Abstract
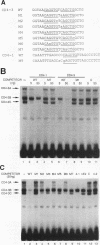
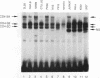
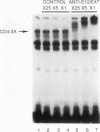
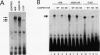
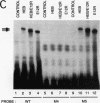
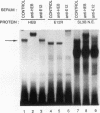
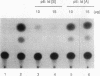
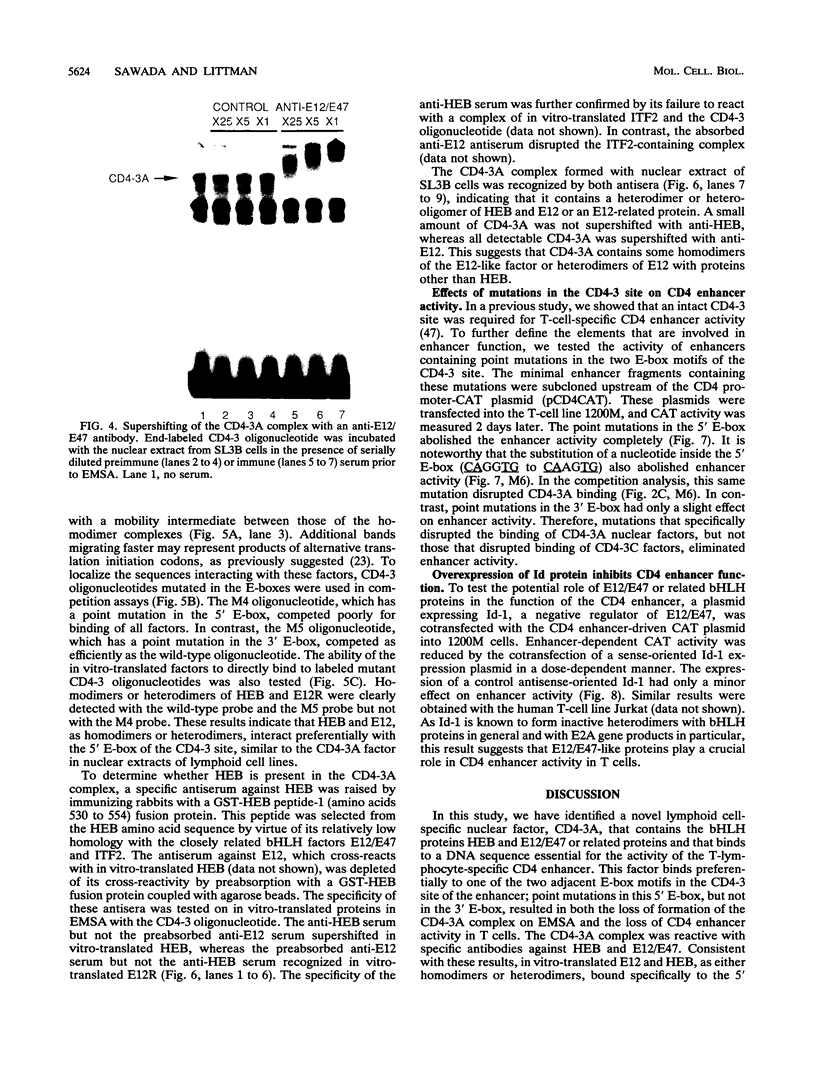
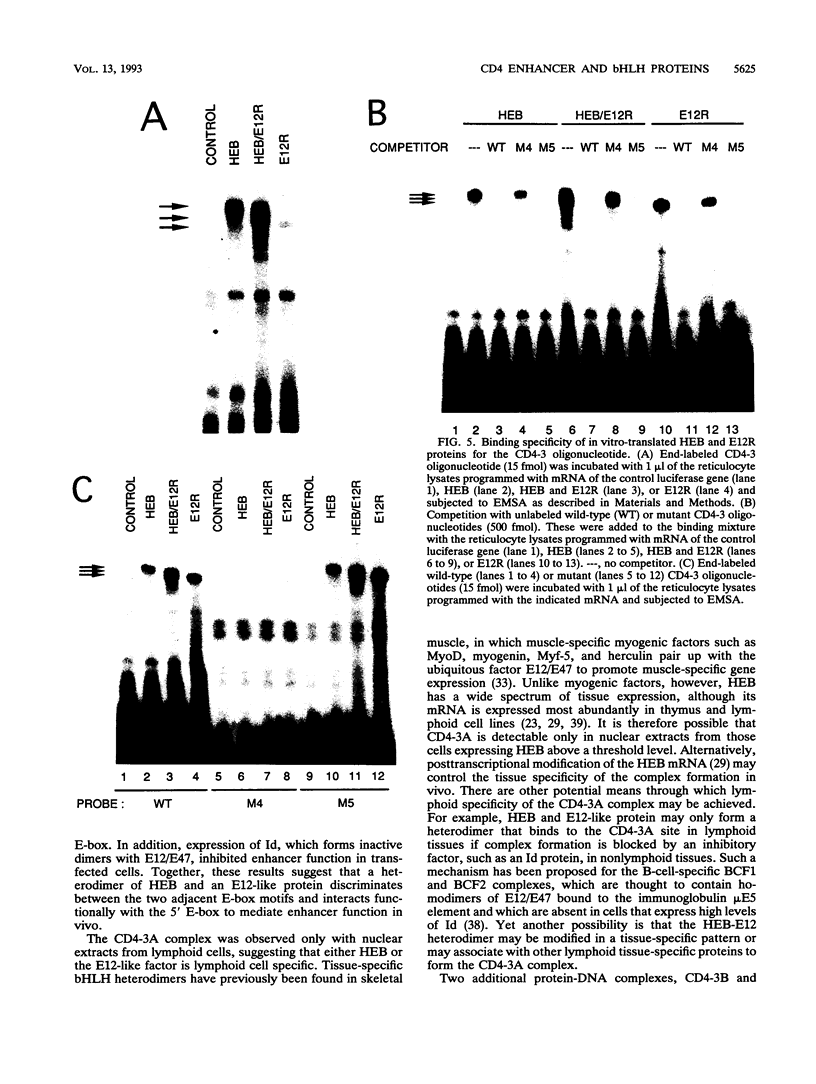
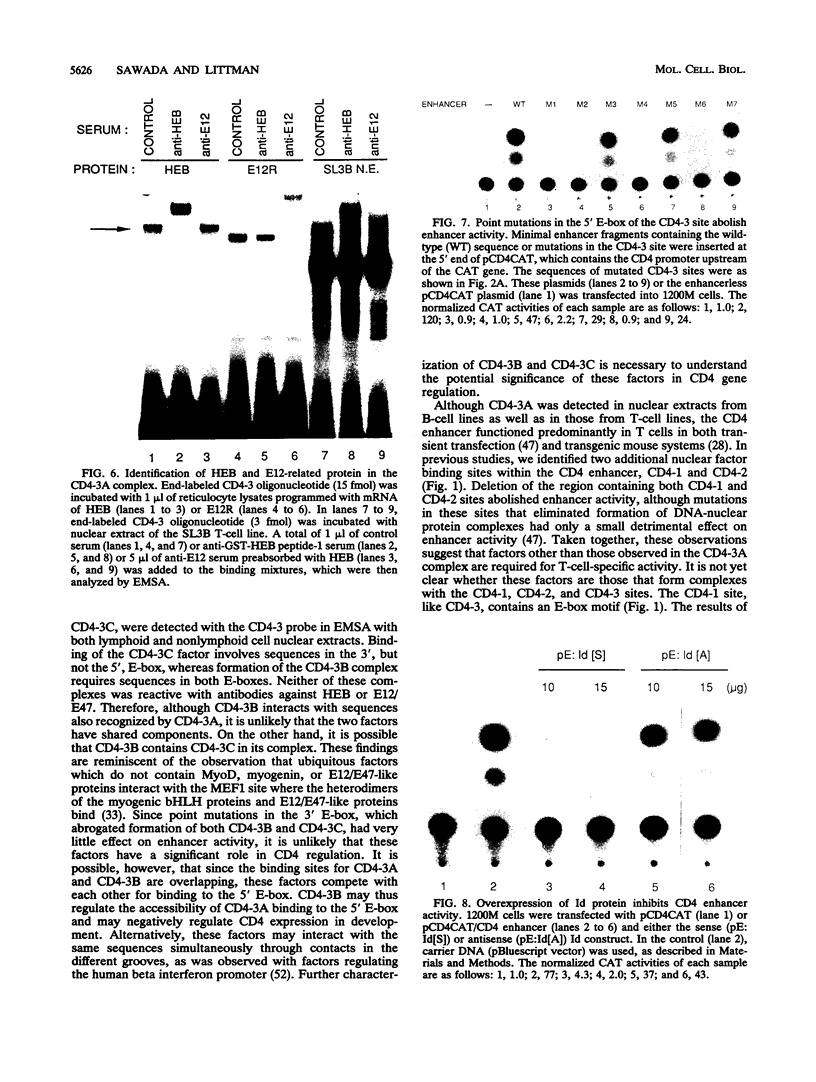
A T-lymphocyte-specific enhancer located 13 kb upstream of the murine CD4 gene was recently shown to be required for the developmentally regulated expression of CD4. We have previously identified three nuclear protein binding sites in this enhancer; one of these sites, CD4-3, is essential for expression and contains two E-box core motifs (CANNTG) adjacent to each other in the sequence TAACAGGTGTCAGCTGGT. In electrophoretic mobility shift assays using the CD4-3 oligonucleotide as a probe, three nuclear protein complexes, termed CD4-3A, -B, and -C, were detected with nuclear extracts from T-cell lines. CD4-3A, which involves nuclear protein binding to the 5' E-box, was detected only with nuclear extracts from lymphoid cells. Specific antisera were used to show that the CD4-3A complex contains a heterodimer or heterooligomer of basic helix-loop-helix transcriptional factors, E12 or a related factor and HEB, which is expressed predominantly in thymus. Consistent with this finding, in vitro-translated E12 and HEB proteins, as homodimers or heterodimers, bound preferentially to the 5' E-box. Point mutations in the 5' E-box, but not in the 3' E-box, abolished CD4 enhancer activity. Furthermore, overexpression of Id, a protein that forms inactive heterodimers with E12/E47, blocked CD4 enhancer activity in T cells. These results suggest that a heterodimer composed of HEB and E12 or a closely related protein plays a critical role in CD4 enhancer function by interacting with the 5' E-box motif of the CD4-3 site in vivo.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich C. J., Hammer R. E., Jones-Youngblood S., Koszinowski U., Hood L., Stroynowski I., Forman J. Negative and positive selection of antigen-specific cytotoxic T lymphocytes affected by the alpha 3 domain of MHC I molecules. Nature. 1991 Aug 22;352(6337):718–721. doi: 10.1038/352718a0. [DOI] [PubMed] [Google Scholar]
