Abstract
Ethanol profoundly influences cerebellar circuit function and motor control. It has recently been demonstrated that functional N-methyl-d-aspartate (NMDA) receptors are postsynaptically expressed at climbing fiber (CF) to Purkinje cell synapses in the adult cerebellum. Using whole cell patch-clamp recordings from mouse cerebellar slices, we examined whether ethanol can affect NMDA receptor signaling in mature Purkinje cells. NMDA receptor-mediated currents were isolated by bath application of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptor antagonist 2,3-dihydroxy-6-nitro-7-sulfamoylbenzol[f]quinoxaline (NBQX). The remaining d-2-amino-5-phosphonovaleric acid (d-APV)-sensitive current was reduced by ethanol at concentrations as low as 10 mM. At a concentration of 50 mM ethanol, the blockade of d-APV-sensitive CF-excitatory postsynaptic currents was significantly stronger. Ethanol also altered the waveform of CF-evoked complex spikes by reducing the afterdepolarization. This effect was not seen when NMDA receptors were blocked by d-APV before ethanol wash-in. In contrast to CF synaptic transmission, parallel fiber (PF) synaptic inputs were not affected by ethanol. Finally, ethanol (10 mM) impaired long-term depression (LTD) at PF to Purkinje cell synapses as induced under control conditions by paired PF and CF activity. However, LTD induced by pairing PF stimulation with depolarizing voltage steps (substituting for CF activation) was not blocked by ethanol. These observations suggest that the sensitivity of cerebellar circuit function and plasticity to low concentrations of ethanol may be caused by an ethanol-mediated impairment of NMDA receptor signaling at CF synapses onto cerebellar Purkinje cells.
Keywords: alcohol, cerebellum, parallel fiber, motor coordination, synaptic plasticity
alcohol impairs the cerebellar contribution to motor control and the fine adjustment of movements (for review, see Valenzuela et al. 2010). For example, acute application of ethanol affects conditioned eyeblink responses in human subjects (Hobson 1966) as well as in rabbits (Hernández et al. 1986). Chronic ethanol exposure reduces cerebellar motor coordination and causes ataxia in mice (Servais et al. 2005). Purkinje cells, which provide the sole output of the cerebellar cortex, are a main target of ethanol (Siggins and French 1979; Rogers et al. 1980; Sorensen et al. 1980). Acute application of ethanol differentially interferes with Purkinje cell activity depending on the ethanol concentration. At low doses, ethanol can increase current-evoked spiking in Purkinje cells, while high doses cause a reduction in firing rates (Chu 1983; Franklin and Gruol 1987; Urrutia and Gruol 1992; Freund et al. 1993a). This complex effect may be due to the broad spectrum of targets of ethanol, such as voltage-gated calcium channels (Walter and Messing 1999; Belmeguenai et al. 2008), GABAergic transmission (Freund et al 1993b; Freund and Palmer 1997; Mameli et al. 2008), and type 1 metabotropic glutamate receptors (mGluR1) function (Carta et al. 2006; Belmeguenai et al. 2008). In addition, ethanol has been shown to alter cerebellar synaptic plasticity. Long-term depression (LTD) at climbing fiber (CF) synapses onto Purkinje cells (Hansel and Linden 2000) is inhibited by 50 mM ethanol (Carta et al. 2006). Similarly, parallel fiber (PF) LTD is blocked by bath application of ethanol (50 mM) in rat cerebellar slices (Belmeguenai et al. 2008; Su et al. 2010). At both types of synapses, it has been suggested that an inhibition of mGluR1 by ethanol contributes to the suppression of LTD (Carta et al. 2006; Belmeguenai et al. 2008; Su et al. 2010).
In cerebellar cultures, ethanol alters the response of Purkinje cells to glutamate application (Franklin and Gruol 1987). In slices prepared from young adult rats, ethanol reduces mGluR1-mediated currents, whereas α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptor signaling remains unaffected (Belmeguenai et al. 2008). It still is conceivable that in culture AMPA receptor signaling is impaired by ethanol as well, since in slices prepared from neonatal rats, hippocampal AMPA receptor transmission is depressed by ethanol (Mameli et al. 2005). In young adult and adult hippocampal pyramidal cells (CA1 and CA3), postsynaptic N-methyl-d-aspartate (NMDA) receptors become a main ethanol target and display a greater ethanol sensitivity than AMPA receptors (Lovinger et al. 1989, 1990; Peoples et al. 1997; Mameli et al. 2005). Until recently, it was assumed that Purkinje cells lack functional NMDA receptors (Farrant and Cull-Candy 1991; Llano et al. 1991). However, it has now been demonstrated that functional NMDA receptors, composed of NR1 and NR2-A/B subunits, are postsynaptically expressed at CF synapses onto Purkinje cells in the mature mouse cerebellum (Piochon et al. 2007; Renzi et al. 2007). NMDA receptor signaling has a late developmental onset with NMDA receptors reaching full expression levels after ∼8 wk (Piochon et al. 2007). In line with these results obtained in cerebellar slices, it has been shown that Purkinje cells in old, but not young, cerebellar cultures express functional NMDA receptors (Lonchamp et al. 2012). The physiological significance of NMDA receptor signaling in Purkinje cells is supported by the observation that in slices prepared from adult mice and rats, postsynaptically expressed NMDA receptors are required for PF-LTD induction (Piochon et al. 2010).
Here, we examine whether ethanol impairs NMDA receptor signaling and synaptic plasticity in cerebellar Purkinje cells. We show that, at concentrations as low as 10 mM, ethanol reduces d-2-amino-5-phosphonovaleric acid (d-APV)-sensitive synaptic responses at CF synapses and impairs PF-LTD induced by paired PF and CF activation. In contrast, LTD induced by pairing PF stimulation and application of depolarizing voltage steps is not affected by ethanol. It has previously been shown that both voltage-gated calcium channels and mGluR1 receptor signaling are affected by ethanol at concentrations of 50 mM but not 20 mM (Belmeguenai et al. 2008). Thus our data suggest that NMDA receptors are a primary ethanol target in Purkinje cells and are selectively impaired by ethanol at low concentrations.
MATERIALS AND METHODS
All animal procedures described were performed in accordance with a protocol approved by the Animal Care and Use Committee of the University of Chicago.
Slice preparation.
Sagittal slices of the cerebellar vermis (190–250 μm) were prepared from C57BL/6 mice (2- to 6-mo-old, unless specified otherwise) and were kept at room temperature in artificial cerebrospinal fluid (ACSF) containing the following (in mM): 124 NaCl, 5 KCl, 1.25 Na2HPO4, 2 CaCl2, 26 NaHCO3, and 10 d-glucose bubbled with 95% O2-5% CO2. Glycine (25 μM) was added to the ACSF to facilitate the NMDA receptor-mediated current. MgSO4 (2 mM) was added when indicated in the text. Throughout recording, slices were continuously perfused with ACSF that was supplemented with picrotoxin (100 μM) to block GABAA receptors. All drugs were purchased from Sigma (St. Louis, MO).
Whole cell patch-clamp recordings.
Patch-clamp recordings from the Purkinje cell soma were performed at room temperature using an EPC-10 amplifier (HEKA Electronics, Lambrecht/Pfalz, Germany). Currents were filtered at 3 kHz, digitized at 25 kHz, and acquired using Patchmaster software (HEKA). Patch pipettes (2–5 MΩ) were filled with a solution containing the following (in mM): 9 KCl, 10 KOH, 120 K-gluconate, 3.48 MgCl2, 10 HEPES, 4 NaCl, 4 Na2ATP, 0.4 Na3GTP, and 17.5 sucrose (pH 7.25–7.35). Purkinje cells were voltage clamped at holding potentials in the range of −65 to −78 mV. To evoke synaptic responses, PFs and CFs were activated using glass electrodes filled with ACSF. For PF stimulation, electrodes were placed in the upper molecular layer to reduce the risk of unintentional CF stimulation. For CF stimulation, electrodes were placed in the granule cell layer, just underneath the recorded Purkinje cells. In all experiments, the paired-pulse ratio was monitored using paired-pulse stimulation (100-ms interval) at a frequency of 0.05 Hz. For LTD induction, we used two different protocols. In the first protocol, 100-Hz PF burst stimulation (8–10 pulses) was followed 150 ms later by single pulse CF stimulation (current-clamp mode). This stimulation pattern was repeated at 1 Hz for 5 min. In the second protocol, depolarizing voltage steps (voltage-clamp; −75 to 0 mV; 250 ms) were paired with PF stimulation (2 pulses; 10-ms interval) at 1 Hz for 3 min. This protocol was applied for a second time after a period of 5 min. Series and input resistances were monitored throughout the experiments by applying hyperpolarizing voltage steps (−10 mV) at the end of each sweep. Recordings were excluded if series or input resistances varied by >15% over the course of the experiments. All values were averaged over time (3 successive responses) and are shown as a percentage of baseline (calculated from the last 5 min of baseline) ± SE. For statistical analysis, we used the Student's t-test (paired/unpaired) and the Mann-Whitney U test, when appropriate.
