Abstract
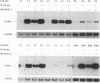
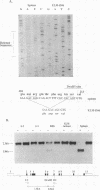
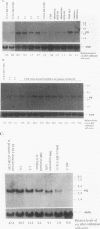
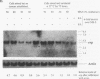
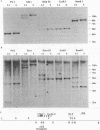
The ELM erythroleukemia is novel in that long-term survival of leukemic cells in culture (ELM-D cells) is dependent on contact with a bone marrow-derived stromal feeder cell layer. However, a number of stroma-independent (ELM-I) mutants that vary in their ability to differentiate in vitro in response to erythropoietin and interleukin-3 have been derived. We have attempted to define the genetic changes responsible for these different phenotypes. At the p53 locus in the primary leukemic cells, one copy of the gene has been lost whereas the other contains an 18-bp depletion, implicating its mutation as an early step in the development of the leukemia. Changes in ets gene expression have also been found. The Fli-1 gene region is rearranged in the primary tumor because of the insertion of a retrovirus inserted upstream of one Fli-1 allele, but this does not result in Fli-1 gene activation in any of the ELM-D or ELM-I cell lines except one. It seems significant that this line is the only one to have lost the ability to differentiate in response to erythropoietin. In addition, up-regulation of erg is associated with stromal cell-independent growth, since all ELM-I mutants have moderate levels of erg mRNA, whereas only low or undetectable levels are found in primary leukemic cells in vivo or in ELM-D cells in vitro. This up-regulation of erg mRNA seems to be important for stromal cell-independent growth, since ELM-D cells show elevated expression of the erg gene after separation from stromal cells. This seems to be made permanent in ELM-I mutants, since they do not down-regulate erg mRNA when grown in contact with stromal cells. We therefore propose that ets family members regulate both the survival and differentiation of erythroid cells.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker S. J., Markowitz S., Fearon E. R., Willson J. K., Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990 Aug 24;249(4971):912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- Bartek J., Iggo R., Gannon J., Lane D. P. Genetic and immunochemical analysis of mutant p53 in human breast cancer cell lines. Oncogene. 1990 Jun;5(6):893–899. [PubMed] [Google Scholar]
- Bartholomew C., Morishita K., Askew D., Buchberg A., Jenkins N. A., Copeland N. G., Ihle J. N. Retroviral insertions in the CB-1/Fim-3 common site of integration activate expression of the Evi-1 gene. Oncogene. 1989 May;4(5):529–534. [PubMed] [Google Scholar]
- Ben-David Y., Bernstein A. Friend virus-induced erythroleukemia and the multistage nature of cancer. Cell. 1991 Sep 6;66(5):831–834. doi: 10.1016/0092-8674(91)90428-2. [DOI] [PubMed] [Google Scholar]
- Ben-David Y., Giddens E. B., Letwin K., Bernstein A. Erythroleukemia induction by Friend murine leukemia virus: insertional activation of a new member of the ets gene family, Fli-1, closely linked to c-ets-1. Genes Dev. 1991 Jun;5(6):908–918. doi: 10.1101/gad.5.6.908. [DOI] [PubMed] [Google Scholar]
- Ben-Ishay Z., Yoffey J. M. Ultrastructural studies of erythroblastic islands of rat bone marrow. II. The resumption of erythropoiesis in erythropoietically depressed rebound marrow. Lab Invest. 1972 Jun;26(6):637–647. [PubMed] [Google Scholar]
- Bergeron D., Poliquin L., Kozak C. A., Rassart E. Identification of a common viral integration region in Cas-Br-E murine leukemia virus-induced non-T-, non-B-cell lymphomas. J Virol. 1991 Jan;65(1):7–15. doi: 10.1128/jvi.65.1.7-15.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer H. E., Cooper S., Lu L., Hangoc G., Anderson D., Cosman D., Lyman S. D., Williams D. E. Effect of murine mast cell growth factor (c-kit proto-oncogene ligand) on colony formation by human marrow hematopoietic progenitor cells. Blood. 1991 May 15;77(10):2142–2149. [PubMed] [Google Scholar]
