Abstract
Primary motor cortex (M1) movement representations reflect acquired motor skills. Representations of muscles and joints used in a skilled task expand. However, it is unknown whether motor restriction in healthy individuals results in complementary reductions in M1 representations. With the use of intracortical microstimulation techniques in squirrel monkeys, detailed maps of movement representations in M1 were derived before and up to 35 wk after restriction of the preferred distal forelimb (DFL) by use of a soft cast. Although total DFL area and movement threshold remained constant, casting resulted in a redistribution of digit and wrist/forearm representations. Digit representations progressively decreased, whereas wrist/forearm representations progressively increased in areal extent. In three of four monkeys, hand preference returned to normal by the end of the postcast recovery period, and postrecovery maps demonstrated reversal of restriction-induced changes. However, in one monkey, a chronic motor impairment occurred in the casted limb. Rehabilitation via a forced-use paradigm resulted in recovery in use and skill of the impaired limb, as well as restoration of normal motor maps. These results demonstrate that plasticity in motor representations can be induced by training or restricting movements of the limb. Physiological changes induced by restriction appear to be reversible, even in the case of adverse motor outcomes. The respective contributions of both disuse and lost motor skills are discussed. These results have relevance for clinical conditions requiring forelimb casting as well as interpreting the differential effects of injury and disuse that are necessarily intertwined after cortical injury, as occurs in stroke.
Keywords: motor cortex, plasticity, disuse, primate, hand
the configuration of the primary motor cortex (M1) motor map is modifiable as a result of various types of manipulations, including limb amputation, peripheral nerve injury, central injury, and motor skill training (Adachi et al. 2007; Franchi 2002; Nudo et al. 1996a, b; Sanes et al. 1990; Schieber and Deuel 1997; Sessle et al. 2007). In particular, a large number of studies, ranging from neurophysiological experiments in animal models to noninvasive imaging studies in humans, have focused on the modifiability of the motor cortex as a function of behavioral experience (Karni et al. 1998; Nudo et al. 2001). As new motor skills are acquired, the muscles and joints used in the task come to be represented over larger cortical territories (Dancause 2006; Monfils et al. 2005; Nudo et al. 1996a). In addition, dendrites arborize, and synapses multiply and change conformation (Kleim et al. 1996, 1998; Xu et al. 2009). The most robust plasticity occurs with the acquisition of increased skill (Plautz et al. 2000; Remple et al. 2001), although forelimb motor activity in the absence of skill acquisition can also induce other structural changes in motor cortex, such as angiogenesis (Kleim et al. 2002).
Relatively few studies have specifically examined the complementary behavioral experience, that is, disuse. In this paper, we use the term “disuse” to refer to the reduction in movement resulting from restriction of the forelimb. This procedure also results in reduction of motor skills, and reduced motor skill may be an independent contributor to motor map topography. The relative contributions of these interacting variables are addressed specifically in discussion. The remainder of the paper simply refers to restriction-induced disuse.
Disuse is implied after central or peripheral injury, and motor map reorganization does, indeed, occur under circumstances that restrict movements (Adachi et al. 2007; Kaas 2000; Navarro et al. 2007; Sanes et al. 1990). However, to date, these experiments have been complicated by the fact that the central nervous system is directly (as in the case of a cortical lesion) or indirectly (as in the case of a peripheral nerve transection) compromised by the experimental manipulation. Furthermore, with few exceptions, the manipulation is irreversible. Thus we have only sparse information regarding the effects of reversible disuse manipulations on motor map integrity at the spatial resolution that invasive neurophysiological procedures in animal models allow. Such information may be very important in understanding the consequences of reversible clinical procedures, such as casting. Furthermore, poststroke rehabilitation interventions [e.g., constraint-induced movement therapy (CIMT)] are based on the premise that the injury creating the stroke results in learned nonuse (or learned disuse) of the impaired limb (Taub et al. 1993). Reversal of learned nonuse is thought to contribute to recovery of function (Taub et al. 1994).
The following longitudinal experiments were designed to evaluate the effects of decreased distal forelimb (DFL) use in the absence of frank nervous system injury by restricting forelimb movements in nonhuman primates with a cast for up to 35 wk. We then examined the effects of disuse on motor map topography by remapping the animals after the casts were removed. Finally, whereas the behavioral effects of long-term casting were generally reversible, one animal experienced a chronic impairment in DFL use. The experimental model of forelimb restriction offers the unique opportunity to observe effects of disuse on cortical motor topography in the absence of any frank tissue destruction. This question has not been investigated previously in animal models, except in the context of disuse following injury.
MATERIALS AND METHODS
A total of 25 neurophysiological mapping sessions were conducted in seven experimentally naive, adult male squirrel monkeys (Saimiri boliviensis boliviensis and Saimiri boliviensis peruviensis). Squirrel monkeys were chosen because the primary motor DFL representation in this species is located within a relatively flat, unfissured sector of M1 that enables easy access for intracortical microstimulation (ICMS) procedures (Nudo et al. 1992). The monkeys ranged in age from 2 to 7 years and were free of any obvious physical or neurological deficits. All procedures were approved by the local Institutional Animal Care and Use Committee. Due to potential adverse effects of restricting the forelimb in a nonhuman primate species and the multiple survival surgeries (ranging from three to eight in animals undergoing forelimb restriction), each requiring 13–20 h to accomplish, veterinary consultation was required at all stages of the study to maintain the general health and well being of the animals and for ultimate decisions regarding the timing of subsequent survival surgeries. Therefore, identical time periods for the various phases of the experiments were not possible in each animal. Nevertheless, the ranges of short-term restriction, long-term restriction, and recovery periods were appropriate for the purposes of our study.
To evaluate the effects of forelimb restriction on motor map topography, subjects were assigned to one of two groups. Monkeys in the experimental group (n = 4) had their preferred DFL restricted by encapsulating it in a cast fashioned from Elastoplast tape. The fingers were first covered by a piece of gauze to minimize fixation of the fingers to the tape adhesive and to make it easier to remove the cast later. The cast extended from the distal phalanges proximally to the midhumerus. Four monkeys served as controls by participating in all aspects of the study except for forelimb restriction. One monkey served in both control and experimental groups (control procedures preceded experimental procedures), and hence, a total of seven monkeys were used.
General Experimental Protocol
First, all subjects were tested on a modified Klüver board task to evaluate forelimb preference (details below). After ∼1 mo, a baseline motor map was generated in the hemisphere contralateral to the preferred hand using ICMS techniques. Then, monkeys were randomly assigned to either the experimental or control group, with the requirement that forelimb preference was balanced for each condition, wherein two right-preferred and two left-preferred monkeys were included in the control and experimental groups. Then, the preferred forelimb of the monkeys in the experimental group was placed in the cast. The forelimbs of the monkeys in the control group were left unrestricted. Both groups of monkeys were returned to their home cages located in the same room.
In three of the four monkeys, the initial restriction period ranged from 38 to 42 days. One monkey had a longer restriction period of 85 days due to postponement of the initial postrestriction mapping surgery because of respiratory concerns (Fig. 1). In the remainder of the paper, “short-term restriction” refers to a period of 38–85 days. At the end of the short-term restriction period, monkeys in the experimental group underwent a second neurophysiological mapping procedure. Monkeys in the control group underwent a second mapping procedure ∼42 days after the baseline procedure.
Fig. 1.
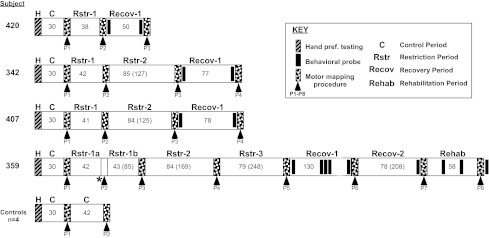
Timeline of experimental manipulations for each monkey. Numbers indicate duration (in days) of each manipulation. Numbers in parentheses indicate cumulative days of restriction or recovery. Dotted lines in case 359, Recov-1, indicate that the time period is not drawn to scale. H, hand preference testing; P, mapping procedure number (see Key for other descriptions). *Aborted mapping procedure.
In three of four subjects in the experimental group, the same arm was recasted at the conclusion of the second mapping procedure for an additional period of time ranging from 84 to 85 days. Thus the cumulative time after restriction ranged from 125 to 169 days and is hereafter called “long-term restriction”. After the second period of restriction, a third motor map was generated. The data from these three motor maps (baseline, short-term restriction, and long-term restriction) formed the basis for the statistical analyses to test the primary hypotheses regarding the effects of restriction on topography of motor maps. In one monkey (case 359), the same arm was recasted a third time for an additional period of 79 days. Subsequently, another motor map was derived. The restriction times are summarized for each of the monkeys in the experimental timeline in Fig. 1.
At the conclusion of the final postrestriction mapping procedure, all monkeys were allowed to recover uncasted for a period ranging from 50 to 130 days. Behavioral data were obtained at the beginning and end of the recovery period to evaluate hand preference and motor skill. After the recovery period, a motor map was derived once again.
One monkey did not return to use of the previously preferred (and restricted) forelimb during this initial recovery period (case 359). This monkey underwent a second recovery period of 78 days. Although functional recovery was still not observed after this time, another motor mapping experiment was performed. At the conclusion of the mapping procedure, the previously unrestricted hand was casted to encourage use of the previously restricted hand. After 58 days of forced use (“rehabilitation”), a final motor map was derived.
Behavioral Assessment
Forelimb preference and motor skill were evaluated using a modified Klüver board task that required the monkeys to remove banana-flavored pellets from a small well. Behavioral evaluation was conducted at baseline, after the maximum restriction period (following the final postrestriction mapping session) and after the recovery period (prior to the postrecovery mapping session). The Klüver board was a Plexiglas plate with five wells, 25, 19.5, 13.5, 11, and 9.5 mm in diameter and 5 mm deep. Food wells of smaller diameters were more challenging for the monkeys, because they required skilled use of one or two digits for successful retrieval (Nudo et al. 1996a).
Each animal was food restricted for 18 h before the hand preference/motor-skill testing procedure was conducted. For each testing period, hand preference was determined for 100 trials over a 2- to 3-day period. The Klüver board was attached to the front of the animal's home cage. The animal was required to support itself with one hand while reaching through the cage bars to retrieve pellets with the other hand. A single, banana-flavored, 45 mg food pellet (BioServe, Beltsville, MD) was placed randomly into one of the five wells for a maximum of 1 min, and the animal was allowed to retrieve it. The time limit was imposed to minimize training effects. For each trial, we recorded the hand used to dislodge or retrieve the pellet. A preference score was derived for each animal, equal to the percentage of pellet retrievals by the preferred hand. In addition, motor skill was assessed based on the average number of finger flexions per retrieval on each well (Nudo et al. 1996a).
