Abstract
Light exerts a variety of effects on mammals. Unexpectedly, one of these effects is the cessation of nocturnal locomotion and the induction of behavioral sleep (photosomnolence). Here, we extend the initial observations in several ways, including the fundamental demonstration that core body temperature (Tc) drops substantially (about 1.5°C) in response to the light stimulation at CT15 or CT18 in a manner suggesting that the change is a direct response to light rather than simply a result of the locomotor suppression. The results show that 1) the decline of locomotion and Tc begin soon after nocturnal light stimulation; 2) the variability in the magnitude and onset of light-induced locomotor suppression is very large, whereas the variability in Tc is very small; 3) Tc recovers from the light-induced decline in advance of the recovery of locomotion; 4) under entrained and freerunning conditions, the daily late afternoon Tc increase occurs in advance of the corresponding increase in wheel running; and 5) toward the end of the subjective night, the nocturnally elevated Tc persists longer than does locomotor activity. Finally, EEG measurements confirm light-induced sleep and, when Tc or locomotion was measured, show their temporal association with sleep onset. Both EEG- and immobility-based sleep detection methods confirm rapid induction of light-induced sleep. The similarities between light-induced loss of locomotion and drop in Tc suggest a common cause for parallel responses. The photosomnolence response may be contingent upon both the absence of locomotion and a simultaneous low Tc.
Keywords: sleep, circadian, light, locomotion, masking, temperature, suprachiasmatic, photosomnolence
the daily light-dark photoperiod is the predominant Zeitgeber-controlling circadian rhythmicity. In mammals, photic information detected by retinal receptors and transmitted through the retinohypothalamic tract (RHT) sets the phase of the master circadian clock residing in the densely retinorecipient suprachiasmatic nucleus (SCN) (23). Through this process, the system of circadian oscillator cells in the SCN is thought to be organized as a pacemaker for the entrainment of numerous circadian rhythms to the prevailing photoperiod.
The daily rhythm in locomotion has been the most frequently used index of circadian rhythmicity, in part, because of its recording simplicity (15). The widespread use of locomotion measures, particularly wheel running, has facilitated tests designed to detect the ability of light to modify circadian rhythm phase and to determine which photoreceptors or visual pathways mediate entrainment. In addition, measurement of the locomotor rhythm has enabled rapid assessment of the ability of various pharmacological or behavioral stimuli to block light-induced phase shifts. Other rhythm indices, such as the well-documented core body temperature (Tc) oscillation (37), are more difficult to obtain and have not been as widely employed as alternatives to the locomotor rhythm index.
The relationship between light and the amount of locomotion has been a persistent theme in circadian rhythm studies. Most notably, when nocturnal rodents are exposed to light during their active phase, the level of activity typically shows a substantial decrease (27). This phenomenon has been historically labeled “negative masking,” now more appropriately referred to as “locomotor suppression” (25). The need for a different descriptor arose from observations that mice or hamsters exposed to nocturnal light during their active phase quickly stop running, enter a quiescent phase, and then go to sleep. The label, “locomotor suppression,” accurately reflects the effect of light on one behavior in the light-induced sequence of behavioral change. The behavioral state change, coincident with the decline in locomotion, has been termed “photosomnolence” and lasts about 30 min in mice and 40 min in hamsters (L. P. Morin, unpublished data; Refs. 24 and 25).
In humans, it has long been known that body temperature (Tb) drops shortly before sleep onset (48), although there is debate as to whether this is a prerequisite to sleep or is a consequence of other behaviors associated with bed time (17), which makes this an attractive area to explore in animal models. Sleep in nocturnal rodents typically occurs during the subjective day. At this time, Tc achieves its nadir, from which it rises to maximum during the subjective night (40). The general temporal association between changes in Tc and sleep or locomotion supports the premise that low Tc is either causal or permissive of sleep. Moreover, the fact that many sleep-active neurons in the medial lateral preoptic area are also temperature sensitive provides an anatomic basis for this relationship (1). In the present studies, we examined the Tc in mice in relationship to the daily photoperiod, in constant lighting conditions and in response to photosomnolence-inducing stimuli. These studies reveal that Tc and activity levels are only loosely related, that Tc frequently changes in the absence of wheel running or other forms of activity and that even very brief light stimuli known to simultaneously induce phase shifts and locomotor suppression (25) also induce abrupt, large declines in Tc following the same general pattern seen for locomotion. Video- and EEG-based sleep assessments confirm that brief (millisecond) light stimulation during the circadian rhythm active phase will initiate a change of state culminating in sleep without the continuing presence of light. The observation that the state change is associated with a corresponding large drop in Tc raises the question of whether light is solely responsible for the observed variety of behavioral changes or whether there is a contributory effect of light-induced temperature change.
METHODS
Male C57BL/J6 mice were purchased from the Jackson Laboratory and singly housed in plastic cages with sawdust bedding under an 12:12-h light-dark (LD) photoperiod. Mice weighed 26–28 g and were 3 to 8 mo old during the experiments. Food and water were continuously available at all times. In the experiments proper, the photoperiod was 12:12-h LD, unless described otherwise. All procedures involving the handling and care of laboratory animals were approved by both the Stony Brook University and Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committees, as pertains to the studies performed in each institution.
Locomotion and Temperature Assessment
Locomotion was monitored by one of three methods: 1) For the running-wheel method, each mouse was housed in a 45 L × 20 W × 20 H cm clear polycarbonate cage containing a 16.5-cm diameter stainless-steel running wheel. Each wheel revolution closed a switch monitored by computer using WinCollectXP software written by Glenn Hudson (Stony Brook University). Data were collected in 1-min bins and plotted in standard actogram format or exported to a spreadsheet for further analysis. 2) For video detection, each animal was housed in a standard polycarbonate cage containing sawdust bedding. Two such cages were positioned side by side forming a 45 L × 45 W cm rectangle and together covered with a lid fashioned from nylon mesh (Small Parts, Miami Lakes, FL; part no. B000FN1Q82). Video images were obtained through the lid by an overhead infrared (IR) USB camera (Sabrent M5011-160, TigerDirect.com) focused on the floor of the cages. The cages were additionally illuminated by IR lighting via a Clover IR01020 LED illuminator (TigerDirect.com) with all IR light passing through a diffusion filter to reduce glare. Video image collection was managed by ANY-maze (Stoelting, Wood Dale, IL) software, which simultaneously determined the position of each animal in its cage, yielding a data record of movement per second across the recorded interval. The critical ANY-maze settings were 10 readings per second and a 95% sensitivity setting for determination of “immobility.” A bout of sleep was defined as an interval of at least 40 s, during which the animal was immobile, according to the ANY-maze software. 3) When high-resolution information regarding simultaneously recorded Tc and activity was needed, telemetric transmitters [PhysioTel model TA-F10; Data Sciences International (DSI), St. Paul, MN], weighing 1.6 g with a 1.1 cc volume, were used to collect the data, and ART v4.3 (DSI) software was used for the analyses.
One method used to assess light-induced suppression of locomotion was the “zero minutes” counting procedure (25). This involved counting the number of minutes, beginning with stimulus onset and continuing for 60 min, with zero wheel revolutions. The effects of the different photic stimuli were compared using ANOVA and post hoc tests. Temperature was assessed by a detection device surgically implanted in mice deeply anesthetized with a cocktail of ketamine (100 mg/kg; Butler Supply, Dublin, OH) and xylazine (10 mg/kg, Lloyd Laboratories, Shenandoah, IA). The device free floated intra-abdominally, but remained where placed during surgery. Ten days postsurgical recovery time was allowed prior to data collection. One type of temperature-sensing device consisted of the DSI transmitters, which provided telemetric transmission of both temperature and activity information. Significant advantages of the TA-F10 devices are that they can be turned on and off when desired and that data are collected at a rate of 100 Hz. iButtons (Thermochron model DS1922LF5; Embedded Data Systems, Lawrenceberg, KY) were also used for chronic temperature measurement. These items have programmable start and stop times and dates, plus a programmable interval between data points (1-s minimum) collected. However, they are limited to 4,096 data points with 0.0625°C resolution (7). Each iButton was sterilized with Cidex (ASP, Irvine, CA), then implanted without any waterproofing. All iButtons survived all procedures without leakage. Because iButtons cannot be reprogrammed in situ and mice were not usable for a second surgical procedure, each was euthanized with pentobarbital sodium at the end of each test, and the iButton was removed, cleaned, and sterilized for further use.
The thermal response time for the iButtons and TA-F10 DSI transmitters was measured in the laboratory by placing a device in a 38°C water bath for 5 min, followed by transfer (<1 s) to a second bath measuring 33°C. The latency to register 90% of the new temperature was measured for each device, and each device was tested twice. The measured 90% response time for the iButtons was 56 s and for the telemetry transmitters, 48 s.
The data collection computers were synchronized to Internet time. The iButtons recorded a single temperature at the preprogrammed time. The wheel-running data consist of the number of revolutions accumulated across the stated bin size. DSI temperature measures represent the average temperature within the stated sampling interval. The DSI motion counts were also accumulated across the sampling interval, and the ANY-maze-based distance data represent the distance traveled during the interval.
Lighting
General lighting in the animal quarters was from overhead fluorescent fixtures (Phillips Cool White 32-W tubes), providing 40–50 μW/cm2 within each cage. Specialized light sources included broad-spectrum white light LED arrays inside each of 5 light-tight chambers. Each LED array was positioned above the wheel end of each cage. LED illumination timing and irradiance (100 μW/cm2) within each chamber were controlled by custom software (LightControl written by Glenn Hudson, Stony Brook University). Light “flashes” were delivered as previously described (26). Briefly, a DynaLite Flash Head (model 2040; DynaLite, Union, NJ) powered by a DynaLite M1000er power supply was mounted in an animal colony room and directed at the center of a cage rack, ∼2 m across the room, provided 2-ms broad-spectrum white light stimuli. As previously described, each flash delivered ∼0.36 J/m2 energy/flash, and the standard stimulus consisted of 10 flashes delivered at equal intervals across 5 min. Light “pulses” (5 min or 1 h duration) were provided by a 100-W incandescent reflector bulb (GE type 6E) with an irradiance of about 40 μW/cm2 (78 lux) in each cage. Light levels, measured immediately in front of each cage with a P-9710 photometer (Gigahertz-Optik, Newburyport, MA), did not vary by more than 2% of the value measured in front of the cage most directly in line with the light source. The animal housing room and LED chambers were painted white. Unless stated otherwise, all stimuli in all experiments were delivered beginning at zeitgeber time (ZT)13.
Experimental Procedures
Experiment 1.
Each mouse (n = 8) had an implanted iButton and access to a running wheel under LD 12:12 h. On day 1 of the experiment, the animals were transferred to an enclosure consisting of 5 light-tight shelves, 5 cages per shelf. The timing of initial photoperiod in the enclosure was identical to that outside the enclosure. Animals were exposed to the initial LD 12:12 h for 5 days, followed by 4 days in constant dark (DD), followed by 5 days in constant light (LL). The primary purpose of this study was to obtain normative data under entrained and freerunning conditions, allowing evaluation of the temporal changes in Tc relative to the photoperiod and changes in wheel running. Each iButton sampled Tc every 5 min, and wheel revolutions were collected in 5-min bins.
