Abstract
The encounter between antigen-presenting cells and T cells is crucial for initiating immune responses to infectious microorganisms. In the spleen, interaction between dendritic cells (DC) and T cells occurs in the periarteriolar lymphoid sheath (PALS) into which DC and T cells migrate from the marginal zone (MZ) along chemokine gradients. However, the importance of DC migration from the MZ into the PALS for immune responses and host resistance to microbial infection has not yet been elucidated. Here we report that following Leishmania donovani infection of mice the migration of splenic DC is regulated by the CCR7 ligands CCL19 / CCL21. DC in plt/plt mutant mice which lack these chemokines are less activated and produce less IL-12, compared to those in wild type mice. Similar findings are seen when mice are treated with Pertussis toxin, which blocks chemokine signalling in vivo. plt/plt mice had increased susceptibility to L. donovani infection compared to wild type mice, as determined by spleen and liver parasite burden. Analysis of splenic cytokine profiles at day 14 post infection demonstrated that IFNγ and IL-4 mRNA accumulation was comparable in wild type and plt/plt mice. In contrast, accumulation of mRNA for IL-10 was elevated in plt/plt mice. In addition, plt/plt mice mounted a delayed hepatic granulomatous response and fewer effector T cells migrated into the liver. Taken together, we conclude that DC migration from the MZ to the PALS is necessary for full activation of DC and the optimal induction of protective immunity against L. donovani.
Keywords: Leishmania, chemokine, dendritic cells, cytokines, granulomas
Introduction
The encounter between antigen-bearing dendritic cells (DC6) and naïve T cells is one of the most important events for inducing immune responses (1). This event mainly occurs in the T cell area of secondary lymphoid organs, and localisation of both DC and T cells is regulated by chemokine signalling (2, 3). In the spleen, the chemokines which attract T and B cells are differentially expressed by stromal cells in the T or B cell zone, respectively. Stromal cells in the T cell area constitutively express both CCL19 (formerly ELC; EBI1-ligand chemokine) and the serine isoform of CCL21-Ser (formerly SLC; secondary lymphoid organ chemokine) (4). Both of these chemokines bind to CCR7, which is expressed on naïve/ central memory T cells and mature DC (5 - 7). Thus following gradients of these chemokines, both DC and T cells migrate into the T cell area where they interact and T cell priming occurs (8). The importance of chemokine-dependent migration is exemplified in mice with genetic deficiency in either chemokines or their receptors (9, 10). The paucity of lymph node T cell (plt) mutation, which arose as a spontaneous recessive mutation in mice, was recently mapped to the chemokine locus on chromosome 4 and results in loss of functional CCL19 and CCL21-Ser genes and in an aberrantly formed lymphoid T cell zone (11). As a consequence of lack of migration in response to these chemokines, plt/plt mice have impaired ability to recruit naïve T cells and DC into the T cell areas of secondary lymphoid organs (9, 10).
Infection of mice with amastigotes of Leishmania donovani is a well-established experimental model of visceral leishmaniasis (12). Injected amastigotes are mostly phagocytosed by macrophages in the marginal zone (MZ) of the spleen, and perhaps rarely by DC (13). We and other groups have previously shown that early production of IL-12, localised by immunochemistry to DC in the T cell area of the spleen (the periarteriolar lymphoid sheath; PALS), plays a key role in the innate and antigen-specific immune response against L. donovani (14, 15). IL-12 plays a key role in skewing naïve T cells into IFN-γ producing Th1 cells and IFN-γ is essential for the activation of macrophages and elimination of parasites (16, 17). These findings suggest that DC in the MZ, which either acquire parasites or parasite-derived antigens, are stimulated to produce IL-12p40 and IL-12p70, migrate into the PALS, and induce effector T cells. However, the sequence of such events, at which site IL-12 induction is initiated, which cytokines regulate DC migration in this context, and the requirement for DC migration in the priming of host protective T cells, all remain as unanswered questions.
To address some of these questions, we have now investigated the course of L. donovani infection using CCL19/21-deficient plt/plt mice. We demonstrate that plt/plt mice are more susceptible to L. donovani infection than normal mice. DC activation is limited early after infection, accompanied by lack of DC migration from the MZ to the PALS. These defects in early DC activation in plt/plt mice were mirrored by enhanced susceptibility to infection, elevated IL-10 mRNA accumulation and in the liver granuloma formation was delayed and effector CD4+ and CD8+ T cells recruitment limited. Taken together, these data indicate that chemokine-dependent encounters between DC and T cells are critical for optimal protection against L. donovani infection.
Materials and Methods
Mice and parasites
plt/plt mice backcrossed to the C57BL/6 (B6) background were provided from Dr. Hans Hengartner and Dr Tobias Junt (University of Zurich) and bred at the London School of Hygiene and Tropical Medicine under barrier conditions. B6 mice were purchased from Charles River (Margate, U.K.) and were housed under specific pathogen-free conditions. L. donovani (LV9) amastigotes were isolated from infected hamsters, as previously described (18). Mice were infected at 6-8 weeks of age by injecting 2 × 107 or 2 × 108 amastigotes i.v. via the lateral tail vain. Mice were killed by cervical dislocation and parasite burden in livers and spleens determined from Giemsa stained impression smears. Parasite burden was expressed in Leishman-Donovan units (LDU) (18). 500 ng of Pertussis toxin (PTX; List Biological Lab, Campell, CA) in saline was administrated by intraperitoneal (i.p.) injection twice, at one day and three days before L. donovani infection. All animal procedures were approved by the LSHTM Animal Procedures Ethics Committee and under licence from the U.K. Home Office.
Flow cytometry
Spleens were harvested and digested in RPMI 1640 (Gibco, Paisley, U.K.) containing 0.05% collagenase (Worthington Biochemical, Lakewood, NJ) and 100 μg/ml DNase I (Sigma, Dorset, U.K.) at 37 °C for 30 minutes. After washing with calcium-free medium, cells were stained for FITC-, PE-labelled, or biotinylated CD11c (HL3), I-Ab (M5/117 and 2G9), CD40 (3/23), CD80 (16-10A1), and CD86 (GL1), (BD PharMingen, San Jose, CA), followed by incubation with allophycocyanin-labelled streptavidin. Cells were analysed using a FACSCalibur (BD Biosciences, Mountain View, CA).