RESULTS
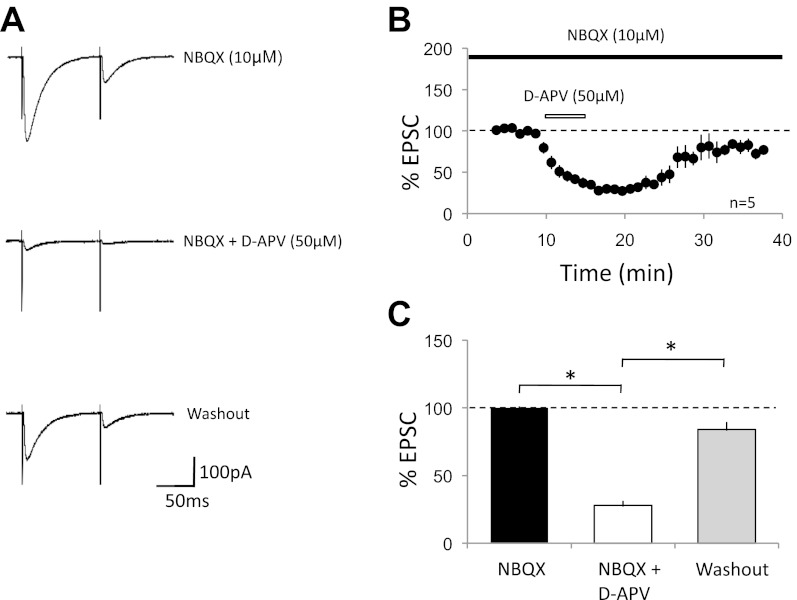
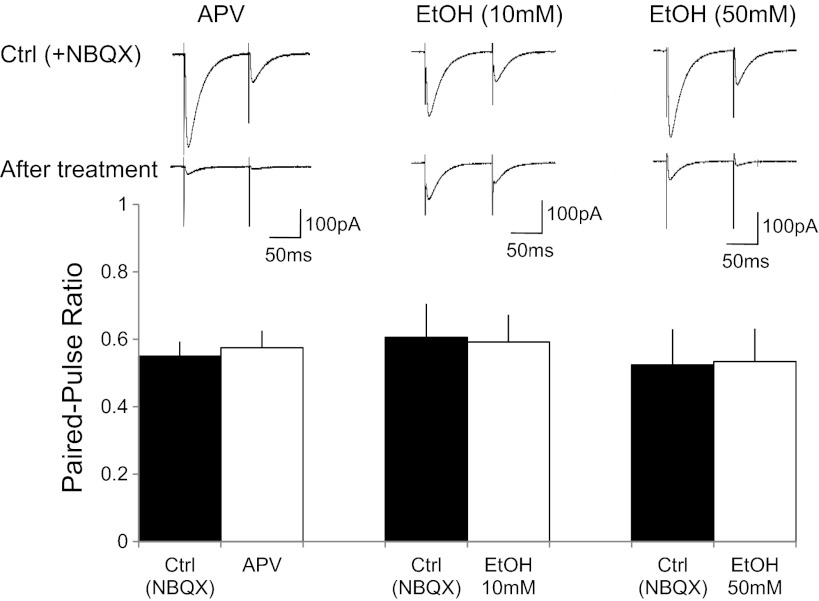
Whole cell voltage-clamp recordings were performed from Purkinje cells in acute cerebellar slices of 2- to 6-mo-old mice in the presence of 100 μM picrotoxin. Two CF-stimulation pulses were applied at an interval of 100 ms. CF-excitatory postsynaptic currents (EPSCs) were distinguished from PF-EPSCs by their typical paired-pulse depression and the characteristic all-or-none response. AMPA receptor-mediated currents were blocked by bath application of 2,3-dihydroxy-6-nitro-7-sulfamoylbenzol[f]quinoxaline (NBQX; 10 μM), unveiling a residual current of 150.8 ± 13.8 pA (EPSC1, n = 5; Fig. 1A; see Piochon et al. 2007). This residual current was significantly reduced by subsequent bath application of the NMDA receptor antagonist d-APV (50 μM; 37.2 ± 9.3 pA; 24.7 ± 3.5%; n = 5; P < 0.05; Fig. 1), suggesting that this current is largely NMDA receptor dependent. Upon washout of d-APV, the residual current recovered to 126.5 ± 31.1 pA (83.9 ± 16.3%; n = 5; P < 0.05; Fig. 1). The paired-pulse ratio of the residual CF-EPSC did not change with d-APV bath application (0.55 ± 0.04 under control conditions; 0.58 ± 0.05 in the presence of d-APV; n = 5; P > 0.05; see Fig. 3), suggesting that the NMDA receptors blocked by d-APV were of postsynaptic origin.
Fig. 1.
N-methyl-d-aspartate (NMDA) receptor-mediated currents at cerebellar climbing fiber-Purkinje cell synapses. A: typical traces. Climbing fiber (CF)-excitatory postsynaptic currents (EPSCs) recorded in the presence of 2,3-dihydroxy-6-nitro-7-sulfamoylbenzol[f]quinoxaline (NBQX; 10 μM; top); the residual current was further inhibited by d-2-amino-5-phosphonovaleric acid (d-APV; 50 μM; middle). After washout of d-APV, the current recovered (bottom). B: time graph of d-APV inhibition of NBQX-resistant CF-EPSCs (EPSC1; n = 5). Black bar represents the presence of NBQX (10 μM) in the bath. White bar indicates the presence of d-APV (50 μM) in the bath. C: bar graph showing the reduction of NBQX-resistant currents by d-APV and the recovery during washout (n = 5; the data were obtained during a 5-min baseline period, during 5 min of maximal reduction by d-APV, and during 5 min of steady-state recovery; *P < 0.05; paired Student's t-test). Error bars are means ± SE.
Fig. 3.
Paired-pulse ratio of CF-EPSCs remains unaffected in the presence of d-APV and ethanol, respectively. Top: typical traces under control conditions and after treatment with d-APV (left), 10 mM EtOH (middle), and 50 mM EtOH (right), respectively. Bottom: bar graphs showing paired-pulse ratio values before and during d-APV application (n = 5), as well as during the application of EtOH at concentrations of 10 mM (n = 6) and 50 mM (n = 9), respectively.