The temporal changes in Tc and wheel running were evaluated during entrained and free-running conditions from the patterns of Tc and wheel running, which were plotted midnight to midnight at a resolution of one data point every 5 min using SigmaPlot 11 (Systat Software, San Jose, CA). Plots of the two variables were placed on the same graph for each individual mouse for two consecutive days in LD and in DD beginning the second day of exposure to each light condition. Then, a horizontal reference line was placed on each graph at a point on the y axis corresponding to 20% of the maximum wheel revolutions/5 min observed over the 2 days. Tc was plotted relative to the minimum observed over the 2 days. Then, in SigmaPlot, the time of the daily evening increase in wheel running was noted, along with the time of the associated rise in Tc. In SigmaPlot, the graph for each animal was enlarged about 540% to encompass the two consecutive daily activity onsets. The tip of the computer monitor cursor arrow was placed on the SigmaPlot graph at the point at which the activity level line intersected the 20% line. SigmaPlot then revealed the corresponding x coordinate of that point. This procedure was repeated for the point at which the line describing the rising evening Tc intersected with the 20% line. The x coordinate information returned by SigmaPlot was converted to minutes, and the phase difference between the time the animal achieved 20% of the maximum wheel revolution per 5 min, and the Tc rise time was calculated. The same process was also applied to the raw data before it was converted to percentages or plotted relative to the lowest temperature, but with phase measurements made at the point that the daily wheel revolutions reached 50 per 5 min.
Raster plots of the iButton data were based on the methods of Paul and Schwartz (31) and were constructed by 1) obtaining the mean Tc for the interval to be plotted (15 days), 2) then subtracting the mean from each recorded temperature and multiplying the result × 100 (a scaling factor), 3) followed by generating the plots with Actiview v 1.3 software (Mini Mitter, Bend, OR). The plots are analogous to wheel-running raster plots but display only temperatures above the mean temperature of the plotted interval.
Experiment 2.
Mice (n = 12) bearing iButtons were entrained to LD 12:12 h in the LED lighting chambers. Data were collected at 5-min intervals. On the test day, the lights went off at the normally scheduled time. Half of the animals were then exposed to a 5-min 100 μW/cm2 pulse sequentially at times circadian time (CT)2100, CT0300, CT0900, and CT1500, otherwise remaining in the dark. The remainder of the animals was exposed to the same light pulse sequentially at the times CT2400, CT0600, CT1200, and CT1800. Data were plotted as the maximal Tc drop relative to the last Tc measured prior to light onset. This experiment was designed to determine whether the effect of light on change in Tc varies, according to circadian time.
Experiment 3.
Mice (n = 9–10/group) bearing iButtons and entrained to LD 12:12 with wheel access were exposed to each of four light stimulus conditions. The conditions were no light control, 10 flashes, 5-min pulse, and the 1-h pulse. Data were collected for 90 min beginning at ZT1230, and the light stimulus began at ZT13. The data from this experiment describe the basic effect of brief or long light stimuli on Tc and wheel running. Temperature data were collected at 2-min intervals.
Experiment 4.
Mice (n = 7) were implanted with DSI telemetry transmitters and placed in standard test cages without a wheel. At the start of a test session, the transmitter was turned on and remained on until the end of the session. Animals were exposed to a 5-min light pulse beginning at ZT13. Activity and Tc data were collected from ZT1230 to ZT1400. Because the activity scores provided by the transmitters are essentially unitless (counts of detected accelerations), the animals were simultaneously evaluated in an apparatus allowing video recording of an activity. The video recordings were then analyzed with ANY-maze software, which converted distance in video pixels to distance in meters. This procedure enabled an estimate of distance traveled by mice for which there was only telemetry-based distance data. The video recording was preserved and evaluated for recording errors (26); then the video record of behavior was compared with the estimates of activity generated by the ANY-maze software and by the DSI transmitters.
Additional mice (n = 6) were implanted with DSI telemetry transmitters and placed in standard running-wheel cages. These animals were entrained to LD 12:12 h, with data collected at 1-min intervals from ZT11–ZT13 on 1 day. The Tc, activity (DSI), and wheel-running levels were evaluated with respect to the transition from light to dark.
The studies in experiment 4 concern the temporal relationship between Tc and locomotor activity. In particular, they focus on three portions of the light-induced response pattern for Tc and locomotion: the transition from late daytime to early nighttime as Tc rises, as Tc and activity fall in response to light, and as Tc and activity recover at the end of the light-induced suppression interval.
Experiment 5.
Mice were deeply anesthetized in preparation for surgical implantation of EEG and EMG electrodes. Two methods were used with four mice each implanted with a biotelemetry transmitter (model TL11M-F20EET; DSI) for chronic recording of sleep, wake, and temperature/activity, and four mice each implanted with EEG electrodes attached to a model 8202-SL 3-channel preamplifier (Pinnacle Technology, Lawrence, KS). In each case, a device was attached to a cleaned skull using a combination of screws and dental acrylic. Data from the latter group were collected through a tether cable from mice having full access to a running wheel constructed as a 6.5-cm-long section cut from a geometric cone consisting of a 9.5-cm-diameter plastic disk at the narrow end and a 16.5-cm-diameter plastic ring at the other. The two pieces were joined by spokes of 1.5-mm-diameter stainless-steel rod ∼10 mm apart at the wide end. The cone section was positioned on its side, such that seven spokes were always horizontal, and the wide opening was on a slant of 45°, enabling the mice to run freely in the wheel without tether entanglement and minimal friction. The EEG leads from the preamplifier were attached to a counterbalanced arm (model MCLA, Instech Laboratories, Plymouth Meeting, PA); use of a commutator proved unnecessary.
In preparation for the experiment, each mouse was placed in the specialized recording cage, and the wire recording leads were connected to the socket attached to the skull. Tests were conducted after at least 7 days adaptation to the cage. Wheel revolution data were continuously collected. Beginning at ∼ZT10 on each test day, EEG/EMG data collection began. Each animal was exposed to a sequence of a no light control condition, a condition of 10 flashes, 2 ms each, equally distributed over 5 min, a 5-min light exposure (50 μW/cm2) condition, and a 1-h light exposure (50 μW/cm2). Groups of mice were randomly assigned to progress through the light treatments in different orders (i.e., not all mice started with the 10 flashes, some started with 5 min, others with 1 h, etc.) to ensure that previous light exposures did not influence subsequent responses. Light stimulus onset occurred at ZT13, and there were at least 3 days between tests.
The EEG/EMG data were collected using either PAL8200 (Pinnacle acquisition software) or Dataquest A.R.T. (DSI acquisition software), and analyzed using Sleep Sign v3.0 analysis software (Kissei Comtec, Nagano, Japan). For staging, non-rapid eye movement (NREM) sleep was identified by a preponderance of high-amplitude, low-frequency (∼1–4 Hz) EEG activity and relatively low and unchanging EMG activity, whereas wakefulness was characterized by a preponderance of low-amplitude, fast EEG activity and highly variable muscle tone on EMG. The primary goals of this experiment were 1) to determine the timing, duration and pattern of EEG-determined sleep in relation to the photic stimuli, 2) to determine the relationship between EEG-determined sleep and the post-stimulus changes in locomotion, and 3) to obtain novel data regarding the timing and pattern of Tc change in relationship to EEG-determined sleep.
RESULTS
Experiment 1. Relationship Between Wheel-Running Activity and Tc Under Entrained and Free-Running Conditions
Both the Tc and running rhythms entrained to the initial 5-day-long LD 12:12-h photoperiod (Fig. 1, A and B). Subsequently, during 4 days of DD, the animals showed free-running Tc rhythms with an average period of 23.46 ± 0.11 h. Upon transfer to 5 days of LL, five of the eight mice showed clear free-running rhythms (τ = 25.31 ± 0.19 h). The remaining animals showed varying degrees of noncircadian rhythmicity, which did not devolve into a stable circadian waveform within the experimental time frame. The patterns of Tc were generally mimicked by the patterns of wheel running (Figs. 1 and 2), where clear similarity in the timing of the activity and elevated Tc can be seen. The correlations between Tc and wheel revolution data (Table 1), determined from 48 consecutive hours of data (576 data points) from the LD (days 3 and 4), DD (days 2 and 3), and LL (days 2 and 3) conditions are statistically significant for all but three data sets (animals T801, T802, T807 in the LL condition), but, on average, only account for about 23.8, 18.9, and 0.06% of the variance in LD, DD, and LL, respectively. Thus, as one would expect, Tc generally correlates with the amount of wheel running, but most of the daily variability in Tc is accounted for by factors other than simply the number of wheel revolutions. The nature of these other factors remains to be determined.
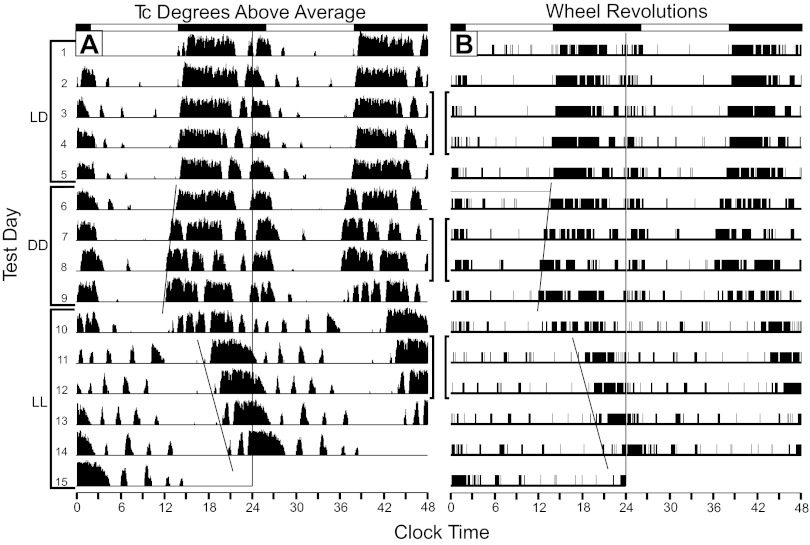
Fig. 1.
Raster plots showing 15 consecutive test days of simultaneously collected Tc (A) and wheel-running (B) data from a mouse exposed to 12:12-h light-dark cycle, constant darkness (DD), then constant light (LL). The days corresponding to each lighting condition are indicated by the left side brackets. The Tc data were collected every 5 min via an iButton. The ordinate for each day's Tc data indicates the extent to which Tc exceeded the average Tc of the entire data collection interval. The two data types were collected and plotted using different analysis/plotting software producing an analog plot for the Tc data and an all-or-none digital plot for the wheel-running data. The identical eye-fitted lines through the daily onsets of rising Tc and wheel-running illustrate equality of the circadian periods of the two rhythms during DD and LL. Brackets between the two plots indicate the days used for further analysis of Tc and wheel-running (see text and Fig. 2).
