Abstract
The uptake and metabolism of long chain fatty acids (LCFA) are critical to many physiological and cellular processes. Aberrant accumulation or depletion of LCFA underlie the pathology of numerous metabolic diseases. Protein-mediated transport of LCFA has been proposed as the major mode of LCFA uptake and activation. Several proteins have been identified to be involved in LCFA uptake. This review focuses on the SLC27 family of fatty acid transport proteins, also known as FATPs, with an emphasis on the gain- and loss-of-function animal models that elucidate the functions of FATPs in vivo and how these transport proteins play a role in physiological and pathological situations.
Keywords: SLC27, FATP, fatty acid transport proteins, fatty acid uptake, fatty acid activation
1. Introduction
One of the primary sources of energy for a cell is long-chain fatty acids (LCFA). Uptake and activation of LCFA is integral to many cellular processes, including membrane synthesis, intracellular signal transduction, energy metabolism, posttranslational modifications, and transcriptional regulation of metabolic genes (Kazantzis and Stahl, 2011). Many obesity-related diseases are due to an abnormal influx of LCFA from adipose stores into highly metabolic tissues such as heart, liver, and muscle, where the aberrant accumulation of lipids leads to insulin resistance, endoplasmic reticulum (ER) stress, and cell death (Kazantzis and Stahl, 2011). Therefore, studying the molecules responsible for cellular uptake of LCFA is key to identifying potential therapies for metabolic diseases.
When LCFA are released from their stores in adipose tissue or from the breakdown of triacylglycerides into the circulation they are bound to albumin, while in the intestine they form mixed micelles with bile acids (Schwenk et al., 2010). Upon crossing of the plasma membrane into the cytoplasm, LCFA become bound to cytoplasmic fatty acid binding proteins (FABPc) (Schwenk et al., 2010). While albumin-bound LCFA do have the ability to passively diffuse through the plasma membrane, the majority of LCFA uptake appears to be protein-mediated (Stahl et al., 2002). Several membrane proteins have been implicated in LCFA uptake, including fatty acid translocase (FAT)/CD36 (Coburn et al., 2000), plasma membrane fatty acid binding proteins (FABPpm) (Huang et al., 2002), long-chain fatty acyl-coenzyme A (CoA) synthetase (ACSL), and fatty acid transport proteins (FATP). This review will focus on the FATP family, also known as solute carrier family 27, which contains members A1 through 6 (SLC27A1-6) family (Table 1), and how they function in vivo in normal and pathological situations.
Table 1.
SLC27 family of transport proteins
| Human gene name | Protein name | Aliases | Predominant substrates | Transport type/coupling ions | Tissue distribution and cellular/subcellular expression | Link to disease | Human gene locus | Sequence accession ID (RefSeq) | Splice variants and their features |
|---|---|---|---|---|---|---|---|---|---|
| SLC27A1 | FATP1 | FATP, ACSVL5 | LCFA, VLCFA | LCFA transport, VLCFA activation | BAT, WAT, heart, skeletal muscle, skin, brain, kidney, endothelial cells | Unknown | 19p13.11 | NM_198580.1 | Unknown |
| SLC27A2 | FATP2 | ACSVL1, VLCS, VLACS, FACVL1 | LCFA, VLCFA | LCFA transport, VLCFA activation | Liver, kidney cortex, placenta | Unknown | 15q21.2 | NM_003645.3 (Variant 1), NM_001159629.1 (Variant 2) | Variant 1 encodes the full-length protein; Variant 2 lacks an in-frame coding exon, resulting in a shorter isoform missing an internal protein segment |
| SLC27A3 | FATP3 | ACSVL3, VLCS-3 | LCFA, VLCFA | LCFA transport, VLCFA activation | Skin, adrenal gland, testis, ovary, brain, lung, endothelial cells | Unknown | 1q21.3 | NM_024330.1 | Unknown |
| SLC27A4 | FATP4 | ACSVL4 | LCFA, VLCFA | LCFA transport, VLCFA activation | Small intestine, skin, placenta, brain, skeletal muscle, WAT, endothelial cells | Restrictive dermopathy (OMIM #275210) | 9q34.11 | NM_005094.3 | Unknown |
| SLC27A5 | FATP5 | ACSVL6, VLCS-H2, VLACSR, FACVL3, BAL, ACSB, BACS | LCFA, bile acids | LCFA transport, bile acid conjugation | Liver | Unknown | 19q13.43 | NM_012254.2 | Unknown |
| SLC27A6 | FATP6 | ACSVL2, VLCS-H1, FACVL2 | LCFA, VLCFA | LCFA transport, VLCFA activation | Heart, skin | Unknown | 5q23.3 | NM_014031.3 (Variant 1), NM_001017372.1 (Variant 2) | Variant 2 has an additional segment in the 5’ UTR but encodes the same protein as variant 1 |
LCFA: Long-chain fatty acid
VLCFA: Very long-chain fatty acid
BAT: Brown adipose tissue
WAT: White adipose tissue
2. The SLC27 family
The SLC27 gene family is comprised of six members, SLC27A1-6, which encode FATP1-6 (Table 1). SLC27A1, encoding FATP1, was the first member of this gene family to be identified through screening of a cDNA library from 3T3-L1 adipocytes for cDNAs that augment LCFA uptake (Schaffer and Lodish, 1994). Three FATPs have been listed in the transporter classification database: FATP1 (TC# 4.C.1.1.9.), FATP4 (TC# 4.C.1.1.1.) and FATP5 (TC# 4.C.1.1.8.). FATPs range from 63-80 kilodaltons (kDa) in size and are integral membrane proteins with at least one transmembrane domain (Fig. 1) (Lewis et al., 2001; Schaffer and Lodish, 1994). The N-terminus is located on the extracellular/luminal side and the C-terminus on the cytosolic side (Fig. 1) (Lewis et al., 2001; Schaffer and Lodish, 1994). All FATP members have a highly conserved, 311-amino acid signature sequence known as the FATP sequence, as well as an AMP binding domain, located on the C-terminus (Fig. 1). This region is responsible for the binding and uptake of LCFA and is commonly found in members of the ACSL family (Faergeman et al., 1997; Hirsch et al., 1998; Milger et al., 2006). A lipocalin motif, which is present in several proteins that are carriers of small hydrophobic molecules, has been identified near the N-terminus of FATP1 (Fig. 1) (Ordovas et al., 2006). Interestingly, FATP4 has been shown to have an ER localization signal domain, which aids in bringing the transport protein into the ER (Fig. 1) (Milger et al., 2006). While FATPs have sequence similarities, the proteins are differentially expressed in a wide variety of tissues and cell types, including adipose tissue, liver, skeletal muscle, heart, intestine, skin, and endothelial cells (Fig. 2).
Figure 1. Protein structure of FATP1.
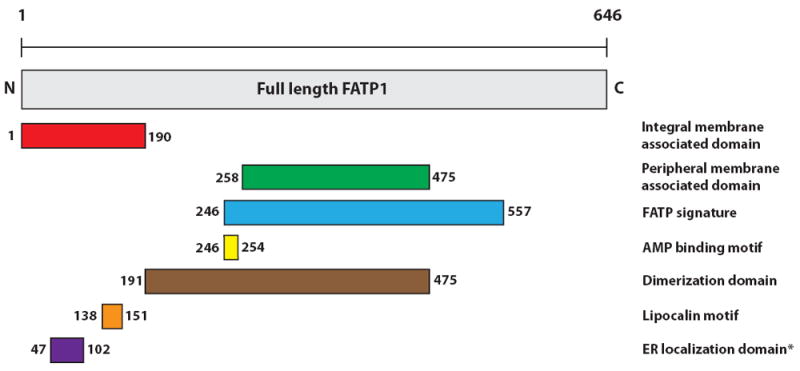
Structural domains of FATP1 are listed on the right. Locations of the domains within FATP1 are indicated by amino acid residue number. *ER localization domain has been identified only in FATP4.
Figure 2. FATP expression pattern in vivo.
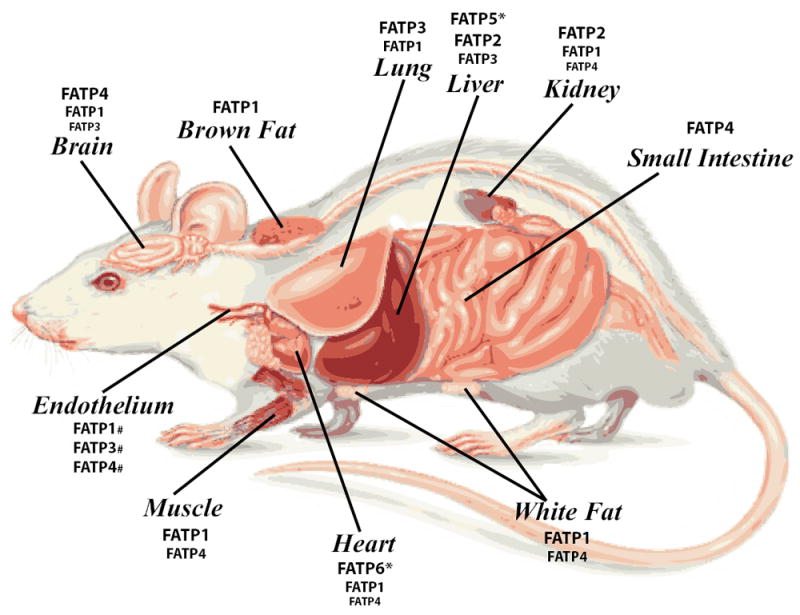
Relative expression levels of FATPs in mammalian tissues. Increasing font size corresponds to greater expression levels. *FATP5 is expressed only in the liver and FATP6 is expressed almost exclusively in the heart. #Relative expression level of each FATP in endothelial cells has not been determined.
3. Fatty acid transport and activation by FATP family members
Gain- and loss-of-function studies have demonstrated a categorical role for FATPs in mediating LCFA uptake (summarized in Tables 2 and 3). However, the precise mechanism by which FATPs function in LCFA uptake is still debatable. It has been proposed that FATPs can function as direct transporters of LCFA, as enzymes that activate LCFA through inherent acyl-CoA synthetase (ACS) activity, or as bifunctional proteins with independent transport and enzymatic activity.
Table 2.
