Abstract
Hypertension and renal damage in Dahl SS rats are associated with increased infiltrating immune cells in the kidney. To examine the role of infiltrating immune cells in this disease process, a zinc finger nuclease targeting bases 672–706 of recombination-activating gene 1 (Rag1) was injected into the pronucleus of Dahl SS (SS/JrHsdMcwi) strain embryos and implanted in pseudopregnant females. This strategy yielded a rat strain with a 13-base frame-shift mutation in the target region of Rag1 and a deletion of immunoreactive Rag1 protein in the thymus. Flow cytometry demonstrated that the Rag1-null mutant rats have a significant reduction in T and B lymphocytes in the circulation and spleen. Studies were performed on SS and Rag1-null rats fed a 4.0% NaCl diet for 3 wk. The infiltration of T cells into the kidney following high-salt intake was significantly blunted in the Rag1-null rats (1.7 ± 0.6 × 105 cells/kidney) compared with the Dahl SS (5.6 ± 0.9 × 105 cells/kidney). Accompanying the reduction in infiltration of immune cells in the kidney, mean arterial blood pressure and urinary albumin excretion rate were significantly lower in Rag1-null mutants (158 ± 3 mmHg and 60 ± 16 mg/day, respectively) than in SS rats (180 ± 11 mmHg and 251 ± 37 mg/day). Finally, a histological analysis revealed that the glomerular and tubular damage in the kidneys of the SS rats fed a high-salt diet was also attenuated in the Rag1 mutants. These studies demonstrate the importance of renal infiltration of immune cells in the pathogenesis of hypertension and renal damage in Dahl SS rats.
Keywords: hypertension, kidney disease, albuminuria, T lymphocytes, B lymphocytes, recombination activating gene 1
infiltration of immune cells into the kidney has been documented in many animal models of hypertension (15, 21, 24, 25, 28). Human data are consistent with these observations (13, 27). Moreover, immunosuppressive therapy attenuates hypertension and renal damage in different animal models of hypertension (7, 17, 21, 22, 23), and immune modulation also affects blood pressure in patients (12, 26), suggesting a causative relationship between the immune system and the disease.
Studies examining the role of the immune system in hypertension and renal disease have largely been dependent upon the use of pharmacological agents (7, 17, 21, 22, 23), although recent studies have utilized mice in which the immune system has been genetically manipulated to study experimental hypertension (11). Experiments in our laboratory have examined the influence of two mechanistically dissimilar immunosuppressive agents on the development of salt-induced hypertension and kidney damage in the Dahl salt-sensitive rat (3, 4), a genetic model of hypertension and renal disease that exhibits many phenotypic characteristics in common with human hypertension (1, 2, 6, 10, 14). A major concern related to the studies that have used pharmacological agents to suppress the immune system, are the potential nonspecific effects of these agents.
Recently, zinc finger nuclease technology (ZFN) was described as an efficient means to generate targeted mutations in rats (8, 9, 19). To eliminate the potential side effects of pharmacological agents and to address the role of the immune system in hypertension and renal disease in a genetic model of disease, a ZFN was designed to target recombination-activating gene 1 (Rag1) in the Dahl SS genetic background. Rag1 is a gene product important for somatic recombination in lymphocytes; genetic deletion of Rag1 in mice leads to a depletion of mature T and B lymphocytes (18). The present studies were performed to develop and validate a ZFN-mediated null mutation of Rag1 in the Dahl SS rat genetic background and to demonstrate the influence of genetic depletion of immune cells in the development of Dahl SS hypertension and kidney disease.
