Abstract
Enhanced odor preference learning and attenuated fear learning characterizes rat pups’ attachment learning Sensitive Period for learning the maternal odor. This period terminates at 10 days old (PN10) with increasing endogenous levels of the stress hormone, corticosterone. Increasing Sensitive Period pups’ corticosterone prematurely terminates the Sensitive Period, while decreasing corticosterone in older pups delays Sensitive Period termination. Here we extend these findings and define the age range corticosterone alters learning and question whether corticosterone permanently terminates the Sensitive Period. Pups were odor-0.5mA shock conditioned with either corticosterone increased (PN5–6; 4 mg/kg vs. saline) or decreased (PN15–16; naturally by maternal presence or corticosterone synthesis blocker, Metyrapone). Finally, PN7–8 pups were conditioned with corticosterone and reconditioned without corticosterone to assess whether the Sensitive Period was permanently terminated. Results indicate developmental limits for corticosterone regulation of pup learning are PN6 through PN15. Furthermore, inducing precocious corticosterone induced fear learning was not permanent, since reconditioning without corticosterone enabled odor preference learning. Results suggest pups are protected from learning aversions to maternal odor until approaching weaning.
Keywords: attachment, infant, rat, sensitive period, avoidance learning, preference learning, maternal presence, corticosterone, olfaction, fear conditioning
INTRODUCTION
The young of many species have temporally defined periods of modified learning used to support learning to attach to the caregiver. This specialized sensitive-period learning was first identified in avian imprinting by Spalding (1873) and later defined by Lorenz (1935). To date, imprinting continues to remain the classic example of early life learning about the caregiver. Avian imprinting occurs during a very brief Sensitive Period after birth when the chick learns to identify its mother and initiates a following response for proximity seeking (Hess & Schaeffer, 1959; Lorenz, 1935). The animal is predisposed for this biological proximity seeking but learns the object of attachment. Since defining imprinting, infant attachment learning during defined developmental Sensitive Periods has been identified in other altricial and non-altricial species, including rats, rabbits, sheep, and nonhuman, and human primates (see reviews, Fifer & Moon, 1995; Harlow & Harlow, 1965; Hensch, 2004; Hudson, 1993; Leon, 1992; Nowak et al., 1997; Pedersen, Williams, & Blass, 1982; Poindron, Terrazas, & Hernandez, 2003; Schaal, Orgeur, & Arnould, 1995). Thus, this attachment learning has a wide phylogenetic representation and permits infants of many species to easily form a repertoire of proximity-seeking behaviors to the caregiver, regardless of the quality of the care they receive (Bowlby, 1969; review: Hofer & Sullivan, 2008; Van der Horst, LeRoy, & van der Veer, 2008).
A Sensitive Period for attachment learning has been identified in rat pups and supports the odor learning required for rat pup attachment to the mother and her odor. Sensitive Period pups were found to learn a preference to odors within and outside the nest when paired with presumably pleasant stimuli such as maternal presence, nursing, milk, and stroking to emulate maternal licking and grooming (Alberts & May, 1984; Brunjes & Alberts, 1979; Johanson & Hall, 1979; Johanson & Teicher, 1980; Levine (2000); Leon, 1975; Pedersen et al., 1982; Polan & Hofer, 1998; Sullivan, Brake, Hofer, & Williams, 1986; Sullivan & Hall, 1988; Sullivan, Hofer, & Brake, 1986; Sullivan, Wilson, Wong, Correa, & Leon, 1990; Teicher & Blass, 1977; Terry & Johanson, 1996). Paradoxically, this odor approach learning occurs equally well with presumably aversive stimuli such as mild shock, tail pinch, or maternal maltreatment of pups (Camp & Rudy, 1988; Haroutunian & Campbell, 1979; Languille, Richer, & Hars, 2009; Roth & Sullivan, 2005; Sullivan, Hofer, et al., 1986; Sullivan, Landers, Yeaman, & Wilson, 2000). This odor learning, supported by both pleasant (Pedersen et al., 1982) and aversive (Raineki, Moriceau, & Sullivan, submitted) stimuli produces an odor that mimics the natural maternal odor and can support both approach responses and nipple attachment. Rat pup Sensitive Period for attachment learning is punctuated by a strong tendency to prefer odors and the suppression of adult-like fear learning.
Rat pup attachment learning requires norepinephrine (NE) dependent learning-induced changes in the olfactory bulb and anterior piriform cortex (Guerin, Didier, Peace, Linster, & Cleland, 2008; Rangel & Leon, 1995; Sullivan, Stackenwalt, Nasr, Lemon, & Wilson, 2000; Sullivan & Wilson, 1991, 1994; Sullivan, Wilson, & Leon, 1989; Wilson & Leon, 1988; Wilson, Sullivan, & Leon, 1987; Yuan, Harley, Darby-King, Neve, & McLean, 2003). However, attachment learning using aversive stimuli also requires suppression of the amygdala (Sullivan, Landers, et al., 2000), a brain area previously identified as a key structure in fear learning (Fanselow & Gale, 2003; Fanselow & LeDoux, 1999; LeDoux, 2003, 2007; Sananes & Campbell, 1989; Schettino & Otto, 2001; Sevelinges, Gervais, Messaoudi, Granjon, & Mouly, 2004).
The infant amygdala requires the stress hormone corticosterone (CORT) for aversion learning, a finding demonstrated through both systemic injection and intraamygdala infusions (Moriceau & Sullivan, 2005; Moriceau, Wilson, Levine, & Sullivan, 2006; Sullivan, Landers, et al., 2000). Until postnatal day (PN)10, the pup exhibits a stress hyporesponsive period (SHRP) where pups show a greatly attenuated shock-induced CORT release (Cirulli, Gottlieb, Rosenfeld, & Levine, 1992; Cirulli, Santucci, Laviola, & Alleva, 1994; Levine, 1962, 2001; Sapolsky & Meaney, 1986; Schapiro, Geller, & Eiduson, 1962). Increases in CORT through either systemic injection or intraamygdala infusion can alter Sensitive Period development and prematurely end Sensitive Period type attachment learning (Moriceau & Sullivan, 2005; Moriceau et al., 2006; Sullivan, Landers, et al., 2000). Although pups develop a natural shock-induced CORT release after PN10, maternal presence blocks this release (Moriceau & Sullivan, 2005, 2006; Shionoya, Moriceau, Bradstock, & Sullivan, 2007; Suchecki, Rosenfeld, & Levine, 1993; Stanton & Levine, 1990) thus preventing the CORT increase required for infant amygdala plasticity and fear learning. During this extended Sensitive Period, rat pups display either Sensitive Period attachment learning with low CORT or adult-like, amygdala-dependent aversion/fear learning with higher CORT levels (Moriceau & Sullivan, 2006; Moriceau et al., 2006; Shionoya et al., 2007). Since this CORT-dependent, dual learning does not occur in adults (McGaugh & Roozendaal, 2002), we explore the age at which this dual learning fails to occur. In this, we begin to define developmental limitations in CORT regulation of amygdala-dependent aversion learning. Finally, we questioned whether the precocious emergence of odor aversion learning from odor-0.5mA shock conditioning with CORT permanently terminates the Sensitive Period.
METHODS
Subjects
Subjects were male and female PN5–6, PN7–8, and PN14–16-day-old rat pups born in the University of Oklahoma animal vivarium of Long-Evans rats (Harlan, IN). This age range was chosen based on pilot data indicating a difference in learning between these age groups. Housing was a 34 × 29 × 17-cm polypropylene cage with pine wood shavings in a temperature (20°C) and light (6:00 a.m. to 6:00 p.m.) controlled room with ad libitum food and water. Day of birth was considered PN0 and animals were culled to 12 pups per litter on PN1. Each experimental condition used no more than one male and one female from a given litter to avoid litter effects. Only pups born in the morning were used to better observe age effects. University of Oklahoma Institutional Animal Care and Use Committee approved all procedures and National Institutes of Health guidelines were followed.
Pup Conditioning
Immediately after being removed from their nest, pups were placed individually in clear, plastic 600 ml beakers and given a 10 min acclimation period to recover from experimenter handling. Pups were placed into one of three conditioning groups: (1) Paired: pups received 11 pairings of a 30 s conditioned stimulus (CS) odor and an unconditioned stimulus (US) of hind limb shock (0.5 mA, 1 s) delivered during the last second of the odor; (2) Unpaired: pups received shock 1.5 min after 30 s odor presentation; (3) Odor only: pups received 11, 30 s odor presentations. Unless otherwise stated, the CS was peppermint odor (Pure peppermint, McCormick; 2 L/min, 1:10 odor vapor) delivered through a flow dilution olfactometer (Laboratory Supplies Co, Inc) controlled at an inter-trial interval (ITI) of 4 min through a Chrontrol (ChronTrol Corporation, San Diego, CA). This air to odor ratio produced an odor intensity that produced a head-up response in pups with an activity rating of 1. The US was a 0.5mA shock (Lafayette Instruments) delivered manually to the hind limb. Pups’ behavior was monitored during conditioning using a behavior activity scale, which rates movements from 0 to 5 based on the number of limbs/head in movement (i.e., 1 = 1 limb or the head moving for at least 3 s). Regardless of conditioning group, all pups displayed a strong response to the shock. During conditioning, ambient temperature was 32°C and 28°C for the younger and older pups respectively. Pups were placed back in the nest immediately following conditioning.