Immunohistochemistry
Immunohistochemistry was performed on 6 μm frozen sections as described before (19). Primary antibodies were purified or biotinylated HL3 (mouse CD11c), 53-6.7 (CD8α), RM4-5 (CD4), Gr-1 (RB6-8C5), (BD PharMingen), CI:A3-1(anti-F4/80), FA-11(CD68), 3D6.112 (anti-CD169), (Serotec, Oxford, UK), C17.8 (anti-IL-12p40), ER-TR9 (anti-SIGN related gene 1; SIGNR1; a gift from G Kraal, Free University, Amsterdam, the Netherlands), or hamster antisera to L. donovani amastigotes . Secondary antibodies were biotinylated rabbit anti-rat IgG (Vector Laboratories, Peterborough, UK), 10% mouse serum-containing goat anti-hamster IgG (for L. donovani; Vector Laboratories), biotinylated rabbit anti-rat Ig (for confocal microscope; DAKO Ltd., Ely, U.K.), Alexa 488-condugated Goat anti-hamster IgG, or Alexa 546-conjugated streptavidin (Molecular Probes, Leiden, Holland). As appropriate, sections were developed with Vector Elite-ABC kit followed by Vector 3-3′ diaminobenzidine substrate kit (Vector Laboratories), or directly viewed using a Zeiss LSM510 confocal microscope. In some experiments, mice were injected intravenously with 200 μl 5% (v/v in 0.9% NaCl) Indian ink (Rowney & Co., Brackwell, UK) to allow visualization of MZ macrophages in the spleen.
Restimulation assay
Spleens from mice infected for 7 days were digested with collagenase as described above. Dead cells and red blood cells were removed using a Histopaque 1083 (Sigma) density gradient. 2 × 105 spleen cells were incubated with or without 2 × 106 paraformaldehyde-fixed L. donovani amastigotes in 10% FCS (Sigma) containing RPMI medium in a 24-well plate at 37 °C for 72 hours. For proliferation assays, the cells were pulsed with 1 μCi of [3H] thymidine for final 6 hours of culture and then harvested onto glass fiber filters. The incorporated [3H] thymidine was determined by liquid scintillation spectrometry(20). Supernatants from the assays were collected after 72 hours incubation. IFN-γ specific ELISA was performed using IFN-γ ELISA Kit (R&D Systems, Abingdon, U. K.) as described previously (20).
Evaluation of granulomatous response
Paraffin-sections of liver tissue, stained with H&E, were prepared by conventional methods. In some cases, frozen sections of liver tissue were stained with hamster anti-L. donovani serum. The granuloma response in the infected livers was graded as follows: (1) an infected Kupffer cell without cellular infiltrate, (2) an immature granuloma composing an infected Kupffer cell surrounded by a few inflammatory cells but without organisation, (3) a mature granuloma having an organised structure, or (4) an empty granuloma, in which amastigotes had been killed as a result of effective antileishmanial immunity(21). At least 25 to 50 high magnification fields per mouse were counted and evaluated.
Real-time Reverse Transcription PCR
RNA was isolated from spleen tissue using an RNeasy Mini Kit with on-column DNase digestion (QIAGEN, Hilden, Germany), according to the manufacturers’ instructions. RNA was reverse transcribed into cDNA as described previously (22). Oligonucleotides (5′–3′) used for the specific amplification of were; IL-4 were CCTCACAGCAACGAAGAACA (sense), and TGGACTCATTCATGGTGCAG (antisense), IL-10 AGGGTTACTTGGGTTGCCAA (sense) and CACAGGGGAGAAATCGATGA (antisense), IL-12p40 GGAAGCACGGCAGCAGAATA (sense) and AACTTGAGGGAGAAGTAGGAATGG (antisense), IFNγ CCTCCTGCGGCCTAGCTC (sense), and TAACAGCCAGAAACAGCCATG (antisense), and for amplification of the house keeping gene hypoxanthine-guanine phosphoribosyl transferase (HPRT) were GTTGGATACAGGCCAGACTTTGTTG (sense) and GATTCAACCTTGCGCTCATCTTAGGC (antisense). The number of cytokines and HPRT cDNA molecules in each sample was calculated by real-time RT-PCR using QuantiTect SYBR green master mix (QIAGEN) and a ABI prism 7000 (Applied Biosystems, Warrington, Cheshire, U.K.), according to the manufacturers’ instructions. Standard curves were constructed with known amounts of cyokines and HPRT cDNA, and the number of cytokine molecules per 1,000 HPRT molecules in each sample was calculated.
Results
L. donovani infection in plt/plt mice
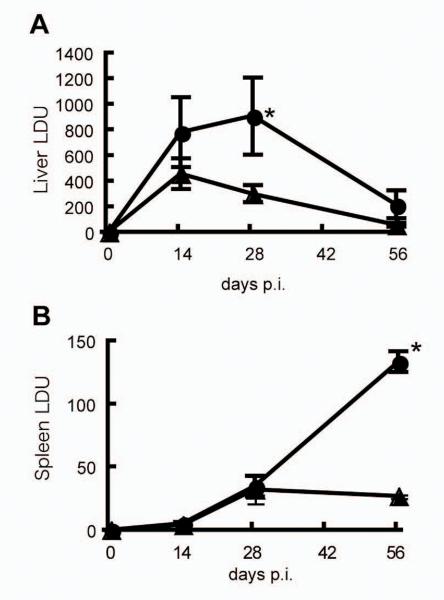
First, we determined the outcome of infection with L. donovani in plt/plt mice compared to wild type B6 mice. Fig. 1A shows the outcome of infection in the liver, an organ associated with acquisition of cell-mediated immunity to L. donovani and thus with the capacity to clear intracellular amastigotes (23). Compared to B6 mice, liver parasite burdens were significantly higher in plt/plt mice at day 28 p.i., and although these mice eventually were able to resolve hepatic infection, even at day 56, amastigotes numbers in the tissue remained somewhat higher than seen in B6 mice. In contrast to the curing response observed in the liver, the spleen is a site of parasite persistence, extending over the 56 day time period studied. Strikingly, although parasite numbers were equivalent in these two mouse strains at day 14 and day 28, plt/plt mice ultimately failed to exert control over parasite growth and at day 56 spleen parasite burden was approximately 5-fold higher in plt/plt mice than in B6 mice (Fig. 1B). These data indicate that plt/plt mice are more susceptible to L. donovani infection than B6 mice.
Figure 1. The course of L. donovani infection in B6 and plt/plt mice.
B6 (.) and plt/plt (.) mice were injected 2 × 107 L. donovani amastigotes and parasite burden was measured in the liver (A) or the spleen (B) at the live parts indicated. The data are expressed as mean ± SEM for 4 to 8 mice pooled from two experiments. * p<0.05.
