Abstract
Staphylococcus aureus is a gram positive bacterium, responsible for both community-acquired and hospital-acquired infection, resulting in a mortality rate of 39%. 43.2% resistance to methicilin and emerging resistance to Fluroquinolone and Oxazolidinone, have evoked the necessity of the establishment of alternative and effective therapeutic approach to treat this bacteria. In this computational study, various database and online software are used to determine some specific targets of Staphylococcus aureus N315 other than those used by Penicillin, Quinolone and Oxazolidinone. For this purpose, among 302 essential proteins, 101 nonhomologous proteins were accrued and 64 proteins which are unique in several metabolic pathways of S. aureus were isolated by using metabolic pathway analysis tools. Furthermore, 7 essentially unique enzymes involved in exclusive metabolic pathways were revealed by this research, which can be potential drug target. Along with these important enzymes, 15 non-homologous proteins located on membrane were identified, which can play a vital role as potential therapeutic targets for the future researchers.
Keywords: Staphylococcus aureus, Essential proteins, Surface, Therapeutic targets
Background
Staphylococcus aureus is a vanguard for both nosocomial and community-acquired infections. It is the primary cause of surgical site infections and lower respiratory tract infections [1] and the second leading cause of cardiovascular infections, pneumonia [1]. Because of evolved resistance to antimicrobial drugs such as penicillin and newer narrow-spectrum β- lactamase–resistant penicillin antimicrobial drugs (e.g., methicillin, oxacillin) infections with S. aureus are especially difficult to treat and this resistance appeared soon after they were introduced into clinical practice respectively in the 1940s and 1960s [2]. Initially resistance to penicillin was restrained to a small number of hospitalized patients, but as use of penicillin increased, resistance spread first to other hospitals and then into the community [3]. Greater than 80% of community- and hospital- acquired S. aureus isolated were resistant to penicillin by the late 1960s [2]. Current report suggests that the spread and evolution of methicillin-resistant S. aureus (MRSA) seems to be following an identical wavelike emergence pattern [3]. In many US hospitals, MRSA is now endemic and even epidemic along with long-term care facilities and communities [4]. National Nosocomial Infections Surveillance system's data suggest that the proportion of S. aureus isolates that are resistant to methicillin has increased to 59.5%-64.4%, in intensive care units [5]. Accurate national estimates of incidence are needed for an understanding of the magnitude of the problem. Nevertheless, national studies examining the effect of S. aureus or MRSA on the healthcare system are greater than 5years old [6]. Noskin et al. estimated that there were 290,000 S. aureus – related whereas Kuehnert et al. estimated a similar number of S. aureus-related hospitalizations for 1999-2000 and reported that 125,969 (43.2%) were likely resistant to methicillin [6]. The infections caused by this pathogen range from mild infections such as skin infections, food poisoning to life threatening infections such as pneumonia, sepsis, osteomylatis and infectious endocarditis. Methicillin-resistant Staphylococcus aureus is considered as superbug which was first reported in 1961 and now-a-days cause mortality rate of 39% while MSSA cause 24% death [7].
The currently known target of Staphyloccus sp. includes PBP (penicillin binding protein) of peptidoglycan biosynthesis pathway. Previously, beta-lactam antibiotics were known to be effective against them, but due to production of altered form of PBP protein as well as their beta-lactamase enzme synthesis those drugs are not effective now. Hence, our current study involves identifying targets apart from PBP [8]. Another antibiotic prescribed is Fluroquinolone. Fluroquinolone target DNA Gyrase A enzyme-essential for the replication, supercoiling of DNA. But according to Stephen et al., a highly significant association between Levofloxacin and Ciprofloxacin treatment and subsequent isolation of MRSA is reported [9]. Linozolid, a new class of antibiotic called Oxazolidinones is used to treat MRSA, which involves the mechanism of binding to the bacterial 23s ribosomal RNA of the 50s subunit and thus inhibiting the formation of Functional 70s initiation complex. But between April 13 & June 26, 2008, 12 patients were identified with LRSA (Linozolid resistant Staphylococcus aureus). All the isolates were detected with a point mutation in 23s rRNA. It was concluded that clinical outbreak of LRSA mediated by the cfr gene was related with extensive usage of Linozolid [10]. In this study, we have tried to search for some potential therapeutic targets other than the targets discussed above. We have implemented an approach considering two important criteria. First of all, the identified target should be essential to that pathogen in the sense that it must be associated with replication as well as viability of the pathogen. Secondly, the target should not be homologous to human. The nonhomolog property of these proteins helps to establish highly selective drug against the pathogen preventing the possibility of the cross-reaction with the human host. This may help to minimize the side effects of the proposed drug [11]. By this approach, we have found some targets which are not only essential & non-human homolog but also bypass the resistant mechanism of existing targets and some targets are involved in the metabolic pathway of pathogen which is not exploited as potential target area [12]. We have also identified the membrane-bound Essential, non-human homolog proteins emphasizing the fact that 60% of the drug target is membranebound [13].
