Abstract
Glutathione-S-transferase is a major phase-II detoxification enzyme in parasitic helminthes. Previous research highlights the importance of GSTs in the establishment of chronic infections in cytotoxic microenvironments. Filarial nematodes depend on these detoxification enzymes for their survival in the host. GST plays an important role in filariasis and other diseases. GST from W.bancrofti and B.malayi are very much different from human GST. This structural difference makes GST potential chemotherapeutic targets for antifilarial treatment. In this study we have checked the efficacy of some well known antifilarial compounds against GST from B.malayi and W.bancrofti. The structure of BmGST was modeled using modeller9v10 and was submitted to PMDB. Molecular docking study reveals arbindazole to be the most potent compounds against GST from both the filarial parasites. Role of some residues playing important role in the binding of compounds within the active site of GST has also been revealed in the present study. The BmGST and WbGST structural information and docking studies could aid in screening new antifilarials or selective inhibitors for chemotherapy against filariasis.
Abbreviations
GST - Glutathione-S-transferase, Bm - Brugia malayi, Wb - Wuchereria bancrofti.
Background
Filariasis, caused by spirunid nematodes, is one of the most prevalent diseases of tropical and subtropical countries and encompasses a number of different pathological conditions [1]. Lymphatic Filariais is an infectious disease that causes serious social and economic burden [2]. Lymphatic filariasis (LF) is a mosquito-borne tropical disease caused by the nematode parasites Wuchereria bancrofti, Brugia malayi and Brugia.timori live in human lymph system [3]. Three physiological races exist in W.bancroofii and B.malayi, depending on the microfilarial periodicity [4]. They are the nocturnally periodic, nocturnally subperiodic and diurnally subperiodic forms. In the Indian sub continent both the parasites exist as nocturnally periodic forms. The vector of W bancrofri is C quinquefmciatus and vector of B. malayi is Mansoni species. There are eight species of filariae which parasitise man along with many other species, which infect other vertebrates. In humans these reside in lymph glands, deep connective tissue, subcutaneous tissues or mesenteries.live in human lymph system. It is the major cause of acute and chronic morbidity in 81 countries in Asia-Pacific, Africa and the Americas. Approximately 1.3 billion people living in these regions are at risk of infection [5].
Lymphatic filariasis is a major health problem in tropical and subtropical countries and control of this infection is still dependent on the application of diethylcarbamazine (DEC) or Ivermectin. Both are mainly a microfilaricides with poor or no activity on adult parasites. In spite of the significant progress made towards control of lymphatic filariasis by advocating single-dose treatment with DEC or Ivermectin, combination of DEC or Ivermectin with albendazole. This mosquito-borne disease has been targeted by the World Health Organization for elimination by the year 2020. The key strategy of the Global Program to Eliminate Lymphatic Filariasis (GPELF) is to eliminate the microfilariae (mf) from the human host through mass drug administration (MDA) of the population at risk of infection with the aim to interrupt transmission. Sensitive and specific diagnostic tools are required to monitor the success of MDA and to establish endpoints for intervention. Glutathione- S-transferase (EC. 2.5.1.18) is a major phase-II detoxification enzyme in parasitic helminthes. Published research highlights the importance of GSTs in the establishment of chronic infections in cytotoxic microenvironments. Filarial nematodes depend on these detoxification enzymes for their survival in the host. The ability of helminth GSTs to effectively neutralize cytotoxic products arising from reactive oxygen species (ROS) attack on cell membranes provides evidence that GSTs have potential to protect the parasite against the host immune response [6].
Inhibition of GST(s) is important from several points of view. These involve applications in studies of the catalytic mechanism, e.g. studying the topological and binding characteristics of the active site. Also, from a therapeutic point of view, inhibition of GST(s) steadily becomes more interesting, since these enzymes appear to be involved in drug resistance and in the biosynthesis of a number of important arachidonic acid metabolites such as prostaglandins and leukotrienes [7]. In the view of present background, we have used bioinformatics approach to compare the efficacy of most commonly used antifilarial compounds against GST of B.malayi and W.bancrofti.
