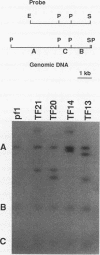
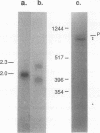
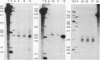
Abstract
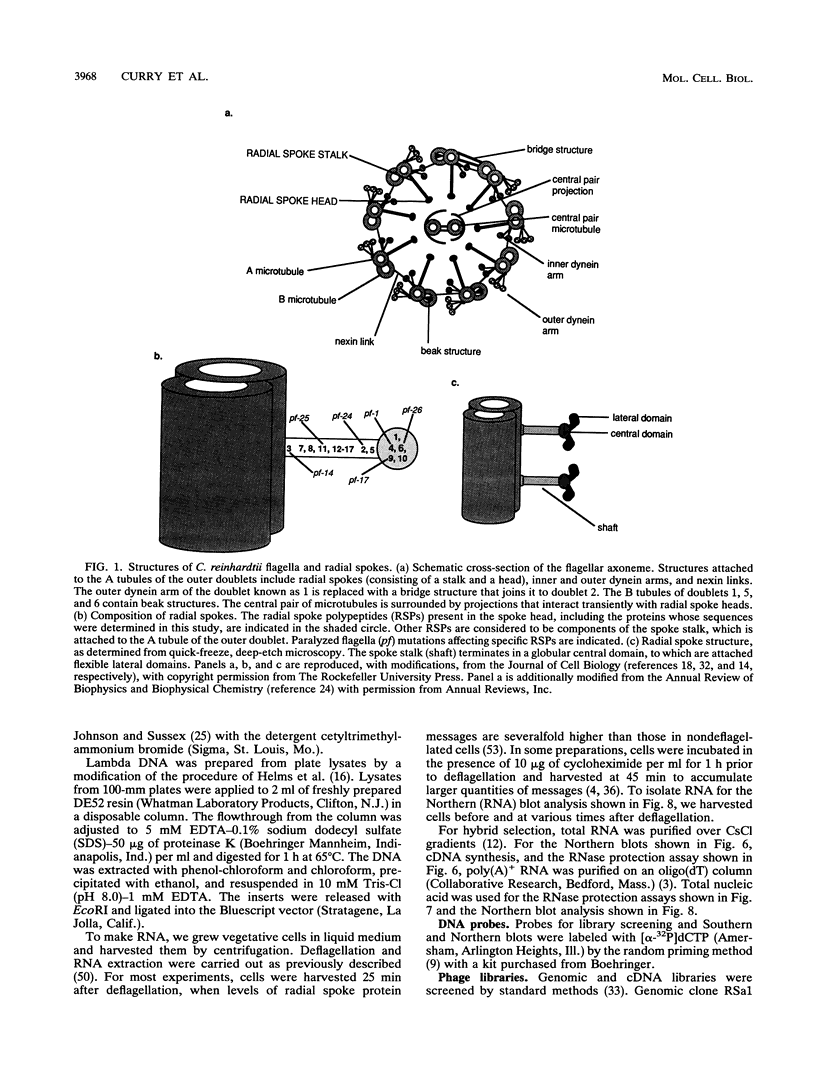
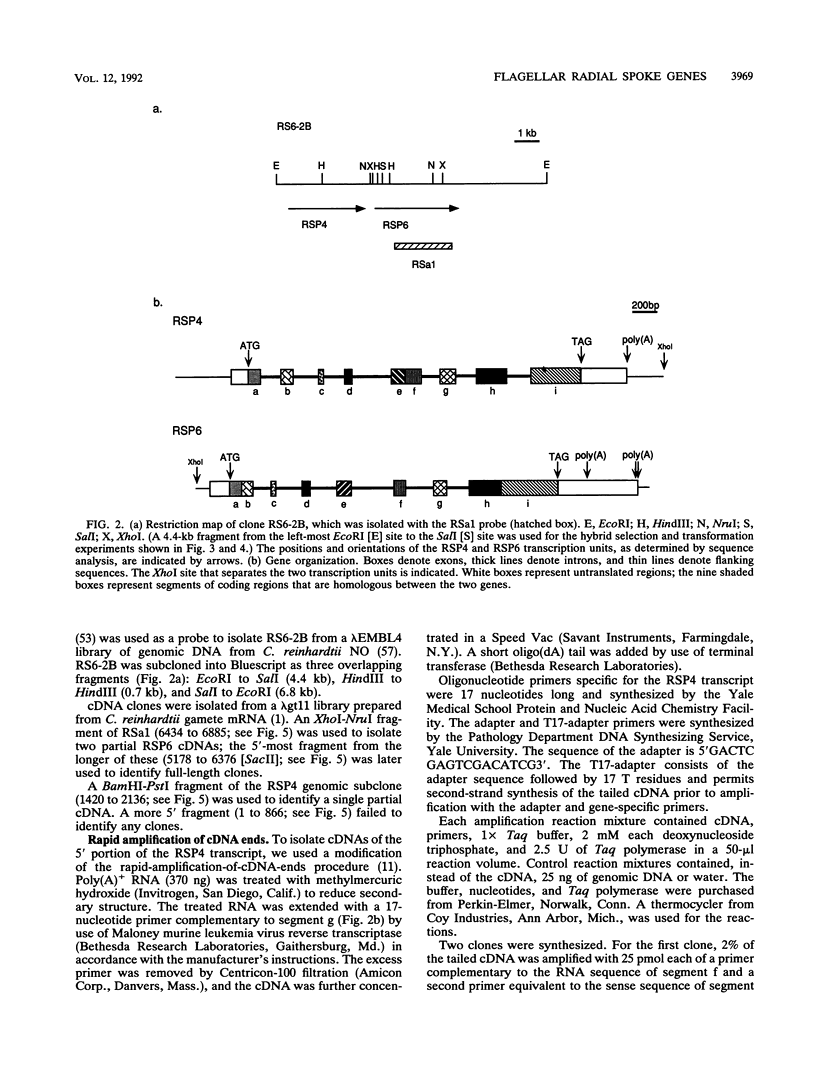
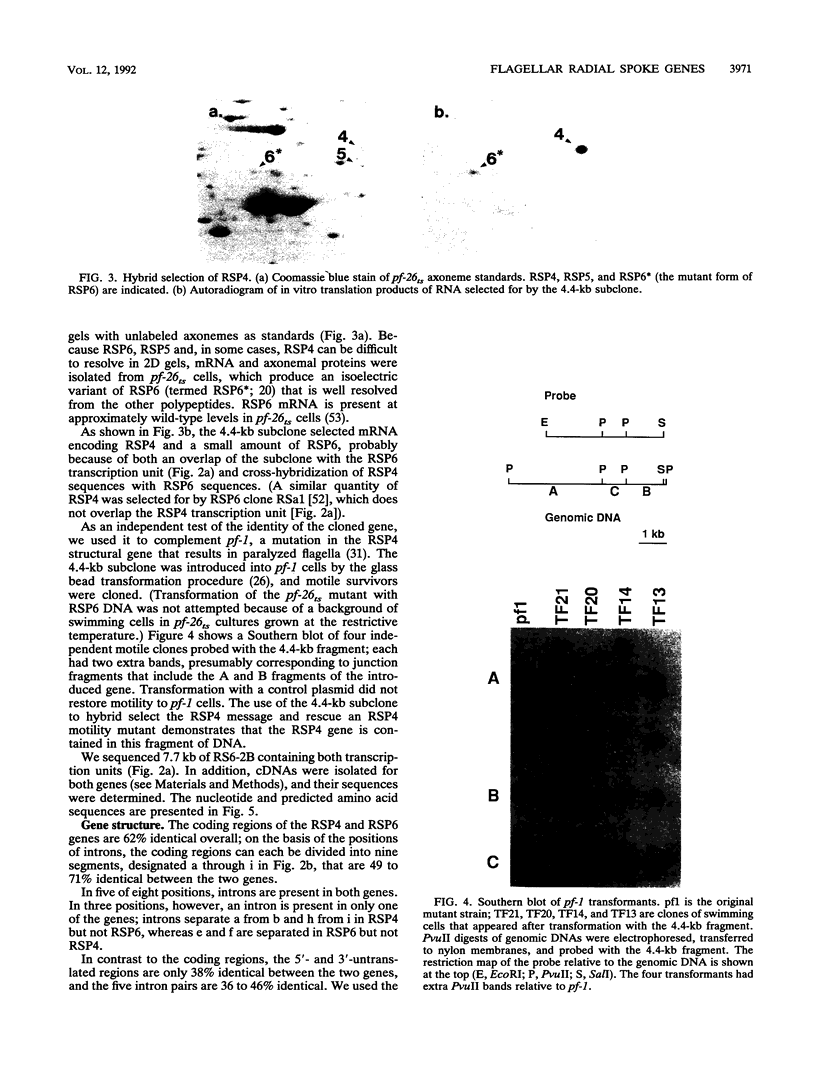
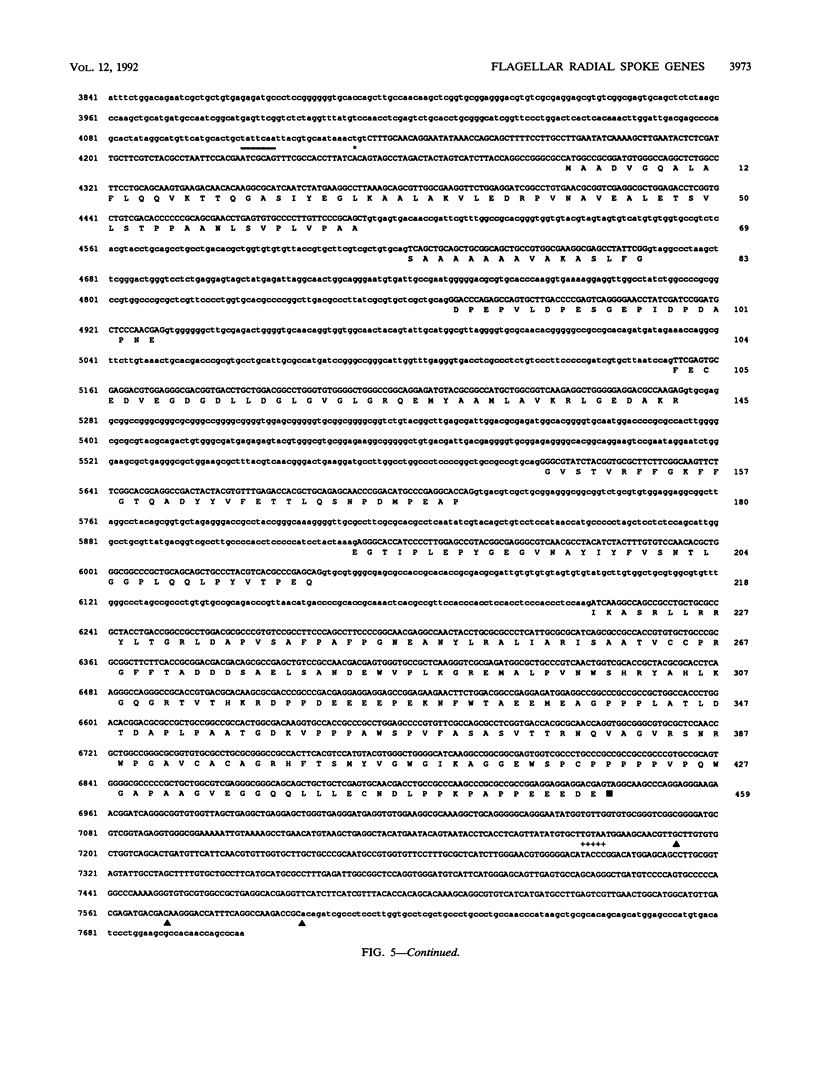
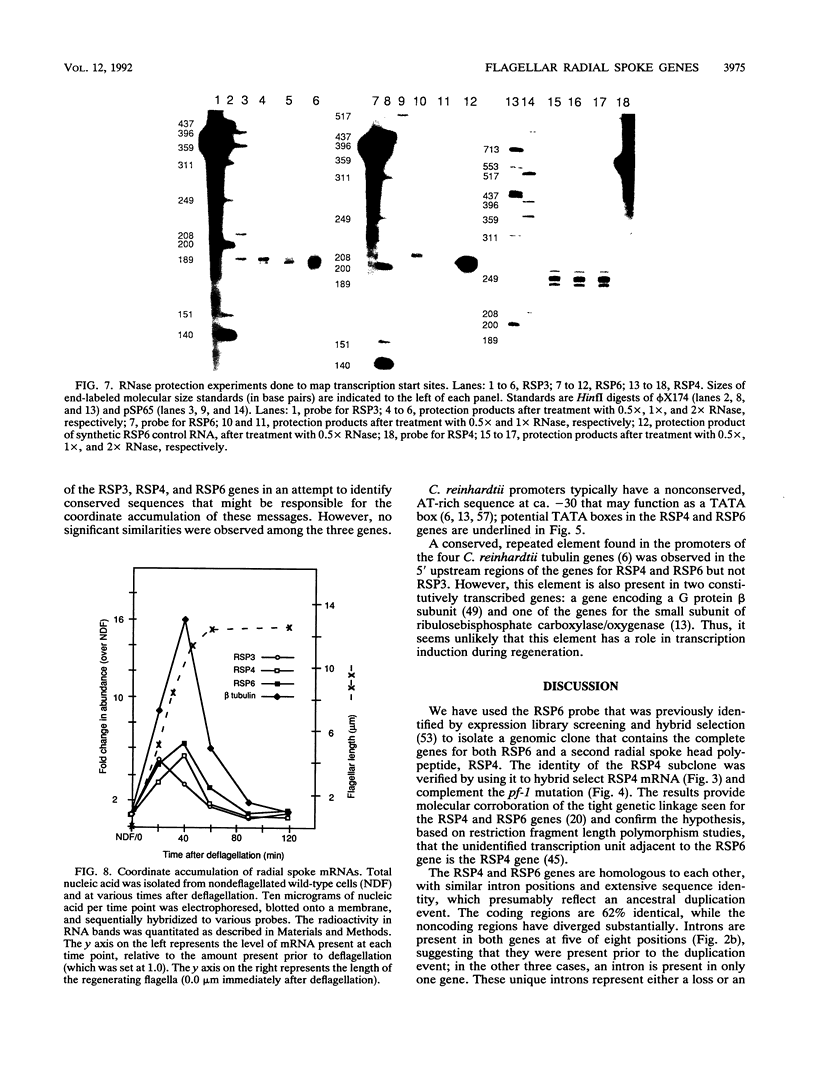
Flagellar radial spokes contribute to the regulation of dynein arm activity and thus the pattern of flagellar bending. We have sequenced the genes for radial spoke protein 4 (RSP4) and RSP6, two of the five proteins that make up the radial spoke head in Chlamydomonas reinhardtii. The two genes, which are tightly linked genetically (B. Huang, G. Piperno, Z. Ramanis, and D.J.L. Luck, J. Cell Biol. 88:80-88, 1981), are separated by only 435 bp. They encode proline-rich polypeptides of 49.8 kDa (RSP4) and 48.8 kDa (RSP6), which are 48% identical to each other but do not resemble any previously sequenced proteins. The transcription start sites of these genes and an additional radial spoke protein gene, that for RSP3, were determined, and patterns of mRNA accumulation during flagellar regeneration were examined for the three radial spoke protein genes. These studies provide the molecular tools for a detailed analysis of radial spoke head function and assembly and for a determination of the mechanism by which the genes required to build a complex organelle are regulated.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair W. S., Apt K. E. Cell wall regeneration in Chlamydomonas: accumulation of mRNAs encoding cell wall hydroxyproline-rich glycoproteins. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7355–7359. doi: 10.1073/pnas.87.19.