Abstract
Left ventricular (LV) twist mechanics and regional strain during cardiac sympathetic efferent activation are poorly understood. The purpose of this study was to compare the effects of left stellate ganglia (LSG) and right stellate ganglia (RSG) stimulation on cardiac twist/untiwst mechanics and regional strain. In nine pigs, echocardiographic imaging and LV pressure-volume measurements were performed before and during unilateral and bilateral stellate ganglion stimulation. LSG and RSG stimulation significantly augmented LV end-systolic pressure by 24% and 22% (P < 0.01), maximal rate of LV pressure change by 167% and 165% (P < 0.01), and time constant of LV relaxation by 20% and 12% (P < 0.01), respectively. RSG stimulation resulted in a greater chronotropic response than LSG stimulation (RSG: 68% vs. LSG: 12%, P < 0.01). Both LSG and RSG stimulation significantly increased global epicardial and endocardial LV rotation and diastolic untwisting rate and reduced the time to peak rotation (P < 0.05). However, LSG stimulation predominantly increased radial and circumferential strain in the LV inferoseptal, inferior, posterior, and lateral regions, whereas RSG stimulation primarily increased radial and circumferential strain in the anteroseptal, anterior, and lateral LV regions. Stimulation of both stellate ganglia led to a uniform increase in all LV segments. Our data suggest that LSG and RSG stimulation lead to a global increase in LV twist, driven by distinct regional strain heterogeneity that may result from myocardial innervation from the LSG and RSG. These findings provide a better understanding of the global and regional functional consequences of regional myocardial innervation from the LSG and RSG.
Keywords: stellate ganglia, sympathetic efferent, autonomic regulation, twist
over a century ago, Hunt (19) first demonstrated that stimulation of the left cardiac nerve increased blood pressure but had little effect on heart rate (HR). Previous studies have shown that stimulation of the left stellate ganglion (LSG) causes a significant inotropic response, whereas stimulation of the right stellate ganglion (RSG) has a predominantly chronotropic effect (13, 34, 36). Electrophysiological studies (33, 44, 51) have shown predominant innervation of the anterior aspect of the right ventricle (RV) and left ventricle (LV) by the RSG, whereas the posterior aspect of the RV and LV is predominantly innervated by the LSG.
Randall et al. (35, 36) examined the patterns of sympathetic nerve projections onto the heart by measuring regional contractile force using a strain gauge during stimulation of the cardiac nerves. They found that although specific regions of the epicardial surfaces receive their major sympathetic supply from a given nerve trunk, all regions examined appeared to be innervated by multiple trunks, originating from both right and left distributing systems. Their data suggest that the overlap of sympathetic projections to the epicardial surface of the heart is necessary for the regulation of cardiac function.
In this study, we aimed to use speckle tracking echocardiography (STE) along with invasive hemodynamic measurements to assess the effects of sympathetic stimulation on the heart. STE is a noninvasive imaging tool that can quantify both global and regional myocardial function by measuring, for example, radial and circumferential strains and the torsional motion of the LV, which closely correlates with global ventricular function (6, 7, 14, 16). Radial strain quantifies transmural thickening of the myocardium, whereas circumferential strain measures shortening along the circumference (2, 27, 37). LV rotation is defined as the average angular displacement of all regions of the myocardium relative to the center of the LV cavity in the short axis (42). When viewed from the apex to base, a counterclockwise rotation corresponds to a positive rotation. LV twist is defined as the difference between apical and basal LV rotation and is a fundamental motion component during LV ejection. LV untwisting is a critical component of early diastolic function (7, 8). Therefore, quantification of LV strain and twist mechanics provides insight into global and regional systolic and diastolic function (24, 30, 41, 42) with a proven correlation to pressure-volume loop parameters (52).
The objective of this study was to compare the effects of LSG and RSG stimulation on LV twist mechanics and regional myocardial strain, which has not been previously reported. We hypothesized that LSG and RSG stimulation results in an enhancement of LV twist and nonuniform changes in LV regional systolic function due to differences in their respective areas of innervation.
METHODS
Surgical preparation.
This protocol was designed in compliance with institutional, state, and National Institutes of Health guidelines for the care and use of laboratory animals. Female Yorkshire pigs (n = 9) weighing 40–45 kg were anesthetized intramuscularly with ketamine (15–25 mg/kg) and xylazine (2 mg/kg) and mechanically ventilated. Anesthesia was maintained by isoflurane (1–2%) and intermittent intravenous boluses of fentanyl (5–10 μg/kg). The ECG was monitored from limb leads. Femoral artery and jugular venous access were used for blood pressure measurements and drug infusion. Animals underwent median sternotomy to expose the heart. The LSG and RSG were dissected free and encircled with custom-made semicircular tin-copper electrodes.
Hemodynamic assessment.
A 5-Fr pigtail 12-pole multielectrode combination conductance-pressure catheter (Millar Instruments, Houston, TX) was placed in the LV via the right carotid artery and connected to a conductance processor (MPVS Ultra, Houston, TX) for continuous measurements of LV pressure and volume (3). Proper electrode position was confirmed by an examination of the segmental volume signals. Total LV volume was calibrated using high-frequency harmonic two-dimensional echocardiographic images (biplane Simpson's method). Hemodynamic indexes were obtained from steady-state pressure-volumen loops. LV performance was assessed by HR, stroke volume (SV), LV end-systolic volume (LVESV), LV end-diastolic volume (LVEDV), cardiac output (CO), and stroke work (SW). Systolic LV function was assessed by ejection fraction (EF), LV end-systolic pressure (LVESP), and the maximum rate of LV pressure change (dP/dtmax). Diastolic LV function was assessed by LV end-diastolic pressure (LVEDP), the isovolumetric relaxation time constant (τ), and the minimal rate of LV pressure change (dP/dtmin) (48, 54).
STE recording and analysis.