Measurement of Limb Circumference
To assess atrophy in restricted limbs, upper-arm and forearm circumference were measured in both left and right forelimbs at two time points following: 1) maximum restriction and 2) recovery. Arm circumference was measured at the midpoint between the tip of the olecranon process and the acromium, whereas forearm circumference was measured at the largest part of the forearm. The ratio of the circumference between the restricted and unrestricted limbs was used as an index of atrophy. This ratio was preferred to comparing circumference with baseline, as male squirrel monkeys display large seasonal fluctuations in body mass (Boinski 1987), rendering longitudinal data in each limb unreliable.
Neurophysiological Techniques
The experiments in this study required multiple survival surgeries. The standardized procedure is summarized briefly below. Each subject was first anesthetized with ketamine hydrochloride (20 mg/kg im), intubated with an endotracheal tube, and catheterized with an intravenous fluid line (Ringer's/5% dextrose, 10 ml/h). Antibiotics (Crysticillan, 60,000 U) were administered at the beginning of each experiment and after 12 h of data collection.
After induction of halothane (1–2%)/nitrous oxide (75%) anesthesia, the monkey was placed in a stereotaxic frame, and Mannitol (8 cc/kg) was administered to reduce the likelihood of brain edema. Then, under sterile conditions, a craniotomy was made over M1. The dura was removed, and a small plastic cylinder was fitted over the craniotomy and filled with warmed, sterile silicone oil (30 k centistokes, dimethylpolysiloxane, Dow Corning 200 fluid) to prevent desiccation and reduce pulsations. Halothane/nitrous oxide was then withdrawn and ketamine gradually administered intravenously until the animal was stabilized. Supplemental doses of ketamine (∼15 mg·kg−1·h−1) were administered throughout the remainder of the experiment as needed to maintain a surgical level of anesthesia. To alleviate instances of extreme muscle tone, acepromazine (diluted to 0.1 mg/cc with saline) was also used in supplemental doses (typically 0.01 mg/kg). Heart rate, blood pressure, CO2, and temperature levels were monitored throughout the experiment. Core temperature (35.5–38°C) was monitored with a homeothermic blanket system for the duration of the mapping experiment (typically 13–20 h).
Before motor mapping commenced, a digital photograph of the cortical surface vasculature was derived. Afterward, a glass micropipette filled with 3.6 M NaCl (impedance range from 750 k to 1 MΩ) was introduced on a fine grid pattern, sited with reference to the surface vasculature (∼250 μm interpenetration distances), and then advanced perpendicular to the cortical surface to a depth of 1,700–1,800 μm. Thresholds for evoking movements are minimal at this depth (Nudo et al. 1992). Motor fields were defined by determining movements elicited by ICMS (<30 μA). The motor response evoked by ICMS was determined by visual observation. Movements evoked at threshold were verified independently by a second observer. The ICMS stimulus consisted of a 40-ms current train of 13, 200 μs-long monophasic cathodal pulses delivered at 350 Hz from an electrically isolated, charge-balanced (capacitively coupled) stimulation circuit. Trains were delivered at a rate of 1/s. Current was monitored by observing the voltage drop across a 10-kΩ resistor in series with the stimulation circuit. These procedures are widely used for mapping the functional topography of motor cortex and are described in detail in several publications (Gould et al. 1986; Nudo et al. 1992, 1996a).
The duration of stimulus trains used here (40 ms) is typical of most ICMS mapping studies. These trains are relatively brief compared with studies by some investigators (Graziano et al. 2002), who demonstrated complex movements with trains of 500 ms in duration. However, the nature of such long-duration trains is still somewhat controversial. Traditionally, it has been thought that whereas direct current spread from ICMS is limited to a few hundred microns using the current levels used here, ICMS can activate polysynaptically a wide area of cortex, and repetitive ICMS with long-duration trains can significantly favor tangential spread of trans-synaptic excitation of corticofugal neurons (Jankowska et al. 1975; Stoney et al. 1968). Furthermore, correlation between spike-triggered averaging results and ICMS results is optimal with a single stimulus pulse, although good correlation is still obtained with brief trains (Cheney and Fetz 1985). Since the purpose of this experiment was to derive detailed maps of motor cortex output topography, train lengths were minimized.
After mapping was completed, the plastic cylinder was removed, the dura replaced with gelfilm, the bone flap secured with acrylic, the skin sutured, and the wound dressed with an antibacterial agent. The monkey was monitored closely until it regained consciousness (15 min–1 h) and was then transferred to a temperature-controlled incubator for recovery.
Analysis of Motor Maps
ICMS elicited visually discriminable movements (e.g., finger flexion, thumb extension, wrist adduction and abduction, forearm pronation, forearm supination, etc.) (Nudo et al. 1992, 1996a). Whereas at most sites, movement of a single joint was observed at near-threshold current levels, at many sites, movement of two joints was observed within 2 μA of the threshold current. Responses at these sites were defined as multijoint responses and were analyzed as separate movement categories, as in previous publications (Nudo et al. 1996a). Data from individual stimulation sites were constructed into a two-dimensional map of movement representations. Coordinates (x–y) of each penetration site were determined by their location on an enlarged digital photograph of the surface vasculature. Next, a custom computer algorithm objectively delineated discrete regions encompassing sites whose stimulation evoked identical movements. A different color was used for each movement category. This resulted in a two-dimensional map comprised of colored regions whose borders are located halfway between sites representing different movement categories. These color-coded representational maps were then analyzed using an image analysis program (NIH Image, v.1.51; National Institutes of Health, Bethesda, MD) that measured the area of the differently colored regions. Subsequent analyses involved calculation of derived measures of percentage total area (based on the area of a particular movement type divided by the total contiguous DFL area).
To test our primary hypothesis regarding changes in motor map topography as a result of restriction, resulting areas (expressed as a percentage of DFL area) were analyzed using a two-way repeated measures ANOVA to determine the changes in areal extent of each of the movement categories in the restriction group compared with the control group (two-tailed tests; significance level, 0.05). The arcsine transformation was used on these values prior to statistical testing, as percentage data form a binomial rather than a normal distribution (Zar 1984). Since only two maps were derived in each of the control animals, it was necessary to perform two separate ANOVA analyses. Thus the effects of short-term restriction were evaluated by comparing control maps [motor mapping procedure number (P)1 vs. P2; n = 4] with experimental maps [P1 vs. P2 (or P3 in case 359); n = 4]. Similarly, the effects of long-term restriction were evaluated by comparing control maps (P1 vs. P2; n = 4) with experimental maps [P1 vs. P3 (or P4 in case 359); n = 3].
Threshold currents required to evoke movements using ICMS in the derivation of baseline and postrestriction maps were compared in a similar fashion. The postrecovery and postrehabilitation maps were secondary outcomes of the study, and as such, the study was not powered to examine these effects. Therefore, these results are reported using descriptive statistics.
ICMS maps, as typically derived, constitute the topographical organization of the motor cortex based on just-threshold current levels. Based on multimuscle and multijoint responses elicited by suprathreshold currents (Cheney and Fetz 1985; Nudo et al. 1992), as well as results from spike-triggered averaging studies, demonstrating multiple muscle facilitation from individual corticospinal neurons (Fetz and Cheney 1980), it is clear that there is more overlap in digit and wrist forearm representations than is illustrated with the present techniques. Nevertheless, the ICMS technique continues to provide valuable information about the normal organization and plasticity of motor cortex at a level of spatial resolution not possible with less-invasive techniques such as transcranial magnetic stimulation (TMS) or functional MRI.
Histological Verification
After the final motor map was generated, the animals were given a lethal injection of sodium pentobarbital (100 mg/kg) and perfused first with 9% saline, 0.2% lidocaine hydrochloride, and 1% heparin, followed with 4% paraformaldehyde. The brain was then removed and immersed in 30% sucrose for 1–2 days. It was cut in 50 μm parasagittal sections. Every-other section was mounted and stained for cresyl violet for cytoarchitectonic analysis (Nudo et al. 1992).
RESULTS
Behavioral Observations During Forelimb Restriction
During the first few days after casting, each of the animals periodically attempted to remove the cast, occasionally requiring recasting. The casted animals climbed less than the uncasted control monkeys, but these effects disappeared after ∼1 wk. Because the casts effectively limited movement of the digits, wrist, and elbow, the monkeys were unable to grasp the cage bars. Also, the casted limb was rarely used for feeding, grooming, or other activities requiring fine manipulation. However, the shoulder maintained full range of movement, and the restricted limb was often used for support in these behaviors. For example, the casted limb was frequently used for postural support during locomotion, especially during climbing and circling. Occasionally, the casted DFL was placed through the cage bars and appeared to be used for support during pellet retrieval. These observations suggest that although the digits were used infrequently, active stabilization of the wrist/forearm of the casted limb may have occurred. In the absence of chronic electromyographic records to indicate the relative use of various muscles in the present study, this conclusion must be weakened accordingly.
Changes in Limb Circumference
After the maximum restriction period in each animal and prior to the recovery period, the circumferences of the restricted and unrestricted forearm and upper arm were compared. The restricted forearm decreased significantly by an average of 5.8% ± 0.8%, whereas upper-arm circumference was not significantly different. The largest decrease was found in case 407, which displayed a 6.7% decrease in forearm circumference. At the end of the recovery period, no significant differences were found in either the forearm or upper arm. Thus forelimb restriction resulted in a statistically significant atrophy in the restricted forearm, although this difference was relatively small and reversible.