Methodology
The systematic identification & characterization of the putative drug target of Staphylococcus aureus N315 was done sequentially by the following methods:
Retrieval of Essential proteins of S. aureus:
At first, according to the Database of essential genes (DEG) [14], 302 essential proteins of S. aureus N315 were retrieved from NCBI [15] in FASTA format.
Identification of non-human homologous essential proteins in S. aureus:
These 302 essential proteins were subjected to BlastP at NCBI server against Homo sapiens with default parameters. To identify human non-homologous essential proteins of S. aureus , proteins having threshold expectation value greater than 10(-4) were taken and for this purpose Refseq protein database was selected [16].
Metabolic pathway analysis:
The human non-homologous essential proteins of Staphylococcus aureus obtained through BlastP were then subjected to metabolic pathway analysis, which was done by KAAS server at KEGG .Functional annotation of genes is provided by KAAS (KEGG Automatic Annotation Server) [17]. This functional annotation was done by this server through BLAST comparison of the genes against the manually curated KEGG GENES database.
Unique pathway identification:
After this, unique metabolic pathways of Staphylococcus aureus N315 were identified through the comparison of metabolic pathways of both Staphylococcus aureus & Homo sapiens by using KEGG (Kyoto Encyclopedia of Genes & Genomes) [18] Genome Database. Among unique metabolic pathways of S. aureus, only those proteins were identified which were human nonhomologue essential proteins.
Surface protein identification:
Sub-cellular localization of metabolic proteins (essential nonhuman homologues) of S. aureus was done by PSORTb [19] to identify the surface membrane proteins which can be used as putative therapeutic targets.
Uncharacterized membrane-bound protein characterization:
Uncharacterized membrane-bound Hypothetical Proteins were characterized by SVM-PROT [20].
Result & Discussion
The aim of this investigation was to determine the potential therapeutic targets for alternative treatment of MRSA and to categorize and analyze the targets. Subtractive genomic approach was used in this approach. The basic principle of subtractive genomic approach is, “a good therapeutic target is a gene/protein essential for the bacterial survival, which cannot be found in host” [21]. In the present study, at first essential proteins of S. aureus N315 were retrieved from ‘Database of Essential Gene (DEG)’. Essential genes can be defined as a minimal gene set which is adequate for survival of a cellular life form under favorable condition [22]. The results obtained by this approach are shown in (Figure 1).
Figure 1.
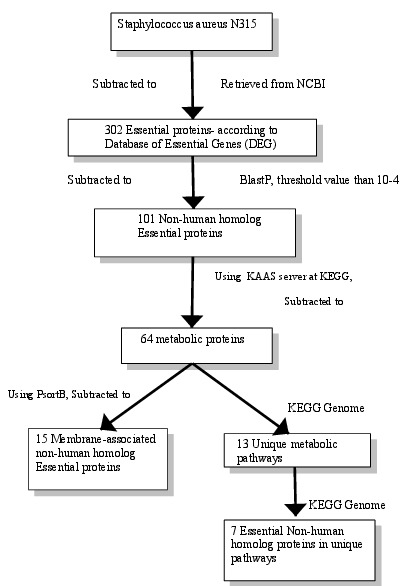
Flowchart- A schematic representation of process analysis and interpretations.