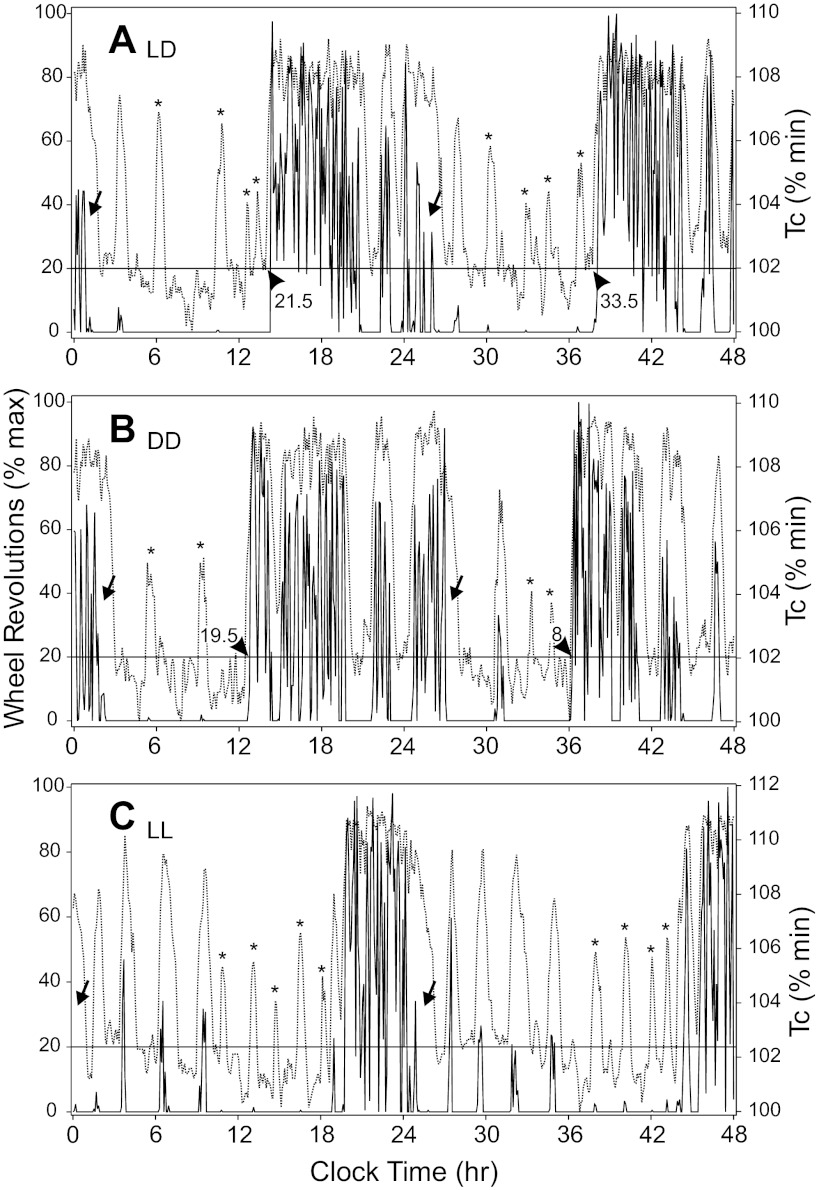
Fig. 2.
Two-day Tc and wheel-running data plots during LD (A), DD (B), and LL (C) for the animal whose raster records of the same measures (bracketed 2-day intervals) are shown in Fig. 1. Arrowheads indicate where Tc (broken lines) rise in advance of the daily nocturnal active phase, as indicated by wheel-running (solid lines). Numbers adjacent to the arrowheads indicate the phase difference, in minutes, between the temperature and wheel-running plots, as measured at the horizontal black line indicating the 20% maximal wheel-running rate (see text). The relatively earlier rise in Tc occurred in all animals under all test conditions. Arrows indicate intervals at the end of the active phase during which Tc remains high, while wheel-running is low. Asterisks identify Tc spikes with very little or no associated wheel-running.
Table 1.
Pearson correlation coefficients (r) indicating the strength of the relationship between the number of wheel revolutions per 5-min bin and Tc during the same bin
| Animal | LD | DD | LL |
|---|---|---|---|
| T800 | 0.585 | 0.663 | 0.582 |
| T801 | 0.322 | 0.267 | 0.062 |
| T802 | 0.584 | 0.401 | 0.006 |
| T803 | 0.476 | 0.288 | 0.131 |
| T804 | 0.631 | 0.646 | 0.488 |
| T805 | 0.393 | 0.307 | 0.111 |
| T806 | 0.696 | 0.647 | 0.679 |
| T807 | 0.217 | 0.259 | 0.085 |
| Mean ± SE | 0.488 ± 0.17a,b | 0.435 ± 0.15c | 0.251 ± 0.09 |
The lighting condition did not significantly affect the correlations. Tc, core body temperature.
P = 0.167 LD vs. DD;
P = 0.007 LD vs. LL;
P = 0.012 DD vs. LL.
Additionally, the data in Table 1 emphasize the fact that the relationship between Tc and wheel running of individuals tends to be maintained across lighting conditions. In Table 1, the LD-DD correlation between Tc and wheel revolutions is 0.850 (P = 0.008), the LD-LL correlation is 0.728 (P = 0.041), and the DD-LL and 0.922 (P = 0.001). In other words, if there is a high correlation between wheel revolutions per 5-min interval and the simultaneous Tc for an animal under LD, the correlation between those two variables tends to remain high for that animal under all lighting conditions.
Of special note is the emergence of an ultradian structure to acute increases in Tc by mice in LL (Figs. 1A and 2C). In fact, 8/8 mice show similar patterns of wheel running, which typically begin very soon after initiation of LL. As is evident (Fig. 2, A–C), these bouts of running tend to be present in both LD and DD but become accentuated during LL. In all instances of such brief wheel-running bouts, each is associated with a large, acute rise in Tc. However, instances in which there is a large, brief increase in Tc without an associated wheel-running bout are common (Fig. 2A). This Tc increase is virtually always initiated in advance of the wheel running and persists beyond the end of the bout. As is also evident, there is little relationship between the amount of wheel running and the size of the acute increase in Tc. When wheel running ceases or is greatly diminished during the late active phase, Tc remains elevated.
Under LD, mice ran 5,842 ± 843 revolutions/24 h. This significantly increased to 10,122 ± 1,572 (P = 0.008) after transfer to DD. Wheel running was subsequently attenuated during LL (4,198 ± 938 revolutions; 58% reduction from DD to LL, P < 0.002). There was no significant difference between the LD and LL wheel revolutions (P = 0.063). There were large-response differences between animals, and one mouse actually increased revolutions during LL compared with its LD performance.
With respect to Tc, there was no change in the overall mean 24 h Tc during DD vs. LD (36.96 ± 0.04 vs. 37.00 ± 0.05°C, P = 0.546). However, subsequent exposure to LL significantly decreased the mean 24-h temperature compared with LD and DD (36.57 ± 0.03; P < 0.001 for each comparison), further indicating a link between light and Tc response. Nevertheless, despite the highly significant effects of lighting condition on elevated or reduced Tc, higher mean Tc in DD only differed from the lower mean Tc in LL by 0.43°C.
The locomotor records revealed entrained and free-running rhythms under LD and DD conditions, respectively. In LL, the pattern was much less stable, with two mice showing a more or less arrhythmic pattern (T800 and T806) and the remainder showing convergence of two running-rhythm components. One component appeared to originate from the late activity phase with a period >24 h and the other from the early activity phase with a period of <24 h. The inactive and active phases of the LD and DD conditions could be analyzed separately, but the “noisiness” of the LL data precluded that possibility.
Under LD conditions, animals ran an average 245 ± 87 revolutions per 12 h light phase and 5,596 ± 840 during the dark. In DD, the revolutions during the subjective day were about 7.5 times higher (1,826 ± 297 revolutions) than in the light during LD. During DD subjective night, mice ran 2,701 revolutions more than during the 12-h dark phase, a 48% increase to 8,297 ± 1,397 revolutions. Under LD, 4.2% of daily wheel revolutions occurred during the light, while under DD, 18.0% occurred during the 12-h subjective day.
By way of comparison, despite the increased locomotion during both the subjective day and the subjective night under DD, mean Tc was not significantly greater during either of those intervals than during the corresponding 12-h intervals of light and dark under the LD condition (light Tc = 36.18 ± 0.06°C vs. subjective day, Tc = 36.33 ± 0.08°C; dark Tc = 37.70 ± 0.06°C vs. subjective night, Tc = 37.61 ± 0.09°C; t = 0.10 and 0.36, respectively). This again suggests that the reduced Tc observed during LL is not strictly coupled to reduced locomotor activity, but rather coupled to increased light exposure.
Calculation of the relative timing of the daily, late-afternoon Tc rise, and the similar rise in wheel running using the two procedures (20% line transect and time at which 50 wheel revolutions were achieved; see methods) produced similar results (Fig. 2). Each method revealed that Tc rose well in advance of the rise in wheel revolutions, averaging 41.9 ± 11.7 min in LD and 24.9 ± 11.7 min in DD (this difference is not statistically significant, t-test). This result provides additional support for the view that change in the amount of locomotion does not necessarily dictate change in Tc.
Experiment 2. Light-Induced Decrease in Tc Across the Circadian Day
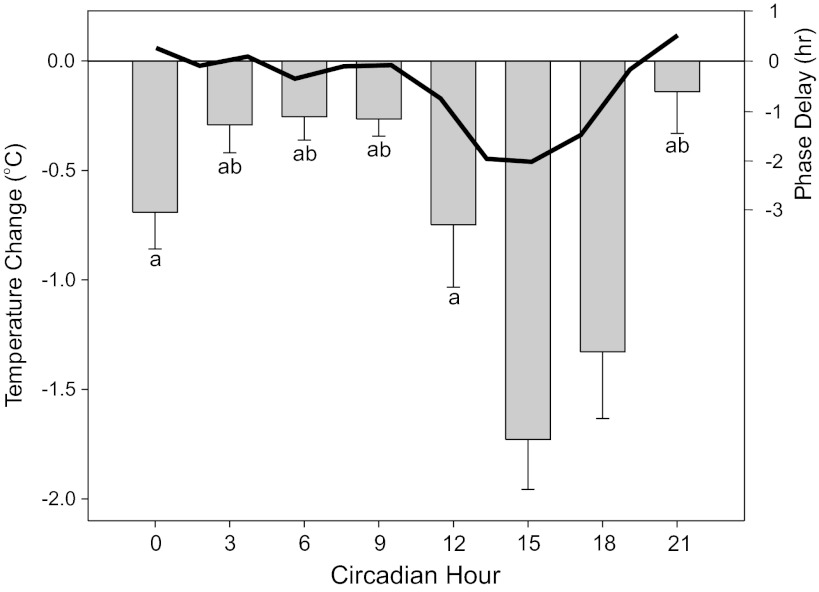
Tests of Tc response magnitude to a 5-min, 100 μW/cm2 light pulse showed substantial variation, according to circadian time (Fig. 3). At no time did Tc increase. During the subjective day, light had little or no effect on Tc but was maximal (about 1.7°C) between CT15 and CT18.
Fig. 3.