Defining the Lower Age Limit: CORT Injection
To determine if a lower age limit exists for CORT effects on Sensitive Period aversion learning, we used an odor-0.5mA shock fear conditioning paradigm for PN5 and PN6 pups. Thirty minutes prior to conditioning, pups were injected with either CORT (4 mg/kg, i.p.) or saline.
Defining the Upper Age Limit: Maternal Presence
To test for an upper limit of CORT effects on Sensitive Period aversion learning, we used maternal presence to attenuate pups’ shock-induced CORT release during odor-0.5mA shock fear conditioning with PN14–16 pups. Radioimmunoassay (RIA) for CORT shows maternal presence reduced CORT levels by 55%. Pups were placed with an anesthetized mother in a Plexiglas chamber (27 cm × 37.5 cm × 28 cm) for conditioning. The mother was the same biological age (±1 day) as the pups’ biological mother. The mother was anesthetized (urethane, 1.5 g/kg, i.p.) to prevent her from interfering with conditioning, as well as to control for maternal behavior and milk letdown. The mother was placed on her side to allow pups access to her nipples during conditioning.
Defining the Upper Age Limit: Metyrapone
To further test the upper limits of CORT effects in older pups (PN14–16), we lowered pup CORT levels by injecting the CORT synthesis blocker, Metyrapone. Radioimmunoassay (RIA) for CORT shows Metyrapone reduced CORT levels by 70%. Metyrapone blocks CORT synthesis at the adrenal gland by inhibiting 11β-hydroxylase, which facilitates conversion of CORT. Ninety minutes prior to conditioning, pups were injected with either Metyrapone (50 mg/kg, i.p.) or saline and returned back to the mother until conditioning.
Determining if a Sensitive Period CORT Injection Permanently Terminate the Sensitive Period
To determine if a CORT-induced Sensitive Period learned odor aversion permanently terminates the Sensitive Period, PN7 pups were odor-0.5mA shock conditioning with either peppermint as described above or citral (Sigma, St. Louis, MO) as the CS. Thirty minutes prior to conditioning, pups were injected with either CORT (4 mg/kg, i.p.) or saline. Pups were reconditioned at PN8 in the same manner except all were injected with saline only. The CS for reconditioning was different from the original conditioning CS.
Radioimmunoassay
RIA using heart and trunk blood was done following conditioning on approximately half the animals, while the remaining pups were given behavioral testing. Shock did not significantly increase CORT in PN5–6-day-old pups. Shock significantly increased CORT in the older pups while maternal presence and Metyrapone reduced CORT levels by 55% and 70% respectively compared to saline pups. This replicates previous results (Barrett & Gonzalez-Lima, 2004; Cordero, Kruyt, Merino, & Sandi, 2002; Dallman, 2000; Hennessy, Maken, & Graves, 2002; Hennessy, Kaiser, & Sachser, 2009; Levine, Dent, & de Kloet, 2000; Moriceau & Sullivan, 2006; Moriceau et al., 2006; Roozendaal, Bohus, & McGaugh, 1996; Suchecki, Mozaffarian, Gross, Rosenfeld, & Levine, 1993; Walker, Perrin, Vale, & River, 1986). For the RIA, immediately after conditioning, blood samples were taken and centrifuged at 14,000 cpm for 6 min. Aliquoted plasma samples were stored at −70°C until RIA analysis. Rat corticosterone Coat-a-Count kit (Radioassay Systems Labs, Carson, CA) was used to analyze duplicate plasma samples (125I corticosterone, sensitivity of 5 ng/ml).
Behavioral Testing
Expression of odor preference or aversion was assessed in pus 1 day following conditioning using a Y-maze. Pups were given 5 trials of a choice between the CS (25 µl on Kimwipe tissue, dried under vent hood for 5 min; peppermint or citral depending on the experiment) and familiar clean wood shaving odor (same as those used in the nest). Odors were placed at the end of each arm of a Plexiglas Y-maze with 8.5 cm × 24 cm × 8 cm choice arms and 8 cm × 8 cm start box. After 5 s in a start box, alley doors were opened and pups were allowed 60 s to choose an arm. Every animal left the start box. In PN5–6 pups, a choice required the pup’s front paws to pass the alley entrance. In older animals, a choice required the pup’s entire body to pass the alley entrance. Pups were placed in a holding cage for 30 s between trials and the maze floor was cleaned with water and dried between each trial. Ambient temperature was monitored and maintained at 28°C. Furthermore, since injected CORT systemically clears within hours of injection, CORT was not present during testing (Koch, Lutz, & Mialhe, 1969; Leeper, Schroeder, & Henning, 1988).
Statistical Analysis
ANOVA followed by post-hoc Fisher tests were used to analyze results. Experimental groups were considered statistically different from control groups as indicated by at least p = .05 in the post-hoc Fisher test.
RESULTS
Lower Limit of CORT Induced-Aversion Learning: PN 6
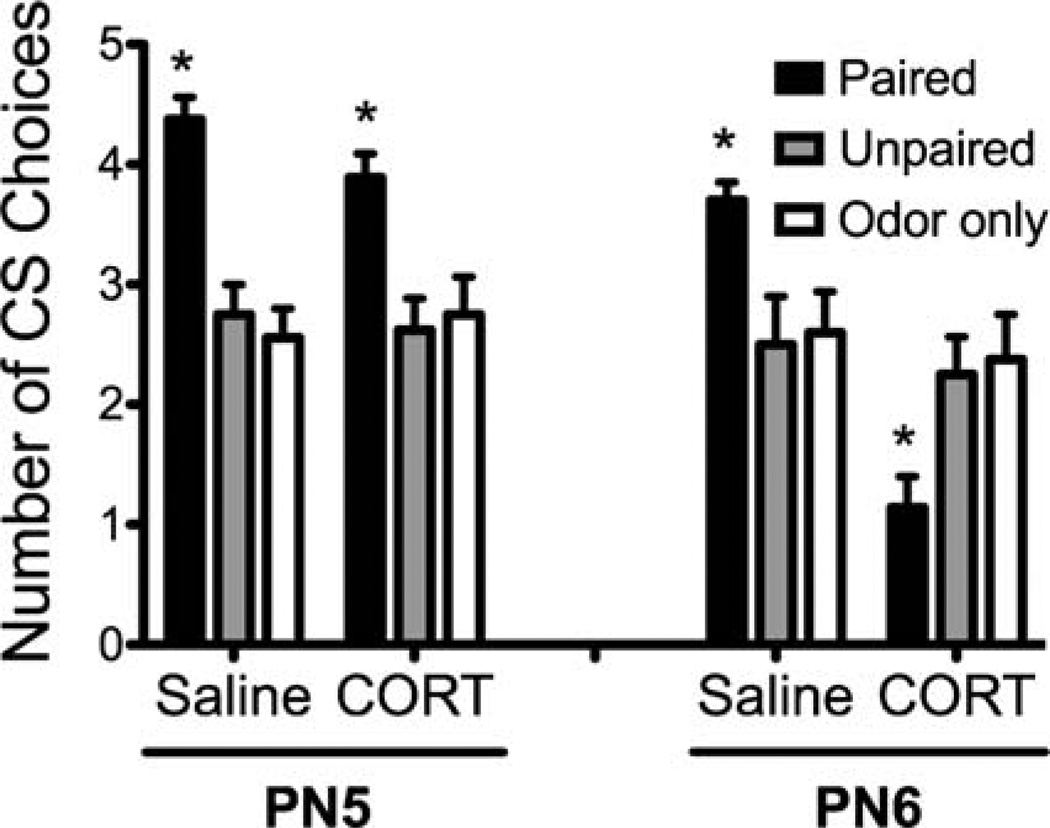
As illustrated in Figure 1, all PN5 and PN6 paired pups injected with saline before odor-shock conditioning expressed an odor preference in the Y-maze test. However, a CORT injection prematurely terminated Sensitive Period learning in the PN6 pups and these pups learned an odor aversion, although CORT did not produce an effect at PN5. ANOVA analysis indicates a significant effect of age (F(1,91) = 18.932, p < .0001), condition (F(2,91) = 8.280, p < .0005) and drug (F(1,91) = 11.751, p < .0009). ANOVA analysis also showed a significant interaction between condition and drug (F(2,91) = 8.013, p < .0006), condition and age (F(2,91) = 8.571, p < .0004), drug and age (F(1,91) = 6.758, p < .02) and between condition, drug and age (F(2,91) = 3.229, p < .05). Post-hoc Fisher tests revealed PN5 and PN6 paired pups with saline and PN5 paired pups with CORT chose the CS odor significantly more than each control group and PN6 paired CORT pups (p <.03). Post-hoc Fisher tests also showed PN6 paired CORT pups were significantly different from all other groups (p < .02) and there were no significant differences within control groups.