Loss-of-function animal studies targeting SLC27 family members
| SLC27 isoform targeted | Animal model | Tissue specificity | Phenotype | Reference |
|---|---|---|---|---|
| SLC27A1/FATP1 | Knockout | Whole body | Reduced skeletal muscle lipid accumulation; improved insulin sensitivity after lipid challenges | (Kim et al., 2004) |
| SLC27A1/FATP1 | Knockout | Whole body | Abolished insulin-stimulated LCFA uptake by muscle and adipose tissue; redistribution of lipids from fat and muscle to liver | (Wu et al., 2006b) |
| SLC27A1/FATP1 | Knockout | Whole body | Defective non-shivering thermogenesis; reduced core body temperature and LCFA uptake by BAT in response to cold | (Wu et al., 2006a) |
| SLC27A2/FATP2 | Knockout | Whole body | Reduced VLCS activity in kidney and in liver peroxisomes/microsomes; reduced VLCFA -oxidation | (Heinzer et al., 2003) |
| SLC27A2/FATP2 | Knockdown | Liver | Protection from hepatosteatosis and improved glucose levels and insulin sensitivity following high-fat diet | (Falcon et al., 2010) |
| SLC27A2/FATP2 | Knockout | Whole body | No difference in litter size, genotype, placenta and embryo weight, and placental fat accumulation at E17.5 compared to control littermates | (Mishima et al., 2011) |
| SLC27A4/FATP4 | wrfr mutant mice | Whole body | Tight, thick, shiny, “wrinkle-free” skin, impaired hair growth, and severe breathing difficulties, neonatal death due to dehydration and restricted movement; retrotransposon insertion in coding exon of Slc27a4 leads to absence of Slc27a4 mRNA and FATP4 protein | (Moulson et al., 2003) |
| SLC27A4/FATP4 | Knockout | Whole body | Lower body weight, facial dysmorphia, rigid and thickened wrinkle-free skin, restricted movement, abnormal breathing and suckling; neonatal death; disturbed epidermal fatty acid composition | (Herrmann et al., 2003) |
| SLC27A4/FATP4 | Heterozygote and Knockout | Whole body | KO is embryonic lethal by E9.5; hets display reduced enterocyte LCFA uptake but no effects on fat absorption | (Gimeno et al., 2003a) |
| SLC27A4/FATP4 | Knockout | Whole body | Reduced VLCFA activation in skin, small intestine, and brain | (Hall et al., 2005) |
| SLC27A4/FATP4 | Inducible knockout | Epidermis | No differences in appearance or behavior; mice still alive 13 weeks after tamoxifen treatment; histology shows hyperproliferative hyperkeratosis; similar phenotype to whole body knockout (Herrmann T 2003) | (Herrmann et al., 2005) |
| SLC27A4/FATP4 | wrfr mutant mice | Whole body | Dermal fibroblast cell line from these mice have reduced fatty acid uptake and VLCFA activity, and have fewer but larger lipid droplets | (Jia et al., 2007) |
| SLC27A4/FATP4 | Knockout | Whole body | KO mice display epidermal hyperplasia; microarray on embryonic skin shows increased expression of epidermal growth factor (EGF) family members; increased EGF receptor and STAT3 epidermal activity | (Lin et al., 2010) |
| SLC27A4/FATP4 | Knockout | Adipocytes | Increased weight gain, visceral adipocyte hypertrophy, and hepatic steatosis on high fat/high LCFA diet | (Lenz et al., 2011) |
| SLC27A4/FATP4 | Knockout | Whole body | No difference in litter size, genotype, placental and embryo weight, and placental fat accumulation at E17.5 compared to control littermates | (Mishima et al., 2011) |
| SLC27A5/FATP5 | Knockout | Whole body | Enlarged hepatocytes; reduced LCFA uptake in hepatocytes and triglyceride levels in liver; increased fatty acid synthetase expression and de novo fatty acid synthesis | (Doege et al., 2006) |
| SLC27A5/FATP5 | Knockout | Whole body | Gallbladders have more unconjugated than conjugated bile acids due to defective reconjugation; failure to gain weight on high-fat diet due to reduced food intake and increased energy expenditure | (Hubbard et al., 2006) |
| SLC27A5/FATP5 | Knockdown | Liver | Reduced fatty acid absorption in liver and increased lipid deposition in heart, muscle, and fat; reversal of hepatic steatosis; improved whole body energy homeostasis | (Doege et al., 2008) |
LCFA: Long-chain fatty acid
VLCFA: Very long-chain fatty acid
VLCS: Very long-chain acyl-CoA synthetase
BAT: Brown adipose tissue
KO: Knockout
Hets: Heterozygotes
Table 3.
Gain-of-function animal studies targeting SLC27 family members
| SLC27 isoform targeted | Animal model | Tissue specificity | Phenotype | Reference |
|---|---|---|---|---|
| SLC27A1/FATP1 | Overexpressing transgenic | Heart | Increased LCFA uptake and accumulation in heart; diastolic dysfunction; metabolic cardiomyopathy | (Chiu et al., 2005) |
| SLC27A1/FATP1 | Overexpressing electrotransfection | Skeletal muscle | Increased fatty acid transport into skeletal muscle and rate of fatty acid oxidation, but no change in intramuscular triacylglycerol stores | (Nickerson et al., 2009) |
| SLC27A1/FATP1 | Overexpressing transgenic | Skeletal muscle | Increased sarcolemmal LCFA transport and oxidation and no change in intramuscular lipid accumulation; no effect on diet-induced insulin resistance | (Holloway et al., 2011) |
| SLC27A4/FATP4 | Overexpressing transgenic in FATP4 whole body knockout | Whole body | Rescue of the neonatal lethal phenotype and most skin defects observed in FATP4 KO mice (Herrmann T 2003) | (Moulson et al., 2007) |
| SLC27A4/FATP4 | Overexpressing transgenic in FATP4 whole body knockout | Keratinocytes and hair follicles | Rescue of the neonatal lethal phenotype and most skin defects observed in FATP4 KO mice (Herrmann T 2003); this rescue requires FATP4 with an intact acyl-CoA synthetase domain; minor alterations in hair types | (Moulson et al., 2007) |
| SLC27A4/FATP4 | Overexpressing transgenic in FATP4 whole body knockout | Keratinocytes and hair follicles | No change in weight gain, intestinal fat and cholesterol absorption, intestinal LCFA uptake and secretion, and hepatic lipid content and secretion; high-fat diet increased TG and fatty acid content in intestine | (Shim et al., 2009) |
| SLC27A4/FATP4 | Overexpressing electrotransfection | Skeletal muscle | Increased fatty acid transport into skeletal muscle and rate of fatty acid oxidation, but no change in fatty acid activation and intramuscular triacylglycerol stores | (Nickerson et al., 2009) |
LCFA: Long-chain fatty acid
KO: Knockout
TG: Triglyceride
3.1 Fatty acid uptake function of FATPs
FATP1 was the first FATP to be identified based on its ability to increase LCFA uptake upon overexpression in cells (Schaffer and Lodish, 1994). Since then, many studies have demonstrated the direct role FATPs play in LCFA uptake in a variety of tissues and cell types (Doege et al., 2006; Falcon et al., 2010; Stahl et al., 1999). In order for FATP1 to function in the uptake of LCFA dimerization is required, but the ability of other FATPs to form homodimers remains to be determined (Richards et al., 2003).
3.2 ACS activity of FATPs
In addition to a role in fatty acid uptake, FATPs have also been shown to display ACS activity. ACS enzymes catalyze the conversion of LCFA to acyl-CoA thioesters in order to activate LCFA. Activated LCFA can then be used by the cell in many metabolic processes, such as fatty acid synthesis and oxidation and phospholipids synthesis (Black and DiRusso, 2007b). Mutations in the yeast homolog of FATP1, Fat1p, resulted in reduced very-long-chain ACS (VLACS) activity (Watkins et al., 1998). Overexpression of wild-type FATP1 in COS1 cells led to increased ACS activity, while mutation of a domain within FATP1 that is highly conserved in ACS proteins abolished ACS activity (Coe et al., 1999). Concomitant with reduced ACS activity, these mutations also led to decreased LCFA uptake, suggesting that FATPs are involved in both fatty acid activation and uptake (Choi and Martin, 1999; Coe et al., 1999; Watkins et al., 1998).
Interestingly, both the yeast and murine forms of FATP1 and ACSL-1, a member of the acyl-CoA synthetase long-chain family, may interact with each other to form a complex (Richards et al., 2006; Zou et al., 2003). Inhibition of ACSL-1 impaired LCFA uptake, suggesting that fatty acid activation is crucial for LCFA uptake and that the interaction of FATP1 and ACSL-1 is responsible for LCFA uptake (Richards et al., 2006). Furthermore, overexpression of FATP4 in cells led to increased ACS activity, and mice lacking FATP4 had reduced LCFA activation in the skin and intestine (Hall et al., 2005; Herrmann et al., 2001). The findings that FATPs have intrinsic ACS activity, together with studies demonstrating the direct role of FATPs in LCFA uptake, support the notion of vectorial acylation, in which LCFA transport is directly coupled to fatty acid activation (Black and DiRusso, 2007a).
3.3. Subcellular localization of FATPs
Studies examining the subcellular localization of FATPs have supported a role for the proteins in both fatty acid uptake as well as activation. FATPs can reside in both the plasma membrane as well as the intracellular space. FATP1 was first identified as an integral membrane protein that localizes to the plasma membrane (Schaffer and Lodish, 1994). FATP4 is localized only to the apical side of intestinal enterocytes, where it is associated with the plasma membrane (Stahl et al., 1999). Another study demonstrated the presence of FATP4 in the ER of intestinal enterocytes (Milger et al., 2006). The differences in FATP4 intestinal location could be attributable to the theory that only a fraction of FATP4 is actually translocating to the membrane from the ER (Stahl et al., 1999). Similar to other solute carriers, such as the glucose transporter family (GLUT), the location of FATPs is not static: FATP1 can translocate from the cytoplasm to the plasma membrane in response to insulin (Stahl et al., 2002). The identification of FATPs in the plasma membrane as well as the cytoplasm lends credence to its role as a bifunctional protein in both fatty acid uptake and activation.
4. FATP family members
4.1 FATP1
FATP1 was the first isoform of the SLC27 family to be identified and is encoded by the SLC27A1 gene (Schaffer and Lodish, 1994). It is a plasma membrane protein that, when overexpressed in 3T3-L1 fibroblasts, leads to increased LCFA uptake (Schaffer and Lodish, 1994). FATP1 is highly expressed in skeletal muscle, heart, white adipose tissue (WAT) and brown adipose tissue (BAT), with an increase in expression upon differentiation of 3T3-L1 cells from preadipocytes to adipocytes (Fig. 2) (Schaffer and Lodish, 1994; Stahl et al., 2002). FATP1 is also expressed, albeit in lower levels, in brain, kidney, lung, and liver, as well as keratinocytes (Schaffer and Lodish, 1994; Schmuth et al., 2005).