Open-chest epicardial echocardiography was performed using a GE Vivid 7 Dimension system (GE Vingmed Ultrasound, Horten, Norway) with a 10-MHz transducer. Image quality was improved by using a section of pig liver as a tissue offset. Echocardiographic and hemodynamic data were acquired simultaneously. Transducer frequencies (6–11.5 MHz), sampling rates (70–110 frames/s), and sector widths were adjusted manually to optimize speckle quality. Three cardiac cycles were recorded for subsequent offline analysis. LV rotation measurements were obtained using speckle tracking software (EchoPAC PC version 8.0, GE Vingmed Ultrasound) at the basal (identified by the mitral valve) and apical (no papillary muscles noted) levels. Transmural layers that included the inner or outer third of the myocardium during end diastole were used to evaluate endocardial and epicardial rotation (18). Counterclockwise rotation was defined as a positive angle and clockwise rotation as a negative angle when viewed from the apex toward the base. We also evaluated peak LV rotation and peak untwisting rate. Short-axis circumferential and radial strain values were evaluated using the same speckle tracing software at the midventricular level, which was identified as the level according with the maximum papillary muscle circumference (8, 16, 30, 47). The accuracy of the image tracking software was verified manually.
Baseline end-systolic strain values along the circumferential direction are presented as negative values (segmental shortening), whereas baseline end-systolic radial strain values are presented as positive values (segmental thickening). Strain changes from baseline to stellate ganglion stimulation are reported as percent changes. All echocardiographic analyses were performed blinded from hemodynamic measurements and sympathetic stimulation protocol.
Experimental protocol.
The exposed LSG and RSG were electrically stimulated for 3 min using a bipolar electrode connected to a Grass stimulator (model S9D, Grass Technologies, West Warwick, RI). Stellate stimulation consisted of repeated square-wave pulses (5-ms duration, 5 Hz) delivered at an amplitude of 5–7 V, titrated to the hemodynamic response, as in previous studies (23, 26). Animals were randomly assigned the order in which they received LSG, RSG, or bilateral stellate ganglion (BSG) stimulation, with a 30-min interval between stimulations to allow return to baseline.
Statistical analysis.
Analyses were performed using Sigma Stat (version 3.1). Data are presented as means ± SD. Hemodynamic and echocardiographic measurements were compared in the experimental stages using repeated-measures ANOVA followed by a post hoc Bonferroni correction. Statistical differences were considered significant at P < 0.05.
Baseline data were also acquired in between stimulation protocols. A preliminary analysis indicated no significant hemodynamic changes between the baseline experiments; hence, only the first baseline experiment was used during the analysis to simplify the reporting of our results.
Inter- and intraobserver variability.
Two independent observers processed the echocardiography images for six randomly selected experiments and evaluated apical rotation and septal and lateral circumferential and radial strains. Each observer analyzed the same images twice. Intraobserver variability was determined by having one observer repeat the measures 1 mo after the initial analysis. Interobserver and intraobserver variability were assessed using intraclass correlation coefficients with 95% confidence intervals.
RESULTS
Hemodynamic responses to stellate ganglion stimulation.
An example of the hemodynamic recordings obtained from one animal is shown in Fig. 1. RSG, LSG, and BSG stimulation caused a rapid increase in arterial pressure, LV pressure, and dP/dtmax (Fig. 1). Positive hemodynamic responses were used to confirm successful stimulation capture of the stellate ganglion, and all animals demonstrated a similar response. Table 1 shows changes in systolic and diastolic hemodynamic parameters. Stimulation of the LSG resulted in significant increases in SV, CO, EF, LVESP, SW, and dP/dtmax and significant decreases in LVESV, LVEDV, dP/dtmin, and τ relative to the pre-LSG stimulation baseline. LSG stimulation did not significantly alter HR, LVEDP, or total peripheral resistance. RSG stimulation significantly increased HR, LVESP, CO, EF, SW, and dP/dtmax and decreased total peripheral resistance, LVESV, LVEDV, dP/dtmin, and τ relative to the pre-RSG stimulation baseline. BSG stimulation also increased HR, LVESP, CO, EF, SW, and dP/dtmax and decreased dP/dtmin and τ.
Fig. 1.
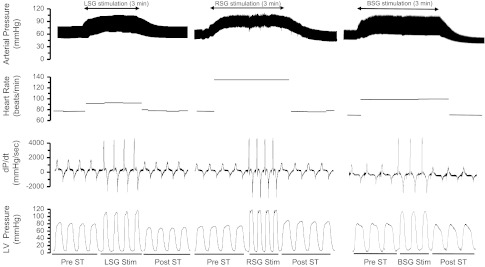
Records obtained in one pig demonstrating changes in heart rate, arterial pressure, left ventricular (LV) pressure, and the rate of change of pressure in the LV (dP/dt) in response to unilateral and bilateral stellate ganglion (BSG) stimulation. ↓ indicates the onset of stellate ganglion stimulation. LSG, left stellate ganglion; RSG, right stellate ganglion.
Table 1.
Hemodynamic responses induced by stellate ganglia stimulation
| Baseline | LSG Stimulation | RSG Stimulation | BSG Stimulation | |
|---|---|---|---|---|
| Heart rate, beats/min | 77.6 ± 3.8 | 86.7 ± 8.6 | 134.1 ± 6.6*† | 102.5 ± 5.8* |
| End-systolic volume, ml | 23.9 ± 2.2 | 13.2 ± 1.7* | 12.8 ± 1.4* | 12.7 ± 1.3* |
| End-diastolic volume, ml | 50.5 ± 4.2 | 43.5 ± 4.3* | 37.6 ± 5.7* | 39.9 ± 2.3* |
| Stroke volume, ml | 26.2 ± 2.3 | 30.1 ± 2.5* | 25.3 ± 1.9 | 28.3 ± 1.0 |
| Cardiac output, liters | 2.04 ± 0.27 | 2.61 ± 0.28* | 3.39 ± 0.31*† | 2.90 ± 0.30* |
| Ejection fraction, % | 51.8 ± 5.3 | 68.5 ± 4.4* | 67.3 ± 5.9* | 69.3 ± 2.74* |
| LV end-systolic pressure, mmHg | 83.1 ± 5.3 | 102.7 ± 8.8* | 105.9 ± 5.1* | 114.0 ± 4.3* |
| LV End-diastolic pressure, mmHg | 4.6 ± 0.6 | 4.9 ± 0.5 | 4.0 ± 0.5 | 3.5 ± 0.2 |
| Total peripheral resistance, mmHg/l | 1,876 ± 120 | 1,988 ± 115 | 1,332 ± 101*† | 2,044 ± 90 |
| Stroke work, mmHgml | 2,174 ± 335 | 3,091 ± 341* | 2,679 ± 221*† | 3,226 ± 382* |
| dP/dtmax, mmHg/s | 1,612 ± 150 | 4,296 ± 230* | 4,518 ± 297* | 5,057 ± 241*† |
| dP/dtmin, mmHg/s | 1,564 ± 160 | 3,782 ± 256* | 3,922 ± 211* | 4,196 ± 243*† |
| τ, ms | 69.2 ± 4.8 | 54.8 ± 5.9* | 56.9 ± 3.2* | 51.7 ± 4.0* |
| End-diastolic volume/LV end-diastolic pressure, ml/mmHg | 11.0 ± 2.3 | 8.7 ± 1.8 | 9.4 ± 2.1 | 11.4 ± 2.6† |
Values are means ± SD. LSG, left stellate ganglion; RSG, right stellate ganglion; BSG, bilateral stellate ganglia; LV, left ventricular; dP/dtmax and dP/dtmin, maximum positive and negative rates of pressure change, respectively; τ, time constant of isovolumic relaxation.
