Abstract
Hemorrhagic transformation is an important complication of acute ischemic stroke, particularly in diabetic patients receiving thrombolytic treatment with tissue plasminogen activator, the only approved drug for the treatment of acute ischemic stroke. The objective of the present study was to determine the effects of acute manipulation of potential targets for vascular protection [i.e., NF-κB, peroxynitrite, and matrix metalloproteinases (MMPs)] on vascular injury and functional outcome in a diabetic model of cerebral ischemia. Ischemia was induced by middle cerebral artery occlusion in control and type 2 diabetic Goto-Kakizaki rats. Treatment groups received a single dose of the peroxynitrite decomposition catalyst 5,10,15,20-tetrakis(4-sulfonatophenyl)prophyrinato iron (III), the nonspecific NF-κB inhibitor curcumin, or the broad-spectrum MMP inhibitor minocycline at reperfusion. Poststroke infarct volume, edema, hemorrhage, neurological deficits, and MMP-9 activity were evaluated. All acute treatments reduced MMP-9 and hemorrhagic transformation in diabetic groups. In addition, acute curcumin and minocycline therapy reduced edema in these animals. Improved neurological function was observed in varying degrees with treatment, as indicated by beam-walk performance, modified Bederson scores, and grip strength; however, infarct size was similar to untreated diabetic animals. In control animals, all treatments reduced MMP-9 activity, yet bleeding was not improved. Neuroprotection was only conferred by curcumin and minocycline. Uncovering the underlying mechanisms contributing to the success of acute therapy in diabetes will advance tailored stroke therapies.
Keywords: minocycline; curcumin; 5,10,15,20-tetrakis(4-sulfonatophenyl)prophyrinato iron (III); nuclear factor-κB; vascular protection
within the next decade, the number of individuals living with diabetes is expected to rise dramatically (8). By 2030, the estimated global prevalence of the disease will exceed 437 million (53), and the vascular damage sustained during the course of the disease will increase the likelihood that these affected individuals will develop micro- and macrovascular complications, including acute ischemic stroke (AIS) (41). Historically, individuals 65 yr and older are disproportionately affected, but for the first time ever, there is an alarming increase in the number of Americans who are diagnosed at a younger age and face a prolonged course of diabetes (46). This trend poses a serious problem because recent findings suggest that the risk of AIS increases 3% with each year of diabetes and triples after 10 yr (5). In addition, diabetes complicates ischemic injury, leading to increased morbidity and poor functional recovery (7), but how diabetes worsens ischemic stroke is not fully delineated. Understanding these subtleties is essential in identifying targets for neurovascular protection and for developing therapeutic strategies tailored to this burgeoning at-risk population.
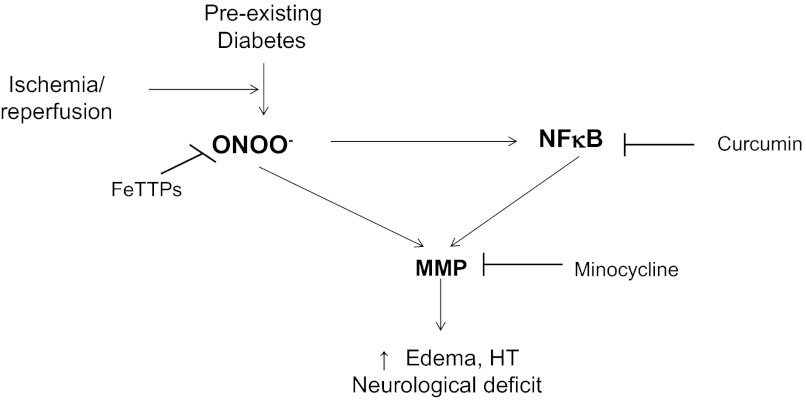
In a series of studies, we showed that there is extensive cerebrovascular remodeling and dysfunctional neovascularization that is characterized by increased matrix metalloproteinase (MMP)-2 and -9 activity in a lean and mild model of type 2 diabetes (T2D) (18, 28). Preexisting diabetes in conjunction with ischemia-reperfusion injury causes a rapid loss of myogenic tone via increased oxidative stress (i.e., peroxynitrite) (35), augments hemorrhagic transformation (HT), and worsens functional outcome despite the relatively smaller infarct size (19). Based on these findings, we asked the following question: “Does acute peroxynitrite scavenging improve stroke outcomes?” Proinflammatory responses initiated during ischemia-reperfusion involve the activation of the nuclear transcription factor NF-κB, which is responsible for upregulating genes coding for cytokines and growth factors and can directly modulate MMP activity (25, 47). Accordingly, the present study investigated the effect of NF-κB inhibition on MMP activity and neurovascular injury and outcomes in diabetic stroke. Moreover, prevention of cerebrovascular remodeling by glycemic control with metformin or MMP inhibition with minocycline started at the onset of diabetes reduces HT and improves outcome (18). Given that MMPs, and especially MMP-9, are associated with the breakdown of the blood-brain barrier (BBB) (3, 24) and ensuing HT in experimental models of stroke that used normoglycemic animals (12, 55), in the present study, we hypothesized that pharmacological inhibition of MMP-9 at reperfusion either directly or indirectly by inhibition of NF-κB or peroxynitrite will prevent HT and improve functional outcomes in diabetes (Fig. 1).
Fig. 1.
Schematic of experimental hypothesis. Excess peroxynitrite formation and inflammation during ischemia-reperfusion injury in preexisting diabetes amplifies the proteolytic activity of matrix metalloproteinases (MMPs), thereby contributing to greater vascular injury [i.e., edema and hemorrhagic transformation (HT)] and neurological deficit. FeTTPs, 5,10,15,20-tetrakis(4-sulfonatophenyl)prophyrinato iron (III).
METHODS
Animals.
In previous studies, our group has shown that 3 h of middle cerebral artery (MCA) occlusion (MCAO) and 21 h of reperfusion coincided with the development of smaller infarcts and greater vascular injury (i.e., edema and HT) in the T2D Goto-Kakizaki (GK) rat model compared with normoglycemic control rats (18, 19, 38). Therefore, we selected this nonobese model of diabetes to study the effects of acute NF-κB and MMP inhibition on edema, HT, and neurological outcomes after transient focal cerebral ischemia. This spontaneously diabetic rat strain was derived from the repeated in-breeding of glucose-intolerant Wistar rats (27). For this reason, we chose to use weight-matched Wistar rats as normoglycemic control rats in these experiments. Glucose intolerance can be observed in GK rats as early as 2 wk of age in these animals, and the onset of moderate hyperglycemia can be as early as 5–6 wk of age (26). During weeks 6–12, plasma insulin levels are elevated and then decrease to levels lower than those observed in Wistar rats (27, 28). In contrast to the severely hyperglycemic streptozotocin-induced rat model of diabetes, whose glucose levels are typically above 300 mg/dl (34, 42), GK rats are moderately hyperglycemic, with plasma glucose levels similar to what has been observed in stroke patients (7, 33, 35). The colonies used for the experiments outlined in this study (in-house bred or purchased from the Tampa colony, Taconic, Hudson, NY) have been shown to be neither hyperlipidemic nor hypertensive (16, 28). Comorbidities, such as kidney or heart damage, are not present in these animals and can be ruled out as potential confounders of the stroke data.
Weight-matched (280–320 g) male normoglycemic Wistar (n = 53, Harlan, Indianapolis, ID) and chronically diabetic GK (n = 46) rats were used in the experiments in this study. Animals were housed at the Georgia Regents University Augusta animal care facility, which is approved by the American Association for Accreditation of Laboratory Animal Care. All protocols were approved by the Institutional Animal Care and Use Committee. Animals were fed standard rat chow and tap water ad libitum. Body weights and blood glucose measurements were taken biweekly. Blood glucose measurements were taken from tail vein samples using a commercially available glucometer (Freestyle, Abbott Diabetes Care, Alameda, CA). Mean arterial pressure (in mmHg) was measured using the tail-cuff method.
Experimental cerebral ischemia.