METHODS
All animal procedures and breeding were performed at the Medical College of Wisconsin under protocols approved by the Institutional Animal Care and Use Committee. The generation of ZFN mutants was performed as previously described (8, 9, 19). ZFN constructs specific for the rat Rag1 gene were designed, assembled, and validated by Sigma-Aldrich (St. Louis, MO), to target bases 672–706 (NCBI reference sequence: NM_053468.1) of Rag1 (target sequence: gtctactgcccaaggaatgtgaccgtggagtggca). In vitro transcribed mRNA encoding the Rag1 ZFNs was diluted in microinjection buffer (1 mmol/l Tris, 0.1 mmol/l EDTA, pH 7.4) at a concentration of 10 ng/μl and injected into the pronucleus of 1-cell SS/JrHsdMcwi (SS) rat embryos, as described previously (8). Two-hundred-and-one embryos were injected and transferred to pseudopregnant Sprague-Dawley females. At 10 days of age, pups were ear punched, and DNA was extracted and screened for ZFN-induced mutations, as described previously. Briefly, DNA extracted from ear tissue was amplified using primers flanking the target sequence (Rag1_F: 5′-CTCATTGCCAGAGTTTTCCG-3′ and Rag1_R: 5′-TGCTGACCCTAGCCTGAGTT-3′). PCR products were heat denatured, reannealed (95°C, 2 min; 95° to 85°C, −2°C/s; 85° to 25°C, −0.1°C/s; 4°C indefinitely), and subjected to cleavage by the Surveyor Nuclease (Cel-I; Transgenomic, Omaha, NE), according to the manufacturer's instructions. Reaction products were separated on a 10% Tris/borate/EDTA polyacrylamide gel (Bio-Rad Laboratories, Hercules, CA) and poststained with 1× GelStar nucleic acid gel stain (Cambrex Bio Science, Baltimore, MD). Among the pups born, positive mutant founders were identified for further study (Fig. 1). PCR products from two positive pups were cloned using the pCR2.1-TOPO kit (Invitrogen, Carlsbad, CA), according to the manufacturer's instructions, and 12 individual white colonies were cultured and miniprepped for Sanger sequencing using M13F and M13R primers provided in the kit. Sequencing of the first mutant revealed a 13-base frameshift deletion of bases 681–693 in exon 1 (caaggaatgtgac), resulting in truncation of the normal 1,040-amino acid RAG1 protein after amino acid P186 and insertion of 34 additional nonsense amino acids before a stop codon is introduced. The second mutant has a 17-base frameshift deletion of bases 687–703 (aatgtgaccgtggagtg), resulting in the insertion of a stop codon within 25 bases of the deletion. Previous reports demonstrated that off-target effects of ZFNs are rare and separated from the target locus by backcrossing (9, 16); the founder rats were backcrossed to an SS. To minimize possible effects of off-target mutations, multiple separate pairs of mutation-carrying progeny were intercrossed to generate F2 populations that were used for phenotyping and breeding to homozygosity. Fluorescent genotyping was performed in subsequent generations using the same Rag1 primers above, with an M13F sequence tag (5′-TGTAAAACGACGGCCAGT-3′) added to the 5′ end of the Rag1_F primer, as previously described (20).
Fig. 1.
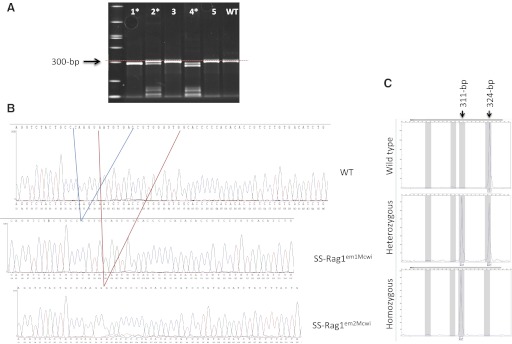
Identification and genotyping of the Rag1 mutant strains. A: surveyor nuclease assay revealed three mutant founders (*), of which pups #1 and #2 were analyzed and bred. WT, wild-type Dahl salt-sensitive rat. B: Sanger sequencing revealed a 13-bp deletion mutation (SS-Rag1em1Mcwi) in pup #1 and a 17-bp deletion mutation (SSRag1em2Mcwi) in pup #2. C: in subsequent generations, fluorescent genotyping could easily distinguish wild-type from heterozygous and homozygous pups for breeding and phenotyping. Shown are representative example GeneMapper 4.0 plots for SSRag1em1Mcwi animal genotypes.
Experiments were performed on age-matched, inbred, male Dahl SS rats (SS/JrHSDMcwi), and Dahl SS rats with the 13-base mutation in Rag1 (SS-Rag1em1Mcwi). The strain with a 17-base mutation (SS-Rag1em2Mcwi) was used in a minimal number of phenotyping experiments to confirm the Rag1-mutant phenotype. The breeders and weanlings were fed purified AIN-76A rodent diet (Dyets, Bethlehem, PA) containing 0.4% NaCl. Within the timeline of these experiments, we noted no differences in survival between the Dahl SS and Rag1-mutant rats.
The experimental methods for phenotyping (isolation of infiltrating mononuclear cells from the kidney, histology, immunohistochemistry, flow cytometry, Western blotting, and surgical preparation of the animals) were all performed using methods previously described (3, 4, 5, 17).
Immune cell isolation and flow cytometry.
Immune cells were isolated from the kidney, spleen, and blood using methods that we previously described (3, 4, 5). Rats were anesthetized with pentobarbital sodium (50 mg/kg ip); the kidneys were flushed with heparinized saline, minced with a razor blade, and incubated in a PBS solution containing collagenase. Mononuclear cells were separated by centrifugation on Histopaque. In some experiments, infiltrating T cells in the kidney were separated by incubating the mononuclear cells with a rat Pan T-cell antibody coupled to magnetic microbeads (MACS rat pan T cells microbeads, Miltenyi Biotec, Auburn, CA); the T lymphocytes were then isolated with a magnetic column (MACS Separation Columns, Miltenyi Biotec) and counted. In other experiments, mononuclear cells were incubated with anti-CD3 (APC-CD3; Becton Dickinson, Franklin Lakes, NJ) for T cells and anti-CD45R (PE-CD45R; Becton Dickinson) for B cells. The cells were analyzed by flow cytometry (FACS Calibur; Becton Dickinson) using Cellquest Pro software (Becton Dickinson).
