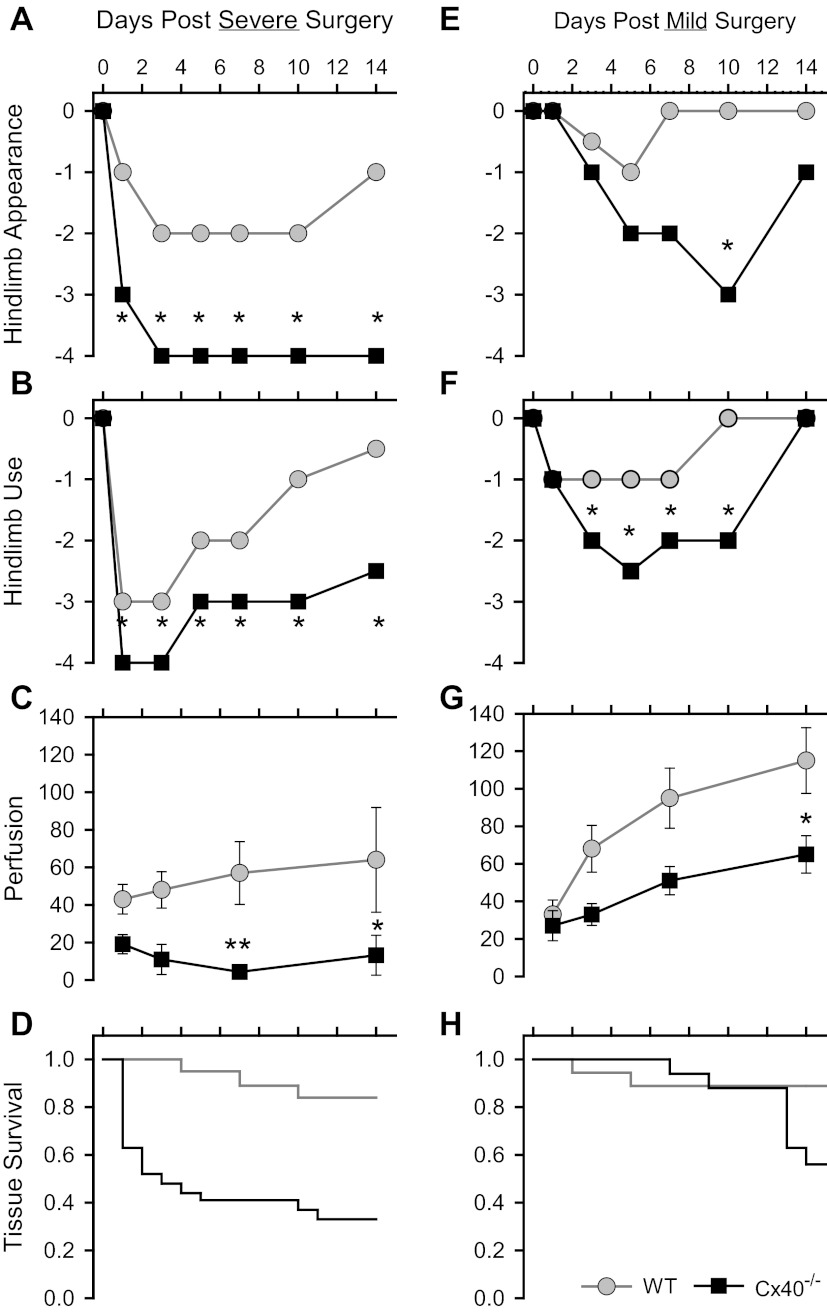
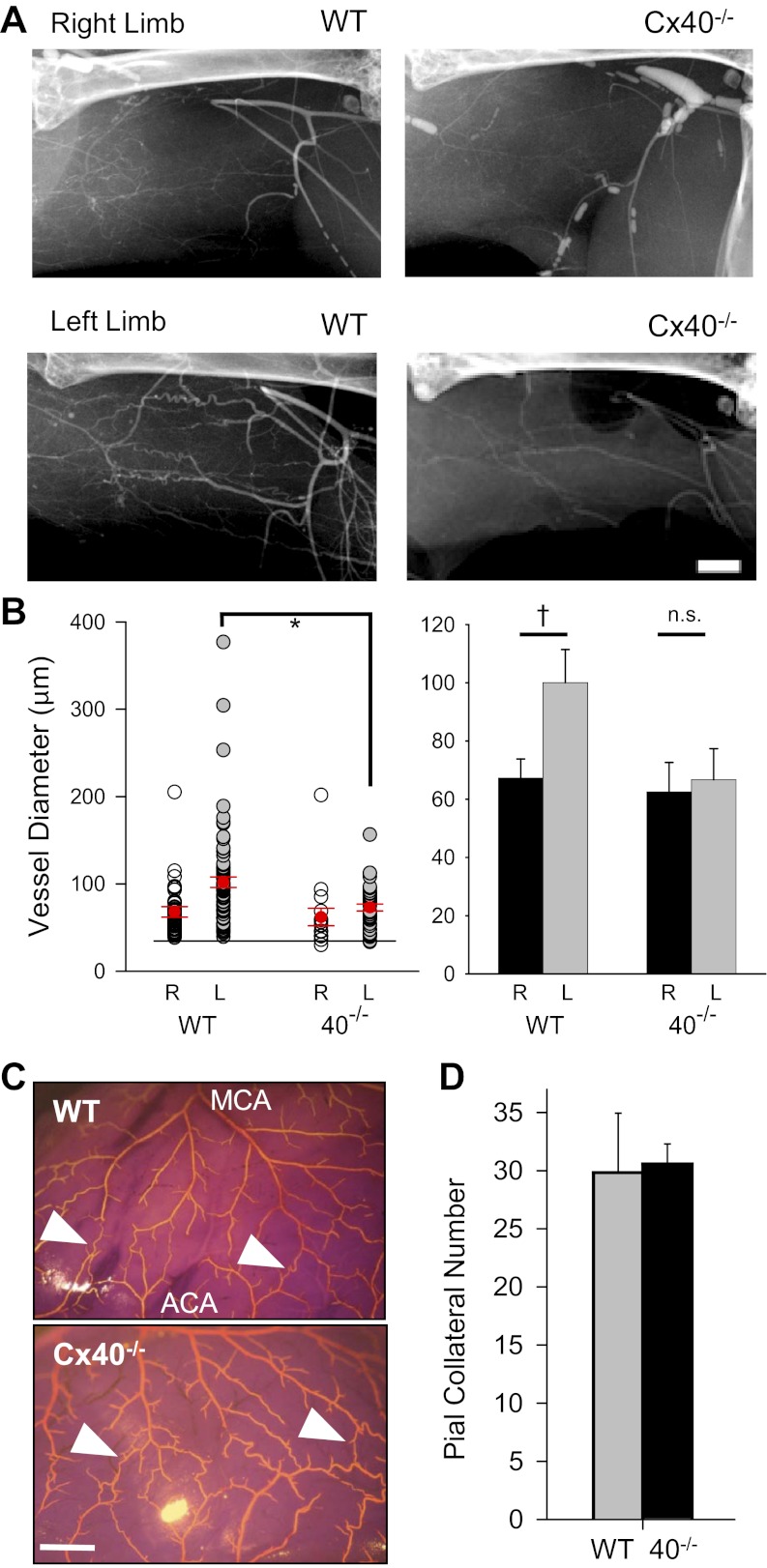
Over a longer postsurgical period (14 days), recovery of hindlimb appearance (Fig. 3, A and E), use (Fig. 3, B and F), distal limb perfusion (Fig. 3, C and G), and tissue survival (Fig. 3, D and H) were significantly compromised in Cx40−/− versus WT mice, especially in mice subjected to the severe surgery. In such mice average tissue survival time for WT (n = 19) and Cx40−/− (n = 27) mice was 12.9 ± 0.7 and 6.5 ± 1.2 days (P < 0.001), respectively; survival time was not different between genotypes after mild surgery. To determine whether impaired arteriogenesis (collateral remodeling) might be a factor in the poor postsurgical recovery of Cx40−/− mice, we used X-ray angiography to examine vessel diameter in the nonischemic and ischemic gracilis muscles of WT and Cx40−/− mice subjected to the mild surgery (Fig. 4A). The mean diameter of detected vessels in the nonischemic (right) hindlimbs of Cx40−/− versus WT mice were not different (P = 0.65); in contrast, the diameter of detected vessels was significantly larger in the remodeled ischemic left limb of WT compared with Cx40−/− mice (P < 0.00015; Fig. 4B, *). Within-genotype paired comparison of vessel diameters in the left versus right limbs also showed significant outward remodeling in the WT (n = 6, P < 0.003) but not in the Cx40−/− (n = 4, P = 0.784) animals (Fig. 4B, right). To verify that differences in sample size did not bias this comparison, we also performed a paired comparison on subsets of the WT data that were matched for sample size and sex to the Cx40−/− dataset (4 animals; 2 male, 2 female). Regardless of which WT males (2 of 4) were included with the two WT females, paired comparison of left and right limbs always revealed a significant increase in vessel diameter in the remodeled WT limb (P = 0.017, 0.0109, 0.0145, 0.0086). Thus our results demonstrate that remodeling is impaired in Cx40−/− but not in WT animals. Because the resolution of X-ray angiography does not permit detection of vessels with diameters smaller than ∼35 μm nor verification of their collateral nature (arterial to arterial connection), we examined collateral density in the pial circulation (in the absence of ischemic stimulus) where collateral vessels can be morphologically distinguished and where smaller collateral vessels are evident (Fig. 4C). No difference in the number of collateral vessels was detected across genotypes in the pial circulation (Fig. 4D). Thus our morphological as well as perfusion data (i.e., Fig. 2, effects of mild vs. severe surgery) suggest that outward remodeling of pre-existing collateral vessels is compromised in Cx40−/− compared with WT mice, with no significant difference in the number of pre-existing (native) collateral vessels.
Fig. 3.
Recovery of the ischemic hindlimb was compromised to a greater extent in Cx40−/− compared with WT mice. Severe surgery (A–D) affected recovery of hindlimb appearance, use, and perfusion, and tissue survival to a greater extent than mild surgery (E–H). Recovery of hindlimb appearance (A, E) and hindlimb use (B, F) over 14 days was compromised to a greater degree after severe compared with mild surgery, with significant differences (*rank sum test, P < 0.05) between genotypes evident throughout the recovery period after severe surgery and only through a portion of the