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker E. J., Keller L. R., Schloss J. A., Rosenbaum J. L. Protein synthesis is required for rapid degradation of tubulin mRNA and other deflagellation-induced RNAs in Chlamydomonas reinhardi. Mol Cell Biol. 1986 Jan;6(1):54–61. doi: 10.1128/mcb.6.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brokaw C. J., Luck D. J., Huang B. Analysis of the movement of Chlamydomonas flagella:" the function of the radial-spoke system is revealed by comparison of wild-type and mutant flagella. J Cell Biol. 1982 Mar;92(3):722–732. doi: 10.1083/jcb.92.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunke K. J., Anthony J. G., Sternberg E. J., Weeks D. P. Repeated consensus sequence and pseudopromoters in the four coordinately regulated tubulin genes of Chlamydomonas reinhardi. Mol Cell Biol. 1984 Jun;4(6):1115–1124. doi: 10.1128/mcb.4.6.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener D. R., Curry A. M., Johnson K. A., Williams B. D., Lefebvre P. A., Kindle K. L., Rosenbaum J. L. Rescue of a paralyzed-flagella mutant of Chlamydomonas by transformation. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5739–5743. doi: 10.1073/pnas.87.15.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fernández E., Schnell R., Ranum L. P., Hussey S. C., Silflow C. D., Lefebvre P. A. Isolation and characterization of the nitrate reductase structural gene of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6449–6453. doi: 10.1073/pnas.86.17.6449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont M., Rahire M. Sequence, evolution and differential expression of the two genes encoding variant small subunits of ribulose bisphosphate carboxylase/oxygenase in Chlamydomonas reinhardtii. J Mol Biol. 1986 Oct 5;191(3):421–432. doi: 10.1016/0022-2836(86)90137-3. [DOI] [PubMed] [Google Scholar]