With a purpose to forsake any cross-reactivity of established drug with the human host, BlastP was done against the 302 essential proteins then 101 non-human homolog proteins were resulted having a threshold expectation value greater than 104. For further specification of the non-human homolog Essential proteins of S. aureus, metabolic pathway analysis was done by KAAS server at KEGG, which resulted in a set of 64 proteins Table 1 (see supplementary material). With a view to further reduce the number of 64 metabolic proteins as probable drug targets, comparative analysis of metabolic pathways of human host & S. aureus at KEGG Genome database was done. This comparison reveals 13 unique pathways that mean these metabolic pathways are present in S. aureus (bacteria), but not in human. Then, each selected unique pathway was tested for the presence of pathogen specific essential proteins. At last, 7 proteins were found to be present on two different unique pathways. Six out of the seven proteins are present in peptidoglycan biosynthesis pathway and one protein- Phosphoenolpyruvate-protein phosphatase is present in Phosphotransferase system (PTS). For further characterization, Computational characterization of hypothetical proteins and prediction of subcellular localization of pathogenic proteins was done which provides a quicker way to identify cell surface drug targets Table 2 (see supplementary material) [22].
Potential Drug targets from unique metabolic pathways of S. aureus:
In this section we analyze some potential therapeutic targets identified from unique metabolic pathways. The Penicillin Binding Proteins (PBPs), involved in the final step of Peptidoglycan Biosynthsis pathway was the one of the first conventional targets of Beta-lactam antibiotics- Penicillins & Cephalosporins. The beta-lactam ring of these drugs has the ability to bind to transpeptidase (TPase) of PBPs and thus prevent normal cross-lingking of peptide chains in the peptidoglycan layer. Unfortunately, expression of betalactamase and an altered form of PBP2 has lead to Methicillin resistant Staphylococcus aureus. Thus one of the approaches of establishing new anti-MRSA agents should be to target on molecule other than PBPs to modulate the methicillin resistance in S. aureus [8].
Our study finds six essential non-human homolog proteins in the peptidoglycan pathway-all excluding the PBP, they are: UDP-N-acetylglucosamine 1-carboxyvinyltransferase(murA), UDP-N-acetylmuramate- -L-alanine ligase (murC), UDP-Nacetylmuramoyl- L-alanyl-D-glutamate synthetase (murD), UDP-N-acetylmuramoylalanyl-D-D-glutamyl-2,6- diaminopimelate-D-alanyl-D-alanyl ligase (murF), protein-Dalanyl- alanine synthetase A (ddl), Undecaprenyl pyrophosphate phosphatase (uppP). Among these six proteins four are from Mur ligase family catalyzing the synthesis of Peptidoglycan precursor (UDP-MurNAc-pentapeptide) - the first step in the Peptidoglycan synthesis, thus fulfilling the prerequisite of being targets other than PBP.
One potential drawback of murA target is that the presence of two different genes-murA1 and murA2- encoding proteins with similar enzymatic activity. Both the genes should be mutated in order to cause bacterial death, but as both murA1 and murA2 has only 60% sequence similarity, the encoded enzymes has different active sites for which a unique murA-specific antibiotic development is difficult. Another potential drug target can be D-alanyl-alanine synthetase A (ddl), which catalyzes the synthesis of D-alanyl:D-alanine dipeptide-a prime building block in peptidoglycan biosynthesis and any disruption in this step would lead to a tender cell wall resulting in cell-death. Another essential protein is Undecaprenyl pyrophosphate phosphatase encoded by uppP. Bacteriocin and drugs of similar mechanism will not be suitable for targeting the S. aureus N315, as uppP is responsible for coding protein conferring resistance to bacteriocin [23].
Potential Drug targets from common metabolic pathways:
In this study, we have found essential metabolic non-human homolog proteins in energy metabolism pathways. In vitro and In vivo studies suggest that inhibitors designed for targeting these proteins can effectively hinder bacterial growth by bypassing the bacterial mutation site for drug resistance. The logic behind this is almost all existing antibiotics target only four pathways (cell wall synthesis, nucleic acid synthesis, protein synthesis, folate synthesis), thus repeated exposure to similar site increases the chance of mutation to the site, resulting in mutant bacteria [24, 25]. Targets of these pathways have shown their potential for anti-bacterial agents (e.g., Oligomycin, Antimycin A) , but not used in case of human, due to harmful effect both to human & bacteria. But the proteins revealed by our study can be considered as non-human homolog, thus reducing the baneful effect of the drug to human host [26].