Insulin is a critical regulator of FATP1 in vitro. The Slc27a1 gene has an insulin response sequence, and insulin decreases FATP1 transcript levels in white adipose tissue (Hui et al., 1998; Man et al., 1996). While insulin did not alter FATP1 protein levels, it did induce the translocation of FATP1 from a perinuclear, intracellular compartment to the plasma membrane (Jain et al., 2009; Stahl et al., 2002). Subsequently, increased plasma membrane levels of FATP1 in response to insulin led to increased postprandial LCFA uptake (Stahl et al., 2002). In addition, treatment of 3T3-L1 adipocytes with TNF-, which antagonizes the effects of insulin, suppressed FATP1 expression and LCFA uptake (Stahl et al., 2002). Furthermore, knockdown of FATP1 in 3T3-L1 adipocytes resulted in reduced basal as well as insulin-stimulated LCFA uptake (Lobo et al., 2007). Taken together, these results suggest that insulin regulates adipocyte LCFA uptake by inducing translocation of FATP1 from an intracellular compartment to the plasma membrane, perhaps due to a posttranslational modification of FATP1.
In addition to insulin, FATP1 expression can be modulated in response to cold and 3-adrenergic receptor (3-AR) stimuli in BAT (Wu et al., 2006a). Stimulation of 3-AR, either by cold exposure or by treatment with the 3-AR agonist isoproterenol, activates a signaling cascade that results in increased expression of the BAT-specific mitochondrial uncoupling protein 1 (UCP1) and heat generation (Lafontan and Berlan, 1993). FATP1, which is expressed on the plasma membrane of BAT, is greatly increased in BAT after mice were exposed to the cold for 12 hours (Wu et al., 2006a). Furthermore, treatment of brown adipocytes with isoproterenol resulted in increased FATP1 expression (Wu et al., 2006a). Taken together, these results show that FATP1 expression in BAT is regulated by 3-AR stimulation.
While insulin and 3-AR stimulation are major regulators of FATP1 expression and function, the transport protein is also regulated by other factors involved in adipocyte differentiation and metabolism. Peroxisome proliferator-activated receptors (PPARs) are members of the steroid hormone receptor family and expressed in highly metabolic tissues (Amri et al., 1995; Kliewer et al., 1994). The Slc27a1 promoter contains a PPAR response element, to which PPAR and PPAR can bind and upregulate FATP1 expression in adipocytes and liver (Frohnert et al., 1999; Martin et al., 1997). In addition to PPARs, a member of the nuclear receptor superfamily that is highly expressed in adipose tissue, liver, and muscle, TR4, was recently shown to regulate Slc27a1 gene expression (Choi et al., 2011). The Slc27a1 promoter contains a TR4 response element, and binding of TR4 to this element induced activity of the promoter (Choi et al., 2011). Adipocytes overexpressing TR4 increased expression of FATP1, along with a subsequent increase in lipid accumulation, and knockdown of TR4 inhibited Fatp1 promoter activity and expression, suggesting that Fatp1 is positively regulated by TR4 to facilitate LCFA uptake and lipid accumulation (Choi et al., 2011).
Genetic studies in mice targeting Slc27a1 suggest a role for FATP1 in lipid metabolism. Mice lacking FATP1 had no change in weight gain and no defects in fatty acid uptake compared to control mice when placed on a high-fat diet (Kim et al., 2004). However, Slc27a1 knockout (KO) mice were protected from high-fat diet-induced accumulation of fatty acid metabolites in skeletal muscle and insulin resistance (Kim et al., 2004). Furthermore, insulin-stimulated LCFA uptake in adipocytes and skeletal muscle from Slc27a1 KO mice was severely blunted compared to control tissues (Wu et al., 2006b). However, serum free-fatty acid levels were increased in Slc27a1 KO mice, as was LCFA uptake in the liver (Wu et al., 2006b). These findings demonstrate that Slc27a1 KO mice have a redistribution of dietary lipids from skeletal muscle and adipose tissue to the liver, protecting them from diet-induced obesity and insulin resistance. Interestingly, Slc27a1 KO mice also displayed normal extracellular FA and glycerol levels (Wu et al., 2006b). These findings, coupled with the result that Slc27a1 KO mice have reduced insulin-stimulated LCFA uptake, suggest that FATP1 is predominantly involved in fatty acid uptake and not efflux, and that insulin regulates fatty acid uptake via FATP1.
Because FATP1 is localized to the plasma membrane of BAT, the requirement for FATP1 in non-shivering thermogenesis was examined (Wu et al., 2006a). An increase in FATP1 expression in response to cold stimuli was coupled with an increase in LCFA uptake in wild-type mice (Wu et al., 2006a). However, this cold-induced increase in LCFA uptake was abolished in Slc27a1 KO mice, and these mice also had significantly smaller BAT lipid droplets (Wu et al., 2006a). Consequently, Slc27a1 KO mice failed to defend their core body temperature when exposed to the cold (Wu et al., 2006a). Slc27a1 KO mice also displayed impaired LCFA uptake and reduced metabolic burst upon adrenergic stimulation, which is required for the ultimate expression of the inner mitochondrial protein and mature brown adipocyte marker UCP-1 (Wu et al., 2006a). Taken together, these findings support a role for FATP1 in non-shivering thermogenesis.
In the heart and vasculature, FATP1 is one of the predominant fatty acid transport proteins (Schaffer and Lodish, 1994). An imbalance between fatty acid import and utilization in cardiomyocytes results in LCFA accumulation, which leads to the development of cardiomyopathy (Chiu et al., 2005). Mice overexpressing FATP1 specifically in the heart had increased fatty acid uptake and lipid accumulation in cardiomyocytes and, consequently, developed lipotoxic cardiomyopathy (Chiu et al., 2005). In rats that underwent a myocardial infarction, where there is a shift away from fatty acid utilization, FATP1 protein levels were decreased, along with myocardial lipid oxidation and incorporation (Heather et al., 2006). Recently, it has been shown that endothelial cells express FATP1 (Mitchell et al., 2011; Sandoval et al., 2008). FATP1 is one of the predominant isoforms expressed in the microvessel endothelial cells of the blood brain barrier, and knockdown of FATP1 in human brain microvessel endothelial cells (HBMEC) decreased fatty acid transport across the plasma membrane (Mitchell et al., 2011). These findings highlight FATP1 as a potential therapeutic target in metabolic cardiomyopathies.
In humans, FATP1 has been shown to be associated with defects in lipid metabolism. SLC27A1 expression was reduced in the skeletal muscle of obese woman, and there was a negative correlation between SLC27A1 expression and body mass index in both obese and lean women (Binnert et al., 2000). Furthermore, several single nucleotide polymorphisms (SNPs) within SLC27A1 have been identified to be associated with lipid metabolism problems. One study in a French population identified an A/G substitution within intron 8, in which the A allele was associated with increased fasting plasma triglyceride levels in healthy women (Meirhaeghe et al., 2000). Supporting this finding, another study in a Swedish population showed that the A allele in this position was associated with elevated fed plasma triglyceride levels in healthy men, as well as variations in LDL size and distribution (Gertow et al., 2003). Overall, these studies demonstrate that there is genetic variability within the human SLC27A1 gene, and the resulting effects on lipid metabolism identify FATP1 as a potential therapeutic target for maintaining lipid homeostasis.
4.2 FATP2
FATP2 is encoded by the SLC27A2 gene and expressed primarily in the liver and kidney (Fig. 2), but has also been shown to be present in trophoblasts of the human placenta (Krammer et al., 2011; Mishima et al., 2011; Steinberg et al., 1999). FATP2 is unique to other FATPs in that it has been shown to function as both a fatty acid transporter and an ACS. FATP2 was first identified as VLACS, with subcellular localization in liver peroxisomes and microsomes (Uchiyama et al., 1996). FATP2 overexpression in hepatic cells increased ACS activity, which drives fatty acid uptake indirectly by trapping activated fatty acids inside the cell and creating a concentration gradient that facilitates fatty acid uptake (Krammer et al., 2011). Overexpression of FATP2 in cell culture also directly increased LCFA uptake and activation (Falcon et al., 2010; Krammer et al., 2011; Steinberg et al., 1999). The role of FATP2 as both a FATP and ACS correlates with its multiple subcellular locations, including peroxisomes, the plasma membrane, and the ER (Falcon et al., 2010; Krammer et al., 2011; Steinberg et al., 1999; Uchiyama et al., 1996).
It was recently identified that SLC27A2 actually has two splice variants in humans encoding FATP2a and FATP2b (Melton et al., 2011). While both variants can mediate fatty acid uptake, only FATP2a has ACS activity, with a preference for VLCFA (Melton et al., 2011).
Studies targeting FATP2 in mice confirm its function as a fatty acid transporter, as well as a peroxisomal VLACS. Mice either lacking FATP2 or with reduced hepatic FATP2 had decreased ACS activity in the liver and kidney, specifically in the peroxisomes and microsomes (Falcon et al., 2010; Heinzer et al., 2003). Knockdown of FATP2 in the liver resulted in only 51% reduced VLACS activity in peroxisomes, suggesting that another enzyme is mediating the remaining hepatic peroxisomal VLACS activity and that FATP2 is not the only VLACS in liver peroxisomes (Falcon et al., 2010). Slc27a2 KO mice also had reduced VLCFA -oxidation, but interestingly intracellular VLCFA levels were unchanged (Heinzer et al., 2003). These results demonstrate that, despite a decrease in VLCFA degradation, VLCFA do not accumulate in Slc27a2 KO mice. Mice with reduced FATP2 specifically in the liver had decreased LCFA uptake by hepatocytes but no change in VLACS activity (Falcon et al., 2010). These results suggest that FATP2 may play a more prominent role in hepatic LCFA uptake than in peroxisomal ACS activity. Additionally, knockdown of FATP2 was able to reverse established, diet-induced hepatosteatosis and improve insulin sensitivity (Falcon et al., 2010). Taken together, these animal studies suggest that FATP2 could be a novel target for NAFLD treatment.
In addition to playing a role in lipid metabolism, FATP2 may also be involved in bile acid synthesis from cholesterol. Overexpression of FATP2 in COS-1 cells led to activation of the precursor of cholic acid, 3,7,12-trihydroxy-5 -cholestanoic acid (THCA), to its CoA derivative, THCA-CoA, demonstrating that FATP2 exhibits THCA-CoA synthetase activity, and that this occurs in the peroxisome (Mihalik et al., 2002). However, FATP2 did not activate cholate to its CoA derivative, demonstrating that FATP2 does not directly activate bile acids (Mihalik et al., 2002). These results suggest that FATP2 may be involved in de novo synthesis of bile acids and not in the reactivation or recycling of bile acids, however this has not been proven in vivo.