Focal cerebral ischemia was achieved using the monofilament suture MCAO model previously described by our group and others (17, 39). Fagan et al. (20) previously reported that the duration of occlusion required to observe HT in 50% of animals was 3 h. For this reason, we chose to use this duration of ischemia to evaluate the end points of this experimental stroke study. Briefly, all animals were anesthetized by inhalation with 5% isoflurane in pure oxygen gas. After induction, 2.5% isoflurane was maintained for the duration of the surgery. The MCA was occluded with an 18- to 25-mm 4-0 surgical nylon monofilament by advancing the suture into the internal carotid artery to block the origin of the MCA. Laser-Doppler imaging (Perimed, North Royalton, OH) was used to confirm successful occlusion and ensure similar levels of blood flow reduction in all groups. After 3 h of occlusion, the suture was removed, and restoration of blood flow was confirmed by laser-Doppler imaging. The peroxynitrite decomposition catalyst 5,10,15,20-tetrakis(4-sulfonatophenyl)prophyrinato iron (III) (FeTPPs; 10 mg/kg ip, Calbiochem, San Diego, CA) (6), the nonspecific MMP inhibitor minocycline (20 mg/kg ip, Sigma-Aldrich, St. Louis, MO) (60), or the Curcuma longa derivative curcumin (250 mg/kg ip in ethyl oleate, Sigma-Aldrich) (37) was administered in a single dose immediately after reperfusion.
Assessment of infarct size, edema, and HT.
Twenty-four hours after MCAO, all animals were anesthetized with pentobarbital sodium (Fatal-Plus, Vortech Pharmaceuticals; Dearborn, MI) and perfused with saline, and brains were extracted after euthanization. The brain was placed in a plastic mold (Braintree Scientific, Braintree, MA) and sliced into 2-mm slices in the coronal plane (labeled as slices A–G, front to back). Macroscopic bleeding was identified in unstained tissue slices. Brain slices were then stained with a 2% solution of 2,3,5-triphenyltetrazolium chloride (TTC; Sigma-Aldrich) to evaluate tissue viability and delineate the infarcted area. Images of the stained brain slices were captured using a digital scanner, and SPOT Advanced 3.4 software (Diagnostic Instruments, Sterling Heights, MI) was used to quantify grossly visible infarction zones. The infarct volume was determined as a percentage of the ipsilateral (ischemic) hemisphere. Edema was calculated as the difference between the ipsilateral and contralateral (nonischemic) hemispheres divided by the infarct volume. HT was evaluated by two different methods, including measurement of macroscopic and microscopic bleeding into the brain. Severity of bleeding was determined using a four-point rubric modified from Crumrine et al. (11). Visual inspection of bleeding was identified in slices B–E only. A blinded investigator scored macroscopic bleeding in each slice (where 0 = normal ischemic damage or hemorrhage, 1 = dispersed individual petechiae, 2 = confluent petechiae, 3 = small diffuse hemorrhage or hematoma, and 4 = large diffuse hemorrhage or hematoma), and the total score for each animal was reported. Microscopic bleeding was quantified using a colorimetric hemoglobin detection assay (QuantiChrom Hemoglobin Assay Kit, BioAssay Systems, Haywood, CA). First, TTC-stained brain samples were homogenized in a 10% glycerol-Tris-buffered saline solution containing Tween 20. Samples were prepared and read at 562 nm using a standard microplate reader, and the hemoglobin concentration was calculated according to the manufacturer's instructions. The color intensity of the three drugs used in the study interfered with the results of the colorimetric assay; therefore, all values were normalized with respect to the concentrations detected in the brains of nonstroked animals receiving the corresponding treatment.
Neurological assessment.
A battery of tests was performed to evaluate neurological function at baseline and at 24 h after stroke (just before euthanization). These included the seven-point beam walk described by Feeney et al. (22) and measurement of grip strength using a digital grip strength meter (Columbus Instruments, Columbus, OH) (30). A modified binary Bederson scoring system was also used to detect pathological postural reflexes (i.e., no resistance to push, circling, forearm flexion, and hindlimb extension).
MMP activity.
For each treatment group, cerebral macrovessels from the nonischemic and ischemic brain hemispheres were isolated, homogenized, and analyzed for MMP-2 and -9 activity using gelatin zymography, as we have previously described (18, 28). Recombinant MMP-2 and -9 proteins (Calbiochem) were run in parallel with all samples, and the band intensity on zymogram gels was normalized to that of the standard to prevent gel-to-gel variability. Gelatinolytic activity was assessed by densitometric analysis (Gel-Pro version 3.1, Media Cybernetics, Carlsbad, CA).
Statistical analysis.
Data are expressed as means ± SE. Data were evaluated for normality, and the appropriate transformations were used when necessary. Due to skewed distributions and small sample sizes, a rank transformation was used before analysis for infarct size and HT. A log transformation was used to stabilize the variance as a function of the mean for MMP-9 activity. Grip strength deficit percent was calculated as grip strength at baseline minus grip strength post-MCAO divided by the baseline value. A 2 × 2 ANOVA was used to study the effect of disease (Wistar vs. GK) and treatment (untreated vs. FeTTPs or curcumin or minocycline) and their interaction on infarct size, edema, HT, neurological assessments, grip strength deficit percent, MMP-9 activity, and blood flow. A significant interaction indicates that a treatment had a different effect on an outcome dependent on disease status. A Tukey's test was used to adjust for the multiple comparisons to assess significant interaction effects from all analyses. For analysis of mortality, a Fisher's exact test was performed, due to small frequencies, within each treatment to examine whether mortality rates were different in control and diabetic mice. Zelens' exact test for homogeneity was used to examine whether the mortality rates were homogeneous across treatments. If mortality was homogeneous across treatments, an exact Cochran-Mantel-Haenszel χ2-test was used to determine whether there was a general difference in mortality between control and diabetic rats. There was not enough variation in blood score in Wistar animals for analysis. Blood score was analyzed for GK animals only as a Mantel-Haenszel test for trend for comparisons of untreated versus FeTTPs or curcumin or minocycline. Statistical significance was determined at α = 0.05. SAS version 9.3 was used for all analyses (SAS Institute, Cary, NC).
RESULTS
Metabolic parameters and mortality in experimental animals.
Average body weights and blood glucose values before MCAO surgery are shown in Table 1. Weight-matched (270–320 g) diabetic animals displayed moderate levels of hyperglycemia compared with normoglycemic control animals. Furthermore, there were no differences in poststroke mortality rates between groups.
Table 1.
Metabolic parameters and mortality in experimental stroke groups
| Untreated |
FeTPPs (10 mg/kg ip) |
Curcumin (250 mg/kg ip) |
Minocycline (20 mg/kg ip) |
|||||
|---|---|---|---|---|---|---|---|---|
| NG | D | NG | D | NG | D | NG | D | |
| Body weight, g | 293 ± 4 | 307.6 ± 3 | 299.4 ± 7 | 307.2 ± 5 | 285 ± 8 | 315.4 ± 5 | 314.6 ± 4 | 298.6 ± 4 |
| Blood glucose, mg/dl | 102 ± 4 | 241 ± 22* | 105 ± 5 | 255 ± 16* | 100 ± 5 | 161 ± 14* | 85 ± 3 | 259 ± 14 |
| Mortality, no. of animals/total (%) | 3/15 (20) | 1/16 (6.3) | 3/8 (37.5) | 0/6 (0) | 2/13 (15.4) | 1/12 (8.3) | 4/17 (23.5) | 2/12 (16.7) |
Results are given as means ± SE. NG, normoglycemia; D, diabetes; FeTPPs, 5,10,15,20-tetrakis(4-sulfonatophenyl)prophyrinato iron (III).
P < 0.05 vs. untreated NG.
Evaluation of HT.
Arterial blood gases in control and diabetic groups after the induction of anesthesia before MCAO were pH 7.5 ± 0.003 versus 7.5 ± 0.003, Pco2 42.9 ± 1.4 versus 51.5 ± 1.5 mmHg, and Po2 522.8 ± 22.1 versus 545.7 ± 22.4 mmHg. Whereas there were no differences in the percent drop in cerebral perfusion right after occlusion, the percent increase in flow in the first 5–10 min of reperfusion was significantly lower in all experimental diabetic groups compared with normoglycemic groups (Table 2).