Circadian pattern of Tc change in response to 5-min light pulses. Change was calculated relative to Tc during the last measurement before light onset. The largest decreases induced by light occurred at CT15 and CT18. Letter identifiers indicate significant differences (P < 0.05) between groups: a indicates significant difference vs. CT15, while b indicates significant difference vs. CT18. The thick black line shows the expected light-type phase response curve for the same mouse strain (after Ref. 32).
The individual pattern of Tc shows that the light pulse at CT12 was administered at a time that Tc was increasing slightly in advance of the daily activity increase. The typical response was a modest (−0.75°C) drop in Tc from the level already attained. This returned Tc to the range of the daily minimum, but not below, suggesting that light cannot reduce Tc below the normal daily minimum.
Experiment 3. Tc and Locomotor Responses to Light Stimuli During the Nocturnal Activity Phase
The patterns of wheel running and Tc were obtained and plotted at 2-min intervals. Wheel running tended to diminish to zero soon after the light stimulus and remained at zero for about 40 min for the 10 flash and 5-min pulse groups (Fig. 4; Table 2). Analysis of the “zero minutes” counted after stimulus onset for each animal showed that the unstimulated control animals averaged 15.1 ± 1.9 min with zero wheel revolutions. There was a major effect of stimulus (F = 39.8, df = 3, P < 0.001), with the results from the photic conditions differing significantly from the control condition (P < 0.004, Holm-Sidak post hoc tests; Table 2). The 1-h pulse yielded significantly larger zero minute counts than the 10 flash and 5-min pulse stimuli (P < 0.004), but the latter two results did not differ. Note that the poststimulus onset test interval was 60 min, and a result greater than 59 min for any test was assigned the value of 60 min.
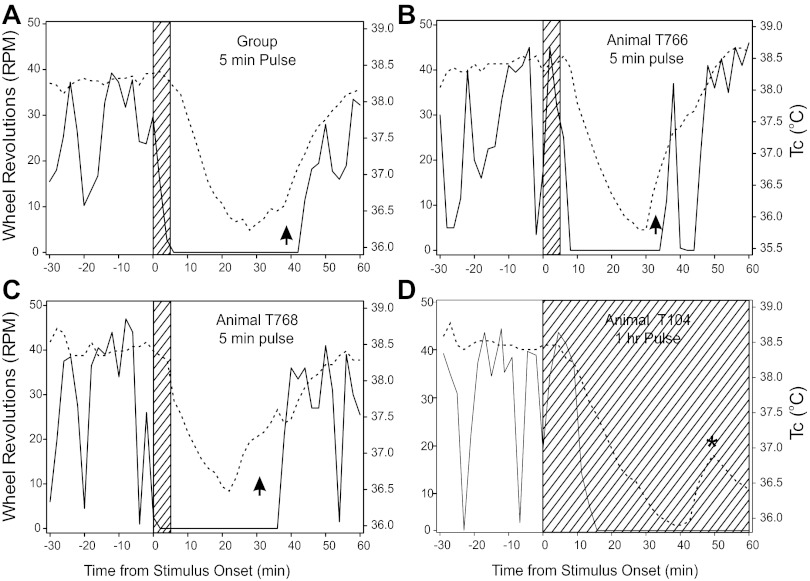
Fig. 4.
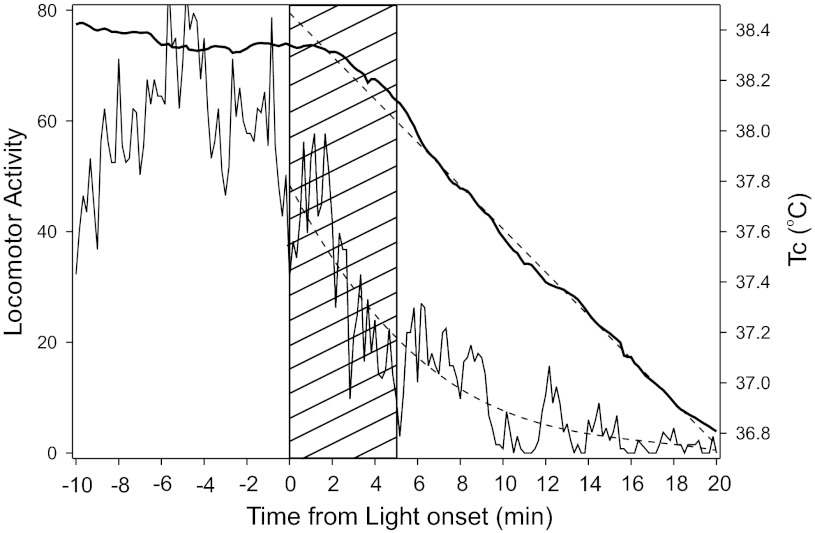
Effect of light stimuli (hatched areas) administered during the early active phase on simultaneous mean Tc (broken lines) and median wheel-running (solid lines). The Tc data were collected at 2-min intervals, with wheel-running data binned to match. A: group data for the 5-min pulse animals showing the time course of Tc change and wheel running. The arrow indicates the interval during which Tc is recovering toward normal in advance of the recovery of normal wheel-running. B, C: examples from two animals, each receiving a 5-min light pulse at time 0. B: wheel-running was rising and continued to rise when the pulse occurred, and its fall was followed by a decline in Tc. C: running was low and falling at stimulus onset, but Tc remained high for several minutes thereafter. The arrows in B and C designate areas during which Tc is rising rapidly without an associated increase in wheel-running. This feature was equally evident in the 10 Flash data (not shown). D: plot illustrating the ability of a long light stimulus (1-h pulse) to prolong the suppression of wheel running and simultaneously prolong the reduction in Tc. The asterisk (*) in D highlights the increase in Tc commonly seen during the longer locomotor suppression interval in mice exposed to the 1-h pulse (see text).
Table 2.
Effect of light stimuli on Tc and wheel-running responses
| Response Latency | Time to Minimum | Turning Point | Recovery Time | Duration** (zero minutes) | |
|---|---|---|---|---|---|
| 10 Flash Tc | 9.3 ± 2.0a | 30.2 ± 1.9a | 32.7 ± 2.4a | 41.4 ± 2.7 | NA |
| 10 Flash WR | 2.33 ± 1.4 | 4.7 ± 1.5 | 42.7 ± 3.2 | 44.6 ± 3.5 | 41.2 ± 2.7e |
| 5-min Pulse Tc | 4.6 ± 0.6b | 33.8 ± 3.4b | 37.4 ± 3.5b | 42.9 ± 3.1 | NA |
| 5-min Pulse WR | 1.6 ± 0.6 | 3.0 ± 1.1 | 43.5 ± 4.1 | 43.9 ± 3.9 | 40.6 ± 3.5e |
| 1-h Pulse Tc | 6.4 ± 2.1c | 35.0 ± 1.7c | 39.1 ± 2.3c | 51.9 ± 3.0d | NA |
| 1-h Pulse WR | 2.8 ± 2.4 | 4.0 ± 2.3 | 58.2 ± 1.8 | 59.2 ± 0.8 | 55.7 ± 2.6 |
Response latency (first minute the measure declines from the last Tc obtained prior to the stimulus); time to minimum (minute at which the smallest response occurs); turning point (first minute with a response greater than the minimum; max = 60); recovery time (minute at which the response achieves half-maximal recovery; max = 60); duration (number of minutes in which zero wheel revolutions occurred; max = 60). a,b,cP < 0.01 vs. corresponding wheel running (WR) result. dP = 0.063 vs. corresponding WR result. eP < 0.05 vs. 1-h pulse result.
Mean duration for unstimulated controls was 15.1 ± 1.9 min.
Tc did not show the same pattern as the wheel running in that it did not decline to its minimum and remain there. Rather, the decline was a fairly steady decrease, followed by a few minutes near the minimum, and then a steady rise back to normal. These characteristics are evident in the group plot (Fig. 4A) for mean Tc (as well as for individuals; Fig. 4, B–D) with Tc declining steadily from 38.32°C during the 10 min prior to the 5-min pulse onset to a minimum poststimulus Tc of 36.08 ± 0.12°C; the remaining groups fell to 36.34 ± 0.21 (10 flashes), 36.12 ± 0.19 (1-h pulse), and 37.79°C ± 0.10 (CON). The CON response was significantly smaller than the other groups, which did not differ from each other.
There was no significant effect of stimulus type on the latency of wheel running to show a decline (Table 2), but there was for Tc (P < 0.025; treatment effect) with the 10 flash group differing from the 1-h pulse group (P < .05). Other significant stimulus effects on Tc included the half-maximal recovery latency (P = 0.032; treatment effect) in which the 1-h latency differed significantly from the 10 flash recovery (P < 0.05). For wheel running, the turning point measure differed significantly across groups (P < 0.001) with the 1-h pulse group differing from the other two groups (P < 0.05 each). The half-maximum recovery latency also differed across groups (P < 0.001), with the 1-h pulse group again differing from the other two groups (P < 0.05 each).
The details of individuals (Fig. 4, B–D), especially during the Tc recovery phase, are variable. Note that animal T766 (Fig. 4B) had a large wheel-running bout after the beginning of Tc recovery, with this bout having little, if any, influence on Tc. Animal T768 (Fig. 4C) had a response pattern similar to the whole group. Most notably, in 100% of animals tested across all stimulus conditions, the point at which Tc turns from its minimum and begins recovery back toward the normal range is significantly earlier than the equivalent locomotor measure (Fig. 4, A–C). Tc began to rise an average 9.25 ± 2.68, 6.13 ± 1.63, and 19.10 ± 2.53 min earlier for the 10 flash, 5 min, and 1-h stimuli, respectively (treatment effect; P = 0.001). The 10 flash and 5-min groups did not differ on this measure, but the 1-h group differed from the others (P < 0.05 each). The locomotion turning point of animal T104 in the 1-h group (asterisk in Fig. 4D) was not attained by the end of the test. The same result occurred in 9/10 mice, and their results were assigned the maximum value of 60 min. Note also that Tc commonly (9/10 animals) showed an incomplete return toward the normal nocturnal level late in the test period during the 1-h stimulus (e.g., Fig. 4D). As indicated by the zero counts and previous results (24), the light-induced wheel-running suppression was longer with a 1-h light exposure than with the 5-min pulse or 10 Flashes. Nevertheless, in every animal, Tc began to rise well in advance of wheel-running resumption, which was greatly delayed in the 1-h pulse group. Typically, the Tc rise achieved a peak late in the 1-h test interval (48.1 ± 2.4 min), then fell back by a small amount (Fig. 4D) before rising again, resuming the typical nocturnal Tc level beyond the 1-h test interval after stimulus onset (not shown).
Experiment 4. Relative Timing of Changes in Tc and Activity
A 5-min light pulse at ZT13 caused the expected drop in locomotor activity and Tc. In this experiment, the data were collected with a 10-s sampling interval to facilitate visualization of the rate of change, while eliminating noise in the temperature records. Onset time for the drop in Tc was determined by eye-fitting a line describing the rate of change, with the line passing through the highest temperature recorded within 5 min of the light stimulus onset. This point, designated as the time to drop, averaged 69 ± 17 s after stimulus onset based on the measures obtained from the individual mice. A variant of this measure was estimated from lines best fitting the group data (Fig. 5), with the latency for Tc change closer to 2 min (cf., response latency, Table 2). Thereafter, Tc declined fairly steadily for ∼18 min at which point it leveled out. The onset time for the decline in locomotion is much more difficult to specify because of the large, abrupt changes in activity level. Qualitatively, about half of the mice contributing to the curve had at least one large amplitude activity bout subsequent to the start of their own Tc decline. These bouts produced a level of activity within the range that occurred prior to the stimulus onset.