Recently, expression of FATP2 was identified in placental trophoblasts (Mishima et al., 2011). Normal embryonic development requires, among many transported nutrients, efficient fatty acid uptake from maternal blood (Haggarty, 2002). Therefore, the presence of FATP2 in the placenta suggested a role for the transport protein in embryonic development (Mishima et al., 2011). However, Slc27a2 KO mice at embryonic day 17.5 (E17.5) were present at normal Mendelian ratios, and Slc27a2 KO embryos and placentas exhibited normal weight, morphology, and triglyceride content (Mishima et al., 2011). These results demonstrate that, despite its expression in the placenta, FATP2 is actually dispensable for embryonic development and intrauterine fetal growth, potentially due to the redundant expression of other FATP family members.
4.3 FATP3
Very little is known about FATP3, which is encoded by the human SLC27A3 gene. Expression analysis has shown mouse Slc27a3 to be highly expressed in the mouse adrenal gland, testis, ovary, and lung (Fig. 2) (Pei et al., 2004). Additionally, while Slc27a3 is weakly expressed in the neonatal and adult brain, it is highly expressed in the embryonic brain (Pei et al., 2004). FATP3 protein has also been shown to be present in both mouse and human epidermal keratinocytes, with more prominent expression on the baso-lateral side of the cells (Schmuth et al., 2005).
In addition, a recent study identified the induction of FATP3 expression by vascular endothelial growth factor B (VEGF-B) in endothelial cells (Hagberg et al., 2010). In cultured and human primary endothelial cells, VEGF-B robustly increased Slc27a3 expression, and this effect was inhibited by the addition of antibodies to VEGFR1 and NRP1, receptors essential for mediating VEGF-B signaling (Hagberg et al., 2010). Furthermore, in the hearts of mice lacking total VEGF-B or the VEGFR1 tyrosine kinase domain, or NRP1 in endothelial cells, Slc27a3 expression was significantly reduced, (Hagberg et al., 2010). These results demonstrate that FATP3 is regulated by VEGF-B via VEGFR1 and NRP1 signaling, and suggest a role for FATP3 in endothelial cell fatty acid metabolism (Hagberg et al., 2010). In tissues such as the liver, which have capillaries with fenestrated, or porous, endothelium, LCFA can directly access the parenchymal cells from the circulation through the fenestrated endothelium (Stremmel et al., 2001). However, endothelial cells in tissues such as the heart, muscle and fat have tight junctions which prevent the direct uptake of LCFA by these tissues (Stremmel et al., 2001). Therefore, the findings that FATPs are present in endothelial cells and are regulated by VEGF-B demonstrate that LCFA may actually cross endothelial cell membranes via FATPs in order to enter tissues such as the heart and muscle.
There is still doubt as to whether FATP3 is truly a fatty acid transporter. Despite decreased ACS activity in FATP3 knockdown cells, there was no difference in LCFA uptake in these cells (Pei et al., 2004). In yeast, FATP3 only weakly promotes LCFA uptake (DiRusso et al., 2005). The subcellular localization of FATP3 is also still debatable: in one study, FATP3 was not detectable in subcellular fractions containing the plasma membrane (Pei et al., 2004); in yeast, the murine homolog of FATP3 did localize to the yeast plasma membrane (DiRusso et al., 2005). The function of FATP3 in vivo has yet to be determined, as no animal models targeting Slc27a3 have been generated.
4.4 FATP4
FATP4, which is encoded by the SLC27A4 gene, was first identified as the major intestinal fatty acid transporter. It is the primary FATP expressed in enterocytes and located specifically on the apical side of the intestinal epithelial cells (Herrmann et al., 2001; Stahl et al., 1999). FATP4 is also expressed at lower levels in brain, kidney, liver and heart, as well as trophoblasts of the placenta and endothelial cells (Fig. 2) (Fitscher et al., 1998; Herrmann et al., 2001; Mishima et al., 2011; Mitchell et al., 2011; Sandoval et al., 2008). Overexpression of FATP4 led to increased LCFA uptake, and knockdown of FATP4 in enterocytes reduced LCFA uptake (Stahl et al., 1999). In endothelial cells, similarly to FATP1, knockdown of FATP4 impaired fatty acid transport across the plasma membrane (Mitchell et al., 2011). These findings show that FATP4 functions similarly as other FATPs in facilitating fatty acid uptake.
While FATP4 is the primary fatty acid transporter in the intestine, FATP4 is also expressed in skin, where it has been shown to play a major role in epidermal development (Herrmann et al., 2001; Schmuth et al., 2005). FATP4 is highly expressed in neonatal keratinocytes but becomes restricted to the sebaceous glands in the adult (Schmuth et al., 2005). Mutant mice with a spontaneous, autosomal recessive mutation, known as “wrinkle-free” or wrfr mice, harbor a retrotransposon insertion in the coding exon of Slc27a4 (Moulson et al., 2003). These mice exhibited tight, thick, shiny, wrinkle-free skin, as well as impaired hair growth and severe breathing difficulties (Moulson et al., 2003). wrfr mice ultimately died shortly after birth due to dehydration and restricted movement (Moulson et al., 2003). The phenotype of wrfr mice mimics a human condition known as restricted dermopathy (Moulson et al., 2003).
Several studies in mice with a targeted mutation in Slc27a4 have confirmed a role for the transport protein in skin and hair development. Mice with a targeted disruption in intron 2 of Slc27a4 displayed features of restrictive dermopathy, including lower body weight, facial dysmorphia, rigid and thick skin, and flexion contracture (Herrmann et al., 2003). The rigid skin restricted movement, leading to severely hindered breathing and suckling and eventually neonatal death (Herrmann et al., 2003). Slc27a4 KO mice also had a disturbed epidermal barrier with an altered lipid composition that formed early but never progressed to completion (Herrmann et al., 2003; Lin et al., 2010). To confirm that these effects are not due to the lack of FATP4 in other tissues where it is expressed, Slc27a4 was selectively deleted only in epidermal keratinocytes after birth (Herrmann et al., 2005). These mice displayed a phenotype similar to that seen in Slc27a4 KO mice, including hyperproliferative hyperkeratosis and a disturbed epidermal barrier (Herrmann et al., 2005). Furthermore, keratinocyte-specific overexpression of FATP4 in Slc27a4 KO mice was able to rescue the neonatally lethal skin defects, resulting in viable and fertile mice (Moulson et al., 2007).
Several members of the epidermal growth factor (EGF) family were upregulated in Slc27a4 KO mice, including Ereg, Areg and Epgn, and epidermal activation of the EGF receptor (EGFR) and the downstream signaling molecule STAT3 were increased (Lin et al., 2010). Treatment of Slc27a4 KO mice with inhibitors of the EGFR pathway resulted in suppressed STAT3 activity, reduced skin thickening, and attenuated epidermal barrier disruptions (Lin et al., 2010). These findings highlight a surprising requirement of FATP4 in epidermal keratinocytes, possibly by negatively influencing EGFR activation and STAT3 signaling during normal epidermal development.
Given its high expression levels in the intestine, it has been hypothesized that FATP4 plays a role in fat absorption. Mice that have a targeted disruption of Slc27a4 located upstream of exon 2 died by E9.5 (Gimeno et al., 2003a). It is of note that the targeted disruption upstream of exon 2 may result in a different form of FATP4 compared to the mutation located in intron 2/exon 3, which may give rise to a truncated form of FATP4 that leads to a less severe phenotype. In any case, mice that are heterozygous for the exon 2 Slc27a4 mutation were viable and, despite lacking any fat absorption defects, had reduced LCFA uptake (Gimeno et al., 2003a). The authors show that FATP4 is highly expressed in epithelial cells of the visceral endoderm, specifically located on the brush-border membrane of extraembryonic endodermal cells (Gimeno et al., 2003a), suggesting a role for FATP4 in fat absorption. To specifically address the requirement of FATP4 in the intestine, FATP4 was overexpressed in the keratinocytes of Slc27a4 KO mice (Shim et al., 2009). These mice, when fed a chow or high-fat diet, displayed no difference in growth, weight, food consumption, intestinal triglyceride absorption, fecal fat losses, cholesterol absorption, and hepatic VLDL secretion or lipid content (Shim et al., 2009). However, they did have lower serum cholesterol levels and higher enterocyte triglyceride and fatty acid content when fed a high-fat diet (Shim et al., 2009). Control mice treated with ezetimibe, a drug that inhibits cholesterol absorption, and mice lacking NPC1L1, a protein that mediates the cholesterol absorption pathway, both had reduced weight gain and saturated fatty acid absorption, which appears to be due to reduced FATP4 expression in the intestine (Labonte et al., 2008). Taken together, these results suggest a role for FATP4 in early embryonic fat absorption, and possibly in adult dietary lipid absorption.
The mechanisms by which FATP4 enhances fatty acid uptake has been somewhat controversial. Cells with either overexpression or knockdown of FATP4 had no change in fatty acid influx (Lobo et al., 2007). Mice with a conditional deletion of Slc27a4 in adipocytes displayed increased weight gain and subcutaneous fat mass, adipocyte hypertrophy, and an altered lipid metabolic profile, but no changes in LCFA uptake when placed on a high-fat diet suggesting that FATP4 in adipocytes is dispensable for fatty acid uptake (Lenz et al., 2011). Overexpression of FATP4 in the keratinocytes of Slc27a4 KO mice resulted in no change in intestinal LCFA uptake and secretion rates (Shim et al., 2009). Instead, an additional role for FATP4 as an ACS has been proposed. Overexpression of FATP4 in cells led to increased ACS activity that is more pronounced for VLCFA (Herrmann et al., 2001). Mice lacking FATP4 had reduced esterified VLCFA in the skin, intestine, and brain (Hall et al., 2005). Rescue of mice lacking FATP4 with keratinocyte-specific overexpression of FATP4 required an intact ACS domain (Moulson et al., 2007). These results support a role for FATP4 in VLCFA activation and a function as an ACS.
