Abstract
Heme is critical for a variety of cellular processes, but excess intracellular heme may result in oxidative stress and membrane injury. Feline leukemia virus subgroup C receptor (FLVCR1), a member of the SLC49 family of 4 paralogous genes, is a cell surface heme exporter, essential for erythropoiesis and systemic iron homeostasis. Disruption of FLVCR1 function blocks development of erythroid progenitors, likely due to heme toxicity. Mutations of SLC49A1 encoding FLVCR1 are noted in patients with a rare neurodegenerative disorder: posterior column ataxia with retinitis pigmentosa. FLVCR2 is highly homologous to FLVCR1 and may function as a cellular heme importer. Mutations of SLC49A2encoding FLVCR2 are observed in Fowler syndrome, a rare proliferative vascular disorder of the brain. The functions of the remaining members of the SLC49 family, MFSD7 and DIRC2 (encoded by the SLC49A3and SLC49A4 genes), are unknown, although the latter is implicated in hereditary renal carcinomas. SLC48A1 (Heme responsive gene-1, HRG-1), the sole member of the SLC48 family, is associated with the endosome and appears to transport heme from the endosome into the cytosol.
Keywords: SLC48, SLC49, Heme, FLVCR, HRG-1, membrane transporters, Diamond-Blackfan anemia
1. Introduction
Heme, a complex of protoporphyrin IX and iron, is ubiquitous in nature and indispensable for myriad cellular processes. For example, it serves as the prosthetic group of hemoproteins, including cytochromes, peroxidases, catalases, synthases, and the oxygen storage and transport proteins, myoglobin and hemoglobin (Hb) [reviews: (Khan and Quigley, 2011; Ponka, 1997; Ryter and Tyrrell, 2000)]. Heme is important for erythropoiesis—it regulates erythroid gene transcription, inhibiting repression of the globin locus by the transcription factor BACH1 (Ogawa et al., 2001); gene translation, inhibiting an erythroid-specific eIF-2a kinase that controls globin translation (Crosby et al., 2000; de Haro et al., 1996); as well as the targeting and stability of heme synthesis proteins (Lathrop and Timko, 1993; Qi et al., 1999). More recent studies indicate that intracellular heme levels also modulate circadian rhythm, microRNA processing, and ion-channel functions (Faller et al., 2007; Imaizumi et al., 2007; Wang et al., 2009).
An excess of free or non-protein–bound heme, however, promotes lipid peroxidation and increases oxidative stress through generation of reactive oxygen species, which can give rise to membrane injury and, ultimately, cell apoptosis (Balla et al., 2003; Halliwell and Gutteridge, 1990; Ryter and Tyrrell, 2000). Thus, intracellular heme levels must be kept within a narrow range, ~0.1 μM (Ryter and Tyrrell, 2000; Sassa, 2004). Control occurs through a coordination among heme biosynthesis, utilization by hemoproteins, compartmentalization into organelles and catabolism, primarily by heme-inducible heme oxygenase–1 (HO-1) (Ponka, 1997). The recent identification of heme transporters moreover suggests further layers of complexity. Despite the lipophilic nature of heme, the presence of anionic carboxylate side chains—responsible for binding to heme carrier proteins—limits diffusion through the membrane (Light and Olson, 1990), indicating the need for specific transmembrane heme transporters at the cell surface (e.g., FLVCR1), in mitochondria (the site of heme synthesis), and in endosome-lysosomes (e.g., HRG-1), where hemoproteins are degraded (Goldman et al., 1998; Keel et al., 2008; Quigley et al., 2004; Ricchelli et al., 1995; Yang et al., 2010); for reviews, see (Khan and Quigley, 2011; Severance and Hamza, 2009; Thöny-Meyer, 2009).
Here we describe the SLC49 and SLC48 transporters. Studies of three of these five transporters indicate they are involved in heme transport. FLVCR1 (encoded by SLC49A1), the founding member of the SLC49 family, has three identifiable paralogs in the human genome, SLC49A2, SLC49A3 and SLC49A4. These genes all encode members of the Major Facilitator Superfamily (MFS) of secondary active (or facilitative transport) permeases, which usually have 12–14 transmembrane domains (TMDs) with the N- and C-termini in the cytosol. The MFS permeases transport small solutes (e.g., sugars, iron, and amino acids) across membranes in response to chemico-osmotic gradients (Pao et al., 1998). In the Transporter Classification (TC) database (www.tcdb.org), the FLVCR family forms the MFS subgroup 2.A.1.28 (Saier et al., 2009). In contrast, the SLC48 family is comprised of only one human gene to date, SLC48A1, which also appears to encode a facilitative transporter, HRG-1. This protein is predicted to have 4 TMDs and thus is not an MFS permease (O’Callaghan et al., 2009; Rajagopal et al., 2008). In the TC database, HRG-1 and its C. elegans ortholog, CeHRG-1 comprise the subgroup 9.A.61 (the heme transporter, heme-responsive gene protein family).
2. SLC49 Family
2.1. SLC49A1, FLVCR1, TC: 2.A.1.28.1
FLVCR1 is the cell surface receptor for Feline leukemia virus subgroup C (FeLV-C) [see also Online Mendelian Inheritance in Man (OMIM) website, www.ncbi.nlm.nih.gov/omim; OMIM ID: 609144]. The retrovirus infects all hematopoietic cells, impairing cell FLVCR1 function due to continual binding of FLVCR1 proteins by FeLV-C envelope synthesized within the cell. Infected cats, however, usually present with severe anemia, a red blood cell (RBC) aplasia that is characterized by an absence of circulating reticulocytes and a paucity of erythroid progenitors in the bone marrow. As these findings indicated a critical function for FLVCR1 during erythropoiesis (Abkowitz et al., 1987; Onions et al., 1982), the feline ortholog of FLVCR1 and FLVCR1 were cloned (Quigley et al., 2000; Tailor et al., 1999).
FLVCR1 is conserved throughout evolution with orthologs present in animals, plants, insects, and bacteria (Lipovich et al., 2002). SLC49A1, the human gene, contains 10 exons, and spans ~40 Kb on chromosome 1q32. Topology predictions suggest the protein has 12 TMD with intracellular N- and C-termini [~108 amino acids (aa) and ~43 aa, respectively] (Fig. 1). By homology, FLVCR is an MFS permease, but the specific transport function of FLVCR1 was not readily apparent.
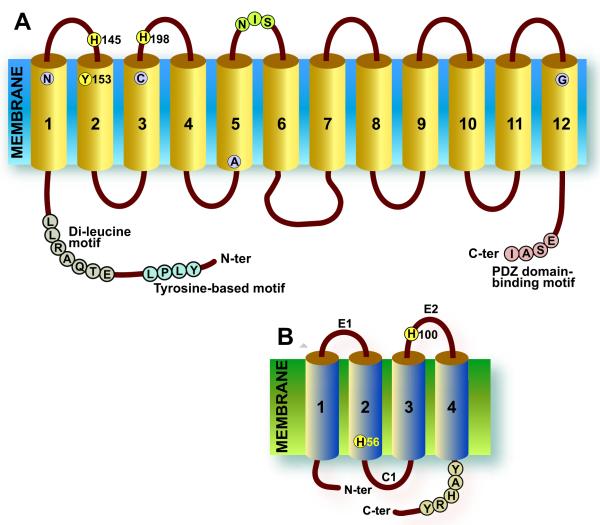
Fig. 1.
(A)Predicted topology of FLVCR1. The protein is predicted to have 12 TMD with both N- and C-termini in the cytosol. The tyrosine-based, di-leucine, and PDZ domain–binding motifs are shown. By analogy to other heme-binding proteins, H145, Y153, and H198 residues are predicted to constitute a heme-binding pocket. A potential N-linked glycosylation signal (NIS) is located in exofacial loop 3. Four mutations identified in patients with PCARP, N121D, C192R, A241T, and G493R are shown in TMD 1, 3, 5, and 12, respectively. Note that the predicted topology of the other SLC49 members—FLVCR2, SLC49A3/MFD7, and SLC49A4/DIRC2—is similar. (B) Predicted topology of HRG-1. The transporter is predicted to have 4 TMD (Rajagopal et al., 2008). The residues H56 and H100 and the YAHRY motif in the C-terminus are predicted to be important for heme transport.
In subsequent studies, FLVCR1 was identified as a mammalian cell heme exporter that appears to protect erythroid progenitors from potential heme toxicity during the heme synthesis phase of erythropoiesis (Quigley et al., 2004). Similar to the phenotype of FeLV-C–infected cats, conditional deletion of murine SLC49A1 in neonatal mice results, within 6 weeks, in a severe macrocytic anemia due to a block in erythroid differentiation (hematocrit = 13.2 ± 1.1% in deleted mice and 49.6 ± 2.0% in controls; n = 11 and 13, respectively) (Keel et al., 2008). Of interest, knockdown of murine FLVCR1 is embryonic lethal with the embryos displaying a phenotype similar to that of patients with Diamond-Blackfan anemia (DBA), a congenital syndrome that includes red cell aplasia (Lipton and Ellis, 2009). Neonatal deletion of Slc49a1 in mice also results in the rapid development of systemic iron overload, which may be due in part to the impaired recycling of heme-iron that occurs following erythrophagocytosis of senescent RBC by SLC49A1-deleted macrophages. Compared to controls, SLC49A1-deleted macrophages have elevated ferritin levels following erythrophagocytosis, suggesting that macrophages normally re-export some RBC heme via FLVCR1. The murine model of FLVCR1 deficiency demonstrates the importance of FLVCR1 in both systemic iron and heme biology.
2.1.1. Tissue distribution and cellular localization
The protein is abundantly expressed at potential sites of heme trafficking, such as the liver and small intestine. Protein expression is also noted in the brain, kidney, lung, spleen, uterus, and placenta (Keel et al., 2008). The subcellular localization of FLVCR1 has not been examined in detail. When GFP-tagged FLVCR1 is ectopically expressed in HEK293T cells, the fusion protein is observed predominantly at the plasma membrane [our unpublished observations; see also (Rey et al., 2008)], consistent with FLVCR1 function as a cell surface heme exporter and its use by FeLV-C for entry into cells (Quigley et al., 2000; Quigley et al., 2004). In addition, incubation of K562 cells with a specific anti-FLVCR1 polyclonal antibody impairs heme export, supporting its functional presence on the cell surface (Quigley et al., 2004).