- Goodenough U. W., Heuser J. E. Substructure of inner dynein arms, radial spokes, and the central pair/projection complex of cilia and flagella. J Cell Biol. 1985 Jun;100(6):2008–2018. doi: 10.1083/jcb.100.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms C., Graham M. Y., Dutchik J. E., Olson M. V. A new method for purifying lambda DNA from phage lysates. DNA. 1985 Feb;4(1):39–49. doi: 10.1089/dna.1985.4.39. [DOI] [PubMed] [Google Scholar]
- Hoffman E. K., Trusko S. P., Freeman N., George D. L. Structural and functional characterization of the promoter region of the mouse c-Ki-ras gene. Mol Cell Biol. 1987 Jul;7(7):2592–2596. doi: 10.1128/mcb.7.7.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoops H. J., Witman G. B. Outer doublet heterogeneity reveals structural polarity related to beat direction in Chlamydomonas flagella. J Cell Biol. 1983 Sep;97(3):902–908. doi: 10.1083/jcb.97.3.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B., Mengersen A., Lee V. D. Molecular cloning of cDNA for caltractin, a basal body-associated Ca2+-binding protein: homology in its protein sequence with calmodulin and the yeast CDC31 gene product. J Cell Biol. 1988 Jul;107(1):133–140. doi: 10.1083/jcb.107.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B., Piperno G., Ramanis Z., Luck D. J. Radial spokes of Chlamydomonas flagella: genetic analysis of assembly and function. J Cell Biol. 1981 Jan;88(1):80–88. doi: 10.1083/jcb.88.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B., Ramanis Z., Luck D. J. Suppressor mutations in Chlamydomonas reveal a regulatory mechanism for Flagellar function. Cell. 1982 Jan;28(1):115–124. doi: 10.1016/0092-8674(82)90381-6. [DOI] [PubMed] [Google Scholar]