Homology modeling along with docking studies was performed in order to understand the interaction of antifilarials compounds against GST from Brugia malayi (BmGST) and Wuchereria bancrofti (WbGST). The structure of GST from W.bancrofti was available with protein data bank, while that of B.malayi was modeled and was further verified using SAVES. The information revealed from this study would aid in structural analysis and screening and designing new antifilarials inhibitors for chemotherapy against filariasis.
Methodology
Preparation of protein:
The three dimensional structure of the GST of W.Bancrofti was retrieved from RCSB protein databank (pdb id: 3T2U). All the heteroatoms and water molecules were removed. The crystal structure of was extracted from rcsb protein databank. The complete amino acid sequence of Glutathione S transferase of Brugia malayi, which contains 301 amino acids, was extracted from the UniProt Database with an accession number of O02636. Blastp search was performed against protein databank to identify template structure. The automated comparative protein modeling program MODELLER9v8 [8] was then used to generate an all-atom model by alignment of the target sequence with the selected template sequence in an alignment file. The models generated and the previously prepared structure of the GST of Wuchereria bancrofti were subjected to three steps energy-minimization processes using Steepest Descent, conjugate Gradient and adopted Basis NR algorithm for 2000 steps and RMS Gradient 0.1, 0.05 and 0.01 respectively.
Structure Validation and Submission:
The overall stereochemical quality of the modelled structure of Brugia malayi's GST was checked using PROCHECK [9]. Quality factors for the enzyme models were calculated using ERRAT2 [10]. The best generated model of BMGST was successfully submitted in Protein Model DataBasewith PMDB id: PM0078779.
Ligand preparation:
The 3D structure of albendazole, BHA, Diethylcarbamazine, Ethacrynic acid and Hexylglutathione were extracted from pubchem compounds database with pubchem id: 2082, 8456, 3052, 3278 and 97536 respectively.
Molecular docking:
Molecular docking study was performed using GOLD (Genetic Optimization for Ligand Docking) 5.0 [11] for analysing the binding affinity and mode of interaction of selecte antifilarial compounds against GST of both B.malayi and W.bancrofti. Docking annealing parameters for Van der Walls and hydrogen bonding were set to 5.0 and 2.5 respectively. The parameters used for genetic algorithm were population size 100, selection pressure 1.2, number of operations 1,00,000, number of islands 5, niche size 2, migrate 10, mutate 100 and cross -over 100. The best ranked poses were further analysed using a consensus scoring function of X-Score [12] to calculate the binding affinity. Interaction figure was generated using discovery studio visualizer.
Result & Discussion
Glutathione-S-transferase(s) (GST) enzyme has been exploited as an important therapeutic target in lymphatic filariasis [13–18]. Glutathione-S-transferase is a 208 residue long homodimer (smaller α/β domain and larger α domain). The N-terminal small domain (residues 1 to 74) is an α/β structure with the folding topology βαβαββα arranged in the order β2, β1, β3 and β4 with β3 anti-parallel to the others, forming a regular β-sheet with a right-handed twist surrounded by three α-helices (Figure 1). The C terminal, large domain 2 (82–208 residues) is α-helical [19]. GST from human filarial parasites is significantly different from human GST in sequence and structure [13]. Hence one can speculate high sequence similarity between the B. malayi and W.bancrofti since both belong to same family, i.e. Onchocercidae. Multiple sequence alignment shows a high level of similarity between GST of B.malayi and W.Bancrofti in comparision to their human counterpart (Figure 2). GST protein of B. malayi (BmGST) and W. bancrofti (WbGST) shares 98% similarity.
Figure 1.
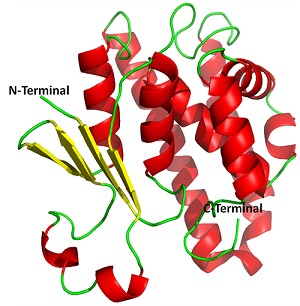
Schematic representation of secondary structure of BmGST. Red regions: α-helices, yellow color: β-sheet and green regions: loop.