Genetic studies in mice highlighting the requirement of FATP4 for fat absorption and epidermal development have led to the identification of several mutations and polymorphisms within the human SLC27A4 gene. A G/A polymorphism within exon 3 of SLC27A4, which gives rise to a Gly209Ser substitution, was shown to be associated with symptoms of insulin resistance (Gertow et al., 2004a). Carriers of the Ser209 allele displayed lower body mass index (BMI), systolic blood pressure, plasma triglyceride levels, and insulin concentrations (Gertow et al., 2004a). In addition, SLC27A4 is upregulated in acquired obesity, and its expression level correlates with obesity and insulin resistance (Gertow et al., 2004b).
Several mutations in SLC27A4 have been identified in patients with ichthyosis prematurity syndrome (IPS), which is characterized by thickened epidermis and respiratory complications and displays symptoms that are similar to the phenotype seen in Slc27a4 KO mice (Herrmann et al., 2003; Klar et al., 2009; Sobol et al., 2011). In a Scandinavian population with IPS, all patients were found to be homozygous or compound heterozygous for a nonsense mutation in exon 3 of SLC27A4, known as p.C168X (Klar et al., 2009). IPS patients with this mutation had no detectable FATP4 in their keratinocytes and increased epidermal lipid droplets, in addition to reduced VLCFA-CoA synthetase activity and VLCFA incorporation into lipids (Klar et al., 2009). Missense mutations have also been identified within SLC27A4 in families from Scandinavia affected by IPS (Sobol et al., 2011). The missense mutations p.V477D and p.R504H are located within the AMP binding domain and the FATP motif of FATP4, domains that are crucial for transport and activation of fatty acids (Sobol et al., 2011; Zou et al., 2002). Identification of mutations and polymorphisms within SLC27A4 that are associated with obesity, insulin resistance, and skin disorders highlight a role for FATP4 in fatty acid metabolism and epidermal formation in human health.
4.5 FATP5
FATP5 is encoded by the SLC27A5 gene and expressed exclusively in the liver, specifically in the basal membrane of hepatocytes where it functions as a fatty acid transport protein (Fig. 2) (Doege et al., 2006). Hepatocytes from mice lacking FATP5 had reduced LCFA uptake, while cells overexpressing FATP5 had increased LCFA uptake (Doege et al., 2006). Slc27a5 KO mice also displayed lower hepatic triglyceride and fatty acid content but, interestingly, increased fatty acid synthetase expression (Doege et al., 2006). Further analysis demonstrated that Slc27a5 KO mice had a redistribution of lipids from the liver to other tissues that metabolize fatty acids (Doege et al., 2006). In addition, Slc27a5 KO mice fed a high-fat diet had normal fat absorption but failed to gain weight due to reduced food intake and increased energy expenditure (Hubbard et al., 2006). These results demonstrate that FATP5 is required for efficient fatty acid uptake by hepatocytes and liver lipid homeostasis and is involved in body weight regulation.
Given this role for FATP5 in normal liver fatty acid uptake, the requirement of FATP5 in the development of non-alcoholic fatty liver disease (NAFLD) was examined (Doege et al., 2008). Knockdown of FATP5 in the livers of mice fed a high-fat diet resulted in reduced hepatic dietary LCFA uptake and decreased weight gain due to reduced food consumption (Doege et al., 2008). These results suggest that knockdown of FATP5 is protective against NAFLD. Furthermore, knockdown of FATP5 in the livers of mice who were already on a high-fat diet and continued on a high-fat diet resulted in significant reduction in lipid droplet deposition, hepatic triglyceride content, and serum glucose levels (Doege et al., 2008). A recent study showed that knockdown of FATP5 in APOB knockdown mice, which have hepatic steatosis, had no effect on liver triglyceride levels (Ason et al., 2011). However, this study only examined the effect of FATP5 knockdown on triglyceride de novo synthesis and not dietary fat uptake. Therefore, these results demonstrate that knockdown of FATP5 is able to reverse already established diet-induced hepatic steatosis in mice. In humans, it was shown that SLC27A5 expression is increased in patients with early-stage NAFLD (Mitsuyoshi et al., 2009). Taken together, these findings from studies in mice and humans suggest that inhibition of FATP5 could be a novel treatment for NAFLD.
In addition to fatty acid transport activity and lipid metabolism, FATP5 also functions in bile acid metabolism. The rat ortholog of FATP5 is bile acid-CoA ligase (BAL), which catalyzes conjugation of bile acids with amino acids (Falany et al., 2002). Human FATP5 activates the primary unconjugated bile acid (cholic acid) to its CoA thioester derivative (cholate), as well as secondary bile acids, via its bile acid-CoA ligase activity (Mihalik et al., 2002; Steinberg et al., 2000). In mice lacking FATP5, the gallbladders had mostly unconjugated forms of bile acids, and of the conjugated forms, had mostly primary and few secondary bile acids, suggesting that FATP5 is required for bile acid reconjugation but not de novo synthesis 2006 (Hubbard et al., 2006). Knockdown of FATP5 in mice with hepatic steatosis from reduced APOB resulted in a significant increase in unconjugated bile acids, particularly cholic acid, and a reduction in conjugated bile acids (Ason et al., 2011). Taken together, these results demonstrate that FATP5 is required for bile acid reconjugation but not de novo synthesis.
Recently, a variant in the human SLC27A5 promoter associated with hepatic steatosis and metabolic syndrome was identified (Auinger et al., 2010). Male subjects harboring the rare A-allele versus the GG-allele within the SLC27A5 promoter had higher postprandial insulin and triglyceride levels (Auinger et al., 2010). These subjects also had higher alanine aminotransferase (ALT) activity, which is an indicator of hepatocellular injury (Auinger et al., 2010). This study demonstrates that, in humans, a polymorphism within SLC27A5 is associated with hepatic injury, insulin resistance, and dyslipidemia (Auinger et al., 2010).
4.6 FATP6
FATP6 is encoded by the SLC27A6 gene and expressed primarily in the heart, specifically in the sarcolemma of cardiomyocytes and plasma membranes juxtaposed to the blood vessels of the heart, where it colocalizes with another fatty acid transport protein, CD36 (Fig. 2) (Gimeno et al., 2003b). FATP6 has been shown to function as a fatty acid transporter in the heart by increasing LCFA uptake (Gimeno et al., 2003b). Given these findings, FATP6 has been speculated to play a role in lipid-related cardiac disorders. Rats that underwent cardiac infarction had reduced FATP6 protein, as well as FATP1, accompanied by reduced fatty acid oxidation and lipid incorporation (Heather et al., 2006). In humans, a T>A polymorphism within the 5’ UTR of SLC27A6 has been associated with lower fasting and fed triacylglycerides, blood pressure, and left ventricular hypertrophy, suggesting that this SNP may be protective against cardio-metabolic diseases (Auinger et al., 2011). Several studies have also shown FATP6 expression in other tissues and cell types, including skin and hair follicle epithelia (Schmuth et al., 2005) and human trophoblasts from the placenta (Mishima et al., 2011). However, the precise role of FATP6 in the heart and other tissues remains to be determined, as animal models targeting Slc27a6 have not been generated.
5. Potential therapeutic applications of FATPs
Mouse models and the identification of polymorphisms in human SLC27 genes associated with illness demonstrate that FATPs may be novel therapeutic targets for the treatment of metabolic diseases such as insulin resistance and type 2 diabetes. A high-throughput screening assay using live yeast cells overexpressing murine FATP2 was developed to identify chemical inhibitors of FATP2 by examining uptake of the fluorescent fatty acid reporter C1-BODIPY-C12 (Li et al., 2005). Using this assay with human FATP2 to screen a standardized small compound library, several groups of compounds were identified to inhibit LCFA uptake, including a family derived from atypical antipsychotic drugs, the tricyclic phenothiazine core derivatives (Li et al., 2008). Interestingly, these drugs can cause severe metabolic side effects, such as weight gain, hypertriglyceridemia, hyperglycemia, and ketoacidosis, and possibly death (Newcomer, 2004). These inhibitors specifically interacted with human FATP2, and treatment of human Caco-2 cells, which spontaneously differentiate into columnar cells similar to intestinal cells and express FATP2 and FATP4, with the identified inhibitors abolished C1-BODIPY-C12 uptake (Li et al., 2008). This assay not only identified potential inhibitory targets of FATP2, but also attributed the severe side effects of antipsychotic drugs to inhibition of FATP2 and LCFA uptake, making this assay useful for screening both new compounds as well as established drugs for potential damaging side effects.
In a larger assay screening 100,000 compounds in yeast expressing human FATP2, 234 hits were identified in a primary screen (Sandoval et al., 2010). Of these hits, 5 compounds were shown to inhibit LCFA uptake in Caco-2 and HepG2 cells, both of which express human FATP2, and this inhibition was reversible (Sandoval et al., 2010). These compounds were specific for FATP2, as they were less effective at inhibiting LCFA uptake in 3T3-L1 adipocytes, which do not express FATP2 (Sandoval et al., 2010). More importantly, these compounds had no effect on cell viability, glucose transport, or ACS activity, demonstrating that FATP2 transport can be selectively inhibited without adverse effects on cell function (Sandoval et al., 2010).
A high-throughput screening assay in mammalian cells was also developed to identify drug targets of human FATP4 and FATP5 (Blackburn et al., 2006; Zhou et al., 2010). In HEK293 cells overexpressing human FATPs, dihydropyrimidinones selectively and potently inhibit FATP4 and not FATP2 or FATP5 (Blackburn et al., 2006). Specifically, two compounds from this class of molecules, identified as j3 and j5, were synthesized and tested for LCFA uptake inhibition (Zhou et al., 2010). Both of these compounds strongly and specifically inhibited uptake of LCFA by human FATP4 in overexpressing cells (Zhou et al., 2010). However, in differentiated 3T3-L1 adipocytes, j3 and j5 also inhibited FATP1, albeit at a weaker level (Zhou et al., 2010). Treatment of primary mouse intestinal enterocytes with the j5 compound robustly inhibited LCFA uptake, and this was specific to FATP4 as it is the only FATP expressed in the intestine (Zhou et al., 2010). Two bile acids, chenodiol and ursodiol, were shown to inhibit human FATP5 with selectivity over FATP4, suggesting that bile acids can inhibit FATP5, which is expressed only in the liver, without affecting FATP4, which is expressed mainly in the intestine (Zhou et al., 2010).
6. Summary
Members of the SLC27 gene family encode LCFA transport proteins which are critical mediators of fatty acid metabolism. Studies targeting Slc27 genes in mice (summarized in Tables 2 and 3), together with the identification of mutations in human SLC27 genes associated with genetic disorders, demonstrate that the SLC27 family members are major contributors to maintaining lipid homeostasis. Therefore, the development of small molecules targeting members of the SLC27 family could lead to significant treatment options for human metabolic diseases.
