Abstract
Bradycardia prolongs action potential (AP) durations (APD adaptation), enhances dispersion of repolarization (DOR), and promotes tachyarrhythmias. Yet, the mechanisms responsible for enhanced DOR and tachyarrhythmias remain largely unexplored. Ca2+ transients and APs were measured optically from Langendorff rabbit hearts at high (150 × 150 μm2) or low (1.5 × 1.5 cm2) magnification while pacing at a physiological (120 beats/min) or a slow heart rate (SHR = 50 beats/min). Western blots and pharmacological interventions were used to elucidate the regional effects of bradycardia. As a result, bradycardia (SHR 50 beats/min) increased APDs gradually (time constant τf→s = 48 ± 9.2 s) and caused a secondary Ca2+ release (SCR) from the sarcoplasmic reticulum during AP plateaus, occurring at the base on average of 184.4 ± 9.7 ms after the Ca2+ transient upstroke. In subcellular imaging, SCRs were temporally synchronous and spatially homogeneous within myocytes. In diastole, SHR elicited variable asynchronous sarcoplasmic reticulum Ca2+ release events leading to subcellular Ca2+ waves, detectable only at high magnification. SCR was regionally heterogeneous, correlated with APD prolongation (P < 0.01, n = 5), enhanced DOR (r = 0.9277 ± 0.03, n = 7), and was gradually reversed by pacing at 120 beats/min along with APD shortening (P < 0.05, n = 5). A stabilizer of leaky ryanodine receptors (RyR2), 3-(4-benzylcyclohexyl)-1-(7-methoxy-2,3-dihydrobenzo[f][1,4]thiazepin-4(5H)-yl)propan-1-one (K201; 1 μM), suppressed SCR and reduced APD at the base, thereby reducing DOR (P < 0.02, n = 5). Ventricular ectopy induced by bradycardia (n = 5/15) was suppressed by K201. Western blot analysis revealed spatial differences of voltage-gated L-type Ca2+ channel protein (Cav1.2α), Na+-Ca2+ exchange (NCX1), voltage-gated Na+ channel (Nav1.5), and rabbit ether-a-go-go-related (rERG) protein [but not RyR2 or sarcoplasmic reticulum Ca2+ ATPase 2a] that correlate with the SCR distribution and explain the molecular basis for SCR heterogeneities. In conclusion, acute bradycardia elicits synchronized subcellular SCRs of sufficient magnitude to overcome the source-sink mismatch and to promote afterdepolarizations.
Keywords: action potential adaptation, arrhythmia, bradycardia, optical mapping, secondary Ca2+ release
bradycardia, defined as heart rate (HR) < 60 beats/min in adult humans (12), is usually a normal adaptive response to conditions of low metabolic demand, such as rest and sleep. Bradycardia due to pathological conditions, e.g., sick sinus syndrome, atrioventricular (AV) nodal disease, or conduction system disease, is common in clinical practice and frequently causes symptoms because of inappropriately low cardiac output. Less frequently, profound bradycardia, usually caused by heart block, may paradoxically cause ventricular tachycardia, often polymorphic ventricular tachycardia indistinguishable from Torsades de Pointes (TdP) that is classically observed in acquired or congenital long QT syndrome (LQTS) (57).
Bradycardia is known to be a cofactor in the initiation of LQT-related arrhythmias in humans and most animal models of LQTS (56, 58); ventricular pacing at relatively high rates is an effective treatment of TdP. Occasionally, severe bradycardia appears to be the only trigger of TdP in patients lacking other obvious preexisting causes of repolarization delay. Polymorphic ventricular tachycardia may also occur as a consequence of relative bradycardia after a prolonged tachycardia, e.g., following radiofrequency ablation of the AV node for atrial fibrillation or ablation of an accessory pathway causing incessant tachycardia (14, 15, 25). This potentially fatal complication occurs frequently enough that pacing at relatively high rates (80–90 beats/min) early after radiofrequency ablation of AV node is recommended (39).
Bradycardia-dependent TdP is more prevalent in women (∼75%) than men (54, 57), which parallels the well-established finding for drug-induced LQT type 2 (LQT2). On the other hand, most episodes of TdP in humans are not preceded by profound bradycardia, and more than one factor is often identified (29, 32).
Effects of bradycardia on action potential duration and Ca2+ handling.
Action potential (AP) duration (APD) adaptation to HR changes is required to maintain an adequate ratio between ventricular filling and ventricular ejection. The mechanisms responsible for APD adaptation have been the topic of extensive investigation. Most studies attributed rate-dependent APD adaptation to changes in intracellular Ca2+ (Cai), which in turn alters the kinetics of Ca2+-dependent inactivation of L-type Ca2+ channels (4, 9, 41). During a transition from slow to fast HR, diastolic Ca2+ rises because of higher influx (a consequence of a greater number of APs per unit time) and decreased efflux because of shorter diastolic intervals. Higher Cai accelerates the Ca2+-dependent inactivation of L-type Ca2+currents (ICa,L) and reduces Ca2+ influx per AP, which lowers the plateau phase and decreases APD. Others have implicated the Na+-Ca2+ exchange (NCX) current (INCX) (21) and the late Na+ current (INa,L) (24). Mathematical simulations of slow HR (SHR) indicated that the long diastolic intervals result in a complete deactivation of the slow component of the delayed rectifying K+ current (IKs) and complete recovery from inactivation of ICa,L, which could theoretically explain APD adaptation in bradycardia (5, 27, 61). Besides ion channel kinetics, changes in ionic concentrations in the cytoplasm (intracellular Na+ and Cai) or extracellular K+ may contribute to APD adaptation (13, 22).
HR and arrhythmias.
HRs outside the physiological range may promote arrhythmias through changes in Ca2+ handling (45). Transitions from slow to fast HR have been extensively studied and shown to create Ca2+ alternans that lead to APD and T-wave alternans, increased dispersion of repolarization (DOR), and arrhythmias (42). In the setting of ischemia (43, 44) and heart failure (64), arrhythmogenic Ca2+ and APD alternans occur at physiological HR. In drug-induced LQT2, SHR promotes sarcoplasmic reticulum (SR) Ca2+ overload, resulting in spontaneous SR Ca2+ release during the AP plateau and early afterdepolarizations (EADs) (18, 38). In intact hearts, bradycardia prolongs APD and enhances DOR (16, 60), which are thought to contribute to LQT2-related arrhythmias.
This project investigates APD adaptation and Ca2+ handling changes during bradycardia in Langendorff-perfused rabbit hearts at low and high magnifications. We show that bradycardia promotes a secondary intracellular Ca2+ (Cai) release at the base of the ventricles, which contributes to further APD prolongation, increased DOR, and triggered activity.
METHODS
Heart preparations.
New Zealand White rabbits (females, 60 to 120 days old) were euthanized with pentobarbital sodium (75 mg/kg intravenously) and anticoagulated with heparin (200 U/kg intravenously). The heart was rapidly dissected and perfused with Tyrode solution containing (in mM) 130 NaCl, 24 NaHCO3, 1.0 MgCl2, 4 KCl, 1.2 NaH2PO4, 50 dextrose, and 1.25 CaCl2 (at pH 7.2–7.4), gassed with 95% O2 plus 5% CO2. Temperature and perfusion pressure were continuously monitored and regulated by a feedback systems at 37 ± 2°C and ∼80 mmHg, respectively. To minimize motion artifact, blebbistatin (5–10 μM; Sigma, St Louis, MO) was added to the perfusate for 5–10 min. The heart was immobilized in a chamber and stained with a voltage-sensitive dye (PGH1: 200 μl of 1 mg/ml dimethyl sulfoxide) and loaded with a Ca2+ indicator (Rhod-2 AM, 200 μl of 1 mg/ml dimethyl sulfoxide). Epicardial bipolar pseudo-EKG was continuously monitored, and the atrioventricular (AV) node was ablated to control HR. Hearts were paced with an epicardial unipolar electrode placed on the lateral wall of the right ventricle, approximately halfway between the apex and base. In pilot experiments, different pacing sites at the apex, base, and posterior were tested and found not to alter the effect of bradycardia on Ca2+ dynamics. For a physiological rate, hearts were paced at a baseline cycle length of 0.5 s [baseline HR (BHR) of 120 beats/min] and for bradycardia at a SHR with a cycle length of 1.2 s (50 beats/min; a profound bradycardia for rabbit hearts). This investigation conformed to the current Guide for Care and Use of Laboratory Animals, published by the National Institutes of Health, and was approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
Optical apparatus.
An optical apparatus consisting of two CMOS cameras (SciMedia, Ultima One, 100 × 100 pixels) has been used for simultaneous measurement of intracellular Ca2+ transients (CaTs) and membrane potential (Vm) changes, as previously described (47). For long uninterrupted recordings at low magnification, a slow scan rate of 250 frames/s was intentionally chosen to record Cai and Vm during two to three sets of transitions from BHR to SHR and SHR to BHR. Brief (4 s) recordings were taken at 2,000 frames/s to verify the kinetics of Cai and Vm upstrokes. Subcellular Vm and Cai signals were measured at high magnification at scan rates of 200 frames/s to achieve a high signal-to-noise ratio without compromising the kinetics of the signals. The anterior surface of the heart was illuminated with a 520 ± 30-nm excitation beam, and the fluorescence emitted by Rhod-2 and PGH1 was separated by a dichroic mirror (660 nm) and was focused on two CMOS cameras, as previously described (47). Some hearts were stained with 4-[β-[2-(di-n-butylamino)-6-naphthyl]vinyl]pyridinium (di-4-ANEPPS) to better visualize transverse tubules at high optical magnification.
Macroscopic and subcellular Cai and Vm mapping.
For macroscopic imaging, each camera viewed 1.5 × 1.5 cm2 from the anterior surface of the heart using a Nikon camera lens (50 mm, 1:1.2). For subcellular imaging, the heart was perfused horizontally in a chamber on the stage of an upright microscope (Olympus BX61W1) using a ×40 water immersion objective (Olympus, ×40/LUMPLFL). Fluorescence microscopy to image intact hearts at the subcellular level offers important advantages compared with previous confocal imaging studies (2, 50). The lack of confocal apertures results in considerably greater light throughput (2 to 3 orders in magnitude), making it possible to measure Cai and Vm signals from two-dimensional fields of view (instead of line scans) with a greater signal-to-noise ratio (>80/1) and temporal resolution (up to 2,000 frames/s). Butterworth low-pass temporal filtering (60- to 100-Hz cutoff) was used if needed, but spatial filtering was not applied to maximize spatial resolution. The limitation of bright field over confocal microscopy is the blurring caused by background scattered light from deeper layers of muscle and the lower voxel resolution. In the present configuration, the ×40 objective has a depth of field of ∼ 0.5 μm and an x-y resolution of 1.5 × 1.5 μm2. The confocal apertures would block scattered light emanating from out-of-focus fluorescence sources and would improve contrast by reducing blurring. More relevant to this study, nonconfocal optics does not significantly alter Cai kinetics in maps of subcellular Ca2+ waves in three dimensions. A recent analysis of spark properties recorded with confocal imaging revealed that the spatial widths and rise times of sparks were similar when recorded in-focus compared with out-of-focus by ±1 μm (51). We estimated that imaging with a ×40 objective represented the summation of Cai or (Vm) signals over a voxel (1.5 × 1.5 × 3.0 μm3) with reduced spatial but an improved temporal resolution compared with confocal microscopy.
