Abstract
We previously reported elevated adipose leptin expression, plasma leptin concentrations, and adrenocortical leptin receptor expression in the long-term hypoxic (LTH) ovine fetus. This study addressed whether leptin antagonist (LA) administration to LTH fetal sheep altered expression of key genes governing cortisol synthesis. Ewes were maintained at high altitude (3,820 meters) from 40 to 130 days gestation (dG), returned to Loma Linda University, and implanted with a maternal tracheal catheter. Reduced Po2 was maintained by nitrogen infusion. On 132 dG, LTH (n = 11) and age-matched, normoxic control (n = 11) fetuses underwent vascular catheter implantation. At 138 dG, fetuses were continuously infused with either saline or the LA (1.5 mg·kg−1·day−1) for 4 days and samples collected for blood gases, ACTH, and cortisol. Fetal adrenal cortex was collected for determination of steriodogenic acute regulatory protein (StAR), ACTH, and leptin receptor, cholesterol side-chain cleavage (CYP11A1), cytochrome P-450 11β-hydroxylase (CYP11B1), 17α-hydroxylase (CYP17), 21-hydroxylase (CYP21), signal transducer and activator of transcription 3 (STAT3), pSTAT3, and 17β-hydroxysteroid dehydrogenase (HSD3B) expression. In the saline-infused LTH fetuses, StAR, ACTH receptor, CYP11A1, and CYP17 expression was significantly lower compared with control (P < 0.05), whereas levels of CYP11B1, CYP21, and HSD3B mRNA were similar between groups. LA infusion restored expression of StAR, pSTAT3, CYP11A1, and CYP17, but not ACTH receptor, to normal ontogenic levels in the LTH group while having no effect on control fetuses. Neither fetal plasma ACTH nor cortisol concentrations were altered by LA infusion. We speculate that while leptin plays a role in governing expression of key enzymes and StAR in response to LTH, other factors play a role in modulating cortisol synthesis in these fetuses.
Keywords: leptin, hypoxia, adrenal, cortisol, steroidogenesis
in the ovine fetus, plasma cortisol undergoes an exponential rise during late gestation coincident with maturation of adrenocortical function [(20); reviewed in Ref. 7]. In this species, as with most mammals including humans (18), this parturient rise in cortisol is essential for maturation of fetal organ systems allowing for survival in the extrauterine environment (19). Fetal stressors can activate the hypothalamic-pituitary-adrenocortical (HPA) axis, resulting in elevated cortisol production and, if sustained, premature maturation of the adrenal cortex. Elevated cortisol is associated with fetal growth restriction and, in ruminants, activation of the parturition cascade and early birth of a small fetus (12, 35).
Acute hypoxia is normally considered a potent activator of the fetal HPA axis, leading to acutely increased cortisol production, and during late gestation, moderate sustained hypoxia (hours to a few days) leads to activation and accelerated maturation of the fetal HPA axis (5, 6, 22). However, we have found that development under conditions of moderate long-term hypoxia (LTH) in sheep results in significant alterations in HPA axis function by late gestation (1, 15, 27, 28). In our model of LTH (1, 15), moderate hypoxia is achieved by maintaining ewes at an altitude of 3,820 meters from approximately day 40 of gestation to near term resulting in fetal Po2 levels of 17–19 mmHg (normoxic ∼23 mmHg). In response to this sustained hypoxic challenge, the HPA axis undergoes a remarkable adaptation with basal fetal plasma cortisol concentrations similar to control normoxic fetuses, despite increased circulating levels of ACTH and elevated hypothalamic paraventricular nuclei expression of corticotropin-releasing hormone (CRH) by late gestation (1, 15, 27). At the level of the adrenal cortex of the LTH fetus, despite the elevated circulating ACTH concentrations, expression of key genes governing cortisol biosynthesis [cholesterol side-chain cleavage (CYP11A1) and 17α-hydroxylase (CYP17)] and the ability to respond to ACTH (ACTH receptor) are decreased (28). These changes indicate that the LTH fetus recognizes this hypoxic condition as a stressor, yet adaptive mechanisms have been invoked to provide for a normal ontogenic maturation of cortisol production. Ultimately, this adaptive response assures a normal gestation length in this species, and importantly, without growth restriction as the LTH fetuses weight at birth is not reduced compared with normoxic controls (16, 13).
Leptin is a 16-kDa polypeptide that is synthesized and secreted primarily by adipose tissue whose major function is governing food intake at the level of the hypothalamus (2). However, in addition to its appetite-regulating function, leptin has been observed to exert inhibitory effects on the HPA axis of several species including fetal sheep (14, 39). When infused into late-gestation fetal sheep, leptin suppressed cortisol (39). In adult bovine adrenocortical cells in vitro, leptin directly suppressed ACTH-stimulated cortisol production concomitant with a reduction in CYP17 expression (4) as well as CYP11A (17). We have demonstrated that circulating leptin is significantly elevated in the late-gestation LTH sheep fetus as is expression of leptin in perirenal fat (8). Furthermore, adrenocortical expression of the biologically active long form of the leptin receptor (OB-Rb) is elevated in the LTH fetus (8). Thus leptin represents a candidate mechanism mediating the adaptive changes observed in the adrenal cortex of the late-gestation LTH sheep fetus. We hypothesized that administration of a leptin receptor antagonist to LTH fetal sheep would increase cortisol production in conjunction with increased expression of the key rate-limiting enzymes mediating cortisol synthesis (CYP11A1 and CYP17) as well as cholesterol transport into the mitochondria via steroidogenic acute regulatory protein (StAR) while leaving other adrenal steroidogenic enzymes such as 21-hydroxylase (CYP21) and 17β-hydroxysteroid dehydrogenase (HSD3B) unchanged.
MATERIALS AND METHODS
Animals
Pregnant ewes were maintained at high altitude (3,820 meters, maternal Po2 ∼60 mmHg, White Mountain Research Station) beginning at approximately day 40 of gestation (term = 146 days). At 123–125 days of gestation, the LTH ewes were transported to Loma Linda University Medical Center Animal Research Facility (elevation: 346 meters) where they were implanted with a nonocclusive tracheal catheter (4.0 mm OD) and an arterial catheter. Maternal Po2 for the LTH group was maintained at ≈60 mmHg (mean Po2 measured in animals at altitude) throughout the studies by adjusting humidified nitrogen gas flow through the tracheal catheter as we have previously described (1, 15). Normoxic, age-matched pregnant ewes were maintained near sea level (≈300 meters) during gestation and transported to Loma Linda University for study.