Uptake of L. donovani amastigotes in the spleen of plt/plt mice
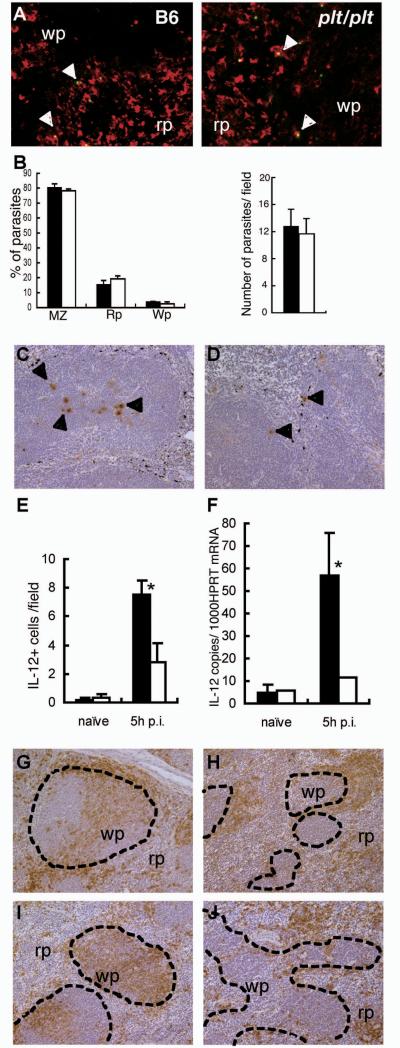
In the spleen, L. donovani were phagocytosed mainly by macrophages in the MZ (13). However, we have previously demonstrated that plt/plt mice are deficient in MZM, a highly phagocytic subset of macrophages in the MZ (40). In order to determine whether the lack of MZM influenced initial splenic infection in plt/plt mice, we determined the number and location of amastigotes in the spleen at 1h post infection, when blood clearance is essentially complete (13) . Double immunohistochemistry to identify amastigotes within subsets of splenic macrophages illustrated that CD68+ macrophages in the MZ but not F4/80+ red pulp macrophages are mainly responsible for uptake of L. donovani in plt/plt mice (Fig. 2A and data not shown). The localisation (Fig. 2B, left panel) and absolute number (Fig. 2B, right panel) of amastigotes in the spleen of plt/plt mice was not altered compared to wild type mice, with uptake being predominantly within the MZ in both strains. Thus, the deficiency of MZM in plt/plt mice does not compromise initial splenic infection and most amastigotes are taken up by alternate CD68+ macrophages in the MZ.
Figure 2. Distribution of parasite-infected macrophages, IL-12p40 production, and DC following L. donovani infection of B6 and plt/plt mice.
The distribution of L. donovani amastigotes (green: arrowheads) and CD68+ macrophages (red) in the spleens of B6 and plt/plt mice (A) at 1h p.i. Original magnification is x800. B. The distribution and number of parasites in B6 (closed bar) or plt/plt (open bar) mice was determined from multiple spleen sections of individual mice. Data are expressed as mean ± SEM for 3-5 mice per strain. The distribution of IL-12p40 producing cells in the spleens of B6 (C) and plt/plt (D) mice at 5h p.i. IL-12p40 (arrowhead) was visualized with immunohistochemistry (brown), and MZ macrophages were labeled with India ink (black). Original magnification is x200. E. The number of IL-12p40 producing cells in B6 (closed bar) or plt/plt (open bar) mice was determined. Data are expressed as mean ± SEM for 3-5 mice per strain* p<0.05. F. IL-12p40 mRNA was detected by real time RT-PCR in the spleen of naïve and 5 h-infected. B6 (closed bar) or plt/plt (open bar) mice.. The values are expressed with mean ± SEM for 3 to 5 mice * p<0.05. G-J. DC were stained using CD11c in naïve B6 (G) and plt/plt (H) and in B6 (I) and plt/plt (J) mice at 5h.p.i. Dotted lines indicated the border of the MZ. Original magnification is x200. Wp; white pulp, Rp; red pulp. Data are representative of one of three experiments.
Early IL-12p40 responses and migration of DC are impaired in plt/plt mice
The pattern of infection observed in plt/plt mice was remarkably similar to that previously described in studies in which IL-12p40 was targeted by neutralising antibodies (14) or in IL-12p40 deficient mice (15). As IL-12p40 is produced solely by dendritic cells in the early phase of immunity to L. donovani (13), we examined whether IL-12p40 responses were intact in plt/plt mice, using immunohistochemistry to identify both the number and localisation of cells producing this cytokine. IL-12p40+ cells were rarely observed in naïve mice of either strain. 5h after infection of B6 mice, IL-12p40+ cells were readily observed in the spleen, mainly localising, as previously described (13), to the deep periarteriolar region (Fig. 2C). In contrast, few IL-12p40+ cells were identified in the spleens of plt/plt mice, and those that were seen were localised in the MZ (Fig. 2D). To quantify this observation in more detail, we scored the number of IL-12p40+ cells in multiple tissue sections from multiple mice. As shown in Fig. 2E, the frequency of IL-12p40+ cells per white pulp section was significantly reduced in plt/plt mice compared to B6 mice. As an independent means of evaluating the IL-12p40 response, we used real time RT-PCR to determine the accumulation of IL-12p40 mRNA in spleen samples from naïve and infected B6 and plt/plt mice. This analysis confirmed at the level of mRNA expression, that the IL-12p40 response of plt/plt mice was indeed significantly reduced (Fig. 2F).
To examine whether altered localisation of IL-12 producing cells in plt/plt mice is associated with abnormal distribution of DC, we visualised DC in the spleen of B6 and plt/plt mice, with or without L. donovani infection. DC in non-infected B6 mice distributed mostly in the MZ, with a few found in the PALS as previously reported (24) (Fig. 2G). In contrast, DC in plt/plt mice were absent from the PALS and accumulated in the MZ and red pulp (9) (Fig. 2H). After 5hr of infection, most DC in B6 mice had migrated into the PALS (Fig. 2I) but the distribution of DC in plt/plt mice was not altered following infection (Fig. 2J). These data demonstrate that DC in plt/plt mice fail to migrate into the PALS and rather accumulate in the MZ.
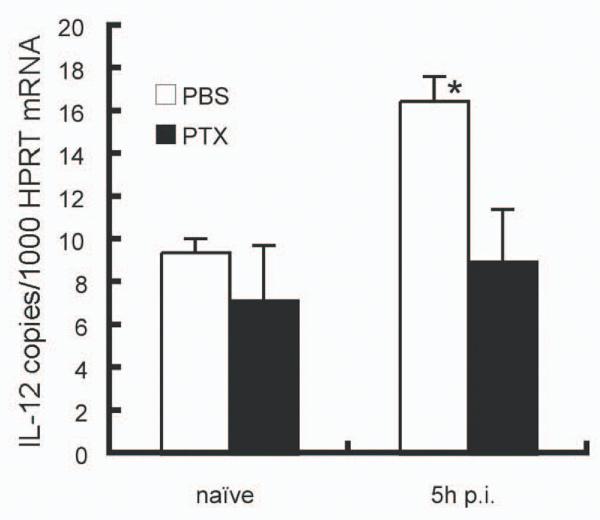
Impaired IL-12 production in PTX treated mice
To further define the importance of chemokine-dependent migration of DC in the introduction of IL-12 production, we treated B6 mice with PTX, which blocks chemokine signalling (25). DC in PTX treated mice failed to migrate from the MZ into the PALS after 5h of L. donovani infection, and showed a scattered distribution throughout the white pulp and red pulp (data not shown). As shown in Figure 3, IL-12 p40 mRNA accumulation did not increase in the spleen of infected B6 mice treated with PTX as compared with control infected mice. These findings further support the notion that chemokine signalling at the early stage of L. donovani infection is critical for DC migration and optimal IL-12 production from splenic DC.