P < 0.05 vs. baseline;
P < 0.05 vs. LSG stimulation.
LV epicardial and endocardial twist responses to stellate ganglion stimulation.
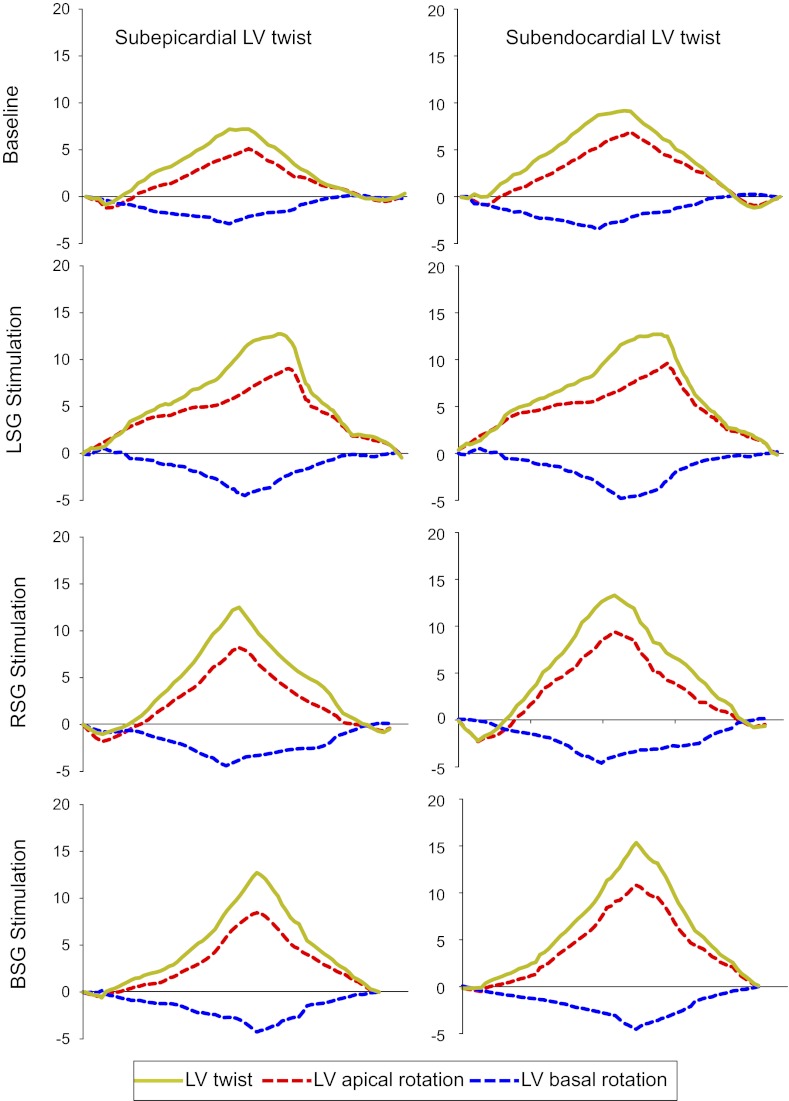
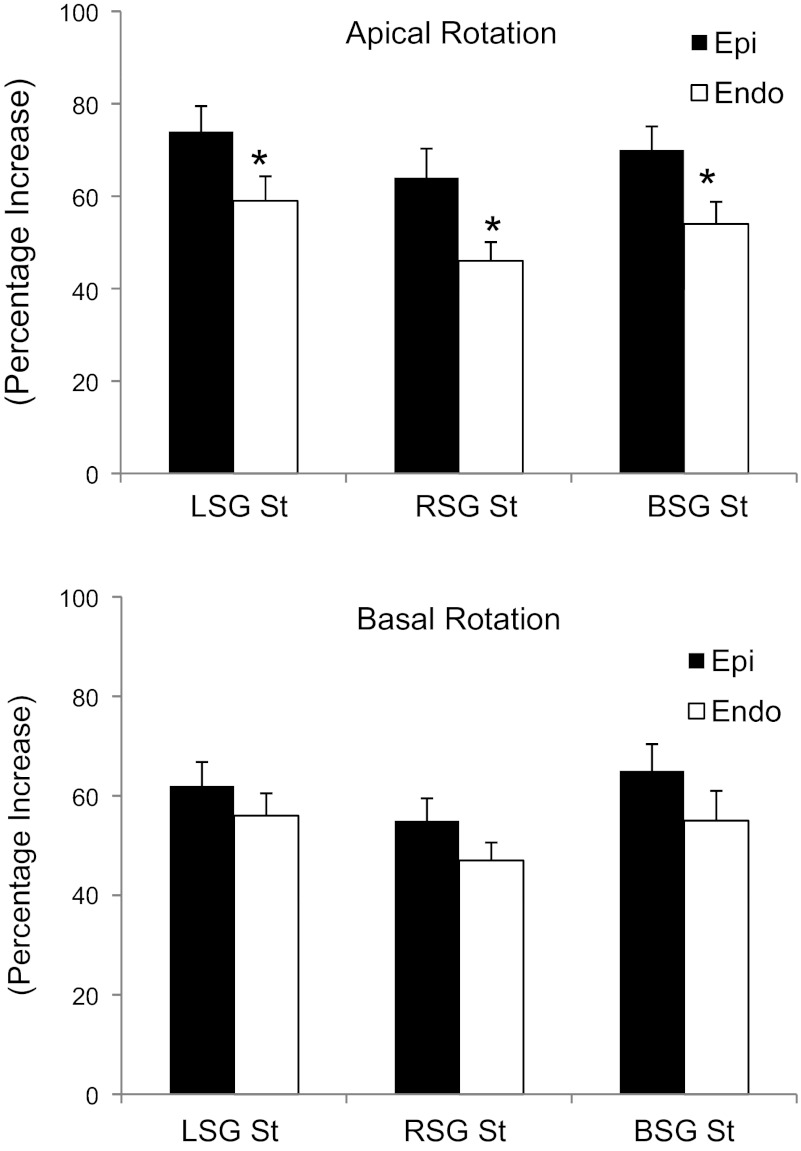
A representative example of LV rotational responses to LSG, RSG, and BSG stimulation is shown in Fig. 2. The results shown in Table 2 demonstrate that LSG, RSG, and BSG stimulation significantly increased both LV apical and basal epicardial and endocardial rotation and twist (P < 0.05) but had no effect on the time to peak rotation normalized by the R-R interval. Endocardial LV twist is greater than epicardial LV twist at baseline, whereas there were no significant differences between epicardial and endocardial LV twist during LSG, RSG, and BSG stimulation. The results shown in Fig. 3 demonstrate that the percent increase in epicardial rotation due to stellate ganglia stimulation was significantly greater than that in endocardial rotation at the apical level. LSG, RSG, and BSG stimulation also increased epicardial and endocardial untwisting rates at both the apical and basal levels with no significant differences between the epicardium and endocardium.