Western blot analysis.
Blotting experiments were performed with 100 μg of total protein obtained from the thymus in a homogenization solution containing 1% Triton-100. Proteins were separated on an 4–15% SDS-PAGE gel, transferred to a nitrocellulose membrane (Bio-Rad), blocked for 1 h at room temperature, and incubated overnight with a polyclonal anti-Rag1 primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and a monoclonal anti-β-actin antibody (Sigma) at 4°C. The bound primary antibodies were detected with horseradish peroxidase-labeled secondary antibodies (anti-rabbit IgG, anti-mouse IgG; Thermo Scientific, Waltham, MA) and visualized by chemiluminescence (HyGLO; Denville Scientific, Plainfield, NJ).
Histological and immunohistochemical analysis.
Kidneys were obtained for histological and immunohistochemical analysis using methods previously described (3, 4, 5). Tissue was fixed in 10% formaldehyde, paraffin embedded (Microm HMP 300), cut in 3-μm sections (Microm HM355S), mounted, and stained with Gomori's one-step trichrome. For immunohistochemistry, slides were deparaffinized and incubated with proteinase K for antigen retrieval. The primary monoclonal antibody used to detect T cells was anti-CD43 (Abcam). A biotinylated horse anti-mouse secondary antibody was used for development with avidin-biotinylated horseradish peroxidase complex (Vectastain ABC kits; Vector Laboratory, Burlingame, CA). Slides were counterstained with aniline blue dye and photographed.
Surgical preparation.
Rats were deeply anesthetized with a mixture of ketamine (75 mg/kg ip), xylazine (10 mg/kg ip), and acepromazine (2.5 mg/kg ip), with supplemental anesthesia administered as needed. Aseptic techniques were used to place polyvinyl catheters in the femoral artery for measurement of arterial pressure. Catheters were tunneled subcutaneously and exteriorized at the back of the neck. In some rats, a telemetry transmitter (Data Sciences International, New Brighton, MN) for measuring arterial blood pressure was aseptically implanted in the carotid artery, with the body of the transmitter implanted subcutaneously in the back of the animal. Animals were maintained on warming trays during and following surgery. Analgesics and antibiotics were administered after surgery to control pain and infection.
Blood pressure and renal disease phenotyping.
The influence of an elevated NaCl intake on the development of hypertension and renal damage was assessed in Dahl SS rats and Rag1-mutant rats. At ∼9 wk of age, the rats were instrumented with telemetry transmitters (Data Sciences International), with the catheter implanted in the carotid artery. The rats were permitted to recover for 3 days; blood pressure was then continuously monitored for 7 days, while the rats were maintained on the 0.4% NaCl chow, and for 21 days following the transition to 4.0% NaCl chow. An overnight urine collection was obtained during the low-salt period and after 7, 14, and 21 days of the high-salt diet. After 3 wk of high-salt intake, the rats were deeply anesthetized, and the kidneys were flushed and prepared for T-cell isolation and counting or for histology and immunohistochemistry. Peripheral blood was obtained from some rats for flow cytometry.
Data are expressed as the means ± SE. Data were assessed for significance using a t-test, a one-way repeated-measures ANOVA with a Tukey post hoc test, or a two-way repeated-measures ANOVA with a Holm-Sidak post hoc test. A probability value of P < 0.05 was considered significant.
RESULTS
Documentation of Rag1 mutation.
As described above, DNA sequencing revealed a 13-base frameshift deletion of bases 681–693 in the SS-Rag1em1Mcwi mutant rats; aside from a small group of confirmatory studies in SS-Rag1em2Mcwi, this mutant strain was studied in the present experiments. Validation studies demonstrated a deficit of Rag1 protein in the thymus of Rag1-null mutant rats compared with Dahl SS rats (Fig. 2). At 12 wk of age (n = 6–8/group), thymus weight was significantly reduced in the Rag1-null mutants (0.30 ± 0.08 g) compared with the Dahl SS rats (0.56 ± 0.03 g). Individual experiments indicated that the total number of mononuclear cells isolated from the thymus was ∼30 times lower in the Rag1-null mutant than in the SS. Body weights (395 ± 10 and 400 ± 10 g) and spleen weights (1.36 ± 0.03 and 1.16 ± 0.09 g) were not different between Dahl SS and Rag1-null rats, respectively. Although there was no difference in spleen weight, the total number of splenocytes was ∼10 times lower in a representative Rag1-null mutant compared with a Dahl SS rats.
Fig. 2.
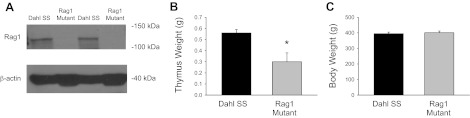
A: Western blot of Rag1 and β-actin protein in homogenates of thymus obtained from Dahl SS and Rag1 mutant rats. Thymus (B) and body weight (C) of 12-wk-old Dahl SS and Rag1 mutant rats. *P < 0.05.