Acknowledgments
This review was supported by NIH grants R01DK089202-01A1 and R01DK066336-08 to A.S. The authors thank Brittney Bivins and Angela Van for critical reading of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amri EZ, Bonino F, Ailhaud G, Abumrad NA, Grimaldi PA. Cloning of a protein that mediates transcriptional effects of fatty acids in preadipocytes. Homology to peroxisome proliferator-activated receptors. J Biol Chem. 1995;270(5):2367–2371. doi: 10.1074/jbc.270.5.2367. [DOI] [PubMed] [Google Scholar]
- Ason B, Castro-Perez J, Tep S, Stefanni A, Tadin-Strapps M, Roddy T, Hankemeier T, Hubbard B, Sachs AB, Michael Flanagan W, Kuklin NA, Mitnaul LJ. ApoB siRNA-induced liver steatosis is resistant to clearance by the loss of fatty acid transport protein 5 (Fatp5) Lipids. 2011;46(11):991–1003. doi: 10.1007/s11745-011-3596-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auinger A, Helwig U, Pfeuffer M, Rubin D, Luedde M, Rausche T, Eddine El Mokhtari N, Folsch UR, Schreiber S, Frey N, Schrezenmeir J. A variant in the heart-specific fatty acid transport protein 6 is associated with lower fasting and postprandial TAG, blood pressure and left ventricular hypertrophy. Br J Nutr. 2011:1–7. doi: 10.1017/S0007114511004727. [DOI] [PubMed] [Google Scholar]
- Auinger A, Valenti L, Pfeuffer M, Helwig U, Herrmann J, Fracanzani AL, Dongiovanni P, Fargion S, Schrezenmeir J, Rubin D. A promoter polymorphism in the liver-specific fatty acid transport protein 5 is associated with features of the metabolic syndrome and steatosis. Horm Metab Res. 2010;42(12):854–859. doi: 10.1055/s-0030-1267186. [DOI] [PubMed] [Google Scholar]
- Binnert C, Koistinen HA, Martin G, Andreelli F, Ebeling P, Koivisto VA, Laville M, Auwerx J, Vidal H. Fatty acid transport protein-1 mRNA expression in skeletal muscle and in adipose tissue in humans. Am J Physiol Endocrinol Metab. 2000;279(5):E1072–1079. doi: 10.1152/ajpendo.2000.279.5.E1072. [DOI] [PubMed] [Google Scholar]
- Black PN, DiRusso CC. Vectorial acylation: linking fatty acid transport and activation to metabolic trafficking. Novartis Found Symp. 2007a;286:127–138. doi: 10.1002/9780470985571.ch11. discussion 138-141, 162-123, 196-203. [DOI] [PubMed] [Google Scholar]
- Black PN, DiRusso CC. Yeast acyl-CoA synthetases at the crossroads of fatty acid metabolism and regulation. Biochim Biophys Acta. 2007b;1771(3):286–298. doi: 10.1016/j.bbalip.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Blackburn C, Guan B, Brown J, Cullis C, Condon SM, Jenkins TJ, Peluso S, Ye Y, Gimeno RE, Punreddy S, Sun Y, Wu H, Hubbard B, Kaushik V, Tummino P, Sanchetti P, Yu Sun D, Daniels T, Tozzo E, Balani SK, Raman P. Identification and characterization of 4-aryl-3,4-dihydropyrimidin-2(1H)-ones as inhibitors of the fatty acid transporter FATP4. Bioorg Med Chem Lett. 2006;16(13):3504–3509. doi: 10.1016/j.bmcl.2006.03.102. [DOI] [PubMed] [Google Scholar]
- Chiu HC, Kovacs A, Blanton RM, Han X, Courtois M, Weinheimer CJ, Yamada KA, Brunet S, Xu H, Nerbonne JM, Welch MJ, Fettig NM, Sharp TL, Sambandam N, Olson KM, Ory DS, Schaffer JE. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ Res. 2005;96(2):225–233. doi: 10.1161/01.RES.0000154079.20681.B9. [DOI] [PubMed] [Google Scholar]
- Choi H, Kim SJ, Park SS, Chang C, Kim E. TR4 activates FATP1 gene expression to promote lipid accumulation in 3T3-L1 adipocytes. FEBS Lett. 2011;585(17):2763–2767. doi: 10.1016/j.febslet.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Choi JY, Martin CE. The Saccharomyces cerevisiae FAT1 gene encodes an acyl-CoA synthetase that is required for maintenance of very long chain fatty acid levels. J Biol Chem. 1999;274(8):4671–4683. doi: 10.1074/jbc.274.8.4671. [DOI] [PubMed] [Google Scholar]
- Coburn CT, Knapp FF, Jr, Febbraio M, Beets AL, Silverstein RL, Abumrad NA. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem. 2000;275(42):32523–32529. doi: 10.1074/jbc.M003826200. [DOI] [PubMed] [Google Scholar]
- Coe NR, Smith AJ, Frohnert BI, Watkins PA, Bernlohr DA. The fatty acid transport protein (FATP1) is a very long chain acyl-CoA synthetase. J Biol Chem. 1999;274(51):36300–36304. doi: 10.1074/jbc.274.51.36300. [DOI] [PubMed] [Google Scholar]
- DiRusso CC, Li H, Darwis D, Watkins PA, Berger J, Black PN. Comparative biochemical studies of the murine fatty acid transport proteins (FATP) expressed in yeast. J Biol Chem. 2005;280(17):16829–16837. doi: 10.1074/jbc.M409598200. [DOI] [PubMed] [Google Scholar]
- Doege H, Baillie RA, Ortegon AM, Tsang B, Wu Q, Punreddy S, Hirsch D, Watson N, Gimeno RE, Stahl A. Targeted deletion of FATP5 reveals multiple functions in liver metabolism: alterations in hepatic lipid homeostasis. Gastroenterology. 2006;130(4):1245–1258. doi: 10.1053/j.gastro.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Doege H, Grimm D, Falcon A, Tsang B, Storm TA, Xu H, Ortegon AM, Kazantzis M, Kay MA, Stahl A. Silencing of hepatic fatty acid transporter protein 5 in vivo reverses diet-induced non-alcoholic fatty liver disease and improves hyperglycemia. J Biol Chem. 2008;283(32):22186–22192. doi: 10.1074/jbc.M803510200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faergeman NJ, DiRusso CC, Elberger A, Knudsen J, Black PN. Disruption of the Saccharomyces cerevisiae homologue to the murine fatty acid transport protein impairs uptake and growth on long-chain fatty acids. J Biol Chem. 1997;272(13):8531–8538. doi: 10.1074/jbc.272.13.8531. [DOI] [PubMed] [Google Scholar]
- Falany CN, Xie X, Wheeler JB, Wang J, Smith M, He D, Barnes S. Molecular cloning and expression of rat liver bile acid CoA ligase. J Lipid Res. 2002;43(12):2062–2071. doi: 10.1194/jlr.m200260-jlr200. [DOI] [PubMed] [Google Scholar]
- Falcon A, Doege H, Fluitt A, Tsang B, Watson N, Kay MA, Stahl A. FATP2 is a hepatic fatty acid transporter and peroxisomal very long-chain acyl-CoA synthetase. Am J Physiol Endocrinol Metab. 2010;299(3):E384–393. doi: 10.1152/ajpendo.00226.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitscher BA, Riedel HD, Young KC, Stremmel W. Tissue distribution and cDNA cloning of a human fatty acid transport protein (hsFATP4) Biochim Biophys Acta. 1998;1443(3):381–385. doi: 10.1016/s0167-4781(98)00231-0. [DOI] [PubMed] [Google Scholar]
- Frohnert BI, Hui TY, Bernlohr DA. Identification of a functional peroxisome proliferator-responsive element in the murine fatty acid transport protein gene. J Biol Chem. 1999;274(7):3970–3977. doi: 10.1074/jbc.274.7.3970. [DOI] [PubMed] [Google Scholar]
- Gertow K, Bellanda M, Eriksson P, Boquist S, Hamsten A, Sunnerhagen M, Fisher RM. Genetic and structural evaluation of fatty acid transport protein-4 in relation to markers of the insulin resistance syndrome. J Clin Endocrinol Metab. 2004a;89(1):392–399. doi: 10.1210/jc.2003-030682. [DOI] [PubMed] [Google Scholar]
- Gertow K, Pietilainen KH, Yki-Jarvinen H, Kaprio J, Rissanen A, Eriksson P, Hamsten A, Fisher RM. Expression of fatty-acid-handling proteins in human adipose tissue in relation to obesity and insulin resistance. Diabetologia. 2004b;47(6):1118–1125. doi: 10.1007/s00125-004-1417-4. [DOI] [PubMed] [Google Scholar]
- Gertow K, Skoglund-Andersson C, Eriksson P, Boquist S, Orth-Gomer K, Schenck-Gustafsson K, Hamsten A, Fisher RM. A common polymorphism in the fatty acid transport protein-1 gene associated with elevated post-prandial lipaemia and alterations in LDL particle size distribution. Atherosclerosis. 2003;167(2):265–273. doi: 10.1016/s0021-9150(02)00454-9. [DOI] [PubMed] [Google Scholar]
- Gimeno RE, Hirsch DJ, Punreddy S, Sun Y, Ortegon AM, Wu H, Daniels T, Stricker-Krongrad A, Lodish HF, Stahl A. Targeted deletion of fatty acid transport protein-4 results in early embryonic lethality. J Biol Chem. 2003a;278(49):49512–49516. doi: 10.1074/jbc.M309759200. [DOI] [PubMed] [Google Scholar]
- Gimeno RE, Ortegon AM, Patel S, Punreddy S, Ge P, Sun Y, Lodish HF, Stahl A. Characterization of a heart-specific fatty acid transport protein. J Biol Chem. 2003b;278(18):16039–16044. doi: 10.1074/jbc.M211412200. [DOI] [PubMed] [Google Scholar]
- Hagberg CE, Falkevall A, Wang X, Larsson E, Huusko J, Nilsson I, van Meeteren LA, Samen E, Lu L, Vanwildemeersch M, Klar J, Genove G, Pietras K, Stone-Elander S, Claesson-Welsh L, Yla-Herttuala S, Lindahl P, Eriksson U. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature. 2010;464(7290):917–921. doi: 10.1038/nature08945. [DOI] [PubMed] [Google Scholar]
- Haggarty P. Placental regulation of fatty acid delivery and its effect on fetal growth--a review. Placenta. 2002;23(Suppl A):S28–38. doi: 10.1053/plac.2002.0791. [DOI] [PubMed] [Google Scholar]
- Hall AM, Wiczer BM, Herrmann T, Stremmel W, Bernlohr DA. Enzymatic properties of purified murine fatty acid transport protein 4 and analysis of acyl-CoA synthetase activities in tissues from FATP4 null mice. J Biol Chem. 2005;280(12):11948–11954. doi: 10.1074/jbc.M412629200. [DOI] [PubMed] [Google Scholar]