Figure 3. IL-12p40 expression in L. donovani infected mice treated with PTX.
IL-12p40 mRNA accumulation was determined by real time RT-PCR in PBS-treated mice (open bar) or mice treated with 500 ng PTX at 1 day and 3days before infection (closed bar). The values are expressed with mean ± SEM for 3 to 5 mice. Data are representative of one of three experiments. * p<0.05.
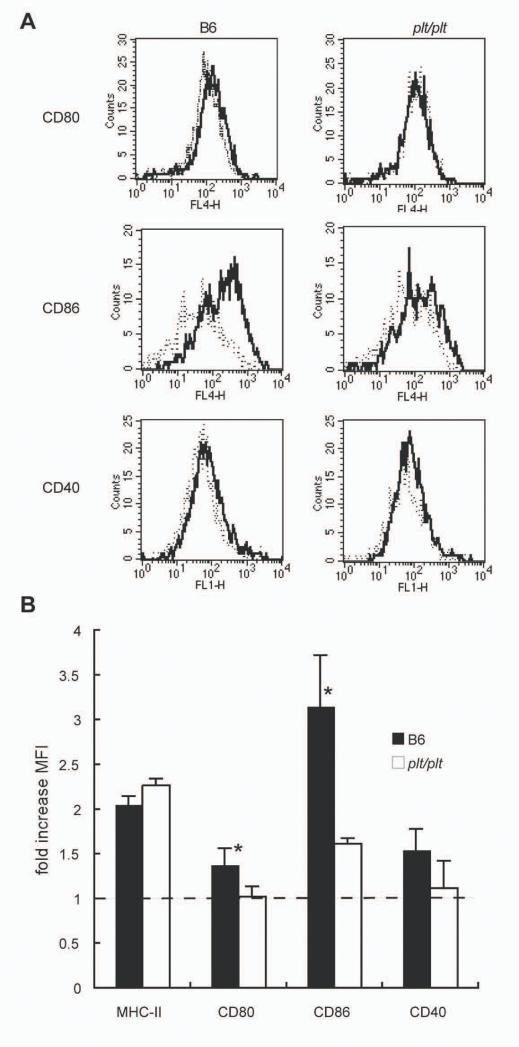
DC activation is impaired in plt/plt mice
IL-12p40 production is only one of a number of alterations that accompany the activation and maturation of splenic DC. To extend this analysis, we evaluated the expression of CD80, CD86 and CD40 on splenic CD11chiMHC-IIhi DC isolated from naïve and infected B6 or plt/plt mice. As shown in Fig. 4, CD11chiMHC-IIhi DC isolated from B6 mice 5h p.i. have slightly, but reproducibly increased levels of expression of CD80 and CD40 compared to naïve B6 mice. In contrast, CD86 shows a more significant response in these mice. DC in plt/plt mice were comparable in their expression of CD11c and MHC-II to those in B6 mice (data not shown, Fig. 4B). However, following infection, no alteration in expression of CD80 and CD40 was observed on DC from plt/plt mice. Further, although CD86 was upregulated, the increase was relatively weak compared to that observed in B6 mice (Fig 4B). Thus, by various criteria, DC in plt/plt mice appear to be muted in their response to L. donovani infection compared to those in B6 mice.
Figure 4. DC activation during early L. donovani infection of B6 and plt/plt mice.
(A) Spleen DC from B6 (left panels) and plt/plt (right panels) were identified as CD11c+ MHC Class IIhi and then analysed for expression of CD80 (top), CD86 (middle) and CD40 (bottom). Histograms show representative staining of naïve (dotted line) and 5hr-infected (solid line) mice. Data are representative of 3 mice from 3 independent experiments. (B) Mean fluorescence intensities (MFI) of MHC-II, CD80, CD86 and CD40 on splenic DC from infected B6 (solid bar) and plt/plt (open bar) mice. Data are shown as fold-increase over DC in naïve mice. The values are expressed with mean ± SEM for 3 independent experiments. * p<0.05.
Immune deviation in plt/plt mice
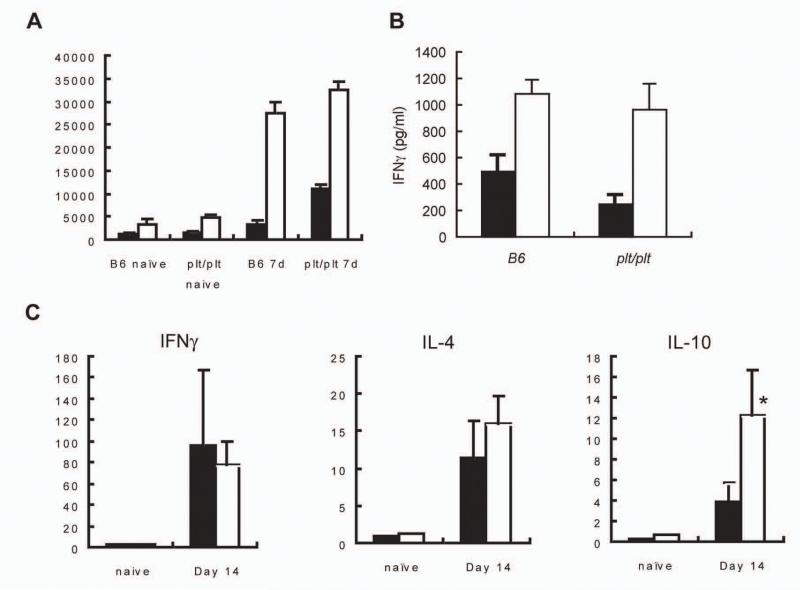
To evaluate whether the restricted activation of DC observed in plt/plt mice was translated into defective T cell priming (26), we isolated spleen cells from naïve and infected B6 and plt/plt mice and restimulated them with L. donovani antigens in vitro. We observed that both the proliferative response (Fig. 5A) and the antigen dependent production of IFNγ (Fig. 5B) were comparable in these two mouse strains. To extend this analysis to other cytokines and to determine without any in vitro bias whether any form of immune deviation had occurred in these mice, we used real time RT-PCR to evaluate the accumulation of mRNA for IFNγ, IL-4 and IL-10 in naïve and infected B6 and plt/plt mice. At day 14 p.i., the level of IFNγ mRNA accumulation was identical in both strains (Fig. 5C) mirroring the data obtained from in vitro cultured spleen cells. IL-4 has also been shown to be co-expressed at the early stages of L. donovani infection (27) and although IL-4 mRNA was detected in infected mice, no difference was observed between B6 and plt/plt mice (Fig.5D). In contrast, when we measured IL-10 mRNA accumulation, it was evident that expression of this cytokine was significantly enhanced in plt/plt mice compared to B6 mice (Fig. 5E). Thus plt/plt mice show immune deviation towards production of a cytokine with known ability to inhibit anti-leishmanial immunity (28).