On day 132 of gestation, fetal catheterization surgeries were performed on normoxic control (n = 11) and LTH fetuses (n = 11) similar to methods previously described (1, 15) with catheters implanted in the fetal tibial vein and arteries as well as the amniotic fluid cavity. All catheters were tunneled under the ewe's skin, exteriorized through the left flank, and stored in a nylon pouch sutured to the skin. All procedures were approved by the Institutional Animal Care and Use Committee at Loma Linda University and followed the guidelines in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Postoperative Care
After surgery, the ewes were maintained in a metabolic cart with food and water provided ad libitum. The ewes received antibiotics (3 ml Crystiben im; 450,000 units penicillin G-procaine, and 450,000 units penicillin G-bensathine; Solvay Animal Health, Mendota Heights, MN) once daily and 1 g Zosyn (Pfizer, NY, NY) twice daily in the amniotic fluid for the first 3 postoperative days. The fetuses received intravenous antibiotics [40 mg Tobramycin iv (Bristol-Myers Squibb, Princeton, NJ), 150 mg Clindamycin iv (Abbott Laboratories)], twice daily for the first 3 days postsurgery. All vascular catheters were flushed two times daily with heparinized saline.
Leptin antagonist infusion.
Starting at 138–140 days gestation, normoxic control or LTH fetal sheep were infused with either saline (control; n = 5–6 per group) or a specific recombinant leptin receptor antagonist (n = 5–6 per group; ovine leptin L39A/D40A/FA1A/I42A; Protein Laboratories Renhovot, Renhovot, Israel). The ovine form of the antagonist has been shown to be effective both in vivo and in vitro (30, 36). For the saline controls, 0.5 ml saline was delivered as an initial bolus followed by continuous saline infusion (0.25 ml/h) for 4 days (terminating at 142–144 days gestation). For the leptin antagonist, an initial bolus (0.5 mg) of recombinant ovine leptin antagonist was delivered in 0.5 ml saline followed by continuous infusion of 1.5 mg·kg−1·day−1 (0.25 ml/h) for 4 days. Beginning immediately before the bolus injections, a pretreatment blood sample was drawn for plasma analysis of ACTH and cortisol. Once daily blood samples were taken throughout the experiment for blood gas analysis and plasma hormone quantification.
Tissue collection.
At the end of the infusion period, both the LTH ewes and age-matched normoxic controls were sedated with pentobarbital, intubated, and maintained under general anesthesia with 1.5–2% halothane in oxygen while the fetuses were delivered through a midline laparotomy and fetal adrenal glands were collected. The adrenals were snap frozen in liquid nitrogen and stored at −80°C until analysis.
Quantitative Real Time PCR Quantification of mRNA
All methods have been previously described and validated by our group (9, 28, 29). Total RNA was prepared from adrenal cortex (n = 6 for each group), and reverse transcription was performed using oligo dT as the primer. Subsequently, PCR reactions were performed in triplicate. The amount of cDNA needed per reaction is gene dependent and was determined by an initial PCR to ascertain that the amount of cDNA is within the linear amplification range for real time (RT)-PCR. Sybr Green was utilized as the fluorophore and a PCR mastermix using hot-start Taq polymerase. We utilized a Bio-Rad iCycler equipped with the real-time optical fluorescent detection system. A general three-step PCR was used with a denaturation at 95°C for 45 s, annealing (oligo specific but typically 55–60°C) for 30 s, and 72°C extension for 45 s. For each RT reaction, we used cyclophillin as a “house keeping” mRNA. An artificial 100-base single-stranded DNA standard was used to generate a standard curve for quantification of starting cDNA concentrations. Primer sequences are listed in Table 1.
Table 1.
Primer sequences used for qRT-PCR
| Gene | Primer Sequence | NCBI Accession No. |
|---|---|---|
| CYP11A1 | Fw: 5-GGCTCACAGAGAATCCACTTTCG-3 | D50057 |
| Rv: 5-TGATGTCCCCTACAAACTTTCCG-3 | ||
| CYP11B1 | Fw: 5-GGAGACACATGGTGTTCGTG-3 | NM_174638.3 |
| Rv: 5-CACCAAGGGCGTGTACTTCT-3 | ||
| CYP17 | Fw: 5-CATCAGAGAAGTGCTCCGAATCC-3 | AF251388 |
| Rv: 5-TCCTGCTCCAAAGGGCAAGTAG-3 | ||
| CYP21 | Fw: 5-TGCCTCGGTGTCTCCTTTTATTG-3 | M11267 |
| Rv: 5-GGTGCCCCTTCACGGAAATG-3 | ||
| HSD3B | Fw: 5-CCTGCTGGAAGGAGACATTCTG-3 | NM_174343 |
| Rv: 5-GTGCTGGTGTGGATAAAGACCG-3 | ||
| MC2R | Fw: 5-ATGAAACACATTCTCAATCTG-3 | NM_001009442 |
| Rv: 5-AACGTTTTCCAAAATCTTGTAC-3 | ||
| OB-Ra | Fw: 5-TGCTGTCACCCAGTGATTACAATC-3 | AY278244 |
| Rv: 5-CAAAGTATGTCCGTTCTCTTCTGA-3 | ||
| OB-Rb | Fw: 5-AACTACAGATGCCCTGCTTTTGAC-3 | U62124 |
| Rv: 5-CCTTTGGTGGAGAATGGTTGC-3 | ||
| CYLCO | Fw: 5-CCATCGTGTGTCAAGGACTTCAT-3 | BT020966 |
| Rv: 5-CTTGCCATCTAGCCAGGGTCTT-3 |
CYP11A1, cholesterol side-chain cleavage; CYP11B1, cytochrome P-450 11β-hydroxylase; CYP17, 17α-hydroxylase; CYP21, 21-hydroxylase; HSD3B, 17β-hydroxysteroid dehydrogenase. See text for additional abbreviations.
Criteria for RT-PCR primers include the following: PCR amplicon must yield a single product; a dilution curve of cDNA must yield a slope that is the same as generated by the standard DNA (100% + 10% “efficiency” where 100% = Δ3 Ct/log cDNA input); and a postamplification melt curve analysis of product must repeatedly demonstrate one product. A control PCR reaction was performed for each mRNA of interest in which the RT was omitted.