Fig. 5.
DSI sensor-determined locomotor activity and change in Tc for a group of mice responding to a 5-min light pulse (hatched area). Thick black line denotes Tc, while thinner black line denotes locomotor activity. Dashed line through the Tc data is a first-order regression line; the dashed line through the locomotor data is a third-order regression line.
At the time of stimulus onset, Tc averaged 38.13°C. The first immobility bout was initiated 6.1 ± 1.7 min later, by which time Tc had dropped an average 0.64°C. There was a significant negative correlation between the change in Tc from stimulus onset and the time to onset of the first immobility bout (r = 0.763; P < 0.05, 2 tails). This is consistent with the fact that temperature begins falling soon after stimulus onset and the longer the delay to immobility, the more Tc will have fallen. Minimum Tc was achieved ∼20.6 ± 1.95 min after the stimulus onset.
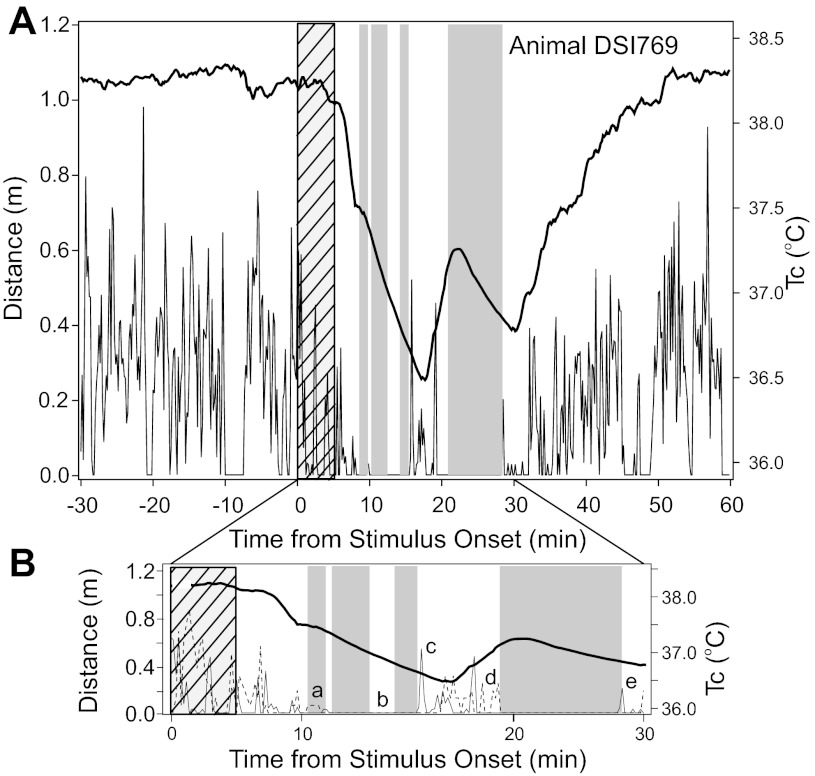
The record of a typical mouse (Fig. 6A) shows the association between the stimulus, change in Tc, bouts of immobility, and distance moved per minute of the test interval relative to stimulus onset. Comparison of the activity data obtained with ANY-maze software and DSI transmitters revealed a number of small discrepancies (Fig. 6B). Neither method perfectly represents mouse behavior subsequent to light stimulation. It may be that a combination of the measurements provides the most accurate index of activity or physical motion that is associated with sleep prevention or wakening. In Fig. 6B, for example, the long immobility bout bracketed by letters d and e demonstrates that the DSI transmitter provided information constraining the start of the bout (letter d), whereas the termination of the bout was apparently more closely associated with an increase in “distance traveled” (letter e) detected by ANY-maze software. In still other instances, none of the measures offer a reason why a bout of immobility has been terminated (letter b in Fig. 6B), and there are occasions during which some form of motion has been detected (letter a), but it is insufficient to terminate or prevent a bout of immobility.
Fig. 6.
A: representative record from mouse DSI769 showing Tc (thick black line), meters traveled (determined by ANY-maze; thin black line), and the ANY-maze immobility sleep index (gray-shaded areas) per minute of the test. The hatched area indicates the interval during which the light pulse was present. B: enlargement of 30 min from the record in A beginning with onset of the 5-min light pulse. The upper, thick black line indicates Tc; the lower, thin black line shows distance traveled (ANY-maze), and the lower, broken line indicates the motion index obtained with the DSI motion sensor. Given the lack of specificity regarding the motion index, there is no ordinate for this measure. B: lower case letters (a–e) identify inconsistencies between the DSI and ANY-maze-determined activity. Notations about the mouse behavior determined from the video are described in parentheses: a, the DSI sensor detects motion without ANY-maze-determined distance traveled, but immobility is not interrupted (mouse is curled up, but twitching, and its body is shifting quickly with total position change <3 cm); b, there is no immobility, but no activity is detected by either method (the mouse is not moving); c, ANY-maze-detected distance traveled occurs without DSI-based motion detection and is coincident with the end of an immobility bout (mouse awakens, moves quickly to a food pellet 15 cm distant and eats); d, the DSI sensor detects motion, but ANY-maze does not detect any distance traveled prior to the start of an immobility bout (mouse moves quickly back to its original resting corner, grooms, settles, and then there is no movement); and e, ANY-maze-detected distance traveled without DSI-based motion detection occurs coincident with the end of an immobility bout (mouse awakens and moves at a normal pace).
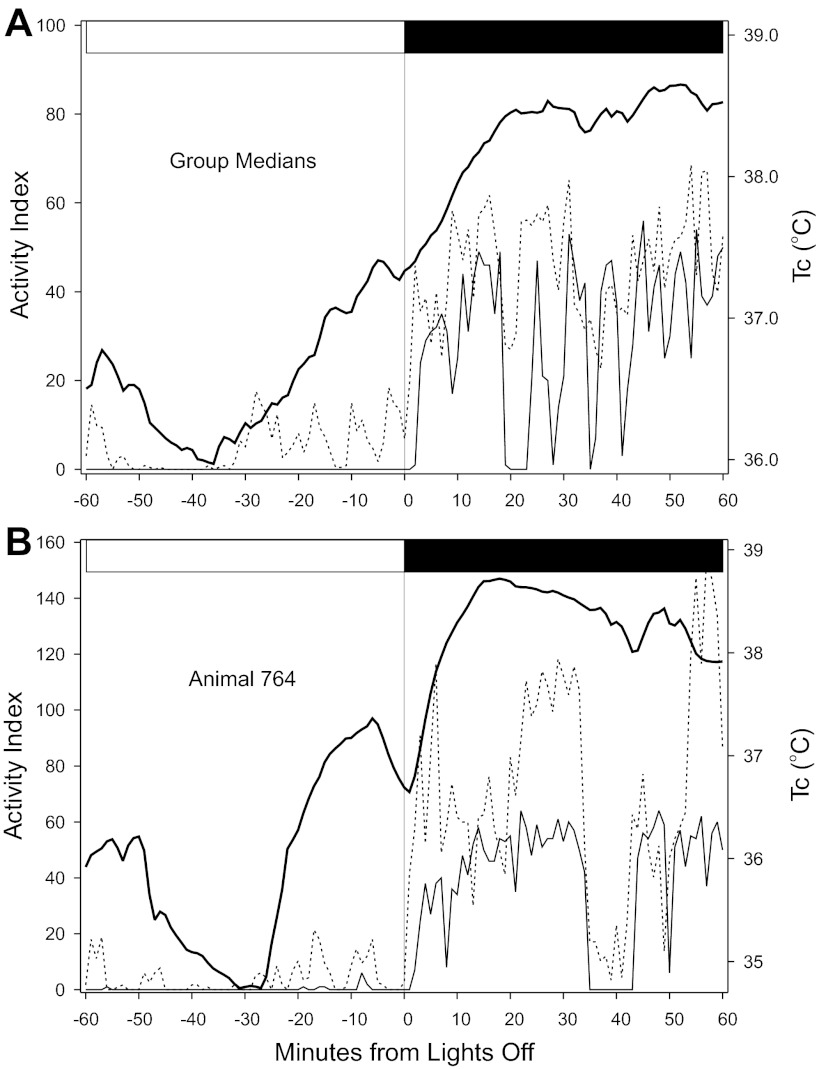
Analysis of the concomitant changes in wheel running, DSI-detected motion, and Tc of well-entrained mice confirms that Tc becomes elevated in advance of the day-night (light-to-dark) transition while wheel running remains low (Fig. 7, A and B). However, the increase in Tc is always associated by an increase in detected motion.
Fig. 7.
Temporal organization of Tc, DSI-detected motion and wheel running during the daily transition from light to dark. A: group median plots. B: representative individual, animal 764. Thick continuous line denotes Tc; broken line denotes detected motion; and thin continuous line denotes wheel-running. The abscissa label, “Activity Index,” indicates the count of wheel revolutions per minute and also indicates the count of accelerations (motions) detected by the DSI transmitter. The horizontal white/black bar at the top of each figure indicates when the light-to-dark transition occurred. The record of animal 764 in B shows an instance in which Tc rose well before lights off and was associated with elevated activity, as indicated by detected motion. This animal also had very low levels of wheel-running at approximately the same time.
On the average, two units of DSI-detected motion correspond to 1 cm of distance traveled, as calculated from the simultaneously determined distance estimate from ANY-maze. In contrast, an animal in a wheel of the size used in the present studies moves ∼52 cm per revolution. It should be noted that the ANY-maze method of calculating locomotor distance is not fully accurate, either. The software must detect a location change greater than one quarter of the animal's length before it documents movement (26).
Experiment 5. Relationship Between Changes in Tc, Locomotor Activity, and Sleep Following Light Stimulation
All mice had at least two variables measured. One was the EEG sleep index, and the other was either a Tc measure (Fig. 8A) or a locomotion index (wheel running; Fig. 8B). In general, all mice showed light-induced sleep. Subsequent to the ZT13 light exposure, locomotor activity was suppressed and the EEG transformed from a low-amplitude, high-frequency state to a higher-amplitude, lower-frequency state, indicative of NREM sleep. NREM activity appeared with an average 14.88 ± 3.59, 22.89 ± 14.04, and 8.95 ± 1.7 min latency after light onset by the 10 flash, 5-min pulse, and 1-h pulse treatments, respectively. In contrast, mice held under normal dark conditions rarely sleep at this time, and sleep latency for these animals was 109.78 ± 40.45 min (P = 0.07 vs. 5 min, P = 0.04 vs. 1 h, P = 0.05 vs. 10 flashes). One mouse slept early in the recording period during the control (no light) condition, and one mouse did not sleep following the 5-min pulse. As a result, the statistical tests are of marginal significance. The EEG-determined state change occurs in parallel with the light-induced decrease in wheel running (Fig. 8B). Response to the 1-h pulse was significantly longer than to the other stimuli. The no-light control condition did not elicit locomotor suppression or EEG-determined sleep within the 1-h poststimulus test interval.