Figure 2.
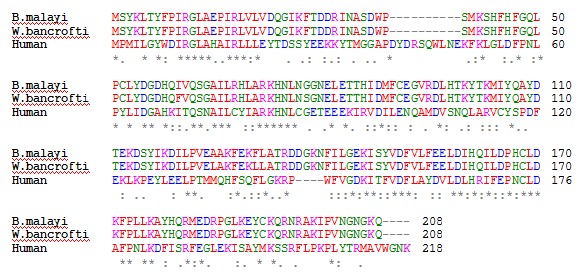
Multiple sequence alignment (ClustalW) of GST sequences of B. malayi, W.bancrofti and Homo sapiens.
The absence of the structure of GST of B.malayi has always been quest for the researchers working in this field. Realising this need, here in this study we have modelled the structure of BmGST. The final constructed model was validated by Ramachandran Plot. As revealed by their Ramachandran plots, 94% of the amino acid residues in the modeled BmGST were found to be present in the most favoured regions while 0% of amino acids were found to be in the disallowed region (Figure 3). All the enzyme structures were modelled using the crystal structure of WbGST (pdb id: 3T2U) as a template. Distribution of amino acid pattern in BmGST, WbGST and HGST reveals the presence of Tyr, Phe, Asp, Lys, His and Ile at most instances (Figure 2). Molecular docking study reveals binding affinity of compounds along with their binding mode within the active site of target enzyme.
Figure 3.
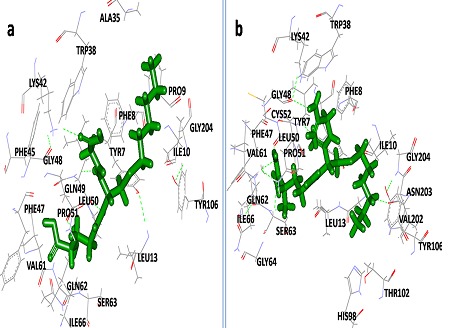
Binding pattern of Hexylglutathione within active site of (a) BmGST and (b) WbGST.
The compounds (albendazole, Diethylcarbamazine and BHA) were docked into GST of each filarial parasite. The compounds were ranked according to their goldfitness score. The top ranked poses were further analysed using a consensus scoring function of X-Score to calculate the binding affinity. This study revealed amino acid residues crucial to the interaction of GST from both the filarial parasite with albindazole, Diethylcarbamazine and BHA. On the basis of goldfitness score and binding free energy, Hexylglutathione was found to the most potent against GST of both B.malayi and W.bancrofti followed by Ethacrynic acid and albendazole. The binding mode of Hexylglutathione within the active site of BmGST and WbGST is shown in (Figure 3). Albindazole has always been a drug of first choice in case of filarial infection. Its effectiveness against GST has also been reported many times in earlier studies [20, 21].
Apart from Hexylglutathione, Butylated hydroxyanisole was found to be the most potent compound candidate against BmGST while Diethylcarbamazine was found to be least effective (binding free energy -6.93 and -6.23 Kcal/mol respectively). Diethylcarbamazine was also found to be least effective against WbGST (binding free energy -6.22 Kcal/mol). BHA, Ethacrynic acid and albendazole were found to be moderate against GST of both the filarial parasites. Table 1 (see supplementary material) depict the binding affinity of various compounds against BmGST and WbGST. The binding pattern of all the compounds within the active site of BmGST and WbGST was found to be more or less very same. Butylated hydroxyanisole (BHA) has been reported for markedly reducing worm viability [14]. Tyr7, Phe8, Gly12, Leu13, Trp38, His98, Thr102, Tyr106 and Thr203 were found to be the most important residues involved in the orientation of compounds within the active site of both BmGST and WbGST. Overall, Hexylglutathione was found to be a modest inhibitor of GSTs of both the filarial parasites.