recovery period after mild surgery [appearance data: severe surgery: WT n = 16 (10 male), Cx40−/− n = 29 (13 male); mild surgery: WT n = 18 (12 male), Cx40−/− n = 17 (10 male); use data: severe surgery: WT n = 20 (12 male), Cx40−/− n = 27 (11 male); mild surgery: WT n = 18 (12 male), Cx40−/− n = 15 (8 male)]. Recovery of distal limb perfusion (C, G) was compromised to a greater extent after both surgery types in Cx40−/− mice than WT mice [repeated-measures ANOVA: (C) P = 0.0073; (G) P = 0.0174; Bonferoni post hoc test: *P < 0.05, **P < 0.01; sample sizes summarized in Fig. 2 legend]. D, H: when compared with WT, survival of distal limb tissue in Cx40−/− mice was compromised after severe surgery but not mild surgery [severe: WT n = 19 (11 male), Cx40−/− n = 27 (11 male); mild: WT n = 18 (12 male), Cx40−/− n = 17 (10 male)].
Fig. 4.
Vascular remodeling is compromised in Cx40−/− mice compared with WT. A: hindlimb vessels were visualized in gracilis muscle angiograms of the right (nonsurgical) and left (surgical) limbs 21 days after mild surgery (scale bar = 1 mm) [WT n = 6 (4 male); Cx40−/− n = 4 (2 male)]. B, left: diameters of detected vessels in the ischemic limbs of WT vs. Cx40−/− mice differed, whereas vessels in the nonischemic right limbs did not differ [left limb (L): WT 102 ± 6 μm (n = 79), Cx40−/− 72 ± 4 μm (n = 35), P = 0.00015, *; right limb (R): WT 68 ± 5 μm (n = 51), Cx40−/− 62 ± 10 μm (n = 16); P = 0.648, not significant (ns); unpaired t-test]. The angiogram detection limit of ∼35 μm is indicated by the lower horizontal line. B, right: paired comparison of left vs. right limb vessel diameters for WT and Cx40−/− animals shows outward remodeling in WT but not Cx40−/− mice [WT (n = 6): right limb, 67 ± 7 μm, left limb, 100 ± 11 μm, P < 0.003, †; Cx40−/− (n = 4): right limb, 62 ± 10 μm, left limb, 67 ± 11 μm; P = 0.78; ns]. C: collateral vessels of smaller size were readily visualized in the pial circulation of the brain and could be distinguished from noncollateral vessels morphologically; examples of collateral vessels interconnecting the major midcerebral and anterior cerebral artery (MCA and ACA, respectively) vessels are indicated with arrowheads (scale bar = 1 mm). D: number of collateral vessels detected in the pial circulation of WT (n = 6, 1 male) and Cx40−/− (n = 6, 3 male) mice was not different.
We also examined the Cx40−/− versus WT mice for possible differences in the magnitude of the inflammatory response evident in the gastrocnemius 14 days after severe surgery. Inflammatory response was evaluated by determining the gastrocnemius area infiltrated by activated macrophages (F4/80 positive) in the ischemic versus nonischemic limbs (Fig. 5A). Despite the absence of obvious external evidence of inflammation in this region of the leg and absence of necrosis in the distal limbs of the evaluated animals, the area of the gastrocnemius of the ischemic limb infiltrated by activated macrophages was significantly (P < 0.006) greater, by ∼15-fold, in Cx40−/− than WT mice (Fig. 5B); no difference in the area infiltrated by activated macrophages was detected in the nonischemic limbs of Cx40−/− versus WT mice. These data suggest the poor recovery of limb function observed in Cx40−/− mice could reflect, in addition to (or potentially in relation to) compromised arteriogenesis, adverse effects of a prolonged and extensive inflammatory response.