Normal Organization of Primary Motor Cortex DFL Representation
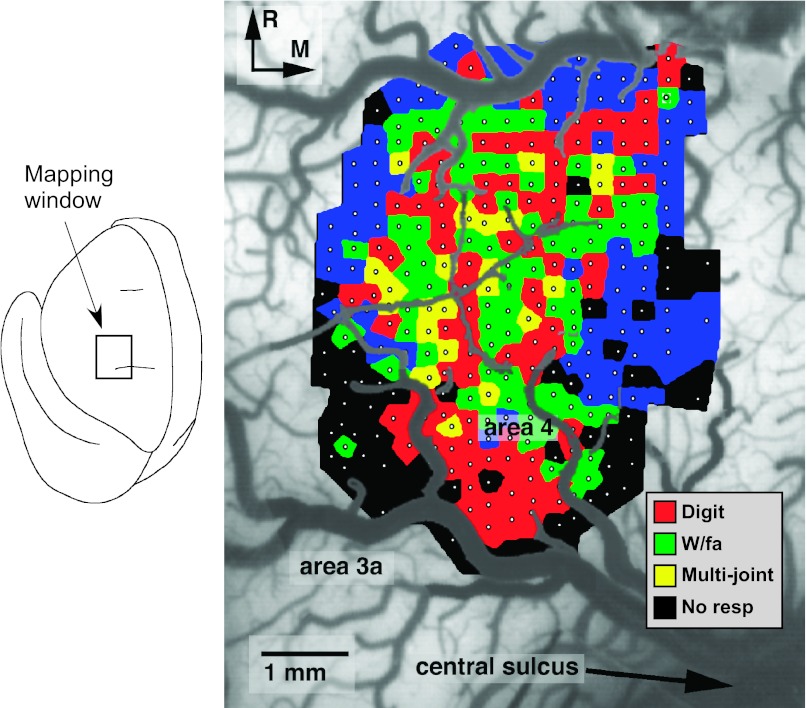
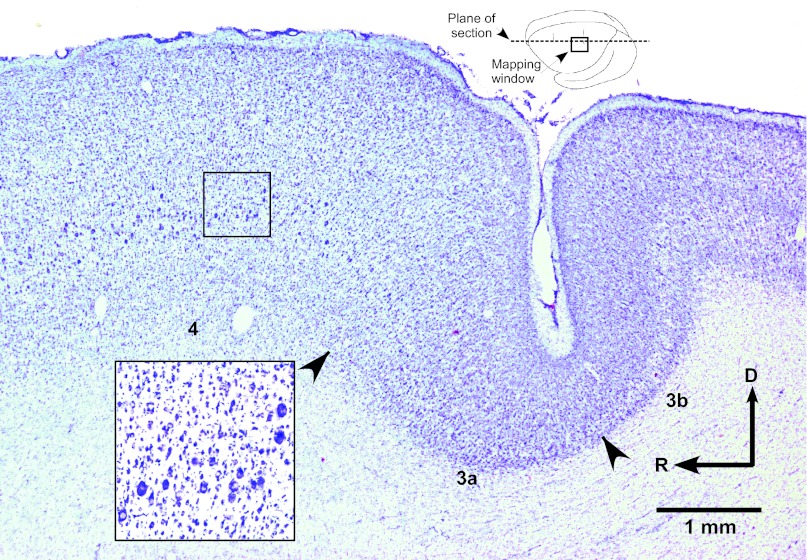
The fractionated representation of DFL movements in M1 was similar to reports published previously (Nudo et al. 1992, 1996a). The DFL representation contained separate digit and wrist/forearm territories, but the entire DFL was largely contiguous (Fig. 2). The DFL was bounded medially, rostrally, and laterally by more proximal (i.e., elbow and shoulder) representations and bounded caudally by a region unresponsive to ICMS at the current levels used in these experiments. With the use of 30 μA as a maximum current level, the responsive region closely corresponded to cytoarchitectonically defined area 4 (Fig. 3), similar to results demonstrated in a previous publication (Nudo et al. 1992).
Fig. 2.
Representation of distal forelimb (DFL) movements in primary motor cortex (M1; area 4) of a representative squirrel monkey (case 342; baseline map). In the case illustrated, movements were evoked by intracortical microstimulation at each of 321 sites (small white dots) located ∼250 μm apart. The DFL representation is comprised of digit (red), wrist/forearm (W/fa; green) movements, as well as combinations of these single-joint movements. Movements of 2 joints that could not be distinguished at threshold current levels (±2 μA) were designated as multijoint movements (yellow). R, rostral; M, medial; No resp, no response ≤30 μA.
Fig. 3.
Photomicrograph of cresyl violet-stained parasagittal section through the M1 DFL representation. Inset at top shows plane of section in dorsolateral view of brain. Movements were evoked at ≤30 μA at ∼1,750 μm from the cortical surface within cortical regions containing large pyramidal cells in layer V, indicative of M1 (see inset at higher magnification). Cytoarchitectonically defined boundaries are indicated by arrowheads. D, dorsal.
In the four control animals, representational maps of DFL movements were relatively stable from one procedure to the next (Fig. 4), as was demonstrated in previous studies (Nudo et al. 1996a). The map–remap data in control cases were used for statistical analysis to compare with animals undergoing restriction (Table 1).
Fig. 4.
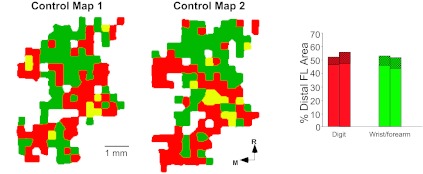
Stability of intracortical microstimulation (ICMS) maps in a representative control case. Maps were derived from ICMS mapping procedures conducted 30 days apart. See Fig. 2 for ICMS map color code. For clarity, proximal movement representations are not shown. Bar graph on right illustrates representational areas for single-joint digit movements (red), single-joint wrist/forearm movements (green), or combinations of digit or wrist/forearm movements with movements of other joints (cross-hatched bars). Whereas site-by-site variation in the movements evoked by ICMS between mapping procedures is common, the general topography as well as the relative representational areas are stable. FL, forelimb.
Table 1.
Change in DFL area in control cases
| Movement | Map 1 (% Total DFL) | Map 2 (% Total DFL; n = 4) |
|---|---|---|
| DFL | 100.0 ± 0.0 | 106.3 ± 8.6 |
| Digit | 56.5 ± 16.5 | 55.1 ± 16.4 |
| Digit (incl) | 63.7 ± 10.3 | 62.4 ± 9.3 |
| Wrist/forearm | 37.6 ± 8.4 | 37.2 ± 7.3 |
| Wrist/forearm (incl) | 44.6 ± 13.0 | 46.1 ± 14.2 |
| Digit + wrist/forearm | 6.0 ± 5.6 | 6.2 ± 6.3 |
Distal forelimb (DFL) values represent the percentage change in total DFL area between maps 1 and 2, averaged across the group. Thus map 1 values are necessarily 100% for each animal. Values for the other movement categories represent the percent total of the DFL representation averaged across the group. Data represent group means ± SD. incl, inclusive.
Changes in DFL Representational Area After Short-Term Restriction (38–85 Days)
In this section, main effects of the initial or short-term period of restriction (38–85 days) are summarized. Statistically significant values represent significant area-by-group interactions, that is, changes in representational areas that were significantly different from those found in sequential control mapping procedures based on a two-way repeated measures ANOVA. It should be noted that threshold currents required to elicit movements using ICMS did not change significantly after short- or long-term restriction (Table 2).
Table 2.
Threshold currents required to evoke movements in M1
| Movement | Baseline | Short-Term Restriction (n = 4) | Long-Term Restriction (n = 3) | F | P |
|---|---|---|---|---|---|
| DFL | 15.5 ± 3.9 | 15.3 ± 5.0 | 14.2 ± 2.3 | 0.10 | 0.908 |
| Digit | 15.9 ± 3.9 | 16.5 ± 4.7 | 15.1 ± 3.2 | 0.10 | 0.903 |
| Wrist/forearm | 15.9 ± 4.4 | 15.2 ± 4,2 | 14.1 ± 1.4 | 0.19 | 0.828 |
| Digit + wrist/forearm | 7.8 ± 1.6 | 8.3 ± 4.3 | 8.6 ± 4.7 | 0.04 | 0.959 |
Threshold currents (μA) required to evoke movements from primary motor cortex (M1) using intracortical microstimulation at baseline and after short- and long-term restriction. No significant changes were found in any of the major movement categories. F, continuous probability distribution; P, probability of type 1 error.
We analyzed representations of two different types of movement categories: 1) single-joint movement representations comprising all sites where ICMS evoked only one particular movement at threshold current levels (e.g., finger flexion) and 2) “inclusive” movement representations comprising all sites where ICMS evoked a particular movement alone or in combination with another movement at threshold current levels. For example, the inclusive finger flexion representation comprised sites where ICMS evoked finger flexion, whether or not another movement was elicited at threshold current. Hereafter, unless indicated specifically as an inclusive movement representation, reported movement representations are single joint.
On average, in the restriction group, the DFL increased from 10.0 to 10.5 mm2, an increase of only 5%. This degree of variation is similar to that found in control cases (average change in control cases = 6.3%). Thus no net change in total DFL (or percent total DFL; Table 3A; F = 0.51; P = 0.500) was found after short-term restriction. Since the total DFL was relatively stable after restriction, the representational areas for more specific movement categories are expressed as a percentage of total DFL.
Table 3.