- Aronheim A., Ohlsson H., Park C. W., Edlund T., Walker M. D. Distribution and characterization of helix-loop-helix enhancer-binding proteins from pancreatic beta cells and lymphocytes. Nucleic Acids Res. 1991 Jul 25;19(14):3893–3899. doi: 10.1093/nar/19.14.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benezra R., Davis R. L., Lockshon D., Turner D. L., Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990 Apr 6;61(1):49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- Biggs J., Murphy E. V., Israel M. A. A human Id-like helix-loop-helix protein expressed during early development. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1512–1516. doi: 10.1073/pnas.89.4.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanar M. A., Rutter W. J. Interaction cloning: identification of a helix-loop-helix zipper protein that interacts with c-Fos. Science. 1992 May 15;256(5059):1014–1018. doi: 10.1126/science.1589769. [DOI] [PubMed] [Google Scholar]
- Cabrera C. V., Alonso M. C. Transcriptional activation by heterodimers of the achaete-scute and daughterless gene products of Drosophila. EMBO J. 1991 Oct;10(10):2965–2973. doi: 10.1002/j.1460-2075.1991.tb07847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarota G., Scheirle A., Takacs B., Doran D. M., Knorr R., Bannwarth W., Guardiola J., Sinigaglia F. Identification of a CD4 binding site on the beta 2 domain of HLA-DR molecules. Nature. 1992 Apr 30;356(6372):799–801. doi: 10.1038/356799a0. [DOI] [PubMed] [Google Scholar]
- Campuzano S., Modolell J. Patterning of the Drosophila nervous system: the achaete-scute gene complex. Trends Genet. 1992 Jun;8(6):202–208. doi: 10.1016/0168-9525(92)90234-u. [DOI] [PubMed] [Google Scholar]
- Chakraborty T., Brennan T. J., Li L., Edmondson D., Olson E. N. Inefficient homooligomerization contributes to the dependence of myogenin on E2A products for efficient DNA binding. Mol Cell Biol. 1991 Jul;11(7):3633–3641. doi: 10.1128/mcb.11.7.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy B. A., Sanders L. K., Lau L. F., Copeland N. G., Jenkins N. A., Nathans D. An Id-related helix-loop-helix protein encoded by a growth factor-inducible gene. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1815–1819. doi: 10.1073/pnas.88.5.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly J. M., Hansen T. H., Ingold A. L., Potter T. A. Recognition by CD8 on cytotoxic T lymphocytes is ablated by several substitutions in the class I alpha 3 domain: CD8 and the T-cell receptor recognize the same class I molecule. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2137–2141. doi: 10.1073/pnas.87.6.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordle S. R., Henderson E., Masuoka H., Weil P. A., Stein R. Pancreatic beta-cell-type-specific transcription of the insulin gene is mediated by basic helix-loop-helix DNA-binding proteins. Mol Cell Biol. 1991 Mar;11(3):1734–1738. doi: 10.1128/mcb.11.3.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. B., Killeen N., Crooks M. E., Raulet D., Littman D. R. Evidence for a stochastic mechanism in the differentiation of mature subsets of T lymphocytes. Cell. 1993 Apr 23;73(2):237–247. doi: 10.1016/0092-8674(93)90226-g. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H. Acquisition of myogenic specificity by replacement of three amino acid residues from MyoD into E12. Science. 1992 May 15;256(5059):1027–1030. doi: 10.1126/science.1317057. [DOI] [PubMed] [Google Scholar]
- Doyle C., Strominger J. L. Interaction between CD4 and class II MHC molecules mediates cell adhesion. Nature. 1987 Nov 19;330(6145):256–259. doi: 10.1038/330256a0. [DOI] [PubMed] [Google Scholar]
- Ellmeier W., Aguzzi A., Kleiner E., Kurzbauer R., Weith A. Mutually exclusive expression of a helix-loop-helix gene and N-myc in human neuroblastomas and in normal development. EMBO J. 1992 Jul;11(7):2563–2571. doi: 10.1002/j.1460-2075.1992.tb05321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung-Leung W. P., Schilham M. W., Rahemtulla A., Kündig T. M., Vollenweider M., Potter J., van Ewijk W., Mak T. W. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell. 1991 May 3;65(3):443–449. doi: 10.1016/0092-8674(91)90462-8. [DOI] [PubMed] [Google Scholar]
- German M. S., Blanar M. A., Nelson C., Moss L. G., Rutter W. J. Two related helix-loop-helix proteins participate in separate cell-specific complexes that bind the insulin enhancer. Mol Endocrinol. 1991 Feb;5(2):292–299. doi: 10.1210/mend-5-2-292. [DOI] [PubMed] [Google Scholar]
- Giese K., Cox J., Grosschedl R. The HMG domain of lymphoid enhancer factor 1 bends DNA and facilitates assembly of functional nucleoprotein structures. Cell. 1992 Apr 3;69(1):185–195. doi: 10.1016/0092-8674(92)90129-z. [DOI] [PubMed] [Google Scholar]
- Glaichenhaus N., Shastri N., Littman D. R., Turner J. M. Requirement for association of p56lck with CD4 in antigen-specific signal transduction in T cells. Cell. 1991 Feb 8;64(3):511–520. doi: 10.1016/0092-8674(91)90235-q. [DOI] [PubMed] [Google Scholar]