FIGURE 1.
Pups were odor-0.5mA shock conditioned at either PN5 or PN6 with either a saline or CORT (4 mg/kg; i.p.) injection. Pups were then tested in a Y-maze the next day. While both PN5 and PN6 pups learned an odor preference, the CORT
Upper Limit of CORT Regulation of Fear Learning: PN15
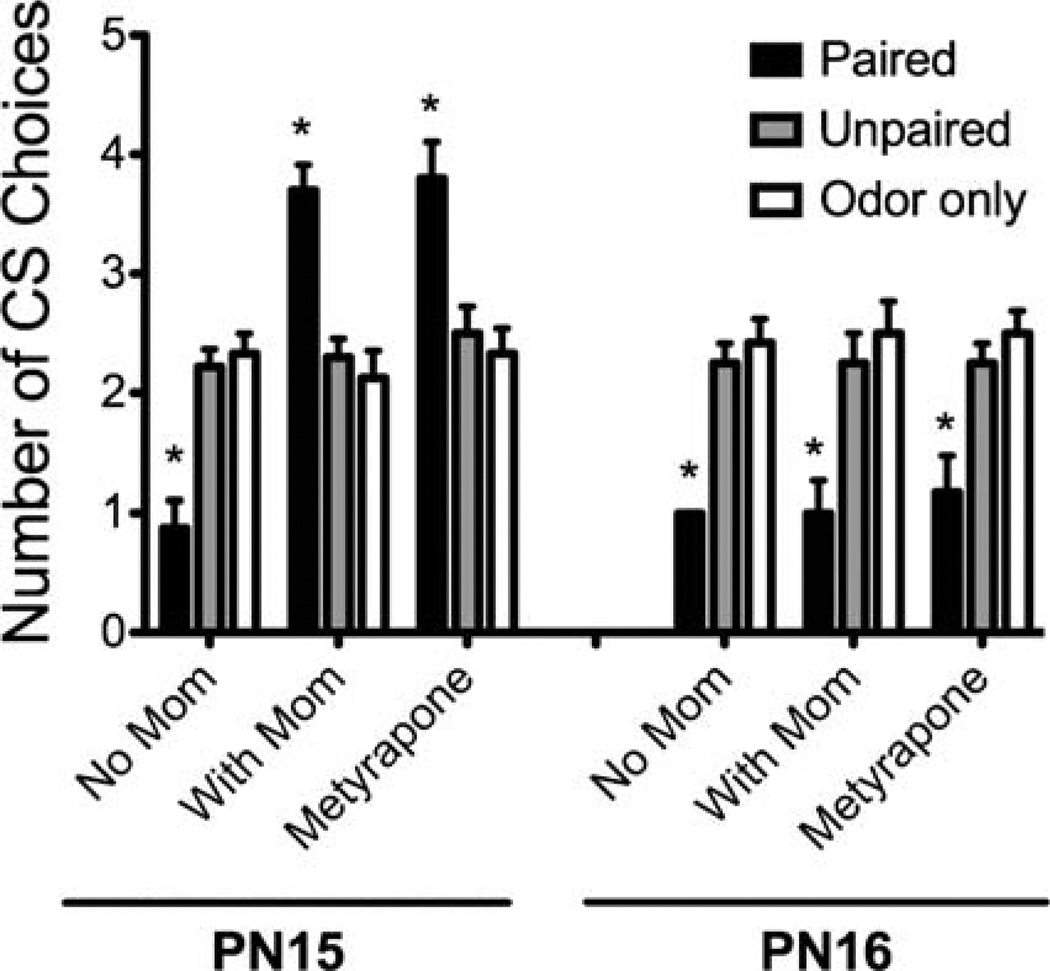
As illustrated in Figure 2, both PN15 and PN16 pups learned conditioned fear without maternal presence. Lowered CORT levels through maternal presence or blocked CORT production, however, was only capable of switching learning to a preference in PN15 pups and not PN16. ANOVA analysis indicates significant differences between age (F(1,123) = 29.402, p < .0001), condition (F(2,123) = 7.297, p < .001) and drug (F(2,123) = 12.547, p < .0001). ANOVA also indicated an interaction between age and condition (F(2,123) = 36.469, p < .0001), condition and drug (F(4,123) = 10.685, p < .0001), drug and age (F(2,123) = 10.066, p < .0001), and between condition, age, and drug (F(4,123) = 10.293, p< .0001). Post-hoc Fisher tests reveal PN15 paired pups with mother present or with CORT blocker displayed a significant preference compared to all other conditioning groups (p < .01). Post-hoc Fisher tests also revealed PN15 and PN16 paired without mother, PN16 paired with mother, and PN16 paired with CORT blocker are not significantly different from each other, but show an aversion compared to all other control groups (p < .001). There were no significant differences across control groups.
FIGURE 2.
Pups were odor-0.5mA shock conditioned at either PN15 or PN16 with either a saline injection or one of two methods to reduce pups’ shock-induced CORT release (maternal presence, Metyrapone (50 mg/kg, i.p.) injection). Pups were then tested in a Y-maze the next day. Although both PN15 and PN16 pups are capable of learning odor aversion, only PN15 pups are capable of learning odor preference with attenuated CORT
Sensitive Period Pups Can Switch Between Approach and Avoidance Learning
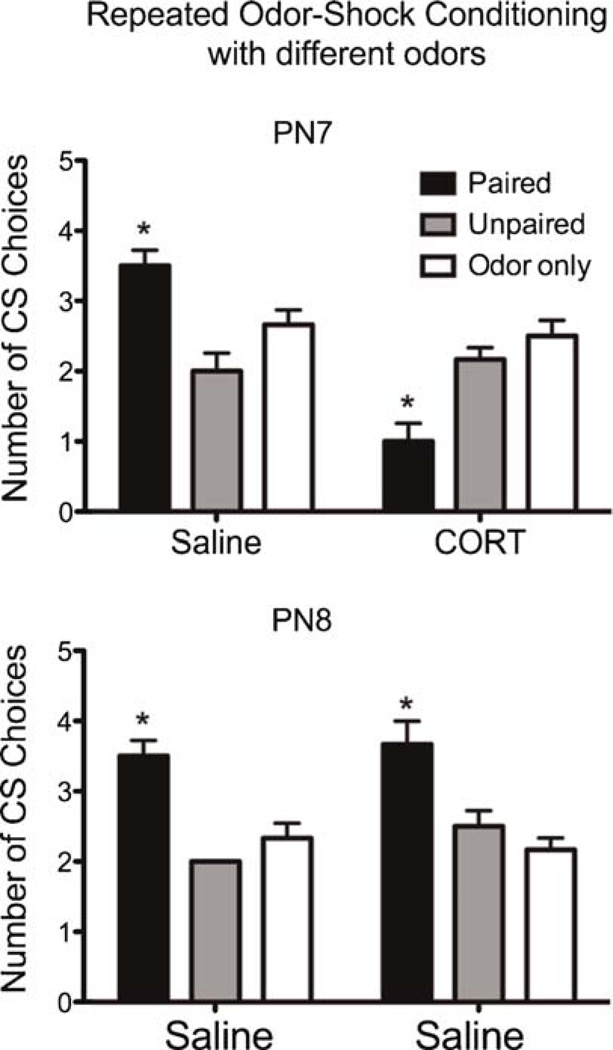
As illustrated in Figure 3, precocious CORT-induced avoidance learning does not permanently terminate Sensitive Period learning. An ANOVA analysis indicates a significant difference at PN7 between drug (F(1,30) = 20.455, p < .0001) and condition and drug (F(2,30) = 20.727, p < .0001) and at PN8 between drug (F(1,30) = 0.882, p < .0001) and condition and drug (F(2,30) = 1.176, p < .0001). Post-hoc Fisher tests also revealed PN7 and PN8 paired pups significantly differ from one another, as well as from control groups (p < .05). There were no significant differences across control groups.
FIGURE 3.
Pups were odor-0.5mA shock conditioned twice, first at PN7 with CORT or saline injection and then conditioned again at PN8 with a saline injection to determine if preference learning would return. The PN7 conditioning with CORT produced an odor aversion, although additional conditioning at PN8 permitted pups to learn an odor preference. *p <.05, error bars indicate SEM; N = 6 pups per group.
DISCUSSION
The present results define the developmental limits of CORT control during rat pup odor-0.5mA shock aversion learning from PN6 through PN15. Specifically, injection of CORT at PN6, but not PN5, permitted odor-shock aversion learning rather than the age-specific preference learning. The ability of CORT to switch preference learning to aversion ended between PN15 and PN16, as indicated by maternal presence (natural attenuation of CORT) or Metyrapone injection (blocking of CORT synthesis), permitting the odor-shock preference learning at PN15, but not PN16. In addition, the present results indicate that the switch to aversion learning induced by elevated CORT does not permanently end the Sensitive Period since odor-shock conditioning with a new odor the next day produced an odor preference.