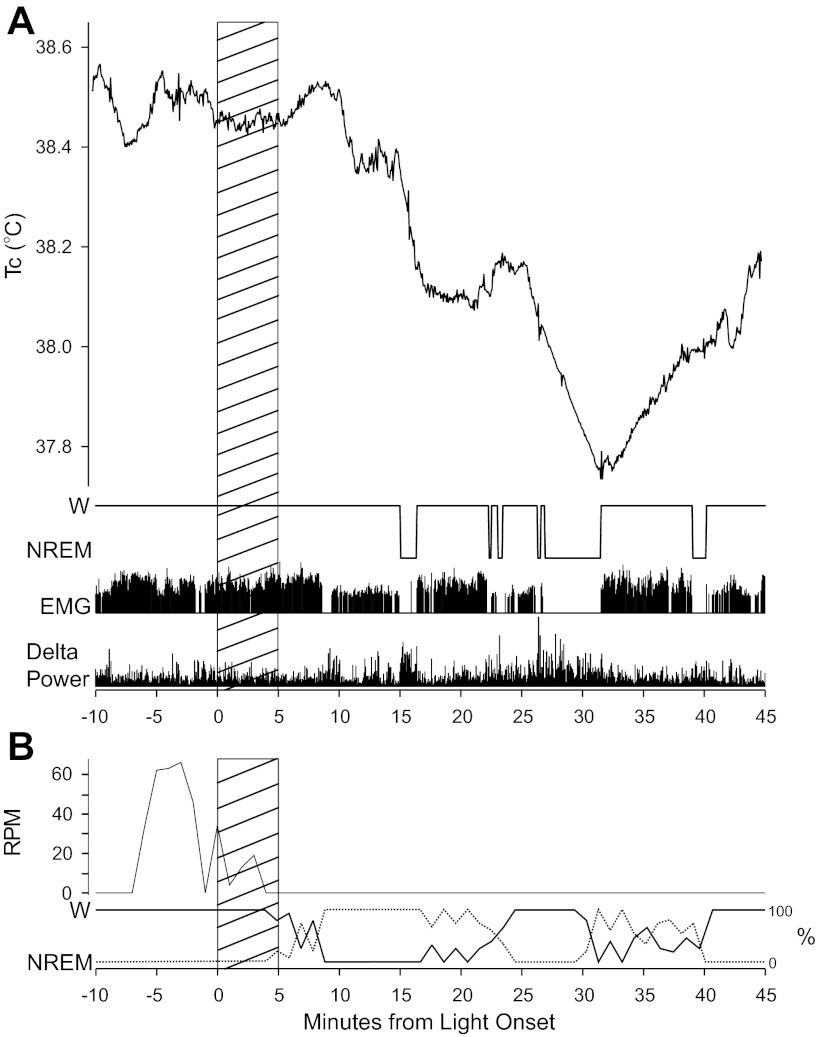
Fig. 8.
Patterns of light-induced sleep determined from mouse EEG. A: Tc, nonrapid eye movement sleep (NREM), wake (W), and corresponding EEG delta power. Tc, depicted in the upper panel, as well as activity levels as measured by the EMG in the third panel, are generally lower following the light stimulus. Delta power, shown in the bottom panel, generally rises shortly before, and is elevated during, sleep (in this case NREM) bouts. B: wheel revolutions/min (rpm) and simultaneously recorded sleep plotted as the percent of each minute containing W or NREM. The hatched areas indicate the interval of the 5-min light pulses.
In mice for which temperature change could be measured simultaneously with the EEG, all (n = 4) experienced a Tc decline during the first 30 min following initiation of light exposure. The change was greater than one standard deviation of the mean Tc during a comparable interval in the control recordings. For some mice, Tc continued to rise briefly following light onset only to fall thereafter. The times when Tc peaked and began to decline are 5.32 ± 1.47 min (5 min), 2.56 ± 0.75 min (1 h), and 3.56 ± 0.31 min (10 flashes), supporting the conclusion from experiments 3 and 4 that the initial decline in Tc occurs at approximately the same time regardless of which light stimulus was used. This Tc peak occurred after the first NREM episode in two instances (both during the 5-min exposure to light), but otherwise generally occurred close to or before NREM onset for the 5 min and 1-h light treatments. Sleep onset may have been more delayed following the 10 flashes. The amount of time between the onset of Tc decline and the onset of the first NREM bout was calculated for each animal and then averaged for each light stimulus: 0.21 ± 1.5 min (5 min), 1.28 ± 1.97 min (1 h), and 9.97 ± 2.15 min (10 flashes).
Similar to the timing of peak Tc, the rate of Tc decline appeared to follow approximately the same time course regardless of the stimulus. Tc reduction magnitudes greater than one standard deviation below the peak occurred at 10.63 ± 2.48 min for the 5-min light treatment, 8.28 ± 2.31 for the 1-h light treatment, and 13.14 ± 2.09 min for the 10 flashes. Relative to sleep onset, however, this magnitude was achieved 5.10 ± 2.48, 4.44 ± 2.95, and 0.50 ± 0.42 min after initiation of the first NREM sleep bout in each animal for the 5-min, 1-h, and flash groups, respectively.
Sleep following light exposure was generally brief and fragmented. Sleep duration was 19.70 min (10 flashes), 18.12 ± 6.0 min (5 min), and 26.86 ± 3.11 min (1 h); however, during this time, the animals were awake an average of 2.7 ± 0.84 min (5 min), 4.43 ± 0.65 min (1 h), 4.0 ± 0.79 min (10 flashes) (n = 8). The wake bouts were as short as 12 s (suggestive of minor wake-related activity such as brief scratching) to as long as 7.5 min (suggestive of greater activity). Sleep bouts lasted longer during the 1-h light treatment, suggesting that the influence of light can be sustained over a longer period of time. Interestingly, even with the fragmented and short sleep duration, animals were, in fact, able to enter REM sleep following each of the respective light treatments. Average REM sleep bout durations: 0.63 ± 0.42 min (5 min), 1.73 ± 1.09 (1 h), and 3.39 ± 1.23 (10 flashes).
DISCUSSION
Behavioral measures have demonstrated that brief exposure of mice to nocturnal light induces locomotor suppression and sleep (25). Here, we show the novel result that light simultaneously induces a drop in Tc. Together, the light-induced absence of locomotion and reduced Tc appear to create an internal environment conducive to sleep, a state change that has now been confirmed with EEG data. The results also demonstrate that the ability of light to induce a drop in Tc varies as a circadian rhythm, with the largest change occurring during the midsubjective night when Tc is normally highest and the animals are most active. The studies provide a new perspective on the relationship between sleep and thermoregulation, and especially on the broader role played by light in regulation of normal physiology and circadian rhythmicity.
Technical Considerations
Activity measurement.
Both temperature and activity data were collected using multiple methods. With regard to animal activity, wheel running, motion analysis of video images, and accelerometers, each provided similar gross information, but with certain subtle differences. Wheel running differs from the alternative activity measures by virtue of the fact that the animal must locomote to a wheel, enter, and begin locomotion in it before activity is registered. The transition from the nonrunning state to the wheel-running state may contain certain types of behavior (such as grooming or eating) in which an animal is not locomoting, but is awake and active.
The ANY-maze video analysis provided two useful indices of activity. One is “immobility,” which is a valid and noninvasive index of sleep (10, 30). This index is dependent upon the software's definition of “movement.” The software definition of what constitutes movement for immobility assessment is slightly different from the definition the same software employs to determine “distance traveled” (see Ref. 26 for discussion). In particular, immobility is signaled if a user-definable percentage of the pixels in the video silhouette of an animal has not changed location from one video frame to the next. In contrast, an animal travels zero distance if the software point of reference has not changed by 25% of the body length. Thus, the two measures will provide highly correlated, but nevertheless different, indices of when an animal is inactive.
Accelerometer-detected motion is not sensitive to change in location and does not have a software-based definition of what constitutes motion. The detector only indicates that a threshold-level acceleration has occurred and does not indicate the magnitude of such accelerations. They are highly correlated with the ANY-maze-determined “distance-traveled” measure, but comparison of the two types of results also reveals numerous small inconsistencies between them. As indicated in Fig. 6B, several types of inconsistencies occur, and it is not clear whether motion detection or distance traveled is the more useful or accurate measure. Direct viewing of the recorded video commonly revealed that the motion detected by accelerometer during the intervals of inconsistency results from awake animals engaging in grooming. Thus, grooming appears to be a common behavior occurring during the interval of transition between the inactive, sleep-prone subjective day and the awake and highly active subjective night. The motion associated with grooming is not detectable with running wheels and may not be detectable with ANY-maze software in either the immobility or distance traveled indices.
Temperature measurement.
One important aspect of the temperature recording method concerns detection of response latencies following a light stimulus. There are two factors involved. One is the rapidity with which heat loss through skin can be detected as a decline in Tc. The other concerns the physical properties of the thermal detector and the extent to which they constrain the rate of temperature change. With respect to the latter, both the DSI wireless transmitters and iButtons suffer from the same difficulty, namely a significant thermal time constant, which has been confirmed as 50–60 s. Therefore, all latency measures obtained in these studies have a built-in error. Reduction of this error will require sensors with smaller mass, allowing more rapid assessment of both core and skin temperature change. It is highly likely that the initial Tc response is reflected initially by a rapid increase in skin temperature occurring as a light-stimulated animal suddenly engages in peripheral vasodilation and heat loss.
Temperature Response to Nocturnal Light
The novel feature of these studies is the observation that a brief light stimulus during the night elicits a short latency, rapid drop in Tc. The light-induced change in Tc is associated with suppression of locomotion and sleep (present data; Refs. 25 and 26), followed by complete recovery to normal. The basic phenomenon occurs in response to light stimuli that are very brief (20 ms over 5 min), brief (5-min pulse), or long (1 h). There is no significant difference in the responses to the 10 flash or 5-min pulse stimuli, with each yielding relatively fixed response durations for both Tc and locomotor suppression. In contrast, the 1-h stimulus prolongs both responses, probably because the stimulus length has two different effects (24). On the one hand, millisecond flashes or pulses as long as about 1,200 s induce the expected fixed duration response; on the other, the continued presence of light has additive effects capable of prolonging the induced drop in Tc, locomotor suppression, and sleep [present data and (24)]. The general pattern of Tc change is similar to the pattern of light-induced suppression of nocturnal locomotion and has many of the same characteristics. In particular, the drop in Tc 1) is triggered by light, 2) has a relatively fixed duration (30–40 min) that endures well beyond the presence of the stimulus, and 3) can be maintained by additional exposure to the stimulus beyond the expected response duration. The most noticeable difference between the effects of light on Tc compared with locomotion is the fact that Tc returns toward normal prior to the recovery of locomotor activity.
