Abstract
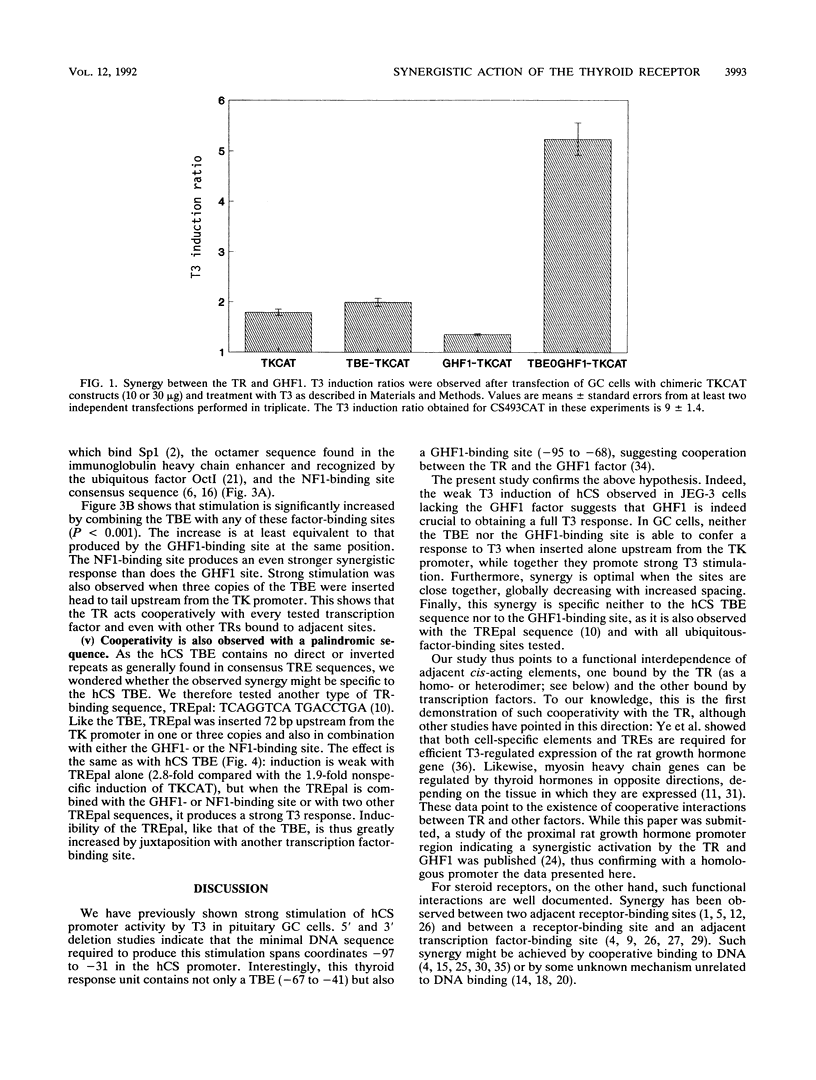
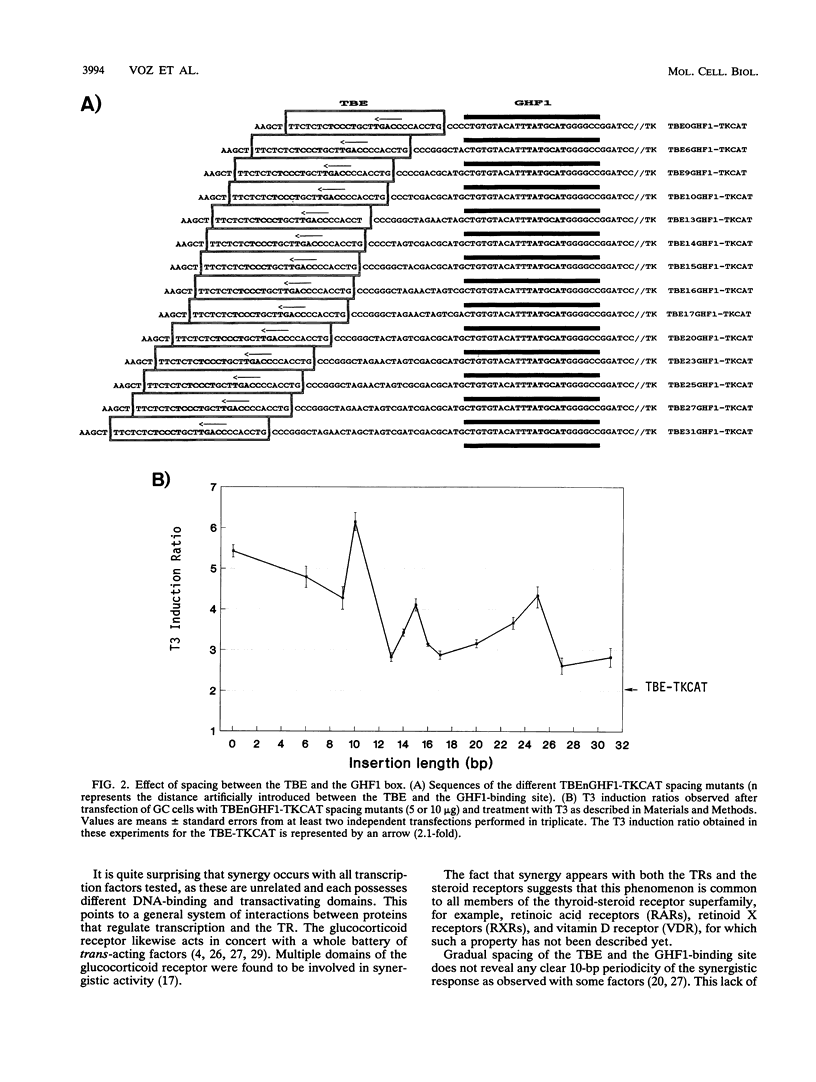
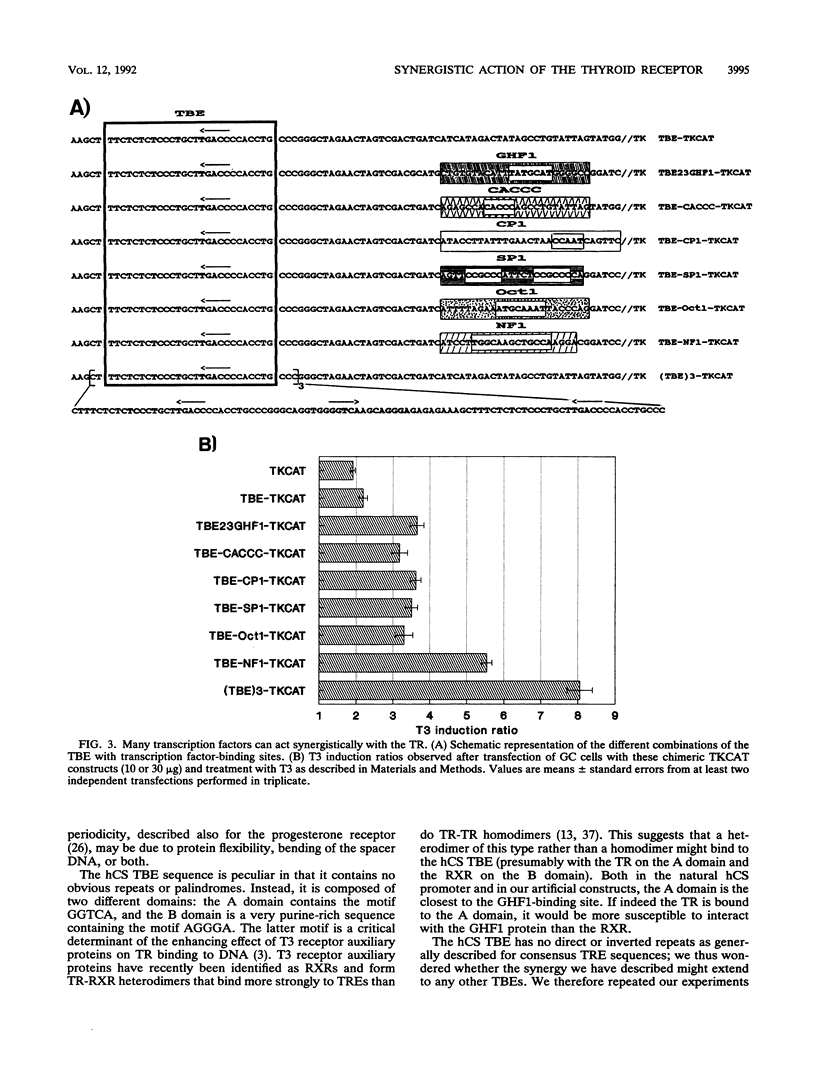
Human placental lactogen B (hCS-B) promoter activity is strongly stimulated by triiodothyronine (T3) in pituitary GC cells through interaction between the thyroid receptor and a thyroid receptor-binding element (TBE) spanning coordinates -67 to -41. This TBE is adjacent to the binding site for pituitary factor GHF1 (-95 to -68) which seems necessary for T3 stimulation of hCS-B promoter activity (M. L. Voz, B. Peers, A. Belayew, and J. A. Martial, J. Biol. Chem. 266:13397-13404, 1991). We here demonstrate actual synergy between the thyroid receptor and GHF1. Indeed, in placental JEG-3 cells devoid of factor GHF1, hCS promoter activity is barely stimulated by T3, while a strong response is observed in pituitary GC cells. In the latter, furthermore, neither the TBE nor the GHF1-binding site alone is sufficient to render the thymidine kinase promoter responsive to T3, while in combination they promote strong T3 stimulation. Close proximity between these sites is required for optimal synergy: T3 stimulation globally decreases with increased spacing. Furthermore, synergy occurs not only with a GHF1-binding site but also with all other factor recognition sequences tested (Sp1, NF1, CP1, Oct1, and CACCC boxes) and even with two other copies of the TBE. Nor is it specific to hCS TBE, since the palindromic sequence TCAGGTCA TGACCTGA (TREpal) also exhibits cooperativity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ankenbauer W., Strähle U., Schütz G. Synergistic action of glucocorticoid and estradiol responsive elements. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7526–7530. doi: 10.1073/pnas.85.20.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera-Saldana H., Takahashi K., Vigneron M., Wildeman A., Davidson I., Chambon P. All six GC-motifs of the SV40 early upstream element contribute to promoter activity in vivo and in vitro. EMBO J. 1985 Dec 30;4(13B):3839–3849. doi: 10.1002/j.1460-2075.1985.tb04156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe J. S., Darling D. S., Chin W. W. 3,5,3'-triiodothyronine receptor auxiliary protein (TRAP) enhances receptor binding by interactions within the thyroid hormone response element. Mol Endocrinol. 1991 Jan;5(1):85–93. doi: 10.1210/mend-5-1-85. [DOI] [PubMed] [Google Scholar]