These results extend and support previous research that indicated infant attachment Sensitive Period learning is maintained through endocrine regulation. Specifically, odor-0.5mA shock conditioning produces an odor preference, whether during the SHRP or with maternal presence after SHRP termination. Switching between learning a preference or an aversion is dependent upon amygdala CORT levels, with low CORT supporting preference learning and elevated CORT supporting aversion learning though engagement of the amygdala (Barr et al., 2009; Moriceau & Sullivan, 2004, 2006; Moriceau et al., 2006; Shionoya et al., 2007).
Lower Limit of CORT Induced-Aversion Learning: PN6
During rat pup SHRP most stressful stimuli, including shock, do not significantly raise the level of the stress hormone CORT (Levine, 1962, 2001; Moriceau & Sullivan, 2006; Sapolsky & Meaney, 1986; Schapiro et al., 1962; Walker et al., 1986). Thus, during the SHRP, pups have insufficient shock-induced CORT release to support amygdala plasticity for aversion learning, and exhibit an odor preference and the learning-induced plasticity of the olfactory bulb (Raineki, Shionoya, Sander, & Sullivan, 2009; Sullivan & Leon, 1986; Sullivan, McGaugh, & Leon, 1991; Sullivan & Wilson, 1991; Sullivan et al., 1990;Wilson & Sullivan, 1990). This Sensitive Period for attachment learning developmentally ends at PN10 (Sullivan, Landers, et al., 2000). However, elevation of CORT through systemic or intra-amygdala injection alters typical development by permitting amygdala plasticity in pups younger than PN10 allowing aversion learning (Barr et al., 2009; Moriceau & Sullivan, 2004, 2006; Moriceau et al., 2006; Shionoya et al., 2007). The present results suggest that Sensitive Period odor preference learning can be prematurely terminated and odor aversions can be learned by pups as young as PN6 provided CORT levels are sufficiently elevated. Since early life stress induces premature elevation of CORT (pups’ endogenous CORT: Bell & Denenberg, 1962; Branchi, 2009; Brunson et al., 2005; Cameron et al., 2005; Coe, Glass, Wiener, & Levine, 1983; Denenberg, 1963; Harlow & Harlow, 1965; Jordan, Hennessy, Gonzalez, & Levine, 1985; Levine, 1952, 1967, 2001; Macri & Wurbel, 2006; Rosenzweig et al., 1969; Sackett, 1972; Suchecki & Tufik, 2000; Tang, Akers, Reeb, Romeo, & McEwen, 2006 or through the mother’s milk: Yeh, 1984), this suggests that early life stress could prematurely end pups’ Sensitive Period for learning the maternal odor. Indeed, we have recently shown that rearing pups with an abusive mother (adaptation of the Baram lab’s stress paradigm using insufficient bedding for maternal nest building: Avishai-Eliner, Gilles, Eghbal-Ahmadi, Bar-El, & Baram, 2001) prematurely ended pups’ Sensitive Period learning at PN6–7 and was caused by CORT activation of the amygdala (Moriceau, Shionoya, Jakubs, & Sullivan, in press).
While the present study did not assess why CORT fails to support aversion learning at PN5, the anatomical data suggests the amygdala may not be sufficiently mature. Amygdala nuclei begin to emerge prior to parturition around E17, neurogenesis peaks between E20-PN7 continuing through adolescence and delineation of amygdala nuclei are present by PN7 (Bouwmeester, Smits, & Van Ree, 2002; Cunningham, Bhattacharyya, & Benes, 2002; Schwob & Price, 1984). CORT receptors are present in the neonatal rat (Cintra et al., 1993; Meaney, Sapolsky, & McEwen, 1985a; Rosenfeld, Sutanto, Levine, & De Kloet, 1988; Rosenfeld, van Eekelen, Levine, & de Kloet, 1993; Sapolsky & Meaney, 1986). The functional significance of this may relate to the fact that birth is associated with elevation of pups’ CORT (Guillet, Saffran, & Michaelson, 1980; Malinowska, Hardy, & Nathanielsz, 1972; Martin, Cake, Hartmann, & Cook, 1977; Meaney et al., 1985a; Meaney, Sapolsky, & McEwen, 1985b; Milkovic & Milkovic, 1963) yet pups must learn the maternal odor at birth to nipple attach and receive the nutrients and warmth required for survival (Leon, 1992; Pedersen et al., 1982). It should also be noted that our understanding of the SHRP has greatly increased in recent years and we now know that there is no absolute SHRP since some stressful stimuli produce a robust CORT increase in pups, such as cold temperature (reviews, Dallman, 2000; Levine et al., 2000).
Upper Limit of CORT Regulation of Fear Learning: PN15
The upper limit for CORT-dependent aversion learning appears to be PN15 since this is the last age suppressing CORT also suppresses fear learning. Specifically, by PN10, shock naturally increases CORT sufficiently to permit the amygdala-dependent fear learning (Moriceau & Sullivan, 2004, 2006; Moriceau et al., 2006; Shionoya et al., 2007). However, in PN10–15 pups, removing the source of CORT (adrenalectomy), intra-amygdala infusion of CORT receptor antagonist, or suppressing CORT through maternal presence can all reinstate the preference learning (Moriceau & Sullivan, 2004, 2006; Moriceau et al., 2006). The ecological significance of lower CORT levels preventing fear learning was demonstrated by maternal presence, (Moriceau & Sullivan, 2006; Raineki et al., submitted; Shionoya et al., 2007) which prevents pups’ shock-induced CORT release (Stanton & Levine, 1990; Suchecki, Mozaffarian, et al., 1993). A causal link between maternal presence, decreased CORT and suppression of amygdala-dependent fear was shown by intraamygdala CORT infusion (Moriceau & Sullivan, 2006) and initiation of the CORT release at the level of the hypothalamic paraventricular nucleus (Shionoya et al., 2007) both of which override the effect of maternal presence and permit the amygdala-dependent fear learning. Furthermore, maternal suppression of pups’ CORT blocked amygdala plasticity and reinstated olfactory bulb changes, although overriding this suppression with either systemic or intra-amygdala CORT reinstated the amygdala plasticity and odor aversion learning (Moriceau & Sullivan, 2004, 2006; Shionoya et al., 2007). Here, we further demonstrated this link by lowering CORT through maternal presence or with Metyrapone and define the upper age limit for this CORT reduction blocking amygdala-dependent fear learning at PN15.
The present study did not assess why PN16 pups fail to obtain preference learning with maternal presence, but as pups leave the nest, learning about the world and avoiding danger is critical for survival and the amygdala fear system is required. These results indicate a change in the role of CORT during aversion learning from necessary in infancy to the more “adult-like” modulatory role at PN16 as the pup matures and approaches independent living in less than 1 week (Akirav et al., 2004; Beylin & Shors, 2003; Hodes & Shors, 2005; Liu, Tsuji, Takeda, Takada, & Matsumiya, 1999; McGaugh & Roozendaal, 2002; McGaugh, Roozendaal, & Cahill, 1999; Sandi, 1998; Shors, 2001).
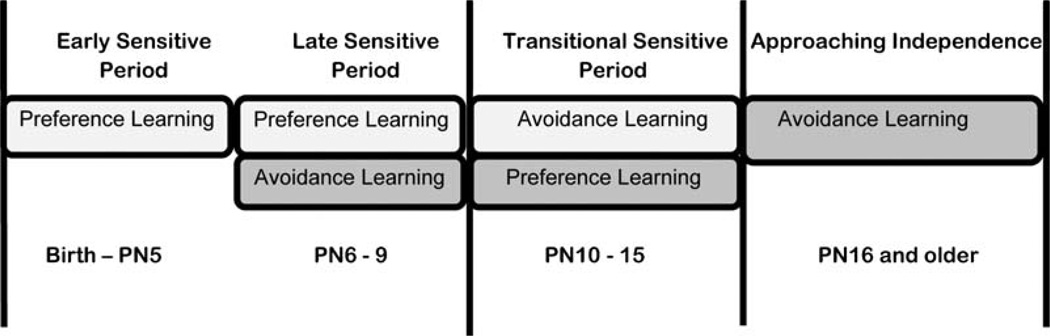
The effects of experience and endocrine control (specifically CORT) on development and pup learning are illustrated in Figure 4. From PN6 through PN15, pups can switch between preference and aversion learning during the later part of the Sensitive Period, as well as during the Transitional Sensitive Period dependent upon CORT level. During this time, pups appear to learn an odor aversion with elevated CORT but an odor preference with low CORT, although how the CORT level is controlled varies with development (Moriceau & Sullivan, 2006; Moriceau et al., 2006; Shionoya et al., 2007). It should also be noted that preference learning, even after an episode of CORT induced odor aversion learning, remains accessible when CORT levels are low again. This suggests that when pups are in the nest with the mother, an odor preference will be learned regardless of the treatment pups receive from the mother, even following experience with fear conditioning outside the nest. Since pups still require the mother’s milk for growth and the mother’s diet may change her maternal odor, continued odor learning is critical for survival.
