Abstract
The mammalian Rh glycoproteins belong to the solute transporter family SLC42 and include RhAG, present in red blood cells, and two non-erythroid members RhBG and RhCG that are expressed in various tissues, including kidney, liver, skin and the GI tract. The Rh proteins in the red blood cell form an “Rh complex” made up of one D-subunit, one CE-subunit and two RhAG subunits. The Rh complex has a well-known antigenic effect but also contributes to the stability of the red cell membrane. RhBG and RhCG are related to the NH4+ transporters of the yeast and bacteria but their exact function is yet to be determined. This review describes the expression and molecular properties of these membrane proteins and their potential role as NH3/NH4+ and CO2 transporters. The likelihood that these proteins transport gases such as CO2 or NH3 is novel and significant. The review also describes the physiological importance of these proteins and their relevance to human disease.
Keywords: SLC42, RhAG, RhBG, RhCG, NH3, NH4+ Transport, CO2 Transport
1. Overview
Rh glycoproteins
Rh glycoproteins, belonging to solute transporter family SLC42, have long been identified and studied in human blood cells for their immunogenic characteristics and importance in pregnancy (Avent and Reid, 2000). Table 1 summarizes some of the known properties of these membrane proteins. In red blood cells, the Rh antigens exist as a hetero oligomeric “Rh complex” of membrane polypeptides that include one D subunit, one CE subunit and two glycosylated RhAG (SLC42A1) subunits each with 12 transmembrane domains TM (Avent et al., 1996; Conroy et al., 2005; Ridgwell et al., 1992). RhAG has been expressed in heterologous systems (e.g. oocytes) independently of RhCE or D subunits. In addition to its antigenic property, the Rh complex is thought to contribute to the membrane stability and structure of red blood cell. Its exact function has not been determined.
Table 1.
SLC42 - The Human Rh Ammonium Transporter Family
| Human Gene Symbol | SLC Symbol | Protein Name | ORF (aa) | Predominent Substrates | Transport type Coupling ions | Tissue distribution & cellular/subcellular expression | Link to disease | Human gene locus | Sequence Accession ID | Splice Variants and their features |
|---|---|---|---|---|---|---|---|---|---|---|
| RHAG (Rh50A) | SLC42A1 | RhAG | 409 | NH4+, NH3+ | H+ | Red blood cells, (cell membrane) | Rhnull-regulator Rhmod, OHSt* |
6p12.3 | AF031548 | |
| RHBG | SLC42A2 | RhBG | 458 | NH4+, NH3, Methyl amine Methyl ammonium |
Electrogenic No coupled ions |
Kidney, Liver, skin, GI tract sweat glands, ovaries (basolateral membrane) | Unknown | 1q21.3 | AF193807 | 5 isoforms: Q9H310-1 Q9H310-2 Q9H310-3 Q9H310-4 Q9H310-5 (No experimental confirmation) |
| RHCG (RhGK) | SLC42A3 | RhCG | 479 | NH4+, NH3 | Electroneutral possibly H+ | Kidney (apical membrane) Brain, Testis Placents, Pancreas, Prostate |
Renal distal tubular acidosis (?) | 15q25 | AF193809 | Unknown |
OHSt: Dominant over-hydrated hereditary stomatocytosis
RhCG
Marini and co-workers made the original observation that human Rh antigens share sequence homology to the MEP NH4+ transporters of the yeast (Marini et al., 1997). A subsequent study, (Marini et al., 2000) identified a kidney homologue originally named RhGK (for Rh glycoprotein kidney) and later RhCG (SLC42A3). The RhCG protein shows close identity to RhAG (50%) and sequence homology (24%) with the MEP/Amt ammonium transporters. RHCG cDNA was amplified from human kidney RNA and successfully expressed in yeast cells (Marini et al., 2000). Liu et al., (Liu et al., 2000) subsequently cloned human (RHCG) and mouse (Rhcg) homologues with cDNAs sequences of 1952 and 2097 bp respectively and overall identity of 68.8%. At the protein level, RHCG encodes a 53 KDa, 479 amino acid polypeptide, whereas Rhcg encodes a 55 KDa, 498 amino acid polypeptide and they are highly conserved (77.2% identity and 90.4% similarity). Hydropathy analysis indicated that they share identical topology of 12 transmembrane domains with intracellular N and C termini (Fig 1). Their 12 TM structure has conserved regions shared with other Rh homologues, especially RhAG, but differ from RhAG with a much-elongated C – terminal and a unique N – terminal. RHCG was mapped to the 15q25 chromosome whereas Rhcg was mapped by linkage analysis to a locus (D7Xrf229) on the long arm of chromosome 7.
Figure 1. A model of the membrane topology of Mus musculus RhCG.
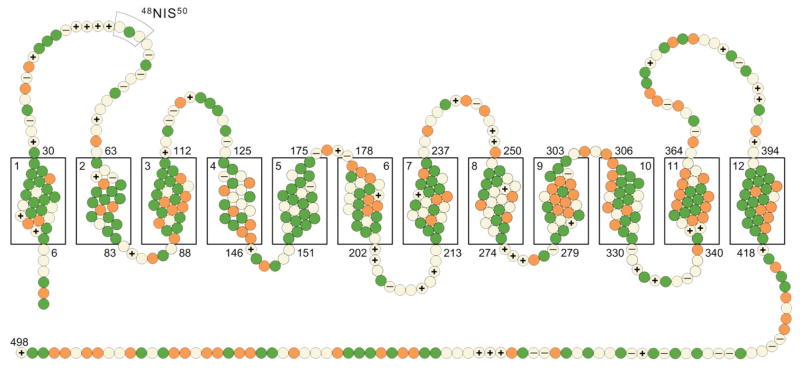
Solid green circles denote the hydrophobic amino acids Phe, Ile, Leu, Met, Val and Trp; orange circles, Gly, Ala and Pro; light yellow circles, polar residues Ser, Cys, Thr, Asn, Gln and Tyr; cicrles marked with + denote the positively charged Lys, Arg and His; and −, negatively charged Asp and Glu. The probability of the location of the transmembrane sequences was predicted by the N-best algorithm (Krogh et al., 2001). The N-glycosylation site (48NIS50), present in the first e4xoloop, is illustrated.