2.1.2. Functional studies
NRK, a “normal rat kidney” epithelial cell line engineered to overexpress human FLVCR1 exports 2-fold more heme than control NRK cells, as measured by quantitative microscopy utilizing the fluorescent heme analog ZnMP; by quantification of the export of radioactively labeled 55Fe-hemin; or by HPLC-based quantification of export of exogenously supplied heme (Quigley et al., 2004). As predicted, FLVCR1-mediated heme export is impaired in K562 cells that are infected with FeLV-C (and thus expressing large amounts of FeLV-C envelope protein, which interferes with FLVCR1 cell surface expression). As noted, incubation of K562 cells with specific anti-FLVCR1 polyclonal antibody also impairs heme export. In addition to exogenous or endogenous heme, FLVCR1 can export protoporphyrin IX or coproporphyrin, but not unconjugated bilirubin (Yang et al., 2010).
Heme export by FLVCR1 requires the presence of an extracellular heme-binding protein, such as albumin or hemopexin (Hpx) in the media, and export is not observed in the absence of a carrier protein (Yang et al., 2010). By analogy to the gram-negative bacterial hemophore HasA (Deniau et al., 2003; Letoffe et al., 2003), CcsBA (Frawley and Kranz, 2009), and other heme-binding proteins, the aa residues His 145, Tyr 153, and His 198 of FLVCR1 are likely involved in the heme transport route and/or heme transfer to extracellular heme-binding proteins (Khan and Quigley, 2011) [predicted motif: H-x(7)-Y-x(44)-H, using the Prosite system and pattern syntax (Hulo et al., 2008)]. The histidine residues, conserved in mammalian FLVCR1 proteins, are predicted to lie in extracellular loops 1 and 2 (E1, E2) and are thus situated to coordinate heme docking and export (Fig. 1). The motif is absent in the other SLC49 paralogs that do not export heme. Observations indicate that Hpx—with a similar arrangement of His residues—may function physiologically to acquire heme bound to FLVCR1. Notably, export of heme by NRK/FLVCR1 cells is 100-fold more efficient when the media contains Hpx rather than albumin (Yang et al., 2010), in keeping with the relative affinities of Hpx and albumin for heme (Kd <1 pM vs. Kd = 5 nM) (Hargrove et al., 1996; Hrkal et al., 1974). Thus, heme may be channeled from the cytosol through FLVCR1, docked at the E1 and E2 histidine residues, and subsequently released to a heme carrier protein.
2.1.3. Regulation
Analysis of the SLC49A1 promoter region that lies within 1 Kb of the translation initiation site indicates four potential STAT5a binding sites, as well as consensus GATA-1–, GATA-2–, c-myb–, and NF-E2–binding sites, providing potential mechanisms for upregulation of transcription during early erythroid commitment and differentiation (Quigley et al., 2004). However, these findings have not been confirmed experimentally. In addition, a consensus binding motif for the heme-regulated transcriptional repressor BACH1 lies 4.2 kb upstream of the translation initiation site, suggesting potential regulation by heme.
Notably there are marked disparities between SLC49A1 transcript and FLVCR1 protein levels in diverse tissues and cell lines, indicating that FLVCR1 is regulated by post-translational mechanisms (Keel et al., 2008; Quigley et al., 2004). The N-terminus of FLVCR1 contains a consensus sequence for both a tyrosine-based motif (YXXφ, where φ denotes a bulky or hydrophobic residue) and a non-canonical di-leucine motif (consensus [DE]XXXL[LI]), (Fig. 1). It is known that sorting motifs within the N- and C-termini of MFS transporters like FLVCR1 are important for trafficking and proper localization, especially in polarized epithelial cells of tissues, such as the intestine and kidney (Muth and Caplan, 2003; Royle and Murrell-Lagnado, 2003; van Beest et al., 2006). The cross-species’ conservation of the di-leucine motif suggests a potential role in FLVCR1 membrane sorting; we therefore mutated both leucines to alanines. As expected, the mutant protein does not localize to the plasma membrane in HEK293 cells (unpublished data). The predicted C-terminal sequences of FLVCR1 in mammals also contain a motif—a class I PSD95/Dlg/ZO1 (PDZ) domain consensus binding sequence (X-S/T-X-φ) that suggests interaction with a PDZ domain–containing protein(s). PDZ domains promote protein-protein interactions and act as scaffolds between transmembrane proteins and the cytoskeleton (Sugiura et al., 2011; van Ham and Hendriks, 2003). Preliminary studies suggest that PTPN3, a non-receptor protein tyrosine phosphatase containing a PDZ domain, interacts with the C-terminus of FLVCR1, modulating transporter function (unpublished data).
2.1.4. Pathological implications
DBA is a rare congenital form of red cell aplasia that usually presents in infancy [see review, (Da Costa et al., 2010)]. The disease is heterogeneous with ~50%–60% of patients exhibiting mutations of the ribosomal protein (RP) genes (Farrar et al., 2011). Up to 40% of patients have associated congenital malformations (cranio-facial or upper limb). Mutations of at least 13 RP genes have been identified, with the RPS19 gene the first characterized (Draptchinskaia et al., 1999) and the most commonly affected gene [see review (Chiabrando and Tolosano, 2010)]. RPS19 deficiency or mutation affects erythroid progenitor development in zebrafish and mouse models and in human primary hematopoietic cells (Danilova et al., 2008; Devlin et al., 2010; Flygare et al., 2005; Jaako et al., 2011). These studies indicate that the block in erythroid development occurs at the CFU-E/pro-erythroblast stage, the same progenitor affected by FLVCR1 deficiency (Keel et al., 2008).
It is postulated that defects in RP genes result in abnormal RP maturation, with a subsequent defect in ribosome function and protein translation. Such defects should preferentially impact cells with high levels of protein synthesis as occurs in maturing erythroid cells. However it is unclear why defective protein translation does not also affect other tissues (Da Costa et al., 2010). Investigators have postulated that perhaps dysfunction of other genes important for erythropoiesis, such as TP53 or SLC49A1 acting in concert with the RP mutation result in the DBA phenotype (Chiabrando and Tolosano, 2010; Jaako et al., 2011).
A small group of patients with non-RPS19-related DBA were reported to have reduced levels of SLC49A1 mRNA in erythroid progenitors (defined as CD71high cells) with a concomitant increase in alternatively spliced isoforms lacking exon 2 (E2) or E3 (± E6 deletion) (Rey et al., 2008). Deletion of E2 should result in a prematurely terminated transcript, whereas E3 is predicted to encode the central cytoplasmic loop, believed to be critical for protein stability (Keel et al., 2008). Of interest, the E3-deleted protein is not present on the cell surface when overexpressed in a murine fibroblast cell line and does not transport heme (Rey et al., 2008). In previous studies, we analyzed 4 patients with non-RPS19–related DBA and found no SLC49A1 mutations, but we did not look for abnormal splicing (Quigley et al., 2005). Interestingly, down-regulation of RPS19 protein in K562 cells also promotes alternative splicing of SLC49A1, suggesting perhaps that the described alternative splicing of SLC49A1 was caused by abnormalities of RP genes not examined in this study. To examine the possibility that non-specific alternative splicing of genes occurs in DBA erythroid cells, cDNAs encoding for the erythropoietin receptor and for a phosphate transporter (Pit1) were sequenced. These genes did not have evidence of abnormal splicing (Rey et al., 2008). Another possibility is that the alternative splicing of SLC49A1 and the resultant defect in heme export causes DBA in these patients. An imbalance between globin and heme synthesis due to either RP (impeding protein, predominantly globin translation) or FLVCR1 dysfunction (impairing heme export) during erythropoiesis may present with the same disease (Chiabrando and Tolosano, 2010). As mentioned, SLC49A1-deleted mice recapitulate not only the erythroid phenotype but also anatomical features including hypertelorism, flattened facies, and digit/hand abnormalities frequently seen in patients with DBA (Keel et al., 2008).
Studies describe homozygous missense mutations within the predicted TMD of FLVCR1 in affected individuals with a rare childhood-onset, autosomal-recessive, neurodegenerative disorder characterized by sensory ataxia and retinitis pigmentosa (PCARP, OMIM ID: 609033) (Ishiura et al., 2011; Rajadhyaksha et al., 2010), (Fig. 1). The selective degeneration of sensory neurons in both the retina (rod photoreceptors) and the posterior columns of the spinal cord (predominantly large-fiber somatic afferent nerves) in this disease suggest that both groups of neurons are affected by a common disease mechanism (and perhaps have a common cell origin). Analysis of murine CNS tissues shows specific high expression of SLC49A1 in the posterior columns and retina (as measured by qPCR) (Rajadhyaksha et al., 2010). The mRNA for the hemoglobin-related protein neuroglobin is >100-fold higher in the retinal photoreceptor layer compared to the CNS; thus, it is proposed that FLVCR1 functions normally to moderate any increases in heme levels during synthesis of this neuroprotective hemoprotein (Rajadhyaksha et al., 2010). Alternatively, FLVCR1 may serve to protect these tissues from heme toxicity related to heme release during local hemorrhages. Notably, there are no reports of anemia in patients with PCARP (or of PCARP in patients with DBA), suggesting that for example, with these mutations FLVCR1-mediated erythroid progenitor heme export is reduced but present.