Mononuclear cell populations in the blood and tissue.
Flow cytometry experiments (n = 5–8/group) demonstrated a significant decrease in the number of circulating CD3+ T cells and CD45R+ B cells in the Rag1 mutant compared with the SS rats (Fig. 3). Further flow cytometry of mononuclear cells isolated from the spleen showed a marked decrease in T cells (CD3+) and B cells (CD45R+) in the spleen of the SS-Rag1em1Mcwi compared with the Dahl SS (Fig. 4). Immunohistochemical images illustrated the typical rodent spleen anatomy in the SS with a central arteriole immediately surrounded by a region containing T lymphocytes bordered by tissue enriched in B lymphocytes. Consistent with the flow cytometry data, both T and B cells were noticeably decreased in the immunohistochemical images of the Rag1-mutant spleen. Individual experiments indicated that the peripheral blood mononuclear cell concentration and total splenocyte and thymocyte counts were ∼2-, 10-, and 30-times greater in the SS than in the Rag1-mutant rat. Despite the near total reduction of circulating CD3+ cells in the circulation, ∼43% of the thymocytes were CD4+, CD8+, or CD4+/CD8+ in the Rag1 mutant. Finally, we observed approximately equal numbers of CD11b+ cells (monocytes and macrophages) in the circulation, spleen, and thymus of the Rag1 mutant and Dahl SS.
Fig. 3.
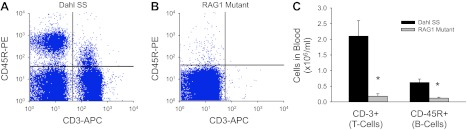
Flow cytometric identification of T lymphocytes (CD3+) and B lymphocytes (CD45R+) in circulating mononuclear cells from a representative Dahl SS (A) and Rag1 rat (B) and quantification of CD3+ and CD45R+ cells in the blood of SS and Rag1 mutant rats (C). *P < 0.05 vs. Dahl SS.
Fig. 4.
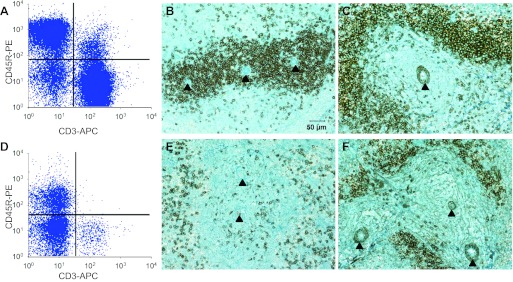
Flow cytometric and immunohistochemical identification of T cells and B cells in the spleen of Dahl SS (A–C) and Rag1 mutant rats (D–F). Flow cytometry indicates a deficit in CD3+ (T cells) and CD45R+ (B cells) in the Rag1 spleen. Immunohistochemical staining with anti-CD43, a T-cell marker, illustrates a deficit of T cells surrounding the central arterioles (indicated with arrowheads) of the spleen of the Rag1 mutant rat (E) compared with the Dahl SS (B). Similarly, staining with anti-CD79, a B-cell marker, shows a reduction in B cells in the spleen of the Rag1 (F) compared with the Dahl SS (C).
Blood pressure and albuminuria in Rag1-mutant rats.
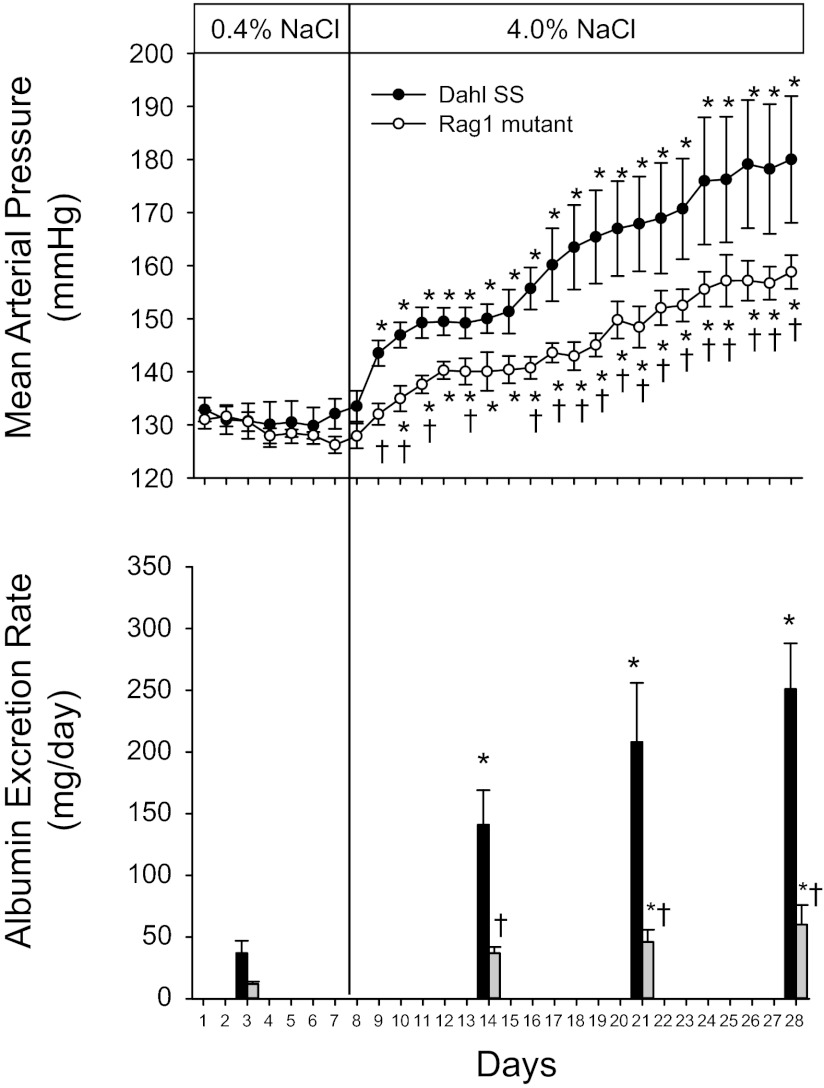