Study protocol.
The AV node was ablated by cauterization to allow control of HR by pacing. Hearts were paced at a cycle length of 0.5 s, which was taken as a BHR, and the cycle length was then lengthened to 1.2 s to impose a bradycardia or a SHR. APs and CaTs were continuously recorded during several (typically 3) transitions from fast to slow and back from slow to fast HR. Changes in HR led to gradual changes in APD until a new steady state was attained. The time course of APD adaptation required continuous recordings of 3–5 min to reach a steady state of APDs during transitions from BHR to SHR (bradycardia) and 5–10 min in going from SHR to BHR.
Pilot experiments were carried out to select the BHR and the SHR that were used in this study. A SHR of 50 beats/min was chosen because it could be maintained reliably without interruptions by an occasional extra beat yet was effective to expose spatial heterogeneities of CaT caused by secondary Ca2+ release (SCR). Similar, but less pronounced, effects were observed at 0.9- and 1.0-s cycle lengths. A basic HR of 120 beats/min was chosen because 1) this HR was well tolerated and did not result in rundown of the preparations, 2) capture with pacing electrodes was reliable during uninterrupted recordings, and 3) spatial heterogeneities of AP and CaT were negligible compared with still faster rates.
Data analysis.
Activation time at each site was calculated from maximum first derivative of the fluorescent signal [(dF/dt)max] of the local AP or CaT upstroke. APD and CaT duration (CaTD) at each site were calculated from the interval between (dF/dt)max and the recovery of Vm and CaT traces to 20% of baseline (APD80 or CaTD80), respectively. Automatic measurement of APD80 and CaTD80 from all pixels (100 × 100 pixels) was used to calculate mean APD80 and CaTD80. The dispersion of APD80 was calculated from the SD of APD80. The time constant (τ) of mean APD (from all 100 × 100 pixels) adaptation to HR changes was calculated by fitting the transitions from BHR to SHR (τf→s) and from SHR to BHR (τs→f) with monoexponential functions. Rate and time-dependent mean APD80 and regional variation in 100 × 100 pixels were statistically evaluated. Bradycardia-dependent APD and CaT prolongation and dispersions before and after 3-(4-benzylcyclohexyl)-1-(7-methoxy-2,3-dihydrobenzo[f][1,4]thiazepin-4(5H)-yl)propan-1-one (K201; 1 μM) perfusion were compared. K201 was synthesized according to a previously reported procedure (63). The amplitudes of SCR (ASCRs) were assessed by calculating areas under curves of normalized CaT from the inflexion time points of Cai downstrokes to the time points of CaTD80 at each pixel. All 10,000 recordings (100 × 100 pixels) were used to generate the scatterplots to correlate spatial heterogeneities of ASCRs to the dispersion of APD80. Regional differences of APD80, base of right ventricle (RVB), and apex of left ventricle (LVA) were compared for statistical significance using two-tailed t-test. Box-whisker diagrams are used to visualize the distribution of the data. The top and bottom whiskers define, respectively, the maximum and minimum values; the top and bottom of the box define the 75th and 25th percentiles, respectively, and the line within the box is the median in the data set.
Western blot analysis.
Female New Zealand White rabbits (3 mo old) were euthanized as described above, the hearts were perfused with Tyrode solution, and ventricular tissue samples (∼50 mg) were dissected from the base (B) and apex (A) of the epicardium. Proteins were isolated as previously described (17), were separated by SDS-PAGE (50 μg/sample), and transferred to polyvinylidene difluoride membranes, which were probed by standard techniques. After immunolabeling, band intensities were measured with ImageJ and normalized with respect to β-actin. Differences between the base and apex were analyzed with two-tailed t-test and considered significant at P < 0.05. Antibodies against rabbit Cav1.2α, sarcoplasmic reticulum Ca2+-ATPase 2 (SERCA2), ERG and β-actin were obtained from Santa Cruz Biotech (Cat. No. SC-103588, SC53010, 15968, and 81178, respectively), NCX1 and ryanodine receptors (RyR2) were obtained from Thermo Scientific (Cat. No. MA3-926 and MA3-916, respectively), and Nav1.5 was obtained from Alomone (Cat. No. ASC-005).
RESULTS
Bradycardia-induced changes in CaT.
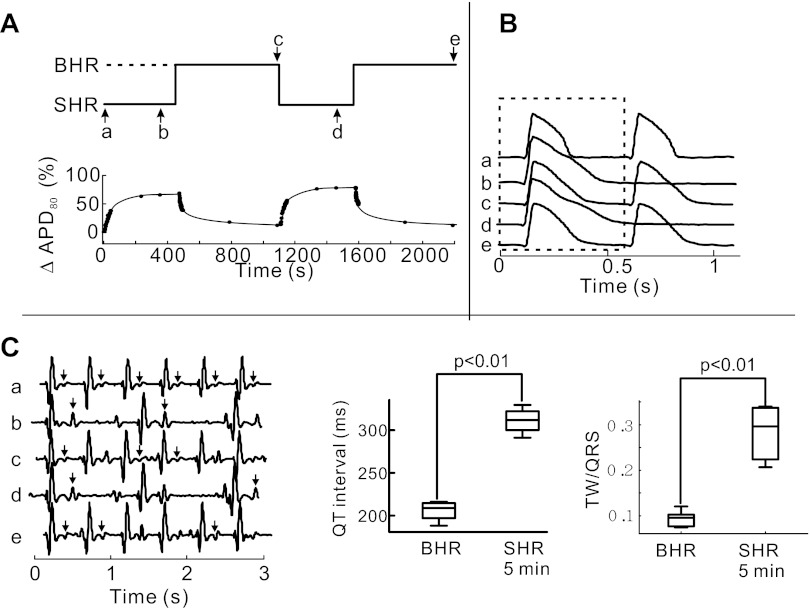
Low-resolution imaging of Vm and Cai signals during HR transition was performed to confirm the reproducibility of rate-dependent changes. APD80 adaptation was reproducible from heart to heart and during repeated cycles (3 to 4 per heart) of transitions from 120 (BHR) to 50 (SHR) beats/min and back. Figure 1A illustrates a continuous sequence of two complete cycles of APD adaptation from BHR to SHR. From a HR of 120 beats/min (not shown), a transition to SHR resulted in a gradual prolongation of APD80 and QT interval which was fully reversed by pacing at a BHR. The mean ΔAPD80 in the first episode of SHR increased by 55.2 ± 10.9%, which was similar to that measured in a second episode of SHR (51.25 ± 8.2%) (P < 0.05, n = 4). Fig. 1, B and C, illustrates optical APs and EKG recordings measured at time points a–e (Fig. 1A). An AP recorded at the onset SHR (trace a) is markedly shorter than at steady-state SHR (trace b). APs measured at the next BHR are shorter than at steady-state SHR. Similarly, T-wave amplitudes were markedly larger at SHR than at BHR or the initiation of SHR (Fig. 1C). The changes in T-wave amplitude relative to the QRS amplitude are a measure of changed repolarization gradient and the ratio of T wave to QRS amplitude was statistically greater at steady-state SHR than BHR (Fig. 1C, right, P < 0.01, n = 15 trials from 5 hearts).
Fig. 1.
Action potential (AP) duration (APD) adaptation during transitions from baseline heart rate (BHR) and slow heart rate (SHR). APD80 was calculated from the interval between the maximum first derivative of the fluorescence signal [(dF/dt)max] and the recovery of membrane potential (Vm) to 20% of baseline. A: repeated cycles of pacing rate changes from SHR to BHR. Time course of heart rate (HR; top trace) and ΔAPD80 (expressed as percent change with respect to APD80 at steady-state BHR) adaptation is shown uninterrupted for 2,200 s. B: optical traces of APs from a single pixel acquired at low magnification at time points a–e, as labeled in A, top trace. C: near-field EKG signals recorded from the left ventricular epicardium at the same time points as depicted in A, top trace. The relative amplitude of T waves (arrows) measured as the ratio of T wave to QRS amplitudes was significantly higher in SHR than BHR, which is indicative of an increase in dispersion of repolarization.