Fig. 2.
Representative example of LV subepicardial and subendocardial LV twist responses to unilateral and BSG stimulation.
Table 2.
Effect of stellate ganglia stimulation on LV twist mechanics
| Baseline | LSG Stimulation | RSG Stimulation | BSG Stimulation | |
|---|---|---|---|---|
| Apical rotation, ° | ||||
| Epicardial | 5.0 ± 0.9 | 8.7 ± 1.5* | 8.2 ± 1.2* | 8.7 ± 1.4* |
| Endocardial | 6.8 ± 1.2† | 9.6 ± 1.8* | 9.1 ± 1.4* | 10.2 ± 1.3* |
| Apical TTP | ||||
| Epicardial | 466 ± 51 | 474 ± 62 | 432 ± 58 | 437 ± 26 |
| Endocardial | 477 ± 47 | 480 ± 58 | 441 ± 56 | 444 ± 24 |
| Basal rotation, ° | ||||
| Epicardial | 2.9 ± 0.7 | 4.5 ± 0.8* | 4.3 ± 0.8* | 4.2 ± 1.1* |
| Endocardial | 3.2 ± 0.5 | 4.6 ± 0.7* | 4.5 ± 0.9* | 4.4 ± 1.0* |
| Basal TTP | ||||
| Epicardial | 438 ± 40 | 451 ± 63 | 406 ± 39 | 417 ± 24 |
| Endocardial | 443 ± 36 | 462 ± 53 | 424 ± 33 | 423 ± 36 |
| LV twist, ° | ||||
| Epicardial | 7.9 ± 0.6 | 13.2 ± 1.2* | 12.5 ± 1.1* | 13.3 ± 1.5* |
| Endocardial | 10.0 ± 0.8† | 14.2 ± 1.3* | 13.6 ± 1.4* | 14.5 ± 1.3* |
| Apical untwisting rate, °/s | ||||
| Epicardial | 36.8 ± 4.3 | 69.6 ± 5.3* | 72.7 ± 6.5* | 75.5 ± 5.5* |
| Endocardial | 41.8 ± 3.9† | 73.1 ± 5.5* | 79.2 ± 6.3* | 80.5 ± 6.2* |
| Basal untwisting rate, °/s | ||||
| Epicardial | 29.1 ± 3.2 | 40.3 ± 4.1* | 48.4 ± 3.5* | 46.4 ± 7.2* |
| Endocardial | 29.2 ± 4.3 | 43.2 ± 5.2* | 52.3 ± 4.2* | 49.5 ± 7.5* |
Values are means ± SD. TTP, time to peak rotation normalized by R-R interval.
P < 0.05 vs. baseline;
P < 0.05, epicardial vs. endocardial.
Fig. 3.
Percent increase in LV epicardial and endocardial rotation during unilateral and BSG stimulation. Epi, epicardium; Endo, endocardium. *P < 0.05, epicardial vs. endocardial rotation.
Heterogeneity of changes in LV strain with stellate ganglion stimulation.
Changes in LV strain during LSG, RSG, and BSG stimulation are shown in Table 3 and Fig. 4, A–D. LSG stimulation significantly increased radial strain in LV septal, inferior, posterior, and lateral segments (Fig. 4A) and significantly increased circumferential strain in LV septal, inferior, and posterior segments (Fig. 4B). In contrast, RSG stimulation significantly increased radial strain in anterior septal, anterior, and lateral segments (Fig. 4C) and significantly increased circumferential strain in anteroseptal, anterior, and lateral segments (Fig. 4D). Interestingly, BSG stimulation uniformly and significantly increased regional radial and circumferential strains in all regions (Fig. 4, E and F).
Table 3.