RhBG
Cloning and biochemical characteristics of Rh type B glycoprotein (SLC42A2) were initially accomplished by Liu et al., (Liu et al., 2001). RHBG and Rhbg have open reading frames of 1377 and 1368 bp respectively, which encode polypeptides of 458 and 455 amino acids respectively. Human and mouse RhBG are 85% identical and 94% similar at the protein level, less similar to RhCG (58% - 53%) and notably different from RhAG (43%). Both proteins have a molecular mass of 49.3 KDa, are negatively charged at physiological pH and have a single N-glycosylation motif. Like RhCG and RhAG, RhBG is a polytopic protein with 12 predicted trans-membrane domains. RHBG resides at 1q21.3 of human chromosome 1 and Rhbg is on mouse chromosome 3 on a site where many markers are similar to those of human 1q21 containing RHBG. The Rh proteins are closely related to the ammonia transporters family (Amt) and seem to have branched off from Amt ancestors in prokaryotes (Huang and Ye, 2010). The two families show different patterns of distribution but they also coexist in a variety of organisms indicating a divergent and independent evolution. Even in organisms that have Amt and Rh proteins their genes cluster independently suggesting a functional distinction between the two families.
Transport of NH3/NH4+
The original link between Rh proteins and NH3/NH4+ transport was provided by studies (Marini et al., 2000) on yeast mutants, termed (Triple-MEPΔ), rendered incapable of NH4+ transport by deletions of 3 endogenous NH4+-transporter genes. Yeast, like many other fungal species as well as bacteria, efficiently scavenge NH4+ which is required as a nitrogen source, without which growth is greatly hampered. Expressing RhAG enabled the Triple-MEPΔ cells to grow in low NH4+ medium and resulted in resistance to toxic concentrations of methyl ammonium suggesting a role in NH4+ transport. Although the evidence for NH4+ transport by Rh proteins is still actively debated, it is intriguing that this class of membrane proteins may be the elusive NH4+ transporter. This is very important in tissues, such as the kidney where transport of NH3/NH4+ is critical for regulation of acid-base balance (Heitman and Agre, 2000).
In mammals, normal acid-base homeostasis is critically dependent on renal excretion of NH4+ in the urine (Knepper et al., 1989). Renal ammoniagenesis and NH4+ transport are highly regulated and NH4+ excretion increases several fold during chronic acidosis (Knepper et al., 1991). In physiological fluids (pH 7.4 – 7.5), NH3/NH4+ exists predominantly as NH4+ (~99%) because the pKa of the equilibrium reaction NH3+H+ ↔ NH4+ is high (~9.2). Whereas NH3, a small neutral molecule, is assumed to mainly diffuse through the cell membrane, NH4+ has to be transported by membrane transporters or channels (Attmane-Elakeb et al., 2001; Good et al., 1984; Knepper et al., 1989). Some K+ transport pathways may serve as NH4+ carriers because of the similarity of the two ions in size and charge.
The renal proximal tubule generates NH3/NH4+ which is then secreted predominantly into the luminal fluid (Hamm and Simon, 1990). This occurs by both NH3 diffusion into the more acid luminal fluid and by NH4+ transport in exchange for sodium on the Na+- H+ exchanger (Knepper et al., 1989). In the loop of Henle, total ammonia may be secreted into the descending limb of Henle but is then reabsorbed in the thick ascending limb (Good and Knepper, 1985; Knepper et al., 1989). NH4+ is reabsorbed from the lumen into the cell via substitution for K+ on both the Na-K-2Cl co-transporter and the apical membrane potassium channel (see (Attmane-Elakeb et al., 2001; Good et al., 1984). In the collecting duct, total ammonia is secreted along the length of the tubule. Most, if not all, of this secretion has been thought to occur by non-ionic diffusion of NH3 driven by the progressively increasing concentrations of ammonia in the medullary interstitium (Hamm, 1986). Concurrent acid secretion along the length of the collecting duct (by H-ATPase for example) keeps luminal concentrations of NH3 low, maintaining a favorable NH3 gradient from interstitium to lumen. The apical membrane of collecting duct cells is one site where rate-limiting rapid NH3 diffusion has to occur. The basolateral membrane, on the other hand, is the site where NH4+ transport occurs presumably by one or more membrane transporters (Hamm et al., 1985; Knepper et al., 1989).
2. Expression of Rh Glycoproteins
In red blood cells, RhAG is expressed in the membrane as a component of the “Rh complex”, as mentioned above. The “Rh Complex” consists of RhAG in association with the nonglycosylated Rh proteins RhD and RhCE in humans or with Rh30 in non-human mammals. RhAG is an erythrocyte-specific protein not found in other tissues (Cartron, 1999; Liu and Huang, 1999). Expression in heterologous systems showed that RhAG was fully glycosylated and properly trafficked to the plasma membranes of oocytes (Westhoff et al., 2002), yeast cells (Marini et al., 2000) and HeLa cells (Benjelloun et al., 2005). Cell surface expression of the “Rh complex” was shown to be linked to RhAG interaction with spectrin-based ankyrin (Nicolas et al., 2006). It was also implied that in red blood cells (RBC) Rh protein, Band-3 and ankyrin form an integral membrane complex that may modulate transport of HCO3− and possibly NH4+ (Nicolas et al., 2006).
Expression of the RHCG and Rhcg genes has been assessed by Northern blot analysis, in situ hybridization and immunohistochemistry (Eladari et al., 2002; Liu et al., 2000; Verlander et al., 2003). These studies indicated that in human adult tissues RhCG was abundantly expressed in the kidneys, brain, testis, placenta, skeletal muscle, liver, GI tract, pancreas, and prostate (Handlogten et al., 2005; Liu et al., 2000; Marini et al., 2000; Weiner et al., 2003). In human fetal tissues, RhCG was expressed only in the kidney. In mouse adult tissues, RhCG was expressed in kidney, testis, liver and other tissues. Using RT – PCR on rat micro dissected tubules, RhCG was found in distal convoluted tubules, the connecting segment (CNT), cortical collecting duct (CCD) and outer medullary collecting duct (OMCD) but not the proximal tubule and the thick ascending limb of Henle’s loop. Immuno-localization studies showed labeling of the apical membrane of cells within the cortex, outer medulla and upper portion of inner medulla. RhCG was present in all CCD cells and the intercalated cells of OMCD and IMCD (inner medullary collecting duct). Another study, however, indicated that RhCG is restricted to the intercalated cells of the CCD cells (Eladari et al., 2002). RhCG was also present in the principal cells of the outer stripe of OMCD. The subcellular distribution of RhCG seemed to differ depending on tissue and species. In the mouse kidney, RhCG was reported to be at the apical membrane (Eladari et al., 2002; Verlander et al., 2003) whereas in the rat RhCG was reported to be either exclusively apical (Eladari et al., 2002) or at both apical and basolateral membranes (Seshadri et al., 2006). In the human kidney, RhCG semed to be restricted to the apical membrane (Eladari et al., 2002; Verlander et al., 2003) but another study reported basolateral expression as well (Han et al., 2006).