2.2. SLC49A2, FLVCR2, TC: 2.A.1.28.4
In previous studies SLC49A2, encoding FLVCR2 (OMIM ID: 610865), was identified as a gene highly homologous to SLC49A1, located on chromosome 14q24 (Quigley et al., 2000). Even the exon sizes are highly conserved between these genes—in keeping with their derivation from a genome block duplication involving chromosomes 1 and 14 [1q24–q42 and 14q21–q32; (Lipovich et al., 2002)]. The predicted MFS proteins encoded by these genes are characterized by 60% aa sequence identity across their 12 TMD. Unlike FLVCR1 however, di-leucine or tyrosine-based motifs are not discernible in the intracellular termini of FLVCR2, but a series of six novel hexad repeats in the N-terminus (P-S-[VS]-[SL]-[VIA]-[HNQ], using the Prosite syntax) and a polyglutamate sequence in the C-terminus, are conserved in mammals. FLVCR2 does not export ZnMP from NRK cells engineered to overexpress the protein (Quigley et al., 2004), but a recent study indicates it imports extracellular heme, and mutations of the SLC49A2gene are now linked to a rare CNS proliferative vasculopathy, Fowler syndrome (Duffy et al., 2010; Meyer et al., 2010).
2.2.1. Tissue distribution and cellular localization
SLC49A2 is ubiquitously expressed with highest transcript levels observed in the placenta, liver, kidney, brain, lung, and hematopoietic tissues including fetal liver and bone marrow (Duffy et al., 2010). A variant transcript, NP_001182212.1 (UniProtKB ID: B7Z485), predicted to encode a protein with a distinct N-terminus peptide of 18 aa, but otherwise identical to aa 224-526 of the canonical isoform, is of unknown significance. FLVCR2 can be utilized by the FeLV-C variant FY981 to enter cells, indicating its expression on the cell surface (Shalev et al., 2009). In addition, in cell lines, FLVCR2 appears to import extracellular heme, supporting its presence on the plasma membrane (Duffy et al., 2010; Shalev et al., 2009). Interestingly, murine FLVCR2 was reported to be expressed in the columnar cells that overlie the fetal blood vessels in the placental yolk sac at day E20, suggesting that, like FLVCR1, it is important for materno-fetal transfer of substrate (Brasier et al., 2004; Keel et al., 2008).
2.2.2. Functional studies
A single report describing the cloning and expression of SLC49A2 and the structure of the predicted protein proposed a role for FLVCR2 in calcium transport, based on its expression in tissues associated with rapid calcium exchange (Brasier et al., 2004). However, to date, there are no functional studies indicating a role for FLVCR2 in calcium metabolism.
Investigations suggest FLVCR2, when overexpressed, is an importer of extracellular heme (Duffy et al., 2010). CHO cells overexpressing FLVCR2 or Xenopus oocytes injected with cRNA encoding FLVCR2 both show a significant (~2-fold) increase in uptake of ZnMP or 55Fe-hemin, respectively. In addition, ZnMP uptake is reduced by ~30% when cells are treated with siRNA against SLC49A2; conversely, the susceptibility of CHO cells to heme toxicity is increased by overexpression of FLVCR2 (and, as expected, decreased by FLVCR1 overexpression) (Duffy et al., 2010). It is estimated that at least two-thirds of Western dietary iron intake is derived from dietary heme; thus, identification of the intestinal heme importer would be of major significance (Carpenter and Mahoney, 1992). Previous studies identified another MFS member, HCP1 (SLC46A1), as the intestinal heme importer using similar transport assays, yet subsequent studies demonstrated that HCP1 functions physiologically as a folate transporter (Qiu et al., 2006; Shayeghi et al., 2005). More recent assays of FLVCR2 overexpression in heme synthesis–deficient yeast cells (the hem1Δ strain, deficient in HEM1, which encodes for 5-aminolevulinate synthase, the first enzyme of the heme synthesis pathway) overexpressing FLVCR2 show no evidence of heme import despite the presence of abundant protein on the cell surface (Yuan&Hamza, 2012). To date there are no studies of conditional knockdown of murine FLVCR2, which may help clarify its potential role in heme import (ES cell lines available, see MGI ID: 2384974 at www.informatics.jax.org).
2.2.3. Pathological implications
Recent papers describe SLC49A2 gene mutations in Fowler syndrome (OMIM ID: 225790), a rare lethal autosomal-recessive cerebral proliferative vasculopathy that results in hydranencephaly-hydrocephaly. There are no visceral malformations in this disease and no evidence of proliferative microangiopathy outside of the CNS. A total of fifteen mutations (10 missense, 2 nonsense, 1 deletion, 1 splice site mutation, and 1 deletion/insertion) were identified in 13 cases of Fowler syndrome (Meyer et al., 2010; Thomas et al., 2010). The effects of these mutations on FLVCR2 localization or function are unknown.
It is postulated that the disordered angiogenesis is a disease of pericytes, which provide mural support to developing capillaries (Bessieres-Grattagliano et al., 2009; Thomas et al., 2010), but why the disease is confined to the CNS is not apparent. Notably, FLVCR2 is widely expressed in the CNS and in endothelial cells in particular (Brasier et al., 2004; Meyer et al., 2010). As heme deficiency appears to affect assembly of mitochondrial electron transport chain complex IV in human lung fibroblasts, heme deficiency related to FLVCR2 dysfunction was proposed as a potential cause of the mitochondrial dysfunction noted in a muscle biopsy from a patient with hydranencephaly-hydrocephaly (Castro-Gago et al., 1999). However, Fowler syndrome is only one of a number of diseases that manifest as hydranencephaly-hydrocephaly syndrome (Aicardi, 1992).
2.3. SLC49A3, MFSD7, TC: 2.A.1.28.2
SLC49A3, originally named major facilitator superfamily domain containing 7 (MFSD7),was identified as 1 of 14 genes that are predictive of time to relapse of ovarian cancer following therapy (Hartmann et al., 2005). A follow-up study identified a promoter region SNP that correlates with reduced risk of invasive ovarian cancer (Peedicayil et al.). The gene spans an 8 Kb region on chromosome 4p16.3 and has 10 exons. As expected for MFS members, the predicted protein has 12 TMD, and has ~30% aa identity to FLVCR1. The topology predictions suggest a short ~20 aa N-terminal segment and a relatively long, ~100 aa, C-terminal segment. No experimental evidence is available as to protein localization or transport function. The EST profile indicates expression in pancreas, mammary gland, ovary, brain, lung and spleen. The murine gene knockout is available, but has not been analyzed to date (see MGI ID: 2442629 at www.informatics.jax.org).
2.4. SLC49A4, DIRC2
The SLC49A4 gene, “FLVCRL3q” (OMIM ID: 602773), was originally discovered using in silico gene cloning from annotated genomic sequences (Lipovich et al., 2002). In subsequent studies it was identified as a gene on 3q21 that spans a recurrent breakpoint—the translocation t(2;3)(q35;q21)— present in affected members of a family with hereditary renal cell carcinoma and was therefore named disrupted in renal cancer 2 (DIRC2) (Bodmer et al., 2002). However, DIRC2 transcript expression was normal, with no aberrant transcripts detected in these tumors, suggesting a position effect of the breakage on a neighboring gene. DIRC2 has since been renamed SLC49A4. Of interest, a recent massively parallel paired-end transcriptome sequencing study to identify novel gene fusions in cancer cell lines describes another translocation, t(2;3)(p14;q21) that involves SLC49A4(Maher et al., 2009). This second translocation, present in a prostate cancer cell line, suggests that SLC49A4 may indeed play an important role in carcinogenesis.
The gene encompasses a region of ~86 Kb on chromosome 3q21.1 and contains 9 exons. The predicted MFS transporter protein is 478 aa in length with ~30% aa identity with FLVCR1 (from aa 100-473). The transcript is expressed ubiquitously, albeit at low levels in the kidney, pancreas, skeletal muscle, placenta, heart, and brain (Bodmer et al., 2002). The murine gene knockout is available, but has not been analyzed to date (MGI ID: 2387188 at www.informatics.jax.org). SLC49A4 is predicted to have 12 TMD, a di-leucine motif in its N-terminus, and 2 potential tyrosine-based motifs in the C-terminus. The protein appears to localize to lysosomes in HeLa cells, with mutation of the N-terminal di-leucine motif (DIRC-LL/AA), resulting in increased cell surface expression (Savalas et al., 2011). Interestingly, SLC49A4 normally undergoes proteolytic cleavage into 2 proteins by a lysosomal cathepsin, whereas the mutated construct, which traffics to the plasma membrane, is not cleaved. Studies of transport by the SLC49A4 -LL/AA construct (not the native protein) expressed on the cell surface of Xenopus oocytes suggests the protein exports an electrogenic metabolite(s) from the lysosomal lumen into the cytosol.
2.5. SLC49 Family—Conclusions
All four SLC49 members are paralogous MFS transporters. FLVCR1 exports heme and its importance is still being recognized. It may prove to play a protective role in disease states characterized by the release of free (toxic) heme from, for example, erythroid cells, including both congenital and acquired hemolytic anemias, malaria, and the myelodysplastic syndromes. The uptake of heme into cells may be mediated by FLVCR2, but confirmatory studies including evaluation of the knockout mouse are needed. The specific transport substrates of SLC49A3/MFSD7 and SLC49A4/DIRC2 are unknown; however, the latter is found in the lysosome, functioning perhaps as two half-transporters, which is of interest since heme derived from cell hemoproteins may be recycled from this organelle.
3. SLC48 Family
3.1. SLC48A1, HRG-1, TC: 9.A.61.1.1
Caenorhabditis elegans and related helminths are heme auxotrophs, lacking endogenous heme synthesis and depending on heme absorption from the environment (Rao et al., 2005). Thus, C. elegans—studied as a model organism for more than 40 years—provides a “clean” background to study both organismal heme transport and intracellular heme trafficking pathways. Genome sequencing reveals that many human genes are conserved in C. elegans. In addition, the ease of genetic manipulation makes it a highly tractable model system to identify heme transporters (Hamza, 2006; Severance and Hamza, 2009).