Western Blot Analysis
Frozen fetal adrenal glands were cut in half along the longitudinal axis and one half of the cortex was microdisected from the capsule and medulla. The remaining half was kept frozen for mRNA analysis. Western blot analysis was performed similarly to what has been described for our laboratory (9, 27). Adrenal cortical tissue was homogenized (4°C, 1 ml, 0.1 M acetic acid, 100 mM sodium chloride, pH 5.0, containing 1 mM pepstatin, 0.4 mM pefablock, 1 μg/ml leupeptin) and centrifuged at 12,000 g for 2 min, and the supernatant was collected. Protein concentrations were determined using a protein quantification kit using BSA as standard (Bio-Rad). Samples [50 μg of protein per cortex (StAR); 5 μg protein (CYP17), or 20 μg protein (CYP11A1)] were electrophoresed by SDS-PAGE (4–10%; Invitrogen), and the proteins were subsequently electrophoretically transferred to nitrocellulose membranes and subjected to Western blot analysis. The membranes were blocked for 1 h with TBS (10 mM Tris·HCl, pH 7.2, 100 mM saline) containing 0.1% Tween 20 (TTBS) and 10% nonfat dry milk. Membranes were then washed twice in TTBS and incubated with primary antibodies [anti-bovine CYP17 generously supplied by Dr. Alan Conley, University of California, Davis, CA; anti-bovine CYP11A1 from Chemicon, Temecula, CA; affinity-purified anti-rat StAR from Abcam, Cambridge, MA; anti-rabbit signal transducer and activator of transcription 3 (STAT3) from Millipore, Billerica, MA; and anti-goat phospho-STAT3 (pSTAT3) from Santa Cruz, Santa Cruz, CA] prepared in TTBS-5% nonfat dry milk overnight at 4°C. The membranes were subsequently washed twice in TTBS and incubated with either a goat-anti-rabbit horseradish peroxidase (HRP)-labeled (for CYP17, CYP11A1, StAR, and STAT3; Perkin-Elmer), rabbit-anti-goat HRP-labeled (pSTAT3; Perkin Elmer), or a goat anti-mouse HRP-labeled (β-actin; Perkin-Elmer) secondary antibody. After final washing with TTBS (twice) was completed, a chemiluminescent detection system (Pierce) was applied and blots were exposed to film (Hypermax; Kodak, Rochester, NY) for varying lengths of time. For controls, we omitted the primary antiserum and used a nonexpressing tissue such as liver or muscle. Films were quantified by densitometry. To ensure that equal protein amounts were loaded for each sample, identical SDS-PAGE was performed with the same protein samples from control and LTH adrenal cortex analyzed by Western blot analysis for β-actin using the above described procedure.
Fetal Plasma Cortisol
Plasma cortisol was measured using an ELISA assay (EA65; Oxford Biomedical Research, Oxford, MI). Cortisol was extracted from plasma using a previously described extraction procedure (extraction efficiency ∼85%) (33). This EIA kit has been previously validated for fetal sheep plasma (33) and used in our laboratory (10). The sensitivity of this assay is 0.1 ng/ml and the interassay coefficient of variation is <10%.
Fetal Plasma ACTH
Because of a limited availability of fetal plasma, we analyzed fetal plasma ACTH1–39 utilizing an ACTH-IRMA (immunoradiometric assay; Diasource Immunoassays,) previously validated in our laboratory (25). This IRMA exhibits no cross-reactivity against α-, β-, or melanocyte-stimulating hormone (γMSH) or β-endorphin and exhibits less than 1% cross reactivity against proopiomelanocortin (POMC) and ACTH precursors (22 kDa proACTH) (25). The limit of detection of this assay is 1.9 pg/ml ACTH, and the intra-assay CV was 5%. Parallelism and recovery have been previously described (25).
Statistical Analysis
Data were analyzed by ANOVA with post hoc tests where appropriate using Prism (GraphPad Software, San Diego, CA). P < 0.05 was indicative of statistical significance. All data are presented as means ± SE.
RESULTS
Fetal Physiological Parameters
Fetal blood gas values for the control and LTH groups are illustrated in Table 2. Since there were no observed differences between days of study, the data are presented as means ± SE for the entire study period for each group. As we have previously described for the LTH model (1, 15), fetal arterial Po2 and Pco2 were significantly lower than control (P < 0.05), whereas pH values were similar in all groups.
Table 2.
Fetal blood gas and physiological data
| Treatment |
pH | PO2, mmHg | PCO2, mmHg | HR, beats/min | MAP, mmHg | Weight, kg |
|---|---|---|---|---|---|---|
| Control (saline) | 7.36 ± 0.01 | 23.36 ± 1.15 | 53.88 ± 1.11 | 137.1 ± 4.8 | 44.1 ± 0.4 | 4.38 ± 0.76 |