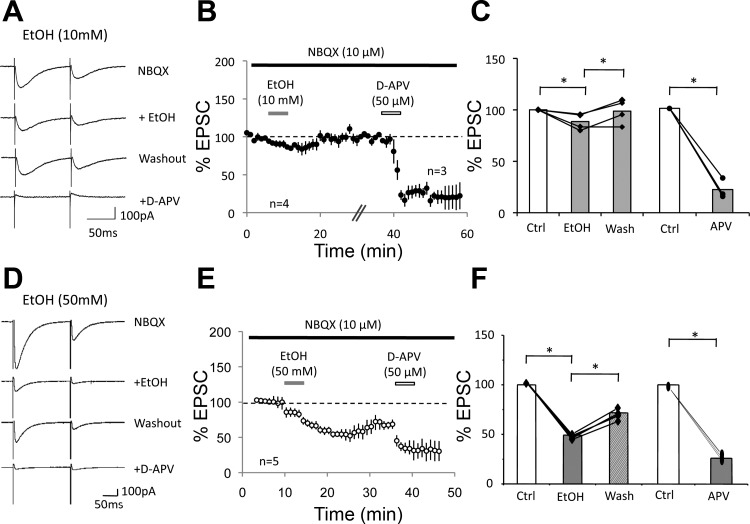
To examine the sensitivity of NMDA receptor-mediated currents in Purkinje cells to ethanol, we repeated the same type of experiment but subsequently added ethanol to the bath. In the presence of NBQX (10 μM), the CF-EPSC amplitude was reduced to 180.9 ± 23.6 pA (n = 6). Subsequent application of d-APV (50 μM) reduced these currents to 26.4 ± 2.5 pA (14.6 ± 3.6%; n = 6; P < 0.05; Fig. 2, A–C). After d-APV washout the current recovered to 87.9 ± 19.6 pA (48.6 ± 6.9%; n = 6; P < 0.05; Fig. 2, A–C). We then applied 10 mM ethanol to the recovered NMDA receptor-mediated current, which reduced this current to 40.2 ± 6.9 pA (45.7 ± 4.6% of the recovered current after d-APV washout; n = 6; P < 0.05; Fig. 2, A–C). Next, we performed this experiment using 50 mM ethanol. In the presence of NBQX (10 μM), the residual current amounted to 195.8 ± 19.3 pA and was inhibited to 24.3 ± 4.5 pA by d-APV (50 μM) bath application (12.4 ± 6.9%; n = 9, P < 0.05; Fig. 2, D–F). Washout of d-APV led to a recovery of this current to 136.1 ± 20.3 pA (69.5 ± 6.8%; n = 9; P < 0.05; Fig. 2, D–F). Application of 50 mM ethanol reduced this current to 18.2 ± 6.9 pA (13.4 ± 5.6% of the recovered current after d-APV washout; n = 9; P < 0.05; Fig. 2, D–F). The reduction of the recovered NMDA current by ethanol was significantly more pronounced at a concentration of 50 mM than at 10 mM (P < 0.05; Fig. 2). These data show that ethanol inhibits NMDA receptor signaling in Purkinje cells in a dose-dependent manner. However, a significant reduction of NMDA current amplitudes was already observed when 10 mM ethanol was bath applied. Ethanol bath application did not significantly alter the rise time (10–90%) or decay time constants of d-APV-sensitive EPSCs (10 mM EtOH: control: rise time, 3.85 ± 0.5 ms; decay time constant, 20.3 ± 0.6 ms; EtOH: rise time, 4.53 ± 0.4 ms; decay time constant, 17.7 ± 1.1 ms; n = 6; P > 0.05; and 50 mM EtOH: control: rise time, 3.61 ± 0.6 ms; decay time constant, 17.9 ± 1.4 ms; EtOH: rise time, 5.07 ± 0.5 ms; decay time constant, 19.2 ± 0.8 ms; n = 9; P > 0.05), which suggests that the residual current recorded in the presence of 50 mM ethanol is either NMDA receptor mediated as well or that a residual non-NMDA receptor current had similar kinetics. Ethanol bath application did not affect the paired-pulse ratio (10 mM EtOH: baseline, 0.61 ± 0.10; EtOH, 0.59 ± 0.08; n = 6; P > 0.05; 50 mM EtOH: baseline, 0.52 ± 0.11; EtOH, 0.53 ± 0.10; n = 9; P > 0.05; Fig. 3), suggesting that EtOH acted postsynaptically.
Fig. 2.
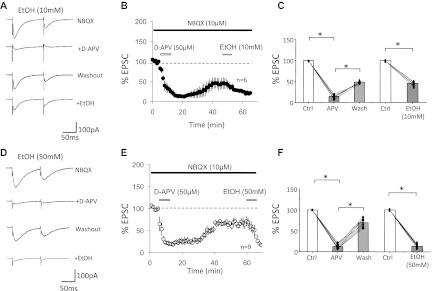
Ethanol inhibits d-APV-sensitive currents. A–C: effects of 10 mM EtOH. A: typical traces. First trace: CF-EPSCs recorded in the presence of NBQX. Second trace: d-APV inhibition of the NBQX-resistant currents. Third trace: recovery from d-APV inhibition. Fourth trace: inhibition of the recovered NMDA receptor-mediated current by 10 mM EtOH. B: time graph showing EtOH inhibition of NMDA receptor-mediated CF-EPSCs (EPSC1; n = 6). Bars indicate periods of drug application. After a stable baseline was established in the presence of NBQX (10 μM; black bar), d-APV (50 μM) was applied until steady inhibition was achieved (white bar). Subsequently, d-APV was washed out and the NMDA receptor-mediated current was allowed to recover before the application of EtOH (10 mM; grey bar). C: bar graph showing the reduction of NBQX-resistant currents by d-APV (50 μM), d-APV washout, and suppression by 10 mM EtOH (n = 6). Amplitude of recovered EPSCs after d-APV washout was normalized to baseline values before calculating the EPSC reduction in the presence of EtOH. D–F: effects of 50 mM EtOH. D: typical traces show the suppression of d-APV-sensitive currents by 50 mM EtOH. E: time graph showing EPSC blockade by d-APV, recovery after washout, and subsequent suppression by 50 mM EtOH (n = 9). F: bar graph showing the reduction of NBQX-resistant currents by d-APV (50 μM), d-APV washout, and suppression by 50 mM EtOH (n = 9; *P < 0.05; paired Student's t-test). Error bars are means ± SE.
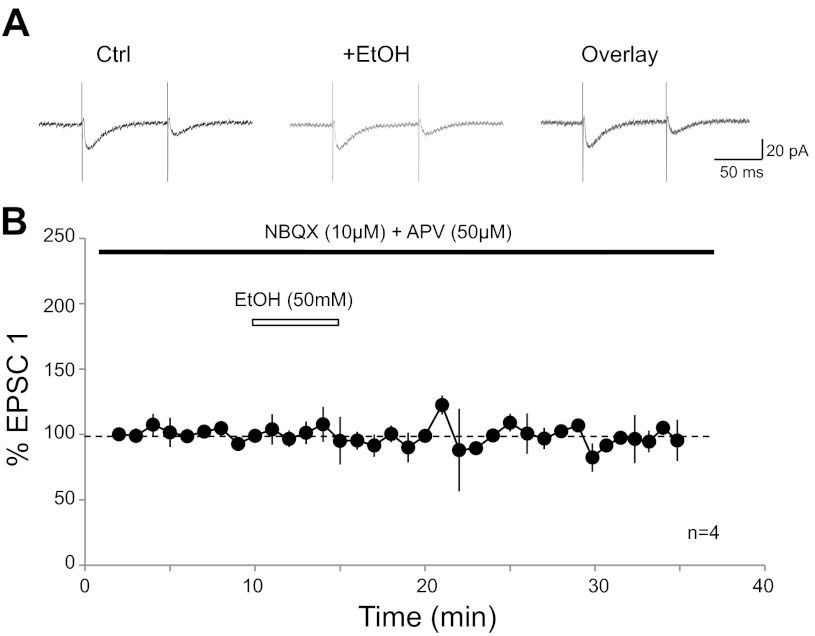
To specifically address the question whether the residual current that can be recorded in the presence of NBQX and d-APV (see Figs. 1, 2, 5) is ethanol sensitive, we measured CF-EPSCs in the presence of NBQX (10 μM) and d-APV (50 μM). Under these recording conditions, CF-EPSCs amounted to 21.3 ± 4.6 pA (n = 4). Bath application of ethanol (50 mM) did not significantly change the amplitude of these residual currents (103.2 ± 7.8%; t = 20–24 min; n = 4; P > 0.05; Fig. 4). These data show that the NBQX/d-APV-insensitive current is not blocked by ethanol (10 mM).