Change in DFL area after forelimb restriction
| Movement | Baseline (% Total DFL) | Short-Term Restriction (% Total DFL; n = 4) | F | P | Long-Term Restriction (% Total DFL; n = 3) | F | P | |
|---|---|---|---|---|---|---|---|---|
| A | DFL | 00.0 ± 0.0 | 103.3 ± 13.9 | 0.51 | 0.500 | 102.9 ± 5.6 | 0.58 | 0.482 |
| B | Digit | 49.6 ± 19.4 | 41.7 ± 18.6 | 4.68 | 0.074 | 34.9 ± 20.6 | 11.87 | 0.018* |
| Digit (incl) | 57.4 ± 16.2 | 50.4 ± 16.5 | 6.09 | 0.049* | 41.6 ± 17.8 | 6.32 | 0.054 | |
| Wrist/forearm | 41.0 ± 15.3 | 48.4 ± 16.7 | 5.45 | 0.058 | 57.1 ± 16.7 | 5.23 | 0.071 | |
| Wrist/forearm (incl) | 50.0 ± 19.5 | 57.1 ± 18.9 | 4.00 | 0.092 | 64.7 ± 21.0 | 13.61 | 0.014* | |
| Digit + wrist/forearm | 7.4 ± 4.5 | 7.6 ± 6.9 | 0.05 | 0.834 | 6.3 ± 4.0 | 0.02 | 0.893 | |
| C | Finger | 34.6 ± 13.1 | 32.5 ± 14.9 | 0.61 | 0.466 | 23.9 ± 12.8 | 6.43 | 0.052 |
| Finger (incl) | 40.6 ± 10.1 | 38.6 ± 10.9 | 0.39 | 0.554 | 29.3 ± 11.6 | 2.81 | 0.155 | |
| Thumb | 14.7 ± 7.5 | 8.9 ± 5.2 | 1.37 | 0.286 | 10.8 ± 8.4 | 0.44 | 0.537 | |
| Wrist | 34.3 ± 8.4 | 38.7 ± 9.3 | 0.11 | 0.757 | 49.4 ± 15.5 | 3.68 | 0.113 | |
| Forearm | 7.4 ± 8.0 | 9.7 ± 8.9 | 7.19 | 0.037* | 7.8 ± 3.6 | 0.94 | 0.377 | |
| Forearm (incl) | 8.9 ± 8.5 | 11.4 ± 8.6 | 8.28 | 0.028* | 8.5 ± 4.8 | 0.84 | 0.403 | |
| D | Finger flexion | 13.8 ± 6.8 | 21.0 ± 10.6 | 7.50 | 0.034* | 17.7 ± 14.1 | 1.00 | 0.364 |
| Finger flexion (incl) | 16.8 ± 5.9 | 24.4 ± 9.4 | 7.70 | 0.032* | 19.4 ± 13.3 | 0.23 | 0.655 | |
| Finger extension | 16.1 ± 10.6 | 8.4 ± 4.9 | 5.37 | 0.060 | 2.4 ± 1.4 | 4.68 | 0.083 | |
| Finger extension (incl) | 17.8 ± 11.9 | 9.8 ± 4.7 | 4.81 | 0.071 | 5.2 ± 1.8 | 4.03 | 0.101 | |
| Finger ulnar | 3.7 ± 4.1 | 2.8 ± 2.0 | 0.03 | 0.863 | 1.8 ± 2.2 | 0.40 | 0.555 | |
| Finger radial | 1.0 ± 0.9 | 0.4 ± 0.4 | 1.04 | 0.353 | 0.0 ± 0.0 | 1.60 | 0.261 | |
| Thumb flexion | 4.4 ± 4.1 | 3.4 ± 3.0 | 0.74 | 0.422 | 3.9 ± 1.2 | 0.32 | 0.594 | |
| Thumb extension | 8.4 ± 6.2 | 5.0 ± 5.1 | 0.73 | 0.426 | 5.8 ± 9.6 | 0.20 | 0.893 | |
| Thumb radial | 1.6 ± 1.5 | 0.6 ± 0.9 | 0.51 | 0.502 | 0.8 ± 0.7 | 0.66 | 0.454 | |
| Thumb ulnar | 0.4 ± 0.7 | 0.1 ± 0.2 | 0.79 | 0.409 | 0.4 ± 0.6 | 2.13 | 0.205 | |
| Wrist flexion | 0.9 ± 0.8 | 1.4 ± 1.6 | 0.43 | 0.537 | 3.4 ± 4.1 | 1.72 | 0.247 | |
| Wrist extension | 17.8 ± 10.6 | 15.4 ± 8.6 | 0.71 | 0.432 | 24.0 ± 12.6 | 0.12 | 0.747 | |
| Wrist abduction | 12.2 ± 7.2 | 17.4 ± 7.9 | 7.27 | 0.036* | 16.4 ± 5.6 | 22.71 | 0.005* | |
| Wrist abduction (incl) | 13.1 ± 7.5 | 18.7 ± 7.5 | 5.96 | 0.050* | 17.2 ± 5.8 | 12.49 | 0.017* | |
| Wrist adduction | 2.7 ± 1.7 | 4.5 ± 4.8 | 0.07 | 0.799 | 5.7 ± 7.1 | 0.23 | 0.651 | |
| Forearm supination | 7.0 ± 7.8 | 8.3 ± 6.9 | 2.61 | 0.158 | 7.4 ± 2.8 | 0.40 | 0.555 | |
| Forearm pronation | 0.4 ± 0.5 | 1.5 ± 2.1 | 3.63 | 0.105 | 0.5 ± 0.8 | 1.27 | 0.310 |
DFL values represent the percentage change in total DFL area between maps 1 and 2, averaged across the group. Thus map 1 values are necessarily 100% for each animal. Values for the other movement categories represent the percent total of the DFL representation averaged across the group. Statistics (F, P) represent the results of repeated measures ANOVAs to determine the changes in areal extent of each of the movement categories in the restriction group compared with the control group (2-tailed tests; significance level, 0.05). Bold and italics, P ≤ 0.10;
P ≤ 0.05. Data represent group means ± SD. Letters (A–D) in the left column refer to hierarchical levels of movement categories referenced in the text.
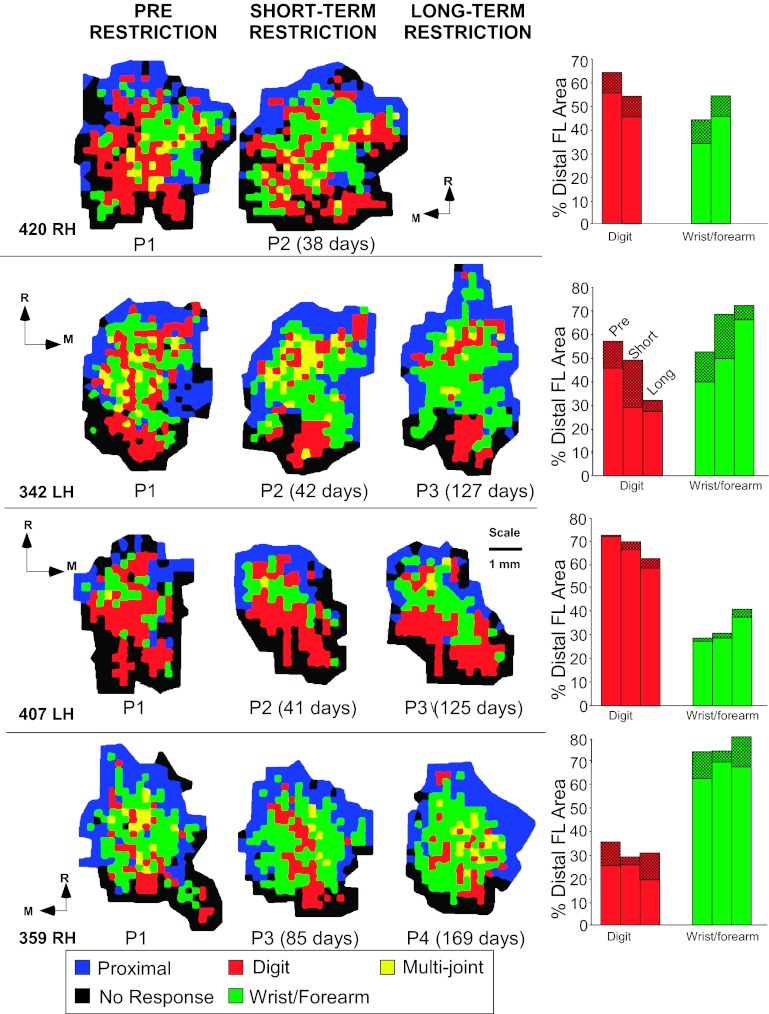
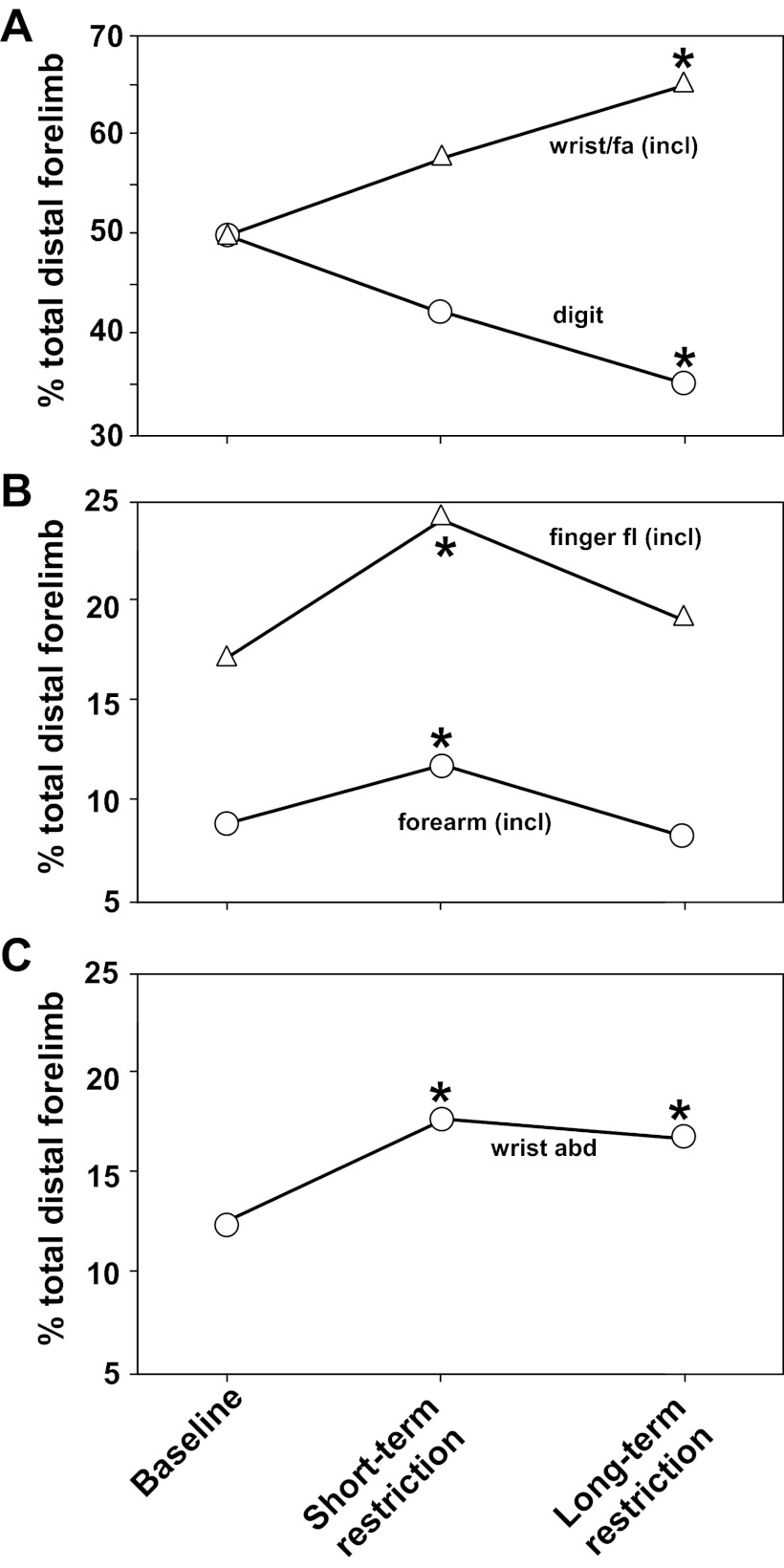
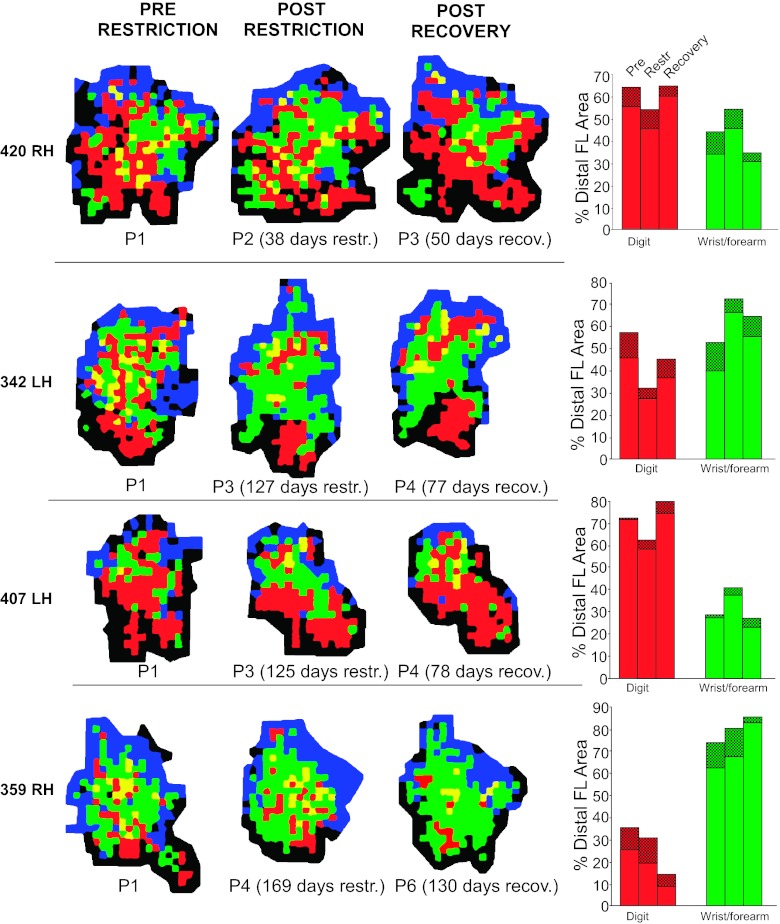
When the DFL was subdivided into more specific movement categories (Table 3B), it was found that digit and wrist/forearm representations were generally redistributed. The digit area (inclusive) decreased in each of the four short-term restriction cases from 57.4% to 50.4% of the total DFL, a statistically significant change (F = 6.09; P = 0.049; Fig. 5). In contrast, wrist/forearm area (%) was larger in each of the initial postrestriction maps, although this change was not statistically significant (F = 5.45; P = 0.058). No systematic change from digit to wrist/forearm was observed in any specific subregion (Fig. 5).