Conclusion
The binding modes exhibited by various docked compounds illustrated the importance of specific residues within the active site region of the targets. Role of some other important aminoacids have also been revealed, that were found to be playing important role in the positioning of inhibitor within the active site. Thus based on above outcomes we conclude that these inhibitors can behave as a lead to promising compounds against the targets selected for our study. However, some experimentally work is still needed for validating these outcomes.
Supplementary material
Acknowledgments
We would like to express our sincere thanks to Vice Chancellor, Integral University, Lucknow, India for providing necessary facilities and encouragement. One of the author (MS) is thankful to UGC, New delhi for granting Maulana Azad National Fellowship. We are also thankful to Dr Mohd Sajid Khan, Dr Salman Khan, for their help and cooperation.
Footnotes
Citation:Saeed et al, Bioinformation 9(5): 233-237 (2013)
References
- 1.Tripathi RP, et al. Curr Med Chem. 2006;13:3319. doi: 10.2174/092986706778773103. [DOI] [PubMed] [Google Scholar]
- 2.Rosenfield PL, et al. Soc Sci Med. 1984;19:1117. doi: 10.1016/0277-9536(84)90317-4. [DOI] [PubMed] [Google Scholar]
- 3.Erickson SM, et al. PLoS Negl Trop Dis. 2009;13:e529. doi: 10.1371/journal.pntd.0000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paily KP, et al. J Parasit Dis. 2009;33:3. doi: 10.1007/s12639-009-0005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ottesen EA, et al. PLoS Negl Trop Dis. 2008;8:e317. doi: 10.1371/journal.pntd.0000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brophy PM, Pritchard DI. Exp Parasitol. 1994;79:89. doi: 10.1006/expr.1994.1067. [DOI] [PubMed] [Google Scholar]
- 7.van Ommen B, et al. Biochem J. 1991;15:661. doi: 10.1042/bj2760661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sánchez R, Sali A. Proteins. 1997;1:50. doi: 10.1002/(sici)1097-0134(1997)1+<50::aid-prot8>3.3.co;2-w. [DOI] [PubMed] [Google Scholar]
- 9.Laskowski RA, et al. J Biomol NMR. 1996;8:477. doi: 10.1007/BF00228148. [DOI] [PubMed] [Google Scholar]
- 10.Colovos C, Yeates TO. Proteins Science. 1993;2:1511. doi: 10.1002/pro.5560020916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones G, et al. J Mol Biol. 1995;245:43. doi: 10.1016/s0022-2836(95)80037-9. [DOI] [PubMed] [Google Scholar]
- 12.Wang R, et al. J Comput Aided Mol Des. 2002;16:11. doi: 10.1023/a:1023880611709. [DOI] [PubMed] [Google Scholar]
- 13.Bhargavi R, et al. Bioinformation. 2005;2:25. doi: 10.6026/97320630001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta S, et al. Exp Parasitol. 2005;109:252. doi: 10.1016/j.exppara.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Morrison CA, et al. Vaccine. 1996;14:1603. doi: 10.1016/s0264-410x(96)00147-8. [DOI] [PubMed] [Google Scholar]
- 16.Grezel D, et al. Eur J Immunol. 1993;23:454. doi: 10.1002/eji.1830230223. [DOI] [PubMed] [Google Scholar]
- 17.Sexton JL, et al. J Immunol. 1990;1:3905. [PubMed] [Google Scholar]
- 18.Veerapathran A, et al. PLoS Negl Trop Dis. 2009;9:e457. doi: 10.1371/journal.pntd.0000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilce MC, Parker MW. Biochim Biophys Acta. 1994;16:1. doi: 10.1016/0167-4838(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 20.Feng JJ, et al. Zhongguo Yao Li Xue Bao. 1995;16:297. [PubMed] [Google Scholar]
- 21.Wojtkowiak A, et al. Parasitol Res. 2006;100:647. doi: 10.1007/s00436-006-0285-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