Figure 5. Cytokine responses in L. donovani-infected B6 and plt/plt mice.
(A) Spleen cells from naïve and d7 infected B6 and plt/plt mice were cultured for 72hr in the absence (black bars) or presence (open bars) of L. donovani amastigote antigen. Proliferation was determined by scintillation counting. (B) IFN was determined in culture dependents from restructured B6 and plt/plt mice in the absence (black bars) or presence (open bars) of L. donovani amastigote antigen. (C-E) mRNA was extracted from the spleens of naïve or day 14 p.i. B6 (dark hatch) or plt/plt (light hatch) mice and accumulation of IFNγ (C), IL-4 (D), and IL-10 (E) mRNA was detected by real time RT-PCR. * p<0.05.
Delayed hepatic granuloma formation in plt/plt mice
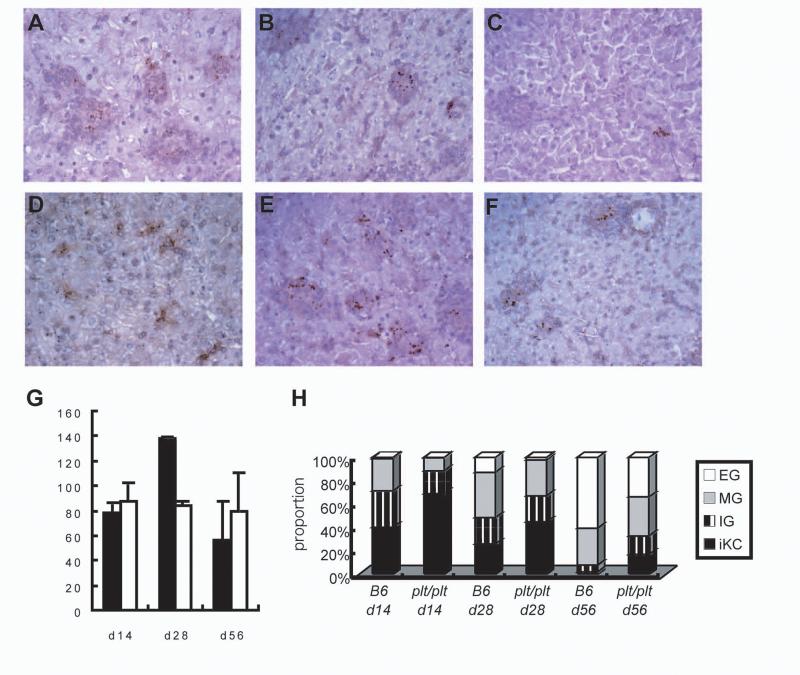
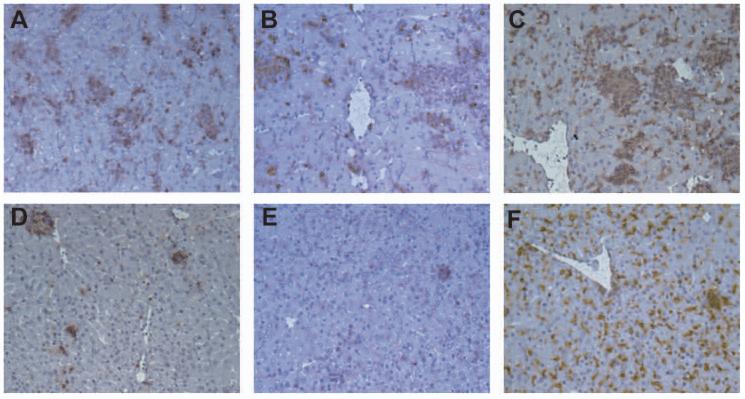
Expression of immunity to L. donovani is most evident in the liver and studies with asplenic mice indicate that T cell priming and differentiation to effector T cells in the spleen contributes significantly to the hepatic immune response (Engwerda et al. unpublished). We therefore wished to determine whether potential changes to T cell priming in the spleen of plt/plt mice was translated into reduced hepatic immunity as expressed by granuloma function. Tissue sections from the livers of infected B6 and plt/plt mice were evaluated throughout the time course of infection and granuloma maturation was quantitated. As shown in Fig 6, granuloma maturation was significantly retarded in plt/plt mice compared to B6 mice. Although amastigotes were abundant in the liver of both strains of mice at day 14 p.i. (Fig. 6A and D and Fig. 1), in plt/plt mice there was minimal initiation of a granulomatous response around infected Kupffer cells (Fig. 6D). At day 28 p.i., granuloma formation was detected in both B6 and plt/plt mice (Fig. 6B and E) and by day 56, empty granulomas were observed in B6 mice but less frequently in plt/plt mice (Fig. 6 C and F). Although absolute number of inflammatory foci was similar (Fig 6G) the delay in maturation was observed throughout the time course studied and at day 56 p.i., almost 20% of infected foci in plt/plt mice had still failed to generate a significant histologic response (Fig. 6H). Immunohistochemistry indicated that the defect in granuloma formation was associated with a lack of infiltration of both CD4+ and CD8+ T cells, whereas the accumulation of F4/80+ macrophages was comparable (Fig. 7). These data suggest therefore that although IFNγ production is equivalent in B6 and plt/plt mice, this response fails to efficiently drive granuloma maturation in plt/plt mice, possibly as a consequence of the elevated IL-10 response.
Figure 6. Granuloma formation in the L. donovani infected livers of B6 and plt/plt mice.
Liver sections at day 14 p.i. (A, D), day 28 p.i. (B, E), and day 56 p.i. (C, F), in B6 (A, B, C) or plt/plt (D, E, F) mice were stained with anti-L. donovani sera. Original magnification is x400. (G.) The number of hepatic granulomas in infected B6 (closed bar) or plt/plt (open bar) mice at each time point. (H) Granuloma maturation during L. donovani infection of B6 or plt/plt mice. Data represent the frequency of infected Kupffer cells (iKC), immature granulomas (IG), mature granulomas (MG), and empty granulomas (EG) per mouse at each time point.
Figure 7. Hepatic T cell and macrophage recruitment during L. donovani infection of B6 and plt/plt mice.