- Brüggemeier U., Kalff M., Franke S., Scheidereit C., Beato M. Ubiquitous transcription factor OTF-1 mediates induction of the MMTV promoter through synergistic interaction with hormone receptors. Cell. 1991 Feb 8;64(3):565–572. doi: 10.1016/0092-8674(91)90240-y. [DOI] [PubMed] [Google Scholar]
- Cato A. C., Heitlinger E., Ponta H., Klein-Hitpass L., Ryffel G. U., Bailly A., Rauch C., Milgrom E. Estrogen and progesterone receptor-binding sites on the chicken vitellogenin II gene: synergism of steroid hormone action. Mol Cell Biol. 1988 Dec;8(12):5323–5330. doi: 10.1128/mcb.8.12.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodosh L. A., Baldwin A. S., Carthew R. W., Sharp P. A. Human CCAAT-binding proteins have heterologous subunits. Cell. 1988 Apr 8;53(1):11–24. doi: 10.1016/0092-8674(88)90483-7. [DOI] [PubMed] [Google Scholar]
- Damm K., Thompson C. C., Evans R. M. Protein encoded by v-erbA functions as a thyroid-hormone receptor antagonist. Nature. 1989 Jun 22;339(6226):593–597. doi: 10.1038/339593a0. [DOI] [PubMed] [Google Scholar]
- Danesch U., Gloss B., Schmid W., Schütz G., Schüle R., Renkawitz R. Glucocorticoid induction of the rat tryptophan oxygenase gene is mediated by two widely separated glucocorticoid-responsive elements. EMBO J. 1987 Mar;6(3):625–630. doi: 10.1002/j.1460-2075.1987.tb04800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day R. N., Koike S., Sakai M., Muramatsu M., Maurer R. A. Both Pit-1 and the estrogen receptor are required for estrogen responsiveness of the rat prolactin gene. Mol Endocrinol. 1990 Dec;4(12):1964–1971. doi: 10.1210/mend-4-12-1964. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Holloway J. M., Devary O. V., Rosenfeld M. G. The thyroid hormone receptor binds with opposite transcriptional effects to a common sequence motif in thyroid hormone and estrogen response elements. Cell. 1988 Jul 29;54(3):313–323. doi: 10.1016/0092-8674(88)90194-8. [DOI] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. All members of the MHC multigene family respond to thyroid hormone in a highly tissue-specific manner. Science. 1986 Feb 7;231(4738):597–600. doi: 10.1126/science.3945800. [DOI] [PubMed] [Google Scholar]