FIGURE 4.
Defining pup’s sensitive period: A graphic illustration of rat pup avoidance learning development prior to weaning. Here, the traditionally defined Sensitive Period age range (PN0–PN10) is divided into early and late to delineate an age range when CORT can induce odor-shock odor aversions. This may occur naturally when the SHRP is prematurely ended through stressful early life rearing. After PN10 until PN15, pups are able to learn both preference and avoidance during fear conditioning depending on maternal presence suppression of endogenous CORT release. As pups approach weaning, only aversion learning is available to pups even with lowered CORT levels.
Integration of the Odor-0.5mA Shock Learning, Odor Malaise Learning, and CORT Effects on Infant Rat Learning
Pups, including fetal rats, can also learn to avoid odors when paired with malaise using LiCl or elevated (≥1.0 mA) shock level (Gruest, Richer, & Hars, 2004; Haroutunian & Campbell, 1979; Molina, Hoffmann, & Spear, 1986; Raineki et al., 2009; Richardson & McNally, 2003; Rudy & Cheatle, 1977; Shionoya et al., 2006; Stickrod, Kimble, & Smotherman, 1982). Shock greater than 1.0 mA in preweanling pups is considered an interceptive stimulus similar to LiCl, both of which produce gastrointestinal distress (Haroutunian & Campbell, 1979; Raineki et al., 2009). Neural correlates of learning suggest odor aversion induced by malaise (LiCl, >1.0 mA) produce plasticity within the olfactory bulb and piriform cortex, while the odor-0.5mA shock conditioning produces plasticity within the amygdala (Raineki et al., 2009; Shionoya et al., 2006), although by weaning age all produce amygdala plasticity (Raineki et al., 2009; Shionoya et al., 2006), similarly to adults (Batsell & Blankenship, 2002; Bermudez-Rattoni, Grijalva, Kiefer, & Garcia, 1986; Ferry & Di Scala, 1997; LeDoux, 2000; Touzani & Sclafani, 2005).
CONCLUSION
The Transitional Sensitive Period naturally begins by PN10 when the pups’ environment expands beyond the nest, yet pups frequently return to the nest to nurse. This developmental period of dual learning allows continued attachment learning to the mother while still learning skills needed for independent life outside the nest. These data suggest that by PN6, the premature CORT increase induced by early life stress can also prematurely induce amygdala-dependent fear learning, which can interfere with attachment learning. Our data also indicates that a single episode of increased CORT does not permanently change attachment learning, insuring continued maternal odor learning. Our data further suggests that pups no longer have access to the odor attachment preference learning by PN16 when survival without the mother is possible although growth is restricted (Greenberg & Ackerman, 1986; Ito, Kikusui, Takeuchi, & Mori, 2006; Kikusui, Nakamura, & Mori, 2008).
The robust effects of early life CORT on attachment learning suggests that some enduring effects of early life stress (i.e., maternal deprivation) may be mediated through variations in mother-infant attachment quality. That is, behavioral effects of CORT may combine with neural organizational effects of CORT to alter typical Sensitive Period learning and contribute to some of the enduring effects of early life experience (Bell & Denenberg, 1962; Branchi, 2009; Cameron et al., 2005; Champagne & Curley, 2009; Coe et al., 1983; Denenberg, 1963; Harlow & Harlow, 1965; Jordan et al., 1985; Levine, 1952, 1967, 2001; Macri & Wurbel, 2006; O’Connor & Cameron, 2006; Rosenzweig et al. 1969; Sackett, 1972; Suchecki & Tufik, 2000; Tang et al., 2006). Together, these data suggest a re-examination of the concept of sensitive periods, as has been discussed previously (Armstrong et al., 2006a,b; Bateson, 1979; Michel & Tyler, 2005; Maurer 2006; Thomas and Johnson, 2006; Tyler 2006).
Acknowledgments
Contract grant sponsor: National Institute of Health
Contract grant numbers: DC003906, DC009910
Contract grant sponsor: NSF
Contract grant number: IOB0544406
Contract grant sponsor: Leon Levy Foundation
This work was funded by grants from National Institute of Health DC003906 and DC009910, NSF IOB0544406, and Leon Levy Foundation to R.M.S. and NSF K20 fellowship grant to K.J.U. This manuscript was written as part of a Master Thesis for Karen J. Upton. KJU would like to graciously acknowledge the contributions of committee members Dr. Regina Sullivan, Dr. Don Wilson, Dr. Maryjohn O’Hair and ad hoc member Dr. Stephanie Moriceau.
Contributor Information
Karen J. Upton, Email: kupton@ou.edu.
Regina M. Sullivan, Email: regina.sullivan@nyumc.org.
REFERENCES
- Akirav I, Kozenicky M, Tal D, Sandi C, Venero C, Richter-Levin G. A facilitative role for corticosterone in the acquisition of a spatial task under moderate stress. Learning & Memory (Cold Spring Harbor) 2004;11(2):188–195. doi: 10.1101/lm.61704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts JR, May B. Nonnutritive, thermotactile induction of filial huddling in rat pups. Developmental Psychobiology. 1984;17(2):161–181. doi: 10.1002/dev.420170207. [DOI] [PubMed] [Google Scholar]
- Armstrong VL, Brunet PM, He C, Nishimura M, Poole HL, Spector FJ. What is so critical? A commentary on the reexamination of critical periods. Developmental Psychobiology. 2006a;48(4):326–331. doi: 10.1002/dev.20135. [DOI] [PubMed] [Google Scholar]
- Armstrong VL, Brunet PM, He C, Nishimura M, Poole HL, Spector FJ. When is a description needed for an explanation: Rejoiner. Developmental Psychobiology. 2006b;48(4):335–336. doi: 10.1002/dev.20135. [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, Gilles EE, Eghbal-Ahmadi M, Bar-El Y, Baram TZ. Altered regulation of gene and protein expression of hypothalamic-pituitary-adrenal axis components in an immature rat model of chronic stress. Journal of Neuroendocrinology. 2001;13(9):799–807. doi: 10.1046/j.1365-2826.2001.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr GA, Moriceau S, Shionoya KS, Muzny K, Gao P, Wang S, Sullivan R. Transitions in infant learning are modulated by dopamine in the amygdala. Nature Neuroscience. 2009;12:1367–1369. doi: 10.1038/nn.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett D, Gonzalez-Lima F. Behavioral effects of metyrapone on Pavlovian extinction. Neuroscience Letters. 2004;371(2–3):91–96. doi: 10.1016/j.neulet.2004.08.046. [DOI] [PubMed] [Google Scholar]
- Batsell WR, Blankenship AG. Beyond potentiation: Synergistic conditioning in flavor-aversion learning. Brain Mind. 2002;3:383–408. [Google Scholar]
- Bateson P. How do sensitive periods arise and what are they for? Animal Behaviour. 1979;27(2):470–486. [Google Scholar]
- Bell RW, Denenberg VH. The interrelationships of shock and critical periods in infancy as they affect adult learning and activity. Animal Behavior. 1962;11:21–27. [Google Scholar]
- Bermudez-Rattoni F, Grijalva CV, Kiefer SW, Garcia J. Flavor-illness aversions: The role of the amygdala in the acquisition of taste-potentiated odor aversions. Physiology & Behavior. 1986;38(4):503–508. doi: 10.1016/0031-9384(86)90417-8. [DOI] [PubMed] [Google Scholar]
- Beylin AV, Shors TJ. Glucocorticoids are necessary for enhancing the acquisition of associative memories after acute stressful experience. Hormones and Behavior. 2003;43(1):124–131. doi: 10.1016/s0018-506x(02)00025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester H, Smits K, Van Ree JM. Neonatal development of projections to the basolateral amygdala from prefrontal and thalamic structures in rat. Journal of Comparative Neurology. 2002;450(3):241–255. doi: 10.1002/cne.10321. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Attachment and Loss. Vol. 1. New York: Basic Books; 1969. [Google Scholar]
- Branchi I. The mouse communal nest: Investigating the epigenetic influences of the early social environment on brain and behavior development. Neuroscience Biobehavior Review. 2009;33:551–559. doi: 10.1016/j.neubiorev.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Brunjes PC, Alberts JR. Olfactory stimulation induces filial preferences for huddling in rat pups. Journal of Comparative & Physiological Psychology. 1979;93(3):548–555. doi: 10.1037/h0077571. [DOI] [PubMed] [Google Scholar]