The changes in arterial blood pressure and albumin excretion rate in SS and SS-Rag1em1Mcwi fed the high-salt diet are illustrated in Fig. 5 (n = 4 or 5/group). The 24-h average daily MAP values measured by telemetry were not different between the SS and Rag1-mutant rats during 7 days of low-salt (0.4% NaCl) intake. Blood pressure rapidly and significantly increased in the Dahl SS rats by the second day of the high-NaCl intake. Following a brief plateau in the blood pressure increase between days 4 and 8 of high salt, MAP continued to increase throughout the experiment. The MAP in the Rag1-null rats was also elevated when dietary NaCl intake was increased, although the rate of MAP increase was significantly less than that observed in the SS rats. In the Rag1 mutants, blood pressure was not significantly different from baseline until day 3 of high salt, and the absolute level of MAP was significantly lower than that in the Dahl SS over the entire period of elevated salt intake. The albumin excretion rate was elevated in the SS rats fed low NaCl compared with the Rag1-null mutants (n = 6–9/group), although the differences did not reach statistical significance. In parallel to the increase in blood pressure, albumin excretion rate was increased on days 7, 14, and 21 of high salt compared with the low-salt value in the SS rats. The albumin excretion rate also increased in the SS-Rag1em1Mcwi rats when NaCl intake was increased, but the albumin excretion rate in the Rag1 mutant rats was significantly less than observed in the SS rats on each day of high NaCl. As an index of renal function, plasma creatine concentration was significantly different between the Rag1 mutants (0.25 ± 0.03 mg/dl) and the SS (0.34 ± 0.04 mg/dl) after 3 wk of the high-salt diet.
Fig. 5.
Changes in mean arterial pressure and albumin excretion rate in Dahl SS and Rag1 mutant rats fed AIN-76A diet containing 0.4% NaCl followed by 21 days of 4.0% NaCl chow. *P < 0.05 vs. values obtained on the final day of 0.4% NaCl chow. †P < 0.05 vs. Dahl SS on the same day.
Renal histological changes in Rag1 mutant rats.
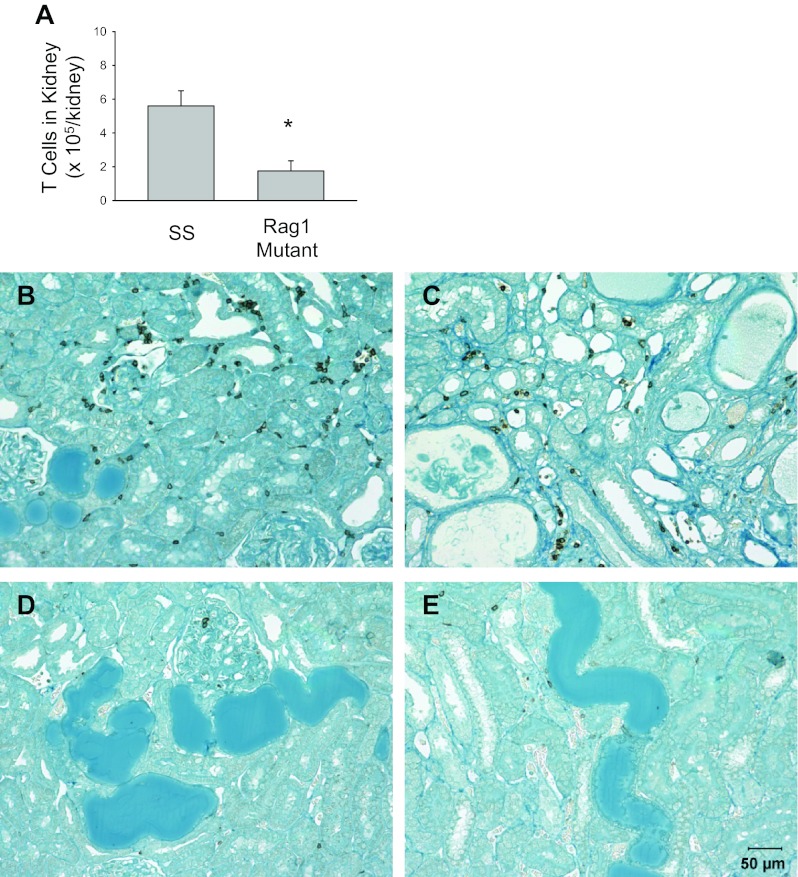
As indicated in Fig. 6, the renal histological damage typically observed in SS rats fed a high-salt diet, including blocked and dilated tubules in the outer medulla, was reduced in the Rag1-null rats (n = 4 or 5/group). Histological scoring demonstrated a significant reduction in the glomerular damage index, as well as a reduction in damaged tubules in the outer medulla. Finally, isolation and counting of infiltrating T cells in the kidney showed significantly fewer infiltrating T cells in the kidneys of the Rag1-null mutant rats compared with the Dahl SS kidney after 3 wk of the high-salt diet (Fig. 7, n = 4/group). An immunohistochemical analysis of the renal cortex and medulla demonstrated fewer CD43-positive cells in the kidneys of the Rag1 mutant rats, even in the regions of the kidney that demonstrated tubular damage.