Membrane-bound protein:
Our study revealed 15 membrane-bound proteins. Special attention was given to membrane proteins, as among all the drug targets- 60% is membrane proteins. Membrane proteins are also easier to study by computational method rather than experimental methods due to their uniform structure & interaction. The high tendency of proteins to construct secondary structure in turn lowers the complexity of the prediction of protein structure by computational method. If a high resolution structure is not found, computer-based structure prediction can be done with membrane protein easily & then structure-based drug prediction can done [13].
Conclusion
Our study working in several points of Staphylococcus aureus N315 genome reveals essential non-homolog proteins, 64 essential non-homolog metabolic proteins- among which the proteins of energy metabolism and 7 metabolic proteins in unique pathways, 15 essential non-homolog metabolic membrane-bound proteins- can be potential drug target. The drug would be specific for the pathogen and not lethal to the host as subtractive genomic approach is applied in this case. Molecular modeling of the targets will help to discover the best possible active sites that can be targeted for drug design. Virtual screening against these novel targets might be useful in the discovery of potential therapeutic agents against Staphylococcus aureus.
Supplementary material
Footnotes
Citation:Hossain et al, Bioinformation 9(4): 187-192 (2013)
References
- 1.Richards MJ, et al. Crit Care Med. 1999;27:887. doi: 10.1097/00003246-199905000-00020. [DOI] [PubMed] [Google Scholar]
- 2.Lowy FD, et al. J Clin Invest. 2003;111:1265. doi: 10.1172/JCI18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chambers HF, et al. Emerg Infect Dis. 2001;7:178. doi: 10.3201/eid0702.010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strausbaugh LJ, et al. Infect Control Hosp Epidemiol. 1996;17:129. doi: 10.1086/647257. [DOI] [PubMed] [Google Scholar]
- 5.Klevens RM, et al. Clin Infect Dis. 2006;42:389. doi: 10.1086/499367. [DOI] [PubMed] [Google Scholar]
- 6.Kuehnert MJ, et al. Emerg Infect Dis. 2005;11:868. doi: 10.3201/eid1106.040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laupland KB, et al. J Infect Dis. 2008;198:336. doi: 10.1086/589717. [DOI] [PubMed] [Google Scholar]
- 8.Hao H, et al. Mol Biosyst. 2012;8:2828. doi: 10.1039/c2mb25188d. [DOI] [PubMed] [Google Scholar]
- 9.Weber SG, et al. Emerg Infect Dis. 2003;9:1415. doi: 10.3201/eid0911.030284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsiodras S, et al. Lancet. 2001;358:207. doi: 10.1016/S0140-6736(01)05410-1. [DOI] [PubMed] [Google Scholar]
- 11.Sakharkar KR, et al. In Silico Biol. 2004;4:355. [PubMed] [Google Scholar]
- 12.Nathan C, et al. Nature. 2004;431:899. doi: 10.1038/431899a. [DOI] [PubMed] [Google Scholar]
- 13.Arinaminpathy Y, et al. Drug Discov Today. 2009;14:1130. doi: 10.1016/j.drudis.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang R, Lin Y. Nucleic Acids Res. 2009;37:D455. doi: 10.1093/nar/gkn858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. http://www.ncbi.nlm.nih.gov.
- 16.Kerfeld CA, et al. PloS Biol. 2011;9:e1001014. doi: 10.1371/journal.pbio.1001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. http://www.genome.jp/kaas-bin/kaas_main.
- 18.Moriya Y, et al. Nucleic Acids Res. 2007;35:W182. doi: 10.1093/nar/gkm321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. http://www.psort.org/psortb/
- 20. http://jing.cz3.nus.edu.sg/cgi-bin/svmprot.cgi.
- 21.Allsop AE, et al. Bioorg Med Chem Lett. 1995;5:443. [Google Scholar]
- 22.Gardy JL, et al. Nat Rev Microbiol. 2006;4:741. doi: 10.1038/nrmicro1494. [DOI] [PubMed] [Google Scholar]
- 23.Butt AM, et al. Plos One. 2012;7:e43080. doi: 10.1371/journal.pone.0043080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freiberg C, et al. Antimicrob Agents Chemother. 2006;50:2707. doi: 10.1128/AAC.00012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stermitz FR, et al. Proc Natl Acad Sci U SA. 2000;97:1433. doi: 10.1073/pnas.030540597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kagawa Y, et al. J Biol Chem. 1966;241:2461. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