| Control (LA) | 7.34 ± 0.01 | 22.00.±1.10 | 54.0 ± 1.17 | 142.4 ± 4.0 | 42.5 ± 0.5 | 4.04 ± 0.40 |
| LTH (saline) | 7.37 ± 0.01 | 18.63 ± 1.19* | 43.70 ± 1.45* | 145.5 ± 1.7 | 43.2 ± 0.6 | 3.69 ± 0.02 |
| LTH (LA) | 7.38 ± 0.01 | 19.54 ± 0.58* | 44.86 ± 1.47* | 140.3 ± 3.3 | 43.2 ± 0.6 | 4.05 ± 0.15 |
Values are means ± SE. LA, leptin antagonist; HR, heart rate; MAP, mean arterial pressure.
P < 0.05 compared with control.
Steroidogenic Enzyme and StAR Expression
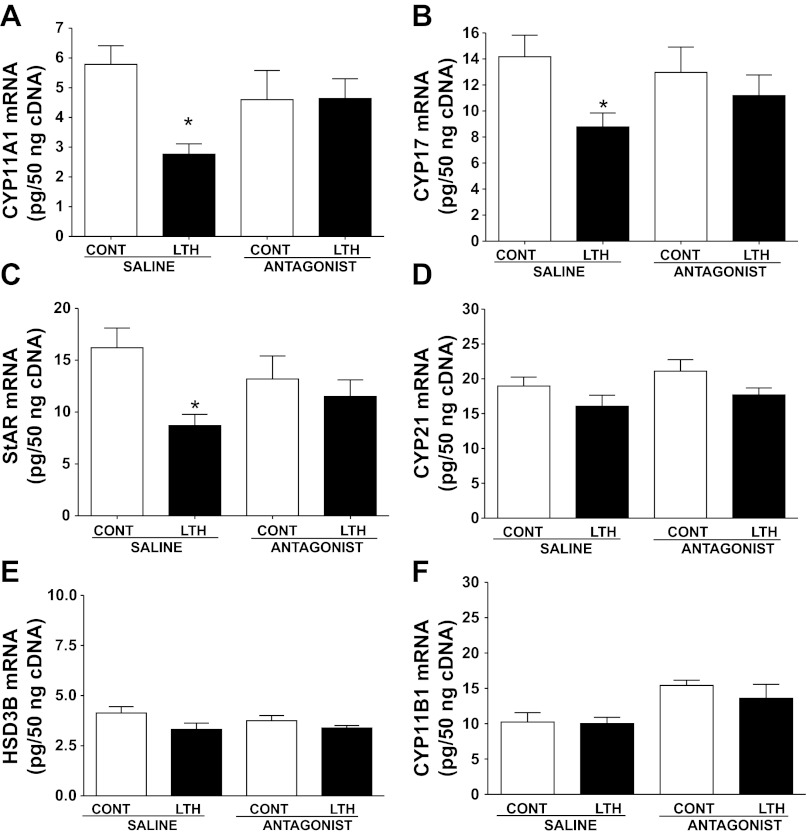
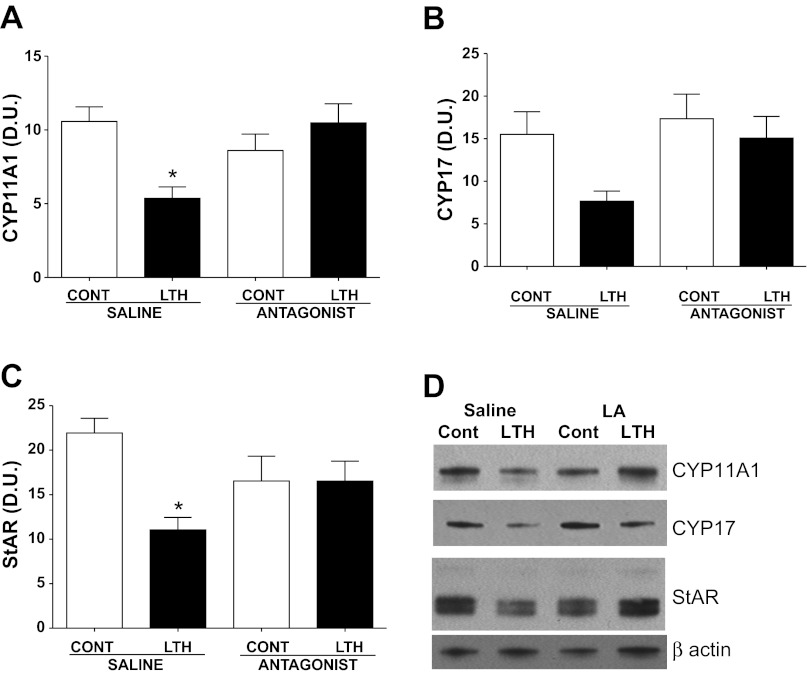
In the saline-infused fetuses, mRNA for CYP11A1, CYP17, and StAR was significantly lower in the LTH adrenal cortex compared with normoxic control fetuses (Fig. 1, A–C). Similar to mRNA, levels of protein for CYP11A1 and StAR were also lower in the saline-infused LTH group (P < 0.05) compared with control (Fig. 2, A and C). A similar pattern was observed for CYP17 but did not reach statistical significance. After the 4-day treatment with the leptin antagonist, expression (mRNA and protein) of CYP11A1, CYP17, and StAR increased in the LTH adrenal cortex to levels not different from either normoxic control saline-infused or leptin antagonist-infused fetuses (Figs. 1, A–C, and Fig. 2). There was no effect of infusion of the leptin antagonist on CYP11A1, CYP17, or StAR in the normoxic control adrenal cortex compared with saline-infused control fetuses.
Fig. 1.
Effect of leptin receptor antagonist infusion on adrenal cortical mRNA in control and long-term hypoxia (LTH) fetuses. LTH fetuses had significantly lower levels of mRNA expression for cholesterol side-chain cleavage (CYP11A1) (A), 17α-hydroxylase (CYP17) (B), and steriodogenic acute regulatory protein (StAR) (C) (P < 0.05, compared with control). After 4 days of leptin antagonist infusion, levels were restored to values similar to control. Antagonist infusion had no effect on mRNA levels in the control fetuses. mRNA values for cytochrome P-450 21-hydroxylase (CYP21), 17-hydroxysteroid dehydrogenase (HSD3B), and 11-hydroxylase (CYP11B1) did not differ among treatment groups (D, E, and F). Values represent means ± SE. *Different from control saline infusion, P < 0.05.
Fig. 2.
Adrenal enzyme and StAR protein levels following leptin receptor antagonist infusion in control and LTH fetuses. Protein was also lower in the LTH fetuses compared with control for CYP11A1 (A) and StAR (C) (*P < 0.05). Leptin antagonist infusion restored protein expression to control values (A and C). CYP17 (B) followed a similar trend that approached statistical significance (P = 0.06). Antagonist infusion had no effect on protein levels in the control fetuses. No differences among the treatment groups were observed in actin (housekeeping protein). A representative bolt for each protein of interest is illustrated in D. Values represent means ± SE. *Different from control saline infusion, P < 0.05.
While the principal focus of this study was on the steroidogenic enzymes (CYP11A1 and CYP17) that we previously demonstrated to be affected by hypoxia (28), we also examined for potential effects of leptin antagonist infusion on the other enzymes involved in the glucocorticoid biosynthetic pathway, particularly in light of a recent study showing that exogenous leptin suppressed CYP21 expression in fetal sheep (37). Levels of mRNA for CYP21, HSD3B, and CYP11B1 were similar between saline-infused LTH and normoxic control fetuses and were not affected by leptin antagonist administration (Fig. 1, D–F). Since we found no differences in mRNA between groups or in response to leptin antagonist treatment, we did not pursue further Western Blot analysis for these genes.
ACTH and Leptin Receptor mRNA
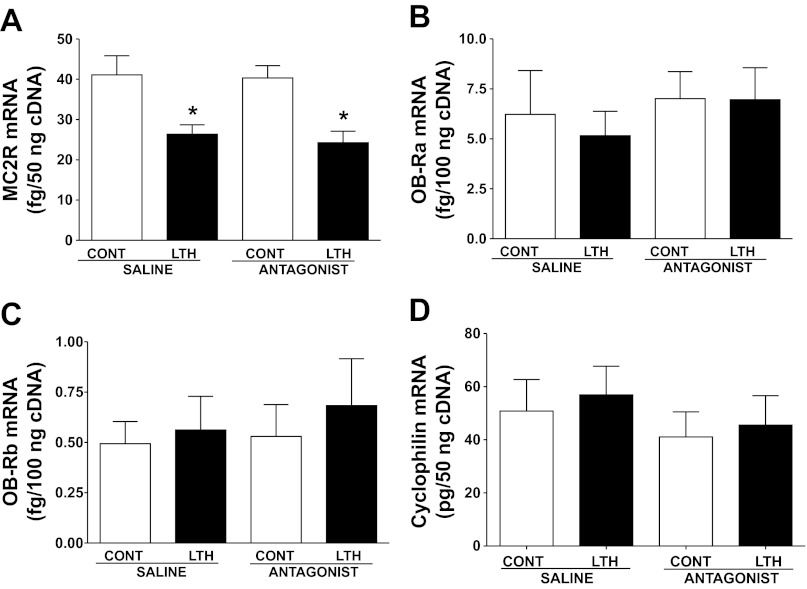
Consistent with our previous finding, MC2R mRNA was lower in the LTH group compared with control (P < 0.05), and levels were unaffected by leptin antagonist infusion in either the control or LTH group (Fig. 3A). In contrast, mRNA levels of both leptin receptor isoforms OB-Ra and OB-Rb were not different among the different treatment groups (Fig. 3, B and C).
Fig. 3.
Effect of LTH and leptin antagonist on ACTH (MC2R, A) and leptin receptor (Ob-R) mRNA. LTH fetuses had significantly lower levels of mRNA for MC2R (*P < 0.05, compared with control), but leptin antagonist treatment had no effect on mRNA (D). There were no differences observed for leptin receptor (OB-Ra, B or OB-Rb, C) mRNA in control and LTH adrenals. Cyclophilin (mRNA housekeeping gene, D) was unaffected by treatment. Values represent means ± SE.