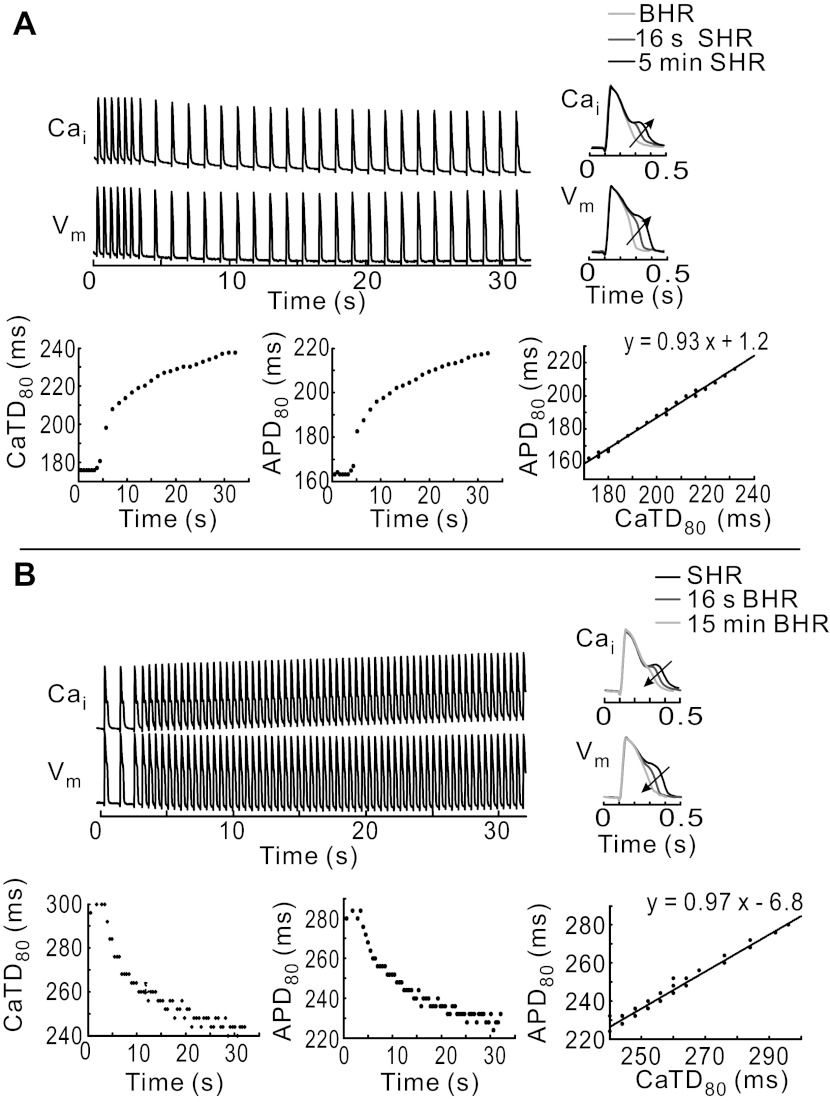
At BHR, the time course of AP and CaT signals exhibited the expected rapid rise and monophasic recovery to baseline. After the transition to SHR, diastolic levels of Cai decreased gradually and APD80 and CaTD80 exhibited the expected time-dependent prolongation (Fig. 2A). Most interesting was the gradual prolongation of CaTD80, which was associated with a slowing down of the CaT downstroke, the appearance of a Cai plateau, and a small secondary Ca2+ peak. This increasingly more pronounced SCR during the AP plateau was associated with APD prolongation, changes in the shape of APs, with a tight linear correlation between CaTD80 and APD80 (Fig. 2A). Reversal to BHR suppressed SCR gradually, altered the shape of AP repolarization, and shortened APD80 and CaTD80 (Fig. 2B). APD80 adaptation curves exhibited monoexponential time courses with significantly different time constants in going from BHR to SHR (τf→s = 48 ± 9.2 s) compared with from SHR to BHR (τs→f = 30.4 ± 4.7 s; P < 0.05, n = 5 hearts). A similar trend was reported for QT adaptation in patients upon sudden HR changes (49).
Fig. 2.
Time course of APD and Ca2+ transient (CaT) duration (CaTD) adaptation. A: changes in AP and CaT dynamics recorded at low magnification during a change in HR from BHR to SHR (top traces). CaTD80 and APD80 are plotted as a function of time (bottom left and bottom middle, respectively), and APD80 vs. CaTD reveals a tight linearly relationship (bottom right). Cai, intracellular Ca2+. Inset: superposition of APs and CaTs from the same pixel on the RVB recorded at different times during adaptation to SHR; arrow points toward steady-state SHR. B: changes in AP and CaT dynamics during the transition from SHR to BHR. APD80 and CaTD80 are plotted as a function of time (bottom left and bottom middle, respectively) and APD80 vs. CaTD80 (bottom right) are linearly related. Inset: superposition of APs and CaTs from the same pixel recorded at different times during adaptation toward BHR; arrow points toward steady-state BHR.
Regional heterogeneity of SCR.
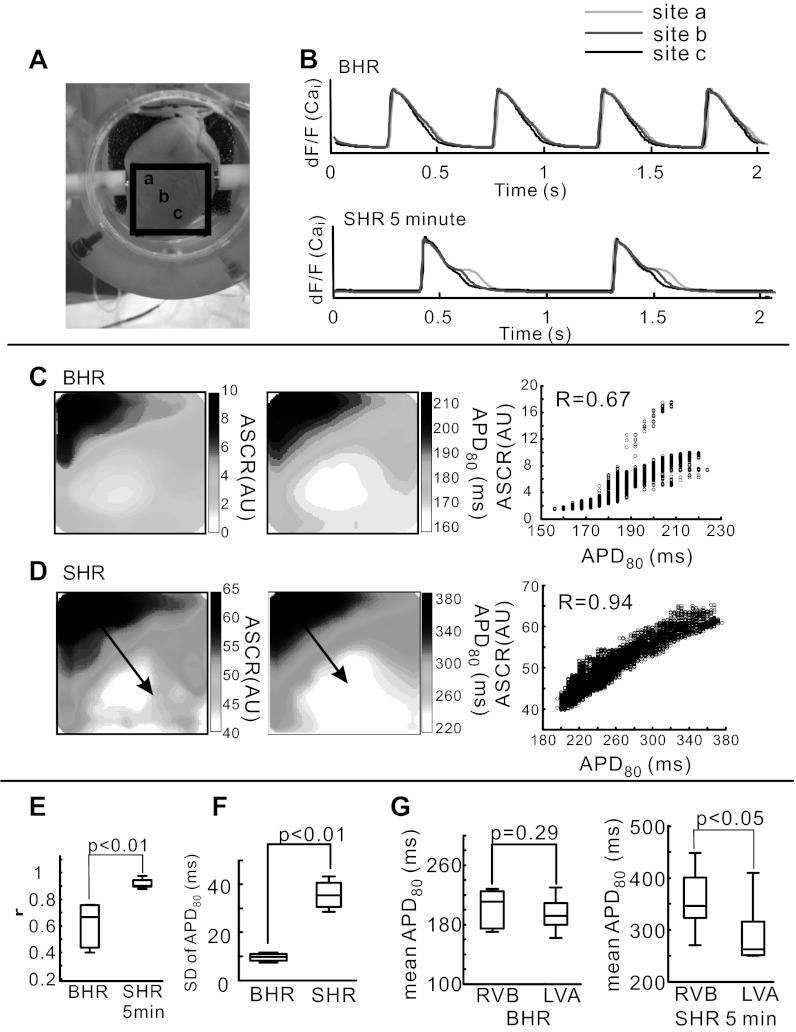
We calculated the spatial correlation between changes in AP and CaT to evaluate the interplay between Vm and Cai. The distribution of SCR was heterogeneous, being more pronounced at the RVB than the LVA. The superposition of CaT measured at BHR and 5 min of SHR exemplify the heterogeneous changes that occur from RVB to LVA (a, b, and c) on the epicardium (Fig. 3, A and B). The delay between the first and second release of Ca2+ (SCR) was measured from the time interval between (dF/dt)max and the inflection point (d2F/dt2 = 0) of the CaT downstroke. When measured for all 10,000 pixels in 5 hearts, SCR occurred 184.4 ± 9.7 ms after the first Ca2+ release, demonstrating a highly reproducible change in Ca2+ dynamics at all sites on the base of the epicardium and from heart to heart. Similarly, the ASCR was assessed by measuring the area under the CaT curve between the inflection point (d2F/dt2 = 0) and CaTD80.
Fig. 3.
Dispersion of amplitudes of SCR (ASCRs) and APD80 in BHR and SHR. A: picture of the anterior surface of the heart with a black box identifying the region of the heart viewed by the CMOS cameras. B: superimposed optical traces of Cai from base of right ventricle (RVB; site a) to apex of left ventricle (LVA; site c) that are identified in A. C: spatial correlation between APD80 (left) and ASCRs (middle) at steady-state BHR. A scatterplot of APD80 and ASCRs represents spatial correlation (right). Spatial correlation coefficient (r) was calculated in 100 × 100 pixels at BHR and SHR. D: as for C, but at 5 min of SHR. E: statistical comparison of r between APD80 and ASCRs at steady-state BHR and 5 min of SHR. F: SD of APD80 at steady-state BHR and 5 min of SHR. SD was used as a measure of dispersion and was calculated from 10,000 recordings (100 × 100 pixels) at BHR baseline and after 5 min of SHR. G: mean APD80 measured at BHR from RVB (24 pixels) and LVA (24 pixels) was not significantly different (left), but after 5 min of SHR, mean APD80 was significantly longer at RVB than LVA (middle) and as a percent change in APD80 (right). AU, arbitrary unit, dF/F, fractional fluorescence change.
The interplay between SCR and Vm was evaluated by correlating ASCR with APD80. Maps of APD80 and ASCRs are shown for BHR and after 5 min of SHR, which increased the dispersion of APD80 and ASCRs (P < 0.01, n = 7 hearts) (Fig. 3, C and D). Most interesting was the statistical analysis that revealed an enhanced correlation between ASCRs and APD80 (r = 0.90 ± 0.03) during SHR compared with BHR (r = 0.66 ± 0.18) (P < 0.01, n = 7 hearts) (Fig. 3E). SHR significantly increased the dispersion of APD80 compared with BHR (P < 0.01, n = 7; Fig. 3F), and mean APD80 (averaged over 24 pixels) was significantly longer at the RVB than the LVA (P < 0.05, n = 7, Fig. 3G).
Subcellular imaging.