- Caron de Fromentel C., Soussi T. TP53 tumor suppressor gene: a model for investigating human mutagenesis. Genes Chromosomes Cancer. 1992 Jan;4(1):1–15. doi: 10.1002/gcc.2870040102. [DOI] [PubMed] [Google Scholar]
- Chen P. L., Chen Y. M., Bookstein R., Lee W. H. Genetic mechanisms of tumor suppression by the human p53 gene. Science. 1990 Dec 14;250(4987):1576–1580. doi: 10.1126/science.2274789. [DOI] [PubMed] [Google Scholar]
- Delattre O., Zucman J., Plougastel B., Desmaze C., Melot T., Peter M., Kovar H., Joubert I., de Jong P., Rouleau G. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992 Sep 10;359(6391):162–165. doi: 10.1038/359162a0. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Coutinho L. H., Spooncer E., Heyworth C. M., Daniel C. P., Schiro R., Chang J., Allen T. D. Stromal cells in haemopoiesis. Ciba Found Symp. 1990;148:76–95. doi: 10.1002/9780470513880.ch6. [DOI] [PubMed] [Google Scholar]
- Diller L., Kassel J., Nelson C. E., Gryka M. A., Litwak G., Gebhardt M., Bressac B., Ozturk M., Baker S. J., Vogelstein B. p53 functions as a cell cycle control protein in osteosarcomas. Mol Cell Biol. 1990 Nov;10(11):5772–5781. doi: 10.1128/mcb.10.11.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolci S., Williams D. E., Ernst M. K., Resnick J. L., Brannan C. I., Lock L. F., Lyman S. D., Boswell H. S., Donovan P. J. Requirement for mast cell growth factor for primordial germ cell survival in culture. Nature. 1991 Aug 29;352(6338):809–811. doi: 10.1038/352809a0. [DOI] [PubMed] [Google Scholar]
- Donehower L. A., Harvey M., Slagle B. L., McArthur M. J., Montgomery C. A., Jr, Butel J. S., Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992 Mar 19;356(6366):215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Feinstein E., Gale R. P., Reed J., Canaani E. Expression of the normal p53 gene induces differentiation of K562 cells. Oncogene. 1992 Sep;7(9):1853–1857. [PubMed] [Google Scholar]
- Flanagan J. G., Chan D. C., Leder P. Transmembrane form of the kit ligand growth factor is determined by alternative splicing and is missing in the Sld mutant. Cell. 1991 Mar 8;64(5):1025–1035. doi: 10.1016/0092-8674(91)90326-t. [DOI] [PubMed] [Google Scholar]
- Itoh K., Ono K., Sawada H., Tezuka H., Sakoda H., Nakane H., Uchiyama T., Uchino H., Mori K. J. Establishment and characterization of a transplantable erythroblastic leukemia in C3H mice. Leuk Res. 1988;12(6):471–478. doi: 10.1016/0145-2126(88)90113-0. [DOI] [PubMed] [Google Scholar]
- Itoh K., Sasaki R., Ono K., Tezuka H., Sakoda H., Sawada H., Hitomi K., Nakane H., Uchiyama T., Uchino H. Stromal cell-dependent growth of leukemic cells from murine erythroblastic leukemia. Jpn J Cancer Res. 1988 Aug;79(8):931–937. doi: 10.1111/j.1349-7006.1988.tb00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K., Tezuka H., Sakoda H., Konno M., Nagata K., Uchiyama T., Uchino H., Mori K. J. Reproducible establishment of hemopoietic supportive stromal cell lines from murine bone marrow. Exp Hematol. 1989 Feb;17(2):145–153. [PubMed] [Google Scholar]
- Jego N., Thomas G., Hamelin R. Short direct repeats flanking deletions, and duplicating insertions in p53 gene in human cancers. Oncogene. 1993 Jan;8(1):209–213. [PubMed] [Google Scholar]
- Johnson P., Chung S., Benchimol S. Growth suppression of Friend virus-transformed erythroleukemia cells by p53 protein is accompanied by hemoglobin production and is sensitive to erythropoietin. Mol Cell Biol. 1993 Mar;13(3):1456–1463. doi: 10.1128/mcb.13.3.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemsz M. J., McKercher S. R., Celada A., Van Beveren C., Maki R. A. The macrophage and B cell-specific transcription factor PU.1 is related to the ets oncogene. Cell. 1990 Apr 6;61(1):113–124. doi: 10.1016/0092-8674(90)90219-5. [DOI] [PubMed] [Google Scholar]
- Lane D. P. Cancer. p53, guardian of the genome. Nature. 1992 Jul 2;358(6381):15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Li J. P., D'Andrea A. D., Lodish H. F., Baltimore D. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature. 1990 Feb 22;343(6260):762–764. doi: 10.1038/343762a0. [DOI] [PubMed] [Google Scholar]