Fig. 5.
d-APV reduces the ethanol-sensitive current. A: typical traces. First trace: NBQX-resistant currents. Second trace: EtOH (10 mM) reduces the residual current. Third trace: the current recovers after EtOH washout. Fourth trace: d-APV (50 μM) reduces the recovered current. B: time graph showing a reduction of the NBQX-resistant CF-EPSCs (EPSC1) by EtOH (10 mM), followed by EtOH washout (n = 4) and d-APV inhibition of this current (n = 3). Bars indicate the periods of drug application. In 2 cells, recording of a new baseline before application of d-APV took longer than shown here (+1 and 25 min, respectively), which is indicated by the hash-marks. C: bar graph summarizing the reduction of the NBQX-resistant current by 10 mM EtOH, recovery of this current after EtOH washout (n = 4), and d-APV inhibition of the recovered current (n = 3; *P < 0.05; paired Student's t-test). The amplitude of recovered EPSCs after EtOH washout was normalized to baseline values before calculating the EPSC reduction in the presence of d-APV. D: typical traces as in A, but EtOH was bath applied at 50 mM. E: time graph showing the inhibition of NBQX-resistant CF-EPSCs by EtOH (50 mM), followed by EtOH washout and d-APV inhibition of this current (n = 5). Bars indicate the periods of drug application. F: bar graph summarizing the inhibition of the NBQX-resistant current by 50 mM EtOH, recovery of this current after EtOH washout, and d-APV inhibition of the recovered current (n = 5; *P < 0.05; paired Student's t-test). Error bars are means ± SE.
Fig. 4.
NBQX/d-APV-insensitive residual current is not blocked by ethanol. A: typical traces show CF-EPSCs in the presence of NBQX (10 μM) and d-APV (50 μM; left) and after ethanol (50 mM) wash-in (middle). Right: overlay of traces. B: time graph showing CF-EPSC amplitude changes upon wash-in of ethanol (50 mM; n = 4). Bars represent the presence of drugs in the bath. Error bars are means ± SE.
We next reversed the application sequence of d-APV and ethanol, respectively. Bath application of 10 mM ethanol caused a reduction in the amplitude of NMDA-mediated currents to 88.6 ± 4.0% (NBQX alone: 248.0 ± 97.8 pA; NBQX + EtOH: 209.1 ± 79.3 pA; t = 8–12 min; n = 4; P < 0.01; Fig. 5, A–C). After washout of ethanol, the current recovered to 98.7 ± 6.0% (228.3 ± 80.9 pA). Subsequent application of d-APV (50 μM) reduced the amplitude to 22.3 ± 5.6% of the recovered current (52.9 ± 36.0 pA; t = 49–53 min; n = 3; P < 0.01; Fig. 5, A–C). Bath application of 50 mM ethanol resulted in a more pronounced reduction in the amplitude of NMDA-mediated currents to 49.2 ± 2.8% (NBQX alone: 159.3 ± 17.7 pA; NBQX + EtOH: 78.3 ± 13.2 pA; n = 5; P < 0.05; Fig. 5, D–F). After washout of ethanol, the current recovered to 71.8 ± 10.6% (114.4 ± 2.3 pA). Subsequent application of d-APV (50 μM) caused a decrease of the recovered current to 25.9 ± 12.6% (29.6 ± 3.8 pA; n = 5; P < 0.05; Fig. 5, D–F), confirming that the ethanol-sensitive current was mediated by NMDA receptors.
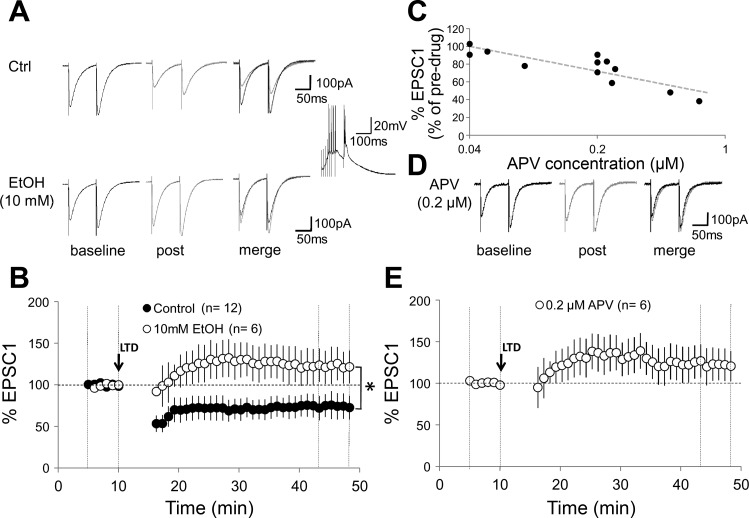
It has recently been shown that activation of Purkinje cell NMDA receptors is required for PF-LTD induction in the mature mouse cerebellum (Piochon et al. 2010). Thus we examined whether LTD induced with the modified protocol described by Piochon et al. (2010) (8–10 PF pulses at 100 Hz, followed after a delay of 150 ms by a single CF pulse; repeated at 1 Hz for 5 min) is affected by ethanol in cerebellar slices prepared from 2- to 6-mo-old mice. PF-EPSCs were evoked by paired-pulse stimulation (100-ms interval) delivered to the outer molecular layer using a glass pipette filled with ACSF. Application of the pairing protocol induced PF-LTD (EPSC1: 75.0 ± 12.8%; n = 12; t = 44–48 min; P < 0.01; Fig. 6, A and B). In the presence of 10 mM ethanol, application of the same induction protocol resulted in long-term potentiation (LTP) instead (128.1 ± 12.7%; n = 6; P < 0.05; Fig. 6, A and B). These alterations were not accompanied by changes in the paired-pulse facilitation (PPF) ratio (data not shown). Under naïve conditions (without prior d-APV application/washout), 10 mM EtOH reduces CF-EPSCs recorded in the presence of NBQX (10 μM) to 88.6% (Fig. 5, A–C). We next asked whether LTD was similarly impaired in the presence of a low concentration of d-APV that would reduce CF-EPSCs to a comparably low degree. We determined that d-APV at a concentration of 0.2 μM reduces CF-EPSCs recorded in the presence of NBQX (10 μM) to 81.3 ± 5.7% (n = 3; Fig. 6C). In the presence of d-APV at 0.2 μM, PF-LTD induction was impaired and a potentiation was observed instead (EPSC1: 121.9 ± 19.8%; n = 6; t = 44–48 min; P < 0.05; Fig. 6, D and E). This change in EPSC amplitudes was significantly different from that observed under control conditions (see above; P < 0.01; Mann-Whitney U-test). The alterations seen in the presence of 0.2 μM d-APV were not accompanied by changes in the PPF ratio (data not shown). These results show that comparably moderate reductions in CF-EPSC amplitude by either ethanol (10 mM) or d-APV (0.2 μM) cause a similar impairment of PF-LTD, supporting the notion that at low concentrations EtOH may affect the LTD/LTP balance by modulating NMDA receptor signaling.
Fig. 6.
Parallel fiber long-term depression (LTD) [paired parallel fiber (PF) and CF activation] is blocked by ethanol in adult mouse Purkinje cells. A: typical traces in 0 mM Mg2+. Top left: PF-EPSCs before tetanization under control conditions. Top middle: PF-EPSCs 30 min after tetanization. Top right: overlay. Bottom traces: corresponding PF-EPSCs in the presence of 10 mM EtOH. Inset: tetanization pattern (8 PF pulses at 100 Hz, followed by single-pulse CF stimulation). This pattern was applied at 1 Hz for 5 min. B: time graph showing LTD under control conditions (n = 12) and LTD blockade in the presence of 10 mM EtOH (n = 6). C: d-APV blockade of CF-EPSCs is concentration dependent. CF-EPSCs were recorded in the presence of NBQX (10 μM). Graph plots the %change in EPSC amplitude against the concentration of d-APV (range: 0.04 to 1 μM). D: typical traces showing PF-EPSCs in the presence of 0.2 μM d-APV before (left) and 30 min after tetanization (middle). Right: overlay of traces. E: time graph showing an impairment of LTD in the presence of 0.2 μM d-APV (n = 6). Arrow indicates the time point of tetanization. Error bars are means ± SE. *P < 0.05, significant differences between the control and EtOH group by Mann-Whitney U-test.