Fig. 5.
Comparison of baseline motor maps with short-term (38–85 days) and long-term (125–169) postrestriction movement maps in M1. Bar graphs on right display representational areas for single-joint digit movements (red), single-joint wrist/forearm movements (green), or combinations of digit or wrist/forearm movements with movements of other joints (cross-hatched bars). Thus inclusive digit and inclusive wrist/forearm areas are represented by the total bar height (shaded + cross-hatched bars). LH, left hemisphere; RH, right hemisphere.
When digit and wrist/forearm categories were subdivided further (Table 3C), it was found that the forearm representational area increased significantly from 7.4% to 9.7% (F = 7.19; P = 0.037). Finally, when movement categories were subdivided still further (Table 3D), it was found that the following two movement categories increased significantly in areal extent: finger flexion (13.8–21.0% of total DFL, F = 7.50; P = 0.034) and wrist abduction (12.2–17.4% of total DFL, F = 7.27; P = 0.036). Finally, the finger extension area was smaller in postrestriction maps, but this difference was not statistically significant (F = 5.41; P = 0.060). Although it may seem paradoxical that digit area (inclusive) decreased while finger flexion area increased, it should be noted that a statistically nonsignificant decrease in both finger extension and thumb representations contributed to the overall digit area decrease.
Changes in DFL Representational Area After Long-Term Restriction (125–169 Cumulative Days)
Because motor maps were derived at the end of each of two forelimb restriction periods in three of the four cases, it was possible to determine whether the effects were progressive over time (Table 3). One set of movement categories showed clear trends that were progressive—a further indication of the reliability of the results in a relatively small sample. Specifically, digit area decreased progressively with restriction duration, decreasing from 50% of the total DFL at baseline, to 42% after short-term restriction, to 35% after long-term restriction (F = 11.87; P = 0.018; Fig. 6A; Table 3B). In contrast, wrist/forearm area progressively increased. That is, the wrist-forearm area (inclusive) increased from 50% at baseline, to 57% after short-term restriction, to 65% after long-term restriction (F = 13.61; P = 0.014; Fig. 6A; Table 3B).
Fig. 6.
Longitudinal patterns of change in DFL representations after short- and long-term restriction. A: wrist/forearm [inclusive; wrist/fa (incl)] and digit areas changed progressively with longer-duration restriction. B: finger flexion [inclusive; finger/fl (incl)] and forearm (inclusive) areas first increased and then decreased back to baseline levels. C: wrist abduction area (wrist abd) increased with short-term restriction and then stabilized.
In a second set of movement categories, representational areas that changed after short-term restriction appeared to revert toward baseline levels after long-term restriction. These included finger flexion and forearm representations (both single-joint and inclusive categories; Fig. 6B; Table 3, C and D). The basis for the reversal of the initial effects of restriction cannot be determined from the present results, but it is possible that the initial expansions were related to attempts to escape from the cast during the initial days to weeks. Such attempts were observed anecdotally, especially during the 1st wk of restriction, but then seemed to subside. Finally, the increase in wrist abduction area (%) that was observed after short-term restriction was maintained after long-term restriction. Wrist abduction increased from 12% at baseline to 17% after short-term restriction and was at 16% after long-term restriction (F = 12.49; P = 0.017; Fig. 6C; Table 3D). Again, the reasons for this pattern of map change are not clear but may be due to attempts to escape from the cast, as noted above, or to a ceiling effect. That is, there may be a limited maximal area within M1 for certain representations.
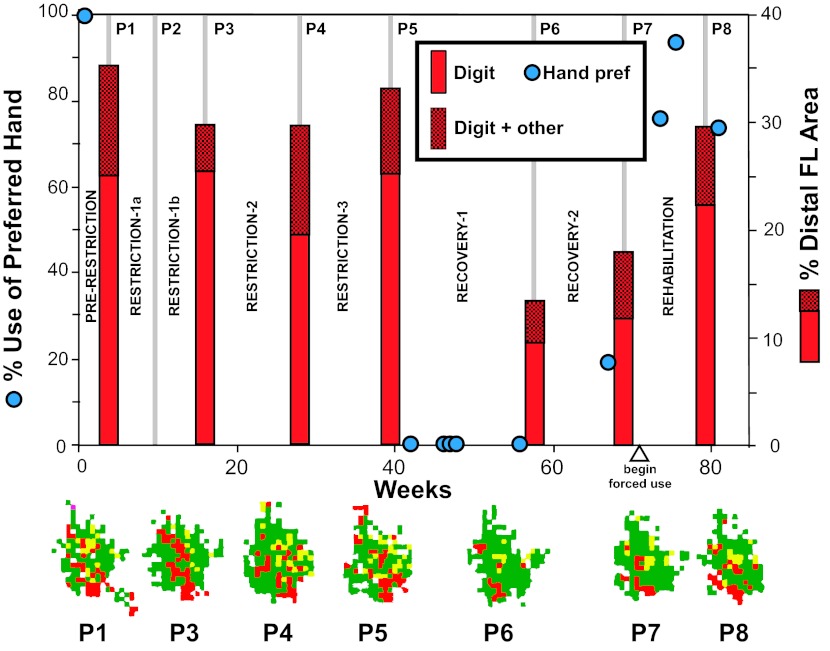
One monkey (case 359) underwent a third restriction period of an additional 79 days, bringing the total restriction period in this monkey to 248 days. The motor map derived after the third restriction period was atypical in that digit and wrist/forearm representations approximated their baseline areas rather than the pattern of redistributed areas seen in the other monkeys, as well as in this monkey after the second restriction period. To further investigate possible anomalies in this map, we examined various multijoint categories in individual maps, that is, sites at which ICMS evoked movements of two joints at approximately the same threshold current level (±2 μA). Typically, such responses comprise a small percentage of the total DFL area. After the maximum restriction period in the other three monkeys, digit + proximal area remained stable at 0.62% ± 0.55%, and wrist/forearm + proximal area decreased somewhat to 0.14% ± 0.25%, both representations within the normal range. In case 359, digit + proximal area increased to 2.0% after the third restriction, still within the normal range of baseline maps. However, wrist/forearm + proximal area increased to 4.86%, an increase of more than 4 SD above the mean of baseline maps and 18 SD above the mean of the other three monkeys. Overall, the area of multijoint representations that included distal + proximal movements represented 6.86% of the motor map in case 359 vs. 1.38% in baseline maps and 0.76% in maximum restriction maps in the other three monkeys.
Behavioral Observations After Cast Removal
In each monkey, after the final postrestriction mapping session, the cast was removed. In the initial few days after cast removal, most monkeys did not immediately use the previously casted (and preferred) forelimb for grasping. By the end of the 1st wk, monkeys showed no obvious difficulty in using the previously casted limb for holding large food biscuits, climbing, and grooming. When assessed on the Klüver board, ∼1 wk after cast removal, hand preference in case 420 was only mildly affected, and the previously casted (and preferred) limb was used in 83% of retrievals. In the other three monkeys (cases 407, 342, and 359), retrievals were made exclusively with the previously uncasted limb.
By the end of the recovery period (50–130 days), cases 407 and 342 returned to use of the previously casted limb for the majority of pellet retrievals. In case 342, the proportion of use with the restricted hand at the end of the recovery period nearly equaled the level of use prior to restriction. In case 407, the proportion of use with the restricted hand remained somewhat lower compared with baseline (reduction from 88% at baseline to 65% after recovery). However, in case 359, the restricted limb was not used for any retrievals at the end of the recovery period. After a second recovery period (78 days), case 359 successfully retrieved pellets with the previously casted limb, but hand preference was still at only 19% (Fig. 7).