- Livingstone L. R., White A., Sprouse J., Livanos E., Jacks T., Tlsty T. D. Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53. Cell. 1992 Sep 18;70(6):923–935. doi: 10.1016/0092-8674(92)90243-6. [DOI] [PubMed] [Google Scholar]
- Macleod K., Leprince D., Stehelin D. The ets gene family. Trends Biochem Sci. 1992 Jul;17(7):251–256. doi: 10.1016/0968-0004(92)90404-w. [DOI] [PubMed] [Google Scholar]
- Mager D., Mak T. W., Bernstein A. Friend leukaemia virus-transformed cells, unlike normal stem cells, form spleen colonies in Sl/sld mice. Nature. 1980 Dec 11;288(5791):592–594. doi: 10.1038/288592a0. [DOI] [PubMed] [Google Scholar]
- Maisel J., Klement V., Lai M. M., Ostertag W., Duesberg P. Ribonucleic acid components of murine sarcoma and leukemia viruses. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3536–3540. doi: 10.1073/pnas.70.12.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minty A. J., Alonso S., Caravatti M., Buckingham M. E. A fetal skeletal muscle actin mRNA in the mouse and its identity with cardiac actin mRNA. Cell. 1982 Aug;30(1):185–192. doi: 10.1016/0092-8674(82)90024-1. [DOI] [PubMed] [Google Scholar]
- Moreau-Gachelin F., Ray D., de Both N. J., van der Feltz M. J., Tambourin P., Tavitian A. Spi-1 oncogene activation in Rauscher and Friend murine virus-induced acute erythroleukemias. Leukemia. 1990 Jan;4(1):20–23. [PubMed] [Google Scholar]
- Moreau-Gachelin F., Tavitian A., Tambourin P. Spi-1 is a putative oncogene in virally induced murine erythroleukaemias. Nature. 1988 Jan 21;331(6153):277–280. doi: 10.1038/331277a0. [DOI] [PubMed] [Google Scholar]
- Moreau-Gachelin F., Wendling F., Bucau-Varlet P., Charon M., Tambourin P. Detection of tumorigenic cells in Friend virus-infected mice: an in vivo methodological investigation. J Natl Cancer Inst. 1981 Jun;66(6):1121–1127. doi: 10.1093/jnci/66.6.1121. [DOI] [PubMed] [Google Scholar]
- Munroe D. G., Peacock J. W., Benchimol S. Inactivation of the cellular p53 gene is a common feature of Friend virus-induced erythroleukemia: relationship of inactivation to dominant transforming alleles. Mol Cell Biol. 1990 Jul;10(7):3307–3313. doi: 10.1128/mcb.10.7.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbantoglu J., Hartley D., Phear G., Tear G., Meuth M. Spontaneous deletion formation at the aprt locus of hamster cells: the presence of short sequence homologies and dyad symmetries at deletion termini. EMBO J. 1986 Jun;5(6):1199–1204. doi: 10.1002/j.1460-2075.1986.tb04347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Leder P. The complete sequence of a chromosomal mouse alpha--globin gene reveals elements conserved throughout vertebrate evolution. Cell. 1979 Nov;18(3):875–882. doi: 10.1016/0092-8674(79)90139-9. [DOI] [PubMed] [Google Scholar]
- Nocka K., Buck J., Levi E., Besmer P. Candidate ligand for the c-kit transmembrane kinase receptor: KL, a fibroblast derived growth factor stimulates mast cells and erythroid progenitors. EMBO J. 1990 Oct;9(10):3287–3294. doi: 10.1002/j.1460-2075.1990.tb07528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohneda O., Yanai N., Obinata M. Combined action of c-kit and erythropoietin on erythroid progenitor cells. Development. 1992 Jan;114(1):245–252. doi: 10.1242/dev.114.1.245. [DOI] [PubMed] [Google Scholar]
- Ostertag W., Melderis H., Steinheider G., Kluge N., Dube S. Synthesis of mouse haemoglobin and globin mRNA in leukaemic cell cultures. Nat New Biol. 1972 Oct 25;239(95):231–234. doi: 10.1038/newbio239231a0. [DOI] [PubMed] [Google Scholar]
- Plumb M., Frampton J., Wainwright H., Walker M., Macleod K., Goodwin G., Harrison P. GATAAG; a cis-control region binding an erythroid-specific nuclear factor with a role in globin and non-globin gene expression. Nucleic Acids Res. 1989 Jan 11;17(1):73–92. doi: 10.1093/nar/17.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesenberry P. J. Stroma-dependent hematolymphopoietic stem cells. Curr Top Microbiol Immunol. 1992;177:151–166. doi: 10.1007/978-3-642-76912-2_12. [DOI] [PubMed] [Google Scholar]