- Brunson KL, Kramar E, Lin B, Chen Y, Colgin LL, Yanagihara TK, et al. Mechanisms of late-onset cognitive decline after early-life stress. Journal of Neuroscience. 2005;25(41):9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron NM, Champagne FA, Parent C, Fish EW, Ozaki-Kuroda K, Meaney MJ. The programming of individual differences in defensive responses and reproductive strategies in the rat through variations in maternal care. Neuroscience Biobehavior Review. 2005;29:843–865. doi: 10.1016/j.neubiorev.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Camp LL, Rudy JW. Changes in the categorization of appetitive and aversive events during postnatal development of the rat. Developmental Psychobiology. 1988;21(1):25–42. doi: 10.1002/dev.420210103. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Curley JP. Epigenetic mechanisms mediating the long-term effects of maternal care on development. Neuroscience Biobehavior Review. 2009;33:593–600. doi: 10.1016/j.neubiorev.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Cintra A, Solfrini V, Bunnemann B, Okret S, Bortolotti F, Gustafsson JA, et al. Prenatal development of glucocorticoid receptor gene expression and immunoreactivity in the rat brain and pituitary gland: A combined in situ hybridization and immunocytochemical analysis. Neuroendocrinology. 1993;57(6):1133–1147. doi: 10.1159/000126480. [DOI] [PubMed] [Google Scholar]
- Cirulli F, Gottlieb SL, Rosenfeld P, Levine S. Maternal factors regulate stress responsiveness in the neonatal rat. Psychobiology. 1992;20(2):143–152. [Google Scholar]
- Cirulli F, Santucci D, Laviola G, Alleva E. Behavioral and hormonal responses to stress in the newborn mouse: Effects of maternal deprivation and chlordiazepoxide. Developmental Psychobiology. 1994;27(5):301–316. doi: 10.1002/dev.420270505. [DOI] [PubMed] [Google Scholar]
- Coe CL, Glass JC, Wiener SG, Levine S. Behavioral, but not physiological, adaptation to repeated separation in mother and infant primates. Psychoneuroendocrinology. 1983;8:401–409. doi: 10.1016/0306-4530(83)90019-7. [DOI] [PubMed] [Google Scholar]
- Cordero MI, Kruyt ND, Merino JJ, Sandi C. Glucocorticoid involvement in memory formation in a rat model for traumatic memory. Stress. 2002;5:73–79. doi: 10.1080/1025389029000124404. [DOI] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: Implications for the development of normal and abnormal function during adolescence. Journal of Comparative Neurology. 2002;453(2):116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Dallman M. Editorial: Moments in time—The neonatal rat Hypothalamo-pituitary-adrenal axis. Endocrinology. 2000;141:1590–1592. doi: 10.1210/endo.141.5.7527. [DOI] [PubMed] [Google Scholar]
- Denenberg VH. Early experience and emotional development. Scientific American. 1963;208:138–146. doi: 10.1038/scientificamerican0663-138. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Gale GD. The amygdala, fear, and memory. Annals of the New York Academy of Sciences. 2003;985:125–134. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23(2):229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Ferry B, Di Scala G. Bicuculline administration into basolateral amygdala facilitates trace conditioning of odor aversion in the rat. Neurobiology of Learning and Memory. 1997;67(1):80–83. doi: 10.1006/nlme.1996.3743. [DOI] [PubMed] [Google Scholar]
- Fifer W, Moon C. The effects of fetal experience with sound. In: Lecanuet J, Fifer W, Krasnegor N, Smotherman W, editors. Fetal development—A psychobiological perspective. Hillsdale, New Jersey: Lawrence Erlbaum; 1995. pp. 351–368. [Google Scholar]
- Greenberg D, Ackerman SH. Reduced fat stores after early weaning: A correlate of vulnerablity to stress ulcers. Physiology and Behavior. 1986;38(3):375–380. doi: 10.1016/0031-9384(86)90108-3. [DOI] [PubMed] [Google Scholar]
- Gruest N, Richer P, Hars B. Emergence of long-term memory for conditioned aversion in the rat fetus. Developmental Psychobiology. 2004;44(3):189–198. doi: 10.1002/dev.20004. [DOI] [PubMed] [Google Scholar]
- Guerin D, Didier A, Peace ST, Linster C, Cleland TA. Noradrenergic neuromodulation in the olfactory bulb modulates odor habituation and spontaneous discrimination. Behavioral Neuroscience. 2008;122(4):816–826. doi: 10.1037/a0012522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillet R, Saffran M, Michaelson SM. Pituitary-adrenal response in neonatal rats. Endocrinology. 1980;106:991–994. doi: 10.1210/endo-106-3-991. [DOI] [PubMed] [Google Scholar]
- Harlow HF, Harlow M. The affectional system. In: Schrier A, Harlow H, Stollnitz F, editors. Behavior of nonhuman primates. Vol. 2. New York: Academic Press; 1965. [Google Scholar]
- Haroutunian V, Campbell BA. Emergence of interoceptive and exteroceptive control of behavior in rats. Science. 1979;205(4409):927–929. doi: 10.1126/science.472715. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Kaiser S, Sachser N. Social buffering of stress response: Diversity, mechanisms, and functions. Frontiers in Neuroendocrinology. 2009;30:470–482. doi: 10.1016/j.yfrne.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Maken DS, Graves FC. Presence of mother and unfamiliar female alters levels of testosterone, progesterone, cortisol, adrenocorticotropin, and behavior in maturing guinea pigs. Hormones & Behavior. 2002;42:42–52. doi: 10.1006/hbeh.2002.1794. [DOI] [PubMed] [Google Scholar]
- Hensch T. Critical period regulation. Annual Review of Neuroscience. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hess EH, Schaeffer HH. Innate behavior patterns as indicators of the ‘Critical Period’. Zeitschrift fur Tierpsychologie. 1959;16:155–160. [Google Scholar]
- Hodes GE, Shors TJ. Distinctive stress effects on learning during puberty. Hormones and Behavior. 2005;48(2):163–171. doi: 10.1016/j.yhbeh.2005.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer MA, Sullivan RM. Towards a neurobiology of attachment. In: Nelson CA, Luciana M, editors. Handbook of developmental cognitive neuroscience. 2nd ed. Cambridge: MIT press; 2008. pp. 787–806. [Google Scholar]
- Hudson R. Olfactory imprinting. Current Opinion in Neurobiology. 1993;3(4):548–552. doi: 10.1016/0959-4388(93)90054-3. [DOI] [PubMed] [Google Scholar]
- Ito A, Kikusui T, Takeuchi Y, Mori Y. Effects of early weaning on anxiety and autonomic responses to stress in rats. Behavioural Brain Research. 2006;171(1):87–93. doi: 10.1016/j.bbr.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Johanson IB, Hall WG. Appetitive learning in 1-day-old rat pups. Science. 1979;205(4404):419–421. doi: 10.1126/science.451612. [DOI] [PubMed] [Google Scholar]
- Johanson IB, Teicher MH. Classical conditioning of an odor preference in 3-day-old rats. Behavioral & Neural Biology. 1980;29(1):132–136. doi: 10.1016/s0163-1047(80)92596-0. [DOI] [PubMed] [Google Scholar]
- Jordan TC, Hennessy MB, Gonzalez CA, Levine S. Social and environmental factors influencing mother-infant separation-reunion in squirrel monkeys. Physiology and Behavior. 1985;34:489–493. doi: 10.1016/0031-9384(85)90038-1. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Nakamura K, Mori Y. A review of the behavioral and neurochemical consequences of early weaning in rodents. Applied Animal Behaviour Science. 2008;110(1):73–83. [Google Scholar]
- Koch B, Lutz B, Mialhe C. Plasma level and metabloism of corticosterone during the postnatal period in the rat. Journal De Physiologie. 1969;61:142. [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. The emotional brain, fear, and the amygdala. Cellular and Molecular Neurobiology. 2003;23(4–5):727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. The amygdala. Current Biology. 2007;17(20):R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Languille S, Richer P, Hars B. Approach memory turns to avoidance memory with age. Behavioural Brain Research. 2009;202(2):278–284. doi: 10.1016/j.bbr.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Leeper LL, Schroeder R, Henning SJ. Kinetics of circulating corticosterone in infant rats. Pediatric Research. 1988;24(5):595–599. doi: 10.1203/00006450-198811000-00011. [DOI] [PubMed] [Google Scholar]
- Leon M. Dietary control of maternal pheromone in the lactating rat. Physiology and Behavior. 1975;14:311–319. doi: 10.1016/0031-9384(75)90039-6. [DOI] [PubMed] [Google Scholar]
- Leon M. Neuroethology of olfactory preference development. Journal of Neurobiology. 1992;23(10):1557–1573. doi: 10.1002/neu.480231012. [DOI] [PubMed] [Google Scholar]
- Levine S. A further study of infantile handling and adult avoidance learning. Journal of Pres. 1952;25:70–80. doi: 10.1111/j.1467-6494.1956.tb01289.x. [DOI] [PubMed] [Google Scholar]
- Levine S. Plasma-free corticosteroid response to electric shock in rats stimulated in infancy. Science. 1962;135:795–796. doi: 10.1126/science.135.3506.795-a. [DOI] [PubMed] [Google Scholar]
- Levine S. Maternal and environmental influences on the adrenocortical response to stress in weanling rats. Science. 1967;156:258–260. doi: 10.1126/science.156.3772.258. [DOI] [PubMed] [Google Scholar]
- Levine S. The ontogeny of the hypothalamic-pituitary-adrenal axis. The influence of maternal factors. Annuals of New York Academy of Science. 1994;746:275–288. doi: 10.1111/j.1749-6632.1994.tb39245.x. [DOI] [PubMed] [Google Scholar]