Effects of stellate ganglia stimulation on LV regional strain
| Radial Strain, % |
Circumferential Strain, % |
|||||||
|---|---|---|---|---|---|---|---|---|
| Segments | Baseline | LSG stimulation | RSG stimulation | BSG stimulation | Baseline | LSG stimulation | RSG stimulation | BSG stimulation |
| Septal | 21.3 ± 1.8 | 26.8 ± 2.0* | 24.7 ± 3.7 | 27.8 ± 2.7* | −12.0 ± 1.1 | −14.8 ± 1.9* | −13.5 ± 1.3 | −14.3 ± 1.7* |
| Anterior septal | 19.6 ± 3.3 | 23.3 ± 3.7 | 27.2 ± 3.8* | 29.5 ± 4.5* | −9.9 ± 1.1 | −11.8 ± 1.9 | −12.5 ± 1.3* | −12.0 ± 0.8* |
| Anterior | 19.8 ± 5.1 | 21.9 ± 5.7 | 27.8 ± 4.0* | 25.2 ± 3.9* | −10.7 ± 1.0 | −11.4 ± 1.8 | −15.0 ± 1.4* | −14.3 ± 1.1* |
| Lateral | 22.0 ± 3.0 | 29.5 ± 4.1* | 27.7 ± 4.3* | 26.6 ± 2.2* | −9.2 ± 0.9 | −11.4 ± 1.0* | −12.9 ± 1.8* | −12.0 ± 1.0* |
| Posterior | 21.8 ± 1.2 | 28.1 ± 2.5* | 24.0 ± 4.2 | 29.2 ± 2.3* | −9.8 ± 0.8 | −14.2 ± 0.9* | −11.8 ± 2.6 | −13.0 ± 0.9* |
| Inferior | 21.3 ± 2.9 | 29.6 ± 2.7* | 23.8 ± 3.4 | 27.7 ± 3.6* | −9.7 ± 1.8 | −14.0 ± 2.1* | −11.5 ± 2.1 | −12.3 ± 1.6* |
Values are means ± SD.
P < 0.05 vs. baseline.
Fig. 4.
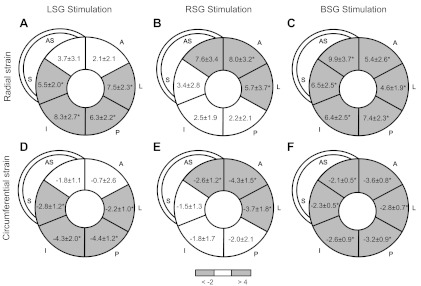
Regional changes in radial (A–C) and circumferential (D–F) LV strain after unilateral and BSG stimulation. Values and grayscale reflect strain (in %) changes from baseline values. S, septal; AS, anteroseptal; A, anterior; L, lateral; P, posterior; I, inferior. *P < 0.05 vs. baseline.
Interobserver and intraobserver variability.
There was good reproducibility and concordance of the various echocardiographic data. Detailed interobserver and intraobserver variability are shown in Table 4. The intraclass correlation coefficient for intraobserver variability was slightly higher than interobserver variability.
Table 4.
Interobserver and intraobserver variability
| Variable | Intraclass Correlation Coefficient | 95% Confidence Intervals |
|---|---|---|
| Interobserver | ||
| Apical rotation | 0.90 | 0.74–0.97 |
| Septal circumferential strain | 0.88 | 0.68–0.95 |
| Lateral circumferential strain | 0.89 | 0.68–0.95 |
| Septal radial strain | 0.89 | 0.73–0.96 |
| Lateral radial strain | 0.90 | 0.74–0.96 |
| Intraobserver | ||
| Apical rotation | 0.93 | 0.81–0.97 |
| Septal circumferential strain | 0.91 | 0.73–0.96 |
| Lateral circumferential strain | 0.91 | 0.75–0.97 |
| Septal radial strain | 0.92 | 0.79–0.97 |
| Lateral radial strain | 0.92 | 0.78–0.97 |
DISCUSSION
The primary finding of the present study is that unilateral stellate ganglion and BSG stimulation significantly increases LV twist but that the underlying regional strain changes that drive the increase in twist are fundamentally different. Interestingly, BSG leads to a more uniform enhancement of wall motion in LV segments compared with LSG and RSG. To our knowledge, this is the first use of STE to demonstrate the regional effects of LSG, RSG, and BSG stimulation on global and regional myocardial function. Our data suggest that both LSG and RSG stimulation lead to a similar global increase in LV twist mechanics, but with distinct regional strain changes, likely representing heterogeneous innervation of the myocardium by the two stellate ganglia.
Prior studies have examined the impact of stellate ganglion stimulation on LV systolic and diastolic function using LV pressure measurements. Furnival et al. (13) demonstrated that the predominant effect of stimulating the left cardiac nerves is inotropic, whereas that of the right cardiac sympathetic nerve is chronotropic. In addition, Burwash and others (9, 17, 50) have demonstrated the generalized inotropic and chronotropic response to sympathetic stimulation with enhanced systolic and diastolic function.
However, our results indicate that both LSG and RSG stimulation have inotropic effects as evidenced by pressure-volume measurements (Table 1). Additionally, we systematically compared the LV rotational mechanics during LSG and RSG stimulation using STE and found that both RSG and LSG stimulation increase epicardial and endocardial LV peak rotation, twist, and untwisting rate. The LV myocardium consists of obliquely oriented muscle fibers that vary from −45° or more on the epicardium to ∼0° in the midwall and to +45° or more on the endocardium (12, 43). Despite the opposing fiber direction, LV twist arises from mainly from shortening of the most superficial epicardial fibers owing to their larger radius compared with fibers of the endocardium (15, 20, 43). Stimulation of the sympathetic ganglia causes the release of norepinephrine from nerve endings in the myocardium (46, 53). The increase in LV rotation with sympathetic stimulation may also be explained by considering the predominance of sympathetic nerve endings in the subepicardial region (10, 11, 21), which favors increased epicardial myocytes.
However, in the present study, we did not see a synergistic effect of BSG on LV twist. We postulate that unilateral stimulation of either the LSG or RSG may increase the LV twist to a maximal extent possible, which is why BSG does not demonstrate a greater effect than unilateral stimulation. Increases in twist and decreases in LVESV are closely linked. Note that LVESV is not further decreased with BSG (compared with LSG or RSG), which further supports the idea that a physiologic maximum has been reached.
One of the novel study findings was that LSG, RSG, and BSG stimulation enhanced LV diastolic function, as evidenced by increased dP/dtmin and shortened τ. In particular, note that dP/dtmin was the highest and τ was the lowest with BSG stimulation, suggesting that diastolic function is more enhanced with BSG stimulation. In accordance with these hemodynamic indications of improved diastolic function, we also found that stellate ganglion stimulation increased the LV untwisting rate. Diastolic dysfunction is known to occur before systolic impairment; hence, therapeutic improvements in diastolic function may play an important role in limiting the progression of LV dysfunction. To our knowledge, this is the first study that demonstrates the effects of stellate ganglion stimulation on LV twist and untwist. This may lead to the clinical assessment of LV rotational STE parameters in the future for the assessment of systolic and diastolic dysfunction and the response to therapy.