RhBG expression was confirmed in non-erythroid tissues only. RhBG was expressed in kidney, skin, sweat glands, GI tract (from duodenum to the colon), liver and, to a lesser extent, ovaries. Using immunohistochemistry in mouse kidney, Verlander et al., (Verlander et al., 2003), localized RhBG to the majority of cells of the connecting segment (CNT) and the cortical collecting duct (CCD). In the OMCD and IMCD, only a subpopulation of cells was labeled. Co-localization studies with carbonic anhydrase II, Na-Cl co-transporter and the anion-exchanger (AE1) demonstrated that RhBG was expressed in all CNT cells and in principal cells of CCD. In CCD, RhBG was also labeled in A-type intercalated cells but not B-type intercalated cells. In OMCD and IMCD only intercalated cells exhibited immunoreactivity to RhBG (Quentin et al., 2003; Verlander et al., 2003). It was demonstrated by double immunostaining that RhCG and RhBG were expressed in the same cell but with distinct apical and basolateral localizations, suggesting different transport properties of the two homologous membrane proteins. In a study on mouse liver, RhBG was localized to the basolateral membrane of hepatocytes surrounding central veins (perivenous) but not in periportal or midzonal hepatocytes (Weiner et al., 2003). RhBG expression has been found to be exclusively restricted to the basolateral membrane of CCD cells (Weiner et al., 2003).
3. Molecular Structure of Rh glycoproteins
An important advancement in the field has been the crystallographic structure of AmtB, the bacterial homologue of Rh (Khademi et al., 2004; Li et al., 2006). The high resolution crystals indicate a trimeric complex of subunits made up of 11 TM helices, each forming a central channel where substrate transport presumably occurs. The crystals showed no conformational changes in the presence or absence of NH4Cl. The proposed tertiary structure indicates that the individual pore of each monomer is composed of an extracellular vestibule followed by a narrow (1.2 A°) and long pore (20 A°) which is terminated at the cytoplasmic side by a wider vestibule. The upper vestibule is lined by aromatic residues that recruit NH4+. The central hydrophobic pore has 2 conserved His residues that promote hydrophobic conduction of substrate. In the absence of resolved structure of RhBG or RhCG the AmtB crystal structure serves as the closest and best model for the Rh protein.
Based on analysis of the 3-dimensional structure of AmtB, a novel transport model was proposed (Fig 2). In AmtB, the upper vestibule recruits NH4+ by a cation - π interactions provided by the residues Trp 148 and Ser 219. As NH4+ moves toward the hydrophobic pore, it gives up its proton predominately on the side of entry (extracellularly) and NH3 is transported through the pore. NH3 in the pore is stabilized by two highly conserved histidines (H168 and H318), which interact with NH3 at 3 specific locations in the pore. On the cytoplasmic side, NH3 acquires an intracellular H+ to re-equilibrate with NH4+.
Figure 2. Proposed architecture of the ammonium pore of RhBG based on the crystal structure of AmtB.
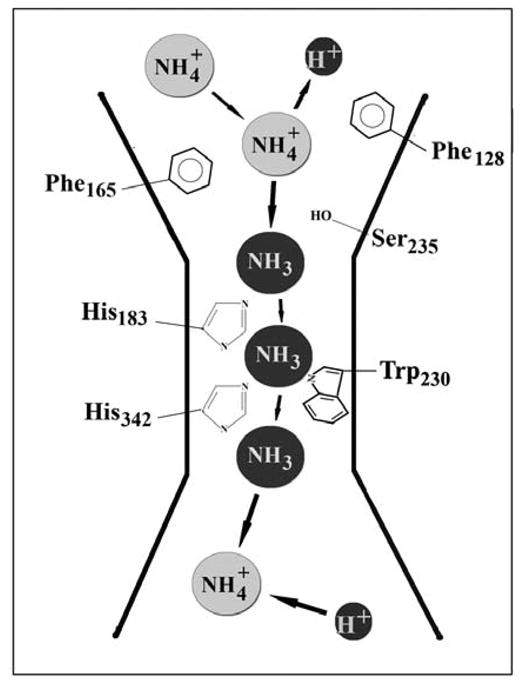
The model shows the equivalent amino acid residues important for transport of NH3/NH4+ based on alignment of RhBG and AmtB sequences.
The degress to which the tertiary structure of Amt B resembles that of Rh glycoproteins remains an open question. Constructing homology models for Rh proteins based on AmtB structure must take into account critical differences in sequences that render proper alignment tricky. Among these differences are putative 12 TM domains in Rh proteins compared to 11 TM domains in Amt B; a long intracellulara C-Terminal α-helix in Rh proteins; and altered critical amino acid residues at sites that that are thought to be key for transport function (Huang and Ye; Lupo et al., 2007). Nevertheless, in the absence of resolved crystal structures or RhCG or RhBG, the structure of AmtB is one of the best models to determine structural determinants of function. Initial studies based on this approach point to important characteristics of function including: a role of C-terminal in gating function and potential binding partners to Rh proteins among cellular proteins (e.g. carbonic anhydrase) and other membrane proteins.
4. Function of Rh Glycoproteins
The role of Rh proteins as NH4+ transporters
Most studies provide strong evidence for a role of the Rh proteins in NH3/NH4+ transport. The initial studies to characterize the functions of RhAG were done in yeast mutant cells deficient in NH4+ transporter genes (Triple mepΔ). Triple mepΔ cells expressing RhAG were able to grow in media containing very low concentrations of NH4+ (Marini et al., 2000). Another study reported that RhAG expression in yeast cells resulted in resistance to toxic concentrations of methyl ammonium (200mM). Also the rate of extracellular NH4+ accumulation was higher in cells expressing RhAG (or RhGK) suggesting that RhAG promotes efflux of NH4+. Measurement of methyl-amine uptake in oocytes expressing RhAG suggested that RhAG serves as an electro neutral counter-transporter of methyl amine (and NH4+) coupled to H+ exit from the cell (Westhoff et al., 2002). As such RhAG would result in transport of net NH3 equivalents. At least one study on HeLa cells suggested that RhAG transports NH3 and NH4+ (Benjelloun et al., 2005). However, RhAG is an erythrocyte membrane protein and its contribution to NH4+ transport in other tissues is unlikely.