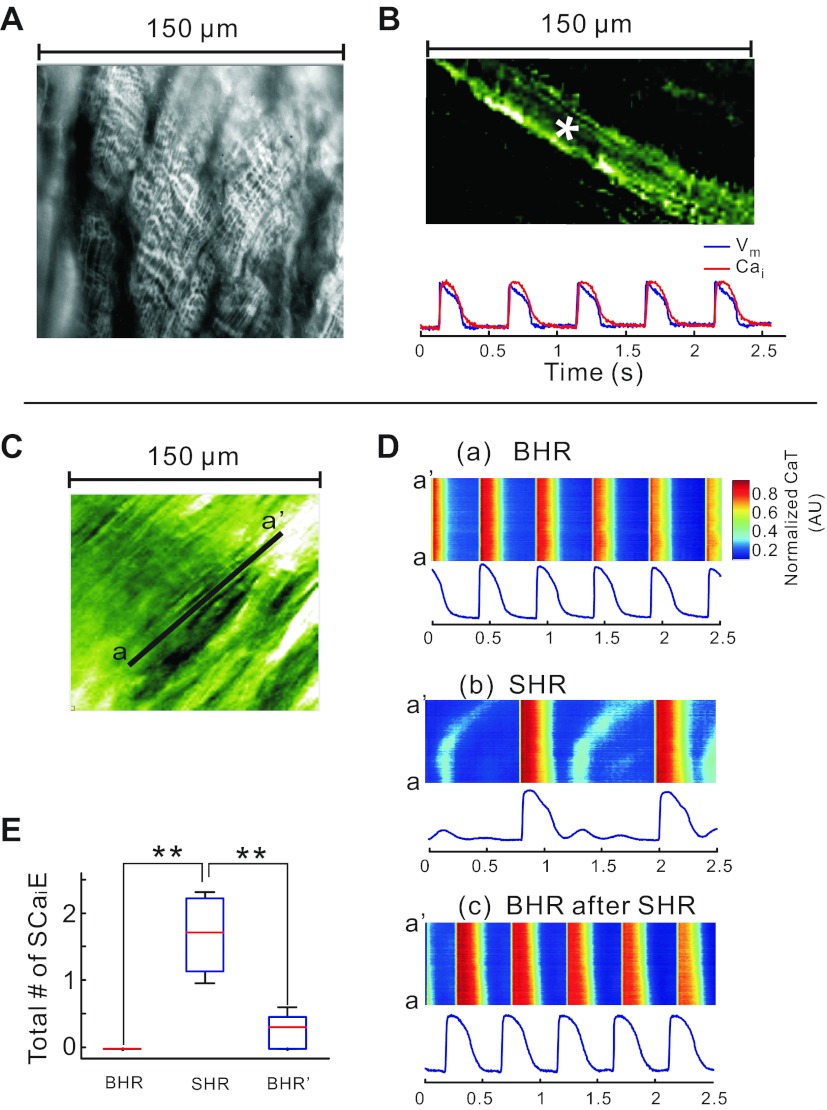
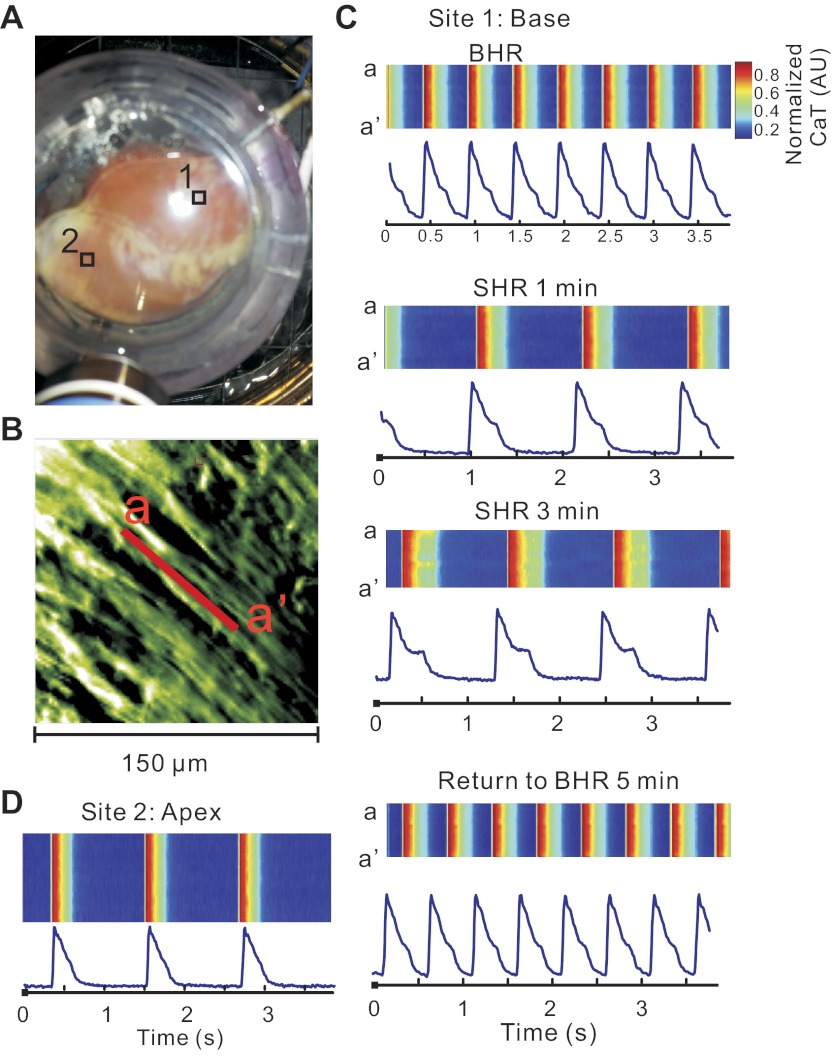
Hearts were mapped at high magnification to elucidate the cellular mechanisms underlying heterogeneities of SCR; this visualized CaT changes in unprecedented detail. When stained with di-4-ANEPPS, the transverse tubules are readily visualized with nonconfocal imaging (Fig. 4A). When stained with PGH1 and Rhod-2 AM, individual myocytes can be identified and subcellular recordings of APs and CaT were measured from a single pixel viewing 1.5 × 1.5 μm2 and a signal-to-noise ratio > 80/1 (Fig. 4B). Changes in CaT were analyzed by converting our two-dimensional data (Fig. 4C, see supplementary movie 1) to line scans, typically used in confocal imaging (Fig. 4D). At BHR, the line scan (a-a′) showed CaT along the longitudinal axis of a ventricular myocyte (Fig. 4D,a). After 5 min at a SHR, the line scans changed in two important ways: the systolic Cai exhibited a SCR and diastolic Cai exhibited subcellular oscillations that were not apparent at low magnification (Fig. 4D,b). Reverting to the BHR for 5 min fully reversed these changes in subcellular Cai (Fig. 4D,c). These subcellular changes in Cai handling seen at SHR were highly reproducible within the same heart and were reproduced in five hearts (Fig. 4E). As illustrated in Fig. 5, SCRs increased in amplitude as a function of time at SHR and were considerably more pronounced at the base than at the apex. Note the absence of SCR at a site on the apex (Fig. 5D), whereas a well-developed SCR was present at basal site (Fig. 5C).
Fig. 4.
Bradycardia-induced subcellular secondary Ca2+ release (SCR) in systole and Ca2+ waves in diastole. A: image of transverse tubules with nonconfocal microscopy; the transverse tubules of striated myocytes from a rabbit heart stained with 4-[β-[2-(di-n-butylamino)-6-naphthyl]vinyl]pyridinium (di-4-ANEPPS) were readily observed using a ×40 objective. B: dual subcellular mapping of APs and CaT from an epicardial myocyte of a Langendorff-perfused rabbit heart stained with PGH-1 and Rhod-2 AM; PGH-1 fluorescence image (top) and optical traces of APs (blue) and CaT (red) from the site marked with an asterisk (top). C: subcellular 150 × 150 μm2 field of view seen through Rhod-2 fluorescence. D: CaTs recorded along the line a–a′ identified in C and displayed in a familiar line scan format: CaT during BHR (top), CaT during SHR (middle), and back to CaT during BHR after 5 min SHR (bottom). CaT fluorescence tracings were recorded from a pixel (1.5 × 1.5 μm2) at the center of the line a–a′. Note that SHR leads to low-amplitude Cai instabilities and subcellular waves during diastole that are not seen at low magnification and disappear at BHR. Subcellular diastolic Cai waves were not synchronized among the neighboring cells. They were averaged out at low magnification. A supplementary movie file is provided to visualize the spatial and temporal heterogeneities of systolic and diastolic Cai (supplementary movie 1). E: statistical comparison of the number of secondary Ca2+ elevations (SCaiE; includes both systolic and diastolic elevations) between BHR, SHR, and return back to BHR. The data are derived from all the pixels in the field of view. **P < 0.001.
Fig. 5.
SHR promotes subcellular SCRs and CaT prolongation at the base but not the apex. A: locations of sites 1 and 2 (RVB and LVA, respectively) is indicated on this photograph of anterior wall of the heart. The high-magnification tracings from these sites are displayed in the other panels. B: subcellular image taken with the ×40 objective from site 1 at the RVB, as indicated in A. C: Cai transients recorded along line a–a′ in RVB shown in B at different HR in the line scan format along with tracings from the pixel located in the middle of line a–a′. Note the increasingly more pronounced SCR in SHR. D: Cai transients recorded along the longitudinal axis of a fiber in LVA at SHR.
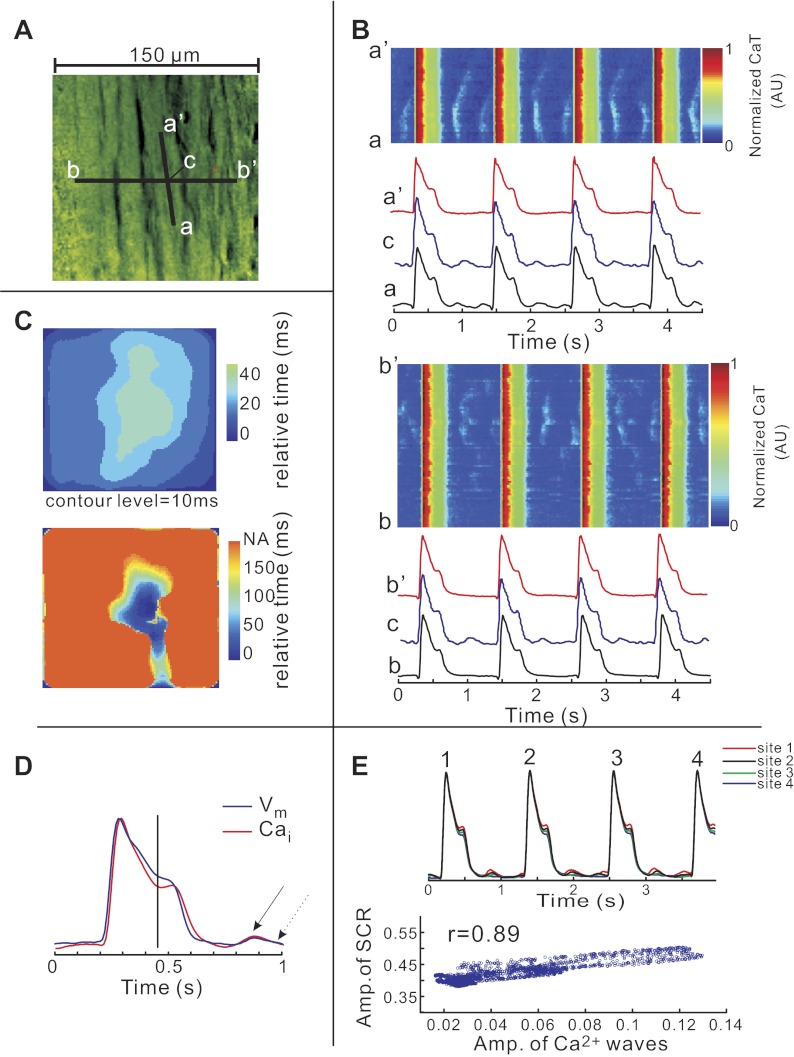
The significance of SCR in bradycardia was investigated with respect to spatial and temporal synchrony among cells at the base of the heart. Figure 6 illustrates the changes in CaT in bradycardia in a two-dimensional recording as a movie (Fig. 6A, see supplementary movie 2) and as line scans along the longitudinal (Fig. 6B, top, along a–a′) and transverse (Fig. 6B, bottom, along b–b′) axis of a myocyte. The video clip shows that SCR occurs synchronously within each cell (<20 ms) and between adjacent cells although with different amplitudes in various cells. In contrast, diastolic Ca2+ waves are temporally and spatially asynchronous and did not propagate across cell borders. An activation map of SCR within a cell showed that SCR was highly synchronous (Fig. 6C, top), whereas Ca2+ waves during diastole propagated slowly and were random within cells (Fig. 6C, bottom). Superimposed Cai and Vm signals from a 10.5 × 10.5 μm2 region of a single myocyte are shown in Fig. 6D. The onset of systolic SCR corresponds to change in AP slope. Diastolic Cai rise and corresponding depolarization are present in the same cell region. Interestingly, the amplitude of systolic SCRs and the diastolic Ca waves in a given cell was tightly correlated, suggesting a related mechanism (r = 0.86 ± 0.021, n = 4 hearts; Fig. 6E).