- Ishii S., Xu Y. H., Stratton R. H., Roe B. A., Merlino G. T., Pastan I. Characterization and sequence of the promoter region of the human epidermal growth factor receptor gene. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4920–4924. doi: 10.1073/pnas.82.15.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. A. Pathway of the microtubule-dynein ATPase and the structure of dynein: a comparison with actomyosin. Annu Rev Biophys Biophys Chem. 1985;14:161–188. doi: 10.1146/annurev.bb.14.060185.001113. [DOI] [PubMed] [Google Scholar]
- Johnson K. A., Sussex I. M. Genomic amplification in the cotyledon parenchyma of common bean. Chromosoma. 1990 Jul;99(3):223–230. doi: 10.1007/BF01731133. [DOI] [PubMed] [Google Scholar]
- Kindle K. L. High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1228–1232. doi: 10.1073/pnas.87.3.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K., Kurosawa Y., Fujita K., Nagatsu T. Human dopamine beta-hydroxylase gene: two mRNA types having different 3'-terminal regions are produced through alternative polyadenylation. Nucleic Acids Res. 1989 Feb 11;17(3):1089–1102. doi: 10.1093/nar/17.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondaiah P., Van Obberghen-Schilling E., Ludwig R. L., Dhar R., Sporn M. B., Roberts A. B. cDNA cloning of porcine transforming growth factor-beta 1 mRNAs. Evidence for alternate splicing and polyadenylation. J Biol Chem. 1988 Dec 5;263(34):18313–18317. [PubMed] [Google Scholar]
- Lee G., Cowan N., Kirschner M. The primary structure and heterogeneity of tau protein from mouse brain. Science. 1988 Jan 15;239(4837):285–288. doi: 10.1126/science.3122323. [DOI] [PubMed] [Google Scholar]
- Lichtenberg B., Mandelkow E. M., Hagestedt T., Mandelkow E. Structure and elasticity of microtubule-associated protein tau. Nature. 1988 Jul 28;334(6180):359–362. doi: 10.1038/334359a0. [DOI] [PubMed] [Google Scholar]