Several studies on RhBG expressed in oocytes or mammalian cells report that it can transport NH3/NH4+. However different results are reported by different labs. Some studies report that NH4+ transport is electroneutral and coupled to the H+ gradient and behaving as a NH4+- H+ exchanger (Ludewig, 2004; Mak et al., 2006; Zidi-Yahiaoui et al., 2005). Recent studies indicate that RhBG mediates electrogenic NH4+ transport and that MA/MA+ is transported differently than NH3/NH4+ (Nakhoul et al.; Nakhoul et al., 2005; Nakhoul et al., 2006). The reported affinity of RhBG to NH4+ is 2–4 mM. This concentration is within reported values of interstitial NH4+ concentrations in the renal medulla which vary between 2.5 and 9 mM and is considerably increased in acidosis. In the kidney, the expression of RhBG at the basolateral membrane and the electrochemical gradient for NH4+, are consistent with electrogenic influx of NH4+ although electroneutral NH3 transport may still occur.
Similar uncertainties about RhCG also persist. Some of the earlier studies did confirm a role in NH3/NH4+ transport. However the questions of electrogenic NH4+ transport (Nakhoul et al., 2005), or electroneutral NH4+- H+ exchange (Ludewig, 2004) or simultaneous NH3 and NH4+ transport (Bakouh et al., 2004) are not yet resolved. Studies on reconstituted RhCG in liposomes demonstrated increased NH3 permeability but had no effect on NH4+ permeability (Mouro-Chanteloup et al.). Surface pH measurements in oocytes expressing RhCG indicate transport of NH3 (Musa-Aziz et al., 2009). The presence of RhCG at the apical membrane of the collecting duct, where electroneutral NH3/NH4+ transport is likely, seems to indicate that the mode of transport by RhCG may be different from that of basolateral RhBG.
The role of Rh glycoproteins as gas channels
Historically it was assumed that gases such as CO2 and NH3 cross cell membranes by diffusing through the lipid bilayer. However the discovery that some membranes have low permeability to NH3 or CO2 challenged this view (Kikeri et al., 1989; Singh et al., 1995; Waisbren et al., 1994) and suggested that specific membrane proteins may facilitate transport of these gases. Rh and related proteins were investigated as potential transporters of CO2 or NH3.
Studies on yeast demonstrated dependence of yeast growth in NH3/NH4+ deficient media on pH and concluded that MEP proteins facilitate diffusion of NH3 and not NH4+(Soupene et al., 2001). Other studies of E. coli also indicated facilitated NH3 diffusion by AmtB (Soupene et al., 2002). It was also suggested that RhAG mediates facilitated transport of NH3 (Ripoche et al., 2004) leading to trapping of NH4+ at higher concentrations in RBC than in plasma.
Other evidence indicates that Rh proteins transport CO2 (Kaplan et al., 2004; Soupene et al., 2004). Direct studies of CO2/HCO3− transport in RBC indicated that DIDS markedly decreased transport of CO2 and suggested that Rh and Band-3 may be coupled functionally. Another study on CO2 permeability in normal and Rhnull RBC concluded that CO2 permeation is mediated in part by Rh/RhAG and by AQP1 (Endeward et al., 2006). Another study demonstrated that expressing RhCG in oocytes increased CO2 permeability compared to H2O-injected oocytes (Bakouh et al., 2006). A recent study proposed that NH3 moves through the monomeric pores of AmtB and RhAG whereas CO2 could move through the central pores of the trimeric structure (Musa-Aziz et al., 2009). Expressing RhCG in oocytes seemed to enhance CO2 transport (Bakouh et al., 2006). A possible role of Rh glycoproteins in transport of gases such as CO2 and NH3 is physiologically critical and studies to resolve the identity of the transported substrate (CO2, NH3 or NH4+) are needed.
Substrate specificity and pH dependence
Candidate substrates that can be transported by Rh proteins include NH4+ (Liu et al., 2000; Ludewig, 2004; Marini et al., 2000); NH3 (Palkova et al., 1997), CO2 (Kustu and Inwood, 2006) or methyl ammonium (Ludewig, 2004; Mak et al., 2006). Methyl amine hydrochloride is often used instead of ammonium chloride because of the similarity of the molecules and because it can be easily radiolabeled. The pH sensitivity of murine RhCG and other Rh glycoproteins is important for several reasons. First, as putative NH4+ transporters, a change in pH will shift the equilibrium of NH3 to NH4+ and thus may affect transport of NH4+. Second, it has been proposed that murine RhCG may function as an NH4+-H+ exchanger that is driven by the H+ gradient (Ludewig, 2004; Mak et al., 2006). (Note that functionally, NH4+-H+ exchange is equivalent to NH3 transport, a notable feature complicating many studies of total ammonia transport. However, NH4+-H+ exchange and NH3 transport are not the same and will respond differently to physiologic stimuli.) Therefore, a pH change will also affect transport of NH4+ by RhCG. Third, pH sensitivity of these transporters is probably highly significant in acid-base disturbances where renal RhBG and RhCG, for example, may play an important role in the adaptive response to acidosis. Finally, based on the crystal structure of AmtB, a model of NH3/NH4+ transport is proposed where deprotonation of NH4+ is critical for NH3 conduction through the pore. This step is also pH sensitive. A recent study showed that NH4+ transport by RhBG in mice was activated by raising extracellular pH but was completely inhibited by decreasing extracellular or intracellular pH (Nakhoul et al.).
5. Physiological Significance and Relevance to Human Diseases
Rh and related proteins are highly expressed in mammalian tissues and primitive species. This suggests that Rh proteins are evolutionarily preserved under high selective pressure, yet their biological function remains poorly defined. Of significance, yet not fully understood, are the following observations: The erythroid RhAG is needed for cell surface expression of the “Rh complex” but it may also be involved in NH3 or CO2 transport. The non-erythroid RhBG and RhCG are expressed in tissues that transport or metabolize NH3/NH4+ and their renal distribution correlates well with NH4+ excretion. However, it was proposed (Li et al., 2007) that they could be linked to other membrane proteins to form a “transport complex” and may transport CO2. It was also suggested that RhBG and RhCG may represent a new class of NH4+ sensing receptors or “ammonium sensors” acting as mediators to regulate different cellular processes (Van Kim et al., 2006).