Most importantly, although both LSG and RSG stimulation lead to a global increase of LV twist, there is a distinct difference in their regional impact on the heart. Previous studies have demonstrated that sympathetic innervation of the ventricular myocardium is heterogeneous by examining regional monophasic action potential duration. These authors (25, 49) found that there is a greater amount of sympathetic nerves in basal regions of the heart compared with apical regions. Norris et al. (29) examined the regional variations in the contractile response of the myocardium and found that nerve fibers arising from the left sympathetic chain innervate the posterior surface of the ventricles and that fibers arising from the right sympathetic chain innervate the anterior ventricular surface. Recently, we (33) demonstrated that LSG stimulation deflects the net vector of repolarization (T-wave vector) posteriorly and inferiorly, whereas RSG stimulation deflects the vector anteriorly and superiorly in porcine hearts. This agrees well with the findings of Yanowitz et al. (51), which demonstrated regional differences in ECGs with unilateral sympathetic stimulation. The data presented in this study along with electrophysiological observations from other groups confirm the functional consequences of heterogeneous sympathetic innervation. Of note, BSG stimulation leads to a uniform increase in strain in all of the LV segments, suggesting that the LSG and RSG can coordinate together to regulate cardiac function.
Clinical implications.
Enhanced sympathetic nerve activity is implicated in ventricular arrhythmogenesis (31, 45, 55). Therefore, modulation of cardiac sympathetic innervation by thoracic epidural anesthesia or removal of LSG, RSG, or both stellate ganglia has been advocated to treat ventricular arrhythmias in patients (1, 5). In conscious dogs with prior anterior myocardial infarction, left stellectomy seemed to protect against ventricular fibrillation during acute myocardial ischemia (40). In an anesthetized dog model without prior myocardial infarction, either left or right stellectomy protected against death by ventricular fibrillation after coronary occlusion (32). Unilateral increases and decreases of sympathetic tone to the heart can induce an asynchronous wall motion pattern affecting cardiac function (38, 39). However, the functional effects of the neural projections of LSG and RSG have not been established. The present study used noninvasive STE imaging to provide greater insights into alteration in global and regional LV function as well as regional cardiac innervation during LSG and RSG stimulation. This may help guide identification of targeted therapies, such as left or right stellectomy, for reducing arrhythmias in patients with ventricular tachycardia storm. Furthermore, targeted stimulation of cardiac sympathetic nerves has recently been suggested as a potential therapeutic approach for improving myocardial contractility in acute heart failure (4, 22, 28). Comprehensive understanding of the functional effects of cardiac sympathetic innervation is essential for such neuromodulation techniques to become successful as innovative therapies for acute heart failure.
There are limitations to the present study. The stellate ganglion was not proximally denervated. Although a central nervous system effect may be present, the primary goal of this study was to assess global and regional effects of LSG and RSG in an intact neural network. The effects of SG stimulation on LV rotational mechanics and regional myocardial strain are not known in pathological conditions such as chronic myocardial ischemia and heart failure accompanied by neural remodeling. The present study was limited to addressing the effects of LSG, RSG, and BSG stimulation on LV global and regional function. Future studies should evaluate RV function during RSG, LSG, or BSG stimulation and also evaluate the cardiac functional consequences of stellate ganglion block, which has been used clinically to treat arrhythmias (5).
In conclusion, we characterized the effects of stellate ganglia stimulation on LV global and regional function. Both LSG and RSG stimulation enhance LV systolic and diastolic function and cardiac twist mechanics; however, LSG and RSG stimulation differentially alter regional strain patterns, suggesting distinct territorial innervation of the LV myocardium. These findings provide a better understanding of regional myocardial innervation from the LSG and RSG and may have significant therapeutic applications.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-084261.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: W.Z., P.B., O.A.A., M.V., K.S., and A.M. conception and design of research; W.Z., K.Y., P.B., O.A.A., J.H., M.V., and A.M. performed experiments; W.Z., K.Y., P.B., O.A.A., D.B.E., J.H., K.S., and A.M. analyzed data; W.Z., K.Y., P.B., O.A.A., D.B.E., J.H., M.V., K.S., and A.M. interpreted results of experiments; W.Z., K.Y., D.B.E., J.H., K.S., and A.M. prepared figures; W.Z., K.Y., D.B.E., and K.S. drafted manuscript; W.Z., K.Y., P.B., O.A.A., D.B.E., J.H., M.V., K.S., and A.M. edited and revised manuscript; W.Z., K.Y., P.B., O.A.A., D.B.E., J.H., M.V., K.S., and A.M. approved final version of manuscript.