Fig. 6.
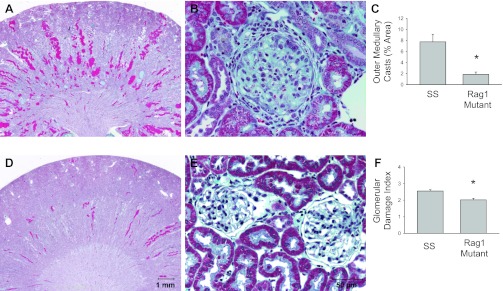
Light microscopy of trichome-stained sections of the kidney (A and D, 1× original magnification) and renal cortex (B and E; 40× magnification) of Dahl SS rats (A and B) or Rag1 mutant rats (D and E) fed 4.0% NaCl chow for 3 wk. The percentage of the renal outer medulla consisting of protein casts (C) and the calculated glomerular injury score (F) in the SS and Rag1 mutant rats are also plotted. *P < 0.05 vs. SS.
Fig. 7.
T-cell counts in the kidney (A) and immunohistochemical images of CD43+ T cells in the renal cortex (B and D) and the outer medulla (C and E) of Dahl SS (B and C) and Rag1 mutant rats (D and E). *P < 0.05 vs. SS.
To confirm the results obtained in SS-Rag1em1Mcwi, a set (n = 4/group) of confirmatory experiments was performed in the 17-base deletion mutant strain (SS-Rag1em2Mcwi) and Dahl SS controls. Body weights were not different between the SS control and the SS-Rag1em2Mcwi (343 ± 16 vs. 354 ± 5 g, respectively), but thymus weight was significantly lower in the SS-Rag1em2Mcwi (0.5 ± 0.1 vs. 0.12 ± 0.01 g). After 21 days of 4.0% NaCl diet, MAP, measured with chronic indwelling catheters, averaged 141 ± 4 mmHg in the Rag1 mutants, significantly different from 181 ± 9 mmHg in Dahl SS. Albumin excretion was significantly lower in the SS-Rag1em2Mcwi than in the SS (19 ± 3 vs. 127 ± 17 mg/day, respectively). Flow cytometry experiments on mononuclear cells isolated from the blood and spleen of the SS-Rag1em2Mcwi were consistent with the observations made in the SS-Rag1em1Mcwi.
DISCUSSION
The present experiments demonstrate that an intact immune system is necessary for the full expression of salt-sensitive hypertension and renal damage in the Dahl SS rat. Rats with a genetic mutation in the Rag1 gene, which resulted in a depletion of mature T and B cells, demonstrated a significant blunting of salt-sensitive hypertension and renal disease. When dietary NaCl intake was increased, the Dahl SS demonstrated a rapid and progressive increase in blood pressure that was accompanied by a significant increase in albumin excretion rate and marked renal histological damage. Mean arterial pressure and albumin excretion rate also increased in the Rag1-mutant rats when fed the elevated NaCl diet, but the increase was significantly attenuated compared with the SS. The glomerular and tubular damage after 3 wk of high-salt intake was also blunted in the Rag1 mutants, as was the number of infiltrating T cells in the kidney. Despite the large differences in blood pressure and renal damage in the rats fed high NaCl, arterial blood pressure and albumin excretion values were not significantly different from age-matched Dahl SS rats when maintained on a low-NaCl diet.