- Klein-Hitpass L., Kaling M., Ryffel G. U. Synergism of closely adjacent estrogen-responsive elements increases their regulatory potential. J Mol Biol. 1988 Jun 5;201(3):537–544. doi: 10.1016/0022-2836(88)90635-3. [DOI] [PubMed] [Google Scholar]
- Leid M., Kastner P., Lyons R., Nakshatri H., Saunders M., Zacharewski T., Chen J. Y., Staub A., Garnier J. M., Mader S. Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently. Cell. 1992 Jan 24;68(2):377–395. doi: 10.1016/0092-8674(92)90478-u. [DOI] [PubMed] [Google Scholar]
- Martinez E., Dusserre Y., Wahli W., Mermod N. Synergistic transcriptional activation by CTF/NF-I and the estrogen receptor involves stabilized interactions with a limiting target factor. Mol Cell Biol. 1991 Jun;11(6):2937–2945. doi: 10.1128/mcb.11.6.2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez E., Wahli W. Cooperative binding of estrogen receptor to imperfect estrogen-responsive DNA elements correlates with their synergistic hormone-dependent enhancer activity. EMBO J. 1989 Dec 1;8(12):3781–3791. doi: 10.1002/j.1460-2075.1989.tb08555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miksicek R., Borgmeyer U., Nowock J. Interaction of the TGGCA-binding protein with upstream sequences is required for efficient transcription of mouse mammary tumor virus. EMBO J. 1987 May;6(5):1355–1360. doi: 10.1002/j.1460-2075.1987.tb02375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M., Baniahmad C., Kaltschmidt C., Renkawitz R. Multiple domains of the glucocorticoid receptor involved in synergism with the CACCC box factor(s). Mol Endocrinol. 1991 Oct;5(10):1498–1503. doi: 10.1210/mend-5-10-1498. [DOI] [PubMed] [Google Scholar]
- När A. M., Boutin J. M., Lipkin S. M., Yu V. C., Holloway J. M., Glass C. K., Rosenfeld M. G. The orientation and spacing of core DNA-binding motifs dictate selective transcriptional responses to three nuclear receptors. Cell. 1991 Jun 28;65(7):1267–1279. doi: 10.1016/0092-8674(91)90021-p. [DOI] [PubMed] [Google Scholar]