Fig. 5.
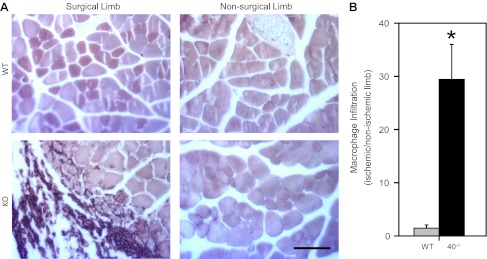
Macrophage infiltration was extensive in the Cx40−/− ischemic hindlimb. A: representative images of gastrocnemius muscle (in cross-section) isolated from mice 14 days after severe surgery and stained to reveal F4/80 antigen, a hallmark of activated macrophages (scale bar = 100 μm). Activated macrophages, stained dark brown, were more obvious in the surgical limbs of Cx40−/− mice. B: macrophage infiltration, quantified as the area of the surgical vs. nonsurgical gastrocnemius occupied by activated macrophages, was significantly (*P < 0.01) greater in Cx40−/− (n = 6, 4 male) compared with WT (n = 4 male) mice. KO, knockout mice.
AT1R-dependent differences in hindlimb ischemic response.
The data presented thus far suggest that in Cx40−/− mice regulation of blood flow distribution in the hindlimb is compromised without obvious impact on limb function before ischemic insult but with serious consequences to the limb after ischemic insult. AT1Rs are involved in regulation of vascular tone, hypertrophy, and hyperplasia of vascular wall cells and also regulate function of immune cells (37). Because these receptors are chronically activated in Cx40−/− mice due to overproduction of renin by the kidney (39), we next asked whether AT1R blockade might be beneficial to recovery of the ischemic limb in Cx40−/− male mice.
Losartan is an orally administered AT1R antagonist used in the treatment of hypertension in human patients (3, 13). To establish an effective oral dosing procedure in mice, we first determined the maximum effect on blood pressure of intravenously delivered drug in WT and Cx40−/− male mice. Comparison of mean systemic blood pressure at 5 min postinjection to preinjection blood pressure (paired t-test) showed that 20 mg/kg losartan infusion reduced systemic blood pressure in Cx40−/− male mice from 139 ± 11 to 113 ± 9 mmHg (n = 6, P < 0.004); this post-losartan blood pressure value in Cx40−/− male mice was not different from preinjection blood pressure (111 ± 6 mmHg; n = 7) of WT male mice but significantly above the postinjection value of WT male mice (87 ± 5 mmHg; P < 0.03). Thus this losartan dose rendered Cx40−/− mice normotensive relative to untreated WT controls. We next tested whether losartan could produce a similar reduction of blood pressure when administered orally. In Cx40−/− male mice treated for 3 days with losartan (150 μg/ml added to their drinking water), blood pressure was 120 ± 10 mmHg (n = 5), a value not different from Cx40−/− male mice after intravenous injection of losartan (P = 0.598).
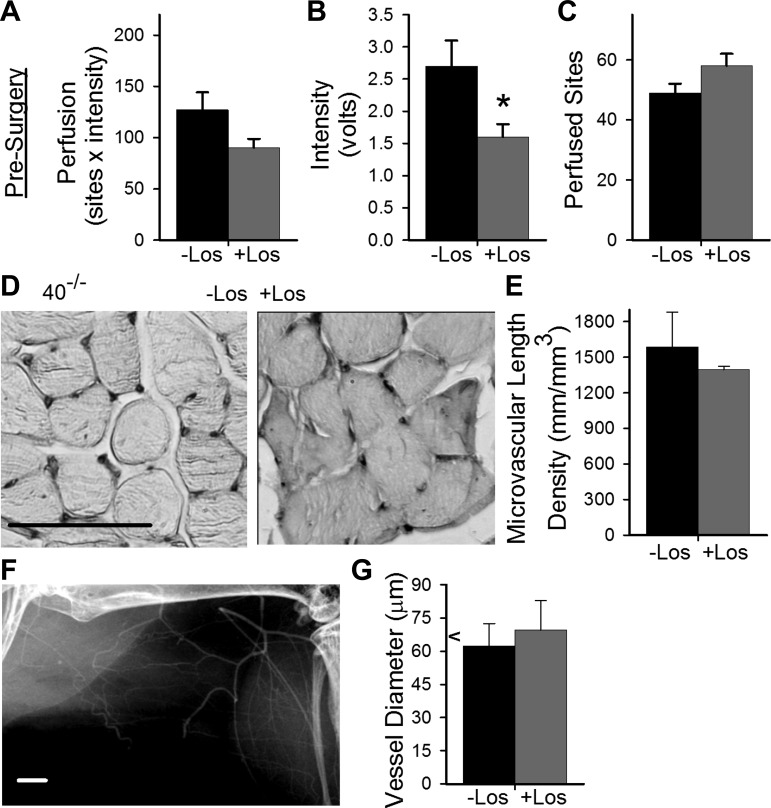
To ascertain whether losartan treatment altered presurgical distal limb perfusion, flow (intensity), or perfused sites, laser Doppler perfusion imaging was performed on treated and untreated Cx40−/− male mice. As shown in Fig. 6, A–C, flow (intensity) was reduced by losartan, as might be expected from the decreased blood pressure. However, this flow reduction was offset by a small increase in perfused sites such that perfusion remained statistically unaffected in losartan-treated (8 wk) Cx40−/− male mice. The absence of effect on perfused sites (indeed, the small increase in sites) suggests the hypertension typical of these mice did not result in vascular pruning. To verify this, we examined microvascular density and vessel diameter in nonischemic limbs of losartan-treated (8 wk) Cx40−/− male mice. As shown in Fig. 6, D and E, microvascular density was unaffected by chronic AT1R blockade. Hindlimb vessel diameter (Fig. 6, F and G) was also not detectably different in losartan-treated Cx40−/− mice.
Fig. 6.
Losartan reduced distal limb perfusion in Cx40−/− (40−/−) mice. A: distal limb perfusion was modestly diminished in losartan (Los)-treated (n = 15) vs. untreated (n = 12) Cx40−/− male mice; however, this difference did not achieve significance (P = 0.07) due to offsetting effects of reduced blood flow (B; *P < 0.02) and the small increase in perfused sites (C; P = 0.09). D and E: microvascular density in losartan-treated (n = 4 males) and untreated (n = 5 males) Cx40−/− mice, quantified in cross-sections of gastrocnemius muscle from the nonsurgical limb (scale bar = 100 μm), was not significantly different. F: angiogram of the gracilis region of the nonsurgical hindlimb of a losartan-treated Cx40−/− mouse. Scale bar = 1 mm. G: no differences between treated and untreated Cx40−/− mice were detected for hindlimb vessel diameter (P = 0.64). Arrowhead on y-axis indicates the mean value observed for the nonsurgical limbs of male WT mice.