- Grosschedl R., Baltimore D. Cell-type specificity of immunoglobulin gene expression is regulated by at least three DNA sequence elements. Cell. 1985 Jul;41(3):885–897. doi: 10.1016/s0092-8674(85)80069-6. [DOI] [PubMed] [Google Scholar]
- Henthorn P., Kiledjian M., Kadesch T. Two distinct transcription factors that bind the immunoglobulin enhancer microE5/kappa 2 motif. Science. 1990 Jan 26;247(4941):467–470. doi: 10.1126/science.2105528. [DOI] [PubMed] [Google Scholar]
- Hu J. S., Olson E. N., Kingston R. E. HEB, a helix-loop-helix protein related to E2A and ITF2 that can modulate the DNA-binding ability of myogenic regulatory factors. Mol Cell Biol. 1992 Mar;12(3):1031–1042. doi: 10.1128/mcb.12.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingold A. L., Landel C., Knall C., Evans G. A., Potter T. A. Co-engagement of CD8 with the T cell receptor is required for negative selection. Nature. 1991 Aug 22;352(6337):721–723. doi: 10.1038/352721a0. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr The T cell receptor as a multicomponent signalling machine: CD4/CD8 coreceptors and CD45 in T cell activation. Annu Rev Immunol. 1992;10:645–674. doi: 10.1146/annurev.iy.10.040192.003241. [DOI] [PubMed] [Google Scholar]
- Jen Y., Weintraub H., Benezra R. Overexpression of Id protein inhibits the muscle differentiation program: in vivo association of Id with E2A proteins. Genes Dev. 1992 Aug;6(8):1466–1479. doi: 10.1101/gad.6.8.1466. [DOI] [PubMed] [Google Scholar]
- Killeen N., Moriarty A., Teh H. S., Littman D. R. Requirement for CD8-major histocompatibility complex class I interaction in positive and negative selection of developing T cells. J Exp Med. 1992 Jul 1;176(1):89–97. doi: 10.1084/jem.176.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen N., Sawada S., Littman D. R. Regulated expression of human CD4 rescues helper T cell development in mice lacking expression of endogenous CD4. EMBO J. 1993 Apr;12(4):1547–1553. doi: 10.1002/j.1460-2075.1993.tb05798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein E. S., Simmons D. M., Swanson L. W., Rosenfeld M. G. Tissue-specific RNA splicing generates an ankyrin-like domain that affects the dimerization and DNA-binding properties of a bHLH protein. Genes Dev. 1993 Jan;7(1):55–71. doi: 10.1101/gad.7.1.55. [DOI] [PubMed] [Google Scholar]