Previous studies from our laboratory have indicated that immunosuppressive agents attenuate the development of salt-sensitive hypertension and renal damage in the Dahl SS rat (3, 4, 5, 17). Similar findings have been made in many other animal models of hypertension and/or renal disease (7, 21, 22, 23). Although the large number of studies with similar conclusions indicate an important role of the immune system in cardiovascular disease, potential nonspecific effects of these pharmacological agents cloud the interpretation of these studies. Moreover, the role of immune cells in the time course of disease development is difficult to ascertain with a pharmacological approach. Previous studies from Dr. David Harrison's group have used genetically mutated mouse models to address the role of T lymphocytes in experimental hypertension (11). Work in the present article describes the development of a rat in which the Rag1 gene has been mutated using ZFN technology in the Dahl SS genetic background, a genetic model of hypertension.
These studies utilized a ZFN strategy to mutate the Rag1 gene in the Dahl SS genetic background. This approach, which has been documented for a number of other genes (8, 9, 19), led to two strains with a mutation in exon 1 of Rag1. The genetic mutation was confirmed by sequencing; and subsequent Western blotting of protein homogenates from the thymus indicated a loss of Rag1-immunoreactive protein in the Rag1-mutant rats. In addition, the mutant rats demonstrated a marked reduction of mature T and B cells in the blood and spleen, with normal body weights, and reduced thymus weights. There were no other developmental abnormalities noted in these animals, although the potential impact of developmental changes and/or alteration in other pathways in the absence of Rag1 protein is a limitation of this gene deletion approach. Qualitatively, the phenotypes observed in the present study are similar to those previously reported in Rag1-/- mice (18) and recently reported in the LEW/Ztm rat with ZFN-mediated disruption of Rag1 (29).
A number of different types of infiltrating immune cells, including macrophages, T lymphocytes, and B lymphocytes, have been identified in the diseased kidney of hypertensive rats. Since the Rag1 mutants have a reduction in both T and B lymphocytes, it is difficult to ascribe the protective effects to a specific cell type. It is, therefore, possible that the blunted hypertensive response in the Rag1-mutant rats was mediated by a reduction in multiple cell types. Further cell-specific approaches will need to be performed before a definitive conclusion can be made regarding the role of this cell type in Dahl SS hypertension. In support of the concept that T cells are mediating the present effects, recent studies in Rag1-/- mice have indicated that T lymphocytes mediate a significant portion of ANG II-mediated hypertension (11). The mechanisms leading to the infiltration of these cells into the kidney in Dahl SS hypertension remain to be determined. An additional important question that is not addressed in this study is the relationship between the elevation in blood pressure, the infiltration of T cells, and renal damage; this present experimental approach does not permit us to ascertain the cause-and-effect relationship(s) between these parameters.
The present data demonstrate that genetic mutation of Rag1 in the Dahl SS genetic background results in a decrease in T and B lymphocytes in the blood and spleen. When the mutant rats were challenged with a high-salt diet, the degree of hypertension and renal damage was reduced, indicating that the infiltration of T and/or B cells in the kidney amplifies the Dahl SS disease phenotype. Since renal infiltration of immune cells has also been observed in hypertensive patients (13, 27), we speculate that these same mechanisms play a role in human hypertension and renal disease.
GRANTS
This work was partially supported by National Institutes of Health Grants HL-29587, DK-62803, DK-96859, and RC2-HL101681.
DISCLOSURES
Sigma and Medical College of Wisconsin (MCW) have a license agreement that could send royalties based on rat sales to MCW. This applies to all authors.
AUTHOR CONTRIBUTIONS
Author contributions: D.L.M., A.M.G., and H.J.J. conception and design of research; D.L.M., H.L., C.G., N.R., and A.M.G. performed experiments; D.L.M., H.L., C.G., and N.R. analyzed data; D.L.M. and N.R. interpreted results of experiments; D.L.M. prepared figures; D.L.M. drafted manuscript; D.L.M., A.M.G., and H.J.J. edited and revised manuscript; D.L.M., H.L., C.G., A.M.G., and H.J.J. approved final version of manuscript.