The presurgery perfusion and vascular density data suggest that AT1R blockade reduced blood pressure without altering the number of vessels available to distribute blood flow, with the consequence that flow was reduced. Any reduction in limb perfusion beyond that already evident presurgically in Cx40−/− mice (vs. WT) would be expected to have additional detrimental effect on recovery of the ischemic hindlimb. Nevertheless, AT1R antagonists have been reported to exert a potent anti-inflammatory effect, which would be expected (17, 26, 37) to have a beneficial effect on recovery of the hindlimb. To determine whether losartan treatment was beneficial or detrimental to recovery of the ischemic hindlimb of Cx40−/− male mice, we next determined whether oral losartan for 3 days or 8 wk before severe surgery and throughout the postsurgical period led to improved recovery of limb use, appearance, perfusion, or tissue survival. As shown in Fig. 7, A–D, after severe surgery none of these markers of ischemic limb function were significantly improved by either 3 day or 8 wk pretreatment with losartan; indeed, the 3-day pretreatment protocol appeared to be detrimental to recovery of these parameters. Furthermore, even with the less damaging mild surgery, 8 wk pretreatment with losartan had no obvious beneficial effect on postsurgical limb recovery (Fig. 7, E–H); instead there was essentially no recovery of distal limb perfusion in losartan-treated Cx40−/− male mice. Failure to recover perfusion in losartan-treated Cx40−/− mice at least as well as in untreated Cx40−/− mice prompted us to compare vessel diameter in the ischemic versus nonischemic limbs of losartan-treated mice. No difference in mean vessel diameter could be detected between the limbs of these animals (paired t-test of surgical to nonsurgical limbs, P = 0.29, n = 4 both groups), suggesting that AT1R blockade did not rescue arteriogenesis in Cx40−/− mice.
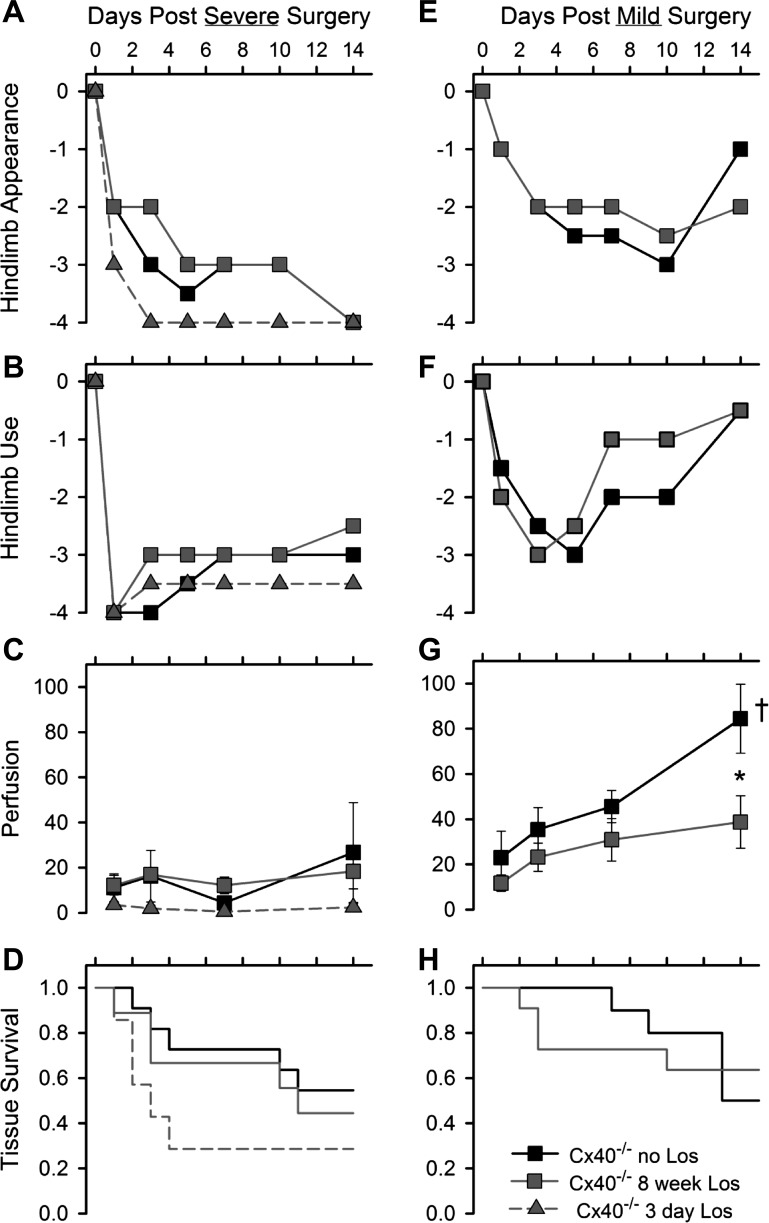
Fig. 7.
Losartan (Los) did not improve recovery of postischemic limb appearance, use, perfusion, or tissue survival. Oral losartan throughout the recovery period and for either 3 days or 8 wk before severe surgery (A–D) or 8 wk before mild surgery (E–H) failed to improve recovery of the postsurgery hindlimb of Cx40−/− male mice. Recovery of hindlimb appearance (A, E) and hindlimb use (B, F) after both surgery types was not different (rank sum test) between losartan-treated and untreated Cx40−/− male mice [appearance data: severe surgery, Cx40−/− no losartan, n = 13, 3-day losartan, n = 7, 8-wk losartan, n = 9; mild surgery: no losartan, n = 10, 8-wk losartan, n = 11; use data, severe surgery: no losartan, n = 11, 3-day losartan, n = 7, 8-wk losartan, n = 9; mild surgery: no losartan, n = 10, 8-wk losartan, n = 11]. C and G: recovery of distal limb perfusion over 14 days was minimal after severe surgery in all Cx40−/− mice (C), irrespective of losartan treatment; in contrast, whereas perfusion improved significantly (†) over time after mild surgery in untreated Cx40−/− mice (†, ANOVA, P = 0.00185), it did not improve in losartan-treated (P = 0.113) Cx40−/− male mice subjected to the mild surgery. Thus recovery of perfusion by day 14 was greater in untreated than treated mice at day 14 (*P < 0.01, t-test). D and H: losartan-treated Cx40−/− male mice were, if anything, more likely to suffer tissue necrosis, although survival times were not different even for 3-day losartan-treated mice (no losartan: 10 ± 2 days and 3-day losartan: 6 ± 2 days, P = 0.104; severe surgery: no losartan, n = 11, 3-day losartan, n = 7, 8-wk losartan, n = 9; mild surgery: no losartan, n = 10, 8-wk losartan, n = 11).
The unexpected detrimental effect of losartan on limb recovery after mild surgery raised the question of whether losartan had been effective in reducing blood pressure in these animals throughout the postsurgical period and whether overall animal health might be adversely affected by losartan in the postsurgical period. Blood pressure of surgical animals measured immediately before euthanization showed that orally administered losartan effectively reduced blood pressure in the postsurgical period (Fig. 8A). Furthermore, comparison of animal weights throughout the recovery period revealed no obvious genotype- or losartan-specific differences, although losartan-treated animals were, on average, heavier than untreated Cx40−/− or WT animals on the day of surgery (Fig. 8B).