Fig. 6.
Spatiotemporal synchronization of SCR and asynchronous diastolic Ca2+ waves. A: high-magnification field of view depicting a longitudinal (a–a′) and a transverse (b–b′) line displayed as line scans in B. A movie is provided to visualize the development of systolic SCR and subcellular diastolic Ca2+ waves (supplementary movie 2). B: Cai transients recorded along the vertical (intracellular) line a–a′ (top) and along the horizontal (intercellular) line b–b′ (bottom) are shown in A in the line scan format. C: maps of onset times of systolic SCR occurring during the AP plateau (top) and of diastolic subcellular Ca2+ waves that occur within one cell but does not cross between cells (bottom). Color scales were selected to optimize visual contrast. NA, no activation. D: superimposed subcellular optical AP and CaT from site c on A (7 × 7 pixels, 10.5 × 10.5 μm2) shows that the speed of AP repolarization decreases after SCR onset (vertical line). Arrows indicate diastolic Ca2+ wave and diastolic Vm depolarization. E: superimposed optical signals of CaT at 4 different sites (top). A scatterplot reveals a tight linearly relationship between amplitudes (Amp) of SCRs and diastolic Ca2+ waves (n = 4 hearts, bottom).
Mechanism of SCR.
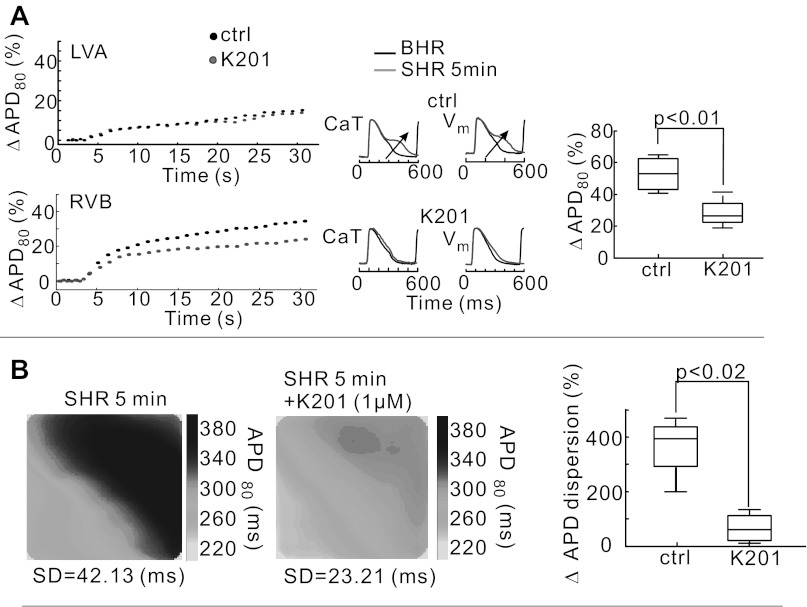
To further elucidate the interplay between Cai and voltage, experiments were carried out to assess whether SCR prolonged APD80 or longer APD80 elicited SCRs. In SHR, it is reasonable to expect that SCRs originate from SR Ca2+ release via RyR2 because the rise of SCRs always preceded those of partial depolarizations determined from inflection time points of AP during the AP plateau (or late-phase voltage humps) by 6.4 ± 1.3 ms. An agent known to stabilize RyR2, K201 (1 μM, n = 5) was tested for its effects on SCR. Plots of mean ΔAPD80 as a function of time are shown during transitions from BHR to SHR from 5 × 5 pixels on the LVA (Fig. 7A, top) and 5 × 5 pixels on the RVB (Fig. 7A, bottom) with and without K201. The graphs show that K201 reduced mean APD80 at pixels on the RVB (P < 0.01, n = 5) but did not significantly alter AP and CaT at the apex. K201 did not alter diastolic Ca2+ levels or the amplitude of CaTs. In the absence of K201 baseline drift recorded a percent change of fluorescence relative to the amplitude of CaT was −1.02 ± 0.26% per min, and in the presence of K201, the slope was −1.14 ± 0.16% per min (P = 0.19, n = 5 hearts). Similarly, the fractional change of fluorescence during a CaT, fractional fluorescence change (ΔF/F) was 0.434 ± 0.11 in the absence and 0.442 ± 0.126 in the presence of K201 (P = 0.24, n = 5).
Fig. 7.
Suppression of SCR by K201. A: change of mean APD80 (expressed as percentage of APD80 at steady-state BHR) as a function of time after transition to SHR recorded from 5 × 5 pixel regions at LVA (top) and RVB (bottom) before and after perfusion with 1 μM K201; traces of Cai and voltage (Vm) are superimposed for BHR and SHR without (top traces) and with K201 (arrow, bottom traces). A, right: summary data (n = 5 hearts) of the change in mean ΔAPD80 from the RVB without (control, Ctrl) and with K201 (1 μM). B: maps of APD80 (in ms) before (left) and after perfusion with K201 (1 μM; middle) at SHR. The percent change in APD80 dispersion between BHR and SHR is compared in the absence (Ctrl) and presence of K201.
K201 did not completely abolish APD adaptation but suppressed the difference in APD prolongation between apex and base. As shown in Fig. 7B, the suppression of SCR by K201 markedly reduced the dispersion of APD80 and DOR at SHR (P < 0.05, n = 5).
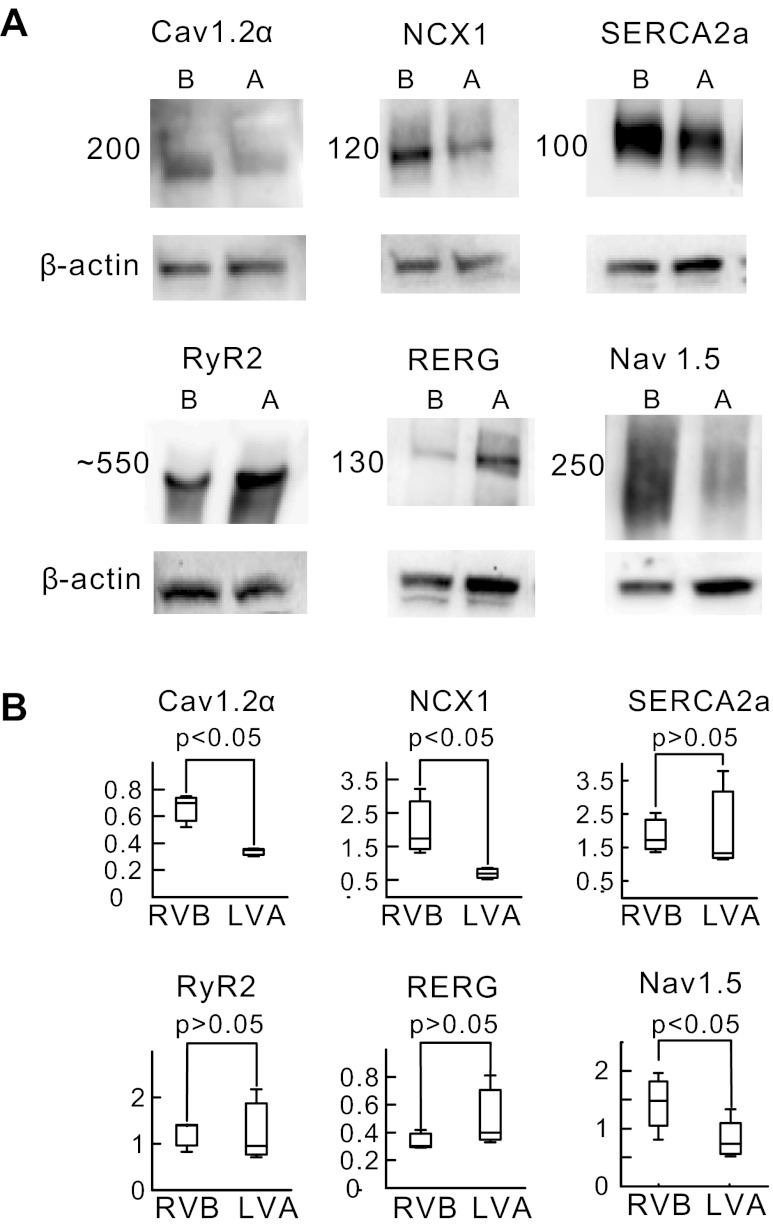
The molecular basis underlying the higher occurrence of SCR and diastolic Ca2+ waves at the base compared with the apex is most likely due to heterogeneities in the expression of ion channels and transporters. As shown in Fig. 8, the expression of the α-subunit of voltage-gated L-type Ca2+ channel protein, Cav1.2α; the predominant NCX protein found in the heart, NCX1, and voltage-gated Na+ channel, Nav1.5 proteins was significantly higher at the base (B) than the apex (A) (P < 0.05; n = 7). In contrast, the levels of SERCA2A and RyR2 were not significantly different and RERG had the opposite distribution, being higher at the apex than the base (P < 0.05; n = 6 hearts).
Fig. 8.
Heterogeneities of ion channels and transporter expression between base and apex Ventricular tissue was dissected from the epicardium of RVB (B) or LVA (A) and processed for Western blots as described in methods. Protein density was normalized with respect to β-actin to compare levels of channel proteins between RVA and LVA. A: relative density of RVB vs. LVA densities for voltage-gated L-type Ca2+ channel (Cav1.2α), Na+-Ca2+ exchange (NCX1; dominant isoform of NCX in heart), sarcoplasmic reticulum Ca2+ ATPase 2a (SERCA2A), ryanodine receptor 2 (RyR2), and rabbit ether-a-go-go-related gene (RERG). B: summary of density histograms shows statistically significant 2-fold upregulation of Cav1.2α, NCX1, and voltage-gated Na+ channel (Nav1.5) at RVB compared with LVA (P < 0.05, n = 7 hearts). There were no significant differences between RVB and LVA for SERCA2A, RyR2, and RERG (P > 0.05, n = 7 hearts).