Table 2.
Percent changes in cerebral perfusion after occlusion and reperfusion in experimental stroke groups
| Untreated |
FeTPPs (10 mg/kg ip) |
Curcumin (250 mg/kg ip) |
Minocycline (20 mg/kg ip) |
|||||
|---|---|---|---|---|---|---|---|---|
| NG | D | NG | D | NG | D | NG | D | |
| Percent decrease Postocclusion | 45 ± 2 | 47 ± 2 | 43 ± 2 | 53 ± 7 | 40 ± 6 | 42 ± 5 | 46 ± 2 | 46 ± 5 |
| Percent increase Postreperfusion | 29 ± 2 | 13 ± 5* | 27 ± 3 | 9 ± 13* | 25 ± 8 | 13 ± 6* | 23 ± 3 | 16 ± 7* |
Results are given as means ± SE.
P < 0.01 vs. NG.
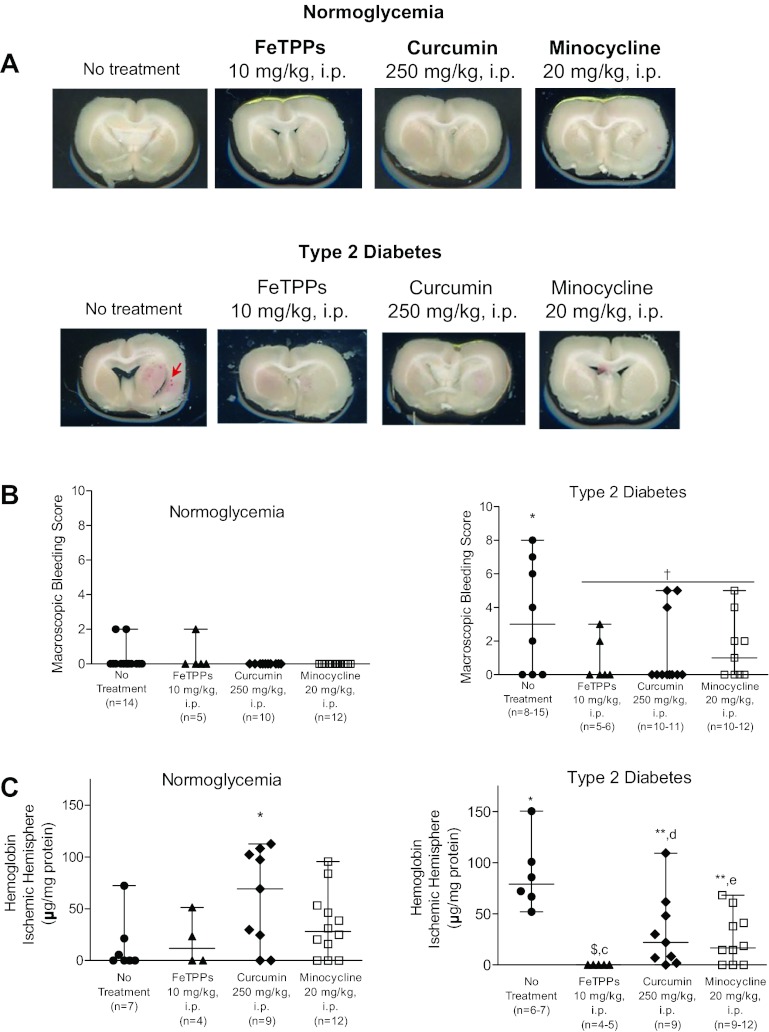
Unstained brain slices from each animal were examined for evidence of stroke-induced macroscopic bleeding (Fig. 2, A and B). Twenty-one hours after ischemia, there was minimal bleeding in normoglycemic groups, primarily in the form of diffuse individual petechiae. Since a large number of animals had no bleeding (a score of 0), vehicle versus treatment groups were not statistically compared. Evaluation of macroscopic bleeding in T2D rats indicated that macroscopic bleeding was more severe compared with untreated normoglycemic rats (P < 0.05; Fig. 2B). There was a trend for decreased macroscopic bleeding by all treatments (P = 0.71). Microscopic bleeding in the ischemic hemisphere was also measured as indicator of HT in all experimental groups (Fig. 2C). Poststroke microscopic bleeding was present in untreated normoglycemic animals. Furthermore, stroke-induced microscopic bleeding was similar in all treated normoglycemic animals except those receiving curcumin therapy. Surprisingly, single-dose administration of curcumin at reperfusion exacerbated bleeding in normoglycemic animals (P < 0.05 vs. untreated animals). Similarly, significantly higher levels of hemoglobin were detected in untreated diabetic animals compared with their normoglycemic counterparts (P < 0.05; Fig. 2C). Administration of FeTPPs at reperfusion reduced microscopic bleeding to undetectable levels. Curcumin and minocycline therapies were also effective in reducing microscopic bleeding in diabetic animals (P < 0.05 vs. untreated diabetic animals). Analysis of the bleeding data using 2 × 2 ANOVA indicated that a disease and drug interaction such that both acute treatment with FeTPPs and minocycline reduced microscopic bleeding in diabetic animals but not in the corresponding normoglycemic animals (P = 0.0003 and P = 0.0011, respectively; Fig. 2C). Curcumin, on the other hand, augmented microscopic bleeding in the ischemic brain hemispheres of control animals but reduced bleeding in diabetic animals (P = 0.0016).
Fig. 2.
Detection of macroscopic and microscopic bleeding as an indicator of vascular integrity in normoglycemic and diabetic animals after stroke. A: representative images of unstained brain slices (slice D was selected for each experimental group). B: small amounts of macroscopic bleeding were detected in unstained brain slices from normoglycemic animals. Results from the visual inspection of macroscopic bleeding in unstained brain slices indicated that poststroke bleeding was more severe in untreated diabetic animals compared with untreated normoglycemic animals, and all treatments showed a trend for reduced macroscopic bleeding. C: quantitative analysis of HT was reported as hemoglobin concentration in the ischemic hemispheres of experimental stroke groups. Results from the colorimetric hemoglobin detection assay were normalized by the absorbance of the drug in the brains of nonstroked animals for each group. Low levels of microscopic bleeding were measured in the ischemic hemispheres of normoglycemic animals. These levels remained unchanged by FeTPPs and minocycline therapies but were worsened in animals receiving curcumin treatment at reperfusion. HT, as measured by hemoglobin concentration, was greater in untreated diabetic animals. Acute treatment with FeTPPs, curcumin, and minocycline at reperfusion reduced bleeding in diabetic animals after ischemic stroke. Results are given as means ± SE; n = 4–13. $All values in this data set were below minimum detection levels; *P < 0.05 vs. untreated normoglycemic animals; **P < 0.05 vs. untreated diabetic animals, †P = 0.071 vs. untreated diabetic animals; cP = 0.0003, dP = 0.0016, and eP = 0.0011, disease by treatment interaction compared with untreated normoglycemic and diabetic animals.
Evaluation of infarct and edema.