Western Blot Analysis for Phospho-STAT3
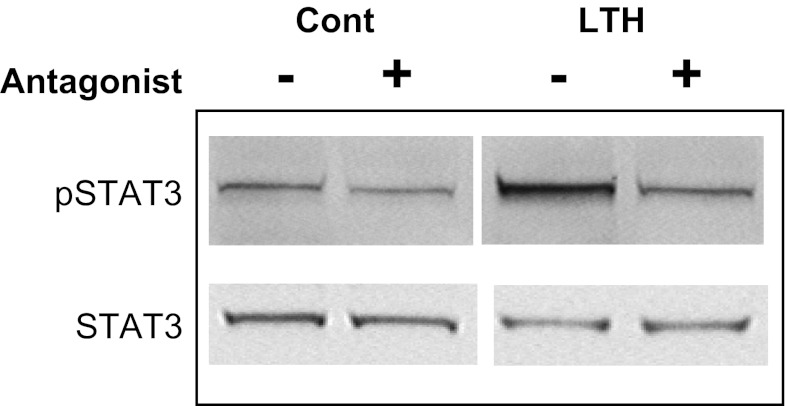
The leptin receptor (OB-Rb) transduces its intracellular signal primarily via activation (phosphorylation) of STAT3. To examine the efficacy of leptin antagonist infusion on OB-Rb signaling in the fetal adrenal cortex, we performed Western Blot analysis for STAT3 and pSTAT3 on all adrenal samples. As shown in the representative blot in Fig. 4, STAT3 levels were similar between control and LTH fetuses and were not affected by leptin antagonist administration. Levels of pSTAT3 were higher in LTH fetuses (saline infused) and administration of the leptin antagonist reduced levels of pSTAT3 by ∼50% (Fig. 4).
Fig. 4.
Effect of leptin antagonist infusion on signal transducer and activator of transcription 3 (STAT3) and phospho-STAT3 (pSTAT3) in control and LTH fetal adrenals. The representative Western blot illustrates that under conditions of LTH, levels of pSTAT3 are elevated compared with control. However, leptin antagonist infusion (+) reduced the level pSTAT3 in the LTH group.
Housekeeping Genes
Leptin antagonist infusion did not alter either cyclophilin (mRNA housekeeping gene, Fig. 3D) or actin (protein housekeeping gene, Fig. 2D) in either LTH or normoxic control fetuses, and there were no differences between LTH and normoxic control fetuses for either of these genes.
Fetal Plasma ACTH1–39 and Cortisol
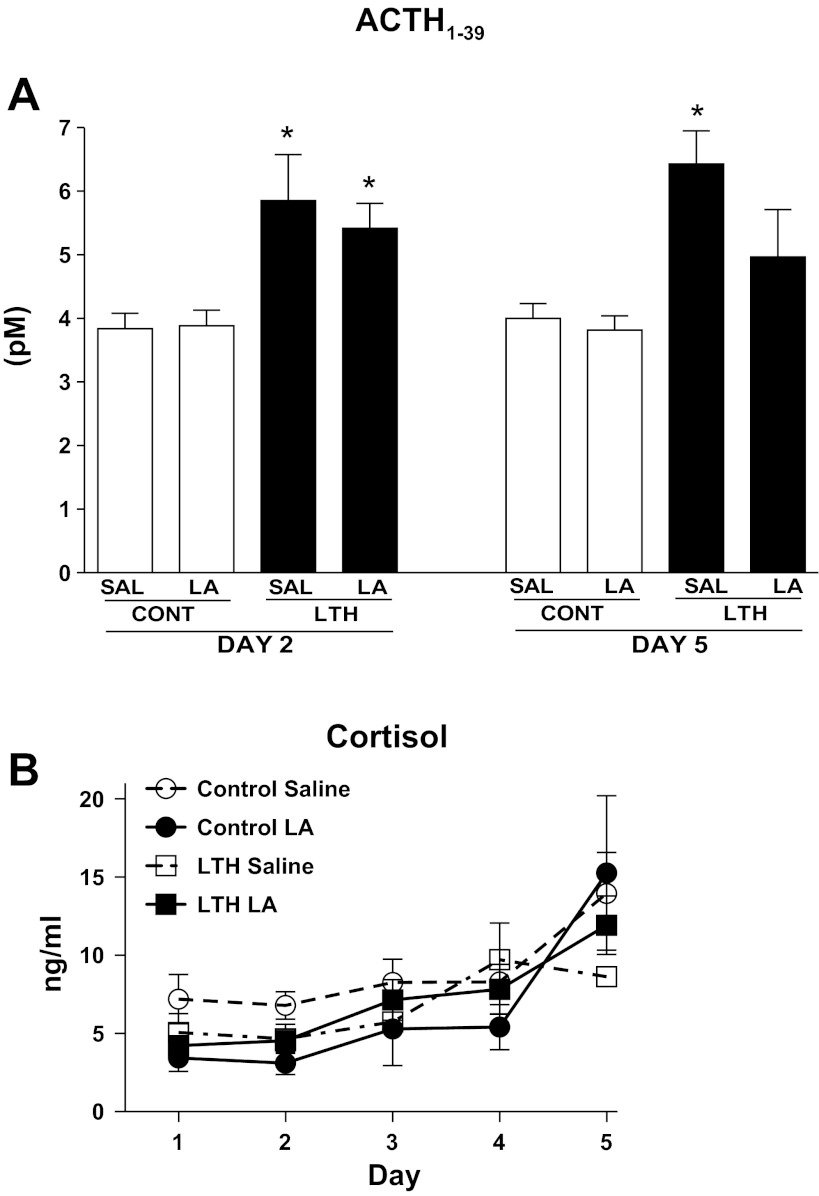
Consistent with our prior studies (9, 25), saline-infused LTH fetuses exhibited significantly (P < 0.05) elevated ACTH1–39 compared with normoxic control (saline) infused fetuses at both 24 h (day 2) and 96 h (day 5) of the study (Fig. 5A). Infusion of the leptin antagonist had no effect on ACTH1–39 at 24 h of infusion for either normoxic or LTH fetuses; similarly, after 96 h of antagonist infusion in the normoxic fetuses, plasma ACTH1–39 was not different from the normoxic saline-infused group (day 2 or day 5). In the LTH fetuses, 96 h of antagonist infusion resulted in fetal plasma ACTH1–39 values that were of intermediate levels, not different from either LTH saline-infused or normoxic saline infused.
Fig. 5.
Effect of leptin receptor antagonist (LA) infusion on plasma ACTH1–39 (A) and cortisol (B) in control and LTH fetuses. A: Saline-infused LTH fetuses exhibited elevated (*P < 0.05) ACTH1–39 compared with normoxic saline-infused fetuses at both day 2 and day 5 of the study. ACTH1–39 was not different in LA-infused fetuses for either group on either day 2 or 5. On day 5 of the study (96 h of LA treatment), ACTH1–39 had declined to levels not different from normoxic control saline fetuses. B: both control and LTH fetuses (LA and saline infused) exhibited the normal ontogenic, late gestation, and rise in plasma cortisol (P < 0.05 gestational age effect). LA infusion did not alter this increase in either group. Values represent mean values ± SE.