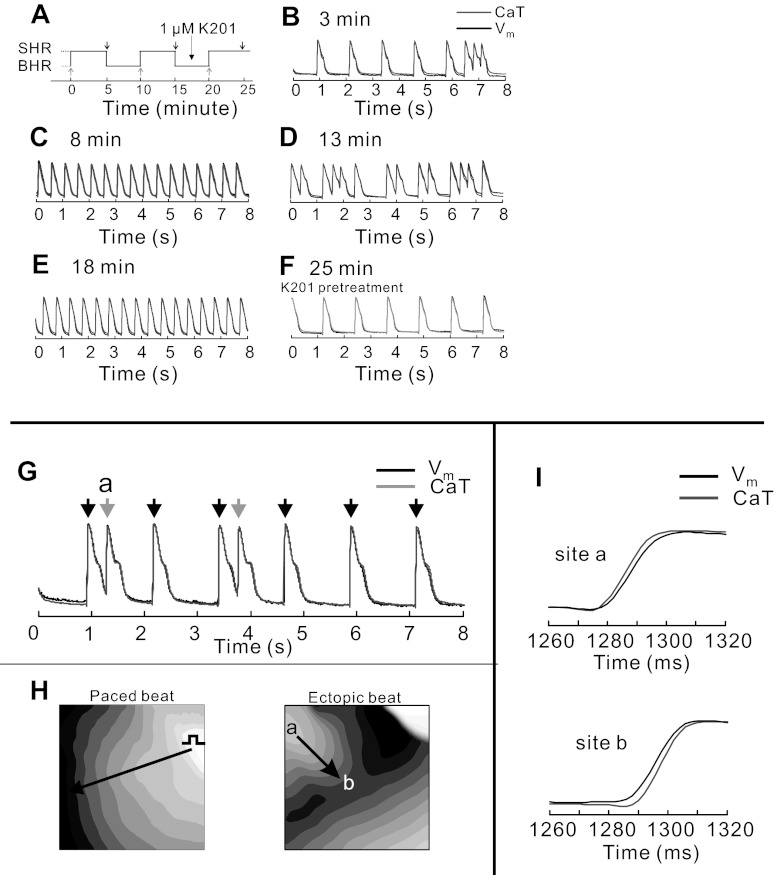
Role of SCR in bradycardia-induced ventricular ectopy.
We performed a systematic evaluation of relationship between SCR and ventricular ectopy. In 5 out of 15 hearts studied at low optical magnification, bradycardia alone was sufficient to produce premature ectopic beats that were eliminated by pacing at BHR or by inhibiting SCR with K201. In the five hearts that developed ectopic beats during bradycardia, ectopic beats appeared after a few minutes (Fig. 9, B and D) and were readily abolished by reverting back to BHR (Fig. 9, C and E). The addition of K201 had no effect on APs and CaT in BHR but blocked ectopic activity after 5 min of SHR (Fig. 9F). The kinetics of AP and CaT upstrokes were analyzed for paced (black arrows) and ectopic beats (gray arrows; Fig. 9G). Activation maps of the paced beats and ectopic beats show that paced beats were initiated at the location of the stimulus electrode and ectopic beats emanated from the RVB, leading to a wavefront that collided with the paced wavefront (Fig. 9H). For all paced beats, Vm preceded Cai (not shown) but Cai preceded Vm for all ectopic beats at their origins, located at the base and most often on the RVB (Fig. 9, H and I, site a). However, as the ectopic beat propagates away from its origin, Vm preceded Cai (site b; Fig. 9, H and I).
Fig. 9.
Role of SCR as a trigger of bradycardia-dependent ectopic beats. A: pacing protocol consisting of 2 cycles of BHR to SHR with 5-min intervals to achieve steady state followed by treatment with K201 and another cycle to examine the effects of RyR2 stabilization. Gray arrows (below trace) indicate the timing of changes in HR from BHR to SHR, and two black arrows (1st two arrows above trace) indicate changes from SHR to BHR. B and D: premature ectopic beats were reproducible during the 2 episodes of bradycardia at 3 min and 13 min of the experiment. C and E: termination of ectopic beats was consistently observed during the episode of BHR, shown here at 8- and 18-min time points. F: after pretreatment with K201 (1 μM), bradycardia-dependent ectopic beats were suppressed. G: APs and CaT measured from the RVB of a heart that developed ectopic activity during SHR (n = 5 out of 15 hearts). The paced beats are labeled with a black arrow and the spontaneous ectopic beats by gray arrows. H: activation maps of paced (left) and ectopic (right) beats. Paced beats were initiated at the stimulating electrode (left), and the first ectopic beat was initiated at site a on the map. Note the origins of the paced and ectopic beats and the collision of the ectopic and paced wavefronts. I: upstrokes of CaT and APs shown at fast sweep speeds to determine their relative kinetics. At the origin of the ectopic beat (site a), Cai preceded Vm (top traces), but farther from the origin of the ectopic wavefront (site b), Vm preceded Cai (bottom traces). During paced beats, Vm preceded Cai, as previously reported. During ectopic beats, Vm followed Cai with a variable delay ranging from 2–12 ms; however, the shift in the Cai to Vm dynamics was unmistakable.
DISCUSSION
HR is an important mechanism used by mammals to adjust cardiac output to changing demands. The adaptive role of tachycardia in a fight-or-flight situation is well accepted, as is the diminished myocardial energy consumption during bradycardia. Several parameters of cardiac contraction have to adjust to dynamic HR changes. At the cellular level, this requires an adaptation of APD and CaT in response to a change in HR.
This study showed that bradycardia changes in Ca2+ dynamics are spatially heterogeneous, being more pronounced at the base of the ventricles. Bradycardia elicits an SCR occurring during the AP plateau, which are synchronous within a given cell and within the myocytes in the high-power field of view. It also leads to diastolic SR Ca2+ release in the form of propagated Ca2+ waves, which are not synchronized among adjacent cells. Ca2+ imaging at low magnification demonstrated a tight correlation of SCR with enhanced APD. Interestingly, high-magnification Ca2+ imaging was required for detection of diastolic Ca2+ waves, since their detection was prevented by their lack of tight temporal synchronization and by spatial averaging over the multiple myocytes comprising a low-magnification pixel.
It is possible that the higher expression of Cav1.2α and NCX1 at the base of the heart reported here and elsewhere may partially explain the spatial heterogeneity of Ca2+ handling during bradycardia, e.g., by causing a higher degree of SR load at the ventricular base.
In principle, ectopic activity induced by bradycardia could be attributed to a spontaneous reactivation of ICa,L, independent of altered Ca2+ dynamics. Alternatively, bradycardia could promote SR Ca2+ overload and produce SCR that activates a depolarizing INCX, with INCX causing the initial depolarization, which results in ICa,L reactivation. In our opinion, compelling evidence implicates SCR as the initial trigger of EADs and ectopic activity in bradycardia. First, SCR frequently occurred with no apparent voltage deflection, and when EADs were present, the SCR onset preceded EAD onset by 5–10 ms at the site of the ectopic focus. Second, pharmacological intervention with K201, a RyR2 stabilizer, suppressed SCR, DOR, EADs, and ectopic activity, yet had no significant effect on APD and CaT at the apex or sites that had no SCR. The effect of K201 on SCR could not be attributed to an indirect effect on Ca2+ load, since K201 did not alter baseline Ca2+ compared with controls. Given the stability and signal quality of Cai measurements, K201 did not alter diastolic Cai.
Triggered activity in bradycardia and impaired repolarization.
Slow ventricular rate is an established proarrhythmic factor known to increase the propensity to TdP, an arrhythmia associated with delayed repolarization (40, 54). Abnormal Ca2+ handling and spontaneous SR Ca2+ release has been suggested as the mechanism of EADs, the form of triggered activity which underlies TdP. (18, 55, 62) We have recently reported that in rabbit hearts with LQT2, TdP elicited by bradycardia and delayed rectifying K+ current (IKr) blockade exhibited Cai oscillations during APD prolongation that preceded the appearance of EADs by minutes (38). The data suggest that Cai oscillations are caused by spontaneous SR Ca2+ release that activates a depolarizing INCX that serves as a trigger to EAD generation. This study was motivated by the need to elucidate the relationship between bradycardia, Ca2+ handling and arrhythmogenesis. We observed that bradycardia alone caused a lesser degree of SCR, sometimes manifested as delayed CaT downstroke rather than CaT reelevation. This could facilitate the generation of EADs when repolarization is impaired and occasionally suffice to trigger EADs on its own.
Bradycardia has long been known to prolong APDs and enhance DOR, setting the stage for functional reentry, but the mechanisms that enhance APD and DOR are not well understood. Here, we report that bradycardia produces the expected gradual increase of APDs and decrease of diastolic Cai. SCR contributes to spatial heterogeneities of APD and enhanced DOR, since its elimination reduces DOR.
Mechanism underlying SCR.
The mechanism whereby bradycardia promotes SCR is uncertain at this moment. In principle, increased diastolic interval during bradycardia should allow more time for Cai removal out of the cell by NCX. However, SR Ca2+ pumps can effectively compete with NCX for the removal of Cai during bradycardia. The amount of Cai transported to the lumen of the SR by SERCA during each cardiac cycle may actually be higher during bradycardia due to 1) longer AP plateau (perhaps a contribution of reduced IKr at the base), 2) longer duration of Ca2+ influx through L-type channels, and 3) the reduced NCX driving force during more positive plateau potentials. If there is a significant diffusion limitation of Ca2+ movement between the uptake and release compartments of the SR, the junctional SR may be replenished after the initial phase of Ca2+-induced Ca2+-release from the nonjunctional compartment during each heartbeat to a degree which allows spontaneous Ca2+ release through RyR2 in at least some myocytes. This process should be augmented by any intervention which prolongs plateau duration, such as IKr blockade.
Cai imaging shows that SCR occurs uniformly within each cell and is temporally synchronous among neighboring cells despite cell-to-cell differences in ASCR. The temporal synchronization can be attributed to similar time needed to overload the lumen of the SR network during long APDs due to incomplete voltage-dependent inactivation of L-type Ca2+ channels.
It is likely that SR loading is accentuated in regions with increased ICa,L density. This is consistent with the observation of SCR occurring preferentially at basal regions of ventricular myocardium. We previously reported that female rabbit hearts express higher protein levels of Cav1.2α and NCX1 and have significantly higher current densities of ICa,L and INCX (25–30%) at the base compared with the apex. The greater Ca2+ influx via ICa,L and reverse mode INCX lead to greater SR Ca2+ overload and a greater propensity to EADs in female myocytes isolated from the base compared with those isolated from the apex, endocardium or isolated from male hearts (17, 52, 65).
SCR prolong APD.