Heme uptake is regulated in C. elegans; when worms grown in optimal-to-low heme conditions (<20 μM) are exposed to ZnMP, there is rapid internalization of ZnMP, yet uptake is significantly impaired when worms are first grown in medium containing 100 μM heme (Rajagopal et al., 2008). Using microarray analyses of C. elegans genes derived from worms during growth under low heme (4 μM) versus optimal (20 μM) versus high heme conditions (500 μM), Hamza’s group identified 288 heme-responsive genes (Severance et al., 2010). To identify heme transporters, they focused on genes upregulated in low heme conditions, sorting them based on the predicted presence of TMD, known transporter function and/or heme- or metal-binding domains. A heme-responsive gene fulfilling these criteria—termed hrg-4—and a paralog, hrg-1 (~30% overall aa identity), were studied in more detail (Rajagopal et al., 2008). CeHRG-4, expressed in worm intestinal cells, appears to be a plasma membrane-associated heme importer, but mammalian orthologs are lacking (Rajagopal et al., 2008). In contrast, the intracellular heme transporter CeHRG-1 (TC: 9.A.61.1.2) has orthologs from arthropods to vertebrates, including zebrafish (Danio rerio), frogs (Xenopus laevis), and mammals.
The HRG-1 proteins differ from heme transporters of the SLC49 family in that they are present predominantly on the endosomal membrane. The human ortholog, HRG-1 or SLC48A1 (OMIM ID: 612187), has ~20% aa identity to CeHRG-1. In silico predictions using protein topology prediction programs (e.g., TMHMM 2.0, SOSUI) indicate the protein has 4 TMD with both N- and C-termini located in the cytosol (Rajagopal et al., 2008; Yuan et al., 2012). Another group however, provide experimental evidence that the N- and C-termini of HRG-1 lie on opposite sides of the endosomal membrane (in studies using an antibody raised against the C-terminus and an antibody to a HA-tag on the N-terminus), with the N-terminus in the cytosol, and predict that the protein contains one transmembrane and three half-transmembrane helices (see Fig. 2, (O’Callaghan et al., 2009)).
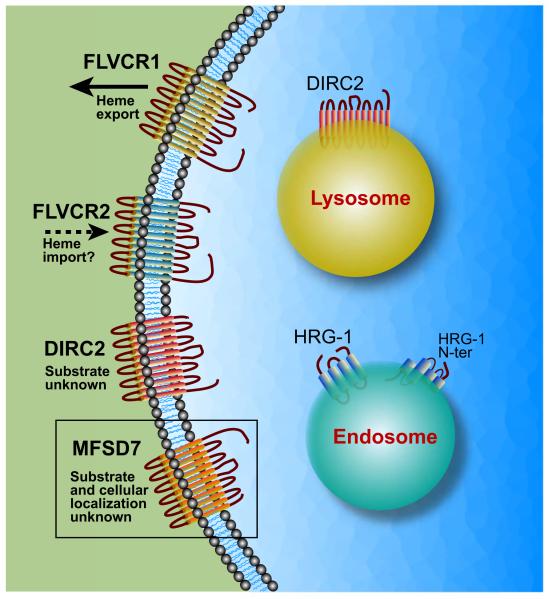
Fig. 2.
Cellular localization of SLC48 and SLC49 family members. SLC49A1/FLVCR1 and SLC49A2/FLVCR2 are plasma membrane proteins. SLC49A4/DIRC2 is predominantly located in the lysosome where it may exist as two half-transporters. The protein can also be found on the cell surface. The cellular location of SLC49A3/MFSD7 is unknown. SLC48A1/HRG-1 is located primarily in the endosome-lysosomal compartment. Alternative predicted topologies for SLC48A1 are shown (O’Callaghan et al., 2009; Rajagopal et al., 2008), see section 3.1. for details.
Further analysis of HRG-1, CeHRG-1 and orthologs identified in zebrafish, chicken, and frog genomes indicates a conserved potential heme-binding histidine residue in predicted TMD2 (H56 in HRG-1 and H90 in CeHRG-1), another highly conserved residue in the second predicted exoplasmic (i.e., endosome lumen) E2 loop (H100 in HRG-1, H135 in CeHRG-1) and, in the C-terminus, a cluster of aromatic and basic residues that may interact with and orient heme side chains (FARKY in CeHRG-1 and YAHRY in HRG-1) (Rajagopal et al., 2008; Yuan et al., 2012). As described above for FLVCR1, the transporter also contains a conserved YXXxØ trafficking motif (but located in the C-terminus).
In the human genome, SLC48A1 is located at 12q13, ~3 Mb from the SLC11A2 gene encoding the principal cellular iron importer, DMT1 (which incidentally is expressed on the cell surface and in endosomes) (Fleming et al., 1997; Gunshin et al., 1997).
3.1.1. Tissue distribution and cellular localization
CeHRG-1 is initially expressed in all C. elegans embryonic somatic cells, yet, with maturation of the embryo, expression is confined to intestinal cells (Sinclair and Hamza, 2010). In D. rerio, slc48a1b mRNA is expressed throughout the developing embryo, including the CNS, the intermediate cell mass, and the developing blood island (Rajagopal et al., 2008). In humans, two mRNA species are identified (1.7 and 3.2 Kb), which differ in the length of the 3′ UTR. The short form is predominantly expressed. Both species are predicted to encode a protein of 146 aa (UniProt ID: Q6P1K1) that is highly expressed in the liver, heart, CNS, kidney, skeletal muscle, and small intestine (O’Callaghan et al., 2009). Although a second isoform of HRG-1 has been proposed (missing aa 1-57 and 2 of the 4 TMD), this may result from sequencing errors (UniProt ID: Q6P1K1-2).
The location of HRG-1 within cells remains controversial. Three groups have examined HRG-1 expression using confocal microscopy. It is agreed that HRG-1 is predominantly expressed in the endosomal compartment, but whether it also traffics to the cell membrane and is also present, as proposed, in lysosomes is not clear (O’Callaghan et al., 2009; Rajagopal et al., 2008; Yanatori et al., 2010). Of relevance, it is known that the cell lysosomal/vacuolar system is heterogeneous and includes, for example, early endosomes that contain endocytosed receptor–ligand complexes and pinocytosed/phagocytosed extracellular contents (Ciechanover, 2005). Reports indicate ~10% of overexpressed HRG-1 protein is present on the plasma membrane and in polarized MDCK cells, HRG-1 appears on the basolateral but not the apical surface (Yanatori et al., 2010). All of these studies analyze overexpressed HRG-1 constructs, not endogenous HRG-1, in various human cell lines (e.g., HEK-293, MCF, HeLa), which can potentially cause mislocalization of the overexpressed protein (Berger, 2002; Padash-Barmchi et al., 2010).
3.1.2. Functional studies
To date, studies of the heme transport function of HRG-1 have been performed in yeast, worms, zebrafish, frog oocytes and mammalian cell lines (O’Callaghan et al., 2009; Rajagopal et al., 2008; Yuan et al., 2012).
(i) S. cerevisiae: Studies by Yuan et al. indicate that C. elegans has at least 4 HRG-1–related family members that transport heme (CeHRG-1, -4, -5 and -6). Thus, to analyze the function of CeHRG-1 or HRG-1 in isolation, they overexpressed the genes in hem1Δ mutant yeast cells, which do not express HRG-1 proteins and, despite a lack of endogenous heme synthesis, absorb exogenous heme poorly (Protchenko et al., 2008). Using 3 established independent assays of yeast heme transport they prove that overexpression of HRG-1 (or CeHRG-1) in hem1Δ mutant yeast cells increases heme import (Yuan et al., 2012). Moreover, by expressing mutant CeHRG-1 or HRG-1 proteins, they demonstrate, using these assays that the HRG-1 histidine residue, H56, conserved from nematodes to humans is critical for heme transport into yeast when environmental heme levels are low. The C-terminus motif (FARKY) and to a lesser extent the E2 histidine residue are also important for heme uptake by CeHRG-1 (HRG-1 not examined). Note that ectopically expressed HRG-1 is found on the yeast vacuolar membrane, an organelle equivalent to the mammalian lysosome.
(ii) C. elegans: Knockdown of CeHRG-1 in the nematode paradoxically appears to increase uptake of ZnMP in the worm intestine (perhaps because the imported ZnMP is unable to escape from the endosome-lysosome) and does not impair uptake of gallium protoporphyrin (a cytotoxic heme analog), suggesting the heme transporter is not present on the cell surface of intestinal cells and/or compensatory uptake by CeHRG-1 paralogs. In addition, the transport function appears specific to heme as a construct comprised of GFP fused to the CeHRG-1 promoter is repressed by heme, but not by protoporphyrin or iron.
(iii) D. rerio: Injection of an antisense morpholino of the D. rerio ortholog of CeHRG-1 (21% aa identity) into D. rerio embryos results in marked anemia and defective embryonic development with hydrocephalus, a curved body axis and a foreshortened yolk tube. Notably, while zebrafish myelopoiesis and megakaryopoiesis appear unaffected, erythropoiesis, as assessed by production of wild-type levels of β e1-globin mRNA, is present in the intermediate cell mass and developing blood islands at 24 h post-fertilization, but absent at 48 h. These results suggest DrHRG-1 is required for maintenance and/or hemoglobinization of D. rerio embryonic erythroid cells. Of note, a murine SLC48A1 (gene trap) knockout is available (MGI:2678417 at www.informatics.jax.org); initial characterization indicates no obvious phenotypic abnormality.
(iv) X. laevis: To assay heme transport directly, X. laevis oocytes were injected with CeHRG-1, HRG-1, or control hKv1 (K+ channel) cRNA and the generation of ionic currents monitored using voltage clamps (Rajagopal et al., 2008). Incubation of oocytes injected with CeHRG-1 or HRG-1 in media containing 20 μM heme results in the generation of significant inward currents (vs. controls), indicating heme-dependant transport across the oocyte plasma membrane.
(v) Mammalian cell lines: Overexpression of HRG-1 in Friend mouse erythroleukemia (MEL), MCF (breast cancer), or HeLa (cervical cancer) cells increases ZnMP import 2-fold (O’Callaghan et al., 2009; Rajagopal et al., 2008), similar to the ZnMP export rate observed in cells overexpressing FLVCR1 (Quigley et al., 2004). In contrast, suppression of SLC48A1 in HeLa cells by siRNA reduces ZnMP uptake by 30%. To demonstrate in vivo binding of heme by HRG-1, cell lysates from HEK293 cells overexpressing HRG-1 were incubated on a hemin-agarose column, washed extensively and then the column-bound protein eluted. CeHRG-4, CeHRG-1, and HRG-1 proteins bound to the column, suggesting they all bind heme. In keeping with the predominant location of HRG-1 in endosome-lysosomes, its binding to heme was significantly reduced by raising the pH (alkalinization), suggesting it is unlikely that HRG-1 binds significant amounts of heme at the (alkaline) cell surface.