- Koland J. G., O'Brien K. M., Cerione R. A. Expression of epidermal growth factor receptor sequences as E. coli fusion proteins: applications in the study of tyrosine kinase function. Biochem Biophys Res Commun. 1990 Jan 15;166(1):90–100. doi: 10.1016/0006-291x(90)91915-f. [DOI] [PubMed] [Google Scholar]
- Kreider B. L., Benezra R., Rovera G., Kadesch T. Inhibition of myeloid differentiation by the helix-loop-helix protein Id. Science. 1992 Mar 27;255(5052):1700–1702. doi: 10.1126/science.1372755. [DOI] [PubMed] [Google Scholar]
- König R., Huang L. Y., Germain R. N. MHC class II interaction with CD4 mediated by a region analogous to the MHC class I binding site for CD8. Nature. 1992 Apr 30;356(6372):796–798. doi: 10.1038/356796a0. [DOI] [PubMed] [Google Scholar]
- Lassar A. B., Davis R. L., Wright W. E., Kadesch T., Murre C., Voronova A., Baltimore D., Weintraub H. Functional activity of myogenic HLH proteins requires hetero-oligomerization with E12/E47-like proteins in vivo. Cell. 1991 Jul 26;66(2):305–315. doi: 10.1016/0092-8674(91)90620-e. [DOI] [PubMed] [Google Scholar]
- Littman D. R. The structure of the CD4 and CD8 genes. Annu Rev Immunol. 1987;5:561–584. doi: 10.1146/annurev.iy.05.040187.003021. [DOI] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Vaessin H., Caudy M., Jan L. Y., Jan Y. N., Cabrera C. V., Buskin J. N., Hauschka S. D., Lassar A. B. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989 Aug 11;58(3):537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- Murre C., Voronova A., Baltimore D. B-cell- and myocyte-specific E2-box-binding factors contain E12/E47-like subunits. Mol Cell Biol. 1991 Feb;11(2):1156–1160. doi: 10.1128/mcb.11.2.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen A. L., Pallisgaard N., Pedersen F. S., Jørgensen P. Murine helix-loop-helix transcriptional activator proteins binding to the E-box motif of the Akv murine leukemia virus enhancer identified by cDNA cloning. Mol Cell Biol. 1992 Aug;12(8):3449–3459. doi: 10.1128/mcb.12.8.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke A. M., Mescher M. F. Cytotoxic T-lymphocyte activation involves a cascade of signalling and adhesion events. Nature. 1992 Jul 16;358(6383):253–255. doi: 10.1038/358253a0. [DOI] [PubMed] [Google Scholar]
- O'Rourke A. M., Rogers J., Mescher M. F. Activated CD8 binding to class I protein mediated by the T-cell receptor results in signalling. Nature. 1990 Jul 12;346(6280):187–189. doi: 10.1038/346187a0. [DOI] [PubMed] [Google Scholar]