- Heather LC, Cole MA, Lygate CA, Evans RD, Stuckey DJ, Murray AJ, Neubauer S, Clarke K. Fatty acid transporter levels and palmitate oxidation rate correlate with ejection fraction in the infarcted rat heart. Cardiovasc Res. 2006;72(3):430–437. doi: 10.1016/j.cardiores.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Heinzer AK, Watkins PA, Lu JF, Kemp S, Moser AB, Li YY, Mihalik S, Powers JM, Smith KD. A very long-chain acyl-CoA synthetase-deficient mouse and its relevance to X-linked adrenoleukodystrophy. Hum Mol Genet. 2003;12(10):1145–1154. doi: 10.1093/hmg/ddg126. [DOI] [PubMed] [Google Scholar]
- Herrmann T, Buchkremer F, Gosch I, Hall AM, Bernlohr DA, Stremmel W. Mouse fatty acid transport protein 4 (FATP4): characterization of the gene and functional assessment as a very long chain acyl-CoA synthetase. Gene. 2001;270(1-2):31–40. doi: 10.1016/s0378-1119(01)00489-9. [DOI] [PubMed] [Google Scholar]
- Herrmann T, Grone HJ, Langbein L, Kaiser I, Gosch I, Bennemann U, Metzger D, Chambon P, Stewart AF, Stremmel W. Disturbed epidermal structure in mice with temporally controlled fatp4 deficiency. J Invest Dermatol. 2005;125(6):1228–1235. doi: 10.1111/j.0022-202X.2005.23972.x. [DOI] [PubMed] [Google Scholar]
- Herrmann T, van der Hoeven F, Grone HJ, Stewart AF, Langbein L, Kaiser I, Liebisch G, Gosch I, Buchkremer F, Drobnik W, Schmitz G, Stremmel W. Mice with targeted disruption of the fatty acid transport protein 4 (Fatp 4, Slc27a4) gene show features of lethal restrictive dermopathy. J Cell Biol. 2003;161(6):1105–1115. doi: 10.1083/jcb.200207080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch D, Stahl A, Lodish HF. A family of fatty acid transporters conserved from mycobacterium to man. Proc Natl Acad Sci U S A. 1998;95(15):8625–8629. doi: 10.1073/pnas.95.15.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway GP, Chou CJ, Lally J, Stellingwerff T, Maher AC, Gavrilova O, Haluzik M, Alkhateeb H, Reitman ML, Bonen A. Increasing skeletal muscle fatty acid transport protein 1 (FATP1) targets fatty acids to oxidation and does not predispose mice to diet-induced insulin resistance. Diabetologia. 2011;54(6):1457–1467. doi: 10.1007/s00125-011-2114-8. [DOI] [PubMed] [Google Scholar]
- Huang H, Starodub O, McIntosh A, Kier AB, Schroeder F. Liver fatty acid-binding protein targets fatty acids to the nucleus. Real time confocal and multiphoton fluorescence imaging in living cells. J Biol Chem. 2002;277(32):29139–29151. doi: 10.1074/jbc.M202923200. [DOI] [PubMed] [Google Scholar]
- Hubbard B, Doege H, Punreddy S, Wu H, Huang X, Kaushik VK, Mozell RL, Byrnes JJ, Stricker-Krongrad A, Chou CJ, Tartaglia LA, Lodish HF, Stahl A, Gimeno RE. Mice deleted for fatty acid transport protein 5 have defective bile acid conjugation and are protected from obesity. Gastroenterology. 2006;130(4):1259–1269. doi: 10.1053/j.gastro.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Hui TY, Frohnert BI, Smith AJ, Schaffer JE, Bernlohr DA. Characterization of the murine fatty acid transport protein gene and its insulin response sequence. J Biol Chem. 1998;273(42):27420–27429. doi: 10.1074/jbc.273.42.27420. [DOI] [PubMed] [Google Scholar]
- Jain SS, Chabowski A, Snook LA, Schwenk RW, Glatz JF, Luiken JJ, Bonen A. Additive effects of insulin and muscle contraction on fatty acid transport and fatty acid transporters, FAT/CD36, FABPpm, FATP1, 4 and 6. FEBS Lett. 2009;583(13):2294–2300. doi: 10.1016/j.febslet.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Jia Z, Moulson CL, Pei Z, Miner JH, Watkins PA. Fatty acid transport protein 4 is the principal very long chain fatty acyl-CoA synthetase in skin fibroblasts. J Biol Chem. 2007;282(28):20573–20583. doi: 10.1074/jbc.M700568200. [DOI] [PubMed] [Google Scholar]
- Kazantzis M, Stahl A. Fatty acid transport proteins, implications in physiology and disease. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbalip.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Gimeno RE, Higashimori T, Kim HJ, Choi H, Punreddy S, Mozell RL, Tan G, Stricker-Krongrad A, Hirsch DJ, Fillmore JJ, Liu ZX, Dong J, Cline G, Stahl A, Lodish HF, Shulman GI. Inactivation of fatty acid transport protein 1 prevents fat-induced insulin resistance in skeletal muscle. J Clin Invest. 2004;113(5):756–763. doi: 10.1172/JCI18917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar J, Schweiger M, Zimmerman R, Zechner R, Li H, Torma H, Vahlquist A, Bouadjar B, Dahl N, Fischer J. Mutations in the fatty acid transport protein 4 gene cause the ichthyosis prematurity syndrome. Am J Hum Genet. 2009;85(2):248–253. doi: 10.1016/j.ajhg.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Forman BM, Blumberg B, Ong ES, Borgmeyer U, Mangelsdorf DJ, Umesono K, Evans RM. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci U S A. 1994;91(15):7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer J, Digel M, Ehehalt F, Stremmel W, Fullekrug J, Ehehalt R. Overexpression of CD36 and acyl-CoA synthetases FATP2, FATP4 and ACSL1 increases fatty acid uptake in human hepatoma cells. Int J Med Sci. 2011;8(7):599–614. doi: 10.7150/ijms.8.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labonte ED, Camarota LM, Rojas JC, Jandacek RJ, Gilham DE, Davies JP, Ioannou YA, Tso P, Hui DY, Howles PN. Reduced absorption of saturated fatty acids and resistance to diet-induced obesity and diabetes by ezetimibe-treated and Npc1l1-/- mice. Am J Physiol Gastrointest Liver Physiol. 2008;295(4):G776–783. doi: 10.1152/ajpgi.90275.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafontan M, Berlan M. Fat cell adrenergic receptors and the control of white and brown fat cell function. J Lipid Res. 1993;34(7):1057–1091. [PubMed] [Google Scholar]
- Lenz LS, Marx J, Chamulitrat W, Kaiser I, Grone HJ, Liebisch G, Schmitz G, Elsing C, Straub BK, Fullekrug J, Stremmel W, Herrmann T. Adipocyte-specific inactivation of Acyl-CoA synthetase fatty acid transport protein 4 (Fatp4) in mice causes adipose hypertrophy and alterations in metabolism of complex lipids under high fat diet. J Biol Chem. 2011;286(41):35578–35587. doi: 10.1074/jbc.M111.226530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SE, Listenberger LL, Ory DS, Schaffer JE. Membrane topology of the murine fatty acid transport protein 1. J Biol Chem. 2001;276(40):37042–37050. doi: 10.1074/jbc.M105556200. [DOI] [PubMed] [Google Scholar]
- Li H, Black PN, Chokshi A, Sandoval-Alvarez A, Vatsyayan R, Sealls W, DiRusso CC. High-throughput screening for fatty acid uptake inhibitors in humanized yeast identifies atypical antipsychotic drugs that cause dyslipidemias. J Lipid Res. 2008;49(1):230–244. doi: 10.1194/jlr.D700015-JLR200. [DOI] [PubMed] [Google Scholar]
- Li H, Black PN, DiRusso CC. A live-cell high-throughput screening assay for identification of fatty acid uptake inhibitors. Anal Biochem. 2005;336(1):11–19. doi: 10.1016/j.ab.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Lin MH, Chang KW, Lin SC, Miner JH. Epidermal hyperproliferation in mice lacking fatty acid transport protein 4 (FATP4) involves ectopic EGF receptor and STAT3 signaling. Dev Biol. 2010;344(2):707–719. doi: 10.1016/j.ydbio.2010.05.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo S, Wiczer BM, Smith AJ, Hall AM, Bernlohr DA. Fatty acid metabolism in adipocytes: functional analysis of fatty acid transport proteins 1 and 4. J Lipid Res. 2007;48(3):609–620. doi: 10.1194/jlr.M600441-JLR200. [DOI] [PubMed] [Google Scholar]
- Man MZ, Hui TY, Schaffer JE, Lodish HF, Bernlohr DA. Regulation of the murine adipocyte fatty acid transporter gene by insulin. Mol Endocrinol. 1996;10(8):1021–1028. doi: 10.1210/mend.10.8.8843418. [DOI] [PubMed] [Google Scholar]
- Martin G, Schoonjans K, Lefebvre AM, Staels B, Auwerx J. Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPARalpha and PPARgamma activators. J Biol Chem. 1997;272(45):28210–28217. doi: 10.1074/jbc.272.45.28210. [DOI] [PubMed] [Google Scholar]
- Meirhaeghe A, Martin G, Nemoto M, Deeb S, Cottel D, Auwerx J, Amouyel P, Helbecque N. Intronic polymorphism in the fatty acid transport protein 1 gene is associated with increased plasma triglyceride levels in a French population. Arterioscler Thromb Vasc Biol. 2000;20(5):1330–1334. doi: 10.1161/01.atv.20.5.1330. [DOI] [PubMed] [Google Scholar]
- Melton EM, Cerny RL, Watkins PA, DiRusso CC, Black PN. Human fatty acid transport protein 2a/very long chain acyl-CoA synthetase 1 (FATP2a/Acsvl1) has a preference in mediating the channeling of exogenous n-3 fatty acids into phosphatidylinositol. J Biol Chem. 2011;286(35):30670–30679. doi: 10.1074/jbc.M111.226316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalik SJ, Steinberg SJ, Pei Z, Park J, Kim DG, Heinzer AK, Dacremont G, Wanders RJ, Cuebas DA, Smith KD, Watkins PA. Participation of two members of the very long-chain acyl-CoA synthetase family in bile acid synthesis and recycling. J Biol Chem. 2002;277(27):24771–24779. doi: 10.1074/jbc.M203295200. [DOI] [PubMed] [Google Scholar]