- Ponglikitmongkol M., White J. H., Chambon P. Synergistic activation of transcription by the human estrogen receptor bound to tandem responsive elements. EMBO J. 1990 Jul;9(7):2221–2231. doi: 10.1002/j.1460-2075.1990.tb07392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales R., Vigneron M., Macchi M., Davidson I., Xiao J. H., Chambon P. In vitro binding of cell-specific and ubiquitous nuclear proteins to the octamer motif of the SV40 enhancer and related motifs present in other promoters and enhancers. EMBO J. 1987 Oct;6(10):3015–3025. doi: 10.1002/j.1460-2075.1987.tb02607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Forman B. M., Horowitz Z. D., Ye Z. S. Regulation of gene expression by thyroid hormone. Annu Rev Physiol. 1989;51:623–639. doi: 10.1146/annurev.ph.51.030189.003203. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Stanley F., Casanova J. Depletion of L-3,5,3'-triiodothyronine and L-thyroxine in euthyroid calf serum for use in cell culture studies of the action of thyroid hormone. Endocrinology. 1979 Jul;105(1):80–85. doi: 10.1210/endo-105-1-80. [DOI] [PubMed] [Google Scholar]
- Schaufele F., West B. L., Baxter J. D. Synergistic activation of the rat growth hormone promoter by Pit-1 and the thyroid hormone receptor. Mol Endocrinol. 1992 Apr;6(4):656–665. doi: 10.1210/mend.6.4.1584227. [DOI] [PubMed] [Google Scholar]
- Schmid W., Strähle U., Schütz G., Schmitt J., Stunnenberg H. Glucocorticoid receptor binds cooperatively to adjacent recognition sites. EMBO J. 1989 Aug;8(8):2257–2263. doi: 10.1002/j.1460-2075.1989.tb08350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüle R., Muller M., Kaltschmidt C., Renkawitz R. Many transcription factors interact synergistically with steroid receptors. Science. 1988 Dec 9;242(4884):1418–1420. doi: 10.1126/science.3201230. [DOI] [PubMed] [Google Scholar]
- Schüle R., Muller M., Otsuka-Murakami H., Renkawitz R. Cooperativity of the glucocorticoid receptor and the CACCC-box binding factor. Nature. 1988 Mar 3;332(6159):87–90. doi: 10.1038/332087a0. [DOI] [PubMed] [Google Scholar]