While AP prolongation caused by bradycardia may be the primary cause for SCR development, it appears likely that once SCRs occur, they may themselves prolong APD. We suppressed SCR with K201 to confirm their causative effect on APD prolongation. K201 has been found to stabilize the closed state of cardiac RyR (26, 33) and suppress the nonvoltage-triggered Ca2+ leak caused by RyR2 mutations or phosphorylation. K201 eliminated SCR and attenuated AP prolongation and DOR during bradycardia. Admittedly, K201 may have off-target effects which must be carefully considered. In guinea pig ventricular myocytes, K201 (1 μM) inhibited INa, ICa,L, and IKr in addition to its effect on RyR2 (30, 31); however, these off-target effect appear to be species dependent. In rabbit myocytes, K201 was specific for RyR2 at 1 μM: it reduced spontaneous SR Ca2+ release and Ca2+ waves without altering ICa,L and SR Ca2+ content (33).
Previous studies on Ca2+ dynamics and arrhythmias.
The role of Ca2+ handling abnormalities as a trigger of cardiac arrhythmias has been extensively investigated using Ca2+ imaging at the subcellular level, with most studies performed on isolated myocytes (8, 10, 46), Purkinje fibers (19, 28, 53), superfused myocardial trabeculae (34, 35, 37, 48), and myocyte monolayers (7, 36). Although intact perfused hearts provide significant advantages compared with isolated preparations to study arrhythmias, technical difficulties (e.g., greater susceptibility to motion artifact, scan rate, and signal-to-noise ratio) have made subcellular Ca2+ imaging challenging in this setting. At least three groups have now used confocal microscopy to study abnormalities of Ca2+ in various models of cardiac arrhythmias, such as ventricular hypertrophy, hypokalemia, rapid pacing, and delayed afterdepolarization after cessation of rapid pacing (1, 2, 6, 23). A common finding was that nonvoltage-triggered diastolic Ca2+ release often produced subcellular Ca2+ waves that appeared to play a crucial role in the genesis of arrhythmias. However, the role of abnormal Ca2+ dynamics will be difficult to analyze in other situations, such as ischemia, repolarization delay, or bradycardia, where longer intervals of data acquisition are needed at both high and low spatial resolution. Whereas confocal imaging in the line-scan mode reaches the necessary high sampling frequency, the field of view is relatively small and cannot be switched from line-scan to macroscopic imaging to track an arrhythmia throughout the intact heart. In addition, most confocal experiments were performed at room temperature because the fluo4 fluorescence diminishes above 30°C but hypothermia can have profound effects on Ca2+ dynamics. The method we used here overcomes some of these technical problems.
Study limitations.
This investigation studied the effects of acute bradycardia and may be less clinically relevant than animal models of chronic bradycardia where considerable tissue remodeling occurs (20, 58, 59). The study focused on the epicardium and did not investigate SCR from different regions of the ventricles. Although our previous studies demonstrated higher levels of ICa,L and INCX occurred at the base of the rabbit epicardium and not on the endocardium (17, 52), we cannot exclude the possibility of SCR in other regions of the heart. In pilot studies, optical maps of APs and CaT at high and low magnification from the endocardium did not find SCR before and during bradycardia. Unfortunately, optical recordings are still limited to the surface.
The study suggests that bradycardia causes an extra APD prolongation at the base by eliciting SCR and INCX augmentation. Several pharmacological agents can be used to suppress SCR by RyR2 inhibition. We used K201 because of its selectivity in rabbit hearts and because alternative agents flecainide and ranolazine also suppress SCR but have significant effects on the INa,L and ICa,L. Pilot studies tested pharmacological blockers of NCX to evaluate more directly its impact on APD prolongation during bradycardia. Unfortunately, the available NCX inhibitors are not sufficiently selective or effective at blocking NCX. Trials with SEA0400 (0.1–2 μM) to block the forward mode of NCX were inconclusive because at these concentrations, inhibition of NCX is partial, and at concentrations > 1 μM, ICa,L is progressively suppressed (11). An alternative approach of lowering external Na+ has been successfully used to inhibit INCX in isolated myocytes but cannot be applied fast enough in perfused hearts. Likewise, caffeine can be effectively used to estimate SR Ca2+ load in isolated myocytes but not in perfused hearts. Nevertheless, these limitations do not detract from the validity of the study which exemplifies what can be done at the intact heart level to fully appreciate heterogeneities and complexities that cannot be exposed in studies with isolated myocytes.
In summary, the study investigates acute abnormalities of myocardial Ca2+ handling caused by bradycardia, which increases propensity to ectopic activity and promotes arrhythmia. A recent elegant study has shown the role of remodeling of Ca2+ handling processes for arrhythmia in chronic bradycardia (58). At the subcellular level, the mechanisms of SCR clearly merit additional investigation. Clinically, it is possible that RyR2 stabilizers and NCX blockers could have a role in acute treatment of bradycardia-induced TdP, at least until pacing therapy can be instituted.
GRANTS
The study was supported in part by National Heart, Lung, and Blood Institute Grants HL-70722 and HL-093074 (to G. Salama) and a Three Rivers Affiliate of the American Heart Association Pre-doctoral Fellowship (to J. J. Kim) and Post-doctoral Fellowship (to R. Papp).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.J.K., J.N., and G.S. conception and design of research; J.J.K., J.N., R.P., R.S., J.J.A., and G.S. performed experiments; J.J.K., J.N., R.P., and G.S. analyzed data; J.J.K. prepared figures; J.J.K., J.N., J.J.A., and G.S. edited and revised manuscript; J.J.K., J.N., R.P., R.S., J.J.A., and G.S. approved final version of manuscript; J.N., J.J.A., and G.S. interpreted results of experiments; J.N. and G.S. drafted manuscript.
Supplementary Material
REFERENCES
- 1. Aistrup GL, Kelly JE, Kapur S, Kowalczyk M, Sysman-Wolpin I, Kadish AH, Wasserstrom JA. Pacing-induced heterogeneities in intracellular Ca2+ signaling, cardiac alternans, and ventricular arrhythmias in intact rat heart. Circ Res 99: e65–e73, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Aistrup GL, Shiferaw Y, Kapur S, Kadish AH, Wasserstrom JA. Mechanisms underlying the formation and dynamics of subcellular calcium alternans in the intact rat heart. Circ Res 104: 639–649, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Altamirano J, Bers DM. Effect of intracellular Ca2+ and action potential duration on L-type Ca2+ channel inactivation and recovery from inactivation in rabbit cardiac myocytes. Am J Physiol Heart Circ Physiol 293: H563–H573, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Arnold L, Page J, Attwell D, Cannell M, Eisner DA. The dependence on heart rate of the human ventricular action potential duration. Cardiovasc Res 16: 547–551, 1982 [DOI] [PubMed] [Google Scholar]
- 6. Baader AP, Buchler L, Bircher-Lehmann L, Kleber AG. Real time, confocal imaging of Ca2+ waves in arterially perfused rat hearts. Cardiovasc Res 53: 105–115, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Baker LC, London B, Choi BR, Koren G, Salama G. Enhanced dispersion of repolarization and refractoriness in transgenic mouse hearts promotes reentrant ventricular tachycardia. Circ Res 86: 396–407, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Berlin JR, Cannell MB, Lederer WJ. Cellular origins of the transient inward current in cardiac myocytes. Role of fluctuations and waves of elevated intracellular calcium. Circ Res 65: 115–126, 1989 [DOI] [PubMed] [Google Scholar]
- 9. Bers DM. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Beuckelmann DJ, Nabauer M, Erdmann E. Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation 85: 1046–1055, 1992 [DOI] [PubMed] [Google Scholar]
- 11. Birinyi P, Acsai K, Banyasz T, Toth A, Horvath B, Virag L, Szentandrassy N, Magyar J, Varro A, Fulop F, Nanasi PP. Effects of SEA0400 and KB-R7943 on Na+/Ca2+ exchange current and L-type Ca2+ current in canine ventricular cardiomyocytes. Naunyn Schmiedebergs Arch Pharmacol 372: 63–70, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Bonow RM, DL; Zipes DP, Libby P. Braunwald's Heart Disease (9th ed.). Philadelphia: Saunders, 2011 [Google Scholar]
- 13. Boyett MR, Jewell BR. A study of the factors responsible for rate-dependent shortening of the action potential in mammalian ventricular muscle. J Physiol 285: 359–380, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brandt RR, Shen WK. Bradycardia-induced polymorphic ventricular tachycardia after atrioventricular junction ablation for sinus tachycardia-induced cardiomyopathy. J Cardiovasc Electrophysiol 6: 630–633, 1995 [DOI] [PubMed] [Google Scholar]
- 15. Brembilla-Perrot B, Jacquemin L, Houplon P, Claudon O, Chivoret G, Vancon AC, Stenger C, Danchin N. Bradycardia-induced polymorphic ventricular tachycardia after radiofrequency catheter modification of atrioventricular junction. J Interv Card Electrophysiol 1: 153–155, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Bryant SM, Wan X, Shipsey SJ, Hart G. Regional differences in the delayed rectifier current (IKr and IKs) contribute to the differences in action potential duration in basal left ventricular myocytes in guinea-pig. Cardiovasc Res 40: 322–331, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Chen G, Yang X, Alber S, Shusterman V, Salama G. Regional genomic regulation of cardiac sodium-calcium exchanger by oestrogen. J Physiol 589: 1061–1080, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choi BR, Burton F, Salama G. Cytosolic Ca2+ triggers early afterdepolarizations and Torsade de Pointes in rabbit hearts with type 2 long QT syndrome. J Physiol 543: 615–631, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Drici MD, Baker L, Plan P, Barhanin J, Romey G, Salama G. Mice display sex differences in halothane-induced polymorphic ventricular tachycardia. Circulation 106: 497–503, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Dunnink A, van Opstal JM, Oosterhoff P, Winckels SK, Beekman JD, van der Nagel R, Cora Verduyn S, Vos MA. Ventricular remodelling is a prerequisite for the induction of dofetilide-induced torsade de pointes arrhythmias in the anaesthetized, complete atrio-ventricular-block dog. Europace 14: 431–436, 2012 [DOI] [PubMed] [Google Scholar]