There was also no effect of treatment on fetal plasma cortisol concentration in either the control or LTH fetuses (Fig. 5B). There was a significant effect of gestational age (P < 0.05) with the normal ontogenic increase in cortisol observed during this latter part of gestation both control and LTH fetuses.
DISCUSSION
We have clearly shown that the ovine fetal adrenal cortex exhibits a unique functional adaptation to development under conditions of long-term moderate hypoxia, becoming refractory to the elevated basal plasma ACTH concentrations achieved in response to this moderate sustained stressor while retaining the capacity for heightened cortisol production in response to a secondary stressor (1, 15, 27, 28). The adaptive changes, which include decreased expression of the key rate-limiting enzymes for cortisol synthesis, allow the fetus to maintain the normal ontogenic levels of fetal plasma cortisol necessary for organ maturation, and in this species, birth. However, the mechanisms governing this discordant adaptation at the adrenal cortex compared with that observed at the hypothalamo-pituitary level remained unresolved.
Leptin is a known pharmacological inhibitor of adrenocortical function, and we (7) have found that the late gestation LTH fetus has elevated fetal plasma leptin resulting from increased adipose expression of this key adipokine. The ability to fully understand the role of leptin in the regulation of the HPA axis has been hampered by the previous lack of a specific leptin receptor antagonist. Nonetheless earlier studies demonstrating that intracerebral leptin infusion in the ovine fetus reduced the amplitude and mean value of plasma ACTH and cortisol pulses near term (14). Peripheral infusion of leptin at pharmacological doses also suppressed ACTH and cortisol in the late-gestation ovine fetus (39). Recently, it was noted that a 96-h infusion of leptin into modestly spontaneously hypoxic ovine fetuses during late gestation also resulted in decreased fetal plasma cortisol associated with decreased ACTH receptor, StAR, and CYP21 mRNA further supporting a pharmacological role for leptin in regulating the fetal HPA axis (37).
In the present study we addressed the role of endogenous leptin on adrenocortical function and expression of key genes governing cortisol synthesis in both normoxic and LTH fetal sheep during late gestation via systemic infusion of an ovine leptin antagonist. In our previous study (26), we observed that LTH reduced expression of two key steroidogenic enzymes, CYP11A1 and CYP17, in the adrenal cortex of fetal sheep as well as lowering the expression of the cholesterol mitochondrial transport protein, StAR and the ACTH receptor (MC2R). However, LTH was without effect on CYP11B1, CYP21, or HSD3B. As we now report, the 4-day infusion of the leptin antagonist exerted a significant impact on expression of both CYP11A1 and CYP17 as well as on StAR, increasing their expression in the cortex to levels comparable to the saline-infused normoxic control fetuses. Infusion of the leptin antagonist was without effect on the MC2R (which remained suppressed), or the other steroidogenic enzymes CYP11B1, CYP21, and HSD3B, whose expression is not affected by the LTH environment. Surprisingly, based on studies on the effects of exogenous leptin to suppress adrenocortical function in late-gestation sheep (39, 37) and on adult bovine adrenocortical cells in vitro (4, 17), administration of the leptin receptor antagonist had no effect on MC2R, StAR, CYP11A1, CYP11B1, CYP17, CYP21, or HSD3B in the normoxic control fetuses.
There was a significant time effect (P < 0.05) on fetal plasma cortisol in all groups, consistent with the beginning of the normal ontogenic rise in late gestation. It should be noted that the study terminated before the period of gestation when the large exponential rise in fetal plasma cortisol is most evident (20, 21, 23). Surprisingly, leptin antagonist treatment did not affect fetal plasma cortisol during the 96-h constant infusion either normoxic or LTH fetal sheep. Thus, although antagonizing the actions of leptin restored expression of the key limiting enzymes for cortisol synthesis, other mechanisms remain in place in these fetuses controlling the production of cortisol. Furthermore, even in LTH fetal sheep, the antagonist only achieved in restoring expression of these enzymes to normal ontogenic levels thus supporting the existence of additional mechanisms via which LTH impacts adrenocortical function. Since LTH fetal adrenocortical cells retain normal signal transduction properties (i.e., cAMP production) in response to ACTH in vitro (38), the mechanisms governing the discordant adrenocortical response to LTH compared with the elevated plasma ACTH achieved would seemingly be post-cAMP and/or via alternative signaling pathways.
Similar to cortisol, infusion of the leptin antagonist did not effect fetal plasma ACTH1–39 concentrations in either the normoxic control fetuses or LTH fetuses when assessed after either 24 or 96 h of antagonist infusion. The relatively constant concentrations of ACTH1–39 during the infusion period are consistent with those previously reported during this window of gestation (21) and are also consistent with the concept that adrenocortical maturation in fetal sheep largely takes place in the presence of relatively steady basal levels of ACTH during late gestation (32). The lack of effect of the leptin antagonist on ACTH1–39, compared with the inhibitory actions of exogenous leptin previously noted in fetal sheep, may relate to the gestational age of the animals in the present study and the mode of administration (peripheral vs. central) of the leptin antagonist. Yuen et al. (39) noted that leptin suppressed both ACTH and cortisol when administered between 136 and 141 days of gestation, yet when administered at 144 days of gestation, a fourfold increase in plasma leptin achieved in that study had no effect on ACTH, yet still suppressed fetal plasma cortisol concentrations by 40%. The lack of effect of the leptin antagonist on ACTH concentrations may thus be related to the age of the fetus. However, for cortisol, the lack of effect may reflect endogenous versus exogenous (potentially pharmacological) actions of the adipose-derived hormone. In the earlier study by Yuen et al. (39), levels of leptin achieved with exogenous infusion exceeded basal levels by more than 10-fold, whereas in the LTH fetuses in the present study, levels were only 3- to 4-fold higher than controls. We have observed (34) a significant decline in expression of the active leptin receptor isoform (OB-Rb) in the near-term fetal sheep adrenal cortex (>140 days of gestation), thus the adrenal may become refractory to the inhibitory actions of leptin as term gestation approaches. In the LTH fetus, such a downregulation could be prerequisite for release of an inhibitory role for leptin allowing for the prepartum cortisol surge triggering organ maturation and birth.
One could also argue that perhaps the dose of leptin receptor antagonist infused was insufficient to exert any major effect on the fetal HPA. However, it is important to emphasize that the dose of antagonist was based on a number of critical parameters. Yuen et al. (39) infused 0.48 mg·kg−1·day−1 of leptin, which resulted in a 10-fold increase in plasma leptin in the ovine fetus. The specific ovine leptin antagonist used in the present study has equal affinity with wild-type leptin (30, 36). Considering the equal affinity and the capacity of a 5- to 10-fold excess of antagonist to completely antagonize wild-type leptin (31), the dose of leptin antagonist used in the present study is more than adequate to be effective. As an index of the effectiveness of the antagonist, we also showed that in the LTH group, the level STAT-3 phosphorylation (pSTAT-3) was reduced following infusion of the leptin antagonist. Since binding of leptin to the OB-Rb activates the JAK/STAT pathway with subsequent activation of STAT-3 via phosphorylation (3), the reduction in pSTAT-3 with the leptin antagonist further strengthens the activity of the antagonist. Alternatively, the effect of LTH on hypothalamic leptin receptors may be responsible at least in part for the lack of effect of the leptin receptor antagonist on ACTH and cortisol. We previously showed that LTH results in a reduction in hypothalamic OB-Ra leptin receptor isoform (8). This receptor isoform is the primary transport receptor for leptin to access the brain (24). This could potentially result in reduced transport of the antagonist to the brain.