Liver sections from B6 (A, B, C) or plt/plt (D, E, F) mice at day 14 p.i were stained for CD4 (A, D), CD8α (B, E), and F4/80 (C, F). Original magnification is x200. Data are representative of one of three experiments.
Discussion
DC are antigen-presenting cells which localise at peripheral tissues throughout the body and migrate into the T cell areas of secondary lymphoid organs for presentation of antigen to T cells. Therefore, migration of DC is thought to be one of the most important events for the induction of protective immunity to pathogens. In the spleen, most blood-borne pathogens are trapped within the MZ, where macrophages and DC are abundantly distributed (29). DC in the MZ, activated following intravenous infection of lipopolysaccharide (LPS) or soluble Toxoplasma antigen (STAg), rapidly move to the T cell area and form tight clusters with T cells (30, 31). These movements of DC are tightly controlled by chemokines and the regulation of chemokine receptor expression. For example, DC migration after LPS administration was not seen in plt/plt mice (9), and injection of STAg into CCR5 deficient mice failed to recruit DC into the PALS (32). Other animal studies also showed that the distribution of DC in the spleen was altered when they were infected with malaria (33), Toxoplasma(34), Salmonella (35), or lymphocytic choriomeningitis virus (LCMV) (36). However, none of these studies with live infectious agents have defined which chemokines are responsible for the observed migration of DC, how altered migration affects the functional behaviour of these DC, or the consequences for host protection.
In a mouse model of visceral leishmaniasis, we and other groups have demonstrated previously that macrophages in the MZ of the spleen phagocytosed the majority of parasites in the first hours after injection (13, 37), whereas IL-12-producing DC were observed deep in the T cell area of the spleen (13). These findings strongly suggested that either a small proportion of infected DC, or DC that had captured parasite-derived antigens in the MZ, migrated to the PALS where they could encounter T cells for priming host protective immune responses. Here we have demonstrated that the chemokines CCL19 / 21 are necessary for the migration of DC in this early phase of L. donovani infection. Many aspects of DC migration and activation in Leishmania infection appear different from that induced by LPS or STAg, however. In contrast to LPS or STAg administration, L. donovani infection induces migration of only a fraction of the DC population and clustering of DC in the PALS is not as evident as with these other stimuli. Whereas DC in wild type mice up-regulated both MHC class II and costimulatory molecules homogeneously at 5 h p.i., DC from plt/plt mice can upregulate MHC Class II to a similar extent but a notably poor response was observed in terms of CD86 expression (Fig. 2). Another study of L. donovani infection using MyD88 deficient mice, which lack this common signalling pathway of Toll-like receptors (TLR), has indicated that activation of DC was severely impaired but that migration from the MZ to the PALS was not affected by loss of TLR signalling (38). These facts suggest there are at least two different stimuli acting on DC in L. donovani infection, which differentially effect DC migration and activation phenotype.
In plt/plt mice although IL-12 producing cells were seen after 5h of infection, the total amount of IL-12p40 mRNA was markedly decreased compared to that induced in B6 mice. In addition, all IL-12p40-producing DC were localised at the MZ in plt/plt mice. These findings indicate that partially activated DC can produce only small amounts of IL-12p40, whereas DC that have migrated into the PALS may become more fully activated and produce large amounts of IL-12p40. Although not studied here, this result may also imply that IL-23, which utilises the IL-12p40 submit (39) may have differential expression in the MZ and PALS. Although plt/plt mice are deficient in MZM (40), our data demonstrates that CD68+ macrophages in the MZ substitute as the main phagocytes for amastigotes in plt/plt mice (Fig. 2) and also suggests that reduced parasite uptake in the spleen does not underlie the defect in DC IL-12p40 production seen in these mice. Animals rendered MZM-deficient by clodronate treatment (data not shown), and mice treated with PTX, an inhibitor of chemokine receptor signalling, also showed markedly decreased IL-12 production from DC at 5 h p.i. (Fig. 3). Thus, DC may be unable to become fully activated until they encounter CD4+ T cells in T cell area. The precise molecular interactions involved in this cell-cell cooperation remain to be elucidated.
In our previous studies, IL-12 production in the early period after infection was shown to determine the course of infection in the spleen and it is striking that the plt/plt mice appear similar to B6 mice in which IL-12 has been neutralised and to B6 IL-12−/− deficient mice (14, 15). The data reported here might, therefore, suggest that the increased susceptibility of plt/plt mice is a direct consequence of the lack of IL-12 production early after infection. The relative importance of IL-12 in determining disease outcome could thus explain why there are discrepancies between the responses of plt/plt mice to Leishmania (this report), murine hepatitis virus (9) and LCMV infection (42) . IL-12 is not required for induction of protective immunity for LCMV (43) and expansion of antigen-specific effector CD8+ T cells in LCMV-infected plt/plt mice is comparable to that of wild type mice. LCMV can also directly activate DC (36), whereas, amastigotes of L. donovani infect very few DC in vivo (13) and DC migration is necessary to achieve full activation (Fig. 2 and 4). Furthermore, loss of CCR7-depending migration of DC is one of the critical factors associated with the chronic stage of L. donovani infection (44), whereas this event is less important for the host protective response agent LCMV infection (42). Alternatively, reduced IL-12 production may merely serve as another indicator of poor activation of DC in plt/plt mice, since our data do not directly address whether decreased IL-12 production is the most significant defect in DC activation, in terms of the ultimately lowered resistance of plt/plt mice to L. donovani infection. Surprisingly, in preliminary experiments we have been unable to enhance resistance in either plt/plt mice or wild type mice by a single administration of 1μg rIL-12 at 5h post infection (Ato et. al. unpublished). These data contrast with the host protective effects of sustained (7d) administration of rIL-12 as reported by others ( 41), suggesting that either IL-12 produced at later times during infection is also important for host resistance, or that other defects in DC activation in plt/plt mice, as noted above, play an important role in determining the outcome of infection.
Given the above findings, it was also surprising to observe that the induction of IFNγ and IL-4, key cytokines involved in optimal host resistance and granuloma development following L. donovani infection(16, 45), was similar in both wild type and plt/plt mice. Thus, the defects reported here in plt/plt mice clearly not block T cell priming completely, as anticipated from other studies (46). In contrast, the accumulation of mRNA for IL-10, a cytokine with notable inhibitory effects on host resistance (28, 47), was increased in plt/plt compared to wild-type mice. IL-10 has multiple cellular sources during active infection with L. donovani, with IL-10 mRNA being most abundant (on a cell per cell basis) in CD4+ T cells, NK cells, DC and macrophages, and to a lesser extent in CD8+ T cells, B cells and neutrophils (Maroof et.al. unpublished). However, the broad expression of IL-10, difficulties associated with identifying IL-10-producing cells directly ex vivo, and a similarly wide cellular distribution of IL-10R expression, together pose significant challenges for identifying functionally relevant cellular interactions mediated through IL-10. These are only likely to addressable in vivo by the future development of mice allowing cell-specific and regulated targeting of IL-10 and its receptor.