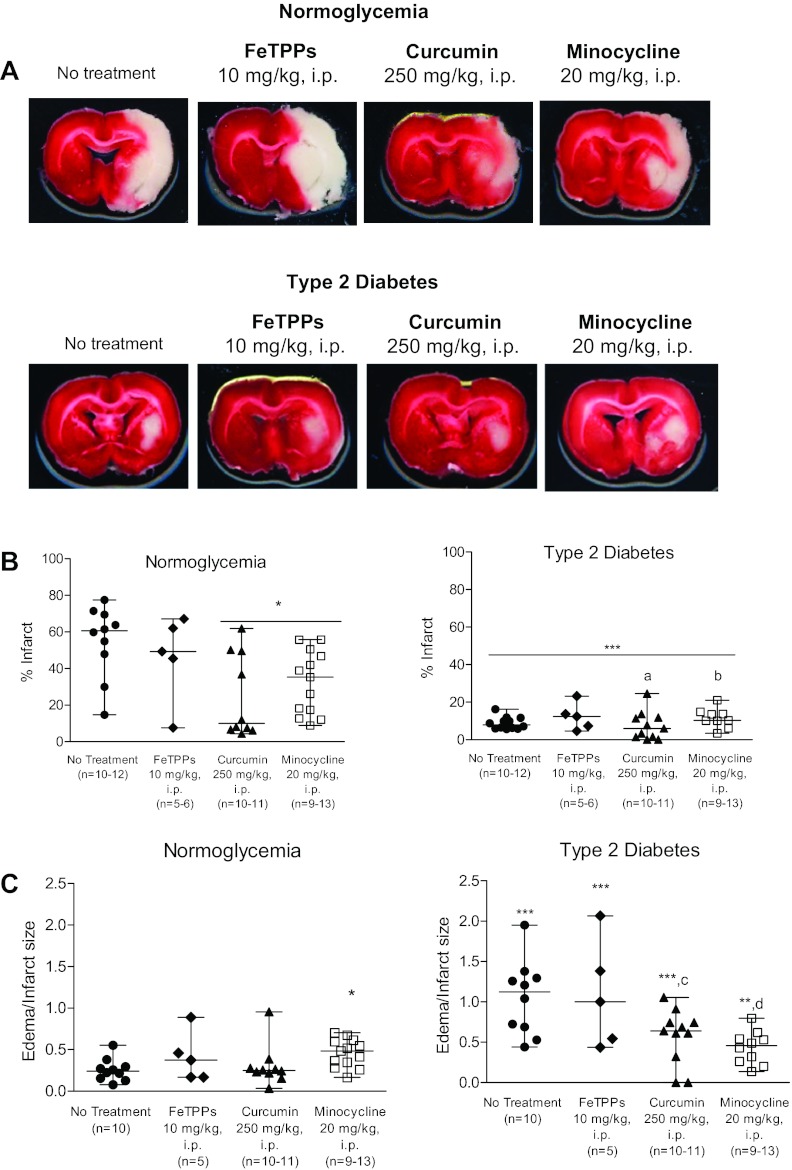
Infarcted regions were identified on TTC-stained brain slices to determine the extent of neuronal damage (Fig. 3A). Infarct size, expressed as a percentage of the total ischemic hemisphere, was determined for all experimental groups (Fig. 3B). FeTPPs therapy did not alter the extent of neuronal damage in normoglycemic animals; however, both curcumin and minocycline treatments reduced infarct size by ∼20% (P < 0.05 vs. untreated normoglycemic animals). Edema formation, expressed as edema/infarct size, was quantified as the percent difference between ischemic and nonischemic hemispheres, correcting for ischemic lesion volume. Stroke-induced edema formation was similar in all normoglycemic treatment groups, with the exception of animals receiving minocycline at reperfusion (Fig. 3C); brain edema in that group was slightly higher (P < 0.05 vs. untreated normoglycemic animals). As we have previously shown, infarcted tissue volume was ∼40% smaller in brains extracted from diabetic animals compared with untreated normoglycemic animals (P < 0.001; Fig. 3B). Furthermore, the infarcted zones were typically confined to the striatum in diabetic animals (Fig. 3A), whereas ischemic lesions in normoglycemic animals were found in both striatal and cortical brain regions (Fig. 3A). Infarct size in diabetic animals did not change with acute FeTPPs, curcumin, or minocycline therapy. Edema was greater in brains from untreated diabetic animals compared with their untreated normoglycemic counterparts (P < 0.001). Edema formation was not reduced by acute FeTPPs treatment; however, curcumin and minocycline therapies mitigated brain edema in diabetic animals. A disease-treatment interaction was detected with curcumin such that it reduced edema in diabetic animals but not normoglycemic animals (P = 0.0013). Similarly, acute minocycline treatment reduced edema in diabetic animals but not in normoglycemic animals (P = 0.0001).
Fig. 3.
Evaluation of infarct volume and edema in normoglycemic and diabetic experimental stroke groups. A: representative digital images of necrotic tissue (white) in 2,3,5-triphenyltetrazolium chloride-stained coronal brain slices (slice D was selected for each group). B: quantification of infarct size. Untreated normoglycemic animals developed infarcts that occupied ∼60% of the total ischemic hemisphere. Single-dose administration of either curcumin or minocycline reduced infarct volume in normoglycemic animals; FeTPPs treatment had no effect. Diabetic animals consistently developed smaller infarcts than normoglycemic animals. Single-dose administration of FeTPPs, curcumin, or minocycline at reperfusion did not alter the magnitude of infarcted brain tissue in diabetic animals. C: ischemic stroke induced brain edema in normoglycemic animals. Acute treatment with FeTTPs or curcumin had no effect on edema formation in normoglycemic animals; however, there was a slight increase in brain edema with minocycline therapy. Edema was greater in untreated diabetic animals compared with untreated normoglycemic animals. Edema formation in animals with preexisting diabetes was not reduced by FeTPPs or curcumin when administered in a single dose at reperfusion. Acute minocycline treatment effectively reduced brain edema in diabetic animals. Results are given as means ± SE; n = 5–13. *P < 0.05 vs. untreated normoglycemic or diabetic animals; ***P < 0.001 vs. untreated normoglycemic animals; aP = 0.0033, bP = 0.016, cP = 0.0013, and dP = 0.0001, disease by treatment interaction compared with untreated control and diabetic animals.
Neurological assessment.
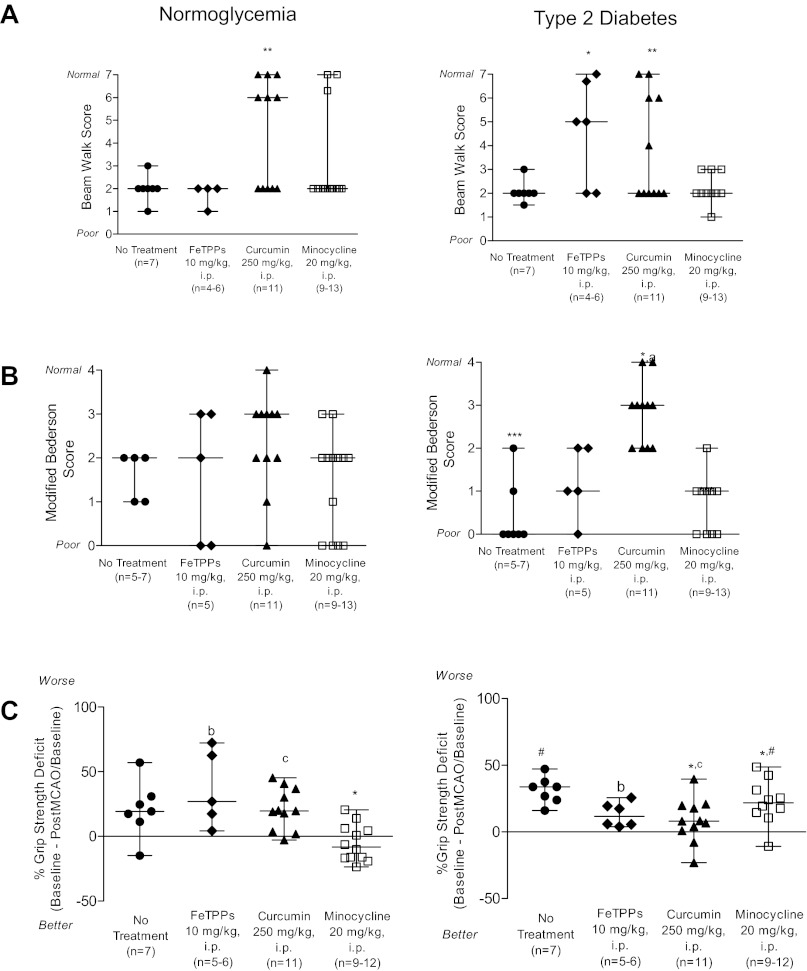
A beam walk test was used to observe vestibulomotor function after stroke in control and diabetic animals (Fig. 4A). Postoperative beam walk performance was poor in untreated normoglycemic animals; these animals could not traverse the beam, but they could stay balanced. Acute treatment with FeTPPs or minocycline did not improve beam walk performance in untreated normoglycemic animals. Curcumin therapy significantly improved poststroke beam walk scores (P < 0.01 vs. untreated animals). A modified Bederson test was used to determine the impact of stroke on global neurological function (Fig. 4B). Abnormal postural responses were observed in all untreated normoglycemic animals after stroke. Administration of FeTPPs, curcumin, or minocycline at reperfusion did not improve modified Bederson scores in normoglycemic animals. Poststroke forelimb strength was evaluated in all experimental groups (Fig. 4C). Untreated normoglycemic animals displayed grip strength deficits after stroke. Acute treatment with FeTPPs or curcumin did not improve postoperative performance. However, minocycline therapy effectively reduced poststroke grip strength deficits in normoglycemic animals. Poststroke beam walk performance in untreated diabetic animals was similar to their untreated normoglycemic counterparts (Fig. 4A). Single-dose administration of FeTPPs and curcumin but not minocycline improved poststroke beam walk performance in diabetic animals (P < 0.05 and P < 0.01 vs. untreated animals). Global neurological function was significantly worse in untreated diabetic animals compared with untreated normoglycemic animals (P < 0.05; Fig. 4B). Acute FeTPPs or minocycline therapies did not improve modified Bederson scores in diabetic animals. Only curcumin treatment effectively improved poststroke Bederson test performance in diabetic animals (P < 0.05). Furthermore, a disease-treatment interaction was detected in which curcumin therapy improved poststroke Bederson test performance in diabetic but not normoglycemic animals (P = 0.0016). Stroke-induced grip strength deficit in untreated diabetic animals was greater than in normoglycemic animals (Fig. 4C). FeTPPs treatment did not correct lost grip strength in diabetic animals; however, curcumin and minocycline improved grip strength deficit in diabetic animals (P < 0.05 vs. untreated normoglycemic animals). These analyses also pointed to differences in response to treatment in control versus diabetic animals. There was a disease-treatment interaction for FeTPPS (P = 0.034) such that the deficit was greater in control animals compared with diabetic animals after treatment (Fig. 4C). Curcumin treatment had no effect in normoglycemic animals but improved the deficit in diabetic animals (P = 0.049).