We recently demonstrated that in addition to enhanced leptin secretion under conditions of LTH, the LTH fetal adrenal has enhanced expression and activity of endothelial nitric oxide synthase (eNOS) (25, 26) and that nitric oxide has an inhibitory effect on cortisol biosynthesis in the LTH adrenal (26). The nitric oxide pathway may therefore also play a key regulatory role, not at the level of enzyme expression, but at enzyme activity (See Ref. 11 for review). As such, NO may continue to play a role in the LTH fetuses, even after restoration of enzyme and StAR expression, preventing a growth-arresting increase in fetal plasma cortisol as well as an early birth due to the actions of fetal cortisol in this species. Enhanced nitric oxide synthesis may work in concert with leptin, and the decreased expression of CYP11A1 and CYP17 potentially prevents a premature cortisol rise in response to LTH to provide a “brake” on “inappropriate” cortisol production in response the this potent stressor.
Perspectives and Significance
In summary, based on our present and previous findings, we propose that the elevated fetal plasma leptin concentrations observed under conditions of LTH plays a key role in the observed suppression of two limiting steroidogenic enzymes as well as StAR in the fetal adrenal cortex. Despite the restoration of expression of these central players in cortisol synthesis, infusion of the leptin antagonist was without effect on plasma cortisol concentrations. Combined with our previous findings of a role for NO in limiting cortisol production in the LTH adrenal cortex in response to ACTH, we propose that multiple mechanisms (e.g., leptin and NO) have been invoked by the conditions of LTH to assure that the late gestation maturation of cortisol biosynthetic capacity and the normal ontogenic increase in fetal plasma cortisol are preserved by acting via distinct sites: gene expression and steroid synthesis. While cortisol is clearly essential for the late gestation maturation of fetal organ systems in preparation for birth in mammals, premature activation of glucocorticoid production in response to fetal stressors leads to fetal growth restriction, including negative impacts on glucocorticoid-responsive organs. We have found that the LTH fetus invokes multiple mechanisms to prevent an early and potentially deleterious rise in fetal plasma cortisol, with the role of leptin being clearly to suppress expression of key genes mediating cortisol synthesis while other mechanisms (e.g., nitric oxide) likely uncouple the ability of the noted elevated plasma ACTH in the LTH fetus to increase cortisol synthesis. Our finding that antagonism of the leptin receptor in the late gestation fetal sheep did not alter function of the HPA axis (ACTH, expression of key steroidogenic genes and cortisol production) does not detract from the potential role of this major adipokine in regulating the HPA axis earlier in gestation. Importantly, leptin may play a role in moderating the critical maturation of this axis with a release from its inhibitory actions as term gestation approaches as suggested from our earlier communication that the level of expression of the Ob-Rb in the adrenal cortex decreases during the final days of gestation (32).
GRANTS
This study was supported by National Institutes of Health Grants PO1HD-31226 and R01HD-51951
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.A.D., K.F., V.E.V., and D.A.M. conception and design of research; C.A.D., K.F., V.E.V., and K.M.K. performed experiments; C.A.D., K.F., V.E.V., K.M.K., K.S., K.H., and D.A.M. analyzed data; C.A.D., K.F., V.E.V., K.M.K., K.S., K.H., and D.A.M. interpreted results of experiments; C.A.D., K.F., K.M.K., K.S., K.H., and D.A.M. prepared figures; C.A.D. and D.A.M. drafted manuscript; C.A.D., K.M.K., and D.A.M. edited and revised manuscript; C.A.D., K.F., V.E.V., K.M.K., K.S., K.H., and D.A.M. approved final version of manuscript.
REFERENCES
- 1. Adachi K, Umezaki H, Kaushal KM, Ducsay CA. Long-term hypoxia alters ovine fetal endocrine and physiological responses to hypotension. Am J Physiol Regul Integr Comp Physiol 287: R209–R217, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Ahima RS, Flier JS. Leptin. Annu Rev Physiol 62: 413–437, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Banks AS, Davis SM, Bates SH, Myers MG., Jr Activation of downstream signals by the long form of the leptin receptor. J Biol Chem 275: 14563–14572, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Bornstein SR, Uhlmann K, Haidan A, Ehrhart-Bornstein M, Scherbaum WA. Evidence for a novel peripheral action of leptin as a metabolic signal to the adrenal gland: leptin inhibits cortisol release directly. Diabetes 46: 1235–1238, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Braems G. Fetal hypoxemia on a molecular level: adaptive changes in the hypothalamic-pituitary-adrenal (HPA) axis and the lungs. Eur J Obstet Gynecol Reprod Biol, 110 Suppl 1: S63–S9: S63–S69, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Braems GA, Matthews SG, Challis JR. Differential regulation of proopiomelanocortin messenger ribonucleic acid in the pars distalis and pars intermedia of the pituitary gland after prolonged hypoxemia in fetal sheep. Endocrinology 137: 2731–2738, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Challis JR, Brooks AN. Maturation and activation of hypothalamic-pituitary adrenal function in fetal sheep. Endocrinol Rev 10: 182–204, 1989 [DOI] [PubMed] [Google Scholar]
- 8. Ducsay CA, Hyatt K, Mlynarczyk M, Kaushal KM, Myers DA. Long-term hypoxia increases leptin receptors and plasma leptin concentrations in the late-gestation ovine fetus. Am J Physiol Regul Integr Comp Physiol 291: R1406–R1413, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Ducsay CA, Hyatt K, Mlynarczyk M, Root BK, Kaushal KM, Myers DA. Long-term hypoxia modulates expression of key genes regulating adrenomedullary function in the late gestation ovine fetus. Am J Physiol Regul Integr Comp Physiol 293: R1997–R2005, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Ducsay CA, Mlynarczyk M, Kaushal KM, Hyatt K, Hanson K, Myers DA. Long-term hypoxia enhances ACTH response to arginine vasopressin but not corticotropin-releasing hormone in the near-term ovine fetus. Am J Physiol Regul Integr Comp Physiol 297: R892–R899, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ducsay CA, Myers DA. eNOS activation and NO function: differential control of steroidogenesis by nitric oxide and its adaptation with hypoxia. J Endocrinol 210: 259–269, 2011 [DOI] [PubMed] [Google Scholar]