The physiological roles of RhBG and RhCG in renal NH3/NH4+ handling are becoming more evident. Studies on RhCG in mice indicated that chronic metabolic acidosis increased RhCG protein expression in the medullary CD (Seshadri et al., 2006) and that the increase was in both intercalated cells and the principal cells (Seshadri et al., 2006). The same study indicated an increase in apical and basolateral RhCG expression and a decrease in cytoplasmic RhCG expression. Metabolic studies demonstrated that mice lacking RhCG had impaired urinary NH4+ excretion in response to acid loads (Biver et al., 2008). The same study concluded that Rhcg knockout mice had reduced apical NH3 permeability and transepithelial NH3/NH4+ transport. A recent study on mice with renal collecting duct-specific Rhcg deletion (CD-KO) (Lee et al., 2009) showed that under basal conditions urinary NH4+ excretion was less in KO mice than in control mice and that after acid loading CD-KO mice developed more severe metabolic acidosis than controls.
Studies on RhBG showed that genetic deletion of pendrin, an apical Cl−-HCO3−, decreased RhBG expression. Although earlier studies on Rhbg knockout mice (Chambrey et al., 2005) did not elicit abnormal acid-base balance or ammonium handling, later studies proved differently. Recently, Bishop et al., (Bishop, 2009) generated intercalated cell-specific Rhbg KO mice and demonstrated that urinary ammonium excretion was significantly less in KO mice vs controls. The same study showed that in control mice, HCl-induced acidosis increased RhBG protein expression significantly in three days.
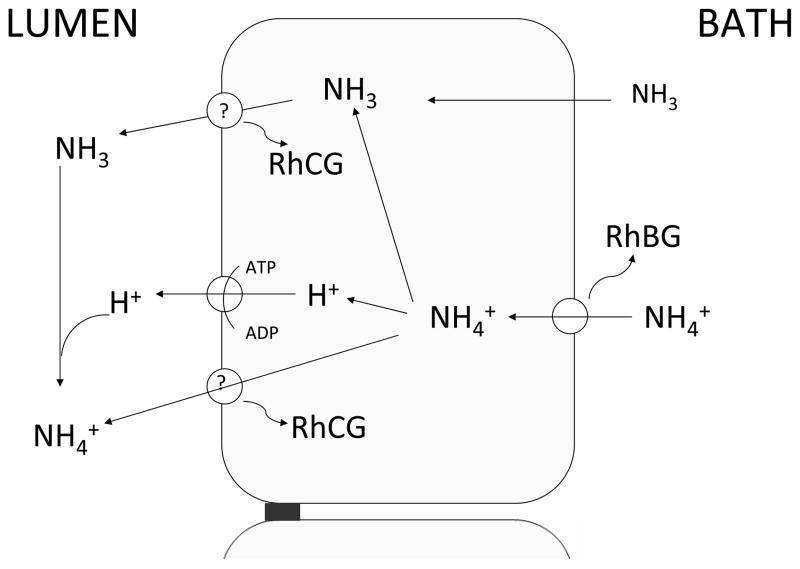
Most studies provide strong evidence for a role of the Rh proteins in NH3/NH4+ transport. Renal NH4+ excretion is critical for acid-base homeostasis and the mechanism includes NH3 and NH4+ transport components. Yet simple diffusion of NH3 is limited and NH4+-specific transporters had not been known to fully account for the significant renal NH3/NH4+ transport. As shown in this figure (Fig 3), it is likely that RhBG and RhCG, working in tandem, may actually function as the elusive NH4+ -specific transporters and/or gas channels for NH3 to explain how the distal nephron achieves transepithelial NH3/NH4+ transport. Characterizing the functions of these proteins is essential to explain these novel mechanisms and will shed light on understanding acid-base homeostasis and its regulation by the mammalian kidney.
Figure 3. Schematic diagram of a medullary collecting duct cell indicating pathways of NH3 and NH4+ transport.
Transporters labeled RhBG and RhCG show the membrane location and putative role of these proteins in transport of NH3 or NH4+.
In relevance to human diseases, the association of Rh proteins with red blood cell (RBC) disorders is well established (Huang et al., 2000; Van Kim et al., 2006). Mutations in the RH locus leading to complete absence of RBC RH antigens cause Rh null syndrome (Cherif-Zahar et al., 1998; Huang et al., 1998). Dominant over-hydrated hereditary stomatocytosis (OHSt) or Rh deficiency syndrome is caused by mutations of RHAG (Cherif-Zahar et al., 1996; Huang and Ye). OHSt is reported to cause increased permeability of RBC to monovalent cations. A recent study (Stewart et al.) reported a heterozygous RhAG missense mutation (F65S) in patients with OHSt that caused loss of function of RhAG for amine transport (NH3/NH4+ and MA/MA+). The physiological importance of RhBG and RhCG are mostly evident in KO mice studies as described above. These studies suggest that loss of function mutation, as shown for Rhcg, may cause distal renal acidosis and male infertility (Biver et al., 2008). Moreover, RhBG and RhCG have been suggested to act as tumor suppressor factors given their down-regulation in human esophageal cancers (Chen et al., 2002) and mouse brain tumors (Johansson et al., 2004).
Acknowledgments
We thank Dr Solange Abdulnour-Nakhoul for her assistance and for reading the manuscript.