Fig. 4.
Poststroke functional deficits in normoglycemic and diabetic animals. A: beam walk performance was impaired in untreated normoglycemic animals after stroke. Neither FeTPPs nor minocycline treatment altered beam walk performance in normoglycemic animals, but curcumin alone was effective in improving poststroke beam walk scores. Modest improvements in poststroke functional deficits in diabetes could be achieved by acute therapies given at reperfusion. Vestibulomotor function impairments in untreated diabetic animals were similar to untreated normoglycemic animals. Neutralizing peroxynitrite using FeTPPs improved beam walk performance in diabetic animals. Countering stroke-induced inflammatory responses using curcumin also improved beam walk scores. B: pathological postural responses were detected in untreated normoglycemic animals after stroke, as indicated by the modified Bederson test scores. Single-dose administration of FeTPPs, curcumin, or minocycline at reperfusion did not alter Bederson test performance in normoglycemic animals. The frequency of pathological postural responses was greater in untreated diabetic animals compared with control animals. Acute treatment with FeTPPs and minocycline failed to prevent neurological impairments diabetic animals. A single dose of curcumin at reperfusion reduced poststroke disability in diabetic animals. C: stroke-induced grip strength deficits were observed in untreated normoglycemic animals. Curcumin and minocycline therapies were effective in preventing the loss of forelimb grip strength in normoglycemic animals; however, FeTPPs had no effect. The deficit in untreated diabetic animals was greater than in untreated normoglycemic animals. There was a disease by treatment interaction such that acute FeTPPs at reperfusion improved the grip strength in diabetic animals but not their normoglycemic counterparts. Curcumin and minocycline curtailed grip strength deficits in diabetic animals. MCAO, middle cerebral artery occlusion. Results are given as means ± SE, n = 4–13. *P < 0.05 vs. untreated animals; **P < 0.01 vs. untreated animals; ***P < 0.005 vs. control animals; #P = 0.0015 vs. normoglycemic animals; aP = 0.0016, bP = 0.034, and cP = 0.049, disease by treatment interaction.
Measurement of Cerebrovascular MMP Activity
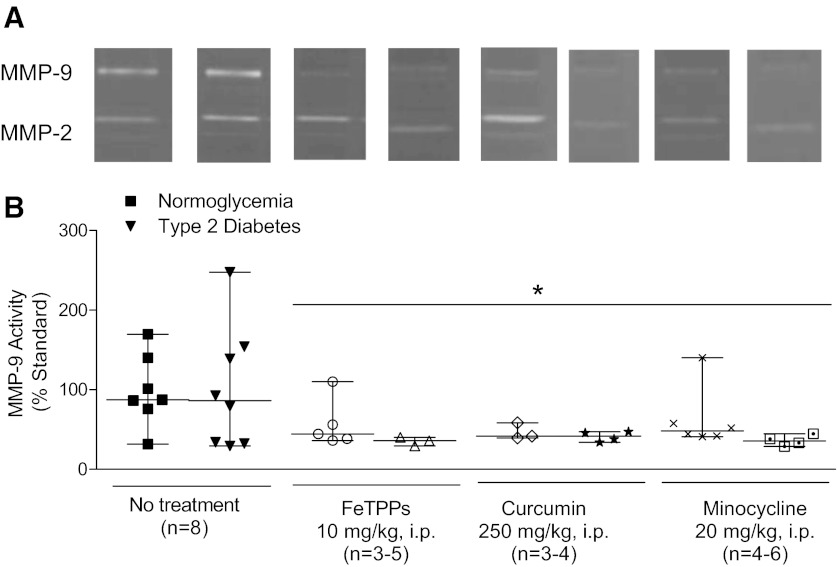
MMP-9 gelatinolytic activity measured in the macrovessels from ischemic brain hemispheres was similar between untreated normoglycemic and diabetic animals. All treatments reduced MMP-9 activity (Fig. 5). There were no changes in MMP-2 activity (not shown).
Fig. 5.
Poststroke MMP-9 activity in cerebral vessels is reduced by single-dose administration of FeTPPs, curcumin, or minocycline at reperfusion. Using gelatin zymography, MMP-9 activity was measured in the macrovessels isolated from the ischemic brain hemisphere. A: representative zymograms. B: average data. Activity was similar in untreated normoglycemic and diabetic animals after transient focal cerebral ischemia. Single-dose administration of FeTPPs, curcumin, and minocycline at reperfusion successfully reduced MMP-9 in all diabetic treatment groups. *P < 0.05 vs. untreated animals.
DISCUSSION
Diabetic patients and experimental animal models sustain greater neurovascular injury and functional deficits in AIS. Disruption of cerebrovascular integrity is associated with increased MMP-9 activity and expression. Therefore, the objective of the present study was to determine whether the acute inhibition of MMP-9 at reperfusion by minocycline or through inhibition of its upstream activators peroxynitrite and NF-κB would improve HT and functional outcomes in diabetic stroke. Major findings from this study indicate that 24 h poststroke, bleeding was improved by a single-dose administration of FeTPPs, curcumin, or minocycline at reperfusion in diabetic animals and that this was associated with reduced MMP-9 activity. Varying degrees of improvement in neurological deficits were observed in diabetic animals receiving these acute therapies. Infarct size, however, was not altered by direct peroxynitrite scavenging, suppression of inflammation, or inhibition of MMPs. The multiple disease and drug interactions noted in this study strongly suggest that these treatments have differential effects on control and diabetic animals, and, as such, therapeutic targets for neurovascular protection may differ in disease states.