- Luck D. J. Genetic and biochemical dissection of the eucaryotic flagellum. J Cell Biol. 1984 Mar;98(3):789–794. doi: 10.1083/jcb.98.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck D., Piperno G., Ramanis Z., Huang B. Flagellar mutants of Chlamydomonas: studies of radial spoke-defective strains by dikaryon and revertant analysis. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3456–3460. doi: 10.1073/pnas.74.8.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes U., Jaussi R., Ziak M., Juretic N., Lindenmann J. M., Christen P. Structure of cDNA of cytosolic aspartate aminotransferase of chicken and its expression in E. coli. Biochimie. 1989 Apr;71(4):411–416. doi: 10.1016/0300-9084(89)90171-5. [DOI] [PubMed] [Google Scholar]
- Mitchell D. R., Rosenbaum J. L. A motile Chlamydomonas flagellar mutant that lacks outer dynein arms. J Cell Biol. 1985 Apr;100(4):1228–1234. doi: 10.1083/jcb.100.4.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G. Functional diversity of dyneins. Cell Motil Cytoskeleton. 1990;17(3):147–149. doi: 10.1002/cm.970170302. [DOI] [PubMed] [Google Scholar]
- Piperno G., Huang B., Luck D. J. Two-dimensional analysis of flagellar proteins from wild-type and paralyzed mutants of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1600–1604. doi: 10.1073/pnas.74.4.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G., Huang B., Ramanis Z., Luck D. J. Radial spokes of Chlamydomonas flagella: polypeptide composition and phosphorylation of stalk components. J Cell Biol. 1981 Jan;88(1):73–79. doi: 10.1083/jcb.88.