This work was supported by grants from NIH-NIDDK (R01-DK-6229) and American Heart Association, Southern Affiliate (0255258B).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Attmane-Elakeb A, Amlal H, Bichara M. Ammonium carriers in medullary thick ascending limb. American Journal of Physiology - Renal Fluid and Electrolyte Physiology. 2001;280 (1):F1–9. doi: 10.1152/ajprenal.2001.280.1.F1. [DOI] [PubMed] [Google Scholar]
- Avent ND, Liu W, Warner KM, Mawby WJ, Jones JW, Ridgwell K, Tanner MJ. Immunochemical analysis of the human erythrocyte Rh polypeptides. Journal of Biological Chemistry. 1996;271 (24):14233–14239. doi: 10.1074/jbc.271.24.14233. [DOI] [PubMed] [Google Scholar]
- Avent ND, Reid ME. The Rh blood group system: a review. [erratum appears in Blood 2000 Apr 1;95(7):2197] Blood. 2000;95 (2):375–387. [PubMed] [Google Scholar]
- Bakouh N, Benjelloun F, Cherif-Zahar B, Planelles G. The challenge of understanding ammonium homeostasis and the role of the Rh glycoproteins. Transfus Clin Biol. 2006;13 (1–2):139–146. doi: 10.1016/j.tracli.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Bakouh N, Benjelloun F, Hulin P, Brouillard F, Edelman A, Cherif-Zahar B, Planelles G. NH3 is involved in the NH4+ transport induced by the functional expression of the human Rh C glycoprotein. J Biol Chem. 2004;279 (16):15975–15983. doi: 10.1074/jbc.M308528200. [DOI] [PubMed] [Google Scholar]
- Benjelloun F, Bakouh N, Fritsch J, Hulin P, Lipecka J, Edelman A, Planelles G, Thomas SR, Cherif-Zahar B. Expression of the human erythroid Rh glycoprotein (RhAG) enhances both NH3 and NH4+ transport in HeLa cells. Pflugers Arch. 2005;450 (3):155–167. doi: 10.1007/s00424-005-1381-y. [DOI] [PubMed] [Google Scholar]
- Bishop J, Verlander JW, Lee H-W, Nelson RD, Handlogten ME, Weiner AJ, Weiner D. Role of the ammonia transporter family member, RhB glycoprotein, in acidosis stimulated renal ammonia excretion. JASN 2009 [Google Scholar]
- Biver S, Belge H, Bourgeois S, Van Vooren P, Nowik M, Scohy S, Houillier P, Szpirer J, Szpirer C, Wagner CA, Devuyst O, Marini AM. A role for Rhesus factor Rhcg in renal ammonium excretion and male fertility. Nature. 2008;456 (7220):339–343. doi: 10.1038/nature07518. [DOI] [PubMed] [Google Scholar]
- Cartron JP. RH blood group system and molecular basis of Rh-deficiency. Baillieres Best Pract Res Clin Haematol. 1999;12 (4):655–689. doi: 10.1053/beha.1999.0047. [DOI] [PubMed] [Google Scholar]
- Chambrey R, Goossens D, Bourgeois S, Picard N, Bloch-Faure M, Leviel F, Geoffroy V, Cambillau M, Colin Y, Paillard M, Houillier P, Cartron JP, Eladari D. Genetic ablation of Rhbg in the mouse does not impair renal ammonium excretion. Am J Physiol Renal Physiol. 2005;289 (6):F1281–1290. doi: 10.1152/ajprenal.00172.2005. [DOI] [PubMed] [Google Scholar]
- Chen BS, Xu ZX, Xu X, Cai Y, Han YL, Wang J, Xia SH, Hu H, Wei F, Wu M, Wang MR. RhCG is downregulated in oesophageal squamous cell carcinomas, but expressed in multiple squamous epithelia. Eur J Cancer. 2002;38 (14):1927–1936. doi: 10.1016/s0959-8049(02)00190-9. [DOI] [PubMed] [Google Scholar]
- Cherif-Zahar B, Matassi G, Raynal V, Gane P, Mempel W, Perez C, Cartron JP. Molecular defects of the RHCE gene in Rh-deficient individuals of the amorph type. Blood. 1998;92 (2):639–646. [PubMed] [Google Scholar]
- Cherif-Zahar B, Raynal V, Gane P, Mattei MG, Bailly P, Gibbs B, Colin Y, Cartron JP. Candidate gene acting as a suppressor of the RH locus in most cases of Rh-deficiency. Nat Genet. 1996;12 (2):168–173. doi: 10.1038/ng0296-168. [DOI] [PubMed] [Google Scholar]
- Conroy MJ, Bullough PA, Merrick M, Avent ND. Modelling the human rhesus proteins: implications for structure and function. Br J Haematol. 2005;131 (4):543–551. doi: 10.1111/j.1365-2141.2005.05786.x. [DOI] [PubMed] [Google Scholar]
- Eladari D, Cheval L, Quentin F, Bertrand O, Mouro I, Cherif-Zahar B, Cartron JP, Paillard M, Doucet A, Chambrey R. Expression of RhCG, a new putative NH(3)/NH(4)(+) transporter, along the rat nephron. J Am Soc Nephrol. 2002;13 (8):1999–2008. doi: 10.1097/01.asn.0000025280.02386.9d. [DOI] [PubMed] [Google Scholar]
- Endeward V, Cartron JP, Ripoche P, Gros G. Red cell membrane CO2 permeability in normal human blood and in blood deficient in various blood groups, and effect of DIDS. Transfus Clin Biol. 2006;13 (1–2):123–127. doi: 10.1016/j.tracli.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Good DW, Knepper MA. Ammonia transport in the mammalian kidney. Am J Physiol. 1985;248 (4 Pt 2):F459–471. doi: 10.1152/ajprenal.1985.248.4.F459. [DOI] [PubMed] [Google Scholar]
- Good DW, Knepper MA, Burg MB. Ammonia and bicarbonate transport by thick ascending limb of rat kidney. Am J Physiol. 1984;247 (1 Pt 2):F35–44. doi: 10.1152/ajprenal.1984.247.1.F35. [DOI] [PubMed] [Google Scholar]
- Hamm LL. Ammonia transportin the rabbit medullary collecting tubule. Kidney International. 1986;29:367A. [Google Scholar]
- Hamm LL, Gillespie C, Klahr S. Ammonium chloride inhibits Na+ and K+ transport in the cortical collecting tubule. Contributions to Nephrology. 1985;47:125–129. doi: 10.1159/000411218. [DOI] [PubMed] [Google Scholar]
- Hamm LL, Simon EE. Ammonia transport in the proximal tubule. Miner Electrolyte Metab. 1990;16 (5):283–290. [PubMed] [Google Scholar]
- Han KH, Croker BP, Clapp WL, Werner D, Sahni M, Kim J, Kim HY, Handlogten ME, Weiner ID. Expression of the ammonia transporter, rh C glycoprotein, in normal and neoplastic human kidney. J Am Soc Nephrol. 2006;17 (10):2670–2679. doi: 10.1681/ASN.2006020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handlogten ME, Hong SP, Zhang L, Vander AW, Steinbaum ML, Campbell-Thompson M, Weiner ID. Expression of the ammonia transporter proteins Rh B glycoprotein and Rh C glycoprotein in the intestinal tract. Am J Physiol Gastrointest Liver Physiol. 2005;288 (5):G1036–1047. doi: 10.1152/ajpgi.00418.2004. [DOI] [PubMed] [Google Scholar]