- Ow D. W., DE Wet J. R., Helinski D. R., Howell S. H., Wood K. V., Deluca M. Transient and stable expression of the firefly luciferase gene in plant cells and transgenic plants. Science. 1986 Nov 14;234(4778):856–859. doi: 10.1126/science.234.4778.856. [DOI] [PubMed] [Google Scholar]
- Parnes J. R. Molecular biology and function of CD4 and CD8. Adv Immunol. 1989;44:265–311. doi: 10.1016/s0065-2776(08)60644-6. [DOI] [PubMed] [Google Scholar]
- Rahemtulla A., Fung-Leung W. P., Schilham M. W., Kündig T. M., Sambhara S. R., Narendran A., Arabian A., Wakeham A., Paige C. J., Zinkernagel R. M. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature. 1991 Sep 12;353(6340):180–184. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- Rudd C. E., Trevillyan J. M., Dasgupta J. D., Wong L. L., Schlossman S. F. The CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5190–5194. doi: 10.1073/pnas.85.14.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter R. D., Benjamin R. J., Wesley P. K., Buxton S. E., Garrett T. P., Clayberger C., Krensky A. M., Norment A. M., Littman D. R., Parham P. A binding site for the T-cell co-receptor CD8 on the alpha 3 domain of HLA-A2. Nature. 1990 May 3;345(6270):41–46. doi: 10.1038/345041a0. [DOI] [PubMed] [Google Scholar]
- Sawada S., Littman D. R. Identification and characterization of a T-cell-specific enhancer adjacent to the murine CD4 gene. Mol Cell Biol. 1991 Nov;11(11):5506–5515. doi: 10.1128/mcb.11.11.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989 Aug 11;17(15):6419–6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw A. S., Amrein K. E., Hammond C., Stern D. F., Sefton B. M., Rose J. K. The lck tyrosine protein kinase interacts with the cytoplasmic tail of the CD4 glycoprotein through its unique amino-terminal domain. Cell. 1989 Nov 17;59(4):627–636. doi: 10.1016/0092-8674(89)90008-1. [DOI] [PubMed] [Google Scholar]
- Shieh S. Y., Tsai M. J. Cell-specific and ubiquitous factors are responsible for the enhancer activity of the rat insulin II gene. J Biol Chem. 1991 Sep 5;266(25):16708–16714. [PubMed] [Google Scholar]
- Sun X. H., Copeland N. G., Jenkins N. A., Baltimore D. Id proteins Id1 and Id2 selectively inhibit DNA binding by one class of helix-loop-helix proteins. Mol Cell Biol. 1991 Nov;11(11):5603–5611. doi: 10.1128/mcb.11.11.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos D., Maniatis T. The high mobility group protein HMG I(Y) is required for NF-kappa B-dependent virus induction of the human IFN-beta gene. Cell. 1992 Nov 27;71(5):777–789. doi: 10.1016/0092-8674(92)90554-p. [DOI] [PubMed] [Google Scholar]