Of the three therapies, single-dose administration of curcumin at reperfusion offered the most benefit by reducing edema, bleeding, and neurological deficit after focal cerebral ischemia in diabetes. A nonspecific NF-κB inhibitor, curcumin has emerged as a promising candidate in the treatment of neurodegenerative diseases because of its antioxidant, anti-inflammatory, and antiapoptotic properties (29, 61). A recent report by King et al. (37) investigated the potential role for curcumin in mitigating neurovascular injury in a model of intracerebral hemorrhage. The researchers demonstrated that curcumin administration (150 mg/kg ip) decreased hematoma size, BBB permeability, and edema postinjury. Evidence in experimental stroke models suggests that a single dose within 30 min to 1 h of focal cerebral ischemia protects against peroxynitrite-mediated BBB disruption (32, 57). Delayed treatment within 4 h of ischemia has also been observed to reduce infarct size and improve neurological outcomes after stroke (14). In the present study, we did not anticipate further reductions in infarct volume in diabetic animals because the lesion size was already very small in untreated animals. However, consistent with earlier studies in normoglycemic animals, curcumin was effective in reducing infarct size in the control group. Given its ability to resolve hematoma within 72 h postinjury in the intracerebral hemorrhage model, we expected only modest improvements in bleeding with a single dose of curcumin at reperfusion. Visual inspection of macroscopic bleeding, however, indicated that curcumin treatment at reperfusion reduced bleeding in both control and diabetic animals. Interestingly, microscopic bleeding was reduced by half in the diabetic group that received curcumin treatment. Even more surprising was the curcumin-mediated increase in microscopic bleeding in the control group despite a reduction in MMP-9 activity. While we do not have an explanation for this finding, it is possible that curcumin is also inhibiting potential vasoprotective mechanisms in otherwise health animals.
NF-κB activation prompts the release of proinflammatory cytokines that promote increases in BBB permeability, thereby allowing neurotoxic blood elements to leak into the infarcted area and exacerbate ischemic injury (43). Improvement of neurological deficits in diabetic animals may be an indirect effect of reducing edema and hemorrhage in these animals. Conversely, failure to attenuate bleeding in control animals may have contributed to worse functional outcomes in this treatment group.
We chose to use minocycline for MMP inhibition in the present study because a phase 2 clinical trial (21) recently showed that minocycline reduces plasma MMP-9 activity in acute ischemic stroke patients, which will be further tested in a phase 3 trial. In the present study, acute minocycline treatment also limited vascular damage after acute stroke but only modestly improved poststroke deficits in diabetic animals. Minocycline is a tetracycline derivative that possesses neuroprotective properties that are distinct from its antimicrobial effects (9, 23, 54). Another study (59) reported that neuroprotection was conferred by block of activation of microglia and proinflammatory cytokines. Minocycline has also been documented to prevent extracellular matrix degradation by inhibiting MMP activity. Using a rat model of focal ischemia, Machado et al. (40) determined that minocycline treatment (a single dose at reperfusion with a followup dose 12 h postocclusion) significantly reduced the expression of MMP-2 and -9 after stroke. Data from our group examining the effects of chronic minocycline treatment diabetic animals on indexes of remodeling and HT showed that MMP-9 activity was reduced and MMP-mediated pathological remodeling was prevented (18). Furthermore, chronic treatment with minocycline reduced HT in diabetic animals. Finally, a recent report by Schildknecht et al. (50) has shown that neuroprotection by minocycline can be attributed to the drug's ability to act as a direct and specific scavenger of peroxynitrite. Thus, in the present study, we anticipated that minocycline would confer neuroprotection by reducing infarct volume in treated control not diabetic rats. We also assumed that acute minocycline treatment would attenuate vascular injury in diabetic animals. Consistent with our hypothesis, single-dose administration of minocycline at reperfusion (20 mg/kg ip) was effective in reducing both macro- and microscopic bleeding and edema in diabetic animals after stroke. Furthermore, macroscopic bleeding in control animals was undetectable, and minocycline had no effect on microscopic bleeding in these animals. Data from the present study also indicate that edema is worsened by acute minocycline treatment in control animals, through an unknown mechanism. Xing et al. (58) demonstrated that at high concentrations, minocycline is cytotoxic to macrovascular endothelial cells, despite its ability to reduce MMP-9 levels. The investigators posited that the dose-dependent cell death was mediated by calpain and caspase activation. In the present study, modest improvements in functional deficits were observed in both treatment groups and may indicate that despite reduced vascular injury in these animals, a single dose of minocycline is not sufficient to reverse stroke-induced behavioral deficits.
Direct peroxynitrite scavenging by FeTPPs reduced stroke-induced macro- and microscopic bleeding and vestibulomotor function deficits in diabetes. Thiyagarajan et al. (57) demonstrated that delayed administration of FeTPPs at 2 and 6 h reduced infarct size, edema, and neurological deficits. The investigators concluded that these effects were mediated by reductions of peroxynitrite and inhibition of apoptosis (52, 57). In the present study, we anticipated decreased infarct volume in FeTPPs-treated control animals. We also predicted that direct peroxynitrite scavenging with FeTPPs would reduce bleeding and edema in diabetic animals by preventing peroxynitrite-mediated loss of myogenic tone, thus confirming data reported in a previous ex vivo study from our group (35). Since hypoxia-induced loss of myogenic tone was not restored by FeTPPs in MCAs isolated from control animals, we hypothesized that this vascular dysfunction would contribute to hyperperfusion and more bleeding. On the contrary, FeTPPs did not have an effect on either macro- or microscopic bleeding in treated control animals. Improvements in neurological deficits were limited to vestibulomotor function in diabetic animals, suggesting that single-dose administration of FeTPPs at reperfusion is not sufficient to reduce neurological impairments.
The present study focused on MMP-9 for a number of reasons. MMPs, particularly MMP-2 and -9, can be activated by oxidative stress, inflammation, or by other MMPs and have biphasic actions in AIS (44). A prolonged opening of the BBB occurs within 24–48 h after stroke and can last for several days (48). During this phase, MMP-9 is associated with increased BBB permeability, vasogenic edema, and hemorrhagic transformation (13). MMP-9 knockout mice are protected from HT (4). In AIS patients, elevated plasma MMP-9 levels are associated with infarct expansion, increased hemorrhage after thrombolytic therapies, and worsened stroke prognosis (36). Data from our group reported that elevated MMP-9 levels were associated with greater HT in a moderately hyperglycemic model of diabetes and that chronic glycemic control or minocycline intervention improved bleeding and stroke outcomes (18). Based on these findings, we anticipated that direct inhibition of MMPs or their upstream regulators (i.e., NF-κB and peroxynitrite) would lower MMP-9 activity in the cerebrovasculature and prevent HT. Evidence from the present study indicates that in diabetes, bleeding and MMP-9 activity were reduced with all therapies. On the other hand, despite inhibition of MMP-9 activity by all treatments, HT was not reduced in any groups in the normoglycemia arm, raising the possibility that mediators of vascular injury may be different in control and disease states. It is also possible that other proteolytic enzymes may be contributing to the improvements observed in this study. One potential candidate could be MMP-3, also known as stromelysin-1, which has been shown to be activated by neurons and microglia in the ischemic brain (31). In a thrombolytic model of MCAO using MMP-3- and MMP-9-deficient mice, Suzuki et al. (56) demonstrated that tissue plasminogen activator-induced intracerebral hemorrhage was attenuated in MMP-3−/− but not MMP-9−/− mice, suggesting that MMP-3 and not MMP-9 is more important in tissue plasminogen activator-induced hemorrhage (56).