- Seed B., Sheen J. Y. A simple phase-extraction assay for chloramphenicol acyltransferase activity. Gene. 1988 Jul 30;67(2):271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- Strähle U., Schmid W., Schütz G. Synergistic action of the glucocorticoid receptor with transcription factors. EMBO J. 1988 Nov;7(11):3389–3395. doi: 10.1002/j.1460-2075.1988.tb03212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. Y., Tsai M. J., O'Malley B. W. Cooperative binding of steroid hormone receptors contributes to transcriptional synergism at target enhancer elements. Cell. 1989 May 5;57(3):443–448. doi: 10.1016/0092-8674(89)90919-7. [DOI] [PubMed] [Google Scholar]
- Tsika R. W., Bahl J. J., Leinwand L. A., Morkin E. Thyroid hormone regulates expression of a transfected human alpha-myosin heavy-chain fusion gene in fetal rat heart cells. Proc Natl Acad Sci U S A. 1990 Jan;87(1):379–383. doi: 10.1073/pnas.87.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesono K., Murakami K. K., Thompson C. C., Evans R. M. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991 Jun 28;65(7):1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanco Ruiz M. M., Bugge T. H., Hirschmann P., Stunnenberg H. G. Functional characterization of a natural retinoic acid responsive element. EMBO J. 1991 Dec;10(12):3829–3838. doi: 10.1002/j.1460-2075.1991.tb04952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voz M. L., Peers B., Belayew A., Martial J. A. Characterization of an unusual thyroid response unit in the promoter of the human placental lactogen gene. J Biol Chem. 1991 Jul 15;266(20):13397–13404. [PubMed] [Google Scholar]
- Wright A. P., Gustafsson J. A. Mechanism of synergistic transcriptional transactivation by the human glucocorticoid receptor. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8283–8287. doi: 10.1073/pnas.88.19.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z. S., Forman B. M., Aranda A., Pascual A., Park H. Y., Casanova J., Samuels H. H. Rat growth hormone gene expression. Both cell-specific and thyroid hormone response elements are required for thyroid hormone regulation. J Biol Chem. 1988 Jun 5;263(16):7821–7829. [PubMed] [Google Scholar]
- Yu V. C., Delsert C., Andersen B., Holloway J. M., Devary O. V., När A. M., Kim S. Y., Boutin J. M., Glass C. K., Rosenfeld M. G. RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell. 1991 Dec 20;67(6):1251–1266. doi: 10.1016/0092-8674(91)90301-e. [DOI] [PubMed] [Google Scholar]