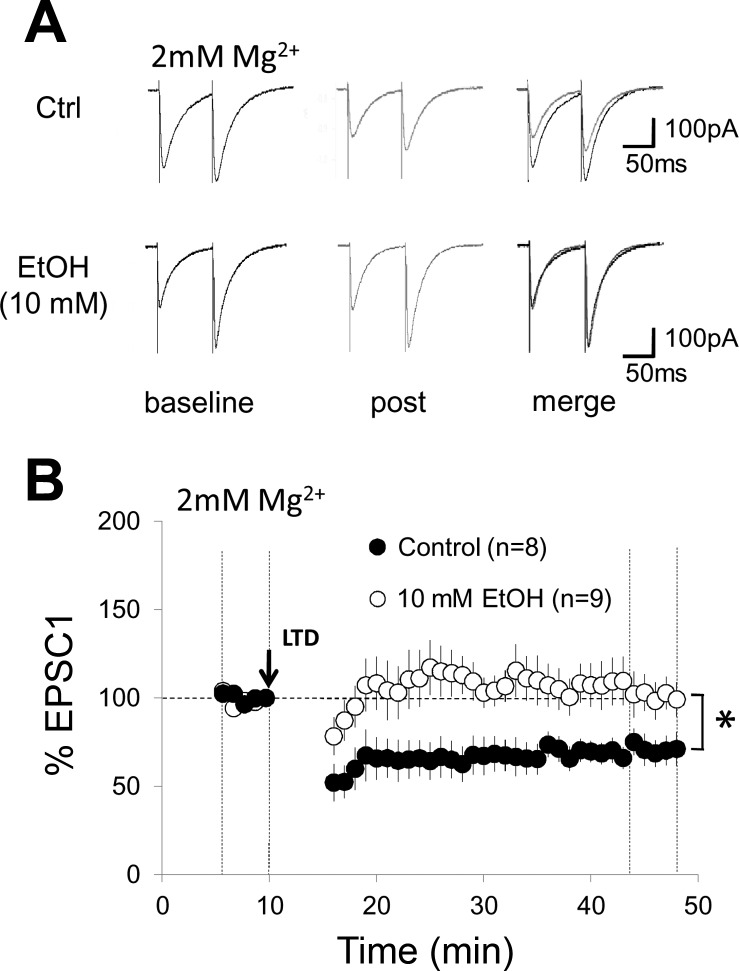
The experiments described above were performed in 0 mM external Mg2+. To examine whether ethanol impairs LTD under more physiological conditions, we repeated these experiments when 2 mM MgSO4 were added to the ACSF. Under control conditions, LTD amounted to 65.5 ± 6.4% (n = 8, P < 0.05; Fig. 7). In the presence of 10 mM ethanol, LTD was blocked (99.2 ± 9.8%; n = 9; P > 0.05; Fig. 7). However, LTD was not reversed to LTP as observed in Mg2+ free solution. These results show that low-dosage ethanol application impairs LTD induction in Mg2+ free solution (facilitating NMDA currents) but also under more physiological conditions (2 mM MgSO4).
Fig. 7.
Parallel fiber LTD (paired PF and CF activation) is blocked by ethanol (10 mM) in 2 mM Mg2+. A: typical traces. Same as in Fig. 6A, but in 2 mM Mg2+. B: same as in Fig. 6B, but the control group (n = 8) and the EtOH group (n = 9) were obtained with 2 mM Mg2+ added to the artificial cerebrospinal fluid. Error bars are means ± SE. *P < 0.05, significant differences between the control and EtOH group by Mann-Whitney U-test.
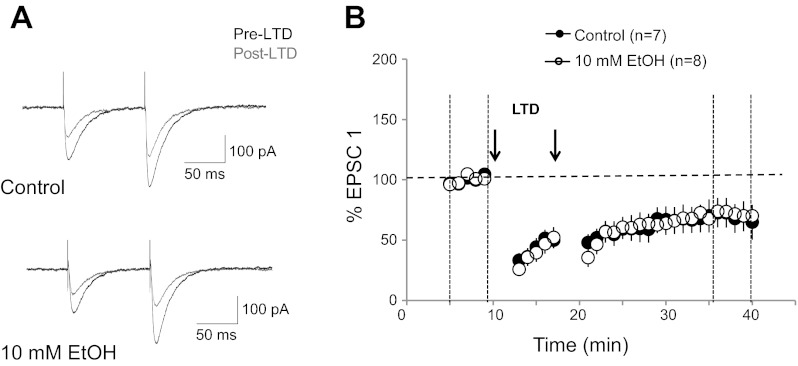
We next asked the question whether 10 mM ethanol also impairs LTD induced by a protocol in which CF activation is replaced by somatic depolarization, thus bypassing the activation of NMDA receptors at CF synapses. LTD was triggered by pairing depolarizing voltage steps [−75 to 0 mV; 250 ms; age: postnatal day (P)24–90] with PF activation (2 pulses at an interval of 10 ms) at 1 Hz for 3 min (2×; 5-min interval; 2 mM MgSO4). Under control conditions, this pairing protocol resulted in a depression of PF response amplitudes (69.7 ± 12.3%; n = 7; t = 36–40 min; P < 0.01; Fig. 8). In the presence of 10 mM ethanol in the bath, LTD could still be induced (71.9 ± 7.2%; n = 8; P < 0.01; Fig. 8). The difference in LTD amplitudes between the two experimental conditions was not significant (P > 0.05; Mann-Whitney U-test). These results show that a form of LTD that does not involve NMDA receptor signaling at CF synapses is not sensitive to 10 mM ethanol.
Fig. 8.
Parallel fiber LTD (paired PF activation and Purkinje cell depolarization) is not impaired by ethanol. A: typical traces. Top: PF-EPSCs before (black traces) and after tetanization (grey traces) under control conditions. Bottom: PF-EPSCs before and after tetanization in the presence of 10 mM EtOH. The LTD protocol consisted of pairing depolarizing voltage steps (−75 to 0 mV; 250 ms) with 2 PF stimuli (10-ms interval) at 1 Hz for 3 min (repeated once after 5 min). B: time graph showing LTD induced under control conditions (n = 7) and in the presence of 10 mM EtOH (n = 8). Arrows indicate the time points of tetanization. Error bars are mean ± SE.
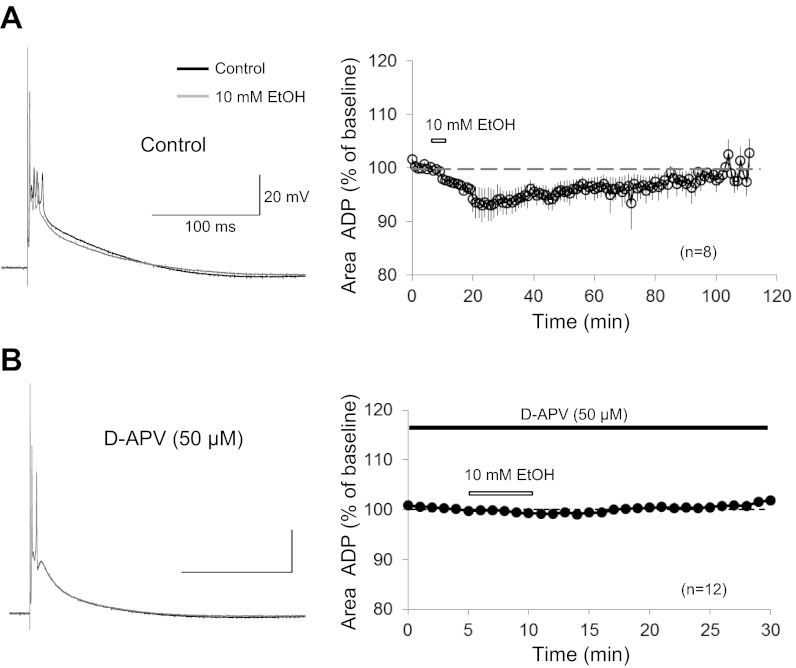
The data shown above indicate that ethanol at low doses (10 mM) impairs cerebellar LTD induced by paired PF and CF activation and identify NMDA receptors at CF synapses as a primary ethanol target at low concentrations. Since the paired synaptic stimulation protocol was applied in current-clamp mode, we determined the effects of 10 mM ethanol on CF-evoked complex spikes recorded in current clamp. It has previously been shown that complex spikes are affected by ethanol: at a concentration of 50 mM, ethanol reduces the late phase of the complex spike waveform. It has been suggested that this effect results from an inhibition of mGluR1 signaling by ethanol (Carta et al. 2006). However, at lower doses (20 mM), ethanol does not have a significant effect on mGluR1 receptors (Belmeguenai et al. 2008). To examine whether ethanol can also affect the complex spike waveform at low doses, we recorded complex spikes (in 0 mM Mg2+) and bath-applied ethanol (10 mM). In the presence of ethanol, the amplitude of the first spike and the early spikelets did not change (Fig. 9A), suggesting that ethanol did not reduce voltage-gated sodium currents (Camacho-Nasi and Treistman 1986; Wu and Kendig 1998). In contrast, the afterdepolarization (ADP) was reduced when ethanol was bath applied (area under the curve; 100-ms period after last spikelet; 93.3 ± 2.7%; t = 20–24 min; n = 8; P < 0.01; Fig. 9A). The ADP recovered after ethanol washout (100.4 ± 0.9%; t = 105–109 min; n = 3; P < 0.01; Fig. 9A). In the presence of d-APV (50 μM), bath application of ethanol (10 mM) did not cause a reduction in the ADP amplitude (99.4 ± 0.8%; t = 10–14 min; n = 12; P > 0.05; Fig. 9B). These data show that the complex spike ADP is reduced by 10 mM ethanol and that at this low concentration this ethanol effect is specifically related to a reduction in NMDA receptor-mediated response components. In some recordings, the ADP reduction observed after ethanol wash-in was accompanied by a reduction in the spikelet number (data not shown). At a concentration of 10 mM, ethanol does not affect mGluR1 receptor signaling or voltage-gated calcium channels (Belmeguenai et al. 2008). The results presented here that 1) 10 mM ethanol reduces the complex spike ADP and 2) this effect is not observed when NMDA receptors are blocked by d-APV thus suggest that at low concentrations NMDA receptors are the main ethanol target and that NMDA receptor blockade may cause the impairment of LTD.