Resolution of hepatic infection is dependent on granuloma formation, in which various effector cells are recruited and produce cytokines. Both CD4+ and CD8+ T cells are required to induce granuloma formation in the liver (21). plt/plt mice have delayed granulomatous responses and an associated reduction in the recruitment of effector T cells. The lack of T cell recruitment is unlikely to be due to a lack of CCL19 /21 in the liver, because CCL21 is detected in afferent lymphatics in the liver of plt/plt mice to the same extent as in the livers of wild mice (data not shown, (11)). Rather, our data suggest that this lack of granuloma maturation is directly linked either to the altered functional development of effector CD4+ and/or CD8+ T cells, or their homing potential in an IL-10-rich environment. These speculations may be supported by the fact that IL-10 suppresses granuloma formation and recruitment of effector cells to the infected liver, independently of IFNγ production (47).
In conclusion, plt/plt mice have a deficiency in DC activation resulting from impaired CCL21/CCL19-dependent migration of DC in these mice which, most likely acting in concert with established defects in T cell migration (9), leads to a demonstrable increase in susceptibility to L. donovani infection. This study, therefore, reveals for the first time the potential link between migration-dependent DC activation and protection against L. donovani infection, substantiating the importance of an appropriate chemokine environment for the generation and expression of optimal host protective immune responses.
Acknowledgments
We thank the staff of the Biological Services Facility for assistance in the breeding and maintenance of mouse colonies.
Footnotes
This work was supported by The Wellcome Trust and the British Medical Research Council. M.A. was a recipient of a Wellcome Trust International Travelling Fellow.
Abbreviations used in this paper: DC, dendritic cells; MZ, marginal zone; PALS, periarteriolar lymphoid sheaths; PTX, pertussis toxin; LDU, Leishman-Donovan unit; p.i., post-infection; LPS, lipopolysaccharide, STAg. soluble Toxoplasma antigen; LCMV, lymphocytic choriomeningitis virus; TLR, Toll-like receptor..
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Cyster JG. Leukocyte migration: scent of the T zone. Curr Biol. 2000;10:R30–33. doi: 10.1016/s0960-9822(99)00253-5. [DOI] [PubMed] [Google Scholar]
- 3.Campbell DJ, Kim CH, Butcher EC. Chemokines in the systemic organization of immunity. Immunol Rev. 2003;195:58–71. doi: 10.1034/j.1600-065x.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 4.Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci U S A. 2000;97:12694–9. doi: 10.1073/pnas.97.23.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ngo VN, Tang HL, Cyster JG. Epstein-Barr virus-induced molecule 1 ligand chemokine is expressed by dendritic cells in lymphoid tissues and strongly attracts naive T cells and activated B cells. J Exp Med. 1998;188:181–91. doi: 10.1084/jem.188.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 7.Kellermann SA, Hudak S, Oldham ER, Liu YJ, McEvoy LM. The CC chemokine receptor-7 ligands 6Ckine and macrophage inflammatory protein-3 beta are potent chemoattractants for in vitro- and in vivo-derived dendritic cells. J Immunol. 1999;162:3859–64. [PubMed] [Google Scholar]
- 8.Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Ait-Yahia S, Briere F, Zlotnik A, Lebecque S, Caux C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–86. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunn MD, Kyuwa S, Tam C, Kakiuchi T, Matsuzawa A, Williams LT, Nakano H. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J Exp Med. 1999;189:451–60. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 11.Nakano H, Gunn MD. Gene duplications at the chemokine locus on mouse chromosome 4: multiple strain-specific haplotypes and the deletion of secondary lymphoid-organ chemokine and EBI-1 ligand chemokine genes in the plt mutation. J Immunol. 2001;166:361–9. doi: 10.4049/jimmunol.166.1.361. [DOI] [PubMed] [Google Scholar]
- 12.Engwerda CR, Kaye PM. Organ-specific immune responses associated with infectious disease. Immunol Today. 2000;21:73–8. doi: 10.1016/s0167-5699(99)01549-2. [DOI] [PubMed] [Google Scholar]
- 13.Gorak PM, Engwerda CR, Kaye PM. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. Eur J Immunol. 1998;28:687–95. doi: 10.1002/(SICI)1521-4141(199802)28:02<687::AID-IMMU687>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 14.Engwerda CR, Murphy ML, Cotterell SE, Smelt SC, Kaye PM. Neutralization of IL-12 demonstrates the existence of discrete organ-specific phases in the control of Leishmania donovani. Eur J Immunol. 1998;28:669–80. doi: 10.1002/(SICI)1521-4141(199802)28:02<669::AID-IMMU669>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 15.Satoskar AR, Rodig S, Telford SR, 3rd, Satoskar AA, Ghosh SK, von Lichtenberg F, David JR. IL-12 gene-deficient C57BL/6 mice are susceptible to Leishmania donovani but have diminished hepatic immunopathology. Eur J Immunol. 2000;30:834–9. doi: 10.1002/1521-4141(200003)30:3<834::AID-IMMU834>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 16.Squires KE, Schreiber RD, McElrath MJ, Rubin BY, Anderson SL, Murray HW. Experimental visceral leishmaniasis: role of endogenous IFN-gamma in host defense and tissue granulomatous response. J Immunol. 1989;143:4244–9. [PubMed] [Google Scholar]
- 17.Taylor AP, Murray HW. Intracellular antimicrobial activity in the absence of interferon-gamma: effect of interleukin-12 in experimental visceral leishmaniasis in interferon-gamma gene-disrupted mice. J Exp Med. 1997;185:1231–9. doi: 10.1084/jem.185.7.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smelt SC, Engwerda CR, McCrossen M, Kaye PM. Destruction of follicular dendritic cells during chronic visceral leishmaniasis. J Immunol. 1997;158:3813–21. [PubMed] [Google Scholar]
- 19.Engwerda CR, Ato M, Stager S, Alexander CE, Stanley AC, Kaye PM. Distinct roles for lymphotoxin-alpha and tumor necrosis factor in the control of Leishmania donovani infection. Am J Pathol. 2004;165:2123–33. doi: 10.1016/s0002-9440(10)63262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stager S, Smith DF, Kaye PM. Immunization with a recombinant stage-regulated surface protein from Leishmania donovani induces protection against visceral leishmaniasis. J Immunol. 2000;165:7064–71. doi: 10.4049/jimmunol.165.12.7064. [DOI] [PubMed] [Google Scholar]