REFERENCES
- 1. Ajijola OA, Lellouche N, Bourke T, Tung R, Ahn S, Mahajan A, Shivkumar K. Bilateral cardiac sympathetic denervation for the management of electrical storm. J Am Coll Cardiol 59: 91–92, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amundsen BH, Helle-Valle T, Edvardsen T, Torp H, Crosby J, Lyseggen E, Stoylen A, Ihlen H, Lima JA, Smiseth OA, Slordahl SA. Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol 47: 789–793, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Baan J, van der Velde ET, de Bruin HG, Smeenk GJ, Koops J, van Dijk AD, Temmerman D, Senden J, Buis B. Continuous measurement of left ventricular volume in animals and humans by conductance catheter. Circulation 70: 812–823, 1984 [DOI] [PubMed] [Google Scholar]
- 4. Bianchi S, Rossi P, Benussi S. Neural autonomic modulation of the heart: a new tool for cardiac surgeons? Ann Thorac Surg 94: 1043, 2012 [DOI] [PubMed] [Google Scholar]
- 5. Bourke T, Vaseghi M, Michowitz Y, Sankhla V, Shah M, Swapna N, Boyle NG, Mahajan A, Narasimhan C, Lokhandwala Y, Shivkumar K. Neuraxial modulation for refractory ventricular arrhythmias: value of thoracic epidural anesthesia and surgical left cardiac sympathetic denervation. Circulation 121: 2255–2262, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burns AT, La Gerche A, Prior DL, Macisaac AI. Left ventricular torsion parameters are affected by acute changes in load. Echocardiography 27: 407–414, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Burns AT, La Gerche A, Prior DL, Macisaac AI. Left ventricular untwisting is an important determinant of early diastolic function. JACC Cardiovasc Imaging 2: 709–716, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Burns AT, La Gerche A, Prior DL, MacIsaac AI. Reduced and delayed untwisting of the left ventricle in patients with hypertension and left ventricular hypertrophy: a study using two-dimensional speckle tracking imaging. Eur Heart J 29: 825; author reply 825–826, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Burwash IG, Morgan DE, Koilpillai CJ, Blackmore GL, Johnstone DE, Armour JA. Sympathetic stimulation alters left ventricular relaxation and chamber size. Am J Physiol Regul Integr Comp Physiol 264: R1–R7, 1993 [DOI] [PubMed] [Google Scholar]
- 10. Crick SJ, Anderson RH, Ho SY, Sheppard MN. Localisation and quantitation of autonomic innervation in the porcine heart II: endocardium, myocardium and epicardium. J Anat 195: 359–373, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crick SJ, Sheppard MN, Ho SY, Anderson RH. Localisation and quantitation of autonomic innervation in the porcine heart I: conduction system. J Anat 195: 341–357, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ennis DB, Nguyen TC, Riboh JC, Wigstrom L, Harrington KB, Daughters GT, Ingels NB, Miller DC. Myofiber angle distributions in the ovine left ventricle do not conform to computationally optimized predictions. J Biomech 41: 3219–3224, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Furnival CM, Linden RJ, Snow HM. Chronotropic and inotropic effects on the dog heart of stimulating the efferent cardiac sympathetic nerves. J Physiol 230: 137–153, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gibbons Kroeker CA, Tyberg JV, Beyar R. Effects of load manipulations, heart rate, and contractility on left ventricular apical rotation. An experimental study in anesthetized dogs. Circulation 92: 130–141, 1995 [DOI] [PubMed] [Google Scholar]
- 15. Greenbaum RA, Ho SY, Gibson DG, Becker AE, Anderson RH. Left ventricular fibre architecture in man. Br Heart J 45: 248–263, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Helle-Valle T, Crosby J, Edvardsen T, Lyseggen E, Amundsen BH, Smith HJ, Rosen BD, Lima JA, Torp H, Ihlen H, Smiseth OA. New noninvasive method for assessment of left ventricular rotation: speckle tracking echocardiography. Circulation 112: 3149–3156, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Henning RJ, Levy MN. Effects of autonomic nerve stimulation, asynchrony, and load on dP/dtmax and on dP/dtmin. Am J Physiol Heart Circ Physiol 260: H1290–H1298, 1991 [DOI] [PubMed] [Google Scholar]
- 18. Hui L, Pemberton J, Hickey E, Li XK, Lysyansky P, Ashraf M, Niemann PS, Sahn DJ. The contribution of left ventricular muscle bands to left ventricular rotation: assessment by a 2-dimensional speckle tracking method. J Am Soc Echocardiogr 20: 486–491, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hunt R. Direct and reflex acceleration of the mammalian heart with some observation on the relations of the inhibitory and accelerator nerves. Am J Physiol 2: 395–470, 1899 [Google Scholar]
- 20. Ingels NB, Jr, Hansen DE, Daughters GT, 2nd, Stinson EB, Alderman EL, Miller DC. Relation between longitudinal, circumferential, and oblique shortening and torsional deformation in the left ventricle of the transplanted human heart. Circ Res 64: 915–927, 1989 [DOI] [PubMed] [Google Scholar]
- 21. Ito M, Zipes DP. Efferent sympathetic and vagal innervation of the canine right ventricle. Circulation 90: 1459–1468, 1994 [DOI] [PubMed] [Google Scholar]
- 22. Kobayashi M, Sakurai S, Takaseya T, Shiose A, Kim HI, Fujiki M, Karimov JH, Dessoffy R, Massiello A, Borowski AG, Van Wagoner DR, Jung EJ, Fukamachi K. Effect of epivascular cardiac autonomic nerve stimulation on cardiac function. Ann Thorac Surg 94: 1150–1156, 2012 [DOI] [PubMed] [Google Scholar]
- 23. Kralios FA, Martin L, Burgess MJ, Millar K. Local ventricular repolarization changes due to sympathetic nerve-branch stimulation. Am J Physiol 228: 1621–1626, 1975 [DOI] [PubMed] [Google Scholar]
- 24. Lester SJ, Tajik AJ, Nishimura RA, Oh JK, Khandheria BK, Seward JB. Unlocking the mysteries of diastolic function: deciphering the Rosetta Stone 10 years later. J Am Coll Cardiol 51: 679–689, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Mantravadi R, Gabris B, Liu T, Choi BR, de Groat WC, Ng GA, Salama G. Autonomic nerve stimulation reverses ventricular repolarization sequence in rabbit hearts. Circ Res 100: e72–e80, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martins JB, Zipes DP. Effects of sympathetic and vagal nerves on recovery properties of the endocardium and epicardium of the canine left ventricle. Circ Res 46: 100–110, 1980 [DOI] [PubMed] [Google Scholar]