The data from the present study would certainly be strengthened by measurements of NF-κB expression, tight junction protein expression in cerebral microvessels, MMP-3 activity, and markers of peroxynitrite generation (e.g., nitrotyrosine or lipid peroxidation). We could not perform those measurements in the present study because of the limited amount of cerebrovascular tissue isolated from our experimental groups. Interpretation of the present findings is also limited by the fact that only a single dose was used and that all end points were measured at 24 h poststroke. We also detected that the percent increase in cerebral blood flow within 5–10 min after suture was removed was significantly less in diabetic animals. It is possible that due to existing vascular dysfunction diabetic animals take longer to fully reestablish blood flow. These limitations, however, do not outweigh the important findings of the present study. In line with current Stroke Therapy Academic Industry Roundtable preclinical recommendations, our study evaluated potential stroke therapies in a moderately hyperglycemic model of T2D with preexisting vascular disease. Experimental stroke studies investigating the therapeutic potential of agents that preserve BBB health and vascular integrity in long-term diabetes are underrepresented in the literature (58, 64, 65). By studying stroke in the context of diabetes, we were able to demonstrate that although MMP-9 was reduced by FeTPPs, curcumin, and minocycline at reperfusion, the targets may differ in control animals and in animals with preexisting diabetes, thus culminating in different stroke outcomes. In conclusion, we have shown that targets of vascular protection in AIS are different in T2D and that future studies are needed to understand why acute treatments at reperfusion conferred greater benefit in our diabetic model of stroke compared with treated control animals. In this way, we will reduce the gap in knowledge of how preexisting diabetes contributes to stroke pathophysiology and will potentially aid in the development of novel therapeutic strategies tailored to the diabetic population.
GRANTS
The work was supported by in part by National Institute of Neurological Disorders and Stroke (NINDS) Grant F31-NS-066746 (to A. I. Kelly-Cobbs). A. Ergul is a research pharmacologist at the Charlie Norwood Veterans Affairs (VA) Medical Center (Augusta, GA). Additional support was provided in part by VA Merit Award BX000347, NINDS Grant NS-054688, and American Heart Association Established Investigator Award 0740002N (to A. Ergul). Funding for this work was also provided, in part, by VA Merit Award BX000891 and NINDS Grant NS-063965 (to S. C. Fagan).
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.I.K.-C., S.C.F., and A.E. conception and design of research; A.I.K.-C., R.P., W.L., B.P., M.C., and S.N.O. performed experiments; A.I.K.-C., B.P., S.H., M.H.J., S.N.O., and A.E. analyzed data; A.I.K.-C., S.H., M.H.J., S.C.F., and A.E. interpreted results of experiments; A.I.K.-C. prepared figures; A.I.K.-C. drafted manuscript; A.I.K.-C., M.C., M.H.J., S.C.F., and A.E. edited and revised manuscript; A.I.K.-C., R.P., W.L., B.P., S.H., M.C., M.H.J., S.N.O., S.C.F., and A.E. approved final version of manuscript.
REFERENCES
- 1. Alvarez-Sabin J, Molina CA, Ribo M, Arenillas JF, Montaner J, Huertas R, Santamarina E, Rubiera M. Impact of admission hyperglycemia on stroke outcome after thrombolysis: risk stratification in relation to time to reperfusion. Stroke 35: 2493–2498, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Aoki T, Sumii T, Mori T, Wang X, Lo EH. Blood-brain barrier disruption and matrix metalloproteinase-9 expression during reperfusion injury: mechanical versus embolic focal ischemia in spontaneously hypertensive rats. Stroke 33: 2711–2717, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab 20: 1681–1689, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Banerjee C, Moon YP, Paik MC, Rundek T, Mora-McLaughlin C, Vieira JR, Sacco RL, Elkind MS. Duration of diabetes and risk of ischemic stroke: the Northern Manhattan study. Stroke 43: 1212–1217, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beauchamp MH, Sennlaub F, Speranza G, Gobeil F, Jr, Checchin D, Kermorvant-Duchemin E, Abran D, Hardy P, Lachapelle P, Varma DR, Chemtob S. Redox-dependent effects of nitric oxide on microvascular integrity in oxygen-induced retinopathy. Free Radic Biol Med 37: 1885–1894, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Bruno A, Kent TA, Coull BM, Shankar RR, Saha C, Becker KJ, Kissela BM, Williams LS. Treatment of hyperglycemia in ischemic stroke (THIS): a randomized pilot trial. Stroke 39: 384–389, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011 (online). http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf [31 January 2013]
- 9. Choi Y, Kim HS, Shin KY, Kim EM, Kim M, Park CH, Jeong YH, Yoo J, Lee JP, Chang KA, Kim S, Suh YH. Minocycline attenuates neuronal cell death and improves cognitive impairment in Alzheimer's disease models. Neuropsychopharmacology 32: 2393–2404, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation 108: 1527–1532, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Crumrine RC, Marder VJ, Taylor GM, Lamanna JC, Tsipis CP, Scuderi P, Petteway SR, Jr, Arora V. Intra-arterial administration of recombinant tissue-type plasminogen activator (rt-PA) causes more intracranial bleeding than does intravenous rt-PA in a transient rat middle cerebral artery occlusion model. Exp Transl Stroke Med 3: 10, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. del Zoppo GJ, Frankowski H, Gu YH, Osada T, Kanazawa M, Milner R, Wang X, Hosomi N, Mabuchi T, Koziol JA. Microglial cell activation is a source of metalloproteinase generation during hemorrhagic transformation. J Cereb Blood Flow Metab 32: 919–932, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. del Zoppo GJ, Milner R, Mabuchi T, Hung S, Wang X, Berg GI, Koziol JA. Microglial activation and matrix protease generation during focal cerebral ischemia. Stroke 38: 646–651, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Dohare P, Garg P, Jain V, Nath C, Ray M. Dose dependence and therapeutic window for the neuroprotective effects of curcumin in thromboembolic model of rat. Behav Brain Res 193: 289–297, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Duplain H, Sartori C, Dessen P, Jayet PY, Schwab M, Bloch J, Nicod P, Scherrer U. Stimulation of peroxynitrite catalysis improves insulin sensitivity in high fat diet-fed mice. J Physiol 586: 4011–4016, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Elgebaly MM, Kelly A, Harris AK, Elewa H, Portik-Dobos V, Ketsawatsomkron P, Marrero M, Ergul A. Impaired insulin-mediated vasorelaxation in a nonobese model of type 2 diabetes: role of endothelin-1. Can J Physiol Pharmacol 86: 358–364, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elgebaly MM, Ogbi S, Li W, Mezzetti EM, Prakash R, Johnson MH, Bruno A, Fagan SC, Ergul A. Neurovascular injury in acute hyperglycemia and diabetes: a comparative analysis in experimental stroke. Transl Stroke Res 2: 391–398, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elgebaly MM, Prakash R, Li W, Ogbi S, Johnson MH, Mezzetti EM, Fagan SC, Ergul A. Vascular protection in diabetic stroke: role of matrix metalloprotease-dependent vascular remodeling. J Cereb Blood Flow Metab 30: 1928–1938, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ergul A, Elgebaly MM, Middlemore ML, Li W, Elewa H, Switzer JA, Hall C, Kozak A, Fagan SC. Increased hemorrhagic transformation and altered infarct size and localization after experimental stroke in a rat model type 2 diabetes. BMC Neurol 7: 33, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fagan SC, Garcia JH. Hemorrhagic transformation in focal cerebral ischemia: influence of time to artery reopening and tissue plasminogen activator. Pharmacotherapy 19: 139–142, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Fagan SC, Waller JL, Nichols FT, Edwards DJ, Pettigrew LC, Clark WM, Hall CE, Switzer JA, Ergul A, Hess DC. Minocycline to improve neurologic outcome in stroke (MINOS): a dose-finding study. Stroke 41: 2283–2287, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Feeney DM, Gonzalez A, Law WA. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science 217: 855–857, 1982 [DOI] [PubMed] [Google Scholar]