Together, these studies indicate that the HRG-1 protein transports heme. Rajagopal et al. (2008) propose a model whereby HRG-1 transports heme present in the endosomal and/or lysosomal compartment into the cytosol. The increase in ZnMP import observed with overexpression in mammalian cells may be due to trafficking of some HRG-1 protein to the cell surface. An alternate possibility is that there is feedback between the various intracellular heme compartments—for example, there may be a relationship between heme levels in the endosomal-lysosomal compartment and the rate of heme import (or export) on the cell surface.
Endocytosis and the trafficking of receptors and cell surface transporters to the cell membrane are dependent on an acidic endosomal environs, which is maintained by the vacuolar H+-ATPase (V-ATPase) rotary proton pump [review, (Forgac, 2007)]. An important example of this function is recycling of the transferrin receptor (TfR1) to the cell surface upon the pH-dependent release of its ferric ions within the acidic endosome. A yeast-two-hybrid study demonstrates that HRG-1 interacts with V-ATPase, increasing assembly of the V-ATPase subunits, V-ATPase activity, endosomal acidity, and TfR1 recycling (O’Callaghan et al., 2009). Of interest, siRNA knockdown of endogenous HRG-1 expression in HeLa cells decreases acidification of endosomes (but not lysosomes—see Section 3.1.1) and, reminiscent of its affects in D. rerio embryonic erythroid cells, decreases cell viability after 48 h, perhaps through effects on TfR1 or growth factor receptor recycling.
There are potential reasons why an intracellular heme transporter interacts with V-ATPase in the endosome. For example, by increasing V-ATPase activity and endosome acidity, HRG-1 may potentiate both release of iron from TfR1 and TfR1 recycling—important for iron acquisition during de novo cell heme synthesis, especially in erythroid progenitors. As heme is more soluble below physiological pH (values <7.0), the recycling of heme from cell hemoproteins within the endosome [e.g., from Hb in macrophages ingesting RBCs (erythrophagocytosis) or endocytosed Hpx-heme complexes] is likely more efficient. SLC48A1 may be a proton symporter, with the V-ATPase-derived proton gradient driving transport of heme into the cytosol. Finally, the increase in recycling of various cell membrane proteins (e.g., growth factor receptors and transporters) as a result of the interaction of HRG-1 and V-ATPase may allow coordination of heme availability with an increase in the availability of other environmental micronutrients (e.g., glucose and biometals) for cell growth or proliferation, as observed in yeast (Tu et al., 2007).
3.1.3. Regulation
As described, CeHRG-1 is specifically expressed in the worm intestine, and is highly upregulated (>60-fold) when environmental heme levels are low (Rajagopal et al., 2008). Fusion of GFP to the proximal 3 Kb of the Cehrg-1 promoter demonstrates that expression is regulated by heme. Subsequent promoter deletion studies identified a repressor site, a 23 bp heme-responsive element in the promoter both necessary and sufficient to mediate a transcriptional response to changing heme levels (called HERE). In addition, analysis of conserved areas of the promoter sequence across Caenorhabditis species genomes identified 5 GATA sites that bind ELT proteins—GATA family transcription factors known to regulate expression of intestinal genes. It is hypothesized that ELT proteins acting at GATA sites in the CeHRG-1 promoter together with other transcription factors (repressors and activators) binding at the HERE site orchestrate HRG-1 expression (Sinclair and Hamza, 2010).
Few studies of mammalian HRG-1 regulation have been performed. The murine Slc48a1 gene appears to be upregulated by insulin-like growth factor (or serum starvation) in an embryonic fibroblast cell line (O’Callaghan et al., 2009). However, induction of “erythroid” differentiation of MEL cells, which results in marked upregulation of iron transporters (e.g., TfR1), heme synthesis enzymes (e.g., ALAS2), and globin genes for hemoglobinization, does not modulate SLC48A1 mRNA levels (Rajagopal et al., 2008). In addition, no change in SLC48A1 expression is observed in a human cell line (HEK293) in response to depletion of cellular iron and inhibition of cell heme synthesis, or to the subsequent repletion of cellular iron or heme (Rajagopal et al., 2008).
The binding of heme to cysteine-proline motifs of the transcription factors BACH1 and BACH2 relieves their transcriptional repression of numerous genes (Sun et al., 2004; Watanabe-Matsui et al., 2011). Recent studies demonstrate that when intracellular heme levels are low, BACH1 mediates repression of anti-oxidant response genes, including those encoding HO-1, the ferritin light and heavy chains and HRG-1 in HEK293 cells (Warnatz et al., 2011). With an increase in intracellular heme, repression by BACH1 is released, resulting in increased expression of SLC48A1 and ~60 other genes involved in heme degradation, redox regulation, cell cycle, and apoptosis pathways. These studies are in contrast to the lack of murine Slc48a1 mRNA regulation by heme in MEL cells observed by Rajagopal et al. (2008). Murine B-cell lymphocyte differentiation involves heme regulation of BACH2. Despite an increase in intracellular heme levels during lymphocyte differentiation, there is no change in Slc48a1 mRNA levels. However, exposure of activated B-lymphocytes to heme does increase Slc48a1 expression (Watanabe-Matsui et al., 2011).
In summary, hrg-1 mRNA expression in nematodes increases in response to low levels of heme, while conversely in mammals the response to heme is tissue dependant, with some studies indicating enhanced expression with increasing intracellular heme. Notably, these studies examine mRNA levels and do not rule out a role for intracellular heme levels in the post-translational regulation of HRG-1 protein expression (e.g., post-translational regulation of FLVCR1 protein is important; see Section 2.1.3).
3.2. SLC48 Family—Conclusions
SLC48A1 is a 4 TMD–containing endosomal heme transporter, present in genomes from arthropods to vertebrates. It likely serves to export endosomal heme that is delivered to the vacuole from the plasma membrane or from cytosolic hemoproteins back into the cytosol, which may be particularly relevant during macrophage erythrophagocytosis. SLC48A1/HRG-1 appears to interact with the V-ATPase that acidifies endosomes, increasing V-ATPase activity, which mediates an increase in cell membrane transporter and receptor recycling. HRG-1 heme-binding decreases in the relatively alkaline pH conditions found at the cell surface; nevertheless, when overexpressed in cell lines HRG-1 is present on the plasma membrane and seems to increase cell heme import directly or indirectly.
4. Therapeutic potential of the SLC48 and SLC49 Families
While the pathophysiologic effects of knockdown or mutation of SLC49A1 in humans, cats, and mice are well described, the therapeutic value of upregulation of FLVCR-mediated heme export is unknown at present. Because FLVCR (with heme oxygenases such as HO-1) should help protect tissues from heme toxicity, it may be of importance in diseases characterized by hemolysis and the release of free (toxic) heme, including hemolytic anemias, sickle cell disease, thalassemias, and malaria, as well as various pathophysiologic states, including ischemia-reperfusion injury, hemorrhage (e.g., CNS), and rhabdomyolysis. However, overexpression of murine FLVCR1 in murine BM results, with time, in a mild microcytic anemia (Keel et al., 2008) that may complicate its therapeutic uses. Of interest, FLVCR is also present on hematopoietic stem cells, thus FeLV-C enveloped (“pseudotyped”) retroviral vectors can be used to target FLVCR on these cells for gene therapy (Doty et al., 2010; Lucas et al., 2005). Further analysis of the links between FLVCR and the pathogenesis of DBA and PCARP may lead to therapies for these diseases.
As discussed, the majority of iron absorbed in Western diets is in the form of heme; therefore, identification of the heme importer in the small intestinal epithelium would be of major therapeutic significance. If further studies support such a role for FLVCR2 then pharmaceutical regulation of its expression or function could be used to modulate (heme) iron absorption in iron deficiency anemia or iron overload diseases (hemochromatosis). The importance of FLVCR2 for angiogenesis may become apparent from analysis of the SLC49A2 knockout mouse. Note that it should be possible to design small molecule inhibitors of FLVCR1 and 2, based on studies of the viral envelopes of the viruses that specifically interact with these proteins [see (Brown et al., 2006; Shalev et al., 2009)].
In addition to C. elegans, the phylogenetically related parasitic nematodes (which infect >2 billion people) are also heme auxotrophs; therefore, CeHRG-1 and related nematode heme transporters may have therapeutic relevance as targets for antihelminthics. Finally, the majority of iron used for erythropoiesis (to create 3 × 1011 RBC daily) stems from the recycling of heme that is derived from senescent RBC Hb breakdown within macrophage endophagolysosomes. Thus if HRG-1 is involved in the transfer of heme across these macrophage organelles, it has the potential to impact systemic iron homeostasis.
Table 1.