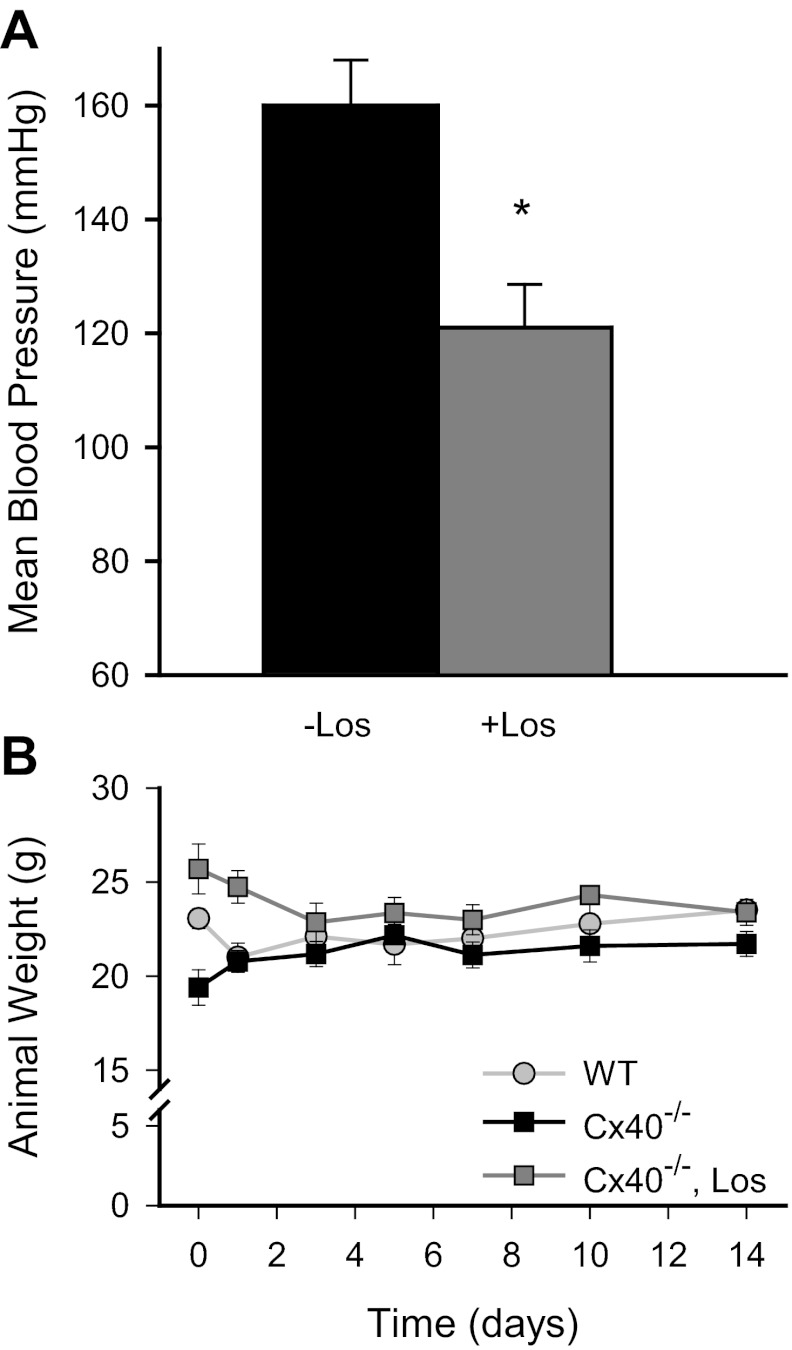
Fig. 8.
Losartan (Los) effectively reduced postsurgical mean arterial blood pressure of Cx40−/− mice without compromising animal health. A: orally administered losartan (8-wk presurgery and throughout recovery period) reduced the blood pressure in Cx40−/− mice subjected to mild surgery. Blood pressure was measured just before termination of these surgical animals (*P < 0.04; untreated n = 7, treated n = 8). Mean blood pressure in losartan-treated, postsurgical Cx40−/− mice was not different from 3-day losartan-treated Cx40−/− mice, nor was it different from Cx40−/− mice dosed acutely with losartan by intravenous injection (P > 0.05 in both cases). B: animal weights were comparable over the recovery period irrespective of genotype or drug treatment; both WT and losartan-treated Cx40−/− animals experienced an initial decline in weight that stabilized at comparable levels within 3 days of surgery (n = 14, 17, and 8 for WT, untreated, and treated Cx40−/− animals, respectively).
Finally, despite the detrimental effects of losartan treatment on recovery of hindlimb perfusion, we determined whether the inflammatory response might nevertheless be reduced in losartan-treated mice. The area of the gastrocnemius infiltrated by activated macrophages in Cx40−/− mice treated for 8 wk before severe surgery and throughout the recovery period with losartan was significantly less than in untreated Cx40−/− male mice (Fig. 9). This result suggests that despite reduction of the inflammatory response, losartan did not improve recovery of the ischemic hindlimb in Cx40−/− male mice.
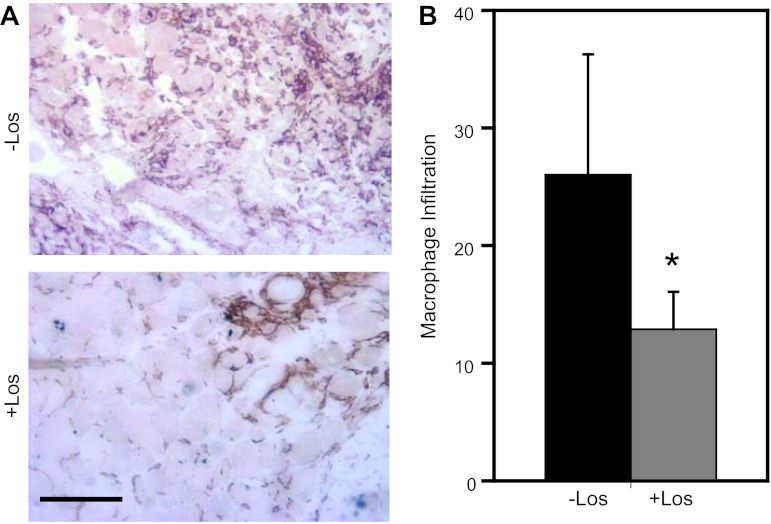
Fig. 9.
Macrophage infiltration of the ischemic hindlimbs of Cx40−/− mice was diminished by losartan (Los) treatment. A: representative images of gastrocnemius muscle (in cross section, stained to reveal F4/80 antigen) isolated 14 days after severe surgery from the surgical limbs of untreated (top) or losartan-treated (bottom) Cx40−/− mice. Note that macrophage staining (dark brown) was more extensive in sections from untreated vs. treated mice (scale bar = 100 μm). B: area of the surgical limb vs. nonsurgical limb infiltrated by macrophages was significantly (*P < 0.05) greater in untreated than in treated Cx40−/− mice (n = 6 for both groups).