- Heitman J, Agre P. A new face of the Rhesus antigen. Nat Genet. 2000;26 (3):258–259. doi: 10.1038/81532. [DOI] [PubMed] [Google Scholar]
- Huang CH, Chen Y, Reid ME, Seidl C. Rhnull disease: the amorph type results from a novel double mutation in RhCe gene on D-negative background. Blood. 1998;92 (2):664–671. [PubMed] [Google Scholar]
- Huang CH, Liu PZ, Cheng JG. Molecular biology and genetics of the Rh blood group system. Semin Hematol. 2000;37 (2):150–165. doi: 10.1016/s0037-1963(00)90040-4. [DOI] [PubMed] [Google Scholar]
- Huang CH, Ye M. The Rh protein family: gene evolution, membrane biology, and disease association. Cell Mol Life Sci. 2010;67 (8):1203–1218. doi: 10.1007/s00018-009-0217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson FK, Brodd J, Eklof C, Ferletta M, Hesselager G, Tiger CF, Uhrbom L, Westermark B. Identification of candidate cancer-causing genes in mouse brain tumors by retroviral tagging. Proc Natl Acad Sci U S A. 2004;101 (31):11334–11337. doi: 10.1073/pnas.0402716101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A, Lieman-Hurwitz J, Tchernov D. Resolving the biological role of the Rhesus (Rh) proteins of red blood cells with the aid of a green alga. Proc Natl Acad Sci U S A. 2004;101 (20):7497–7498. doi: 10.1073/pnas.0402527101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khademi S, O’Connell J, 3rd, Remis J, Robles-Colmenares Y, Miercke LJ, Stroud RM. Mechanism of ammonia transport by Amt/MEP/Rh: structure of AmtB at 1.35 A. Science. 2004;305 (5690):1587–1594. doi: 10.1126/science.1101952. [DOI] [PubMed] [Google Scholar]
- Kikeri D, Sun A, Zeidel ML, Hebert SC. Cell membranes impermeable to NH3. Nature. 1989;339 (6224):478–480. doi: 10.1038/339478a0. [DOI] [PubMed] [Google Scholar]
- Knepper MA, Desai SS, Hornbuckle K, Packer RK. Regulation of renal medullary ammonium accumulation. Contrib Nephrol. 1991;92:119–123. doi: 10.1159/000420087. [DOI] [PubMed] [Google Scholar]
- Knepper MA, Packer R, Good DW. Ammonium transport in the kidney. Physiol Rev. 1989;69 (1):179–249. doi: 10.1152/physrev.1989.69.1.179. [DOI] [PubMed] [Google Scholar]
- Krogh A, Larsson B, Von Heijne G, Sonnhammer ELL. Predicting transmembrane protein topology with a hidden Markov model. Application to complete genomes. J Molecular Biology. 2001;305 (3):567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- Kustu S, Inwood W. Biological gas channels for NH3 and CO2: evidence that Rh (Rhesus) proteins are CO2 channels. Transfus Clin Biol. 2006;13 (1–2):103–110. doi: 10.1016/j.tracli.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Lee HW, Verlander JW, Bishop JM, Igarashi P, Handlogten ME, Weiner ID. Collecting duct-specific Rh C glycoprotein deletion alters basal and acidosis-stimulated renal ammonia excretion. Am J Physiol Renal Physiol. 2009;296 (6):F1364–1375. doi: 10.1152/ajprenal.90667.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Jayachandran S, Nguyen HH, Chan MK. Structure of the Nitrosomonas europaea Rh protein. Proc Natl Acad Sci U S A. 2007;104 (49):19279–19284. doi: 10.1073/pnas.0709710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XD, Lupo D, Zheng L, Winkler F. Structural and functional insights into the AmtB/Mep/Rh protein family. Transfus Clin Biol. 2006;13 (1–2):65–69. doi: 10.1016/j.tracli.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Liu Z, Chen Y, Mo R, Hui C, Cheng JF, Mohandas N, Huang CH. Characterization of human RhCG and mouse Rhcg as novel nonerythroid Rh glycoprotein homologues predominantly expressed in kidney and testis. Journal of Biological Chemistry. 2000;275 (33):25641–25651. doi: 10.1074/jbc.M003353200. [DOI] [PubMed] [Google Scholar]
- Liu Z, Huang CH. The mouse Rhl1 and Rhag genes: sequence, organization, expression, and chromosomal mapping. Biochem Genet. 1999;37 (3–4):119–138. doi: 10.1023/a:1018726303397. [DOI] [PubMed] [Google Scholar]
- Liu Z, Peng J, Mo R, Hui C, Huang CH. Rh type B glycoprotein is a new member of the Rh superfamily and a putative ammonia transporter in mammals. J Biol Chem. 2001;276 (2):1424–1433. doi: 10.1074/jbc.M007528200. [DOI] [PubMed] [Google Scholar]
- Ludewig U. Electroneutral ammonium transport by basolateral rhesus B glycoprotein. J Physiol. 2004;559 (Pt 3):751–759. doi: 10.1113/jphysiol.2004.067728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupo D, Li XD, Durand A, Tomizaki T, Cherif-Zahar B, Matassi G, Merrick M, Winkler FK. The 1.3-A resolution structure of Nitrosomonas europaea Rh50 and mechanistic implications for NH3 transport by Rhesus family proteins. Proc Natl Acad Sci U S A. 2007;104 (49):19303–19308. doi: 10.1073/pnas.0706563104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak DO, Dang B, Weiner ID, Foskett JK, Westhoff CM. Characterization of ammonia transport by the kidney Rh glycoproteins RhBG and RhCG. Am J Physiol Renal Physiol. 2006;290 (2):F297–305. doi: 10.1152/ajprenal.00147.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini AM, Matassi G, Raynal V, Andre B, Cartron JP, Cherif-Zahar B. The human Rhesus-associated RhAG protein and a kidney homologue promote ammonium transport in yeast. Nat Genet. 2000;26 (3):341–344. doi: 10.1038/81656. [DOI] [PubMed] [Google Scholar]
- Marini AM, Urrestarazu A, Beauwens R, Andre B. The Rh (rhesus) blood group polypeptides are related to NH4+ transporters. Trends Biochem Sci. 1997;22 (12):460–461. doi: 10.1016/s0968-0004(97)01132-8. [DOI] [PubMed] [Google Scholar]