SLC48 and SLC49 heme and FLVCR1-related transporter families
| Human gene name |
Prote in name |
Aliases | Predomina nt substrates |
Transport type/coupli ng ions |
Tissue distribution and cellular/subcellul ar expression |
Link to disease |
Human gene locus |
Sequence accession ID |
Splice variants and their specific feautres |
|---|---|---|---|---|---|---|---|---|---|
| SLC49A 1 |
FLVCR 1 |
FLVCR, MFSD7B, AXPC1, PCARP |
Heme | unknown | Ubiquitous, high expression in intestine, liver, kidney, brain and bone marrow. Located on the plasma membrane. |
Posterior column ataxia and retinitis pigmentos a (PCARP) syndrome , Diamond- Blackfan anemia (DBA). |
1q32.3 | NM_014053 | Four splice variants observed in DBA, lacking exon 2 or 3 ± exon 6, an additional splice variant lacking exon 6 seen in both control and DBA erythroid cells. |
| SLC49A 2 |
FLVCR 2 |
MFSD7C, CCT, EPV, PVHH, FLVCRL1 4q |
Heme | unknown | Liver, kidney, brain, lung, placenta, fetal liver, bone marrow. Located on the plasma membrane. |
Fowler syndrome |
14q24.3 |
NM_017791.2 NM_001195283 .1 |
A variant transcript differs in the 5′-UTR and uses an alternate start codon, which should encode a shorter hypothetical protein. |
| SLC49A 3 |
MFSD7 | LP2561, FLJ22269 |
unknown | unknown | Pancreas, spleen, brain, lung, ovary, breast. |
Ovarian cancer |
4p16.3 | NM_032219.2 | Isoforms differ from the canonical sequence as follows: Isoform #2: aa 242 missing. Isoform #3: aa 46-67 and 99-195 missing. Note: No experimenta l confirmation of these isoforms. |
| SLC49A 4 |
DIRC2 | RCC4, FLJ14784 |
unknown | unknown | Pancreas, kidney, liver, placenta, heart. Predominantly localizes to the lysosome. |
Renal cell and prostate cancers. |
3q21.1 | NM_032839.2 | Isoform #2 differs from the canonical sequence as follows: aa 380-411: alternative sequence and aa 412- 478: missing. Note: No experimenta l confirmation . |
| SLC48A 1 |
HRG-1 | Heme | C/H+? | Liver, heart, CNS, kidney, skeletal muscle, small intestine. Localizes predominantly to the endosome, also observed on the cell surface when overexpressed. |
12q13.1 1 |
NM_017842 | Two isoforms differ in the length of the 3′-UTR. Short form is predominant ly expressed. |
Acknowledgements
We thank Dr. N. Mahmud for stimulating discussions. We would also like to acknowledge the help of Ms. E. Quigley with editing of the manuscript. Work in the JQ laboratory is funded by grants from the National Institutes of Health and the Roche Foundation for Anemia research (RoFAR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Anwar A. Khan, Department of Medicine, Section of Hematology/Oncology, University of Illinois at Chicago, Chicago, IL, USA
John G. Quigley, Department of Medicine, Section of Hematology/Oncology, University of Illinois at Chicago, Chicago, IL, USA
References
- Abkowitz JL, Holly RD, Grant CK. Retrovirus-induced feline pure red cell aplasia. Hematopoietic progenitors are infected with feline leukemia virus and erythroid burst-forming cells are uniquely sensitive to heterologous complement. J Clin Lnvest. 1987;80(4):1056–1063. doi: 10.1172/JCI113160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aicardi J. Diseases of the nervous system in childhood / Jean Aicardi ; with contributions from Martin Bax, Christopher Gillberg, Helene Ogier. Mac Keith Press; London: 1992. [Google Scholar]
- Balla J, Vercellotti GM, Nath K, Yachie A, Nagy E, Eaton JW, Balla G. Haem, haem oxygenase and ferritin in vascular endothelial cell injury. Nephrol Dial Transplant. 2003;18(Suppl 5):v8–12. doi: 10.1093/ndt/gfg1034. [DOI] [PubMed] [Google Scholar]
- Berger EG. Ectopic localizations of Golgi glycosyltransferases. Glycobiology. 2002;12(2):29R–36R. doi: 10.1093/glycob/12.2.29r. [DOI] [PubMed] [Google Scholar]
- Bessieres-Grattagliano B, Foliguet B, Devisme L, Loeuillet L, Marcorelles P, Bonniere M, Laquerriere A, Fallet-Bianco C, Martinovic J, Zrelli S, Leticee N, Cayol V, Etchevers HC, Vekemans M, Attie-Bitach T, Encha-Razavi F. Refining the clinicopathological pattern of cerebral proliferative glomeruloid vasculopathy (Fowler syndrome): report of 16 fetal cases. Eur J Med Genet. 2009;52(6):386–392. doi: 10.1016/j.ejmg.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Bodmer D, Eleveld M, Kater-Baats E, Janssen L, Janssen B, Weterman M, Schoenmakers E, Nickerson M, Linehan M, Zbar B, van Kessel AG. Disruption of a novel MFS transporter gene, DLRC2, by a familial renal cell carcinoma-associated t(2;3)(q35;q21) Hum Mol Genet. 2002;11(6):641–649. doi: 10.1093/hmg/11.6.641. [DOI] [PubMed] [Google Scholar]
- Brasier G, Tikellis C, Xuereb L, Craigie J, Casley D, Kovacs CS, Fudge NJ, Kalnins R, Cooper ME, Wookey PJ. Novel hexad repeats conserved in a putative transporter with restricted expression in cell types associated with growth, calcium exchange and homeostasis. Exp Cell Res. 2004;293(1):31–42. doi: 10.1016/j.yexcr.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Brown JK, Fung C, Tailor CS. Comprehensive mapping of receptor-functioning domains in feline leukemia virus subgroup C receptor FLVCR1. J Virol. 2006;80(4):1742–1751. doi: 10.1128/JVI.80.4.1742-1751.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter CE, Mahoney AW. Contributions of heme and nonheme iron to human nutrition. Crit Rev Food Sci Nutr. 1992;31(4):333–367. doi: 10.1080/10408399209527576. [DOI] [PubMed] [Google Scholar]
- Castro-Gago M, Alonso A, Pintos-Martinez E, Beiras-Lglesias A, Campos Y, Arenas J, Novo-Rodriguez ML, Eiris-Punal J. Congenital hydranencephalic-hydrocephalic syndrome associated with mitochondrial dysfunction. J Child Neurol. 1999;14(2):131–135. doi: 10.1177/088307389901400213. [DOI] [PubMed] [Google Scholar]
- Chiabrando D, Tolosano E. Diamond Blackfan Anemia at the Crossroad between Ribosome Biogenesis and Heme Metabolism. Adv Hematol. 2010;2010:790632. doi: 10.1155/2010/790632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A. Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol. 2005;6(1):79–87. doi: 10.1038/nrm1552. [DOI] [PubMed] [Google Scholar]
- Crosby JS, Chefalo PJ, Yeh L, Ying S, London LM, Leboulch P, Chen JJ. Regulation of hemoglobin synthesis and proliferation of differentiating erythroid cells by heme-regulated eLF-2alpha kinase. Blood. 2000;96(9):3241–3248. [PubMed] [Google Scholar]
- Da Costa L, Moniz H, Simansour M, Tchernia G, Mohandas N, Leblanc T. Diamond-Blackfan anemia, ribosome and erythropoiesis. Transfus Clin Biol. 2010;17(3):112–119. doi: 10.1016/j.tracli.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilova N, Sakamoto KM, Lin S. Ribosomal protein S19 deficiency in zebrafish leads to developmental abnormalities and defective erythropoiesis through activation of p53 protein family. Blood. 2008;112(13):5228–5237. doi: 10.1182/blood-2008-01-132290. [DOI] [PubMed] [Google Scholar]
- de Haro C, Mendez R, Santoyo J. The eLF-2alpha kinases and the control of protein synthesis. FASEB J. 1996;10(12):1378–1387. doi: 10.1096/fasebj.10.12.8903508. [DOI] [PubMed] [Google Scholar]
- Deniau C, Gilli R, Lzadi-Pruneyre N, Letoffe S, Delepierre M, Wandersman C, Briand C, Lecroisey A. Thermodynamics of heme binding to the HasA(SM) hemophore: effect of mutations at three key residues for heme uptake. Biochemistry. 2003;42(36):10627–10633. doi: 10.1021/bi030015k. [DOI] [PubMed] [Google Scholar]
- Devlin EE, Dacosta L, Mohandas N, Elliott G, Bodine DM. A transgenic mouse model demonstrates a dominant negative effect of a point mutation in the RPS19 gene associated with Diamond-Blackfan anemia. Blood. 2010;116(15):2826–2835. doi: 10.1182/blood-2010-03-275776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RT, Sabo KM, Chen J, Miller AD, Abkowitz JL. An all-feline retroviral packaging system for transduction of human cells. Hum Gene Ther. 2010;21(8):1019–1027. doi: 10.1089/hum.2010.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draptchinskaia N, Gustavsson P, Andersson B, Pettersson M, Willig TN, Dianzani L, Ball S, Tchernia G, Klar J, Matsson H, Tentler D, Mohandas N, Carlsson B, Dahl N. The gene encoding ribosomal protein S19 is mutated in Diamond-Blackfan anaemia. Nat Genet. 1999;21(2):169–175. doi: 10.1038/5951. [DOI] [PubMed] [Google Scholar]
- Duffy SP, Shing J, Saraon P, Berger LC, Eiden MV, Wilde A, Tailor CS. The Fowler syndrome-associated protein FLVCR2 is an importer of heme. Mol Cell Biol. 2010;30(22):5318–5324. doi: 10.1128/MCB.00690-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faller M, Matsunaga M, Yin S, Loo JA, Guo F. Heme is involved in microRNA processing. Nat Struct Mol Biol. 2007;14(1):23–29. doi: 10.1038/nsmb1182. [DOI] [PubMed] [Google Scholar]
- Farrar JE, Vlachos A, Atsidaftos E, Carlson-Donohoe H, Markello TC, Arceci RJ, Ellis SR, Lipton JM, Bodine DM. Ribosomal protein gene deletions in Diamond-Blackfan anemia. Blood. 2011 doi: 10.1182/blood-2011-08-375170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming MD, Trenor CC, 3rd, Su MA, Foernzler D, Beier DR, Dietrich WF, Andrews NC. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet. 1997;16(4):383–386. doi: 10.1038/ng0897-383. [DOI] [PubMed] [Google Scholar]