Circadian Rhythmicity in the Tc Response to Light
Exposure of mice to light while housed in DD reveals a circadian rhythm in the Tc response, with the greatest induced drop in Tc occurring during the interval of CT15–CT18 and minimal response from CT3–CT9. Prolonged light during the late subjective night suppresses locomotion in an irradiance-dependent manner (24). However, an ordinary examination of locomotor suppression in response to light pulses has not been fruitful because of the high variability in wheel running at that time (unpublished data). Faced with the same problem, Redlin and Mrosovsky (36) placed hamsters in novel wheels and assessed light-induced suppression of novel wheel running. This method yielded a fairly clear, unimodal curve with a maximal locomotor suppression at about CT12 and a minimum at about CT0/24. In other words, the locomotor suppression curve is shifted about 6 h earlier than observed here for the light-induced temperature drop. Whether this is because of methodological (Tc vs. novel wheel locomotion measures) or species differences (hamster vs. mouse) is not clear at the present time.
There are several possible explanations for the rhythmic Tc response to light. Light has little effect on circadian rhythms during the subjective day (6), and a similar regulatory mechanism may also limit photosomnolence and Tc responses. A physiological limitation may be related to the fact that Tc is already reduced during the subjective day (40). Additional Tc reduction may not be possible under normal physiological conditions. A third possibility concerns the relationship between Tc and sleep. If the lower Tc during the subjective day is a result of normal sleep, then it may not be possible to further reduce Tc because photosomnolence cannot occur.
The relationship between the Tc response curve and the phase response curve (PRC) for circadian rhythm regulation is worth noting. The typical mouse PRC (see Fig. 3) has a phase delay portion, which is large and a phase advance portion which is very small (32). The Tc response curve here presented for the mouse is consistent with the PRC from the same strain to the extent that the largest Tc changes elicited are in response to light at CT15 and CT18, with very little change occurring at other circadian times. The present Tc response curve differs from a recently published locomotor suppression curve for the CD1 mouse, which found a reduced activity to 1-h pulses only at ZT14 and not at ZT18 (44). In contrast, an 11-h light period beginning at ZT13 is known to suppress late-night wheel running by C57BL/6J mice in an irradiance-dependent manner (24).
Relationship Between Tc, Locomotion, and Sleep
Mice typically sleep during the daytime when both body temperature and activity are low (18, 38). In fact, there has been a long-standing conjecture that the low body temperature facilitates sleep (19, 28), and it is generally accepted that sleep, regardless of the time of day, is accompanied by lower Tc (14, 29). The reduced Tc is associated predominantly with NREM, with small increases in Tc occurring during REM (2, 29, 52). Moreover, warming the hypothalamic preoptic area elicits a fall in Tc and is associated with sleep induction (20, 41, 42). In addition, a well-developed hypothesis (18, 49) contends that whole body heat loss (leading to a drop in Tc) is detected as a rise in skin temperature (Tsk), increasing sleep propensity in response to the elevated Tsk. A rise in ambient temperature toward the preferred neutral Tsk increases sleep propensity, with the reverse result occurring if ambient temperature is lowered (see Ref. 49 for references). A causal relationship between Tsk and sleep propensity has been documented in humans, with warmer skin facilitating greater sleep (34, 35, 49), and a compelling recent paper has clearly shown sleep state modulation by skin thermoreception in the mouse (52).
The present data are consistent with the holistic view that mice are most likely to sleep (as indicated by both behavioral and EEG-derived evidence) when they do not or cannot engage in locomotion and their body temperatures are reduced. The absolute change in Tc does not appear to be causal of sleep because the initial light-induced sleep bout normally occurs with a relatively short latency while Tc is dropping. At this point in the response sequence, Tc has only declined about half of its maximal change. In humans, a decline in Tc precedes sleep onset, which is most closely associated with the interval of the most rapid change (3, 5, 28, 48), rather than a specific temperature or amount of change. Nor does the loss of activity appear to cause the drop in Tc. About 50% of the light-exposed animals briefly show high rates of activity as Tc is dropping. Light exposure during the subjective night appears to trigger parallel functional changes in physiology and behavior yielding low Tc and inactivity. The exact nature of these changes remains to be determined, but it seems likely that the presence of either locomotion or higher Tc would seriously interfere with photosomnolence. This interference would be similar to the observation that failure to suppress locomotor activity interferes with the photic induction of circadian rhythm phase shifts (9, 21, 22, 33).
The observed parallel regulation of Tc and photosomnolence is consistent with neurophysiological observations that a high percentage of preoptic neurons contributing to sleep or thermoregulation contribute jointly to the regulation of both activities (45, 46). It is also consistent with the idea that light causes an abrupt drop in the set point for body temperature. If this occurs, the error signal between brain and set point would be positive, triggering abrupt heat loss, such as seen in the present data. This signal would simultaneously facilitate sleep (12, 43). Emergence from the interval of photosomnolence may occur, as the set point becomes elevated, generating an error signal requiring an associated increase in Tc. In a similar fashion, but driven by the circadian clock, the set point of animals emerging from the period of daytime sleepiness may rise in anticipation of arousal and nocturnal locomotion. Whether the consequent increase in Tc is causally linked to the timing and level of nocturnal arousal remains to be seen.
A remarkable aspect of photosomnolence is that it can be reliably evoked at ZT13, a time at which sleep bouts of any duration are virtually nonexistent in mice, especially in mice showing nearly constant robust locomotor activity in a wheel. In our experiments, nocturnal light exposure led to sleep whether the mouse had access to a wheel or not, suggesting that photosomnolence does not come about merely as a result of radical locomotor suppression from rigorous wheel running to quiescence, but that sleep is a direct result of nocturnal light. On the other hand, the cessation of locomotor activity is so closely associated with sleep onset that, while there is no empirical proof that the former must precede the latter, there are also virtually no instances in which locomotor activity occurs during actual sleep.
Temporal Relationship Between Activity and Tc
Both Tc and locomotion are expressed as circadian rhythms, which entrain to the prevailing photoperiod. It has been inferred that the daily Tc rhythm is not simply a product of increased metabolism induced by locomotor activity (reviewed by Refs. 39 and 40), and the current data are consistent with that view. The statistical significance of the correlation between the two variables is quite high. However, Refinetti (38) reported correlations between Tc and indices of both daytime and nighttime activity that were much higher than observed here for entire days. In hamsters, the activity level accounted for about 85% of the Tc variability, whereas the present mouse data show much lower correlations between Tc and wheel running and consequent lower ability of one variable to predict the other (at best, 23.8% of the variance accounted for). Most important is the fact that although both Tc and locomotor activity robustly oscillate with a circadian rhythm baseline, there is little additional correlation between the variables. Brown and Refinetti (4) conclude that mechanisms facilitating heat loss are more important to generating the Tc rhythm than those concerned with heat generation, such as locomotion. The present data appear to reflect a compromise between the initial Refinetti report and the subsequent study, indicating no correlation between Tc and activity measures (4, 38).
An alternate perspective on the relationship between Tc and activity has been presented by Weinert and Waterhouse (50, 51). Their results, in contrast to the present data, show a dependence of Tc on activity. It is possible that this difference reflects differing analytical methods, although it is more likely a strain difference because the activity and temperature records shown for individual Haz:ICR mice (51) differ substantially from similar data for C57BL/J6 mice (Figs. 1 and 2). In particular, the C57BL/J6 mice do not show much of any activity during the early daylight hours, unlike the Haz:ICR mice, which have a large activity peak at that time; the C57BL/J6 mice have very sharp spikes in Tc during the daytime, unlike the broader Tc peaks of the Haz:ICR mice, and the Haz:ICR mice have a significant time lag between the bouts of daytime activity and the next broad Tc peak. In contrast, the C57BL/J6 mice tend to have little or no activity associated with the daytime Tc spikes, and even when activity is manifest, the increased Tc anticipates the activity bouts. It is worth noting that at least one rodent species with a robust daily locomotor rhythm, the root vole, does not even have a corresponding circadian Tc rhythm (11). It is apparent that heat generated by locomotor activity can have additive, substitutive, or partial effects on body temperature (47).
There are several daily instances in which a difference between Tc and simultaneously recorded activity fail to support the view that activity level governs Tc. First, when the Tc and wheel running are assessed across the evening light-to-dark photoperiod transition, Tc consistently begins to increase a few minutes in advance of the wheel running. This characteristic has been reported in two mouse species, hamsters, and chipmunks and persists under conditions of restricted activity (8, 11, 13, 47). That this effect is not a consequence of locomotor suppression by the prevailing photoperiod is supported by the fact that the earlier onset phase of the Tc rhythm persists under DD conditions (present data). Moreover, there appears to be a hierarchical organization of arousal from daytime sleepiness revealed by the presence of elevated Tc and an associated small amount of motion beginning 20–30 min prior to the daily offset of light. Well-entrained wheel running typically begins shortly after lights off when Tc is already elevated and the animal is active. These changes may be driven by a more basic rhythm in general metabolism, many aspects of which are regulated by the SCN (16).
The second instance indicating that activity level does not govern Tc occurs during the late night hours when locomotor activity declines to very low levels, but Tc remains elevated for an hour or more. The third instance is evident in the ultradian-type rhythm peaks in Tc that are present during the subjective day, regardless of the lighting conditions. Many of these peaks are associated with small amounts of wheel running, but many have none at all. Thus, wheel running per se is not likely to cause the acute, brief increases in daytime Tc, but as is evident from the records showing more general indices of motion, there is likely to be some activity (many times, it is grooming) associated with the spike in Tc. As with general locomotion, it is unclear whether the increase in grooming is causal of the short-lived elevation in Tc or is a parallel consequence of more general arousal reflected by a change in metabolism.
The fourth instance in which activity level is not clearly related to the measured Tc occurs within the first few minutes after light stimulus onset in the standard test situation. During this time, the level of activity (regardless of how it is measured) commonly remains in the prestimulus range while Tc is beginning to fall. This is a clear indication that the two actions are at least partially independent. As indicated above, Tc is already dropping rapidly at the time of onset of major sleep bouts in humans (3, 5, 28, 48). The fifth, and perhaps the clearest of the instances in which Tc changes independent of the concurrent activity level, is evident as Tc begins to recover from its light-induced nadir. At this time, it nearly always does so in advance of any change in activity. This attempted recovery of Tc is most evident in mice exposed to the long 1-h light pulse. Collectively, these instances strongly support the view that the level of movement does not cause the observed changes in Tc.
Perspectives and Significance
The present studies highlight an effect of environmental lighting on behavioral state. The studies show that brief light exposure induces EEG sleep and a simultaneous drop in Tc. The results provide further emphasis on the interrelationship between thermoregulation and sleep and raise the question as to whether the observed drop in Tc may itself modulate other known actions of light, such as circadian rhythm phase shifts. It remains to be determined whether the light-induced sleep is a response to the magnitude or rate of Tc change or to an error signal. If the former, the mice may sleep in response to a perception of being cool, with reduced Tc supportive of sleep. If the latter, the mice may perceive themselves as too warm because current brain temperature is higher than the current threshold for metabolic heat production and the animals, therefore, lose heat to reduce the perceived error signal.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.M.S. and H.S.G. performed experiments; K.M.S., H.S.G., and L.P.M. analyzed data; K.M.S., H.S.G., and L.P.M. approved final version of manuscript; H.S.G. and L.P.M. conception and design of research; H.S.G. and L.P.M. interpreted results of experiments; H.S.G. and L.P.M. prepared figures; H.S.G. and L.P.M. drafted manuscript; H.S.G. and L.P.M. edited and revised manuscript.