- Levine S. Primary social relationships influence the development of the hypothalamic-pituitary-adrenal axis in the rat. Physiology & Behavior. 2001;73(3):255–260. doi: 10.1016/s0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- Levine S, Dent GW, de Kloet ER. Stress hyporesponsive period. In: Fink G, editor. Encyclopedia of stress. Vol. 3. San Diego: Academic Press; 2000. pp. 518–526. [Google Scholar]
- Liu L, Tsuji M, Takeda H, Takada K, Matsumiya T. Adrenocortical suppression blocks the enhancement of memory storage produced by exposure to psychological stress in rats. Brain Research. 1999;821(1):134–140. doi: 10.1016/s0006-8993(99)01085-9. [DOI] [PubMed] [Google Scholar]
- Lorenz K. The companion in the bird’s world. The fellow-member of the species as releasing factor of social behavior. Journal fur Ornithologie Beiblatt (Leipzig) 1935;83:137–213. [Google Scholar]
- Macri S, Wurbel H. Developmental plasticity of HPA and fear responses in rats: A critical review of the Developmental Psychobiology Age Limits of Sensitive Period Learning 461 maternal mediation hypothesis. Hormones and Behavior. 2006;50(5):667–680. doi: 10.1016/j.yhbeh.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Malinowska KW, Hardy RN, Nathanielsz PW. Plasma adrenocorticosteroid concentrations immediately after birth in the rat, rabbit and guinea pig. Cellular and Molecular Life Sciences. 1972;28:1366–1367. doi: 10.1007/BF01965349. [DOI] [PubMed] [Google Scholar]
- Martin CE, Cake MH, Hartmann PE, Cook IF. Relationship between foetal corticosteroids, maternal progesterone and parturition in the rat. Acta Endocrinologica. 1977;84(1):167–176. doi: 10.1530/acta.0.0840167. [DOI] [PubMed] [Google Scholar]
- Maurer D. Introduction to four articles on sensitive periods. Developmental Psychobiology. 2006;48(4):325. [Google Scholar]
- McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Current Opinion in Neurobiology. 2002;12(2):205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B, Cahill L. Modulation of memory storage by stress hormones and the amygdaloid complex. In: Gazzaniga M, editor. Cognitive neuroscience. 2nd ed. Cambridge: MIT Press; 1999. [Google Scholar]
- Meaney MJ, Sapolsky RM, McEwen BS. The development of the glucocorticoid receptor system in the rat limbic brain. I. Ontogeny and autoregulation. Brain Research. 1985a;350(1–2):159–164. doi: 10.1016/0165-3806(85)90259-7. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Sapolsky RM, McEwen BS. The development of the glucocorticoid receptor system in the rat limbic brain. II. An autoradiographic study. Brain Research. 1985b;350(1–2):165–168. doi: 10.1016/0165-3806(85)90260-3. [DOI] [PubMed] [Google Scholar]
- Michel GF, Tyler AN. Critical period: A history of the transition from questions of when, to what, to how. Developmental Psychobiology. 2005;46(3):156–162. doi: 10.1002/dev.20058. [DOI] [PubMed] [Google Scholar]
- Milkovic K, Milkovic S. Functioning of the pituitary adrenocortical axis in rats at and after birth. Endocrinology. 1963;73:535–539. doi: 10.1210/endo-73-5-535. [DOI] [PubMed] [Google Scholar]
- Molina JC, Hoffmann H, Spear NE. Conditioning of aversion to alcohol orosensory cues in 5- and 10-day rats: Subsequent reduction in alcohol ingestion. Developmental Psychobiology. 1986;19(3):175–183. doi: 10.1002/dev.420190304. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Shionoya K, Jakubs K, Sullivan RM. Early life stress disrupts attachment learning: The role of amygdala corticosterone, locus coeruleus CRF and olfactory bulb NE. Journal of Neuroscience. doi: 10.1523/JNEUROSCI.4106-09.2009. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Corticosterone influences on mammalian neonatal sensitive-period learning. Behavioral Neuroscience. 2004;118(2):274–281. doi: 10.1037/0735-7044.118.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Neurobiology of infant attachment. Developmental Psychobiology. 2005;47(3):230–242. doi: 10.1002/dev.20093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nature Neuroscience. 2006;9(8):1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Wilson DA, Levine S, Sullivan RM. Dual circuitry for odor-shock conditioning during infancy: Corticosterone switches between fear and attraction via amygdala. Journal of Neuroscience. 2006;26(25):6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak RF, Murphy TM, Lindsay DR, Alster P, Andersson R, Uvnas-Moberg K. Development of a preferential relationship with the mother by the newborn lamb: Importance of the sucking activity. Physiology & Behavior. 1997;62(4):681–688. doi: 10.1016/s0031-9384(97)00079-6. [DOI] [PubMed] [Google Scholar]
- O’Connor J, Cameron J. Translating research findings on early experience to prevention: Animal and human evidence on early attachment relationships. American Journal of Preventive Medicine. 2006;31:175–181. doi: 10.1016/j.amepre.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Pedersen PE, Williams CL, Blass EM. Activation and odor conditioning of suckling behavior in 3-day-old albino rats. Journal of Experimental Psychology: Animal Behavior Processes. 1982;8(4):329–341. [PubMed] [Google Scholar]
- Poindron P, Terrazas A, Hernandez H. Exclusive mother-young bonding in sheep and goats: Physiological determinants and consequences. Revista Mexicana de Psicologia. 2003;20(2):265–281. [Google Scholar]
- Polan HJ, Hofer MA. Olfactory preference for mother over home nest shavings by newborn rats. Developmental Psychobiology. 1998;33(1):5–20. [PubMed] [Google Scholar]
- Raineki C, Moriceau S, Sullivan RM. Developing a neurobehavioral animal model for infant attachment to an abusive caregiver. doi: 10.1016/j.biopsych.2009.12.019. (in press Biological Psychiatry). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Shionoya K, Sander K, Sullivan RM. Ontogeny of odor-LiCl vs. odor-shock learning: Similar behaviors but divergent ages of functional amygdala emergence. Learning & Memory. 2009;16(2):114–121. doi: 10.1101/lm.977909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel S, Leon M. Early odor preference training increases olfactory bulb norepinephrine. Developmental Brain Research. 1995;85(2):187–191. doi: 10.1016/0165-3806(94)00211-h. [DOI] [PubMed] [Google Scholar]
- Richardson R, McNally GP. Effects of an odor paired with illness on startle, freezing, and analgesia in rats. Physiology & Behavior. 2003;78(2):213–219. doi: 10.1016/s0031-9384(02)00974-5. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Bohus B, McGaugh JL. Dose-dependent suppression of adrenocortical activity with metyrapone: Effects on emotion and memory. Psychoneuroendocrinology. 1996;21:681–693. doi: 10.1016/s0306-4530(96)00028-5. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P, Sutanto W, Levine S, De Kloet ER. Ontogeny of type I and type II corticosteroid receptors in the rat hippocampus. Brain Research. 1988;470(1):113–118. doi: 10.1016/0165-3806(88)90206-4. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P, van Eekelen JA, Levine S, de Kloet ER. Ontogeny of corticosteroid receptors in the brain. Cellular and Molecular Neurobiology. 1993;13(4):295–319. doi: 10.1007/BF00711575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL, Diamond MC, Wu SY, Slagle RW, Saffran E. Influences of environmental complexity and visual stimulation on development of occipital cortex in rat. Brain Research. 1969;14:427–445. doi: 10.1016/0006-8993(69)90120-6. [DOI] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Memory of early maltreatment: Neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biological Psychiatry. 2005;57(8):823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Cheatle MD. Odor-aversion learning in neonatal rats. Science. 1977;198(4319):845–846. doi: 10.1126/science.918668. [DOI] [PubMed] [Google Scholar]
- Sackett GP. Exploratory behavior of rhesus monkeys as a function of rearing condition and sex. Developmental Psychology. 1972;6:260–270. [Google Scholar]
- Sananes CB, Campbell BA. Role of the central nucleus of the amygdala in olfactory heart rate conditioning. Behavioral Neuroscience. 1989;103(3):519–525. [PubMed] [Google Scholar]
- Sandi C. The role and mechanisms of action of glucocorticoid involvement in memory storage. Journal of Neural Transplantation and Plasticity. 1998;6(3):41–52. doi: 10.1155/NP.1998.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: Neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Research Reviews. 1986;11(1):65–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Schaal B, Orgeur P, Arnould C. Olfactory preferences in newborn lambs: Possible influences of prenatal experience. Behaviour. 1995;132(5):351–365. [Google Scholar]