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G., Ramanis Z., Smith E. F., Sale W. S. Three distinct inner dynein arms in Chlamydomonas flagella: molecular composition and location in the axoneme. J Cell Biol. 1990 Feb;110(2):379–389. doi: 10.1083/jcb.110.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G., Ramanis Z. The proximal portion of Chlamydomonas flagella contains a distinct set of inner dynein arms. J Cell Biol. 1991 Feb;112(4):701–709. doi: 10.1083/jcb.112.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju K., Anwar R. A. Primary structures of bovine elastin a, b, and c deduced from the sequences of cDNA clones. J Biol Chem. 1987 Apr 25;262(12):5755–5762. [PubMed] [Google Scholar]
- Ranum L. P., Thompson M. D., Schloss J. A., Lefebvre P. A., Silflow C. D. Mapping flagellar genes in Chlamydomonas using restriction fragment length polymorphisms. Genetics. 1988 Sep;120(1):109–122. doi: 10.1093/genetics/120.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds G. A., Basu S. K., Osborne T. F., Chin D. J., Gil G., Brown M. S., Goldstein J. L., Luskey K. L. HMG CoA reductase: a negatively regulated gene with unusual promoter and 5' untranslated regions. Cell. 1984 Aug;38(1):275–285. doi: 10.1016/0092-8674(84)90549-x. [DOI] [PubMed] [Google Scholar]
- Rogers J. H. The role of introns in evolution. FEBS Lett. 1990 Aug 1;268(2):339–343. doi: 10.1016/0014-5793(90)81282-s. [DOI] [PubMed] [Google Scholar]
- SAGER R., GRANICK S. Nutritional studies with Chlamydomonas reinhardi. Ann N Y Acad Sci. 1953 Oct 14;56(5):831–838. doi: 10.1111/j.1749-6632.1953.tb30261.x. [DOI] [PubMed] [Google Scholar]
- Schloss J. A. A Chlamydomonas gene encodes a G protein beta subunit-like polypeptide. Mol Gen Genet. 1990 May;221(3):443–452. doi: 10.1007/BF00259410. [DOI] [PubMed] [Google Scholar]