Fig. 7.
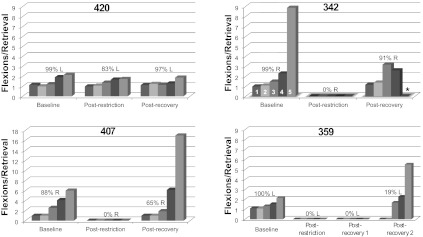
Hand preference and motor skill on the pellet retrieval task 1 wk after removal of the cast (postrestriction) and at the end of the recovery period (postrecovery). Only case 420 maintained the original hand preference in the 1st wk following long-term restriction. Motor skill, as indicated by the number of flexions per retrieval, also returned to normal levels in this animal. By the end of the recovery period, 3 of the 4 monkeys used the originally preferred hand for retrievals. Case 359 was an exception. Motor skill was equivalent or exceeded baseline levels in cases 342 and 420. A noticeable decrement in skill was observed in cases 359 and 407, primarily on well 5. After a 2nd recovery period, case 359 used the previously casted forelimb on a small proportion of trials, but motor skill was still impaired. Percentages indicate hand preference (L, left; R, right) as defined in materials and methods. Numbers 1–5 for case 342 indicate flexions/retrieval for each well size: 1 = 25 mm; 2 = 19.5 mm; 3 = 13.5 mm; 4 = 11 mm; 5 = 9.5 mm. *No pellets were retrieved from well 5 (case 342).
Motor skill with the preferred hand, defined by the number of flexions per pellet retrieval, was approximately equal at baseline and postrecovery assessments in case 420. In cases 342 and 407, flexions per retrieval remained elevated compared with baseline. For example, on the smallest well, case 407 required approximately three times as many flexions per retrieval during the postrecovery assessment compared with baseline. Case 342 failed to retrieve pellets from the smallest well, and flexions per retrieval were somewhat elevated on the other wells. At the end of a 130-day recovery period in case 359, attempts at retrieval of food pellets from the Klüver board typically were initiated with the originally preferred forelimb but successfully completed with the previously nonpreferred hand. As a result, motor skill with the previously preferred limb could not be assessed since no retrievals were made with this limb.
Based on persistently altered hand preference as well as impaired motor skill, case 359 was subjected to a second recovery period of 78 days. At the end of this second recovery period, 19% of the retrievals were made with the previously casted limb, and thus flexions per retrieval could be assessed. No retrievals were made with this hand from wells 1 and 2, whereas flexions per retrieval were somewhat elevated on wells 3–5. Specifically, flexions per retrieval on well 5 were ∼2.5× baseline levels. Furthermore, review of the video records in this case revealed that digits 4 and 5 of the previously casted limb were not used at all during retrieval of pellets or other food objects, remaining motionless in a slightly flexed posture throughout the reach and retrieval. Such abnormal digit movements were not observed at any time in the other three monkeys.
In summary, at the end of an initial recovery period, hand preference returned in three of four monkeys. Subtle deficits in motor skill were observed in two monkeys. In one monkey, more severe and long-lasting deficits were observed, as documented by the failure to retrieve pellets after the initial recovery period and persistent deficits in hand preference, motor skill, and digit kinematics after a second recovery period.
Alterations in Representational Maps After Recovery Period
Movement maps were again derived in each of the four monkeys after the recovery period. Figure 8 shows baseline maps, maps after long-term restriction, and maps derived after the recovery period, 50–130 days after cast removal. For the three monkeys that demonstrated return to use of their previously preferred and restricted hand, physiological motor maps recovered to near-baseline conditions. That is, digit area lost during restriction was regained after the recovery period. Likewise, the increase in wrist/forearm area was reversed. The subject (case 359), which did not return to consistent use of the preferred hand, displayed a substantial decrease in digit area and concomitant increase in wrist/forearm area after 130 days of recovery.
Fig. 8.
Representations of DFL movements after a 50- to 130-day recovery period following cast removal. See Fig. 2 for ICMS map color code. In 3 of 4 monkeys, digit area increased, whereas wrist/forearm area decreased, approximating prerestriction sizes. Hand preference in these 3 monkeys returned to baseline levels within 1 wk of cast removal. In the 4th monkey (case 359), a large reduction in digit area and expansion in wrist/forearm area was observed. This monkey's hand preference had not recovered at the time of the postcast removal mapping procedure.
Rehabilitation of Impaired Forelimb in Case 359 and Relationship to Map Changes
In an attempt to rehabilitate the impaired forelimb in case 359, we placed the previously uncasted forelimb in a cast similar to that used for the original casting procedure for a period of 58 days. This procedure, in effect, forced the animal to use the previously preferred and restricted forelimb for pellet retrievals, as well as for grasping food items and grabbing the cage bars during climbing. Two behavioral probes during the rehabilitation period (with cast temporarily removed for 1 day) and one final behavioral probe following a postrehabilitation map show that there was an increase in use of the previously preferred forelimb as a result of forced use (Fig. 9). Hand preference increased to 76% after 19 days and 93% after 31 days of forced use. The final behavioral probe conducted 14 days after the postrehabilitation map revealed that the use of the previously preferred forelimb was at 73%. Motor skill also recovered, as evidenced by a decreased number of finger flexions/retrieval. Flexions per retrieval on the smallest well (well 5) improved to 2.0 during the postrehabilitation behavioral probe, similar to baseline performance.
Fig. 9.
Relationship between behavior and physiology in case 359 over a period of 80 wk. During the 1st 2 restriction periods, digit area contracted, whereas wrist/forearm area expanded, as in other cases. However, the relationship between behavioral use and map area deviated from expectations through the 3rd restriction period. No pellet retrievals were made with the previously restricted limb during the recovery period. The subsequent mapping procedure revealed a substantially reduced digit area in the motor map. Forced use of the previously preferred (and restricted) limb (rehabilitation) resulted in recovery of original hand preference, as well as recovery of normal distribution of digit and wrist/forearm representations in the motor map. Each of the 7 motor maps is illustrated below the graph. See Fig. 2 for ICMS map color code. P1–P8 (thin gray vertical bars) refer to the 8 surgical procedures also shown in the experimental timeline in Fig. 1.
The motor map derived after 58 days of rehabilitation revealed a substantial expansion in digit area and concomitant reduction in wrist/forearm area. Thus the percentage total areas of the movement representations affected by forelimb restriction returned to near-baseline conditions after rehabilitation, paralleling behavioral recovery. Figure 9 illustrates changes in hand preference throughout the entire experiment in relation to the size of the digit representation in M1 for case 359.
DISCUSSION
Representational Plasticity After Forelimb Restriction
The most significant findings in this unique study of long-term forelimb restriction (up to 35 wk) on M1 motor representations in healthy, nonhuman primates are that 1) whereas total DFL area and the currents required to evoke movements remain remarkably constant, digit and wrist/forearm representations are progressively redistributed, favoring a reduction of digit and an expansion of wrist/forearm representations; 2) restriction-induced changes in the topography of motor maps are reversible, either spontaneously or with the aid of interventions to encourage re-use of the limb; and 3) under certain conditions, long-lasting impairment in motor performance can result from extremely long-term restriction. These results complement the inverse redistribution of digit and wrist/forearm representations after skill training.
Mechanisms Underlying Redistribution of DFL Representations
Acquisition of motor behaviors requiring skilled use of the digits (pellet retrieval) results in progressive expansion of digit representations and concomitant reduction in more proximal representations (Kleim and Jones 2008; Nudo et al. 1996a). Likewise, acquisition of novel motor behaviors using the forearm (forearm pronation and supination) results in reduction of finger representations and enlargement in wrist/forearm representations (Nudo et al. 1996a). We have argued that alterations in motor maps are strictly skill dependent, not use dependent (Plautz et al. 2000). This conclusion was based on the Plautz et al. (2000) result demonstrating that large-well repetitive practice (nonskilled use of digits) did not result in significant changes in motor maps, whereas a comparable amount of small-well repetitive practice (skilled use of digits) resulted in widespread redistribution of digit and wrist/forearm. Furthermore, the cessation of skill training resulted in a reversal of motor maps to baseline conditions. Thus at least superficially, it would seem that the restriction experience in the present study resembles skill learning in that digit and wrist/forearm representations are redistributed progressively and that this effect is reversible.
It would seem logical that restriction of the forelimb results in the loss of baseline digit skills and concomitant contraction of digit representations. This view is supported by reduced motor performance scores (hand preference, flexions per retrieval) exhibited by three of four monkeys after restriction. Furthermore, the casting procedure restricted joint movements differentially. Movements of more proximal joints, such as those of the shoulder, and stabilization of the wrist and elbow joints were favored. It is possible that new motor skills were developed for postural support of the restricted limb, favoring more proximal representations at the expense of digit representations. Although increased proximal skill may have contributed to the map changes, the behavioral constraints were different from the digit-training paradigm in that digit training resulted in highly stereotyped, repetitive movements, whereas proximal movements associated with the restricted limb were less constrained and more variable.
It is possible that cortical network interactions also were altered. Skilled movement of the DFL is an integrated response to cortical processing across a wide expanse of motor cortex (Devanne et al. 2002). Skilled use of the digits involves coordination with wrist/forearm movements, and hence, during acquisition of a task requiring skilled use of the digits, digit and wrist/forearm movement combinations are represented over expanded territories, suggesting formation of functional synergies between digit and wrist/forearm areas (Nudo et al. 1996a). Wrist/forearm stabilization for postural support requires substantial coordination with more proximal elbow and shoulder movements. During restriction, normal motor skill with the digits is no longer maintained. Thus a cortically mediated mechanism may lie in the potential for digit representations to become decoupled effectively from the rest of motor cortex and thus at a competitive disadvantage.
It is important to note that the restriction was conducted over very long time periods compared with earlier studies examining skill acquisition (weeks rather than days). Thus it is possible that simple disuse of the digits, in addition to reduced digit skill or increased proximal skill, contributed to the map redistribution in the present study. Consistent with this view, forearm circumference was reduced significantly, whereas upper-arm circumference did not change. Therefore, whereas the relative contributions of use and skill after forelimb restriction cannot be untwined at present, it is likely that both play a role.