Fig. 9.
Ethanol reduces the complex spike afterdepolarization (ADP). A, left: typical traces. Complex spikes before (black trace) and after application of EtOH (10 mM; grey trace). Right: time graph showing changes in the ADP amplitude after bath application of 10 mM EtOH (t = 80 min: n = 8; t = 105 min: n = 3). B: in the presence of d-APV (50 μM), EtOH did not affect the complex spike waveform. Left: typical traces. Complex spikes before (black trace) and after application of ethanol (10 mM; grey trace). Right: time graph showing the ADP amplitude upon EtOH application (10 mM) when d-APV is present in the bath (n = 12; black bar). White bars indicate the presence of EtOH in the bath. Error bars are means ± SE.
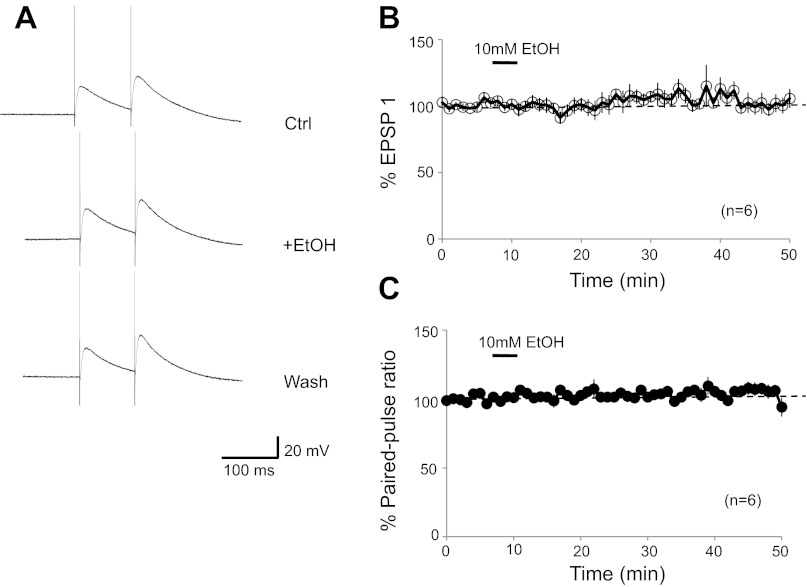
We have previously shown that ethanol (50 mM) does not affect PF synaptic transmission in P18–30 rats (Belmeguenai et al. 2008). To exclude the possibility that in the more mature animals tested here LTD could be impaired by ethanol effects on PF responses, we monitored PF synaptic transmission in 2-mo-old mice. Ethanol bath application (10 mM) had no effect on the PF-excitatory postsynaptic potential (EPSP) amplitude (EPSP1: 97.5 ± 3.9%; t = 15–19 min; n = 6; P > 0.05; Fig. 10, A and B) and did not affect the paired-pulse facilitation ratio (103.1 ± 2.6%; P > 0.05; Fig. 10, A and C). Thus LTD induced by paired PF and CF activation was not blocked by 10 mM ethanol because of ethanol affects at PF synapses but rather because of its reduction of NMDA receptor signaling at CF synapses.
Fig. 10.
Ethanol does not affect parallel fiber synaptic transmission in the mature cerebellum. A: typical traces: PF-excitatory postsynaptic potentials (EPSPs) before (top), during (middle), and after bath application of EtOH (10 mM; bottom). B: time graph showing changes in the PF-EPSP amplitude upon bath application of EtOH (10 mM; n = 6). C: time graph showing the corresponding paired-pulse facilitation ratio (n = 6). Bars represent the presence of EtOH in the bath. Error bars are means ± SE.
DISCUSSION
The results presented here show that ethanol at low doses (10 mM) impairs cerebellar synaptic plasticity and that at this concentration NMDA receptors are a main ethanol target. It has previously been demonstrated that in Purkinje cells voltage-gated calcium channels and mGluR1 receptors are affected by ethanol. Like NMDA receptors, both provide sources for calcium influx that are likely involved in LTD induction. However, voltage-gated calcium channels and mGluR1 receptors are characterized by a lower ethanol sensitivity than NMDA receptors and are affected by ethanol at a concentration of 50 mM but not at 20 mM (Carta et al. 2006; Belmeguenai et al. 2008). Slow currents triggered by mGluR1 receptor activation can be suppressed by 10 mM ethanol, but only when ethanol is applied internally (added to the pipette saline). A reduction in mGluR1 signaling by externally applied ethanol was only observed with higher doses of ethanol (50 mM; Su et al. 2010). The high ethanol sensitivity of NMDA receptors reported here falls into the same range as that observed in the hippocampus, where a significant reduction in the amplitude of NMDA receptor-mediated field EPSPs was observed when ethanol was applied at a concentration of 25 mM, and a reduction that did not reach statistical significance was observed at 1 mM (Lovinger et al. 1990). At 50 mM, ethanol blocks the d-APV-sensitive current in Purkinje cells almost entirely (Fig. 2, D–F). This pronounced reduction might be due to the fact that, in addition to its effects on the phosphorylation state of NMDA receptors and potential consequences for their biophysical properties (Miyakawa et al. 1997; Alvestad et al. 2003; Yaka et al. 2003; Hicklin et al. 2011), ethanol can induce internalization of NMDA receptors, thus lowering the receptor density in the membrane (Suvarna et al. 2005). This possibility is further strengthened by the observation that the effects of ethanol (50 mM) were only partially reversible. At 10 mM ethanol, CF responses returned to baseline following ethanol washout (Fig. 5). The reduction of CF response amplitudes was more pronounced when ethanol was applied after previous d-APV application/washout (Fig. 2) compared with the reduction that was seen when ethanol was applied first (Fig. 5). This unexpected result was obtained when using ethanol at concentrations of 10 and 50 mM, respectively. Future studies will have to address whether this effect may be due to changes in the subunit composition or surface expression of NMDA receptors or any other factor that might regulate ethanol sensitivity.
Throughout our recordings, we observed a small excitatory current that was resistant to bath application of NBQX and d-APV. This residual current was not affected by ethanol (50 mM). We did not attempt to further characterize or identify this current.
Taken together, our data show that in Purkinje cells NMDA receptors are the primary ethanol target at low-dose ethanol exposure. In the United States, the legal blood alcohol threshold is 0.08 g/dl, which is equivalent to a concentration of 17 mM. While we acknowledge that it is difficult to compare the relevance of alcohol concentrations used in vitro to those in living organisms, it can be noted that NMDA receptor signaling and LTD are impaired at low doses that are in the same range as those reached by mild alcohol consumption in humans.