- 21. Eisner DA, Dibb KM, Trafford AW. The mechanism and significance of the slow changes of ventricular action potential duration following a change of heart rate. Exp Physiol 94: 520–528, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Faber GM, Rudy Y. Action potential and contractility changes in [Na+]i overloaded cardiac myocytes: a simulation study. Biophys J 78: 2392–2404, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fujiwara K, Tanaka H, Mani H, Nakagami T, Takamatsu T. Burst emergence of intracellular Ca2+ waves evokes arrhythmogenic oscillatory depolarization via the Na+-Ca2+ exchanger: simultaneous confocal recording of membrane potential and intracellular Ca2+ in the heart. Circ Res 103: 509–518, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Guo D, Lian J, Liu T, Cox R, Margulies KB, Kowey PR, Yan GX. Contribution of late sodium current INa-L to rate adaptation of ventricular repolarization and reverse use-dependence of QT-prolonging agents. Heart Rhythm 8: 762–769, 2011 [DOI] [PubMed] [Google Scholar]
- 25. Haverkamp W, Hordt M, Breithardt G, Borggrefe M. Torsade de pointes secondary to d,l-sotalol after catheter ablation of incessant atrioventricular reentrant tachycardia—evidence for a significant contribution of the “cardiac memory”. Clin Cardiol 21: 55–58, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hilliard FA, Steele DS, Laver D, Yang Z, Le Marchand SJ, Chopra N, Piston DW, Huke S, Knollmann BC. Flecainide inhibits arrhythmogenic Ca2+ waves by open state block of ryanodine receptor Ca2+ release channels and reduction of Ca2+ spark mass. J Mol Cell Cardiol 48: 293–301, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hund TJ, Rudy Y. Rate dependence and regulation of action potential and calcium transient in a canine cardiac ventricular cell model. Circulation 110: 3168–3174, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Izopet J, Sandres-Saune K, Salama G, Pasquier C, Puel J, Rostaing L. [HCV nosocomial infections in hemodialysis]. Ann Biol Clin (Paris) 59: 7–8, 2001 [PubMed] [Google Scholar]
- 29. Kay GN, Plumb VJ, Arciniegas JG, Henthorn RW, Waldo AL. Torsade de pointes: the long-short initiating sequence and other clinical features: observations in 32 patients. J Am Coll Cardiol 2: 806–817, 1983 [DOI] [PubMed] [Google Scholar]
- 30. Kimura J, Kawahara M, Sakai E, Yatabe J, Nakanishi H. Effects of a novel cardioprotective drug, JTV-519, on membrane currents of guinea pig ventricular myocytes. Jpn J Pharmacol 79: 275–281, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Kiriyama K, Kiyosue T, Wang JC, Dohi K, Arita M. Effects of JTV-519, a novel anti-ischaemic drug, on the delayed rectifier K+ current in guinea-pig ventricular myocytes. Naunyn Schmiedebergs Arch Pharmacol 361: 646–653, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Lewis BH, Antman EM, Graboys TB. Detailed analysis of 24 hour ambulatory electrocardiographic recordings during ventricular fibrillation or torsade de pointes. J Am Coll Cardiol 2: 426–436, 1983 [DOI] [PubMed] [Google Scholar]
- 33. Loughrey CM, Otani N, Seidler T, Craig MA, Matsuda R, Kaneko N, Smith GL. K201 modulates excitation-contraction coupling and spontaneous Ca2+ release in normal adult rabbit ventricular cardiomyocytes. Cardiovasc Res 76: 236–246, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Menshikova EV, Salama G. Cardiac ischemia oxidizes regulatory thiols on ryanodine receptors: captopril acts as a reducing agent to improve Ca2+ uptake by ischemic sarcoplasmic reticulum. J Cardiovasc Pharmacol 36: 656–668, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Miedouge M, Devys A, Simon M, Rouzaud P, Salama G, Reyre J, Pujazon M, Carles P, Serre G. High levels of cytokeratin 19 fragments but no evidence of cytokeratins 1, 2, 10/11, 14 or filaggrin in the serum of squamous cell lung carcinoma patients. Tumour Biol 22: 19–26, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Miedouge M, Rouzaud P, Salama G, Pujazon MC, Vincent C, Mauduyt MA, Reyre J, Carles P, Serre G. Evaluation of seven tumour markers in pleural fluid for the diagnosis of malignant effusions. Br J Cancer 81: 1059–1065, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miura M, Boyden PA, ter Keurs HE. Ca2+ waves during triggered propagated contractions in intact trabeculae. Am J Physiol Heart Circ Physiol 274: H266–H276, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Nemec J, Kim JJ, Gabris B, Salama G. Calcium oscillations and T-wave lability precede ventricular arrhythmias in acquired long QT type 2. Heart Rhythm 7: 1686–1694, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nowinski K, Gadler F, Jensen-Urstad M, Bergfeldt L. Transient proarrhythmic state following atrioventricular junction radiofrequency ablation: pathophysiologic mechanisms and recommendations for management. Am J Med 113: 596–602, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Oka Y, Itoh H, Ding WG, Shimizu W, Makiyama T, Ohno S, Nishio Y, Sakaguchi T, Miyamoto A, Kawamura M, Matsuura H, Horie M. Atrioventricular block-induced Torsades de Pointes with clinical and molecular backgrounds similar to congenital long QT syndrome. Circ J 74: 2562–2571, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Papp Z, Peineau N, Szigeti G, Argibay J, Kovacs L. Calcium-dependent modulation of the plateau phase of action potential in isolated ventricular cells of rabbit heart. Acta Physiol Scand 167: 119–129, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Pruvot EJ, Katra RP, Rosenbaum DS, Laurita KR. Role of calcium cycling versus restitution in the mechanism of repolarization alternans. Circ Res 94: 1083–1090, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Qian YW, Clusin WT, Lin SF, Han J, Sung RJ. Spatial heterogeneity of calcium transient alternans during the early phase of myocardial ischemia in the blood-perfused rabbit heart. Circulation 104: 2082–2087, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Qian YW, Sung RJ, Lin SF, Province R, Clusin WT. Spatial heterogeneity of action potential alternans during global ischemia in the rabbit heart. Am J Physiol Heart Circ Physiol 285: H2722–H2733, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Salama G. Arrhythmia genesis: aberrations of voltage or Ca2+ cycling? Heart Rhythm 3: 67–70, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Salama G, Choi BR. Images of action potential propagation in heart. News Physiol Sci 15: 33–41, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Salama G, Hwang SM. Simultaneous optical mapping of intracellular free calcium and action potentials from Langendorff perfused hearts. Curr Protoc Cytom 12: 12.–17., 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Salama G, Menshikova EV, Abramson JJ. Molecular interaction between nitric oxide and ryanodine receptors of skeletal and cardiac sarcoplasmic reticulum. Antioxid Redox Signal 2: 5–16, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Seethala S, Shusterman V, Saba S, Mularski S, Nemec J. Effect of beta-adrenergic stimulation on QT interval accommodation. Heart Rhythm 8: 263–270, 2011 [DOI] [PubMed] [Google Scholar]
- 50. Shimozono S, Fukano T, Nagai T, Kirino Y, Mizuno H, Miyawaki A. Confocal imaging of subcellular Ca2+ concentrations using a dual-excitation ratiometric indicator based on green fluorescent protein. Sci STKE 2002: pl4, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Shkryl VM, Blatter LA, Rios E. Properties of Ca2+ sparks revealed by four-dimensional confocal imaging of cardiac muscle. J Gen Physiol 139: 189–207, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sims C, Reisenweber S, Viswanathan PC, Choi BR, Walker WH, Salama G. Sex, age, and regional differences in L-type calcium current are important determinants of arrhythmia phenotype in rabbit hearts with drug-induced long QT type 2. Circ Res 102: e86–e100, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stuyvers BD, Dun W, Matkovich S, Sorrentino V, Boyden PA, ter Keurs HE. Ca2+ sparks and waves in canine purkinje cells: a triple layered system of Ca2+ activation. Circ Res 97: 35–43, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Subbiah RN, Gollob MH, Gula LJ, Davies RW, Leong-Sit P, Skanes AC, Yee R, Klein GJ, Krahn AD. Torsades de pointes during complete atrioventricular block: genetic factors and electrocardiogram correlates. Can J Cardiol 26: 208–212, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Szabo B, Sweidan R, Rajagopalan CV, Lazzara R. Role of Na+:Ca2+ exchange current in Cs+-induced early afterdepolarizations in Purkinje fibers. J Cardiovasc Electrophysiol 5: 933–944, 1994 [DOI] [PubMed] [Google Scholar]
- 56. Thomsen MB, Verduyn SC, Stengl M, Beekman JD, de Pater G, van Opstal J, Volders PG, Vos MA. Increased short-term variability of repolarization predicts d-sotalol-induced torsades de pointes in dogs. Circulation 110: 2453–2459, 2004 [DOI] [PubMed] [Google Scholar]
- 57. Topilski I, Rogowski O, Rosso R, Justo D, Copperman Y, Glikson M, Belhassen B, Hochenberg M, Viskin S. The morphology of the QT interval predicts torsade de pointes during acquired bradyarrhythmias. J Am Coll Cardiol 49: 320–328, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Tsuji Y, Hojo M, Voigt N, El-Armouche A, Inden Y, Murohara T, Dobrev D, Nattel S, Kodama I, Kamiya K. Ca2+-related signaling and protein phosphorylation abnormalities play central roles in a new experimental model of electrical storm. Circulation 123: 2192–2203, 2011 [DOI] [PubMed] [Google Scholar]
- 59. Tsuji Y, Opthof T, Yasui K, Inden Y, Takemura H, Niwa N, Lu Z, Lee JK, Honjo H, Kamiya K, Kodama I. Ionic mechanisms of acquired QT prolongation and torsades de pointes in rabbits with chronic complete atrioventricular block. Circulation 106: 2012–2018, 2002 [DOI] [PubMed] [Google Scholar]
- 60. Verduyn SC, Vos MA, van der Zande J, van der Hulst FF, Wellens HJ. Role of interventricular dispersion of repolarization in acquired torsade-de-pointes arrhythmias: reversal by magnesium. Cardiovasc Res 34: 453–463, 1997 [DOI] [PubMed] [Google Scholar]
- 61. Viswanathan PC, Shaw RM, Rudy Y. Effects of IKr and IKs heterogeneity on action potential duration and its rate dependence: a simulation study. Circulation 99: 2466–2474, 1999 [DOI] [PubMed] [Google Scholar]
- 62. Volders PG, Vos MA, Szabo B, Sipido KR, de Groot SH, Gorgels AP, Wellens HJ, Lazzara R. Progress in the understanding of cardiac early afterdepolarizations and torsades de pointes: time to revise current concepts. Cardiovasc Res 46: 376–392, 2000 [DOI] [PubMed] [Google Scholar]
- 63. Wehrens XH, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, Coromilas J, Landry DW, Marks AR. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science 304: 292–296, 2004 [DOI] [PubMed] [Google Scholar]
- 64. Wilson LD, Jeyaraj D, Wan X, Hoeker GS, Said TH, Gittinger M, Laurita KR, Rosenbaum DS. Heart failure enhances susceptibility to arrhythmogenic cardiac alternans. Heart Rhythm 6: 251–259, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yang X, Chen G, Papp R, Defranco DB, Zeng F, Salama G. Oestrogen upregulates L-type Ca2+ channels via oestrogen-receptor- by a regional genomic mechanism in female rabbit hearts. J Physiol 590: 493–508, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.