With the aid of detailed ICMS maps of the entire expanse of the DFL area, it now appears that the distribution of component digit and wrist/forearm representations is quite sensitive to behavioral manipulations. After training on a task requiring skilled digit manipulation, digit representations expanded (Nudo et al. 1996a). In the present study, after restriction of the DFL, digit representations contracted, whereas wrist/forearm representations expanded. Digit area decreased from ∼50% before restriction to ∼35% after long-term restriction. Wrist/forearm area increased by a concomitant amount. Whereas it is possible that this magnitude of change represents an upper limit, this conclusion is still in doubt, since the changes in digit and wrist/forearm areas appear to be progressive. Thus longer restriction periods may result in further redistribution of digit and wrist/forearm representations.
Comparison with Human Studies
Since long-term limb immobilization in humans often results in muscle weakness and atrophy, TMS studies have begun to examine physiological changes at cortical and spinal levels to understand neural adaptation in these structures (Clark et al. 2008, 2010; Kaneko et al. 2003; Liepert et al. 1995; Lundbye-Jensen and Nielsen 2008; Ricci et al. 2008; Roberts et al. 2007; Siebner and Rothwell 2003; Zanette et al. 1997, 2004). Whereas there are several important differences in experimental design (most human studies performed on individuals with fractures; immobilization limited to 5–6 wk), and the spatial resolution of TMS is relatively poor compared with ICMS, a comparison with the present results is warranted based on the current paucity of physiological data on cortical organization after restriction, except for results from TMS studies.
Motor threshold.
TMS studies after immobilization of either the upper (Zanette et al. 1997) or lower (Liepert et al. 1995) limb subsequent to fracture or in healthy subjects placed in upper-limb casts (Clark et al. 2008, 2010) demonstrate that motor thresholds are relatively stable. However, in one study, whereas thresholds were unchanged in flexor carpi radialis, they decreased by a small amount in abductor pollicus brevis (Zanette et al. 2004). Furthermore, one TMS study reported no change in “resting” motor threshold (analogous to ICMS results) but a transient decrease in “active” motor threshold during contraction (Clark et al. 2008). This suggests that threshold changes may be state dependent.
It should be noted that motor threshold in typical TMS studies is defined only at the optimal scalp position, limiting the interpretation. The present result using ICMS to define movement thresholds at hundreds of stimulation sites extends these findings to subpopulations of neurons located throughout the DFL representation. It is possible that in ICMS studies, in particular, variability in motor threshold due to such factors as anesthetic state may prevent the identification of any trends across multiple mapping procedures (Nudo et al. 1992, 1996a). Despite these caveats, both TMS in human studies and ICMS studies in nonhuman primates indicate that the ability to evoke skeletal muscle contraction with stimulation of motor cortex clearly does not diminish substantially after long-term forelimb restriction.
Cortical area.
Changes in cortical area defined by TMS have been reported, but results may be a function of the way that areas are defined or duration of immobilization. When the total area from which motor-evoked potentials (MEPs) could be evoked was calculated, a significant reduction in the size of the representation in one muscle was reported but only when the immobilization period was longer than 4–6 wk (Liepert et al. 1995). When MEP amplitudes were recorded at each site after 1 mo of immobilization, no change in the TMS map area was found for four muscles (Zanette et al. 1997, 2004). The present ICMS study allows us to distinguish individual movement representations. Despite stability of the DFL, the component digit and wrist/forearm representations are quite sensitive to behavioral manipulations, a level of spatial detail that is not easily obtainable using TMS.
Intracortical facilitation and inhibition.
TMS studies of intracortical facilitation and inhibition, typically using paired-pulse TMS techniques, may provide clues regarding intracortical network mechanisms that may underlie redistribution of representations after restriction. Following wrist-hand immobilization subsequent to fracture, intracortical facilitation is enhanced significantly, whereas intracortical inhibition (short interval; at rest) is reduced (Zanette et al. 2004), suggesting cortical hyperexcitability. In healthy subjects, no changes were found at rest, but increased intracortical inhibition (long interval) was found during contraction, consistent with a prolonged silent period (Clark et al. 2010). Thus immobilization results in changes in the balance between intracortical facilitation and inhibition in motor cortex. These local changes in cortical excitability may contribute to the map changes demonstrated in the present study and may be the basis for decoupling of distal representations proposed above.
Spinal excitability.
Spinal excitability measures (F-wave or H-reflex parameters) are important in determining whether changes in cortical motor maps are due to spinal and peripheral excitability changes. Whereas F-wave parameters are unchanged after immobilization (Liepert et al. 1995; Zanette et al. 1997, 2004), increased H-reflex amplitudes (at rest) have been demonstrated after 1–3 wks of immobilization (Clark et al. 2010; Lundbye-Jensen and Nielsen 2008). These changes are presumably due to a reduction in presynaptic inhibition of Ia afferents (Lundbye-Jensen and Nielsen 2008). It is unlikely that such changes at the spinal level underlie map plasticity observed in the present study, since ICMS thresholds would be expected to decrease under such circumstances.
Adverse Consequences of Limb Immobilization
Whereas most studies of limb immobilization are complicated by their purpose in treating orthopedic injuries, a few studies have been performed in healthy volunteers for periods up to 3 wk (Clark et al. 2008, 2010; Lundbye-Jensen and Nielsen 2008; Weibull et al. 2011). Immobilization resulted in significant reduction in contraction torque and strength and alteration of neurophysiological properties, requiring ∼1 wk for recovery. Even short-term casting (72 h) resulted in changes in grip strength, dexterity, and tactile discrimination (Weibull et al. 2011). Nevertheless, casting up to 3 wk in duration is deemed benign, and any functional deficits are typically transient.
Casting of the forelimb in three of four healthy squirrel monkeys for 5–18 wk resulted in a small but significant atrophy in the forearm, as well as changes in hand preference that resolved spontaneously within 7–11 wk. In one of the three monkeys, whose previously restricted hand was used, motor skill, as measured by the number of flexions required to retrieve small food pellets, was somewhat impaired. However, in a fourth monkey casted for 35 wk (case 359), a more severe and chronic deficit was observed, as evidenced by a reversal in hand preference, decreased motor skill, and inability to flex the ulnar digits. No evidence of peripheral injury was found upon physical examination. Recovery in limb preference was achieved only after a rehabilitation period, facilitated by placing the uncasted limb in a restraining cast, paralleling a return of the normal map pattern.
Whereas the pathogenesis of the behavioral impairments and anomalous map changes in case 359 cannot be determined from the present data, one possible explanation is that limb immobilization can induce a dystonic condition. This hypothesis has been proposed to explain dystonia in four patients who developed focal dystonia after immobilization (Okun et al. 2002). Conditions that may lead to maladaptive plasticity in the cortex (repetitive, spatially and temporally correlated, stereotyped peripheral inputs) exist in limb immobilization, possibly leading to immobilization dystonia. Studies using a nonhuman primate model of focal hand dystonia have suggested that certain repetitive behaviors may result in dystonic disorders associated with maladaptive plastic changes in somatosensory maps (Byl 2004). Monkeys in the present study were restricted to a small subset of forelimb movements and stereotyped joint kinematics. At least in one monkey, this may have resulted in a form of dystonia, as well as maladaptive motor maps.
Relevance for Understanding Plasticity After Stroke
After stroke-like injury to the motor cortex, map changes in spared motor areas are well known (Cramer 2008; Nudo and Milliken 1996). Behavioral experience and neural injury interact, such that changes in the spared cortex are a product of both injury-related mechanisms, such as diaschisis, and behaviorally driven changes, such as disuse (Woodlee and Schallert 2004). In fact, postinjury disuse forms the mechanistic basis for one of the most popular forms of stroke-rehabilitative therapy, CIMT (Taub et al. 2006). The theoretical constructs of this therapy were based on earlier deafferentation experiments in monkeys (Taub et al. 1977). Whereas the deafferented limb was not used spontaneously, after training, the monkeys could be shaped to reach and grasp for food objects. It was proposed that the monkeys had undergone “learned nonuse” of the deafferented limb. The same logic was used subsequently to describe the propensity for human stroke survivors to use the less-affected limb at the exclusion of the more paretic limb. Both “forced use”, in which a nonparetic limb is immobilized to encourage use of the paretic limb, and CIMT, which combines forced use with repetitive training, result in improved motor performance scores with the impaired limb (Wolf 2007). TMS-based maps of distal muscle representations in stroke survivors are reduced in size in the motor cortex opposite of the paretic limb but expand significantly after CIMT (Liepert et al. 1998; Sawaki et al. 2008). Although the results were obtained after stroke, they parallel the results observed in the present study using ICMS after forelimb restriction.
The present results represent a unique dissociation of the effects of neural injury and disuse; even in the absence of neuronal injury, disuse can contribute to alterations in cortical representations. After cortical injury, spared motor areas may be more sensitive to behavioral experience, and experience-injury interactions may result in both adaptive as well as maladaptive plasticity in the remaining cerebral cortex (Jones et al. 2009).
Conclusions
The present ICMS study in nonhuman primates demonstrates that after long periods of forelimb immobilization (up to 35 wk), component representations within the DFL area are redistributed, with expansion of wrist-forearm and contraction of digit representations. These immobilization-induced changes are, in general, reversible. Due to the greater spatial resolution inherent in ICMS mapping compared with noninvasive TMS mapping, this study greatly extends the results of previous human studies after limb immobilization after fracture or in normal subjects.
GRANTS
Support for this work was provided by Grants NS-30853 (R. J. Nudo) and NS-09366 (G. W. Milliken) from the National Institute of Neurological Disorders and Stroke.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: G.W.M. and R.J.N. conception and design of research; G.W.M., E.J.P., and R.J.N. performed experiments; G.W.M., E.J.P., and R.J.N. analyzed data; G.W.M., E.J.P., and R.J.N. interpreted results of experiments; G.W.M. and R.J.N. prepared figures; G.W.M. drafted manuscript; G.W.M., E.J.P., and R.J.N. edited and revised manuscript; G.W.M., E.J.P., and R.J.N. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Grey Gardner, Ramin Raiszedeh, Cami Knox, Laura Weisheit, and Gerald Falchook for assisting in data collection and Patrick Nudo for manuscript proofreading.
Present address of G. W. Milliken: Dept. of Psychology, College of Charleston, Charleston, SC 29424.
Present address of E. J. Plautz: Dept. of Neurology and Neurotherapeutics, University of Texas Southwestern Medical Center, Dallas, TX 75390.