- Milger K, Herrmann T, Becker C, Gotthardt D, Zickwolf J, Ehehalt R, Watkins PA, Stremmel W, Fullekrug J. Cellular uptake of fatty acids driven by the ER-localized acyl-CoA synthetase FATP4. J Cell Sci. 2006;119(Pt 22):4678–4688. doi: 10.1242/jcs.03280. [DOI] [PubMed] [Google Scholar]
- Mishima T, Miner JH, Morizane M, Stahl A, Sadovsky Y. The expression and function of fatty acid transport protein-2 and -4 in the murine placenta. PLoS One. 2011;6(10):e25865. doi: 10.1371/journal.pone.0025865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RW, On NH, Del Bigio MR, Miller DW, Hatch GM. Fatty acid transport protein expression in human brain and potential role in fatty acid transport across human brain microvessel endothelial cells. J Neurochem. 2011;117(4):735–746. doi: 10.1111/j.1471-4159.2011.07245.x. [DOI] [PubMed] [Google Scholar]
- Mitsuyoshi H, Yasui K, Harano Y, Endo M, Tsuji K, Minami M, Itoh Y, Okanoue T, Yoshikawa T. Analysis of hepatic genes involved in the metabolism of fatty acids and iron in nonalcoholic fatty liver disease. Hepatol Res. 2009;39(4):366–373. doi: 10.1111/j.1872-034X.2008.00464.x. [DOI] [PubMed] [Google Scholar]
- Moulson CL, Lin MH, White JM, Newberry EP, Davidson NO, Miner JH. Keratinocyte-specific expression of fatty acid transport protein 4 rescues the wrinkle-free phenotype in Slc27a4/Fatp4 mutant mice. J Biol Chem. 2007;282(21):15912–15920. doi: 10.1074/jbc.M701779200. [DOI] [PubMed] [Google Scholar]
- Moulson CL, Martin DR, Lugus JJ, Schaffer JE, Lind AC, Miner JH. Cloning of wrinkle-free, a previously uncharacterized mouse mutation, reveals crucial roles for fatty acid transport protein 4 in skin and hair development. Proc Natl Acad Sci U S A. 2003;100(9):5274–5279. doi: 10.1073/pnas.0431186100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer JW. Abnormalities of glucose metabolism associated with atypical antipsychotic drugs. J Clin Psychiatry. 2004;65(Suppl 18):36–46. [PubMed] [Google Scholar]
- Nickerson JG, Alkhateeb H, Benton CR, Lally J, Nickerson J, Han XX, Wilson MH, Jain SS, Snook LA, Glatz JF, Chabowski A, Luiken JJ, Bonen A. Greater transport efficiencies of the membrane fatty acid transporters FAT/CD36 and FATP4 compared with FABPpm and FATP1 and differential effects on fatty acid esterification and oxidation in rat skeletal muscle. J Biol Chem. 2009;284(24):16522–16530. doi: 10.1074/jbc.M109.004788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordovás L, Roy R, Zaragoza P, Rodellar C. Structural and functional characterization of the bovine solute carrier family 27 member 1 (SLC27A1) gene. Cytogenet Genome Res. 2006;115(2):115–22. doi: 10.1159/000095230. [DOI] [PubMed] [Google Scholar]
- Pei Z, Fraisl P, Berger J, Jia Z, Forss-Petter S, Watkins PA. Mouse very long-chain Acyl-CoA synthetase 3/fatty acid transport protein 3 catalyzes fatty acid activation but not fatty acid transport in MA-10 cells. J Biol Chem. 2004;279(52):54454–54462. doi: 10.1074/jbc.M410091200. [DOI] [PubMed] [Google Scholar]
- Richards MR, Harp JD, Ory DS, Schaffer JE. Fatty acid transport protein 1 and long-chain acyl coenzyme A synthetase 1 interact in adipocytes. J Lipid Res. 2006;47(3):665–672. doi: 10.1194/jlr.M500514-JLR200. [DOI] [PubMed] [Google Scholar]
- Richards MR, Listenberger LL, Kelly AA, Lewis SE, Ory DS, Schaffer JE. Oligomerization of the murine fatty acid transport protein 1. J Biol Chem. 2003;278(12):10477–10483. doi: 10.1074/jbc.M212469200. [DOI] [PubMed] [Google Scholar]
- Sandoval A, Chokshi A, Jesch ED, Black PN, Dirusso CC. Identification and characterization of small compound inhibitors of human FATP2. Biochem Pharmacol. 2010;79(7):990–999. doi: 10.1016/j.bcp.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval A, Fraisl P, Arias-Barrau E, Dirusso CC, Singer D, Sealls W, Black PN. Fatty acid transport and activation and the expression patterns of genes involved in fatty acid trafficking. Arch Biochem Biophys. 2008;477(2):363–371. doi: 10.1016/j.abb.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Schaffer JE, Lodish HF. Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell. 1994;79(3):427–436. doi: 10.1016/0092-8674(94)90252-6. [DOI] [PubMed] [Google Scholar]
- Schmuth M, Ortegon AM, Mao-Qiang M, Elias PM, Feingold KR, Stahl A. Differential expression of fatty acid transport proteins in epidermis and skin appendages. J Invest Dermatol. 2005;125(6):1174–1181. doi: 10.1111/j.0022-202X.2005.23934.x. [DOI] [PubMed] [Google Scholar]
- Schwenk RW, Holloway GP, Luiken JJ, Bonen A, Glatz JF. Fatty acid transport across the cell membrane: regulation by fatty acid transporters. Prostaglandins Leukot Essent Fatty Acids. 2010;82(4-6):149–154. doi: 10.1016/j.plefa.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Shim J, Moulson CL, Newberry EP, Lin MH, Xie Y, Kennedy SM, Miner JH, Davidson NO. Fatty acid transport protein 4 is dispensable for intestinal lipid absorption in mice. J Lipid Res. 2009;50(3):491–500. doi: 10.1194/jlr.M800400-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobol M, Dahl N, Klar J. FATP4 missense and nonsense mutations cause similar features in Ichthyosis Prematurity Syndrome. BMC Res Notes. 2011;4:90. doi: 10.1186/1756-0500-4-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl A, Evans JG, Pattel S, Hirsch D, Lodish HF. Insulin causes fatty acid transport protein translocation and enhanced fatty acid uptake in adipocytes. Dev Cell. 2002;2(4):477–488. doi: 10.1016/s1534-5807(02)00143-0. [DOI] [PubMed] [Google Scholar]
- Stahl A, Hirsch DJ, Gimeno RE, Punreddy S, Ge P, Watson N, Patel S, Kotler M, Raimondi A, Tartaglia LA, Lodish HF. Identification of the major intestinal fatty acid transport protein. Mol Cell. 1999;4(3):299–308. doi: 10.1016/s1097-2765(00)80332-9. [DOI] [PubMed] [Google Scholar]
- Steinberg SJ, Mihalik SJ, Kim DG, Cuebas DA, Watkins PA. The human liver-specific homolog of very long-chain acyl-CoA synthetase is cholate:CoA ligase. J Biol Chem. 2000;275(21):15605–15608. doi: 10.1074/jbc.C000015200. [DOI] [PubMed] [Google Scholar]
- Steinberg SJ, Wang SJ, McGuinness MC, Watkins PA. Human liver-specific very-long-chain acyl-coenzyme A synthetase: cDNA cloning and characterization of a second enzymatically active protein. Mol Genet Metab. 1999;68(1):32–42. doi: 10.1006/mgme.1999.2883. [DOI] [PubMed] [Google Scholar]
- Stremmel W, Pohl L, Ring A, Herrmann T. A new concept of cellular uptake and intracellular trafficking of long-chain fatty acids. Lipids. 2001;36(9):981–989. doi: 10.1007/s11745-001-0809-2. [DOI] [PubMed] [Google Scholar]
- Uchiyama A, Aoyama T, Kamijo K, Uchida Y, Kondo N, Orii T, Hashimoto T. Molecular cloning of cDNA encoding rat very long-chain acyl-CoA synthetase. J Biol Chem. 1996;271(48):30360–30365. doi: 10.1074/jbc.271.48.30360. [DOI] [PubMed] [Google Scholar]
- Watkins PA, Lu JF, Steinberg SJ, Gould SJ, Smith KD, Braiterman LT. Disruption of the Saccharomyces cerevisiae FAT1 gene decreases very long-chain fatty acyl-CoA synthetase activity and elevates intracellular very long-chain fatty acid concentrations. J Biol Chem. 1998;273(29):18210–18219. doi: 10.1074/jbc.273.29.18210. [DOI] [PubMed] [Google Scholar]
- Wu Q, Kazantzis M, Doege H, Ortegon AM, Tsang B, Falcon A, Stahl A. Fatty acid transport protein 1 is required for nonshivering thermogenesis in brown adipose tissue. Diabetes. 2006a;55(12):3229–3237. doi: 10.2337/db06-0749. [DOI] [PubMed] [Google Scholar]
- Wu Q, Ortegon AM, Tsang B, Doege H, Feingold KR, Stahl A. FATP1 is an insulin-sensitive fatty acid transporter involved in diet-induced obesity. Mol Cell Biol. 2006b;26(9):3455–3467. doi: 10.1128/MCB.26.9.3455-3467.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Madrid P, Fluitt A, Stahl A, Xie XS. Development and validation of a high-throughput screening assay for human long-chain fatty acid transport proteins 4 and 5. J Biomol Screen. 2010;15(5):488–497. doi: 10.1177/1087057110369700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z, DiRusso CC, Ctrnacta V, Black PN. Fatty acid transport in Saccharomyces cerevisiae. Directed mutagenesis of FAT1 distinguishes the biochemical activities associated with Fat1p. J Biol Chem. 2002;277(34):31062–31071. doi: 10.1074/jbc.M205034200. [DOI] [PubMed] [Google Scholar]
- Zou Z, Tong F, Faergeman NJ, Borsting C, Black PN, DiRusso CC. Vectorial acylation in Saccharomyces cerevisiae. Fat1p and fatty acyl-CoA synthetase are interacting components of a fatty acid import complex. J Biol Chem. 2003;278(18):16414–16422. doi: 10.1074/jbc.M210557200. [DOI] [PubMed] [Google Scholar]