- Mouro-Chanteloup I, Cochet S, Chami M, Genetet S, Zidi-Yahiaoui N, Engel A, Colin Y, Bertrand O, Ripoche P. Functional reconstitution into liposomes of purified human RhCG ammonia channel. PLoS One. 5(1):e8921. doi: 10.1371/journal.pone.0008921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa-Aziz R, Jiang L, Chen LM, Behar KL, Boron WF. Concentration-dependent effects on intracellular and surface pH of exposing Xenopus oocytes to solutions containing NH3/NH4(+) J Membr Biol. 2009;228 (1):15–31. doi: 10.1007/s00232-009-9155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhoul NL, Abdulnour-Nakhoul SM, Boulpaep EL, Rabon E, Schmidt E, Hamm LL. Substrate specificity of Rhbg: ammonium and methyl ammonium transport. Am J Physiol Cell Physiol. 299(3):C695–705. doi: 10.1152/ajpcell.00019.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhoul NL, Abdulnour-Nakhoul SM, Schmidt E, Doetjes R, Rabon E, Hamm LL. pH Sensitivity of Ammonium Transport by Rhbg. Am J Physiol Cell Physiol. doi: 10.1152/ajpcell.00211.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhoul NL, Dejong H, Abdulnour-Nakhoul SM, Boulpaep EL, Hering-Smith K, Hamm LL. Characteristics of renal Rhbg as an NH4(+) transporter. Am J Physiol Renal Physiol. 2005;288 (1):F170–181. doi: 10.1152/ajprenal.00419.2003. [DOI] [PubMed] [Google Scholar]
- Nakhoul NL, Schmidt E, Abdulnour-Nakhoul SM, Hamm LL. Electrogenic ammonium transport by renal Rhbg. Transfus Clin Biol. 2006;13 (1–2):147–153. doi: 10.1016/j.tracli.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Nicolas V, Mouro-Chanteloup I, Lopez C, Gane P, Gimm A, Mohandas N, Cartron JP, Le Van Kim C, Colin Y. Functional interaction between Rh proteins and the spectrin-based skeleton in erythroid and epithelial cells. Transfus Clin Biol. 2006;13 (1–2):23–28. doi: 10.1016/j.tracli.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Palkova Z, Janderova B, Gabriel J, Zikanova B, Pospisek M, Forstova J. Ammonia mediates communication between yeast colonies. Nature. 1997;390 (6659):532–536. doi: 10.1038/37398. [DOI] [PubMed] [Google Scholar]
- Quentin F, Eladari D, Cheval L, Lopez C, Goossens D, Colin Y, Cartron JP, Paillard M, Chambrey R. RhBG and RhCG, the Putative Ammonia Transporters, Are Expressed in the Same Cells in the Distal Nephron. J Am Soc Nephrol. 2003;14 (3):545–554. doi: 10.1097/01.asn.0000050413.43662.55. [DOI] [PubMed] [Google Scholar]
- Ridgwell K, Spurr NK, Laguda B, MacGeoch C, Avent ND, Tanner MJ. Isolation of cDNA clones for a 50 kDa glycoprotein of the human erythrocyte membrane associated with Rh (rhesus) blood-group antigen expression. Biochemical Journal. 1992;287 (Pt 1):223–228. doi: 10.1042/bj2870223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripoche P, Bertrand O, Gane P, Birkenmeier C, Colin Y, Cartron JP. Human Rhesus-associated glycoprotein mediates facilitated transport of NH(3) into red blood cells. Proc Natl Acad Sci U S A. 2004;101 (49):17222–17227. doi: 10.1073/pnas.0403704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri RM, Klein JD, Kozlowski S, Sands JM, Kim YH, Han KH, Handlogten ME, Verlander JW, Weiner ID. Renal expression of the ammonia transporters, Rhbg and Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol. 2006;290 (2):F397–408. doi: 10.1152/ajprenal.00162.2005. [DOI] [PubMed] [Google Scholar]
- Singh SK, Binder HJ, Geibel JP, Boron WF. An apical permeability barrier to NH3/NH4+ in isolated, perfused colonic crypts. Proc Natl Acad Sci U S A. 1995;92 (25):11573–11577. doi: 10.1073/pnas.92.25.11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soupene E, Chu T, Corbin RW, Hunt DF, Kustu S. Gas Channels for NH(3): Proteins from Hyperthermophiles Complement an Escherichia coli Mutant. J Bacteriol. 2002;184 (12):3396–3400. doi: 10.1128/JB.184.12.3396-3400.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soupene E, Inwood W, Kustu S. Lack of the Rhesus protein Rh1 impairs growth of the green alga Chlamydomonas reinhardtii at high CO2. Proc Natl Acad Sci U S A. 2004;101 (20):7787–7792. doi: 10.1073/pnas.0401809101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soupene E, Ramirez RM, Kustu S. Evidence that fungal MEP proteins mediate diffusion of the uncharged species NH(3) across the cytoplasmic membrane. Molecular and Cellular Biology. 2001;21 (17):5733–5741. doi: 10.1128/MCB.21.17.5733-5741.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AK, Shmukler BE, Vandorpe DH, Rivera A, Heneghan JF, Li X, Hsu A, Karpatkin M, O’Neill AF, Bauer DE, Heeney MM, John K, Kuypers FA, Gallagher PG, Lux SE, Brugnara C, Westhoff CM, Alper SL. Loss-of-function and gain-of-function phenotypes of stomatocytosis mutant RhAG F65S. Am J Physiol Cell Physiol. 301(6):C1325–1343. doi: 10.1152/ajpcell.00054.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kim CL, Colin Y, Cartron JP. Rh proteins: key structural and functional components of the red cell membrane. Blood Rev. 2006;20 (2):93–110. doi: 10.1016/j.blre.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Verlander JW, Miller RT, Frank AE, Royaux IE, Kim YH, Weiner ID. Localization of the ammonium transporter proteins RhBG and RhCG in mouse kidney. Am J Physiol Renal Physiol. 2003;284 (2):F323–337. doi: 10.1152/ajprenal.00050.2002. [DOI] [PubMed] [Google Scholar]
- Waisbren SJ, Geibel JP, Modlin IM, Boron WF. Unusual permeability properties of gastric gland cells. Nature. 1994;368 (6469):332–335. doi: 10.1038/368332a0. [DOI] [PubMed] [Google Scholar]
- Weiner ID, Miller RT, Verlander JW. Localization of the ammonium transporters, Rh B glycoprotein and Rh C glycoprotein, in the mouse liver. Gastroenterology. 2003;124 (5):1432–1440. doi: 10.1016/s0016-5085(03)00277-4. [DOI] [PubMed] [Google Scholar]
- Westhoff CM, Ferreri-Jacobia M, Mak DO, Foskett JK. Identification of the erythrocyte Rh blood group glycoprotein as a mammalian ammonium transporter. J Biol Chem. 2002;277 (15):12499–12502. doi: 10.1074/jbc.C200060200. [DOI] [PubMed] [Google Scholar]
- Zidi-Yahiaoui N, Mouro-Chanteloup I, D’Ambrosio AM, Lopez C, Gane P, Le van Kim C, Cartron JP, Colin Y, Ripoche P. Human Rhesus B and Rhesus C glycoproteins: properties of facilitated ammonium transport in recombinant kidney cells. Biochem J. 2005;391 (Pt 1):33–40. doi: 10.1042/BJ20050657. [DOI] [PMC free article] [PubMed] [Google Scholar]