REFERENCES
- Adachi K, Lee JC, Hu JW, Yao D, Sessle BJ. Motor cortex neuroplasticity associated with lingual nerve injury in rats. Somatosens Mot Res 24: 97–109, 2007 [DOI] [PubMed] [Google Scholar]
- Boinski S. Habitat use by squirrel monkeys (Saimiri oerstedi) in Costa Rica. Folia Primatol (Basel) 49: 151–167, 1987 [DOI] [PubMed] [Google Scholar]
- Byl NN. Focal hand dystonia may result from aberrant neuroplasticity. Adv Neurol 94: 19–28, 2004 [PubMed] [Google Scholar]
- Cheney PD, Fetz EE. Comparable patterns of muscle facilitation evoked by individual corticomotoneuronal (CM) cells and by single intracortical microstimuli in primates: evidence for functional groups of CM cells. J Neurophysiol 53: 786–804, 1985 [DOI] [PubMed] [Google Scholar]
- Clark BC, Issac LC, Lane JL, Damron LA, Hoffman RL. Neuromuscular plasticity during and following 3 wk of human forearm cast immobilization. J Appl Physiol 105: 868–878, 2008 [DOI] [PubMed] [Google Scholar]
- Clark BC, Taylor JL, Hoffman RL, Dearth DJ, Thomas JS. Cast immobilization increases long-interval intracortical inhibition. Muscle Nerve 42: 363–372, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SC. A window into the molecular basis of human brain plasticity. J Physiol 586: 5601, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancause N. Neurophysiological and anatomical plasticity in the adult sensorimotor cortex. Rev Neurosci 17: 561–580, 2006 [DOI] [PubMed] [Google Scholar]
- Devanne H, Cohen LG, Kouchtir-Devanne N, Capaday C. Integrated motor cortical control of task-related muscles during pointing in humans. J Neurophysiol 87: 3006–3017, 2002 [DOI] [PubMed] [Google Scholar]
- Fetz EE, Cheney PD. Postspike facilitation of forelimb muscle activity by primate corticomotoneuronal cells. J Neurophysiol 44: 751–772, 1980 [DOI] [PubMed] [Google Scholar]
- Franchi G. Time course of motor cortex reorganization following botulinum toxin injection into the vibrissal pad of the adult rat. Eur J Neurosci 16: 1333–1348, 2002 [DOI] [PubMed] [Google Scholar]
- Gould HJ, 3rd, Cusick CG, Pons TP, Kaas JH. The relationship of corpus callosum connections to electrical stimulation maps of motor, supplementary motor, and the frontal eye fields in owl monkeys. J Comp Neurol 247: 297–325, 1986 [DOI] [PubMed] [Google Scholar]
- Graziano MS, Taylor CS, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron 34: 841–851, 2002 [DOI] [PubMed] [Google Scholar]
- Jankowska E, Padel Y, Tanaka R. The mode of activation of pyramidal tract cells by intracortical stimuli. J Physiol 249: 617–636, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Allred RP, Adkins DL, Hsu JE, O'Bryant A, Maldonado MA. Remodeling the brain with behavioral experience after stroke. Stroke 40: S136–S138, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH. The reorganization of somatosensory and motor cortex after peripheral nerve or spinal cord injury in primates. Prog Brain Res 128: 173–179, 2000 [DOI] [PubMed] [Google Scholar]
- Kaneko F, Murakami T, Onari K, Kurumadani H, Kawaguchi K. Decreased cortical excitability during motor imagery after disuse of an upper limb in humans. Clin Neurophysiol 114: 2397–2403, 2003 [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, Ungerleider LG. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci USA 95: 861–868, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol 80: 3321–3325, 1998 [DOI] [PubMed] [Google Scholar]
- Kleim JA, Cooper NR, VandenBerg PM. Exercise induces angiogenesis but does not alter movement representations within rat motor cortex. Brain Res 934: 1–6, 2002 [DOI] [PubMed] [Google Scholar]
- Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res 51: S225–S239, 2008 [DOI] [PubMed] [Google Scholar]
- Kleim JA, Lussnig E, Schwarz ER, Comery TA, Greenough WT. Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. J Neurosci 16: 4529–4535, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepert J, Miltner WH, Bauder H, Sommer M, Dettmers C, Taub E, Weiller C. Motor cortex plasticity during constraint-induced movement therapy in stroke patients. Neurosci Lett 250: 5–8, 1998 [DOI] [PubMed] [Google Scholar]
- Liepert J, Tegenthoff M, Malin JP. Changes of cortical motor area size during immobilization. Electroencephalogr Clin Neurophysiol 97: 382–386, 1995 [DOI] [PubMed] [Google Scholar]
- Lundbye-Jensen J, Nielsen JB. Central nervous adaptations following 1 wk of wrist and hand immobilization. J Appl Physiol 105: 139–151, 2008 [DOI] [PubMed] [Google Scholar]
- Monfils MH, Plautz EJ, Kleim JA. In search of the motor engram: motor map plasticity as a mechanism for encoding motor experience. Neuroscientist 11: 471–483, 2005 [DOI] [PubMed] [Google Scholar]
- Navarro X, Vivo M, Valero-Cabre A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol 82: 163–201, 2007 [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Jenkins WM, Merzenich MM, Prejean T, Grenda R. Neurophysiological correlates of hand preference in primary motor cortex of adult squirrel monkeys. J Neurosci 12: 2918–2947, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol 75: 2144–2149, 1996 [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci 16: 785–807, 1996a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Plautz EJ, Frost SB. Role of adaptive plasticity in recovery of function after damage to motor cortex. Muscle Nerve 24: 1000–1019, 2001 [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science 272: 1791–1794, 1996b [DOI] [PubMed] [Google Scholar]
- Okun MS, Nadeau SE, Rossi F, Triggs WJ. Immobilization dystonia. J Neurol Sci 201: 79–83, 2002 [DOI] [PubMed] [Google Scholar]
- Plautz EJ, Milliken GW, Nudo RJ. Effects of repetitive motor training on movement representations in adult squirrel monkeys: role of use vs. learning. Neurobiol Learn Mem 74: 27–55, 2000 [DOI] [PubMed] [Google Scholar]
- Remple MS, Bruneau RM, VandenBerg PM, Goertzen C, Kleim JA. Sensitivity of cortical movement representations to motor experience: evidence that skill learning but not strength training induces cortical reorganization. Behav Brain Res 123: 133–141, 2001 [DOI] [PubMed] [Google Scholar]
- Ricci R, Ramsey D, Johnson K, Borckardt JJ, Vallejo M, Roberts DR, George MS. A pilot feasibility study of daily rTMS to modify corticospinal excitability during lower limb immobilization. Ther Clin Risk Manag 4: 1127–1134, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DR, Ricci R, Funke FW, Ramsey P, Kelley W, Carroll JS, Ramsey D, Borckardt JJ, Johnson K, George MS. Lower limb immobilization is associated with increased corticospinal excitability. Exp Brain Res 181: 213–220, 2007 [DOI] [PubMed] [Google Scholar]
- Sanes JN, Suner S, Donoghue JP. Dynamic organization of primary motor cortex output to target muscles in adult rats. I. Long-term patterns of reorganization following motor or mixed peripheral nerve lesions. Exp Brain Res 79: 479–491, 1990 [DOI] [PubMed] [Google Scholar]
- Sawaki L, Butler AJ, Leng X, Wassenaar PA, Mohammad YM, Blanton S, Sathian K, Nichols-Larsen DS, Wolf SL, Good DC, Wittenberg GF. Constraint-induced movement therapy results in increased motor map area in subjects 3 to 9 months after stroke. Neurorehabil Neural Repair 22: 505–513, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber MH, Deuel RK. Primary motor cortex reorganization in a long-term monkey amputee. Somatosens Mot Res 14: 157–167, 1997 [DOI] [PubMed] [Google Scholar]
- Sessle BJ, Adachi K, Avivi-Arber L, Lee J, Nishiura H, Yao D, Yoshino K. Neuroplasticity of face primary motor cortex control of orofacial movements. Arch Oral Biol 52: 334–337, 2007 [DOI] [PubMed] [Google Scholar]
- Siebner HR, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res 148: 1–16, 2003 [DOI] [PubMed] [Google Scholar]
- Stoney SD, Jr, Thompson WD, Asanuma H. Excitation of pyramidal tract cells by intracortical microstimulation: effective extent of stimulating current. J Neurophysiol 31: 659–669, 1968 [DOI] [PubMed] [Google Scholar]
- Taub E, Crago JE, Burgio LD, Groomes TE, Cook EW, 3rd, DeLuca SC, Miller NE. An operant approach to rehabilitation medicine: overcoming learned nonuse by shaping. J Exp Anal Behav 61: 281–293, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub E, Heitmann RD, Barro G. Alertness, level of activity, and purposive movement following somatosensory deafferentation in monkeys. Ann N Y Acad Sci 290: 348–365, 1977 [DOI] [PubMed] [Google Scholar]
- Taub E, Miller NE, Novack TA, Cook EW, 3rd, Fleming WC, Nepomuceno CS, Connell JS, Crago JE. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil 74: 347–354, 1993 [PubMed] [Google Scholar]
- Taub E, Uswatte G, Mark VW, Morris DM. The learned nonuse phenomenon: implications for rehabilitation. Eura Medicophys 42: 241–256, 2006 [PubMed] [Google Scholar]
- Weibull A, Flondell M, Rosen B, Bjorkman A. Cerebral and clinical effects of short-term hand immobilisation. Eur J Neurosci 33: 699–704, 2011 [DOI] [PubMed] [Google Scholar]
- Wolf SL. Revisiting constraint-induced movement therapy: are we too smitten with the mitten? Is all nonuse “learned”? and other quandaries. Phys Ther 87: 1212–1223, 2007 [DOI] [PubMed] [Google Scholar]
- Woodlee MT, Schallert T. The interplay between behavior and neurodegeneration in rat models of Parkinson's disease and stroke. Restor Neurol Neurosci 22: 153–161, 2004 [PubMed] [Google Scholar]
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 462: 915–919, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanette G, Manganotti P, Fiaschi A, Tamburin S. Modulation of motor cortex excitability after upper limb immobilization. Clin Neurophysiol 115: 1264–1275, 2004 [DOI] [PubMed] [Google Scholar]
- Zanette G, Tinazzi M, Bonato C, di Summa A, Manganotti P, Polo A, Fiaschi A. Reversible changes of motor cortical outputs following immobilization of the upper limb. Electroencephalogr Clin Neurophysiol 105: 269–279, 1997 [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. Englewood Cliffs, NJ: Prentice-Hall, 1984, p. 718 [Google Scholar]