- 12. Gluckman PD. Editorial: nutrition, glucocorticoids, birth size, and adult disease. Endocrinology 142: 1689–1691, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Harvey LM, Gilbert RD, Longo LD, Ducsay CA. Changes in ovine fetal adrenocortical responsiveness after long-term hypoxemia. Am J Physiol Endocrinol Metab 264: E741–E747, 1993 [DOI] [PubMed] [Google Scholar]
- 14. Howe DC, Gertler A, Challis JR. The late gestation increase in circulating ACTH and cortisol in the fetal sheep is suppressed by intracerebroventricular infusion of recombinant ovine leptin. J Endocrinol 174: 259–266, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Imamura T, Umezaki H, Kaushal KM, Ducsay CA. Long-term hypoxia alters endocrine and physiologic responses to umbilical cord occlusion in the ovine fetus. J Soc Gynecol Investig 11: 131–140, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Kamitomo M, Longo LD, Gilbert RD. Right and left ventricular function in fetal sheep exposed to long-term high-altitude hypoxemia. Am J Physiol Heart Circ Physiol 262: H399–H405, 1992 [DOI] [PubMed] [Google Scholar]
- 17. Kruse M, Bornstein SR, Uhlmann K, Paeth G, Scherbaum WA. Leptin down-regulates the steroid producing system in the adrenal. Endocrinol Res 24: 587–590, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Liggins GC. Adrenocortical-related maturational events in the fetus. Am J Obstet Gynecol 126: 931–941, 1976 [DOI] [PubMed] [Google Scholar]
- 19. Liggins GC. The role of cortisol in preparing the fetus for birth. Reprod Fertil Dev 6: 141–150, 1994 [DOI] [PubMed] [Google Scholar]
- 20. Magyar DM, Fridshal D, Elsner CW, Glatz T, Eliot J, Klein AH, Lowe KC, Buster JE, Nathanielsz PW. Time-trend analysis of plasma cortisol concentrations in the fetal sheep in relation to parturition. Endocrinology 107: 155–159, 1980 [DOI] [PubMed] [Google Scholar]
- 21. Magyar DM, Elsner CW, Fridshal D, Eliot J, Klein A, Glatz T, Lowe KC, Nathanielsz PW, Buster JE. Time-trend analysis of plasma 11-desoxycorticosterone, corticosterone, cortisol, and aldosterone in fetal and maternal sheep during the last 18 days of gestation. J Steroid Biochem 14: 1091–1099, 1981 [DOI] [PubMed] [Google Scholar]
- 22. Matthews SG, Challis JR. Regulation of CRH and AVP mRNA in the developing ovine hypothalamus: effects of stress and glucocorticoids. Am J Physiol Endocrinol Metab 268: E1096–E1107, 1995 [DOI] [PubMed] [Google Scholar]
- 23. McDonald TJ, Nathanielsz PW. Bilateral destruction of the fetal paraventricular nuclei prolongs gestation in sheep. Am J Obstet Gynecol 165: 764–770, 1991 [DOI] [PubMed] [Google Scholar]
- 24. Merino B, Diez-Fernandez C, Ruiz-Gayo M, Somoza B. Choroid plexus epithelial cells co-express the long and short form of the leptin receptor. Neurosci Letters 393: 269–272, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Monau TR, Vargas VE, King N, Yellon SM, Myers DA, Ducsay CA. Long-term hypoxia increases endothelial nitric oxide synthase expression in the ovine fetal adrenal. Reprod Sci 16: 865–874, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Monau TR, Vargas VE, Zhang L, Myers DA, Ducsay CA. Nitric oxide inhibits ACTH-induced cortisol production in near-term, long-term hypoxic ovine fetal adrenocortical cells. Reprod Sci 17: 955–962, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Myers DA, Bell PA, Hyatt K, Mlynarczyk M, Ducsay CA. Long-term hypoxia enhances proopiomelanocortin processing in the near-term ovine fetus. Am J Physiol Regul Integr Comp Physiol 288: R1178–R1184, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Myers DA, Hyatt K, Mlynarczyk M, Bird IM, Ducsay CA. Long-term hypoxia represses the expression of key genes regulating cortisol biosynthesis in the near-term ovine fetus. Am J Physiol Regul Integr Comp Physiol 289: R1707–R1714, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Myers DA, Hanson K, Mlynarczyk M, Kaushal KM, Ducsay CA. Long-term hypoxia modulates expression of key genes regulating adipose function in the late-gestation ovine fetus. Am J Physiol Regul Integr Comp Physiol 294: R1312–R1318, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Niv-Spector L, Gonen-Berger D, Gourdou I, Biener E, Gussakovsky EE, Benomar Y, Ramanujan KV, Taouis M, Herman B, Callebaut I, Djiane J, Gertler A. Identification of the hydrophobic strand in the A-B loop of leptin as major binding site III: implications for large-scale preparation of potent recombinant human and ovine leptin antagonists. Biochem J 391: 221–230, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peelman F, Van Beneden K, Zabeau L, Iserentant H, Ulrichts P, Defeau D, Verhee A, Catteeuw D, Elewaut D, Tavernier J. Mapping of the leptin binding sites and design of a leptin antagonist. J Biol Chem 279: 41038–41046, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Poore KR, Young IR, Canny BJ, Thorburn GD. Studies on the role of ACTH in the regulation of adrenal responsiveness and the timing of parturition in the ovine fetus. J Endocrinol 158: 161–171, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Reimsnider SK, Wood CE. Does reduction of circulating prostaglandin E2 reduce fetal hypothalamic-pituitary-adrenal axis activity? J Soc Gynecol Investig 12: e13–e19, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Scott J, Hyatt K, Myers DA. Developmental changes in adrenal leptin receptor expression and adrenocortical response to leptin in the ovine fetus. J Soc Gynecol Investig 12: 239A, 2005 [Google Scholar]
- 35. Seckl JR. Glucocorticoids and small babies. Q J Med 87: 259–262, 1994 [PubMed] [Google Scholar]
- 36. Solomon G, Niv-Spector L, Gonen-Berger D, Callebaut I, Djiane J, Gertler A. Preparation of leptin antagonists by site-directed mutagenesis of human, ovine, rat, and mouse leptin's site III: implications on blocking undesired leptin action in vivo. Ann NY Acad Sci 1091: 531–539, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Su Y, Carey LC, Rose JC, Pulgar VM. Leptin alters adrenal responsiveness by decreasing expression of ACTH-r, StAR and p450c21 in hypoxemic fetal sheep. Reprod Sci 19: 1075–1084, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vargas VE, Kaushal KM, Monau T, Myers DA, Ducsay CA. Long-term hypoxia enhances cortisol biosynthesis in near-term ovine fetal adrenal cortical cells. Reprod Sci 18: 277–285, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yuen BS, Owens PC, Symonds ME, Keisler DH, McFarlane JR, Kauter KG, McMillen IC. Effects of leptin on fetal plasma adrenocorticotropic hormone and cortisol concentrations and the timing of parturition in the sheep. Biol Reprod 70: 1650–1657, 2004 [DOI] [PubMed] [Google Scholar]