It has previously been shown that CF-LTD and PF-LTD, but not PF-LTP, are prevented by ethanol at a concentration of 50 mM (Carta et al. 2006; Belmeguenai et al. 2008). Here, we revisited the question of whether ethanol can impair PF-LTD to specifically examine whether ethanol can have this effect at low doses, at which NMDA receptors, but not voltage-gated calcium channels or mGluR1 receptors are affected. Our results show that 10 mM ethanol blocks LTD. It remains possible that relevant factors other than NMDA receptors are affected by 10 mM ethanol. However, the recent demonstration that Purkinje cell NMDA receptors are required for LTD induction (Piochon et al. 2010) suggests that the blockade of NMDA receptor-mediated currents at this ethanol concentration contribute to the suppression of LTD. This notion is supported by two additional observations. First, LTD was similarly impaired in the presence of 0.2 μM d-APV, which reduces NMDA receptor-mediated CF-EPSCs to a comparable degree as 10 mM ethanol does, suggesting that at this concentration ethanol indeed may act primarily through the reduction of NMDA receptor signaling. Second, LTD is not ethanol sensitive when it is induced by a protocol in which depolarizing voltage steps substitute for CF stimulation, thus bypassing NMDA receptor activation at CF synapses. In previous studies, it was suggested that the antagonistic effect of ethanol on NMDA currents is enhanced in the presence of Mg2+ ions (Martin et al. 1991; Morrisett et al. 1991). In our hands, 10 mM ethanol blocked LTD in 2 mM MgSO4, but we even observed a reversal toward LTP in Mg2+ free solution (Fig. 6). Under control conditions, the Mg2+ concentration did not affect the ability of PF synapses to express LTD. It seems that during application of the LTD induction protocol sufficient depolarization is reached to remove the Mg2+ block from NMDA channels. However, ethanol seems to unveil a potentiation mechanism that is suppressed when Mg2+ ions are present in the ACSF.
In young rats (P15–26), it has been found that LTD induction requires the activation of NMDA receptors located at PF terminals (Casado et al. 2002). However, this finding has been controversial as later studies reported that NMDA receptor blockade neither affects calcium transients in PF terminals (Shin and Linden 2005; Qiu and Knöpfel 2007) nor the frequency of miniature EPSCs (Shin and Linden 2005). In our previous study using 2- to 6-mo-old mice, d-APV bath application neither changed the PPF ratio at PF synapses nor the paired-pulse depression ratio at CF synapses, suggesting that in the mature mouse cerebellum presynaptic NMDA receptors are not activated at these synapses (Piochon et al. 2010). In the present study, in which we again used 2- to 6-mo-old mice, we confirmed that d-APV bath application reduces CF-EPSC amplitudes (Fig. 1) but does not affect the paired-pulse depression ratio at CF synapses (Fig. 3). These data suggest that the NMDA receptors studied here are postsynaptically expressed. Moreover, we demonstrate in mature Purkinje cells that ethanol (10 mM) does not affect PF synaptic responses (Fig. 10) but reduces CF responses (Figs. 2, 5, and 9). The latter effect was not seen when NMDA receptors were blocked by d-APV (50 μM; Fig. 9). Together, these data show that at this concentration ethanol selectively reduces NMDA receptor signaling at CF synapses.
At high doses, ethanol has relatively unspecific effects on signaling mechanisms affecting, among others, voltage-gated calcium channels (Walter and Messing 1999; Belmeguenai et al. 2008), mGluR1 receptors (Carta et al. 2006; Belmeguenai et al. 2008), and NMDA receptors (Lovinger et al. 1989, 1990; Schummers and Browning 2001; this study). It is therefore surprising that ethanol selectively suppresses cerebellar LTD, but not LTP (Belmeguenai et al. 2008). A possible explanation is that at cerebellar PF synapses onto Purkinje cells, larger calcium transients are required for LTD than for LTP induction (Coesmans et al. 2004). In contrast to LTD, LTP requires neither mGluR1 (Belmeguenai et al. 2008) nor NMDA receptor activation for induction (Piochon et al. 2010). It is thus likely that ethanol does not block LTP (at concentrations as high as 50 mM), because it suppresses signaling pathways that are specifically involved in LTD induction and because LTP is less sensitive to a reduction in the amplitude of calcium transients. At low doses, ethanol effects become more specific. At a concentration of 10 mM, ethanol reduces NMDA receptor-mediated currents (shown here) but does not affect voltage-gated calcium currents or mGluR1 receptors (Belmeguenai et al. 2008). These data support the notion that NMDA receptors are a primary target of ethanol and emphasize the key role of NMDA receptors in cerebellar LTD. Recent data suggest that LTD alone cannot account for cerebellar motor learning but that other plasticity mechanisms, such as LTP, might play a critical role as well (Schonewille et al. 2010, 2011). It is thus conceivable that motor coordination deficits resulting from alcohol consumption (for review, see Valenzuela et al. 2010) are partially due to ethanol-mediated alterations in the LTD / LTP balance and the resulting deficits in the fast adjustment of PF synaptic strength.
GRANTS
This work was supported by a National Institute on Alcohol Abuse and Alcoholism Grant AA-016540 to (C. Hansel).
DISCLOSURES
Conflict of interest statement: No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Q.H., H.T., G.G., C.P., and C.H. conception and design of research; Q.H., H.T., and G.G. performed experiments; Q.H., H.T., and G.G. analyzed data; Q.H., H.T., G.G., C.P., and C.H. interpreted results of experiments; Q.H. and H.T. prepared figures; Q.H., H.T., G.G., C.P., and C.H. approved final version of manuscript; C.H. drafted manuscript; C.H. edited and revised manuscript.
ACKNOWLEDGMENTS
We are grateful to members of the Hansel laboratory for discussions and invaluable comments on the manuscript.
REFERENCES
- Alvestad RM, Grosshans DR, Coultrap SJ, Nakazawa T, Yamamoto T, Browning MD. Tyrosine dephosphorylation and ethanol inhibition of N-methyl-d-aspartate receptor function. J Biol Chem 278: 11020–11025, 2003 [DOI] [PubMed] [Google Scholar]
- Belmeguenai A, Botta P, Weber JT, Carta M, De Ruiter M, De Zeeuw CI, Valenzuela CF, Hansel C. Alcohol impairs long-term depression at the cerebellar parallel fiber-Purkinje cell synapse. J Neurophysiol 100: 3167–3174, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho-Nasi P, Treistman SN. Ethanol effects on voltage-dependent membrane conductances: comparative sensitivity of channel populations in Aplysia neurons. Cell Mol Neurobiol 6: 263–279, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF. Alcohol potently modulates climbing fiber-Purkinje neuron synapses: role of metabotropic glutamate receptors. J Neurosci 26: 1906–1912, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casado M, Isope P, Ascher P. Involvement of presynaptic N-methyl-d-aspartate receptors in cerebellar long-term depression. Neuron 33: 123–130, 2002 [DOI] [PubMed] [Google Scholar]
- Chu NS. Effects of ethanol on rat cerebellar Purkinje cells. Int J Neurosci 21: 265–277, 1983 [DOI] [PubMed] [Google Scholar]
- Coesmans M, Weber JT, De Zeeuw CI, Hansel C. Bidirectional parallel fiber plasticity in the cerebellum under climbing fiber control. Neuron 44: 691–700, 2004 [DOI] [PubMed] [Google Scholar]
- Farrant M, Cull-Candy SG. Excitatory amino acid receptor-channels in Purkinje cells in thin cerebellar slices. Proc Biol Sci 244: 179–184, 1991 [DOI] [PubMed] [Google Scholar]
- Franklin CL, Gruol DL. Acute ethanol alters the firing pattern and glutamate response of cerebellar Purkinje neurons in culture. Brain Res 416: 205–218, 1987 [DOI] [PubMed] [Google Scholar]