REFERENCES
- 1. Campese VM. Salt sensitivity in hypertension. Hypertension 23: 531–550, 1994 [DOI] [PubMed] [Google Scholar]
- 2. Cowley AW, Jr, Roman RJ. The role of the kidney in hypertension. JAMA 275: 1581–1589, 1996 [PubMed] [Google Scholar]
- 3. De Miguel C, Das S, Lund H, Mattson DL. T-lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 298: R1136–R1142, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Miguel C, Lund H, Di F, Mattson DL. Infiltrating T lymphocytes in the kidney increase oxidative stress and lead to hypertension and renal disease. Am J Physiol Renal Physiol 300: F734–F742, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Miguel C, Lund H, Mattson DL. High dietary protein exacerbates hypertension and renal damage in Dahl salt-sensitive (SS) rats by increasing infiltrating immune cells. Hypertension 57: 269–274, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feldman HI, Klag MJ, Chiapella AP, Whelton PK. End-stage renal disease in US minority groups. Am J Kidney Dis 19: 397–410, 1992 [DOI] [PubMed] [Google Scholar]
- 7. Franco M, Martinez F, Quiroz Y, Galicia O, Bautista R, Johnson RJ, Rodriguez-Iturbe B. Renal angiotensin II concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 293: R251–R256, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Geurts AM, Cost GJ, Remy S, Cui X, Tesson L, Usal C, Menoret S, Jacob HJ, Anegon I, Buelow R. Generation of gene-specific mutated rats using zinc-finger nucleases. Methods Mol Biol 597: 211–225, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Geurts AM, Cost GJ, Freyvert Y, Zeitler B, Miller JC, Choi VM, Jenkins SS, Wood A, Cui X, Meng X, Vincent A, Lam S, Michalkiewicz M, Schilling R, Foeckler J, Kalloway S, Weiler H, Ménoret S, Anegon I, Davis GD, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Jacob HJ, Buelow R. Knockout rats via embryo microinjection of zinc-finger nucleases. Science 24: 433, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grim CE, Wilson TW, Nicholson GD, Hassell TA, Fraser HS, Grim CM, Wilson DM. Blood pressure in blacks. Hypertension 15: 803–809, 1990 [DOI] [PubMed] [Google Scholar]
- 11. Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Herrera J, Ferrebuz A, García MacGregor E, Rodriguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol 17: 218–225, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Hughson GC, Gobe MD, Hoy WE, Manning RD, Douglas-Denton R, Bertram JF. Associations of glomerular number and birth weight with clinicopathological features of African Americans and Whites. Am J Kidney Dis 52: 18–28, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Lackland DT, Keil JE. Epidemiology of hypertension in African Americans. Sem Nephrol 16: 63–70, 1996 [PubMed] [Google Scholar]
- 15. Mai M, Geiger H, Hilgens KF, Veelken R, Mann JFE, Daemmrich J, Luft FC. Early changes in hypertension induced renal injury. Hypertension 22: 754–765, 1993 [DOI] [PubMed] [Google Scholar]
- 16. Mashimo T, Takizawa A, Voigt B, Yoshimi K, Hiai H, Kuramoto T, Serikawa T. Generation of knockout rats with x-linked severe combined immunodeficiency (x-scid) using zinc-finger nucleases. PLos One 5: e8870, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 48: 149–156, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. Rag-1-deficient mice have no mature B and T lymphocytes. Cell 68: 869–877, 1992 [DOI] [PubMed] [Google Scholar]
- 19. Moreno C, Hoffman M, Stodola TJ, Didier DN, Lazar J, Geurts AM, North PE, Jacob HJ, Greene AS. Creation and characterization of a renin knockout rat. Hypertension 57: 614–619, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moreno C, Kennedy K, Andrae JW, Jacob HJ. Genome-wide scanning with SSLPs in the rat. Methods Mol Med 108: 131–138, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Muller DN, Shagdarsuren E, Park JK, Dechend R, Mervaala E, Hampich F, Fiebeler A, Ju X, Finckenberg P, Theuer J, Viedt C, Kreuzer J, Heidecke H, Haller H, Zenke M, Luft FC. Immunosuppressive treatment protects against Angiotensin II-induced renal damage. Am J Pathol 161: 1679–1693, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pechman KR, Basile DP, Lund H, Mattson DL. Immune suppression blocks sodium-sensitive hypertension following recovery from ischemic acute renal failure. Am J Physiol Regul Integr Comp Physiol 294: R1234–R1239, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Quiroz Y, Pons H, Gordon KL, Rincón J, Chávez M, Parra G, Herrera-Acosta J, Gómez-Garre D, Larfo R, Egido J, Johnson RJ, Rodríguez-Iturbe B. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from nitric oxide synthase inhibition. Am J Physiol Renal Physiol 281: F38–F47, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Rodriguez-Iturbe B, Johnson RJ. The role of inflammatory cells in the kidney in the induction and maintenance of hypertension. Nephrol Dial Transplant 21: 260–263, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Rodríguez-Iturbe B, Pons H, Quiroz Y, Gordon K, Rincón J, Chávez M, Parra G, Herrera-Acosta J, Gomez-Garre D, Largo R, Egido J, Johnson RJ. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int 59: 2222–2232, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Seaberg EC, Muñoz A, Lu M, Detels R, Margolick JB, Riddler SA, Williams CM, Phair JP, Multicenter Cohort Study AIDS Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS 19: 953–960, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Sommers SC, Relman AS, Smithwick RH. Histologic studies of kidney biopsy specimens from patients with hypertension. Am J Pathol 34: 685–715, 1958 [PMC free article] [PubMed] [Google Scholar]
- 28. Stewart T, Jung FF, Manning J, Vehaskari VM. Kidney immune cell infiltration and oxidative stress contribute to prenatally programmed hypertension. Kidney Int 68: 2180–2188, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Zschemisch NH, Glage S, Wedekind D, Weinstein EJ, Cui X, Dorsch M, Hedrick HJ. Zinc finger nuclease mediated disruption of Rag1 in the LEW/Ztm rat. BMC Immunol 13: 60, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]