- Rao V. N., Reddy E. S. A divergent ets-related protein, elk-1, recognizes similar c-ets-1 proto-oncogene target sequences and acts as a transcriptional activator. Oncogene. 1992 Jan;7(1):65–70. [PubMed] [Google Scholar]
- Reddy E. S., Rao V. N., Papas T. S. The erg gene: a human gene related to the ets oncogene. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6131–6135. doi: 10.1073/pnas.84.17.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy E. S., Rao V. N. erg, an ets-related gene, codes for sequence-specific transcriptional activators. Oncogene. 1991 Dec;6(12):2285–2289. [PubMed] [Google Scholar]
- Ryan J. J., Danish R., Gottlieb C. A., Clarke M. F. Cell cycle analysis of p53-induced cell death in murine erythroleukemia cells. Mol Cell Biol. 1993 Jan;13(1):711–719. doi: 10.1128/mcb.13.1.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze S., Paul R., Gliniak B. C., Kabat D. Role of the PU.1 transcription factor in controlling differentiation of Friend erythroleukemia cells. Mol Cell Biol. 1992 Jul;12(7):2967–2975. doi: 10.1128/mcb.12.7.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sels F. T., Langer S., Schulz A. S., Silver J., Sitbon M., Friedrich R. W. Friend murine leukaemia virus is integrated at a common site in most primary spleen tumours of erythroleukaemic animals. Oncogene. 1992 Apr;7(4):643–652. [PubMed] [Google Scholar]
- Steel K. P., Davidson D. R., Jackson I. J. TRP-2/DT, a new early melanoblast marker, shows that steel growth factor (c-kit ligand) is a survival factor. Development. 1992 Aug;115(4):1111–1119. doi: 10.1242/dev.115.4.1111. [DOI] [PubMed] [Google Scholar]
- Stephen C. W., Lane D. P. Mutant conformation of p53. Precise epitope mapping using a filamentous phage epitope library. J Mol Biol. 1992 Jun 5;225(3):577–583. doi: 10.1016/0022-2836(92)90386-x. [DOI] [PubMed] [Google Scholar]
- Toksoz D., Zsebo K. M., Smith K. A., Hu S., Brankow D., Suggs S. V., Martin F. H., Williams D. A. Support of human hematopoiesis in long-term bone marrow cultures by murine stromal cells selectively expressing the membrane-bound and secreted forms of the human homolog of the steel gene product, stem cell factor. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7350–7354. doi: 10.1073/pnas.89.16.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. F., Strauss E., Orkin S. H. Functional analysis and in vivo footprinting implicate the erythroid transcription factor GATA-1 as a positive regulator of its own promoter. Genes Dev. 1991 Jun;5(6):919–931. doi: 10.1101/gad.5.6.919. [DOI] [PubMed] [Google Scholar]
- Urness L. D., Thummel C. S. Molecular interactions within the ecdysone regulatory hierarchy: DNA binding properties of the Drosophila ecdysone-inducible E74A protein. Cell. 1990 Oct 5;63(1):47–61. doi: 10.1016/0092-8674(90)90287-o. [DOI] [PubMed] [Google Scholar]
- Wasylyk B., Wasylyk C., Flores P., Begue A., Leprince D., Stehelin D. The c-ets proto-oncogenes encode transcription factors that cooperate with c-Fos and c-Jun for transcriptional activation. Nature. 1990 Jul 12;346(6280):191–193. doi: 10.1038/346191a0. [DOI] [PubMed] [Google Scholar]
- Witte O. N. Steel locus defines new multipotent growth factor. Cell. 1990 Oct 5;63(1):5–6. doi: 10.1016/0092-8674(90)90280-r. [DOI] [PubMed] [Google Scholar]
- Xin J. H., Cowie A., Lachance P., Hassell J. A. Molecular cloning and characterization of PEA3, a new member of the Ets oncogene family that is differentially expressed in mouse embryonic cells. Genes Dev. 1992 Mar;6(3):481–496. doi: 10.1101/gad.6.3.481. [DOI] [PubMed] [Google Scholar]
- Yin Y., Tainsky M. A., Bischoff F. Z., Strong L. C., Wahl G. M. Wild-type p53 restores cell cycle control and inhibits gene amplification in cells with mutant p53 alleles. Cell. 1992 Sep 18;70(6):937–948. doi: 10.1016/0092-8674(92)90244-7. [DOI] [PubMed] [Google Scholar]
- Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991 Jul 25;352(6333):345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]