- Schloss J. A., Silflow C. D., Rosenbaum J. L. mRNA abundance changes during flagellar regeneration in Chlamydomonas reinhardtii. Mol Cell Biol. 1984 Mar;4(3):424–434. doi: 10.1128/mcb.4.3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silflow C. D., Chisholm R. L., Conner T. W., Ranum L. P. The two alpha-tubulin genes of Chlamydomonas reinhardi code for slightly different proteins. Mol Cell Biol. 1985 Sep;5(9):2389–2398. doi: 10.1128/mcb.5.9.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. D., Mitchell D. R., Rosenbaum J. L. Molecular cloning and expression of flagellar radial spoke and dynein genes of Chlamydomonas. J Cell Biol. 1986 Jul;103(1):1–11. doi: 10.1083/jcb.103.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. D., Velleca M. A., Curry A. M., Rosenbaum J. L. Molecular cloning and sequence analysis of the Chlamydomonas gene coding for radial spoke protein 3: flagellar mutation pf-14 is an ochre allele. J Cell Biol. 1989 Jul;109(1):235–245. doi: 10.1083/jcb.109.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witman G. B., Plummer J., Sander G. Chlamydomonas flagellar mutants lacking radial spokes and central tubules. Structure, composition, and function of specific axonemal components. J Cell Biol. 1978 Mar;76(3):729–747. doi: 10.1083/jcb.76.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngblom J., Schloss J. A., Silflow C. D. The two beta-tubulin genes of Chlamydomonas reinhardtii code for identical proteins. Mol Cell Biol. 1984 Dec;4(12):2686–2696. doi: 10.1128/mcb.4.12.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer W. E., Schloss J. A., Silflow C. D., Youngblom J., Watterson D. M. Structural organization, DNA sequence, and expression of the calmodulin gene. J Biol Chem. 1988 Dec 25;263(36):19370–19383. [PubMed] [Google Scholar]