- Freund RK, Palmer MR. Beta adrenergic sensitization of gamma-aminobutyric acid receptors to ethanol involves a cyclic AMP/protein kinase A second-messenger mechanism. Pharmacol Exp Ther 280: 1192–1200, 1997 [PubMed] [Google Scholar]
- Freund RK, Wang Y, Palmer MR. Differential effects of ethanol on the firing rates of Golgi-like neurons and Purkinje neurons in cerebellar slices in vitro. Neurosci Lett 164: 9–12, 1993a [DOI] [PubMed] [Google Scholar]
- Freund RK, Van Horne CG, Harlan T, Palmer MR. Electrophysiological interactions of ethanol with GABAergic mechanisms in the rat cerebellum in vivo. Alcohol Clin Exp Res 17: 321–328, 1993b [DOI] [PubMed] [Google Scholar]
- Hansel C, Linden DJ. Long-term depression of the cerebellar climbing fiber-Purkinje neuron synapse. Neuron 26: 473–482, 2000 [DOI] [PubMed] [Google Scholar]
- Hernández LL, Valentine JD, Powell DA. Ethanol enhancement of Pavlovian conditioning. Behav Neurosci 100: 494–503, 1986 [DOI] [PubMed] [Google Scholar]
- Hicklin TR, Wu PH, Radcliffe RA, Freund RK, Goebel-Goody SM, Correa PR, Proctor WR, Lombroso PJ, Browning MD. Alcohol inhibition of the NMDA receptor function, long-term potentiation, and fear learning requires striatal-enriched protein tyrosine phosphatase. Proc Natl Acad Sci USA 108: 6650–6655, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson GN. Ethanol inhibition of formation of conditioned eyeblink responses in man. Psychopharmacologia 9: 93–100, 1966 [DOI] [PubMed] [Google Scholar]
- Llano I, Marty A, Armstrong CM, Konnerth A. Synaptic- and agonist-induced excitatory currents of Purkinje cells in rat cerebellar slices. J Physiol 434: 183–213, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonchamp E, Gambino F, Dupont JL, Doussau F, Valera A, Poulain B, Bossu JL. Pre and post synaptic NMDA effects targeting Purkinje cells in the mouse cerebellar cortex. PLos One 7: e30180, 1–11, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science 243: 1721–1724, 1989 [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. NMDA receptor-mediated synaptic excitation selectively inhibited by ethanol in hippocampal slice from adult rat. J Neurosci 10: 1372–1379, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Zamudio PA, Carta M, Valenzuela CF. Developmentally regulated actions of alcohol on hippocampal glutamatergic transmission. J Neurosci 25: 8027–8036, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Botta P, Zamudio PA, Zucca S, Valenzuela CF. Ethanol decreases Purkinje neuron excitability by increasing GABA release in rat cerebellar slices. J Pharmacol Exp Ther 327: 910–917, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D, Morrisett RA, Bian XP, Wilson WA, Swartzwelder HS. Ethanol inhibition of NMDA mediated depolarizations is increased in the presence of Mg2+. Brain Res 546: 227–234, 1991 [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Yagi T, Kitazawa H, Yasuda M, Kawai N, Tsuboi K, Niki H. Fyn-kinase as a determinant of ethanol sensitivity: relation to NMDA-receptor function. Science 278: 698–701, 1997 [DOI] [PubMed] [Google Scholar]
- Morrisett RA, Martin D, Oetting TA, Lewis DV, Wilson WA, Swartzwelder HS. Ethanol and magnesium ions inhibit N-methyl-d-aspartate-mediated synaptic potentials in an interactive manner. Neuropharmacology 11: 1173–1178, 1991 [DOI] [PubMed] [Google Scholar]
- Peoples RW, White G, Lovinger DM, Weight FF. Ethanol inhibition of N-methyl-d-aspartate-activated current in mouse hippocampal neurones: whole-cell patch-clamp analysis. Br J Pharmacol 6: 1035–1042, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piochon C, Irinopoulou T, Brusciano D, Bailly Y, Mariani J, Levenes C. NMDA receptor contribution to the climbing fiber response in the adult mouse Purkinje cell. J Neurosci 27: 10797–10809, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piochon C, Levenes C, Ohtsuki G, Hansel C. Purkinje cell NMDA receptors assume a key role in synaptic gain control in the mature cerebellum. J Neurosci 30: 15330–15335, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu DL, Knöpfel T. An NMDA receptor/nitric oxide cascade in presynaptic parallel fiber-Purkinje neuron long-term potentiation. J Neurosci 27: 3408–3415, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzi M, Farrant M, Cull-Candy SG. Climbing-fibre activation of NMDA receptors in Purkinje cells of adult mice. J Physiol 585: 91–101, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Siggins GR, Schulman JA, Bloom FE. Physiological correlates of ethanol intoxication tolerance, and dependence in rat cerebellar Purkinje cells. Brain Res 196: 183–198, 1980 [DOI] [PubMed] [Google Scholar]
- Schonewille M, Belmeguenai A, Koekkoek SK, Houtman SH, Boele HJ, Van Beugen BJ, Gao Z, Badura A, Ohtsuki G, Amerika WE, Hosy E, Hoebeek FE, Elgersma Y, Hansel C, De Zeeuw CI. Purkinje cell-specific knockout of the protein phosphatase PP2B impairs potentiation and cerebellar motor learning. Neuron 67: 618–628, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonewille M, Gao Z, Boele HJ, Vinueza Veloz MF, Amerika WE, Simek AA, De Jeu MT, Steinberg JP, Takamiya K, Hoebeek FE, Linden DJ, Huganir RL, De Zeeuw CI. Reevaluating the role of LTD in cerebellar motor learning. Neuron 70: 43–50, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schummers J, Browning MD. Evidence for a role for GABAA and NMDA receptors in ethanol inhibition of long-term potentiation. Mol Brain Res 94: 9–14, 2001 [DOI] [PubMed] [Google Scholar]
- Servais L, Bearzatto B, Delvaux V, Noël E, Leach R, Brasseur M, Schiffmann SN, Cheron G. Effect of chronic ethanol ingestion on Purkinje and Golgi cell firing in vivo and on motor coordination in mice. Brain Res 1055: 171–179, 2005 [DOI] [PubMed] [Google Scholar]
- Shin JH, Linden DJ. An NMDA receptor/nitric oxide cascade is involved in cerebellar LTD but is not localized to the parallel fiber terminal. J Neurophysiol 94: 4281–4289, 2005 [DOI] [PubMed] [Google Scholar]
- Siggins GR, French E. Central neurons are depressed by iontophoretic and micropressure application of ethanol and tetrahydropapaveroline. Drug Alcohol Depend 4: 239–243, 1979 [DOI] [PubMed] [Google Scholar]
- Sorensen S, Palmer M, Dunwiddie T, Hoffer B. Electrophysiological correlates of ethanol-induced sedation in differentially sensitive lines of mice. Science 210: 1143–1145, 1980 [DOI] [PubMed] [Google Scholar]
- Su LD, Sun CL, Shen Y. Ethanol acutely modulates mglur1-dependent long-term depression in cerebellum. Alcohol Clin Exp Res 34: 1140–1145, 2010 [DOI] [PubMed] [Google Scholar]
- Suvarna N, Borgland SL, Wang J, Phamluong K, Auberson YP, Bonci A, Ron D. Ethanol alters trafficking and functional N-methyl-d-aspartate receptor NR2 subunit ratio via H-Ras. J Biol Chem 36: 31450–31459, 2005 [DOI] [PubMed] [Google Scholar]
- Urrutia A, Gruol DL. Acute alcohol alters the excitability of cerebellar Purkinje neurons and hippocampal neurons in culture. Brain Res 569: 26–37, 1992 [DOI] [PubMed] [Google Scholar]
- Valenzuela CF, Lindquist B, Zamudio-Bulcock PA. A review of synaptic plasticity at Purkinje neurons with a focus on ethanol-induced cerebellar dysfunction. Int Rev Neurobiol 91: 339–372, 2010 [DOI] [PubMed] [Google Scholar]
- Walter HJ, Messing RO. Regulation of neuronal voltage-gated calcium channels by ethanol. Neurochem Int 35: 95–101, 1999 [DOI] [PubMed] [Google Scholar]
- Wu JV, Kendig JJ. Differential sensitivities of TTX-resistant and TTX-sensitive sodium channels to anesthetic concentrations of ethanol in rat sensory neurons. J Neurosci Res 54: 433–443, 1998 [DOI] [PubMed] [Google Scholar]
- Yaka R, Phamluong K, Ron D. Scaffolding of Fyn kinase to the NMDA receptor determines brain region sensitivity to ethanol. J Neurosci 23: 3623–3632, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]