ACKNOWLEDGMENTS
This research was supported by National Institutes of Health Grant NS061804 to L. P. Morin and NS067476 to H. S. Gompf. Maxim Grachev and Steven Mirabella provided outstanding technical assistance, and two anonymous reviewers contributed excellent ideas.
REFERENCES
- 1. Alam MN, McGinty D, Szymusiak R. Neuronal discharge of preoptic/anterior hypothalamic thermosensitive neurons: relation to NREM sleep. Am J Physiol Regul Integr Comp Physiol 269: R1240–R1249, 1995 [DOI] [PubMed] [Google Scholar]
- 2. Alfoldi P, Rubicsek G, Cserni G, Obal F. Brain and core temperatures and peripheral vasomotion during sleep and wakefulness at various ambient-temperatures in the rat. Pflügers Arch 417: 336–341, 1990 [DOI] [PubMed] [Google Scholar]
- 3. Aschoff J. Circadian control of body temperature. In: Physiological and Behavioral Temperature Regulation., edited by Hardy JD, Gagge AP, Stolwijk J. A. J. Springfield, IL: Charles C. Thomas, 1970, p. 905–919 [Google Scholar]
- 4. Brown CM, Refinetti R. Daily rhythms of metabolic heat production, body temperature, and locomotor activity in golden hamsters. J Therm Biol 21: 227–230, 1996 [Google Scholar]
- 5. Campbell SS, Broughton RJ. Rapid decline in body-temperature before sleep—fluffing the physiological pillow. Chronobiol Int 11: 126–131, 1994 [DOI] [PubMed] [Google Scholar]
- 6. Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents. II. The variability of phase response curves. J Comp Physiol A 106: 253–266, 1976 [Google Scholar]
- 7. Davidson AJ, Aujard F, London B, Menaker M, Block GD. Thermochron iButtons: An inexpensive method for long-term recording of core body temperature in untethered animals. J Biol Rhythms 18: 430–432, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Decoursey PJ, Pius S, Sandlin C, Wethey D, Schull J. Relationship of circadian temperature and activity rhythms in two rodent species. Physiol Behav 65: 457–463, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Edelstein K, De la Iglesia HO, Schwartz WJ, Mrosovsky N. Behavioral arousal blocks light-induced phase advances in locomotor rhythmicity but not light-induced Per1 and fos expression in the hamster suprachiasmatic nucleus. Neuroscience 118: 253–261, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Fisher SP, Godinho SI, Hankins MW, Foster RG, Peirson SN. Rapid assessment of sleep/wake behaviour in mice. J Biol Rhythms 27: 48–58, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gebczynski AK, Taylor JRE. Daily variation of body temperature, locomotor activity and maximum nonshivering thermogenesis in two species of small rodents. J Therm Biol 29: 123–131, 2004 [Google Scholar]
- 12. Glotzbach SF, Heller HC. Central nervous regulation of body temperature during sleep. Science 194: 537–539, 1976 [DOI] [PubMed] [Google Scholar]
- 13. Golombek DA, Ortega G, Cardinali DP. Wheel running raises body-temperature and changes the daily cycle in golden-hamsters. Physiol Behav 53: 1049–1054, 1993 [DOI] [PubMed] [Google Scholar]
- 14. Heller HC. Temperature, thermoregulation and sleep. In: Principles and Practice of Sleep Medicine, edited by Kryger MH, Roth T, Dement WC. Philadelphia, PA: Elsevier Saunders, 2005, p. 292–304 [Google Scholar]
- 15. Jud C, Schmutz I, Hampp G, Oster H, Albrecht U. A guideline for analyzing circadian wheel-running behavior in rodents under different lighting conditions. Biol Proced Online 7: 101–116, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kalsbeek A, Scheer FA, Perreau-Lenz S, La Fleur SE, Yi CX, Fliers E, Buijs RM. Circadian disruption and SCN control of energy metabolism. FEBS Lett 585: 1412–1426, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krauchi K. The thermophysiological cascade leading to sleep initiation in relation to phase of entrainment. Sleep Med Rev 11: 439–451, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Krauchi K, Cajochen C, Werth E, Wirz-Justice A. Physiology: warm feet promote the rapid onset of sleep. Nature 401: 36–37, 1999 [DOI] [PubMed] [Google Scholar]
- 19. McGinty D, Szymusiak R. Hypothalamic regulation of sleep and arousal. Front Biosci 8: s1074–s1083, 2003 [DOI] [PubMed] [Google Scholar]
- 20. McGinty D, Szymusiak R, Thomson D. Preoptic/anterior hypothalamic warming increases EEG delta frequency activity within non-rapid eye movement sleep. Brain Res 667: 273–277, 1994 [DOI] [PubMed] [Google Scholar]
- 21. Mistlberger RE, Antle MC. Behavioral inhibition of light-induced circadian phase resetting is phase and serotonin dependent. Brain Res 786: 31–38, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Mistlberger RE, Holmes MM. Morphine-induced activity attenuates phase shifts to light in C57BL/6J mice. Brain Res 829: 113–119, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Morin LP. Circadian Visual System of Mammals. In: How Animals See the World, edited by Lazareva OF, Shimizu T, Wasserman EA. New York: Oxford University, 2012, p. 389–415 [Google Scholar]
- 24. Morin LP, Lituma PJ, Studholme KM. Two components of nocturnal locomotor suppression by light. J Biol Rhythms 25: 197–207, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morin LP, Studholme KM. Millisecond light pulses make mice stop running, then display prolonged sleep-like behavior in the absence of light. J Biol Rhythms 24: 497–508, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morin LP, Studholme KM. Separation of function for classical and ganglion cell photoreceptors with respect to circadian rhythm entrainment and induction of photosomnolence. Neuroscience 199: 213–224, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mrosovsky N. Masking: history, definitions, and measurement. Chronobiol Int 16: 415–429, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Murphy PJ, Campbell SS. Nighttime drop in body temperature: A physiological trigger for sleep onset? Sleep 20: 505–511, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Obal F, Jr, Rubicsek G, Alfoldi P, Sary G, Obal F. Changes in the brain and core temperatures in relation to the various arousal states in rats in the light and dark periods of the day. Pflügers Arch 404: 73–79, 1985 [DOI] [PubMed] [Google Scholar]
- 30. Pack AI, Galante RJ, Maislin G, Cater J, Metaxas D, Lu S, Zhang L, Von SR, Kay T, Lian J, Svenson K, Peters LL. Novel method for high-throughput phenotyping of sleep in mice. Physiol Genomics 28: 232–238, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Paul MJ, Schwartz WJ. On the chronobiology of cohabitation. Cold Spring Harb Symp Quant Biol 72: 615–621, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Pendergast JS, Friday RC, Yamazaki S. Photic entrainment of period mutant mice is predicted from their phase response curves. J Neurosci 30: 12179–12184, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ralph MR, Mrosovsky N. Behavioral inhibition of circadian responses to light. J Biol Rhythms 7: 353–359, 1992 [DOI] [PubMed] [Google Scholar]
- 34. Raymann R, Swaab DF, Van Someren EJW. Cutaneous warming promotes sleep onset. Am J Physiol Regul Integr Comp Physiol 288: R1589–R1597, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Raymann R, Swaaband DF, Van Someren EJW. Skin deep: enhanced sleep depth by cutaneous temperature manipulation. Brain 131: 500–513, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Redlin U, Mrosovsky N. Masking of locomotor activity in hamsters. J Comp Physiol A 184: 429–437, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Refinetti R. The circadian rhythm of body temperature. Front Biosci 15: 564–594, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Refinetti R. Contribution of locomotor activity to the generation of the daily rhythm of body temperature in golden hamsters. Physiol Behav 56: 829–831, 1994 [DOI] [PubMed] [Google Scholar]
- 39. Refinetti R. Entrainment of circadian rhythm by ambient temperature cycles in mice. J Biol Rhythms 25: 247–256, 2010 [DOI] [PubMed] [Google Scholar]
- 40. Refinetti R, Menaker M. The circadian rhythm of body temperature. Physiol Behav 51: 613–637, 1992 [DOI] [PubMed] [Google Scholar]
- 41. Roberts WW, Bergquist EH, Robinson TC. Thermoregulatory grooming and sleep-like relaxation induced by local warming of preoptic area and anterior hypothalamus in opossum. J Comp Physiol Psychol 67: 182–188, 1969 [DOI] [PubMed] [Google Scholar]
- 42. Roberts WW, Robinson TC. Relaxation and sleep induced by warming of preoptic region and anterior hypothalamus in cats. Exp Neurol 25: 282–294, 1969 [DOI] [PubMed] [Google Scholar]
- 43. Sakaguchi S, Glotzbach SF, Heller HC. Influence of hypothalamic and ambient temperatures on sleep in kangaroo rats. Am J Physiol Regul Integr Comp Physiol 237: R80–R88, 1979 [DOI] [PubMed] [Google Scholar]
- 44. Shuboni DD, Cramm S, Yan L, Nunez AA, Smale L. Acute behavioral responses to light and darkness in nocturnal Mus musculus and diurnal Arvicanthis niloticus. J Biol Rhythms 27: 299–307, 2012 [DOI] [PubMed] [Google Scholar]
- 45. Suntsova N, Guzman-Marin R, Kumar S, Alam MN, Szymusiak R, McGinty D. The median preoptic nucleus reciprocally modulates activity of arousal-related and sleep-related neurons in the perifornical lateral hypothalamus. J Neurosci 27: 1616–1630, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Szymusiak R, McGinty D. Hypothalamic regulation of sleep and arousal. Ann N Y Acad Sci 1129: 275–286, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Vaanholt LM, Garland T, Daan S, Visser GH. Wheel-running activity and energy metabolism in relation to ambient temperature in mice selected for high wheel-running activity. J Comp Physiol B 177: 109–118, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Van den Heuvel CJ, Noone JT, Lushington K, Dawson D. Changes in sleepiness and body temperature precede nocturnal sleep onset: Evidence from a polysomnographic study in young men. J Sleep Res 7: 159–166, 1998 [DOI] [PubMed] [Google Scholar]
- 49. Van Someren EJW. Sleep propensity is modulated by circadian and behavior-induced changes in cutaneous temperature. J Therm Biol 29: 437–444, 2004 [Google Scholar]
- 50. Weinert D, Waterhouse J. The circadian rhythm of core temperature: Effects of physical activity and aging. Physiol Behav 90: 246–256, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Weinert D, Waterhouse J. Diurnally changing effects of locomotor activity on body temperature in laboratory mice. Physiol Behav 63: 837–843, 1998 [DOI] [PubMed] [Google Scholar]
- 52. Whitten TA, Martz LJ, Guico A, Gervais N, Dickson CT. Heat synch: inter- and independence of body-temperature fluctuations and brain-state alternations in urethane-anesthetized rats. J Neurophysiol 102: 1647–1656, 2009 [DOI] [PubMed] [Google Scholar]