- Travis A., Amsterdam A., Belanger C., Grosschedl R. LEF-1, a gene encoding a lymphoid-specific protein with an HMG domain, regulates T-cell receptor alpha enhancer function [corrected]. Genes Dev. 1991 May;5(5):880–894. doi: 10.1101/gad.5.5.880. [DOI] [PubMed] [Google Scholar]
- Turner J. M., Brodsky M. H., Irving B. A., Levin S. D., Perlmutter R. M., Littman D. R. Interaction of the unique N-terminal region of tyrosine kinase p56lck with cytoplasmic domains of CD4 and CD8 is mediated by cysteine motifs. Cell. 1990 Mar 9;60(5):755–765. doi: 10.1016/0092-8674(90)90090-2. [DOI] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Bolen J. B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988 Oct 21;55(2):301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- Voronova A., Baltimore D. Mutations that disrupt DNA binding and dimer formation in the E47 helix-loop-helix protein map to distinct domains. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4722–4726. doi: 10.1073/pnas.87.12.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman M. L., Fischer W. H., Jones K. A. A thymus-specific member of the HMG protein family regulates the human T cell receptor C alpha enhancer. Genes Dev. 1991 Apr;5(4):656–669. doi: 10.1101/gad.5.4.656. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Davis R., Tapscott S., Thayer M., Krause M., Benezra R., Blackwell T. K., Turner D., Rupp R., Hollenberg S. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991 Feb 15;251(4995):761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Dwarki V. J., Verma I., Davis R., Hollenberg S., Snider L., Lassar A., Tapscott S. J. Muscle-specific transcriptional activation by MyoD. Genes Dev. 1991 Aug;5(8):1377–1386. doi: 10.1101/gad.5.8.1377. [DOI] [PubMed] [Google Scholar]
- Wilson R. B., Kiledjian M., Shen C. P., Benezra R., Zwollo P., Dymecki S. M., Desiderio S. V., Kadesch T. Repression of immunoglobulin enhancers by the helix-loop-helix protein Id: implications for B-lymphoid-cell development. Mol Cell Biol. 1991 Dec;11(12):6185–6191. doi: 10.1128/mcb.11.12.6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Babin J., Feldhaus A. L., Singh H., Sharp P. A., Bina M. HTF4: a new human helix-loop-helix protein. Nucleic Acids Res. 1991 Aug 25;19(16):4555–4555. doi: 10.1093/nar/19.16.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]