- 23. Friedlander RM. Apoptosis and caspases in neurodegenerative diseases. N Engl J Med 348: 1365–1375, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Fujimura M, Gasche Y, Morita-Fujimura Y, Massengale J, Kawase M, Chan PH. Early appearance of activated matrix metalloproteinase-9 and blood-brain barrier disruption in mice after focal cerebral ischemia and reperfusion. Brain Res 842: 92–100, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Fukuda S, Fini CA, Mabuchi T, Koziol JA, Eggleston LL, Jr, del Zoppo GJ. Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke 35: 998–1004, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goto Y. [What do spontaneously diabetic animals suggest?]. Nihon Naika Gakkai Zasshi 77: 1177–1185, 1988 [DOI] [PubMed] [Google Scholar]
- 27. Goto Y, Kakizaki M, Masaki N. Production of spontaneous diabetic rats by repetition of selective breeding. Tohoku J Exp Med 119: 85–90, 1976 [DOI] [PubMed] [Google Scholar]
- 28. Harris AK, Hutchinson JR, Sachidanandam K, Johnson MH, Dorrance AM, Stepp DW, Fagan SC, Ergul A. Type 2 diabetes causes remodeling of cerebrovasculature via differential regulation of matrix metalloproteinases and collagen synthesis: role of endothelin-1. Diabetes 54: 2638–2644, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci 65: 1631–1652, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ishrat T, Sayeed I, Atif F, Stein DG. Effects of progesterone administration on infarct volume and functional deficits following permanent focal cerebral ischemia in rats. Brain Res 1257: 94–101, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jian Liu K, Rosenberg GA. Matrix metalloproteinases and free radicals in cerebral ischemia. Free Radic Biol Med 39: 71–80, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Jiang J, Wang W, Sun YJ, Hu M, Li F, Zhu DY. Neuroprotective effect of curcumin on focal cerebral ischemic rats by preventing blood-brain barrier damage. Eur J Pharmacol 561: 54–62, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Johnston KC, Hall CE, Kissela BM, Bleck TP, Conaway MR. Glucose Regulation in Acute Stroke Patients (GRASP) trial: a randomized pilot trial. Stroke 40: 3804–3809, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kamada H, Yu F, Nito C, Chan PH. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood-brain barrier dysfunction. Stroke 38: 1044–1049, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kelly-Cobbs AI, Prakash R, Coucha M, Knight RA, Li W, Ogbi SN, Johnson M, Ergul A. Cerebral myogenic reactivity and blood flow in type 2 diabetic rats: role of peroxynitrite in hypoxia-mediated loss of myogenic tone. J Pharmacol Exp Ther 342: 407–415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kelly MA, Shuaib A, Todd KG. Matrix metalloproteinase activation and blood-brain barrier breakdown following thrombolysis. Exp Neurol 200: 38–49, 2006 [DOI] [PubMed] [Google Scholar]
- 37. King MD, McCracken DJ, Wade FM, Meiler SE, Alleyne CH, Jr, Dhandapani KM. Attenuation of hematoma size and neurological injury with curcumin following intracerebral hemorrhage in mice. J Neurosurg 115: 116–123, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li W, Prakash R, Kelly-Cobbs AI, Ogbi S, Kozak A, El-Remessy AB, Schreihofer DA, Fagan SC, Ergul A. Adaptive cerebral neovascularization in a model of type 2 diabetes: relevance to focal cerebral ischemia. Diabetes 59: 228–235, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20: 84–91, 1989 [DOI] [PubMed] [Google Scholar]
- 40. Machado LS, Kozak A, Ergul A, Hess DC, Borlongan CV, Fagan SC. Delayed minocycline inhibits ischemia-activated matrix metalloproteinases 2 and 9 after experimental stroke. BMC Neurosci 7: 56, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mankovsky BN, Ziegler D. Stroke in patients with diabetes mellitus. Diabetes Metab Res Rev 20: 268–287, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Martini SR, Kent TA. Hyperglycemia in acute ischemic stroke: a vascular perspective. J Cereb Blood Flow Metab 27: 435–451, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Matsuo Y, Onodera H, Shiga Y, Shozuhara H, Ninomiya M, Kihara T, Tamatani T, Miyasaka M, Kogure K. Role of cell adhesion molecules in brain injury after transient middle cerebral artery occlusion in the rat. Brain Res 656: 344–352, 1994 [DOI] [PubMed] [Google Scholar]
- 44. McColl BW, Rose N, Robson FH, Rothwell NJ, Lawrence CB. Increased brain microvascular MMP-9 and incidence of haemorrhagic transformation in obese mice after experimental stroke. J Cereb Blood Flow Metab 30: 267–272, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Misko TP, Highkin MK, Veenhuizen AW, Manning PT, Stern MK, Currie MG, Salvemini D. Characterization of the cytoprotective action of peroxynitrite decomposition catalysts. J Biol Chem 273: 15646–15653, 1998 [DOI] [PubMed] [Google Scholar]
- 45a. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 333: 1581–1587, 1995 [DOI] [PubMed] [Google Scholar]
- 46. Peters A, Laffel L. Diabetes care for emerging adults: recommendations for transition from pediatric to adult diabetes care systems: a position statement of the American Diabetes Association, with representation by the American College of Osteopathic Family Physicians, the American Academy of Pediatrics, the American Association of Clinical Endocrinologists, the American Osteopathic Association, the Centers for Disease Control and Prevention, Children with Diabetes, The Endocrine Society, the International Society for Pediatric and Adolescent Diabetes, Juvenile Diabetes Research Foundation International, the National Diabetes Education Program, and the Pediatric Endocrine Society (formerly Lawson Wilkins Pediatric Endocrine Society). Diabetes Care 34: 2477–2485, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Romanic AM, White RF, Arleth AJ, Ohlstein EH, Barone F. Matrix metalloproteinase expression increases after cerebral focal ischemia. Stroke 29: 1020–1030, 1997 [DOI] [PubMed] [Google Scholar]
- 48. Rosenberg GA, Yang Y. Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg Focus 22: E4, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Salvemini D, Wang ZQ, Stern MK, Currie MG, Misko TP. Peroxynitrite decomposition catalysts: therapeutics for peroxynitrite-mediated pathology. Proc Natl Acad Sci USA 95: 2659–2663, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schildknecht S, Pape R, Muller N, Robotta M, Marquardt A, Burkle A, Drescher M, Leist M. Neuroprotection by minocycline caused by direct and specific scavenging of peroxynitrite. J Biol Chem 286: 4991–5002, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sharma SS, Dhar A, Kaundal RK. FeTPPS protects against global cerebral ischemic-reperfusion injury in gerbils. Pharmacol Res 55: 335–342, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Sharma SS, Munusamy S, Thiyagarajan M, Kaul CL. Neuroprotective effect of peroxynitrite decomposition catalyst and poly(adenosine diphosphate-ribose) polymerase inhibitor alone and in combination in rats with focal cerebral ischemia. J Neurosurg 101: 669–675, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87: 4–14, 2010 [DOI] [PubMed] [Google Scholar]
- 54. Stirling DP, Khodarahmi K, Liu J, McPhail LT, McBride CB, Steeves JD, Ramer MS, Tetzlaff W. Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J Neurosci 24: 2182–2190, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Suofu Y, Clark JF, Broderick JP, Kurosawa Y, Wagner KR, Lu A. Matrix metalloproteinase-2 or -9 deletions protect against hemorrhagic transformation during early stage of cerebral ischemia and reperfusion. Neuroscience 212: 180–189, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Suzuki Y, Nagai N, Umemura K, Collen D, Lijnen HR. Stromelysin-1 (MMP-3) is critical for intracranial bleeding after t-PA treatment of stroke in mice. J Thromb Haemost 5: 1732–1739, 2007 [DOI] [PubMed] [Google Scholar]
- 57. Thiyagarajan M, Sharma SS. Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci 74: 969–985, 2004 [DOI] [PubMed] [Google Scholar]
- 58. Xing C, Levchenko T, Guo S, Stins M, Torchilin VP, Lo EH. Delivering minocycline into brain endothelial cells with liposome-based technology. J Cereb Blood Flow Metab 32: 983–988, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci USA 96: 13496–13500, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang W, Narayanan M, Friedlander RM. Additive neuroprotective effects of minocycline with creatine in a mouse model of ALS. Ann Neurol 53: 267–270, 2003 [DOI] [PubMed] [Google Scholar]
- 61. Zhao J, Yu S, Zheng W, Feng G, Luo G, Wang L, Zhao Y. Curcumin improves outcomes and attenuates focal cerebral ischemic injury via antiapoptotic mechanisms in rats. Neurochem Res 35: 374–379, 2010 [DOI] [PubMed] [Google Scholar]