DISCUSSION
We showed previously that tissue survival and recovery were compromised in Cx40-deficient mice subjected to the severe form of hindlimb ischemia that eliminates flow to the major collateral vessels supplying the distal hindlimb (15). Generally, survival and recovery of ischemic tissue is determined, in large part, by the magnitude and area of blood flow reduction, the speed and extent of blood flow recovery, and a balanced inflammatory response (18, 19, 30, 32, 34). Cx40−/− mice exhibit several phenotypic differences from WT mice that could adversely affect these survival/recovery determinants, to include hypertension (9, 10), elevated blood renin/ANG II levels (25, 39), loss of coordinated vasodilatory response (9), high incidence of severe spontaneous vasospasm (10), an overall proinflammatory state (6), and altered gene expression in the vascular wall and kidney (6, 24, 35). In the current study we used two models of hindlimb ischemia, severe and mild, in WT and Cx40−/− mice to determine which of these phenotypic deficits might contribute significantly to the poor recovery of ischemic tissue in Cx40−/− mice.
Genotype-specific differences in distal limb blood flow distribution.
Laser Doppler perfusion imaging revealed significantly fewer (∼20%) perfused sites and overall less perfusion (∼22%) in Cx40−/− versus WT mice despite apparently comparable microvascular densities. That fewer sites displayed detectable flow before any surgical intervention suggests that regulation of the moment-to-moment distribution of flow is compromised in Cx40−/− mice. The available literature indicates that local constriction and dilation responses to a variety of signaling molecules occur normally in Cx40−/− mice, indicating that the cellular signaling machinery necessary for such responses is intact. However, the evoked cellular responses are not shared between cells of the vessel wall to produce coordinated changes in vascular tone, with the consequence that blood flow distribution is compromised in Cx40−/− mice (9, 10, 30). Thus, contrary to conclusions drawn in a previous report (4) in which a similar approach was used, our data suggest that blood flow distribution and perfusion of the distal limb are reduced in the unmanipulated Cx40−/− mouse, most likely as a consequence of failure to distribute local signaling events to distant, especially upstream, sites. Notably, in the presurgical animal, this reduced perfusion of the distal limb (and presumably other peripheral tissues) had no obvious consequence to limb use or appearance, suggesting limb perfusion and flow distribution in the unmanipulated Cx40−/− animal remains sufficient for limb function at levels indistinguishable from WT animals.
The number of sites in the distal limb with detectable flow 24 h after severe surgery was reduced to a greater degree in Cx40−/− versus WT mice (48% vs. 28% reduction). At this time point, significant arteriogenesis and angiogenesis have not occurred (31, 36), suggesting that the acute regulatory mechanisms that compromise flow distribution in unmanipulated Cx40−/− mice also compromise flow distribution in the early ischemic setting, with apparently significant consequences on the severity of induced ischemic injury. This genotype-specific decrease in flow distribution 24 h after severe surgery was not observed after mild surgery. The severe and mild forms of ischemia-inducing surgery differ in several potentially significant ways. In the severe surgery the femoral artery is ligated proximal to the gracilis artery branch, whereas in the mild surgery it is ligated distal to this branch. The gracilis artery feeds the major collateral vessels connecting the femoral and saphenous arteries; thus the area of the distal limb with detected flow (perfused sites) was expected and observed to be significantly less 24 h after severe vs. mild surgery in both genotypes (12). That a genotype-specific difference in perfusion was not observed 24 h after mild surgery highlights the importance of these collaterals in restoring flow to the distal limb and, moreover, suggests that the collateral vessels are likely similar in number and response to flow-dependent dilatory signals across genotypes, despite their compromised ability (9) to propagate signals upstream in the Cx40−/− mice. Other differences between the severe and mild surgery models include ligation of the femoral vein along with the femoral artery in the severe surgery; the position of the distal ligation site, midway between knee and ankle in the severe surgery rather than proximal to the knee and popliteal artery branch in the mild surgery; and resection of the entire length of vessels between ligation sites in the severe surgery. How these differences might impact the surgical outcome at 24 h in a genotype-specific manner remains to be explored.
Perhaps the most striking difference between genotypes, irrespective of the mild versus severe forms of ischemia-inducing surgery, was the poor recovery of hindlimb perfusion observed for Cx40−/− mice. Recovery of perfusion over 14–28 days in the surgical limb of WT mice has been attributed to vascular remodeling, predominantly arteriogenesis—the outward remodeling of preexisting collateral vessels that occurs over a period of 3 or more weeks (7, 8, 12). Lending further support to this conclusion are our data (14) demonstrating that increased collateral number and outward remodeling capabilities of Cx37−/− mice protect the hindlimbs of this genotype from the adverse effects of the severe and mild forms of ischemia-inducing surgery. The angiography data presented herein indicated that arteriogenesis was compromised in Cx40−/− mice, since vessel diameter did not differ in Cx40−/− mice between the ischemic and nonischemic limbs 21 days after mild surgery (P > 0.05). In contrast, a significant increase in vessel diameter was readily observed for the WT animal (P < 0.003) over this same period. The absence of significant vascular remodeling in Cx40−/− mice correlated with virtually no recovery of limb appearance or use after severe surgery and only partial recovery of these parameters after mild surgery.
AT1R blockade-dependent differences in postsurgical outcome of Cx40−/− mice.
Sustained high ANG II levels result in hypertension and associated vascular remodeling, as well as a pro-inflammatory, prothrombogenic, and pro-oxidative state throughout the vasculature (28, 37). Acute administration of, as well as chronic pretreatment with, ANG II exacerbates ischemia/reperfusion injury in the brain as well as other tissues (28), effects that are, in large part, prevented with blockers of AT1 receptors. These receptors are expressed by numerous cell types including endothelial and vascular smooth muscle cells, platelets, leukocytes, and other bone marrow-derived cell types involved in the inflammatory and remodeling response (26, 37); recent evidence highlights the central role of AT1R on bone marrow-derived cells in mediating the inflammatory response triggered by ischemia/reperfusion (28). Because Cx40−/− mice chronically overproduce renin with consequent chronic elevation of ANG II, these and other data prompted us to hypothesize that short- or long-term administration of an AT1R antagonist would promote tissue recovery and repair in Cx40−/− mice.