- 27. Marwick TH. Measurement of strain and strain rate by echocardiography: ready for prime time? J Am Coll Cardiol 47: 1313–1327, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Meyer C, Rana OR, Saygili E, Gemein C, Becker M, Nolte KW, Weis J, Schimpf T, Knackstedt C, Mischke K, Hoffmann R, Kelm M, Pauza D, Schauerte P. Augmentation of left ventricular contractility by cardiac sympathetic neural stimulation. Circulation 121: 1286–1294, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Norris JE, Randall WC. Responses of the canine myocardium to stimulation of thoracic cardiac nerves. Am J Physiol Heart Circ Physiol 232: H485–H494, 1977 [DOI] [PubMed] [Google Scholar]
- 30. Notomi Y, Lysyansky P, Setser RM, Shiota T, Popovic ZB, Martin-Miklovic MG, Weaver JA, Oryszak SJ, Greenberg NL, White RD, Thomas JD. Measurement of ventricular torsion by two-dimensional ultrasound speckle tracking imaging. J Am Coll Cardiol 45: 2034–2041, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Ogawa M, Zhou S, Tan AY, Song J, Gholmieh G, Fishbein MC, Luo H, Siegel RJ, Karagueuzian HS, Chen LS, Lin SF, Chen PS. Left stellate ganglion and vagal nerve activity and cardiac arrhythmias in ambulatory dogs with pacing-induced congestive heart failure. J Am Coll Cardiol 50: 335–343, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Puddu PE, Jouve R, Langlet F, Guillen JC, Lanti M, Reale A. Prevention of postischemic ventricular fibrillation late after right or left stellate ganglionectomy in dogs. Circulation 77: 935–946, 1988 [DOI] [PubMed] [Google Scholar]
- 33. Ramirez RJ, Ajijola OA, Zhou W, Holmstrom B, Luning H, Laks MM, Shivkumar K, Mahajan A. A new electrocardiographic marker for sympathetic nerve stimulation: modulation of repolarization by stimulation of stellate ganglia. J Electrocardiol 44: 694–699, 2011 [DOI] [PubMed] [Google Scholar]
- 34. Randall WC, Rohse WG. The augmentor action of the sympathetic cardiac nerves. Circ Res 4: 470–475, 1956 [DOI] [PubMed] [Google Scholar]
- 35. Randall WC, Szentivanyi M, Pace JB, Wechsler JS, Kaye MP. Patterns of sympathetic nerve projections onto the canine heart. Circ Res 22: 315–323, 1968 [DOI] [PubMed] [Google Scholar]
- 36. Randall WC, Wechsler JS, Pace JB, Szentivanyi M. Alterations in myocardial contractility during stimulation of the cardiac nerves. Am J Physiol 214: 1205–1212, 1968 [DOI] [PubMed] [Google Scholar]
- 37. Reant P, Labrousse L, Lafitte S, Bordachar P, Pillois X, Tariosse L, Bonoron-Adele S, Padois P, Deville C, Roudaut R, Dos Santos P. Experimental validation of circumferential, longitudinal, and radial 2-dimensional strain during dobutamine stress echocardiography in ischemic conditions. J Am Coll Cardiol 51: 149–157, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Schlack W, Schafer S, Thamer V. Left stellate ganglion block impairs left ventricular function. Anesth Analg 79: 1082–1088, 1994 [DOI] [PubMed] [Google Scholar]
- 39. Schlack W, Thamer V. Unilateral changes of sympathetic tone to the heart impair left ventricular function. Acta Anaesthesiol Scand 40: 262–271, 1996 [DOI] [PubMed] [Google Scholar]
- 40. Schwartz PJ, Stone HL. Left stellectomy in the prevention of ventricular fibrillation caused by acute myocardial ischemia in conscious dogs with anterior myocardial infarction. Circulation 62: 1256–1265, 1980 [DOI] [PubMed] [Google Scholar]
- 41. Sengupta PP, Khandheria BK, Narula J. Twist and untwist mechanics of the left ventricle. Heart Fail Clin 4: 315–324, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Sengupta PP, Tajik AJ, Chandrasekaran K, Khandheria BK. Twist mechanics of the left ventricle: principles and application. JACC Cardiovasc Imaging 1: 366–376, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Streeter DD, Jr, Spotnitz HM, Patel DP, Ross J, Jr, Sonnenblick EH. Fiber orientation in the canine left ventricle during diastole and systole. Circ Res 24: 339–347, 1969 [DOI] [PubMed] [Google Scholar]
- 44. Ueda H, Yanai Y, Murao S, Harumi K, Mashima S, Kuroiwa A, Sugimoto T, Shimomura D. Electrocardiographic and vectorcardiographic changes produced by electrical stimulation of the cardiac nerves. Jpn Heart J 28: 359–372, 1964 [DOI] [PubMed] [Google Scholar]
- 45. Vaseghi M, Shivkumar K. The role of the autonomic nervous system in sudden cardiac death. Prog Cardiovasc Dis 50: 404–419, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vaseghi M, Zhou W, Shi J, Ajijola O, Hadaya J, Shivkumar K, Mahajan A. Sympathetic innervation of the anterior left ventricular wall by the right and left stellate ganglia. Heart Rhythm 9: 1303–1309, 2012 [DOI] [PubMed] [Google Scholar]
- 47. Wang J, Khoury DS, Yue Y, Torre-Amione G, Nagueh SF. Left ventricular untwisting rate by speckle tracking echocardiography. Circulation 116: 2580–2586, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Weiss JL, Frederiksen JW, Weisfeldt ML. Hemodynamic determinants of the time-course of fall in canine left ventricular pressure. J Clin Invest 58: 751–760, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Winter J, Tanko AS, Brack KE, Coote JH, Ng GA. Differential cardiac responses to unilateral sympathetic nerve stimulation in the isolated innervated rabbit heart. Auton Neurosci 166: 4–14, 2012 [DOI] [PubMed] [Google Scholar]
- 50. Wong CW, Wang CH. Left stellate stimulation increases left ventricular ejection fraction in patients with essential palmar hyperhidrosis. J Auton Nerv Syst 78: 64–67, 1999 [DOI] [PubMed] [Google Scholar]
- 51. Yanowitz F, Preston JB, Abildskov JA. Functional distribution of right and left stellate innervation to the ventricles. Production of neurogenic electrocardiographic changes by unilateral alteration of sympathetic tone. Circ Res 18: 416–428, 1966 [DOI] [PubMed] [Google Scholar]
- 52. Zhou W, Mahajan A. Assessment of left ventricular systolic and diastolic function by 2D speckle tracking echocardiography. Circulation 118: S605–606, 2008 [Google Scholar]
- 53. Zhou W, Vaseghi M, Ramirez RJ, Patel S, Shivkumar K, Mahajan A. Interstitial norepinephrine levels and local electrophysiological properties of the myocardium during sympathetic nerve activation. FASEB J 25: 1098.1, 2011 [Google Scholar]
- 54. Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure–abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med 350: 1953–1959, 2004 [DOI] [PubMed] [Google Scholar]
- 55. Zipes DP, Barber MJ, Takahashi N, Gilmour RF., Jr Influence of the autonomic nervous system on the genesis of cardiac arrhythmias. Pacing Clin Electrophysiol 6: 1210–1220, 1983 [DOI] [PubMed] [Google Scholar]