- Flygare J, Kiefer T, Miyake K, Utsugisawa T, Hamaguchi L, Da Costa L, Richter J, Davey EJ, Matsson H, Dahl N, Wiznerowicz M, Trono D, Karlsson S. Deficiency of ribosomal protein S19 in CD34+ cells generated by siRNA blocks erythroid development and mimics defects seen in Diamond-Blackfan anemia. Blood. 2005;105(12):4627–4634. doi: 10.1182/blood-2004-08-3115. [DOI] [PubMed] [Google Scholar]
- Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol. 2007;8(11):917–929. doi: 10.1038/nrm2272. [DOI] [PubMed] [Google Scholar]
- Frawley ER, Kranz RG. CcsBA is a cytochrome c synthetase that also functions in heme transport. Proc Natl Acad Sci U S A. 2009;106(25):10201–10206. doi: 10.1073/pnas.0903132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman BS, Beck DL, Monika EM, Kranz RG. Transmembrane heme delivery systems. Proc Natl Acad Sci U S A. 1998;95(9):5003–5008. doi: 10.1073/pnas.95.9.5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388(6641):482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Hamza L. Lntracellular trafficking of porphyrins. ACS Chem Biol. 2006;1(10):627–629. doi: 10.1021/cb600442b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrove MS, Barrick D, Olson JS. The association rate constant for heme binding to globin is independent of protein structure. Biochemistry. 1996;35(35):11293–11299. doi: 10.1021/bi960371l. [DOI] [PubMed] [Google Scholar]
- Hartmann LC, Lu KH, Linette GP, Cliby WA, Kalli KR, Gershenson D, Bast RC, Stec J, Lartchouk N, Smith DL, Ross JS, Hoersch S, Shridhar V, Lillie J, Kaufmann SH, Clark EA, Damokosh AL. Gene expression profiles predict early relapse in ovarian cancer after platinum-paclitaxel chemotherapy. Clin Cancer Res. 2005;11(6):2149–2155. doi: 10.1158/1078-0432.CCR-04-1673. [DOI] [PubMed] [Google Scholar]
- Hrkal Z, Vodrazka Z, Kalousek L. Transfer of heme from ferrihemoglobin and ferrihemoglobin isolated chains to hemopexin. Eur J Biochem. 1974;43(1):73–78. doi: 10.1111/j.1432-1033.1974.tb03386.x. [DOI] [PubMed] [Google Scholar]
- Hulo N, Bairoch A, Bulliard V, Cerutti L, Cuche BA, de Castro E, Lachaize C, Langendijk-Genevaux PS, Sigrist CJ. The 20 years of PROSLTE. Nucleic Acids Res. 2008;36(Database issue):D245–249. doi: 10.1093/nar/gkm977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi T, Kay SA, Schroeder JL. Circadian rhythms. Daily watch on metabolism. Science. 2007;318(5857):1730–1731. doi: 10.1126/science.1151360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiura H, Fukuda Y, Mitsui J, Nakahara Y, Ahsan B, Takahashi Y, Lchikawa Y, Goto J, Sakai T, Tsuji S. Posterior column ataxia with retinitis pigmentosa in a Japanese family with a novel mutation in FLVCR1. Neurogenetics. 2011;12(2):117–121. doi: 10.1007/s10048-010-0271-4. [DOI] [PubMed] [Google Scholar]
- Jaako P, Flygare J, Olsson K, Quere R, Ehinger M, Henson A, Ellis S, Schambach A, Baum C, Richter J, Larsson J, Bryder D, Karlsson S. Mice with ribosomal protein S19 deficiency develop bone marrow failure and symptoms like patients with Diamond-Blackfan anemia. Blood. 2011 doi: 10.1182/blood-2011-08-371963. [DOI] [PubMed] [Google Scholar]
- Keel SB, Doty RT, Yang Z, Quigley JG, Chen J, Knoblaugh S, Kingsley PD, De Domenico L, Vaughn MB, Kaplan J, Palis J, Abkowitz JL. A heme export protein is required for red blood cell differentiation and iron homeostasis. Science. 2008;319(5864):825–828. doi: 10.1126/science.1151133. [DOI] [PubMed] [Google Scholar]
- Khan AA, Quigley JG. Control of intracellular heme levels: Heme transporters and heme oxygenases. Biochim Biophys Acta. 2011;1813(5):668–682. doi: 10.1016/j.bbamcr.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathrop JT, Timko MP. Regulation by heme of mitochondrial protein transport through a conserved amino acid motif. Science. 1993;259(5094):522–525. doi: 10.1126/science.8424176. [DOI] [PubMed] [Google Scholar]
- Letoffe S, Debarbieux L, Lzadi N, Delepelaire P, Wandersman C. Ligand delivery by haem carrier proteins: the binding of Serratia marcescens haemophore to its outer membrane receptor is mediated by two distinct peptide regions. Mol Microbiol. 2003;50(1):77–88. doi: 10.1046/j.1365-2958.2003.03686.x. [DOI] [PubMed] [Google Scholar]
- Light WR, 3rd, Olson JS. Transmembrane movement of heme. J Biol Chem. 1990;265(26):15623–15631. [PubMed] [Google Scholar]
- Lipovich L, Hughes AL, King MC, Abkowitz JL, Quigley JG. Genomic structure and evolutionary context of the human feline leukemia virus subgroup C receptor (hFLVCR) gene: evidence for block duplications and de novo gene formation within duplicons of the hFLVCR locus. Gene. 2002;286(2):203–213. doi: 10.1016/s0378-1119(02)00457-2. [DOI] [PubMed] [Google Scholar]
- Lipton JM, Ellis SR. Diamond-Blackfan anemia: diagnosis, treatment, and molecular pathogenesis. Hematol Oncol Clin North Am. 2009;23(2):261–282. doi: 10.1016/j.hoc.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas ML, Seidel NE, Porada CD, Quigley JG, Anderson SM, Malech HL, Abkowitz JL, Zanjani ED, Bodine DM. Lmproved transduction of human sheep repopulating cells by retrovirus vectors pseudotyped with feline leukemia virus type C or RD114 envelopes. Blood. 2005;106(1):51–58. doi: 10.1182/blood-2004-11-4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher CA, Palanisamy N, Brenner JC, Cao X, Kalyana-Sundaram S, Luo S, Khrebtukova L, Barrette TR, Grasso C, Yu J, Lonigro RJ, Schroth G, Kumar-Sinha C, Chinnaiyan AM. Chimeric transcript discovery by paired-end transcriptome sequencing. Proc Natl Acad Sci U S A. 2009;106(30):12353–12358. doi: 10.1073/pnas.0904720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer E, Ricketts C, Morgan NV, Morris MR, Pasha S, Tee LJ, Rahman F, Bazin A, Bessieres B, Dechelotte P, Yacoubi MT, Al-Adnani M, Marton T, Tannahill D, Trembath RC, Fallet-Bianco C, Cox P, Williams D, Maher ER. Mutations in FLVCR2 are associated with proliferative vasculopathy and hydranencephaly-hydrocephaly syndrome (Fowler syndrome) Am J Hum Genet. 2010;86(3):471–478. doi: 10.1016/j.ajhg.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muth TR, Caplan MJ. Transport protein trafficking in polarized cells. Annu Rev Cell Dev Biol. 2003;19:333–366. doi: 10.1146/annurev.cellbio.19.110701.161425. [DOI] [PubMed] [Google Scholar]
- O’Callaghan KM, Ayllon V, O’Keeffe J, Wang Y, Cox OT, Loughran G, Forgac M, O’Connor R. Heme-binding protein HRG-1 is induced by insulin-like growth factor L and associates with the vacuolar H+-ATPase to control endosomal pH and receptor trafficking. J Biol Chem. 2009;285(1):381–391. doi: 10.1074/jbc.M109.063248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K, Sun J, Taketani S, Nakajima O, Nishitani C, Sassa S, Hayashi N, Yamamoto M, Shibahara S, Fujita H, Lgarashi K. Heme mediates derepression of Maf recognition element through direct binding to transcription repressor Bach1. EMBO J. 2001;20(11):2835–2843. doi: 10.1093/emboj/20.11.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onions D, Jarrett O, Testa N, Frassoni F, Toth S. Selective effect of feline leukaemia virus on early erythroid precursors. Nature. 1982;296(5853):156–158. doi: 10.1038/296156a0. [DOI] [PubMed] [Google Scholar]
- Padash-Barmchi M, Browne K, Sturgeon K, Jusiak B, Auld VJ. Control of Gliotactin localization and levels by tyrosine phosphorylation and endocytosis is necessary for survival of polarized epithelia. J Cell Sci. 2010;123(Pt 23):4052–4062. doi: 10.1242/jcs.066605. [DOI] [PubMed] [Google Scholar]
- Pao SS, Paulsen LT, Saier MH., Jr. Major facilitator superfamily. Microbiol Mol Biol Rev. 1998;62(1):1–34. doi: 10.1128/mmbr.62.1.1-34.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peedicayil A, Vierkant RA, Hartmann LC, Fridley BL, Fredericksen ZS, White KL, Elliott EA, Phelan CM, Tsai YY, Berchuck A, Lversen ES, Jr., Couch FJ, Peethamabaran P, Larson MC, Kalli KR, Kosel ML, Shridhar V, Rider DN, Liebow M, Cunningham JM, Schildkraut JM, Sellers TA, Goode EL. Risk of ovarian cancer and inherited variants in relapse-associated genes. PLoS One. 5(1):e8884. doi: 10.1371/journal.pone.0008884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponka P. Tissue-specific regulation of iron metabolism and heme synthesis: distinct control mechanisms in erythroid cells. Blood. 1997;89(1):1–25. [PubMed] [Google Scholar]
- Protchenko O, Shakoury-Elizeh M, Keane P, Storey J, Androphy R, Philpott CC. Role of PUG1 in inducible porphyrin and heme transport in Saccharomyces cerevisiae. Eukaryot Cell. 2008;7(5):859–871. doi: 10.1128/EC.00414-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, Hamza L, O’Brian MR. Heme is an effector molecule for iron-dependent degradation of the bacterial iron response regulator (Lrr) protein. Proc Natl Acad Sci U S A. 1999;96(23):13056–13061. doi: 10.1073/pnas.96.23.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman LD. Ldentification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell. 2006;127(5):917–928. doi: 10.1016/j.cell.2006.09.041. [DOI] [PubMed] [Google Scholar]