AT1R antagonist (telmisartan, candesartan, losartan) provided in the drinking water over several months has been reported to reverse the cerebral arteriolar remodeling and ischemia-induced vasculogenesis defects present in spontaneously hypertensive rats (11, 40) and to restore connexin expression patterns to those observed in normotensive Wistar Kyoto rats (22). Similar reversal of wall thickness and restoration of connexin expression profile were observed in candesartan-treated Cx40−/− mice (1). Our data show that despite normalization of blood pressure in losartan-treated Cx40−/− mice, distal limb perfusion, blood flow distribution, microvascular density, and mean hindlimb vessel diameter did not differ in the nonsurgical limbs of treated versus untreated Cx40−/− male mice. Similarly, 24 h after mild or severe hindlimb ischemia surgery, hindlimb appearance, use and perfusion did not differ between treated and untreated Cx40−/− male mice. However, during the recovery period of mild-surgery mice, losartan-treated mice consistently had less distal limb perfusion than untreated mice, a difference that gained significance by day 14 of recovery. The absence of a difference in distal hindlimb perfusion between losartan-treated and untreated mice before and 24 h after mild surgery is consistent with a very recent report (20) demonstrating 1) unchanged local dilatory responses in Cx40−/− mice and 2) Cx40 deficiency, not hypertension or hypertension-induced changes in connexin expression, is responsible for compromised vasoconduction in Cx40−/− mice. The consistently lower perfusion observed during the postsurgery recovery period in losartan-treated compared to untreated mice highlights the importance of Cx40 to regulated flow distribution and suggests that AT1R contribute to, or are necessary for, the limited recovery of perfusion that occurs in Cx40−/− mice. Whether AT1R blockade before surgery but not during recovery might be beneficial to Cx40−/− mouse remains to be explored.
Despite the absence of a beneficial effect of pre- and postsurgery losartan treatment on recovery of distal limb perfusion, the area of the hindlimb infiltrated by activated macrophages was significantly less in losartan-treated compared with untreated Cx40−/− mice. AT1R are expressed by endothelial and vascular smooth muscle cells in the vascular wall as well as by circulating platelets and leukocytes; AT1R activation results in a proinflammatory state in both blood cells and endothelial cells (28). Nagai and colleagues (28) demonstrated that ischemia/reperfusion injury in the brain involved AT1R on both endothelium and blood cells, with endothelial AT1R involved primarily in blood cell recruitment and AT1R on immune system blood cells exerting a significant disruptive effect on the barrier function of the endothelium and tissue necrosis. Although ischemic injury in the brain and hindlimb no doubt differ in significant ways, if the Nagai et al. data are predictive of what occurs in the hindlimb, then AT1R blockade in the Cx40−/− mouse may be, at least in part, protective to the endothelium and tissue by reducing blood cell (macrophage) recruitment, but nevertheless unable to compensate for other deficits of vascular function characteristic of Cx40−/− mice. Furthermore, Cx40 deficiency and the high levels of ANG II characteristic of Cx40-deficient mice may act synergistically to promote a pro-inflammatory state that in the setting of ischemia predisposes the Cx40−/− animal to significant irreversible ischemic injury and tissue necrosis.
Summary.
Cx40−/− mice are at greater risk than WT animals for irreversible ischemic injury. AT1R blockade, to counteract effects of chronic ANG II elevation, failed to significantly improve postischemic outcome in Cx40−/− animals despite normalizing blood pressure and reducing the inflammatory response. Whether increased vascular wall permeability and edema associated with reduced (absent) Cx40 expression (41, 42) or impaired ischemia-induced AT1R-dependent arteriogenesis and angiogenesis (33) also contribute to the increased risk of these animals remains to be explored, but our data implicate compromised regulation of flow distribution, consequent to the loss of coordinated vasodilatory response over distances, and compromised arteriogenesis as major contributors to the increased risk of irreversible ischemic injury in this genotype.
GRANTS
This study was supported, in part, by the American Heart Association (550158Z, 0715532Z, and 09PRE2060122 to J. S. Fang) and grants from the National Heart, Lung, and Blood Institute (R01HL064232 to A. M. Simon, R01HL058732 to J. M. Burt, and T32HL007249 to J. M. Burt-support to S. N. Angelov).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.S.F. and S.N.A. performed experiments; J.S.F. and J.M.B. analyzed data; J.S.F., A.M.S., and J.M.B. interpreted results of experiments; J.S.F. and J.M.B. prepared figures; J.S.F. and J.M.B. drafted manuscript; J.S.F., S.N.A., A.M.S., and J.M.B. edited and revised manuscript; J.S.F., S.N.A., A.M.S., and J.M.B. approved final version of manuscript; A.M.S. and J.M.B. conception and design of research.
ACKNOWLEDGMENTS
We thank Dr. David Kurjiaka for assistance with blood pressure measurements and Miranda Good for occasional assistance.
REFERENCES
- 1. Alonso F, Krattinger N, Mazzolai L, Simon A, Waeber G, Meda P, Haefliger JA. An angiotensin II- and NF-kappaB-dependent mechanism increases connexin 43 in murine arteries targeted by renin-dependent hypertension. Cardiovasc Res 87: 166–176, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol 11: 805–815, 1981 [DOI] [PubMed] [Google Scholar]
- 3. Brady AJ. Perindopril versus angiotensin II receptor blockade in hypertension and coronary artery disease: implications of clinical trials. Clin Drug Investig 27: 149–161, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Buschmann I, Pries A, Styp-Rekowska B, Hillmeister P, Loufrani L, Henrion D, Shi Y, Duelsner A, Hoefer I, Gatzke N, Wang H, Lehmann K, Ulm L, Ritter Z, Hauff P, Hlushchuk R, Djonov V, van Veen T, le Noble F. Pulsatile shear and Gja5 modulate arterial identity and remodeling events during flow-driven arteriogenesis. Development 137: 2187–2196, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Cao Y. Monotherapy versus combination therapy of angiogenic and arteriogenic factors for the treatment of ischemic disorders. Curr Mol Med 9: 967–972, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Chadjichristos CE, Scheckenbach KE, van Veen TA, Richani Sarieddine MZ, de WC, Yang Z, Roth I, Bacchetta M, Viswambharan H, Foglia B, Dudez T, Van Kempen MJ, Coenjaerts FE, Miquerol L, Deutsch U, Jongsma HJ, Chanson M, Kwak BR. Endothelial-specific deletion of connexin40 promotes atherosclerosis by increasing CD73-dependent leukocyte adhesion. Circulation 121: 123–131, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Chalothorn D, Faber JE. Formation and maturation of the native cerebral collateral circulation. J Mol Cell Cardiol 49: 251–259, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clayton JA, Chalothorn D, Faber JE. Vascular endothelial growth factor-A specifies formation of native collaterals and regulates collateral growth in ischemia. Circ Res 103: 1027–1036, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Wit C, Roos F, Bolz SS, Kirchhoff S, Kruger O, Willecke K, Pohl U. Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circ Res 86: 649–655, 2000 [DOI] [PubMed] [Google Scholar]