- 21.Murray HW. Tissue granuloma structure-function in experimental visceral leishmaniasis. Int J Exp Pathol. 2001;82:249–67. doi: 10.1046/j.1365-2613.2001.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svensson M, Maroof A, Ato M, Kaye PM. Stromal cells direct local differentiation of regulatory dendritic cells. Immunity. 2004;21:805–16. doi: 10.1016/j.immuni.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Engwerda CR, Ato M, Kaye PM. Macrophages, pathology and parasite persistence in experimental visceral leishmaniasis. Trends Parasitol. 2004;20:524–30. doi: 10.1016/j.pt.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Leenen PJ, Voerman JS, Radosevic K, van Rooijen N, van Ewijk W. Mouse spleen dendritic cells. Phagocytic activity and expression of macrophage markers. Adv Exp Med Biol. 1997;417:91–5. [PubMed] [Google Scholar]
- 25.Cyster JG, Goodnow CC. Pertussis toxin inhibits migration of B and T lymphocytes into splenic white pulp cords. J Exp Med. 1995;182:581–6. doi: 10.1084/jem.182.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:13–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 27.Stager S, Alexander J, Kirby AC, Botto M, Rooijen NV, Smith DF, Brombacher F, Kaye PM. Natural antibodies and complement are endogenous adjuvants for vaccine-induced CD8+ T-cell responses. Nat Med. 2003;9:1287–92. doi: 10.1038/nm933. [DOI] [PubMed] [Google Scholar]
- 28.Murphy ML, Wille U, Villegas EN, Hunter CA, Farrell JP. IL-10 mediates susceptibility to Leishmania donovani infection. Eur J Immunol. 2001;31:2848–56. doi: 10.1002/1521-4141(2001010)31:10<2848::aid-immu2848>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 29.Kraal G. Cells in the marginal zone of the spleen. Int Rev Cytol. 1992;132:31–74. doi: 10.1016/s0074-7696(08)62453-5. [DOI] [PubMed] [Google Scholar]
- 30.De Smedt T, Pajak B, Muraille E, Lespagnard L, Heinen E, De Baetselier P, Urbain J, Leo O, Moser M. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J Exp Med. 1996;184:1413–24. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reis e Sousa C, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186:1819–29. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aliberti J, Reis e Sousa C, Schito M, Hieny S, Wells T, Huffnagle GB, Sher A. CCR5 provides a signal for microbial induced production of IL-12 by CD8 alpha+ dendritic cells. Nat Immunol. 2000;1:83–7. doi: 10.1038/76957. [DOI] [PubMed] [Google Scholar]
- 33.Leisewitz AL, Rockett KA, Gumede B, Jones M, Urban B, Kwiatkowski DP. Response of the splenic dendritic cell population to malaria infection. Infect Immun. 2004;72:4233–9. doi: 10.1128/IAI.72.7.4233-4239.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaussabel D, Pajak B, Vercruysse V, Bisseye C, Garze V, Habib M, Goldman M, Moser M, Vray B. Alteration of migration and maturation of dendritic cells and T-cell depletion in the course of experimental Trypanosoma cruzi infection. Lab Invest. 2003;83:1373–82. doi: 10.1097/01.lab.0000087587.93781.6f. [DOI] [PubMed] [Google Scholar]
- 35.Kirby AC, Yrlid U, Svensson M, Wick MJ. Differential involvement of dendritic cell subsets during acute Salmonella infection. J Immunol. 2001;166:6802–11. doi: 10.4049/jimmunol.166.11.6802. [DOI] [PubMed] [Google Scholar]
- 36.Sevilla N, Kunz S, McGavern D, Oldstone MB. Infection of dendritic cells by lymphocytic choriomeningitis virus. Curr Top Microbiol Immunol. 2003;276:125–44. doi: 10.1007/978-3-662-06508-2_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melby PC, Tabares A, Restrepo BI, Cardona AE, McGuff HS, Teale JM. Leishmania donovani: evolution and architecture of the splenic cellular immune response related to control of infection. Exp Parasitol. 2001;99:1–25. doi: 10.1006/expr.2001.4640. [DOI] [PubMed] [Google Scholar]
- 38.De Trez C, Brait M, Leo O, Aebischer T, Torrentera FA, Carlier Y, Muraille E. Myd88-dependent in vivo maturation of splenic dendritic cells induced by Leishmania donovani and other Leishmania species. Infect Immun. 2004;72:824–32. doi: 10.1128/IAI.72.2.824-832.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–25. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 40.Ato M, Nakano H, Kakiuchi T, Kaye PM. Localization of marginal zone macrophages is regulated by C-C chemokine ligands 21/19. J Immunol. 2004;173:4815–20. doi: 10.4049/jimmunol.173.8.4815. [DOI] [PubMed] [Google Scholar]
- 41.Murray HW, Haraprishad J. Interleukin 12 is effective treatment for an established systemic intracellular infection: expereimental visceral leishmaniasis. J. Exp. Med. 1995;181:387–91. doi: 10.1084/jem.181.1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Junt T, Nakano H, Dumrese T, Kakiuchi T, Odermatt B, Zinkernagel RM, Hengartner H, Ludewig B. Antiviral immune responses in the absence of organized lymphoid T cell zones in plt/plt mice. J Immunol. 2002;168:6032–40. doi: 10.4049/jimmunol.168.12.6032. [DOI] [PubMed] [Google Scholar]
- 43.Oxenius A, Karrer U, Zinkernagel RM, Hengartner H. IL-12 is not required for induction of type 1 cytokine responses in viral infections. J Immunol. 1999;162:965–73. [PubMed] [Google Scholar]
- 44.Ato M, Stager S, Engwerda CR, Kaye PM. Defective CCR7 expression on dendritic cells contributes to the development of visceral leishmaniasis. Nat Immunol. 2002;3:1185–91. doi: 10.1038/ni861. [DOI] [PubMed] [Google Scholar]
- 45.Stager S, Alexander J, Carter KC, Brombacher F, Kaye PM. Both interleukin-4 (IL_4) and IL-4 receptor α signalling contribute to the development of hepatic granulomas with optimal antileishmanial activity. Infect. Immun. 2003;71:4804–7. doi: 10.1128/IAI.71.8.4804-4807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mori S, Nakano H, Aritomi K, Wang CR, Gunn MD, Kakiuchi T. Mice lacking expression of the chemokines CCL21-ser and CCL19 (plt mice) demonstrate delayed but enhanced T cell immune responses. J Exp Med. 2001;193:207–18. doi: 10.1084/jem.193.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murray HW, Moreira AL, Lu CM, DeVecchio JL, Matsuhashi M, Ma X, Heinzel FP. Determinants of response to interleukin-10 receptor blockade immunotherapy in experimental visceral leishmaniasis. J Infect Dis. 2003;188:458–64. doi: 10.1086/376510. [DOI] [PubMed] [Google Scholar]