- Schapiro S, Geller E, Eiduson S. Corticoid response to stress in the steroid-inhibited rat. Proceedings of the Society for Experimental Biology and Medicine (New York, NY) 1962;109:935–937. doi: 10.3181/00379727-109-27383. [DOI] [PubMed] [Google Scholar]
- Schettino LF, Otto T. Patterns of Fos expression in the amygdala and ventral perirhinal cortex induced by training in an olfactory fear conditioning paradigm. Behavioral Neuroscience. 2001;115(6):1257–1272. doi: 10.1037//0735-7044.115.6.1257. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Price JL. The development of lamination of afferent fibers to the olfactory cortex in rats, with additional observations in the adult. Journal of Comparative Neurology. 1984;223(2):203–222. doi: 10.1002/cne.902230205. [DOI] [PubMed] [Google Scholar]
- Sevelinges Y, Gervais R, Messaoudi B, Granjon L, Mouly AM. Olfactory fear conditioning induces field potential potentiation in rat olfactory cortex and amygdala. Learning & Memory. 2004;11(6):761–769. doi: 10.1101/lm.83604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shionoya K, Moriceau S, Bradstock P, Sullivan RM. Maternal attenuation of hypothalamic paraventricular nucleus norepinephrine switches avoidance learning to preference learning in preweanling rat pups. Hormones and Behavior. 2007;52(3):391–400. doi: 10.1016/j.yhbeh.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shionoya K, Moriceau S, Lunday L, Miner C, Roth TL, Sullivan RM. Developmental switch in neural circuitry underlying odor-malaise learning. Learning & Memory. 2006;13(6):801–808. doi: 10.1101/lm.316006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ. Acute stress rapidly and persistently enhances memory formation in the male rat. Neurobiology of Learning and Memory. 2001;75(1):10–29. doi: 10.1006/nlme.1999.3956. [DOI] [PubMed] [Google Scholar]
- Spalding DA. Instinct with original observations on young animals. Macmillan’s Magazine. 1873;27:282–293. [Google Scholar]
- Stanton ME, Levine S. Inhibition of infant glucocorticoid stress response: Specific role of maternal cues. Developmental Psychobiology. 1990;23(5):411–426. doi: 10.1002/dev.420230504. [DOI] [PubMed] [Google Scholar]
- Stickrod G, Kimble DP, Smotherman WP. In utero taste/odor aversion conditioning in the rat. Physiology & Behavior. 1982;28(1):5–7. doi: 10.1016/0031-9384(82)90093-2. [DOI] [PubMed] [Google Scholar]
- Suchecki D, Rosenfeld P, Levine S. Maternal regulation of the hypothalamic-pituitary-adrenal axis in the infant rat: The roles of feeding and stroking. Brain Research. 1993;75:185–192. doi: 10.1016/0165-3806(93)90022-3. [DOI] [PubMed] [Google Scholar]
- Suchecki D, Mozaffarian D, Gross G, Rosenfeld P, Levine S. Effects of maternal deprivation on the ACTH stress response in the infant rat. Neuroendocrinology. 1993;57(2):204–212. doi: 10.1159/000126361. [DOI] [PubMed] [Google Scholar]
- Suchecki D, Tufik S. Long-term effects of maternal deprivation on the corticosterone response to stress in rats. Behavioral Brain Research. 2000;111:99–106. doi: 10.1152/ajpregu.1997.273.4.R1332. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Brake SC, Hofer MA, Williams CL. Huddling and independent feeding of neonatal rats can be facilitated by a conditioned change in behavioral state. Developmental Psychobiology. 1986;19(6):625–635. doi: 10.1002/dev.420190613. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Hall WG. Reinforcers in infancy: Classical conditioning using stroking or intra-oral infusions of milk as UCS. Developmental Psychobiology. 1988;21(3):215–223. doi: 10.1002/dev.420210303. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Hofer MA, Brake SC. Olfactory-guided orientation in neonatal rats is enhanced by a conditioned change in behavioral state. Developmental Psychobiology. 1986;19(6):615–623. doi: 10.1002/dev.420190612. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Landers M, Yeaman B, Wilson DA. Good memories of bad events in infancy. Nature. 2000;407(6800):38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Leon M. Early olfactory learning induces an enhanced olfactory bulb response in young rats. Brain Research. 1986;392(1–2):278–282. doi: 10.1016/0165-3806(86)90256-7. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, McGaugh JL, Leon M. Norepinephrine-induced plasticity and one-trial olfactory learning in neonatal rats. Developmental Brain Research. 1991;60(2):219–228. doi: 10.1016/0165-3806(91)90050-s. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Stackenwalt G, Nasr F, Lemon C, Wilson DA. Association of an odor with activation of olfactory bulb noradrenergic beta-receptors or locus coeruleus stimulation is sufficient to produce learned approach responses to that odor in neonatal rats. Behavioral Neuroscience. 2000;114(5):957–962. doi: 10.1037/0735-7044.114.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA. Neural correlates of conditioned odor avoidance in infant rats. Behavioral Neuroscience. 1991;105(2):307–312. doi: 10.1037//0735-7044.105.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA. The locus coeruleus, norepinephrine, and memory in newborns. Brain Research Bulletin. 1994;35(5–6):467–472. doi: 10.1016/0361-9230(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Leon M. Norepinephrine and learning-induced plasticity in infant rat olfactory system. Journal of Neuroscience. 1989;9(11):3998–4006. doi: 10.1523/JNEUROSCI.09-11-03998.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Wong R, Correa A, Leon M. Modified behavioral and olfactory bulb responses to maternal odors in preweanling rats. Developmental Brain Research. 1990;53(2):243–247. doi: 10.1016/0165-3806(90)90013-o. [DOI] [PubMed] [Google Scholar]
- Tang AC, Akers KG, Reeb BC, Romeo RD, McEwen BS. Programming social, cognitive, and neuroendocrine development by early exposure to novelty. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(42):15716–15721. doi: 10.1073/pnas.0607374103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Blass EM. First suckling response of the newborn albino rat: The roles of olfaction and amniotic fluid. Science. 1977;198(4317):635–636. doi: 10.1126/science.918660. [DOI] [PubMed] [Google Scholar]
- Terry LM, Johanson IB. Effects of altered olfactory experiences on the development of infant rats’ responses to odors. Developmental Psychobiology. 1996;29(4):353–377. doi: 10.1002/(SICI)1098-2302(199605)29:4<353::AID-DEV4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Thomas MSC, Johnson MH. The computational modeling of sensitive periods. Developmental Psychobiology. 2006;48(4):337–344. doi: 10.1002/dev.20134. [DOI] [PubMed] [Google Scholar]
- Touzani K, Sclafani A. Critical role of amygdala in flavor but not taste preference learning in rats. European Journal of Neuroscience. 2005;22(7):1767–1774. doi: 10.1111/j.1460-9568.2005.04360.x. [DOI] [PubMed] [Google Scholar]
- Tyler AN. When is a description not an explanation: A response to Armstrong et al. (2006) Developmental Psychobiology. 2006;48(4):332–334. [Google Scholar]
- Van der Horst FCP, LeRoy H, Van der Veer R. “When Strangers Meet”: John Bowlby and Harry Harlow on attachment behavior. Integrative Psychological & Behavioral Science. 2008;42(4):370–388. doi: 10.1007/s12124-008-9079-2. [DOI] [PubMed] [Google Scholar]
- Walker CD, Perrin M, Vale W, River C. Ontogeny of the stress response in the rat: Role of the pituitary and the hypothalamus. Endocrinology. 1986;118:1445–1451. doi: 10.1210/endo-118-4-1445. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Leon M. Noradrenergic modulation of olfactory bulb excitability in the postnatal rat. Brain Research. 1988;470(1):69–75. doi: 10.1016/0165-3806(88)90202-7. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Olfactory associative conditioning in infant rats with brain stimulation as reward. I. Neurobehavioral consequences. Developmental Brain Research. 1990;53(2):215–221. doi: 10.1016/0165-3806(90)90009-n. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM, Leon M. Single-unit analysis of postnatal olfactory learning: Modified olfactory bulb output response patterns to learned attractive odors. Journal of Neuroscience. 1987;7(10):3154–3162. doi: 10.1523/JNEUROSCI.07-10-03154.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh KY. Corticosterone concentrations in the serum and milk of lactating rats: Parallel changes after induced stress. Endocrinology. 1984;115:1364–1370. doi: 10.1210/endo-115-4-1364. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Harley CW, Darby-King A, Neve RL, McLean JH. Early odor preference learning in the rat: Bidirectional effects of cAMP response element-binding protein (CREB) and mutant CREB support a causal role for phosphorylated CREB. Journal of Neuroscience. 2003;23(11):4760–4765. doi: 10.1523/JNEUROSCI.23-11-04760.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]