- 10. de Wit C, Roos F, Bolz SS, Pohl U. Lack of vascular connexin 40 is associated with hypertension and irregular arteriolar vasomotion. Physiol Genomics 13: 169–177, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Dupuis F, Atkinson J, Liminana P, Chillon JM. Comparative effects of the angiotensin II receptor blocker, telmisartan, and the angiotensin-converting enzyme inhibitor, ramipril, on cerebrovascular structure in spontaneously hypertensive rats. J Hypertens 23: 1061–1066, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Faber JE, Zhang H, Lassance-Soares RM, Prabhakar P, Najafi AH, Burnett MS, Epstein SE. Aging causes collateral rarefaction and increased severity of ischemic injury in multiple tissues. Arterioscler Thromb Vasc Biol 31: 1748–1756, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fabia MJ, Abdilla N, Oltra R, Fernandez C, Redon J. Antihypertensive activity of angiotensin II AT1 receptor antagonists: a systematic review of studies with 24 h ambulatory blood pressure monitoring. J Hypertens 25: 1327–1336, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Fang JS, Angelov SN, Simon AM, Burt JM. Cx37 deletion enhances vascular growth and facilitates ischemic limb recovery. Am J Physiol Heart Circ Physiol 301: H1872–H1881, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fang JS, Angelov SN, Simon AM, Burt JM. Cx40 is required for, and cx37 limits, postischemic hindlimb perfusion, survival and recovery. J Vasc Res 49: 2–12, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gellhaus A, Dong X, Propson S, Maass K, Klein-Hitpass L, Kibschull M, Traub O, Willecke K, Perbal B, Lye SJ, Winterhager E. Connexin43 interacts with NOV: a possible mechanism for negative regulation of cell growth in choriocarcinoma cells. J Biol Chem 279: 36931–36942, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Groth W, Blume A, Gohlke P, Unger T, Culman J. Chronic pretreatment with candesartan improves recovery from focal cerebral ischaemia in rats. J Hypertens 21: 2175–2182, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Harari OA, Liao JK. NF-kappaB and innate immunity in ischemic stroke. Ann N Y Acad Sci 1207: 32–40, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Herskowitz A, Mangano Inflammatory cascade DT. A final common pathway for perioperative injury? Anesthesiology 85: 957–960, 1996 [DOI] [PubMed] [Google Scholar]
- 20. Jobs A, Schmidt K, Schmidt VJ, Lubkemeier I, van Veen TA, Kurtz A, Willecke K, de WC. Defective Cx40 maintains Cx37 expression but intact Cx40 is crucial for conducted dilations irrespective of hypertension. Hypertension 60: 1422–1429, 2012 [DOI] [PubMed] [Google Scholar]
- 21. Kang EY, Ponzio M, Gupta PP, Liu F, Butensky A, Gutstein DE. Identification of binding partners for the cytoplasmic loop of connexin43: a novel interaction with beta-tubulin. Cell Commun Adhes 1–10, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kansui Y, Fujii K, Nakamura K, Goto K, Oniki H, Abe I, Shibata Y, Iida M. Angiotensin II receptor blockade corrects altered expression of gap junctions in vascular endothelial cells from hypertensive rats. Am J Physiol Heart Circ Physiol 287: H216–H224, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Kardami E, Dang X, Iacobas DA, Nickel BE, Jeyaraman M, Srisakuldee W, Makazan J, Tanguy S, Spray DC. The role of connexins in controlling cell growth and gene expression. Prog Biophys Mol Biol 94: 245–264, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Krattinger N, Capponi A, Mazzolai L, Aubert JF, Caille D, Nicod P, Waeber G, Meda P, Haefliger JA. Connexin40 regulates renin production and blood pressure. Kidney Int 72: 814–822, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Kurtz L, Schweda F, de Wit C, Kriz W, Witzgall R, Warth R, Sauter A, Kurtz A, Wagner C. Lack of connexin 40 causes displacement of renin-producing cells from afferent arterioles to the extraglomerular mesangium. J Am Soc Nephrol 18: 1103–1111, 2007 [DOI] [PubMed] [Google Scholar]
- 26. MacKenzie A. Endothelium-derived vasoactive agents, AT1 receptors and inflammation. Pharmacol Ther 131: 187–203, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Munzenmaier DH, Greene AS. Opposing actions of angiotensin II on microvascular growth and arterial blood pressure. Hypertension 27: 760–765, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Nagai M, Terao S, Vital SA, Rodrigues SF, Yilmaz G, Granger DN. Role of blood cell-associated angiotensin II type 1 receptors in the cerebral microvascular response to ischemic stroke during angiotensin-induced hypertension. Exp Transl Stroke Med 3: 15, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Paiardi S, Rodella LF, De CC, Porteri E, Boari GE, Rezzani R, Rizzardi N, Platto C, Tiberio GA, Giulini SM, Rizzoni D, Agabiti-Rosei E. Immunohistochemical evaluation of microvascular rarefaction in hypertensive humans and in spontaneously hypertensive rats. Clin Hemorheol Microcirc 42: 259–268, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Pries AR, Hopfner M, le NF, Dewhirst MW, Secomb TW. The shunt problem: control of functional shunting in normal and tumour vasculature. Nat Rev Cancer 10: 587–593, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Prior BM, Lloyd PG, Ren J, Li H, Yang HT, Laughlin MH, Terjung RL. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol Heart Circ Physiol 287: H2434–H2447, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Salvemini D, Mazzon E, Dugo L, Riley DP, Serraino I, Caputi AP, Cuzzocrea S. Pharmacological manipulation of the inflammatory cascade by the superoxide dismutase mimetic, M40403. Br J Pharmacol 132: 815–827, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sasaki K, Murohara T, Ikeda H, Sugaya T, Shimada T, Shintani S, Imaizumi T. Evidence for the importance of angiotensin II type 1 receptor in ischemia-induced angiogenesis. J Clin Invest 109: 603–611, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shireman PK. The chemokine system in arteriogenesis and hind limb ischemia. J Vasc Surg 45, Suppl A: A48–A56, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Simon AM, McWhorter AR. Decreased intercellular dye-transfer and downregulation of non-ablated connexins in aortic endothelium deficient in connexin37 or connexin40. J Cell Sci 116: 2223–2236, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Sullivan CJ, Doetschman T, Hoying JB. Targeted disruption of the Fgf2 gene does not affect vascular growth in the mouse ischemic hindlimb. J Appl Physiol 93: 2009–2017, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Egido J. Inflammation and angiotensin II. Int J Biochem Cell Biol 35: 881–900, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Talhouk RS, Zeinieh MP, Mikati MA, El-Sabban ME. Gap junctional intercellular communication in hypoxia-ischemia-induced neuronal injury. Prog Neurobiol 84: 57–76, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Wagner C, de WC, Kurtz L, Grunberger C, Kurtz A, Schweda F. Connexin40 is essential for the pressure control of renin synthesis and secretion. Circ Res 100: 556–563, 2007 [DOI] [PubMed] [Google Scholar]
- 40. You D, Cochain C, Loinard C, Vilar J, Mees B, Duriez M, Levy BI, Silvestre JS. Hypertension impairs postnatal vasculogenesis: role of antihypertensive agents. Hypertension 51: 1537–1544, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Zahler S, Hoffmann A, Gloe T, Pohl U. Gap-junctional coupling between neutrophils and endothelial cells: a novel modulator of transendothelial migration. J Leukoc Biol 73: 118–126, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Zhang J, Wang W, Sun J, Li Q, Liu J, Zhu H, Chen T, Wang H, Yu S, Sun G, Chen W, Yi D. Gap junction channel modulates pulmonary vascular permeability through calcium in acute lung injury: an experimental study. Respiration 80: 236–245, 2010 [DOI] [PubMed] [Google Scholar]