- Quigley JG, Burns CC, Anderson MM, Lynch ED, Sabo KM, Overbaugh J, Abkowitz JL. Cloning of the cellular receptor for feline leukemia virus subgroup C (FeLV-C), a retrovirus that induces red cell aplasia. Blood. 2000;95(3):1093–1099. [PubMed] [Google Scholar]
- Quigley JG, Gazda H, Yang Z, Ball S, Sieff CA, Abkowitz JL. Lnvestigation of a putative role for FLVCR, a cytoplasmic heme exporter, in Diamond-Blackfan anemia. Blood Cells Mol Dis. 2005;35(2):189–192. doi: 10.1016/j.bcmd.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Quigley JG, Yang Z, Worthington MT, Phillips JD, Sabo KM, Sabath DE, Berg CL, Sassa S, Wood BL, Abkowitz JL. Ldentification of a human heme exporter that is essential for erythropoiesis. Cell. 2004;118(6):757–766. doi: 10.1016/j.cell.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Rajadhyaksha AM, Elemento O, Puffenberger EG, Schierberl KC, Xiang JZ, Putorti ML, Berciano J, Poulin C, Brais B, Michaelides M, Weleber RG, Higgins JJ. Mutations in FLVCR1 cause posterior column ataxia and retinitis pigmentosa. Am J Hum Genet. 2010;87(5):643–654. doi: 10.1016/j.ajhg.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal A, Rao AU, Amigo J, Tian M, Upadhyay SK, Hall C, Uhm S, Mathew MK, Fleming MD, Paw BH, Krause M, Hamza L. Haem homeostasis is regulated by the conserved and concerted functions of HRG-1 proteins. Nature. 2008;453(7198):1127–1131. doi: 10.1038/nature06934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao AU, Carta LK, Lesuisse E, Hamza L. Lack of heme synthesis in a free-living eukaryote. Proc Natl Acad Sci U S A. 2005;102(12):4270–4275. doi: 10.1073/pnas.0500877102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey MA, Duffy SP, Brown JK, Kennedy JA, Dick JE, Dror Y, Tailor CS. Enhanced alternative splicing of the FLVCR1 gene in Diamond Blackfan anemia disrupts FLVCR1 expression and function that are critical for erythropoiesis. Haematologica. 2008;93(11):1617–1626. doi: 10.3324/haematol.13359. [DOI] [PubMed] [Google Scholar]
- Ricchelli F, Gobbo S, Jori G, Moreno G, Salet C. Temperature-induced changes in fluorescence properties as a probe of porphyrin microenvironment in lipid membranes. 1. The partition of hematoporphyrin and protoporphyrin in liposomes. Eur J Biochem. 1995;233(1):159–164. doi: 10.1111/j.1432-1033.1995.159_1.x. [DOI] [PubMed] [Google Scholar]
- Royle SJ, Murrell-Lagnado RD. Constitutive cycling: a general mechanism to regulate cell surface proteins. Bioessays. 2003;25(1):39–46. doi: 10.1002/bies.10200. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Tyrrell RM. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med. 2000;28(2):289–309. doi: 10.1016/s0891-5849(99)00223-3. [DOI] [PubMed] [Google Scholar]
- Saier MH, Jr., Yen MR, Noto K, Tamang DG, Elkan C. The Transporter Classification Database: recent advances. Nucleic Acids Res. 2009;37(Database issue):D274–278. doi: 10.1093/nar/gkn862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S. Why heme needs to be degraded to iron, biliverdin LXalpha, and carbon monoxide? Antioxid Redox Signal. 2004;6(5):819–824. doi: 10.1089/ars.2004.6.819. [DOI] [PubMed] [Google Scholar]
- Savalas LR, Gasnier B, Damme M, Lubke T, Wrocklage C, Debacker C, Jezegou A, Reinheckel T, Hasilik A, Saftig P, Schroder B. Disrupted in renal carcinoma 2 (DLRC2), a novel transporter of the lysosomal membrane, is proteolytically processed by cathepsin L. Biochem J. 2011;439(1):113–128. doi: 10.1042/BJ20110166. [DOI] [PubMed] [Google Scholar]
- Severance S, Hamza L. Trafficking of heme and porphyrins in metazoa. Chem Rev. 2009;109(10):4596–4616. doi: 10.1021/cr9001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance S, Rajagopal A, Rao AU, Cerqueira GC, Mitreva M, El-Sayed NM, Krause M, Hamza L. Genome-wide analysis reveals novel genes essential for heme homeostasis in Caenorhabditis elegans. PLoS Genet. 2010;6(7):e1001044. doi: 10.1371/journal.pgen.1001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev Z, Duffy SP, Adema KW, Prasad R, Hussain N, Willett BJ, Tailor CS. Ldentification of a feline leukemia virus variant that can use THTR1, FLVCR1, and FLVCR2 for infection. J Virol. 2009;83(13):6706–6716. doi: 10.1128/JVI.02317-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayeghi M, Latunde-Dada GO, Oakhill JS, Laftah AH, Takeuchi K, Halliday N, Khan Y, Warley A, McCann FE, Hider RC, Frazer DM, Anderson GJ, Vulpe CD, Simpson RJ, McKie AT. Ldentification of an intestinal heme transporter. Cell. 2005;122(5):789–801. doi: 10.1016/j.cell.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Sinclair J, Hamza L. A novel heme-responsive element mediates transcriptional regulation in Caenorhabditis elegans. J Biol Chem. 2010;285(50):39536–39543. doi: 10.1074/jbc.M110.167619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Shimizu T, Kijima A, Minakata S, Kato Y. PDZ adaptors: their regulation of epithelial transporters and involvement in human diseases. J Pharm Sci. 2011;100(9):3620–3635. doi: 10.1002/jps.22575. [DOI] [PubMed] [Google Scholar]
- Sun J, Brand M, Zenke Y, Tashiro S, Groudine M, Lgarashi K. Heme regulates the dynamic exchange of Bach1 and NF-E2-related factors in the Maf transcription factor network. Proc Natl Acad Sci U S A. 2004;101(6):1461–1466. doi: 10.1073/pnas.0308083100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tailor CS, Willett BJ, Kabat D. A putative cell surface receptor for anemia-inducing feline leukemia virus subgroup C is a member of a transporter superfamily. J Virol. 1999;73(8):6500–6505. doi: 10.1128/jvi.73.8.6500-6505.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S, Encha-Razavi F, Devisme L, Etchevers H, Bessieres-Grattagliano B, Goudefroye G, Elkhartoufi N, Pateau E, Lchkou A, Bonniere M, Marcorelle P, Parent P, Manouvrier S, Holder M, Laquerriere A, Loeuillet L, Roume J, Martinovic J, Mougou-Zerelli S, Gonzales M, Meyer V, Wessner M, Feysot CB, Nitschke P, Leticee N, Munnich A, Lyonnet S, Wookey P, Gyapay G, Foliguet B, Vekemans M, Attie-Bitach T. High-throughput sequencing of a 4.1 Mb linkage interval reveals FLVCR2 deletions and mutations in lethal cerebral vasculopathy. Hum Mutat. 2010;31(10):1134–1141. doi: 10.1002/humu.21329. [DOI] [PubMed] [Google Scholar]
- Th6ny-Meyer L. Heme Transport and Lncorporation into Proteins. Tetrapyrroles. 2009:149–159. [Google Scholar]
- Tu BP, Mohler RE, Liu JC, Dombek KM, Young ET, Synovec RE, McKnight SL. Cyclic changes in metabolic state during the life of a yeast cell. Proc Natl Acad Sci U S A. 2007;104(43):16886–16891. doi: 10.1073/pnas.0708365104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beest M, Robben JH, Savelkoul PJ, Hendriks G, Devonald MA, Konings LB, Lagendijk AK, Karet F, Deen PM. Polarisation, key to good localisation. Biochim Biophys Acta. 2006;1758(8):1126–1133. doi: 10.1016/j.bbamem.2006.03.007. [DOI] [PubMed] [Google Scholar]
- van Ham M, Hendriks W. PDZ domains-glue and guide. Mol Biol Rep. 2003;30(2):69–82. doi: 10.1023/a:1023941703493. [DOI] [PubMed] [Google Scholar]
- Wang S, Publicover S, Gu Y. An oxygen-sensitive mechanism in regulation of epithelial sodium channel. Proc Natl Acad Sci U S A. 2009;106(8):2957–2962. doi: 10.1073/pnas.0809100106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnatz HJ, Schmidt D, Manke T, Piccini L, Sultan M, Borodina T, Balzereit D, Wruck W, Soldatov A, Vingron M, Lehrach H, Yaspo ML. The BTB and CNC homology 1 (BACH1) target genes are involved in the oxidative stress response and in control of the cell cycle. J Biol Chem. 2011;286(26):23521–23532. doi: 10.1074/jbc.M111.220178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe-Matsui M, Muto A, Matsui T, Ltoh-Nakadai A, Nakajima O, Murayama K, Yamamoto M, Lkeda-Saito M, Lgarashi K. Heme regulates B-cell differentiation, antibody class switch, and heme oxygenase-1 expression in B cells as a ligand of Bach2. Blood. 2011;117(20):5438–5448. doi: 10.1182/blood-2010-07-296483. [DOI] [PubMed] [Google Scholar]
- Yanatori L, Tabuchi M, Kawai Y, Yasui Y, Akagi R, Kishi F. Heme and non-heme iron transporters in non-polarized and polarized cells. BMC Cell Biol. 2010;11(1):39. doi: 10.1186/1471-2121-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Philips JD, Doty RT, Giraudi P, Ostrow JD, Tiribelli C, Smith A, Abkowitz JL. Kinetics and specificity of FLVCR export function and its dependence on hemopexin. J Biol Chem. 2010 doi: 10.1074/jbc.M110.119131. JBC Papers in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Protchenko O, Philpott CC, Hamza L. Topologically Conserved Residues Direct Heme Transport in HRG-1-related Proteins. J Biol Chem. 2012;287(7):4914–4924. doi: 10.1074/jbc.M111.326785. [DOI] [PMC free article] [PubMed] [Google Scholar]